SUMMARY
Protein restriction (PR) is important for the benefits of dietary restriction on longevity and stress resistance, but relevant nutrient sensors and downstream effectors in mammals remain poorly defined. We used PR-mediated protection from hepatic ischemia reperfusion injury to probe genetic requirements for evolutionarily conserved nutrient sensors GCN2 and mTORC1 in stress resistance. One week of PR reduced free amino acids and circulating growth factors, activating GCN2 and mTORC1 repressor TSC complex. However, while GCN2 was dispensable for PR-induced protection, hepatic TSC1 was required. PR improved hepatic insulin sensitivity in a TSC1-dependent manner prior to ischemia, facilitating increased pro-survival signaling and reduced apoptosis after reperfusion. These benefits were partially abrogated by pharmacological PI3K inhibition or genetic deletion of the insulin receptor in hepatocytes. In conclusion, improved insulin sensitivity upon short-term PR required TSC1, facilitated increased pro-survival signaling after injury, and contributed partially to PR-mediated resistance to clinically relevant ischemia reperfusion injury.
INTRODUCTION
Dietary restriction (DR) is loosely defined as reduced food intake without malnutrition and refers to a variety of dietary interventions including both simple reduction of calorie intake and restriction of specific macronutrients. Beneficial health effects of DR were reported in the 1930s, when DR was shown to extend longevity in rats (McCay et al., 1935). DR has since been shown to increase maximal and average lifespan in multiple species including yeasts, worms, flies, fish and rodents (Fontana et al., 2010). In addition to the metabolic and cardiovascular improvements seen in mammals, DR also improves resistance to a variety of stressors such as chemotherapeutic agents (Cheng et al., 2014; Raffaghello et al., 2008) and ischemia reperfusion injury (IRI) to the kidney, liver, heart and the brain (Mitchell et al., 2010; Robertson and Mitchell, 2013; Varendi et al., 2014).
Restriction of calories from any macronutrient source - proteins, lipids or carbohydrates – can result in DR benefits, including lifespan extension. Therefore, the term DR has been used interchangeably with caloric restriction (CR). However, accumulating evidence suggests that specific macronutrients – namely amino acids – play a role in DR benefits beyond their caloric value (Levine et al., 2014; Piper et al., 2011; Solon-Biet et al., 2014). Numerous studies in rodents have shown that total protein restriction and restriction of individual essential amino acids (EAA) tryptophan and methionine increase longevity in rats and mice (Gallinetti et al., 2013). In addition to longevity extension, dietary protein or individual EAA restriction can both mediate other DR-like effects, including improved insulin sensitivity upon leucine restriction (Xiao et al., 2011) and protection against IRI in liver and kidney upon tryptophan restriction (Peng et al., 2012). While EAA, protein, and total calorie restriction induce shared phenotypes, including decreased adiposity, decreased circulating growth hormones (insulin, IGF-1) and improved insulin sensitivity, whether or not these are common effectors of associated benefits remains to be tested.
Two evolutionarily conserved signal transduction pathways sense amino acids: general control nonderepressible 2 (GCN2) and mechanistic target of rapamycin complex 1 (mTORC1). GCN2 senses the absence of any individual amino acid via binding to uncharged cognate tRNAs and activates the amino acid starvation response by phosphorylating Ser51 of the translation initiation factor eIF2α. This results in global translational suppression together with translational derepression of specific mRNAs such as Atf4 (Gallinetti et al., 2013). GCN2 is required for mediating the beneficial effects of short-term individual EAA deficiency against hepatic and renal IRI (Peng et al., 2012).
mTORC1 is a complex of the serine/threonine kinase mTOR, Raptor, and mLst8 as core essential components and integrates various growth stimulating signals including amino acids, energy and growth factors (Dibble and Manning, 2013). Intracellular amino acids, and in particular the branched chain amino acid leucine, are sensed by mTORC1 via an upstream mechanism involving the Rag GTPases (Sancak et al., 2008) and a regulatory complex referred to as the Ragulator (Efeyan et al., 2012). Leucine sufficiency allows recruitment of mTORC1 to the lysosomal surface via the Ragulator. There, mTORC1 can be activated by the small GTPase Rheb, which in turn is negatively regulated by the GTPase-activating protein (GAP) tuberous sclerosis complex 2 (TSC2), which functions in a complex with TSC1 and TBC1D7 (the TSC complex) (Dibble et al., 2012; Huang and Manning, 2008). The TSC complex is a critical negative regulator of mTORC1 that integrates signals from energy levels and growth and endocrine factors, such as insulin. Although growth factor-based TSC-dependent inhibition of mTORC1 is experimentally separable from amino acid-based Ragulator-dependent inhibition in vitro (Menon et al., 2014), whether the TSC complex plays a role in controlling mTORC1 in response to dietary protein restriction in vivo remains unknown.
Inhibition of mTORC1 (Harrison et al., 2009; Lamming et al., 2012; Miller et al., 2011; Miller et al., 2013; Wu et al., 2013) or its direct target S6K (Selman et al., 2009), has been implicated in DR-like benefits including lifespan and healthspan extension; however, the requirement for mTORC1 inhibition in DR-mediated stress resistance, and the potential role of the TSC complex, remain unknown. Although S. cerevisiae and C. elegans lack TSC homologs, overexpression of TSC1 and TSC2 in Drosophila extends longevity, consistent with a beneficial role of reduced mTORC1 signaling (Kapahi et al., 2004). A protective role of the TSC complex via autophagy induction in an mTORC1-dependent manner has also been suggested in a recent study of neuroprotection against ischemia (Papadakis et al., 2013).
Ischemia reperfusion injury (IRI) is a multifactorial acute stress involving occlusion of blood flow to an organ or tissue for a certain period of time (ischemia) and subsequent return of blood flow (reperfusion). During the ischemic period, cells are deprived of oxygen and nutrients, resulting in ATP depletion, loss of membrane potential and accumulation of toxic byproducts. Subsequent reperfusion introduces additional damage via the actions of inflammatory mediators, leading to both apoptotic and necrotic cell death (Jaeschke, 2003). IRI underlies a variety of clinically significant events ranging from heart attack and stroke to surgical interventions requiring temporary restriction of blood flow. Here we investigated the contributions of the TSC/mTORC1 and GCN2 pathways in sensing dietary protein restriction and mediating protective effects against hepatic IRI using mice deficient in GCN2 or lacking hepatocyte-specific expression of the essential TSC complex component TSC1.
RESULTS
Dietary protein restriction mediates stress resistance independent of GCN2
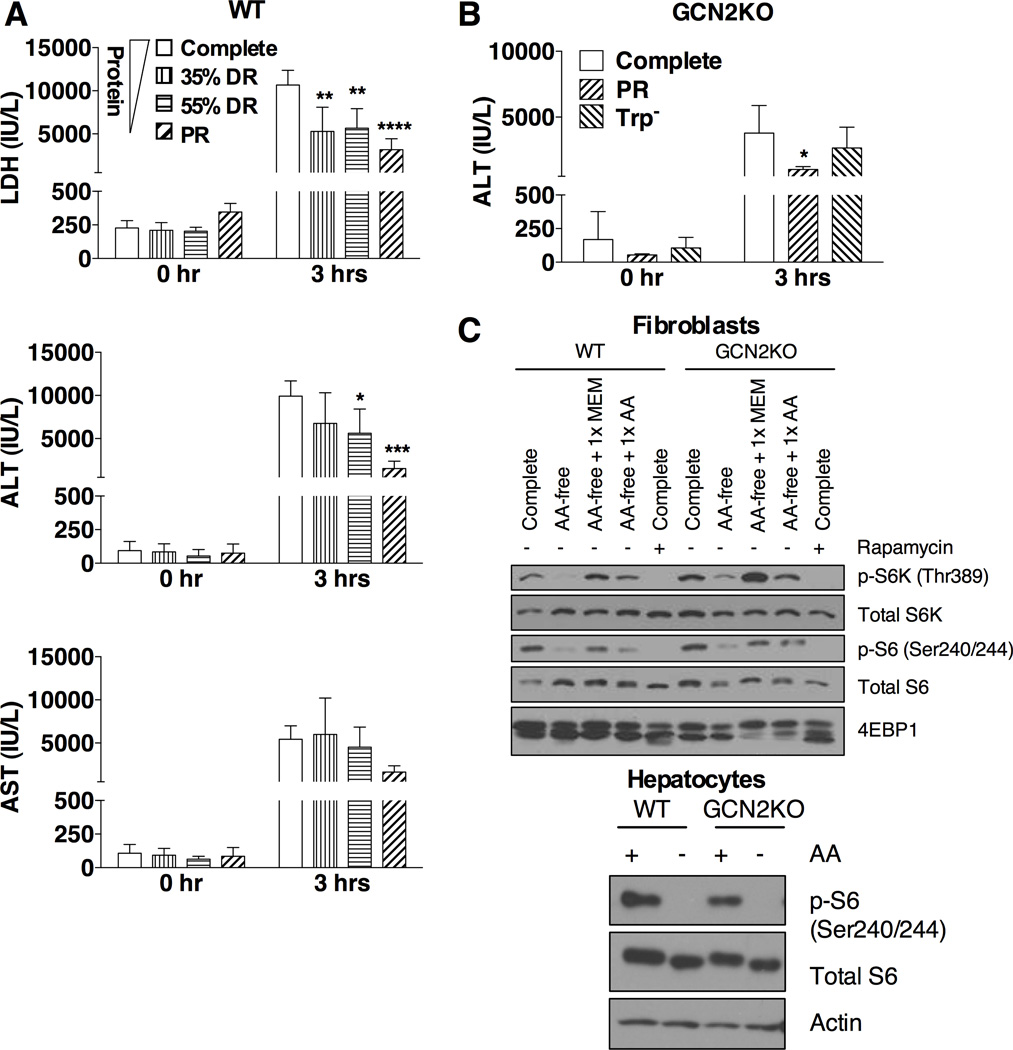
To determine an optimal 1 wk dietary preconditioning regimen against hepatic IRI, we focused on restricting dietary protein intake either by restricting intake of a complete diet (35% or 55% restriction) or by removing protein from the diet (protein restriction, PR) (Figure S1A). Food restriction was calculated on the basis of food intake of ad libitum-fed mice on a complete diet and normalized by animal weight (Figure S1B). Because mice on a protein free diet often display temporary aversion to food intake (Peng et al., 2012), the PR diet was restricted by 35% to normalize daily food intake between experiments. Hepatic IRI was induced by clamping the hepatic artery, portal vein and the bile duct for 30–35 minutes, followed by reperfusion for up to 24 hrs. Liver damage was assessed by measuring the release of cellular enzymes into the bloodstream. Maximal protection correlated with the least protein intake rather than restriction of calories (Figure 1A). Therefore, we used the protein restriction (PR) regimen to further characterize the responsible signaling pathways and downstream effector mechanisms.
Figure 1. Dietary protein restriction mediates stress resistance independent of GCN2.
(A) Serum LDH, ALT and AST activity before ischemia (0 hr) and 3 hrs after reperfusion in male mice preconditioned on the indicated diets for 1 wk prior to hepatic IRI; n = 5 mice/group. Complete: ad libitum access to complete diet; 35% DR: 65% of ad libitum food allowance on complete diet; 55% DR: 45% of ad libitum food allowance on complete diet, PR: 65% of ad libitum food allowance on protein-free diet. Asterisks indicate the significance of the difference vs. the Complete group by 1-way ANOVA with Dunnett’s test for multiple comparisons, multiplicity adjusted p-values; *p < 0.05, **p < 0.005, ***p < 0.0005, ****p < 0.0001.
(B) Serum ALT activity before ischemia (0 hr) and 3 hrs after reperfusion in female GCN2KO mice preconditioned on the indicated diets for 1 wk prior to hepatic IRI; n = 4–5 mice/group. Complete: ad libitum access to complete diet; PR: 65% of ad libitum food allowance on protein-free diet; Trp−: 65% of ad libitum food allowance on tryptophan-free diet. Asterisk indicates the significance of the difference vs. the Complete group by student’s t-test; *p < 0.05.
(C) Immunoblots of markers of mTORC1 signaling in extracts from primary dermal fibroblasts or hepatocytes isolated from WT or GCN2KO mice and treated with the indicated media in the presence of 10% dialyzed FBS (2 hrs for MDFs, 3 hrs for hepatocytes). Complete: complete DMEM; AA-free: amino acid-free DMEM; MEM: minimum essential medium amino acid mix; AA: all amino acids added back to 1× DMEM concentration; Rapamycin: 20 nM.
Data in all panels are shown as means ± SD. See also Figure S1.
Recently, we showed that GCN2 is required for mediating the protective effects of single EAA restricted diets against renal and hepatic IRI (Peng et al., 2012). To test whether GCN2 is required for mediating the benefits of total protein restriction against hepatic IRI, we preconditioned Gcn2 knockout (GCN2KO) mice on complete, tryptophan restricted (Trp−) or PR diets for one wk. Damage assessment 3 hrs after reperfusion revealed no significant difference between complete and Trp− diets; however, GCN2KO mice preconditioned on the PR regimen were significantly protected (Figure 1B, S1C). Thus, GCN2 was dispensable for preconditioning by PR, indicating the likely involvement of another amino acid sensing pathway.
The mTORC1 pathway can also sense amino acids, and is inhibited in the absence of specific amino acids, including leucine. We thus tested whether the mTORC1 pathway is still responsive to amino acid deprivation in vitro in two different GCN2KO cell types, primary dermal fibroblasts and primary hepatocytes (Figure 1C). Phosphorylation of downstream targets of mTORC1, including S6K1, its target S6, and 4E-BP1 (as scored by mobility shifting), were proportionately reduced in both wild type (WT) and GCN2KO cells upon total amino acid withdrawal. We conclude that the mTORC1 pathway can sense amino acid restriction independent of GCN2, and could thus be a potential mediator of PR preconditioning effects.
The TSC complex is required for inhibition of mTORC1 upon PR in vivo
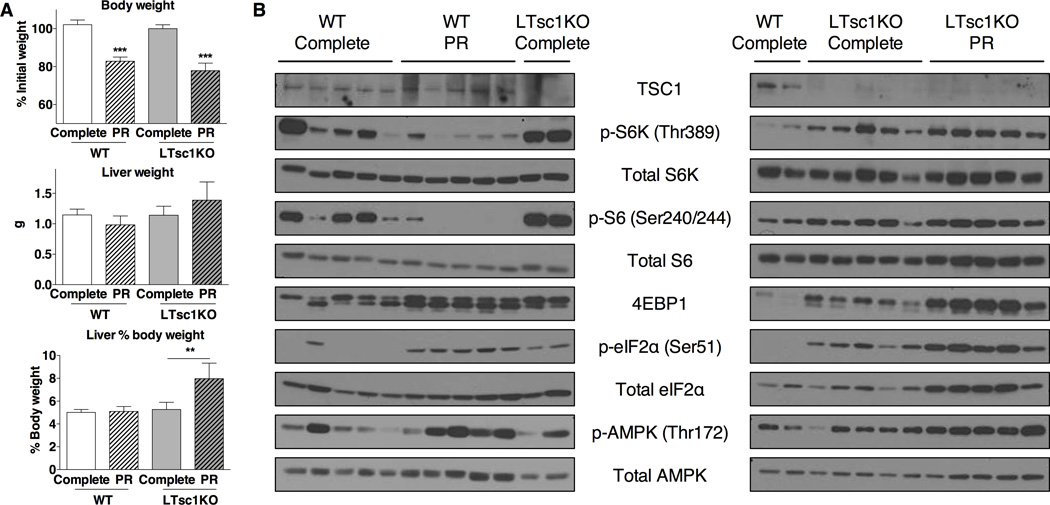
We next determined the effects of PR on hepatic mTORC1 activity in vivo using both WT and liver-specific Tsc1 knockout (LTsc1KO) mice, a model of constitutive mTORC1 activation in the liver (Kenerson et al., 2011; Menon et al., 2012; Sengupta et al., 2010; Yecies et al., 2011). WT and LTsc1KO mice were fed a complete or PR diet for one wk (Figure S2A). Both genotypes lost approximately 20% of their body weight (Figure 2A, S2B), including loss of adipose mass (Figure S2C). In WT mice on PR, livers tended to weigh less, and were thus maintained as a percentage of body weight; in LTsc1KO mice on PR, liver weights did not decrease, resulting in disproportionately larger livers (Figure 2A), consistent with failure of LTsc1KO mice to reduce mTORC1 signaling upon PR.
Figure 2. The TSC complex is required for inhibition of mTORC1 upon PR in vivo.
(A) Body and liver weights of the indicated genotype after 1 wk on the indicated diet; n = 4–5 mice/group. Body weight on the last day of treatment is represented as percentage of initial weight (top). Liver weights on the last day of treatment are represented as absolute values (middle) and percentages of body weight (bottom). Asterisks indicate the significance of the difference between diets within genotype by student’s t-test; **p < 0.01, ***p < 0.00001.
(B) Immunoblots of markers of mTORC1, GCN2 and AMPK activation in liver extracts from WT and LTsc1KO male mice after 1 wk on the indicated diet.
Data in all panels are shown as means ± SD. See also Figure S2.
To confirm differential regulation of hepatic mTORC1 signaling upon PR, we looked in liver extracts at downstream mTORC1 targets and related nutrient/energy sensing pathways. PR increased phosphorylation of eIF2α (Figure 2B, S2D) and expression of downstream markers of the GCN2-dependent amino acid starvation response, including the ATF4 target gene Asns (Figure S2E). Higher baseline eIF2α phosphorylation in LTsc1KOs could be due in part to higher endoplasmic reticulum (ER) stress (Menon et al., 2012). In WT livers, we observed a reduction in S6K and S6 phosphorylation upon PR (Figure 2B, S2D). Total levels of 4EBP1, which is also an ATF4 target gene (Yamaguchi et al., 2008), increased in both genotypes upon PR; therefore changes in phosphorylation of this mTORC1 target are difficult to interpret (Figure 2B, S2E). mTORC1 signaling in Tsc1KO livers was greatly increased relative to WT, and unaffected by PR, if not slightly elevated (Figure 2B, S2D). Interestingly, even though AMPK phosphorylation in some WT livers was increased upon PR, the observed changes were not statistically significant likely due to biological variability; there was no evidence of increased AMPK phosphorylation upon PR in LTsc1KO livers (Figure 2B, S2D).
Differential reduction of essential amino acids and growth factors in vivo upon PR
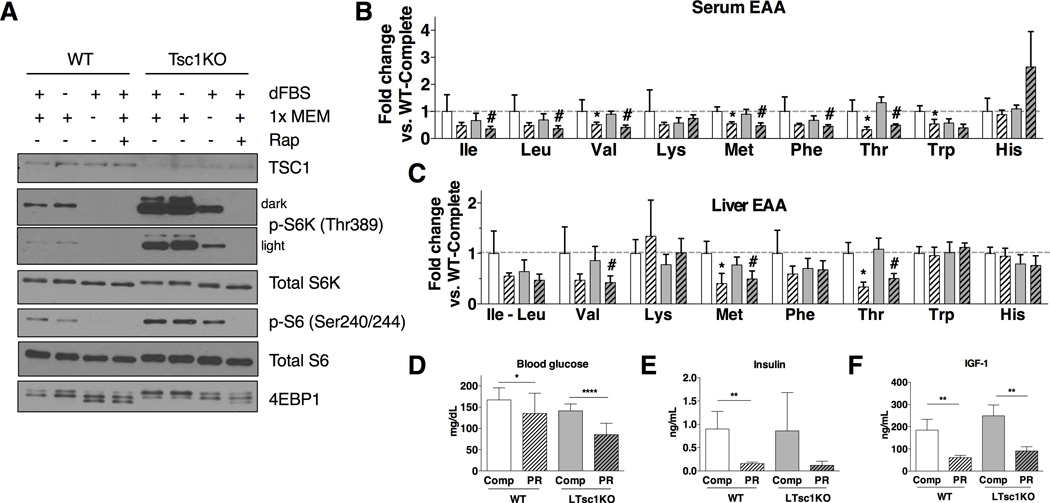
To test the role of amino acid deficiency on cell-autonomous mTORC1 signaling, primary WT or TSC1KO hepatocytes were cultured for 2 hrs in media lacking amino acids. In WT hepatocytes, this led to the reduction of S6K and S6 phosphorylation below the level of detection (Figure 3A). Although Tsc1KO hepatocytes had much higher baseline levels of p-S6K, they also responded to amino acid deprivation in vitro, confirming the ability of amino acid deprivation to reduce mTORC1 activity independent of the TSC complex.
Figure 3. Differential reduction of essential amino acids and growth factors in vivo upon PR.
(A) Immunoblots of mTORC1 targets from extracts of primary hepatocytes isolated from WT or LTsc1KO mice and starved of amino acids in the presence of 10% dialyzed serum (dFBS) for 2 hrs prior to lysis, coincident with 1× MEM addition +/− 20nM rapamycin (Rap) as indicated.
(B, C) Free EAA levels in serum (B) and liver (C) of WT or LTsc1KO mice on the indicated diet expressed as fold change relative to the WT complete diet group; n = 4–5 mice/group. Asterisks/number signs indicate the significance of the difference between diets within genotype by student’s t-test; */#p < 0.05.
(D–F) Blood glucose, serum insuin (E) and IGF-1 (F) levels of WT or LTsc1KO mice on the indicated diet; n = 4–5 mice/group for each experiment, with data pooled from 3 separate experiments (D). Asterisks indicate the significance of the difference between diets within genotype by student’s t-test; *p < 0.05, **p < 0.01, ****p < 0.0000001.
Data in all panels are shown as means ± SD. See also Figure S3.
If amino acid deficiency could at least partially abrogate mTORC1 signaling in Tsc1KO hepatocytes in vitro, why did PR fail to reduce mTORC1 signaling at all in LTsc1KO livers in vivo? To answer this, we first measured free amino acids in the serum and in liver. One wk of PR reduced most free EAA, including leucine, in the serum and liver in both WT and LTsc1KO animals, but only by approximately 20–30% on average (Figure 3B, C); non-essential amino acids were not uniformly affected (Figure S3A, B). Blood glucose was similarly reduced upon PR in both genotypes by 20–40% (Figure 3D). However, circulating levels of growth factors were reduced by more than 5 fold for insulin and 3 fold for IGF-1 upon PR (Figure 3E, F). Taken together, these data suggest that buffering of free amino acid concentrations in vivo upon PR prevents Rag/Ragulator-based mTORC1 inhibition in LTsc1KO livers, and confirms LTsc1KO mice as a suitable model of constitutive hepatic mTORC1 activation, despite intact systemic changes in upstream mTORC1 regulators in response to diet.
The TSC complex is required for benefits of PR against acute hepatic stress
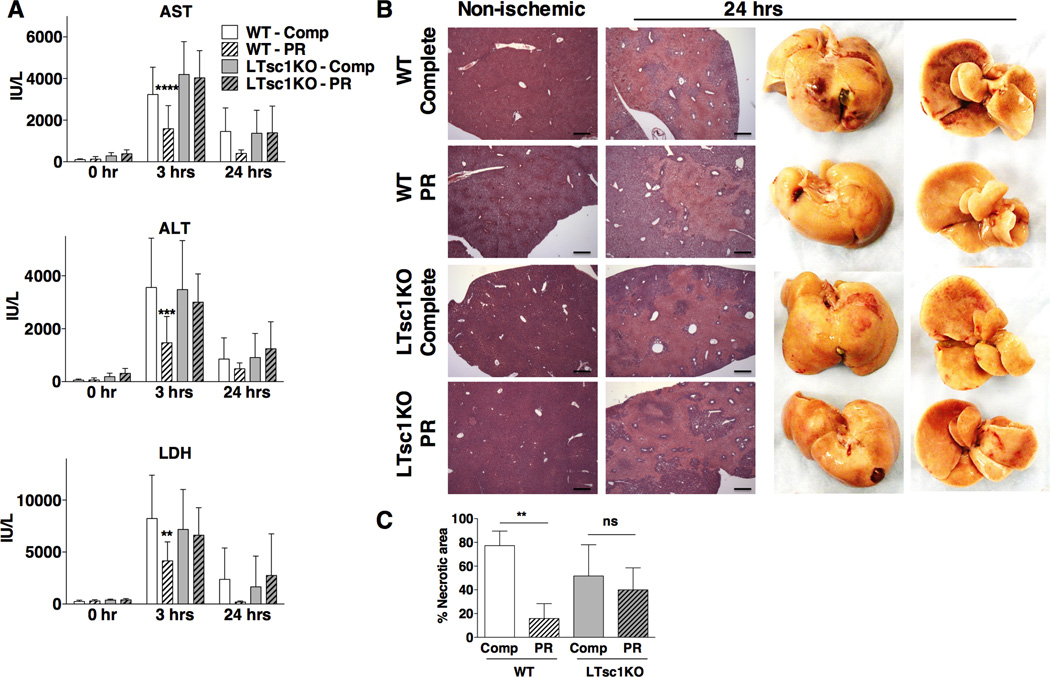
To determine the requirement for the TSC complex and suppression of mTORC1 on PR-mediated stress resistance, we tested the response of female WT and LTsc1KO mice to hepatic IRI. We observed a significant reduction of serum damage markers LDH, ALT and AST in WT animals on PR, but this benefit was lost in LTsc1KOs (Figure 4A, S4A). As a control, changes in the serum levels of these enzymes were not due to differential enzyme activity levels in undamaged livers (Figure S4B). H&E-stained sections from formalin-fixed livers harvested 24 hrs after reperfusion from all 4 groups were analyzed for necrotic cell death, which appeared as lightly stained areas with loss of hepatocyte morphology (Figure 4B). Blind scoring in serial sections confirmed the reduction of necrotic tissue in WT livers upon PR, and the loss of this benefit in LTsc1KOs (Figure 4C). The same relative differences in hemorrhagic necrosis could also be observed at the macroscopic level in whole livers (Figure 4B). Body temperatures of LTsc1KO and WT mice upon PR were similarly reduced (Figure S4C), ruling out the possibility of differential temperatures in protection by PR in WT mice. Finally, PR-mediated protection from hepatic IRI was partially abrogated in LTsc1KO males (Figure S4D). Taken together, these results indicate the requirement for the TSC complex in the protective effect of PR against hepatic IRI.
Figure 4. The TSC complex is required for benefits of PR against acute hepatic stress.
(A) Serum LDH, ALT and AST activity before ischemia (0 hr) and 3 or 24 hrs after reperfusion in female WT or LTsc1KO mice preconditioned on the indicated diets for 1 wk prior to hepatic IRI; n = 4–6 mice/group for each experiment, with data pooled from 4 separate experiments for 0 and 3 hrs and a single experiment for 24 hrs. Asterisks indicate the significance of the difference between diets within genotype by student’s t-test; **p < 0.001, ***p < 0.00001, ****p < 1 × 10−8.
(B) Representative images of H&E stained sections from non-ischemic (left) or ischemic livers 24 hrs after reperfusion (right) from WT or LTsc1KO mice preconditioned on the indicated diets for 1 wk prior to hepatic IRI. Lightly stained areas with loss of hepatocyte morphology indicate necrotic tissue. Right: representative images of whole livers 24 hrs after IRI and fixed with formalin, with areas of hemorrhagic necrosis appearing as dark red. Scale bar: 400 µm.
(C) Quantification of hepatic cell death expressed as percentages of necrotic region/field in 3–5 mice/group, 3 sections/sample; Asterisks indicate the significance of the difference between diets within genotype by student’s t-test; **p < 0.01, ns: not significant.
Data in all panels are shown as means ± SD. See also Figure S4.
In order to investigate potential differences in protective mechanisms by total protein (PR) and tryptophan restriction (Trp−), we tested the response of female LTsc1KO mice to one wk of preconditioning on a Trp− diet. Interestingly, we observed that LTsc1KO mice were still responsive to Trp− diet in terms of protection from hepatic IRI (Figure S4E). Taken together, these data indicate that beneficial effects of total protein restriction and tryptophan restriction have different genetic requirements, suggesting a parallel rather than epistatic relationship between TSC/mTORC1 and GCN2 amino acid sensing pathways in protection from hepatic IRI.
Rapamycin is a partial mTORC1 inhibitor that increases longevity in mice (Harrison et al., 2009; Miller et al., 2011; Miller et al., 2013). We tested the ability of rapamycin to induce benefits against hepatic IRI similar to PR. To this end, WT mice were injected with rapamycin at varying concentrations for up to one wk in the absence of any dietary intervention. Despite reducing mTORC1 activity, none of the rapamycin regimens tested were beneficial against hepatic IRI (Figure S4F, G). Interestingly, we observed that thrice wkly rapamycin treatment partially abrogated PR benefits and reduced mTORC2 activity as assessed by NDRG1 phosphorylation (Figure S4G, H), suggesting that the effects of rapamycin on mTORC2 might be detrimental in the context of hepatic IRI.
The TSC complex is required for improved hepatic insulin sensitivity upon PR
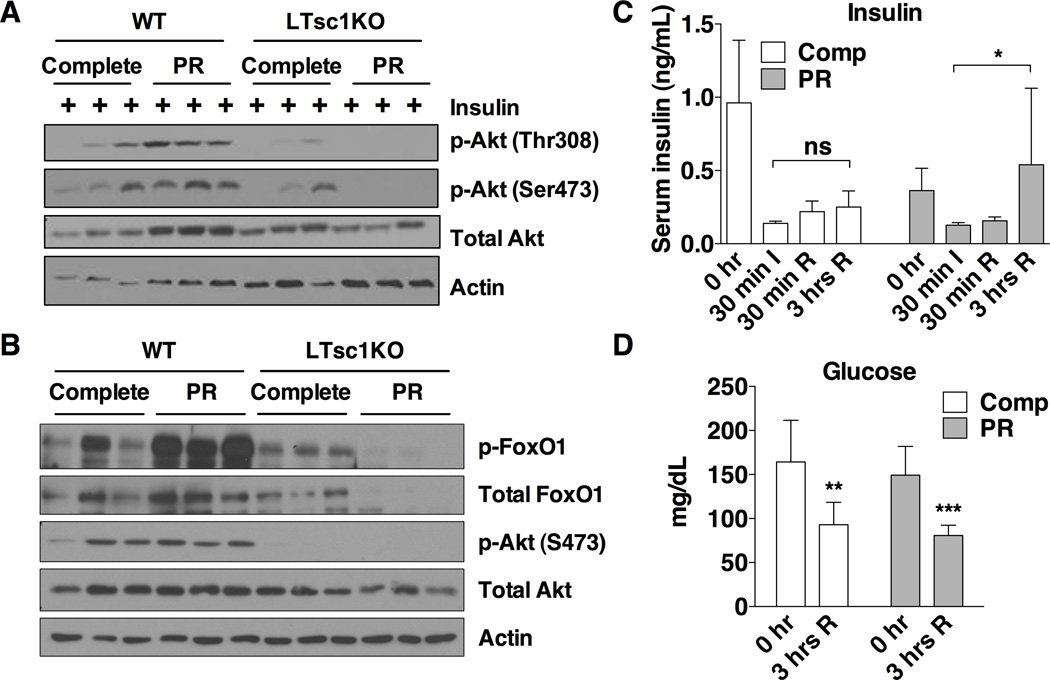
By what downstream mechanism does TSC complex activation and mTORC1 repression improve stress resistance? Improved insulin sensitivity upon DR is associated with a wide range of health benefits including stress resistance and extended longevity (Fontana et al., 2010). We thus tested the ability of PR to increase hepatic insulin sensitivity in a TSC1-dependent manner. One wk of PR reduced fasting insulin, resulting in a significantly reduced HOMA-IR (Figure S5A–C) consistent with improved insulin sensitivity. To directly test the effects of PR on hepatic insulin sensitivity, we measured the levels of Akt phosphorylation in extracts from WT livers following stimulation with insulin. WT mice on PR for one wk had higher levels of pAkt-S473 and pAkt-Thr308 compared to controls (Figure S5D). Levels of Akt phosphorylation in Tsc1KO livers upon insulin stimulation were lower than in WT livers, consistent with previous findings (Yecies et al., 2011), but did not improve upon PR in Tsc1KOs as they did in WT controls (Figure 5A). Together, these findings show that the TSC complex is required for improvement in hepatic insulin sensitivity upon PR.
Figure 5. The TSC complex is required for improved hepatic insulin sensitivity upon PR.
(A) Insulin sensitivity as determined by immunoblotting for markers of Akt pathway activation in liver extracts from mice fasted for 6 hrs and then stimulated with 0.5 U/kg insulin by portal vein injection 3 min before harvest.
(B) Akt activation status as determined by immunoblotting of liver extracts 3 hrs after reperfusion from mice preconditioned for 1 wk on the indicated diet prior to induction of hepatic IRI.
(C) Serum insulin levels from tail blood of WT mice preconditioned for 1 wk on the indicated diet taken prior to ischemia (0 hr), at the end of the ischemic period (30 min I, n=3–4), 30 min after reperfusion period (30 min R, n=3) or 3 hrs after reperfusion (3 hrs R, n=11–12). Asterisk indicates the significance of the indicated comparison according to a Kruskal-Wallis test followed by Dunn’s multiple comparisons test; *p < 0.05; ns: not significant.
(D) Blood glucose levels of mice on the indicated diets for 1 wk before hepatic IRI, measured before (0hr) and 3 hrs after reperfusion; n = 8–10 mice/group. Asterisks indicate the significance of the difference between 0 and 3 hr values by student’s t test within diet group; **p < 0.001, ***p < 0.0001.
Data in all panels are shown as means ± SD. See also Figure S5.
In the context of DR, improved insulin sensitivity is associated with reduced anabolic hormone levels (as observed here, Figure 3E, F) and, consequently, reduced insulin/IGF-1 signaling. Reduced insulin/IGF-1 signaling is an evolutionarily conserved mechanism regulating longevity and stress resistance in part by controlling cytoprotective gene expression (Fontana et al., 2010). To evaluate a potential impact of increased cytoprotective gene expression, we measured expression of genes controlled by both FoxO and NRF2 transcription factors. However, no significant increases in the levels of these genes were observed upon PR, nor did TSC1 ablation have consistent effects (Figure S5E, F).
In contrast to the potential benefits of reduced insulin/IGF-1 signaling named above, increased growth factor signaling after injury can trigger anti-apoptotic survival signaling and improve functional recovery (Fuglesteg et al., 2009; Jonassen et al., 2001; Liu et al., 2007). In order to address the potential protective effects of increased insulin/IGF-1 signaling after injury in the context of protection from hepatic IRI, we determined the status of insulin/Akt signaling at various time points during ischemia and after reperfusion. Akt signaling was dramatically reduced during ischemia and returned rapidly within 15 minutes of reperfusion (Figure S5G). We next looked at insulin/Akt signaling in the context of PR. During ischemia, Akt signaling was maintained at higher levels in PR mice (Figure S5H). Although no consistent differences were detected between PR and control mice 30 minutes after reperfusion (Figure S5H), phosphorylation of the Akt target FoxO1 was clearly increased 3 hrs after reperfusion in livers of PR vs. Complete diet groups (Figure 5B). Importantly, this increase in FoxO1 phosphorylation was absent in LTsc1KO livers (Figure 5B).
To explain this boost in insulin/Akt signaling 3 hrs after reperfusion specifically in WT mice preconditioned on PR, we measured serum insulin at multiple time points during the procedure. In WT mice, serum insulin levels fell dramatically below preoperative levels during the ischemic period independent of diet, and increased gradually following reperfusion (Figure 5C). Importantly, the recovery of circulating insulin was significantly higher 3 hrs after reperfusion in PR animals than in control mice. This increase in insulin was unlikely to be secondary to an increase in blood glucose, since glucose levels remained significantly lower than initial levels 3 hrs after reperfusion (Figure 5D). As a result of the differential insulin sensitivity between WT and LTsc1KO mice, only WT mice displayed the boost in Akt activity upon PR (Figure 5B).
Increased prosurvival signaling and reduced apoptosis contribute to PR-mediated protection
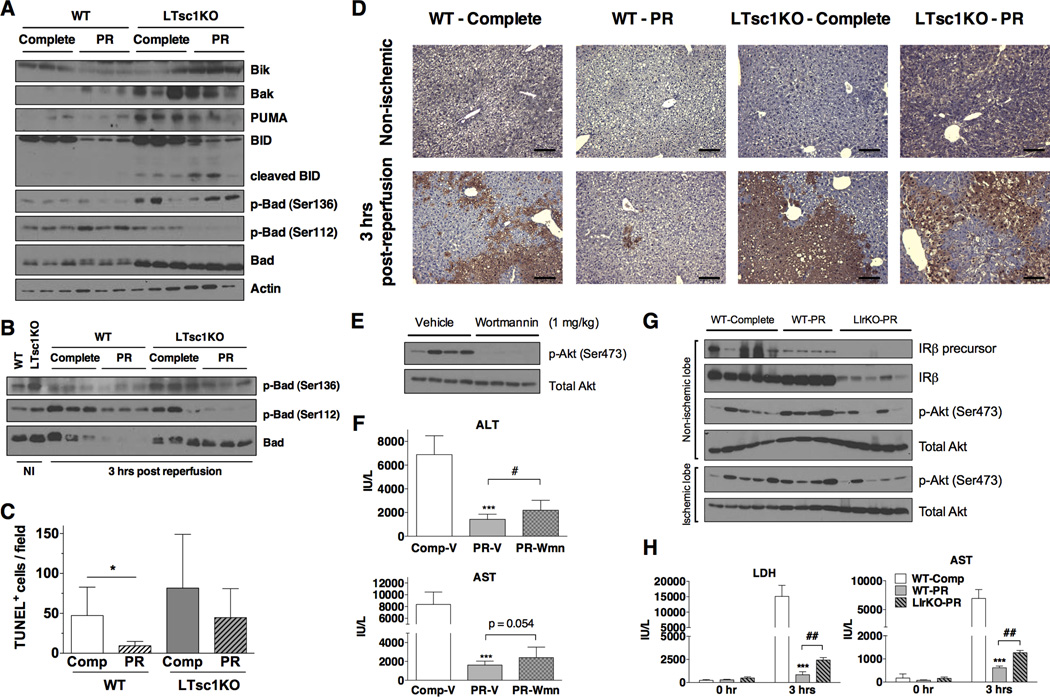
FoxO1 regulates apoptosis via transcription of pro-apoptotic Bcl-2 family members including Bim, PUMA and BNIP3 (Zhang et al., 2011). Although hepatic IRI is largely driven by necrotic cell death, inhibition of apoptosis protects from injury (Datta et al., 2013). We examined protein levels of pro-apoptotic Bcl-2 family members Bik, Bak, PUMA, BID and Bad in WT and LTsc1KO animals on PR or control diets for one wk. In WT livers, Bik and BID were reduced upon PR, consistent with a decrease in the pro-apoptotic environment (Figure 6A). In LTsc1KO livers, on the other hand, pro-apoptotic Bcl-2 family members were generally expressed at higher levels than in WT livers, and were not reduced upon PR (Bik, Bak and Bad, Figure 6A). Phosphorylation of Bad at Ser112 and Ser136 can inhibit its binding to Bcl-2 and Bcl-xL and thus reduce its pro-apoptotic activity (Danial, 2008). Phosphorylation of Bad at Ser112 was decreased upon PR in LTsc1KOs, unlike WTs (Figure 6A). Three hrs after reperfusion, total Bad levels were reduced in WTs upon PR, but remained the same in LTsc1KOs with reduced phosphorylation (Figure 6B).
Figure 6. Increased prosurvival signaling and reduced apoptosis contribute to PR-mediated protection.
(A, B) Immunoblots of pro-apoptotic Bcl-2 family members in liver extracts from WT or LTsc1KO mice preconditioned on the indicated diets for 1 wk and harvested without ischemia (A) or 3 hrs after reperfusion (B). NI: non-ischemic.
(C, D) TUNEL staining of non-ischemic or ischemic livers from the indicated diet-genotype combinations 3 hrs after reperfusion. (C) Quantification was performed by blind scoring of the number of TUNEL+ nuclei/200× field in 10 random fields from each sample, with n = 5 mice/group. Asterisks indicate the significance of the difference between diets within genotype by student’s t test; *p < 0.05. (D) Representative images of TUNEL-stained sections. Scale bar: 100 µm.
(E) Immunoblot of Akt phosphorylation in liver extracts from WT mice injected with vehicle or 1 mg/kg wortmannin 1 hr prior to harvest.
(F) Serum ALT and AST activity 3 hrs after reperfusion in WT mice preconditioned on the indicated diets for 1 wk and injected with vehicle or 1 mg/kg wortmannin (Wmn) 1 hr prior to hepatic IRI; n = 8–10 mice/group. Statistical significance was assessed by student’s t-test between Complete-V and P-RV groups (***p < 0.0005) or between PR-V and PR-Wmn groups (#p < 0.05).
(G) Immunoblot of liver extracts from WT mice on complete diet, or WT and LIrKO mice preconditioned with PR for 1 wk and harvested 3 hrs after reperfusion.
(H) Serum LDH and AST activity before ischemia (0 hr) and 3 hrs after reperfusion in male WT and LIrKO mice preconditioned on the indicated diets for 1 wk prior to hepatic IRI; n = 4–5/group. Statistical significance was assessed by student’s t-test between WT-Complete and WT-PR groups (***p < 0.0005) or between WT-PR and LIrKO-PR groups (##p < 0.001).
Data in all panels are shown as means ± SD. See also Figure S6.
To determine if the observed increase in insulin/Akt signaling and decrease in pro-apoptotic factors resulted in reduced apoptosis in WT livers upon PR, sections from livers harvested 3 hrs post-reperfusion were analyzed by TUNEL staining. As expected, WT livers preconditioned with PR had significantly less TUNEL positive cells compared to controls, and this protective effect of diet was absent in LTsc1KO livers (Figure 6C, D).
In order to directly test the role of differential post-reperfusion insulin signaling in PR-mediated hepatic IRI protection, we tested the effects of the PI3K inhibitor wortmannin (Ren et al., 2011). Treatment 1 hr prior to induction of ischemia increased blood glucose (Figure S6A), reduced in vivo Akt phosphorylation (Figure 6E), and partially abrogated PR-mediated benefits against hepatic IRI (Figure 6F, S6B). Because of the relatively short half-life of wortmannin in vivo, hepatic Akt/FoxO signaling was restored by 3 hrs after reperfusion (Figure S6C), which may explain the partial effect. Finally, we tested the response of liver-specific insulin receptor knockout (LIrKO) mice (Michael et al., 2000) to PR-mediated hepatic IRI preconditioning. LIrKO mice had lower levels of Akt phosphorylation in ischemic liver lobes at 3 hrs post reperfusion (Figure 6G), and their response to PR was worse than WT mice on PR (Figure 6H, S6D).
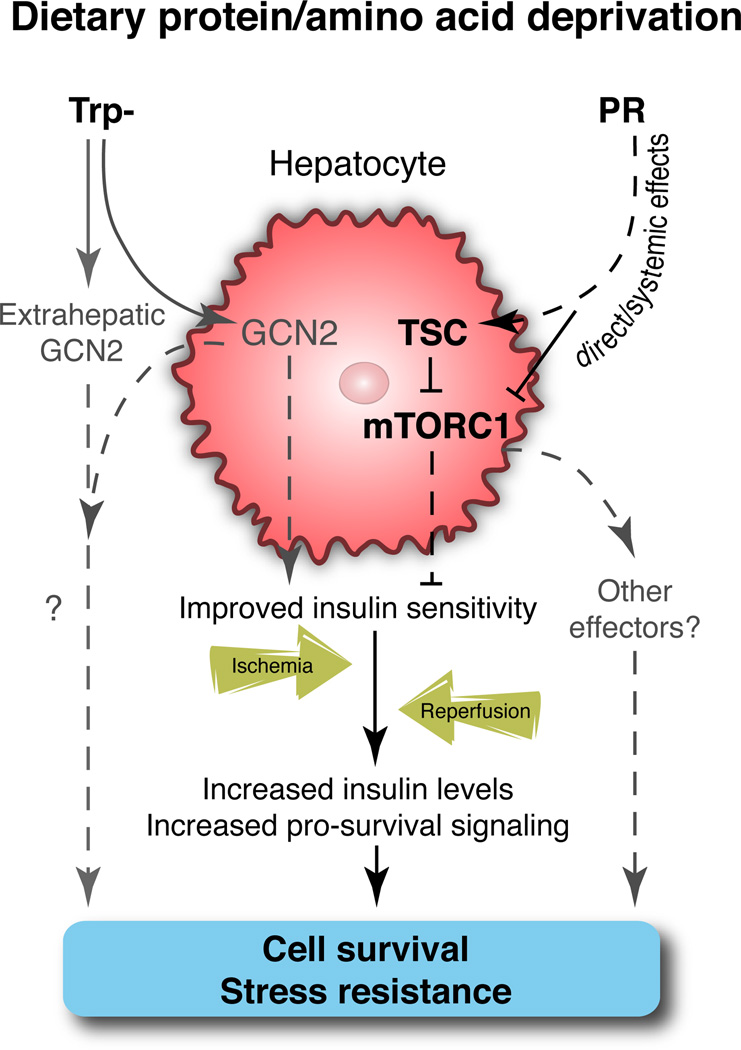
A model for the collective action of PR on increased insulin sensitivity and relative insulin levels in WT animals after ischemia and reperfusion, resulting in increasing pro-survival signaling and reduced cell death, is presented in Figure 7. These latter effects were dependent on the expression of TSC1 in hepatocytes and were partially blocked by genetic and pharmacological inhibition of insulin/Akt signaling.
Figure 7. Model of TSC/mTORC1 function and insulin sensitivity in PR-mediated hepatic stress resistance.
A model for the effects of dietary protein or tryptophan restriction on improved hepatic insulin sensitivity prior to an acute ischemia event. After reperfusion, increased insulin levels and increased hepatic insulin sensitivity facilitate pro-survival signaling and contribute to protection from injury.
DISCUSSION
Previously we reported a genetic requirement for Gcn2 in mediating surgical stress resistance upon single EAA restriction (Peng et al., 2012). Here we found that GCN2 was no longer required when total protein was removed from the diet, suggesting involvement of a redundant nutrient-sensing pathway. We thus tested the role of the mTORC1 pathway using mice deficient in Tsc1 with constitutive mTORC1 activation specifically in the liver. The role of the mammalian TSC complex in DR benefits has not been previously tested. Here we found that two major benefits of PR, namely improved hepatic insulin sensitivity and stress resistance, both required the TSC complex.
Consistent with intact Ragulator-based mechanisms of mTORC1 inhibition in vitro, TSC1KO hepatocytes had higher baseline levels of mTORC1 activity but were still responsive to amino acid deprivation. Nonetheless, hepatic mTORC1 activity in vivo was completely recalcitrant to total dietary protein restriction in LTsc1KO vs. WT livers, despite reduced serum and liver free amino acids and activation of the GCN2-dependent amino acid starvation response in both genotypes. Our interpretation of these data is that the concentration of leucine or other critical amino acids required to trigger Ragulator-based mTORC1 inhibition can be achieved by removal of free amino acids in vitro, but cannot be achieved in vivo due to the buffering capacity on the organismal level against drastic changes in steady-state levels of free amino acids. What level of free amino acids are required to activate Ragulator in hepatocytes in vivo, and whether longer periods of dietary protein deprivation, or combined protein and calorie deprivation such as fasting, ever result in a drop below these levels, remains to be determined.
While female LTsc1KO mice almost entirely lost the preconditioning benefit of PR against hepatic IRI, parallel experiments in males showed that despite similar functional protection by PR as in females, this protection was only partially lost in LTsc1KO males. Although the reason for this apparent sex-based difference in the genetic requirement for Tsc1 is unclear, we note that the lifespan extension benefits of ablation of the downstream mTORC1 target S6K and mTOR+/−mLST8+/− models are also preferentially observed in females (Lamming et al., 2012; Selman et al., 2009).
In addition to total protein restriction, we also tested the effects of tryptophan restriction on LTsc1KO mice. Interestingly, LTsc1KO mice exhibited a response comparable to WT mice. Together with the previous findings indicating a requirement for the amino acid deprivation sensor GCN2 in protection afforded by tryptophan restriction (Peng et al., 2012), these results suggest that GCN2 and mTORC1 may act in parallel pathways, eventually converging on the same beneficial outcome. Whether or not this occurs through a common downstream mechanism remains to be determined. However, the lack of improvement of insulin sensitivity in GCN2KO mice in response to leucine restriction (Xiao et al., 2011) is consistent with the possibility of improved hepatic insulin sensitivity as a potential point of convergence.
Inhibition of TOR signaling has been implicated in longevity, metabolism and stress resistance, including the response to DR in lower organisms, but its downstream effector mechanisms in mammals are not well established (Kaeberlein, 2013; Kaeberlein et al., 2005; Zid et al., 2009). Improved insulin sensitivity is a metabolic hallmark of multiple different DR regimens, including PR (Fontana et al., 2010; Toyoshima et al., 2010), and is potentially controlled in part by mTORC1-based feedback inhibition of IRS-1 (Howell and Manning, 2011). Here, we found that improved hepatic insulin sensitivity upon PR required the TSC complex. Interestingly, PR appeared to reduce hepatic insulin sensitivity in LTsc1KOs, although the reason for this remains unclear.
The fact that both increased and decreased signaling through the evolutionarily conserved insulin/IGF-1 pathway can improve cellular stress resistance and organismal survival is paradoxical (Rincon et al., 2004; Tang, 2006). Reduced insulin signaling, due either to reduced insulin levels as with DR, or in constitutive genetic models of reduced insulin/IGF-1 signaling, can extend longevity and increase stress resistance in part through activation of cytoprotective gene expression normally inhibited by insulin/Akt signaling. For example, in C. elegans, activation of FoxO/Daf-16 targets involved in stress resistance has been implicated in DR mediated longevity (Greer et al., 2009). On the other hand, increased insulin action is desirable under certain circumstances, for example to promote cell survival after ischemic insult. Consistent with this, increased insulin/PI3K/Akt signaling has been shown to play a protective role in both the rat isolated heart ischemia model (Fuglesteg et al., 2009; Jonassen et al., 2001; Liu et al., 2007) and other hepatic IRI models (Kamo et al., 2013) where a protective effect of reduced apoptosis has been described (Datta et al., 2013).
Our model of improved insulin sensitivity upon PR provides an answer to this apparent paradox. During the preconditioning period prior to injury, reduced insulin levels and reduced insulin-dependent mTORC1 activity were required, not to increase expression of cytoprotective FOXO or NRF2 target genes, but to facilitate improved insulin sensitivity via a decrease in mTORC1 feedback inhibition. This occurred in WT livers on PR, but was prevented in LTsc1KO livers due to constitutive mTORC1 activation. After injury, increased insulin signaling led to increased inhibitory FoxO1 phosphorylation, reduced apoptosis and improved outcome in insulin-sensitive WT livers. Inhibition of post-reperfusion insulin signaling either pharmacologically via the PI3K inhibitor wortmannin, or genetically with LIrKO mice, partially abrogated PR-mediated protection. Whether or not IGF-1 plays a role similar to insulin remains to be tested. Although hepatic IGF-1R expression is low, liver-specific deletion compromises regeneration (Desbois-Mouthon et al., 2006); furthermore, IGF-1 can activate the insulin receptor directly. Thus whether insulin alone or a combination of insulin and IGF-1, our model predicts decreased growth factor signaling prior to injury (resulting in increased insulin/IGF-1 sensitivity) and increased signaling after injury are both required for maximal protection. It is important to note that because abrogation of insulin signaling only partially blocked PR-mediated protection, additional mechanisms contributing significantly to protection remain to be identified.
Understanding the mechanistic basis of DR is crucial to the development of potential interventions that mimic its beneficial effects. Genetic and pharmacological approaches targeting nutrient sensing pathways have yielded similar results to DR in terms of longevity extension and other beneficial health effects, suggesting a causal role for the downregulation of these pathways in DR benefits (Fontana et al., 2010; Kaeberlein, 2013). For example, rapamycin extends lifespan in rodents, although what aspect of mTORC1 inhibition is responsible remains unclear (Harrison et al., 2009; Miller et al., 2011; Miller et al., 2013). Despite longevity extension, rapamycin has negative side effects, including increased inflammation and reduced insulin sensitivity (Lamming et al., 2013) and fails to recapitulate many other DR phenotypes (Miller et al., 2013). In our model, rapamycin not only failed to recapitulate benefits of PR, but partially abrogated PR benefits, possibly due to its aforementioned negative effects on insulin signaling via mTORC2 inhibition (Lamming et al., 2013).
Hepatic IRI presents a major challenge during various clinical contexts such as hepatic resection and liver transplantation surgeries, as well as trauma (Datta et al., 2013; Klune and Tsung, 2010). Ischemic damage to other tissues, including heart, brain and kidney, occurs regularly as an unintended complication of cardiovascular surgery, leading to significant morbidity and mortality (Mitchell et al., 2013). Understanding the mechanistic basis of DR-mediated protection could lead to preventative strategies against these clinically relevant stressors.
EXPERIMENTAL PROCEDURES
Animals and Diets
All animal experiments were performed with the approval of the appropriate institutional animal care and use committee. WT C57Bl/6J mice were purchased from Jackson Laboratories at 8–10 wks of age. LTsc1KO and GCN2KO mice were backcrossed on the C57Bl/6J background as described previously (Menon et al., 2012; Peng et al., 2012; Yecies et al., 2011). Irfl/fl mice as described previously (Michael et al., 2000) were purchased from the Jackson Laboratories (B6.129S4(FVB)-Insrtm1Khn/J, Stock #006955). Experimental LTsc1KO and littermate control animals were generated by crossing Tsc1fl/fl (WT) mice with Tsc1fl/fl|Albumin-Cre +/− (LTsc1KO) mice; GCN2KO and control mice were generated by crossing heterozygotes; LIrKO and control mice were generated by crossing Irfl/fl (WT) mice with Irfl/fl|Albumin-Cre+/− (LIrKO) mice. Animals were kept under standard housing conditions with ad libitum access to food (Purina 5058) except for the indicated dietary treatments. Female mice between 10–16 wks of age were used unless otherwise indicated. LTsc1KO mice were used prior to the age of 16 wks in order to avoid any potential complications of hepatocellular carcinomas, which appear later in life (Menon et al., 2012).
Hepatic Ischemia Reperfusion Injury and Serum Damage Assays
Hepatic IRI was performed by placing a microvascular clamp (Roboz) over the portal triad for 35 minutes for female and 30 minutes for male mice as described previously (Peng et al., 2012). After surgery, mice were given ad libitum access to control diet starting at 3 hrs after reperfusion. Serum ALT and AST levels were measured using Infinity Reagents (Thermo Scientific) and serum LDH levels using Pointe Scientific reagents with kinetic absorbance assays in a 384-well format with a BioTek Synergy II plate reader and calculated according to manufacturer’s instructions. For histology, median lobes were formalin fixed, paraffin embedded, cut into 5-micron sections and stained with hematoxylin and eosin or TUNEL (Promega). Percentage of necrotic area or TUNEL positivity in each section was scored blindly.
Primary Dermal Fibroblast and Primary Hepatocyte Isolation and Treatments
Mouse dermal fibroblasts were isolated from tail skin by mincing into fine slices followed by overnight collagenase treatment (Invitrogen Collagenase II). Cells were cultured in DMEM with 20% FBS at 3% oxygen tension. Primary hepatocytes were isolated via portal vein collagenase treatment (Liberase, Roche) followed by Percoll gradient centrifugation and cultured in William’s E media with 5% FBS. Amino acid free DMEM was a special formulation of Invitrogen DMEM-12320. For the amino acid add-back, either MEM amino acid solution (GIBCO) was used at 1× concentration, or individual amino acids were added back to AA-free medium up to their original concentrations in DMEM, and supplemented with 10% dialyzed FBS (Invitrogen). Rapamycin was purchased from Calbiochem.
Serum Measurements and Amino Acid Analysis
Blood glucose was measured with an Easy Step glucometer (Home Aide Diagnostics). Serum insulin and IGF-1 levels were measured with ELISA assay kits (Alpco). Serum and liver free amino acid analyses were performed by mass spectrometry and normalized by volume and total protein content, respectively.
Western Blotting
Cells and tissues were homogenized in NP-40 lysis buffer, cleared by centrifugation, separated by SDS-PAGE and transferred to PVDF membranes for blotting. Primary antibodies were purchased from Cell Signaling Technology except anti-p-BadSer136 and IRβ (Santa Cruz), and secondary antibodies from Dako.
Statistical Analyses
All data are represented as means ± standard deviations (SD). Statistical significance was assessed by student’s t test for pairwise comparisons or by one-way analysis of variance (ANOVA) with post-hoc tests as indicated using Microsoft Excel or GraphPad Prism. P-values smaller than 0.05 were considered statistically significant. Quantification of western blot images was done by ImageJ software.
Supplementary Material
ACKNOWLEDGEMENTS
We thank members of the Mitchell and Manning laboratories for helpful discussions, Chih-Hao Lee for critical feedback on the manuscript and the laboratory of Gokhan Hotamisligil for the use of the Piccolo blood chemistry analyzer. This work was supported by grants from NIH (DK090629, AG036712 to JRM and 5T32CA00938230 to CH), Harvard GSAS Merit Fellowship (EH) and the Ellison Medical Foundation (BDM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Cheng CW, Adams GB, Perin L, Wei M, Zhou X, Lam BS, Da Sacco S, Mirisola M, Quinn DI, Dorff TB, Kopchick JJ, Longo VD. Prolonged Fasting Reduces IGF-1/PKA to Promote Hematopoietic-Stem-Cell-Based Regeneration and Reverse Immunosuppression. Cell stem cell. 2014;14:810–823. doi: 10.1016/j.stem.2014.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danial NN. BAD: undertaker by night, candyman by day. Oncogene. 2008;27(Suppl 1):S53–S70. doi: 10.1038/onc.2009.44. [DOI] [PubMed] [Google Scholar]
- Datta G, Fuller BJ, Davidson BR. Molecular mechanisms of liver ischemia reperfusion injury: insights from transgenic knockout models. World J Gastroenterol. 2013;19:1683–1698. doi: 10.3748/wjg.v19.i11.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desbois-Mouthon C, Wendum D, Cadoret A, Rey C, Leneuve P, Blaise A, Housset C, Tronche F, Le Bouc Y, Holzenberger M. Hepatocyte proliferation during liver regeneration is impaired in mice with liver-specific IGF-1R knockout. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2006;20:773–775. doi: 10.1096/fj.05-4704fje. [DOI] [PubMed] [Google Scholar]
- Dibble CC, Elis W, Menon S, Qin W, Klekota J, Asara JM, Finan PM, Kwiatkowski DJ, Murphy LO, Manning BD. TBC1D7 Is a Third Subunit of the TSC1-TSC2 Complex Upstream of mTORC1. Molecular cell. 2012;47:535–546. doi: 10.1016/j.molcel.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dibble CC, Manning BD. Signal integration by mTORC1 coordinates nutrient input with biosynthetic output. Nat Cell Biol. 2013;15:555–564. doi: 10.1038/ncb2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efeyan A, Zoncu R, Sabatini DM. Amino acids and mTORC1: from lysosomes to disease. Trends Mol Med. 2012 doi: 10.1016/j.molmed.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L, Partridge L, Longo VD. Extending healthy life span--from yeast to humans. Science. 2010;328:321–326. doi: 10.1126/science.1172539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuglesteg BN, Tiron C, Jonassen AK, Mjos OD, Ytrehus K. Pretreatment with insulin before ischaemia reduces infarct size in Langendorff-perfused rat hearts. Acta Physiol (Oxf) 2009;195:273–282. doi: 10.1111/j.1748-1716.2008.01901.x. [DOI] [PubMed] [Google Scholar]
- Gallinetti J, Harputlugil E, Mitchell JR. Amino acid sensing in dietary-restriction-mediated longevity: roles of signal-transducing kinases GCN2 and TOR. Biochem J. 2013;449:1–10. doi: 10.1042/BJ20121098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer EL, Banko MR, Brunet A. AMP-activated protein kinase and FoxO transcription factors in dietary restriction-induced longevity. Annals of the New York Academy of Sciences. 2009;1170:688–692. doi: 10.1111/j.1749-6632.2009.04019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, Pahor M, Javors MA, Fernandez E, Miller RA. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell JJ, Manning BD. mTOR couples cellular nutrient sensing to organismal metabolic homeostasis. Trends Endocrinol Metab. 2011;22:94–102. doi: 10.1016/j.tem.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Manning BD. The TSC1-TSC2 complex: a molecular switchboard controlling cell growth. Biochem J. 2008;412:179–190. doi: 10.1042/BJ20080281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeschke H. Molecular mechanisms of hepatic ischemia-reperfusion injury and preconditioning. Am J Physiol Gastrointest Liver Physiol. 2003;284:G15–G26. doi: 10.1152/ajpgi.00342.2002. [DOI] [PubMed] [Google Scholar]
- Jonassen AK, Sack MN, Mjos OD, Yellon DM. Myocardial protection by insulin at reperfusion requires early administration and is mediated via Akt and p70s6 kinase cell-survival signaling. Circ Res. 2001;89:1191–1198. doi: 10.1161/hh2401.101385. [DOI] [PubMed] [Google Scholar]
- Kaeberlein M. mTOR Inhibition: From Aging to Autism and Beyond. Scientifica. 2013;2013:849186. doi: 10.1155/2013/849186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeberlein M, Powers RW, 3rd, Steffen KK, Westman EA, Hu D, Dang N, Kerr EO, Kirkland KT, Fields S, Kennedy BK. Regulation of yeast replicative life span by TOR and Sch9 in response to nutrients. Science. 2005;310:1193–1196. doi: 10.1126/science.1115535. [DOI] [PubMed] [Google Scholar]
- Kamo N, Ke B, Busuttil RW, Kupiec-Weglinski JW. PTEN-mediated Akt/beta-catenin/Foxo1 signaling regulates innate immune responses in mouse liver ischemia/reperfusion injury. Hepatology. 2013;57:289–298. doi: 10.1002/hep.25958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapahi P, Zid BM, Harper T, Koslover D, Sapin V, Benzer S. Regulation of lifespan in Drosophila by modulation of genes in the TOR signaling pathway. Curr Biol. 2004;14:885–890. doi: 10.1016/j.cub.2004.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenerson HL, Yeh MM, Yeung RS. Tuberous sclerosis complex-1 deficiency attenuates diet-induced hepatic lipid accumulation. PloS one. 2011;6:e18075. doi: 10.1371/journal.pone.0018075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klune JR, Tsung A. Molecular biology of liver ischemia/reperfusion injury: established mechanisms and recent advancements. Surg Clin North Am. 2010;90:665–677. doi: 10.1016/j.suc.2010.04.003. [DOI] [PubMed] [Google Scholar]
- Lamming DW, Ye L, Katajisto P, Goncalves MD, Saitoh M, Stevens DM, Davis JG, Salmon AB, Richardson A, Ahima RS, Guertin DA, Sabatini DM, Baur JA. Rapamycin-induced insulin resistance is mediated by mTORC2 loss and uncoupled from longevity. Science. 2012;335:1638–1643. doi: 10.1126/science.1215135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamming DW, Ye L, Sabatini DM, Baur JA. Rapalogs and mTOR inhibitors as anti-aging therapeutics. The Journal of clinical investigation. 2013;123:980–989. doi: 10.1172/JCI64099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine ME, Suarez JA, Brandhorst S, Balasubramanian P, Cheng CW, Madia F, Fontana L, Mirisola MG, Guevara-Aguirre J, Wan J, Passarino G, Kennedy BK, Wei M, Cohen P, Crimmins EM, Longo VD. Low protein intake is associated with a major reduction in IGF-1, cancer, and overall mortality in the 65 and younger but not older population. Cell metabolism. 2014;19:407–417. doi: 10.1016/j.cmet.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HT, Zhang HF, Si R, Zhang QJ, Zhang KR, Guo WY, Wang HC, Gao F. Insulin protects isolated hearts from ischemia/reperfusion injury: cross-talk between PI3-K/Akt and JNKs. Sheng Li Xue Bao. 2007;59:651–659. [PubMed] [Google Scholar]
- McCay CM, Crowel MF, Maynard LA. The effect of retarded growth upon the length of the life span and upon the ultimate body size. J Nutr. 1935;10:63–79. [PubMed] [Google Scholar]
- Menon S, Dibble CC, Talbott G, Hoxhaj G, Valvezan AJ, Takahashi H, Cantley LC, Manning BD. Spatial Control of the TSC Complex Integrates Insulin and Nutrient Regulation of mTORC1 at the Lysosome. Cell. 2014;156:771–785. doi: 10.1016/j.cell.2013.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon S, Yecies JL, Zhang HH, Howell JJ, Nicholatos J, Harputlugil E, Bronson RT, Kwiatkowski DJ, Manning BD. Chronic Activation of mTOR Complex 1 Is Sufficient to Cause Hepatocellular Carcinoma in Mice. Sci Signal. 2012;5:ra24. doi: 10.1126/scisignal.2002739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael MD, Kulkarni RN, Postic C, Previs SF, Shulman GI, Magnuson MA, Kahn CR. Loss of insulin signaling in hepatocytes leads to severe insulin resistance and progressive hepatic dysfunction. Molecular cell. 2000;6:87–97. [PubMed] [Google Scholar]
- Miller RA, Harrison DE, Astle CM, Baur JA, Boyd AR, de Cabo R, Fernandez E, Flurkey K, Javors MA, Nelson JF, Orihuela CJ, Pletcher S, Sharp ZD, Sinclair D, Starnes JW, Wilkinson JE, Nadon NL, Strong R. Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. J Gerontol A Biol Sci Med Sci. 2011;66:191–201. doi: 10.1093/gerona/glq178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RA, Harrison DE, Astle CM, Fernandez E, Flurkey K, Han M, Javors MA, Li X, Nadon NL, Nelson JF, Pletcher S, Salmon AB, Sharp ZD, Van Roekel S, Winkleman L, Strong R. Rapamycin-Mediated Lifespan Increase in Mice is Dose and Sex-Dependent and Appears Metabolically Distinct from Dietary Restriction. Aging cell. 2013 doi: 10.1111/acel.12194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JR, Beckman JA, Nguyen LL, Ozaki CK. Reducing elective vascular surgery perioperative risk with brief preoperative dietary restriction. Surgery. 2013;153:594–598. doi: 10.1016/j.surg.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JR, Verweij M, Brand K, van de Ven M, Goemaere N, van den Engel S, Chu T, Forrer F, Muller C, de Jong M, van IW, JN IJ, Hoeijmakers JH, de Bruin RW. Short-term dietary restriction and fasting precondition against ischemia reperfusion injury in mice. Aging cell. 2010;9:40–53. doi: 10.1111/j.1474-9726.2009.00532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadakis M, Hadley G, Xilouri M, Hoyte LC, Nagel S, McMenamin MM, Tsaknakis G, Watt SM, Drakesmith CW, Chen R, Wood MJ, Zhao Z, Kessler B, Vekrellis K, Buchan AM. Tsc1 (hamartin) confers neuroprotection against ischemia by inducing autophagy. Nat Med. 2013;19:351–357. doi: 10.1038/nm.3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng W, Robertson L, Gallinetti J, Mejia P, Vose S, Charlip A, Chu T, Mitchell JR. Surgical stress resistance induced by single amino acid deprivation requires Gcn2 in mice. Sci Transl Med. 2012;4:118ra111. doi: 10.1126/scitranslmed.3002629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper MD, Partridge L, Raubenheimer D, Simpson SJ. Dietary restriction and aging: a unifying perspective. Cell metabolism. 2011;14:154–160. doi: 10.1016/j.cmet.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffaghello L, Lee C, Safdie FM, Wei M, Madia F, Bianchi G, Longo VD. Starvation-dependent differential stress resistance protects normal but not cancer cells against high-dose chemotherapy. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:8215–8220. doi: 10.1073/pnas.0708100105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren F, Duan Z, Cheng Q, Shen X, Gao F, Bai L, Liu J, Busuttil RW, Kupiec-Weglinski JW, Zhai Y. Inhibition of glycogen synthase kinase 3 beta ameliorates liver ischemia reperfusion injury by way of an interleukin-10-mediated immune regulatory mechanism. Hepatology. 2011;54:687–696. doi: 10.1002/hep.24419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rincon M, Muzumdar R, Atzmon G, Barzilai N. The paradox of the insulin/IGF-1 signaling pathway in longevity. Mechanisms of ageing and development. 2004;125:397–403. doi: 10.1016/j.mad.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Robertson LT, Mitchell JR. Benefits of short-term dietary restriction in mammals. Experimental gerontology. 2013;48:1043–1048. doi: 10.1016/j.exger.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancak Y, Peterson TR, Shaul YD, Lindquist RA, Thoreen CC, Bar-Peled L, Sabatini DM. The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science. 2008;320:1496–1501. doi: 10.1126/science.1157535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selman C, Tullet JM, Wieser D, Irvine E, Lingard SJ, Choudhury AI, Claret M, Al-Qassab H, Carmignac D, Ramadani F, Woods A, Robinson IC, Schuster E, Batterham RL, Kozma SC, Thomas G, Carling D, Okkenhaug K, Thornton JM, Partridge L, Gems D, Withers DJ. Ribosomal protein S6 kinase 1 signaling regulates mammalian life span. Science. 2009;326:140–144. doi: 10.1126/science.1177221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta S, Peterson TR, Laplante M, Oh S, Sabatini DM. mTORC1 controls fasting-induced ketogenesis and its modulation by ageing. Nature. 2010;468:1100–1104. doi: 10.1038/nature09584. [DOI] [PubMed] [Google Scholar]
- Solon-Biet SM, McMahon AC, Ballard JW, Ruohonen K, Wu LE, Cogger VC, Warren A, Huang X, Pichaud N, Melvin RG, Gokarn R, Khalil M, Turner N, Cooney GJ, Sinclair DA, Raubenheimer D, Le Couteur DG, Simpson SJ. The ratio of macronutrients, not caloric intake, dictates cardiometabolic health, aging, and longevity in ad libitum-fed mice. Cell metabolism. 2014;19:418–430. doi: 10.1016/j.cmet.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang BL. SIRT1, neuronal cell survival and the insulin/IGF-1 aging paradox. Neurobiology of aging. 2006;27:501–505. doi: 10.1016/j.neurobiolaging.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Toyoshima Y, Tokita R, Ohne Y, Hakuno F, Noguchi T, Minami S, Kato H, Takahashi S. Dietary protein deprivation upregulates insulin signaling and inhibits gluconeogenesis in rat liver. J Mol Endocrinol. 2010;45:329–340. doi: 10.1677/JME-10-0102. [DOI] [PubMed] [Google Scholar]
- Varendi K, Airavaara M, Anttila J, Vose S, Planken A, Saarma M, Mitchell JR, Andressoo JO. Short-term preoperative dietary restriction is neuroprotective in a rat focal stroke model. PloS one. 2014;9:e93911. doi: 10.1371/journal.pone.0093911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JJ, Liu J, Chen EB, Wang JJ, Cao L, Narayan N, Fergusson MM, Rovira II, Allen M, Springer DA, Lago CU, Zhang S, DuBois W, Ward T, deCabo R, Gavrilova O, Mock B, Finkel T. Increased mammalian lifespan and a segmental and tissue-specific slowing of aging after genetic reduction of mTOR expression. Cell Rep. 2013;4:913–920. doi: 10.1016/j.celrep.2013.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao F, Huang Z, Li H, Yu J, Wang C, Chen S, Meng Q, Cheng Y, Gao X, Li J, Liu Y, Guo F. Leucine deprivation increases hepatic insulin sensitivity via GCN2/mTOR/S6K1 and AMPK pathways. Diabetes. 2011;60:746–756. doi: 10.2337/db10-1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi S, Ishihara H, Yamada T, Tamura A, Usui M, Tominaga R, Munakata Y, Satake C, Katagiri H, Tashiro F, Aburatani H, Tsukiyama-Kohara K, Miyazaki J, Sonenberg N, Oka Y. ATF4-mediated induction of 4E-BP1 contributes to pancreatic beta cell survival under endoplasmic reticulum stress. Cell metabolism. 2008;7:269–276. doi: 10.1016/j.cmet.2008.01.008. [DOI] [PubMed] [Google Scholar]
- Yecies JL, Zhang HH, Menon S, Liu S, Yecies D, Lipovsky AI, Gorgun C, Kwiatkowski DJ, Hotamisligil GS, Lee CH, Manning BD. Akt stimulates hepatic SREBP1c and lipogenesis through parallel mTORC1-dependent and independent pathways. Cell metabolism. 2011;14:21–32. doi: 10.1016/j.cmet.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Tang N, Hadden TJ, Rishi AK. Akt, FoxO and regulation of apoptosis. Biochim Biophys Acta. 2011;1813:1978–1986. doi: 10.1016/j.bbamcr.2011.03.010. [DOI] [PubMed] [Google Scholar]
- Zid BM, Rogers AN, Katewa SD, Vargas MA, Kolipinski MC, Lu TA, Benzer S, Kapahi P. 4E-BP extends lifespan upon dietary restriction by enhancing mitochondrial activity in Drosophila. Cell. 2009;139:149–160. doi: 10.1016/j.cell.2009.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.