Abstract
Although osteoblast lineage cells, especially osteocytes, are thought to be a primary mechanosensory cell in bone, the identity of the mechano-receptor and downstream mechano-signaling pathways remain largely unknown. Here we show using osteoblastic cell model of mechanical stimulation with fluid shear stress that in the absence of integrin αv, phosphorylation of the Src substrate p130Cas and JNK was impaired, culminating in an inhibition of nuclear translocation of YAP/TAZ and subsequent transcriptional activation of target genes. Targeted deletion of the integrin αv in osteoblast lineage cells results in an attenuated response to mechanical loading in terms of Sost gene expression, indicative of a role for integrin αv in mechanoreception in vivo. Thus, integirn αv may be integral to a mechanosensing machinery in osteoblastic cells and involved in activation of a Src-JNK-YAP/TAZ pathway in response to mechanical stimulation.
Keywords: osteoblast, integrin αv, mechanotransduction, p130Cas, YAP/TAZ
Introduction
Mechanical forces play important roles in the regulation of skeletal development and homeostasis [1,2], which is represented by a gradual development of bone atrophy (over months and years) in the elderly due to declining daily activities or sedentary life style, and by a rapid bone loss (over days or weeks) even in young healthy individuals placed under microgravity. It is recognized that disuse bone atrophy is caused, at the tissue level, by aberrant activation of osteoclastic bone resorption and suppression of osteoblastic bone formation. We have previously made a transgenic mouse model that allows for an inducible ablation of osteocytes, and found that when osteocytes were ablated in young adult mice, bone loss caused by unloading through tail suspension was inhibited, suggesting that osteocytes play pivotal roles in the sensing and/or transduction of mechanical signals, thereby controlling osteoclast and osteoblast activities on the bone surface [3]. However, the identity of the mehcano-receptor that converts mechanical forces into biochemical signals remains to be determined [4,5].
Integrins are a family of transmembrane receptors that mediate physical attachment between a cell and its extracellular matrix and transmit signals, outside-in as well as inside-out, in response to environmental cues [6], and integrin-mediated signaling pathways have been implicated in mechanotransduction especially in cardiovascular cells [7,8]. Integrins in mechanosensing in bone have been studied mainly using in vitro models. It was shown, for example, that mechanical stimulation by stretching activates ERK in an integrin-dependent manner in MLO-Y4 osteocytic cells [9], and that αvβ3 integrin signaling enhances the response to hypotonic swelling with increased cytosolic calcium in rat osteocyte cultures [10]. However, signaling pathways downstream of integrins in skeletal mechanotransduction are not fully understood. Taking advantage of the availability of the integrin αv floxed mouse [11], we have now examined the roles of integrin αv in mechanical response in vitro as well as in vivo.
Material and Methods
Reagents
Anti-integrin αv polyclonal antibody was purchased from Millipore (Billerica, MA, USA). Anti-total Src, anti-SrcY416, anti-SrcY527, anti-JNK, anti-phospho-JNK, anti-p38, anti-phospho-p38, anti-p44/42 ERK and anti-phospho-p44/42 ERK polyclonal antibodies were purchased from Cell Signaling Technology (Beverly, MA, USA), anti-YAP/TAZ polyclonal antibody from Santa Cruz biotechnology (Santa Cruz, CA, USA), and anti-β-actin monoclonal antibody from Biovision (Milpitas, CA, USA). Anti-p130Cas (Cas3) polyclonal antibody and anti-CasY165 antibody were a kind gift from Dr. Yasuhiro Sawada (National University of Singapore) [12]. SP600125, a specific JNK inhibitor, was purchased from Wako Pure Chemicals (Osaka, Japan). All other chemicals were obtained from Sigma (St. Louis, MO, USA).
Mice
Integrin αv (Itgav) flox mice have been described [11] and genotyping was performed according to the reported method [13]. Mice hemizygous for Osterix-GFP(x02237)Cre transgene [14] and Src knockout mice [15] were obtained from the Jackson Laboratory.
Mice were raised under standard laboratory conditions at 24 ± 2 °C and 50-60% humidity, and allowed free access to tap water and commercial standard rodent chow (CE-2) containing 1.20% calcium, 1.08% phosphate, and 240 JU/100 g vitamin D3 (Clea Japan Inc., Tokyo, Japan). Loading on the forearm was performed according to the published method [16]; under isoflurane-induced anesthesia, the right forearms was loaded for a single session (2.5-2.8N, 2 Hz, 120 cycles), and the left forearms were not loaded and served as a control. Mice were sacrificed 24 h later and RNA was extracted from the ulnae for gene expression analysis.
All experiments were performed in strict accordance with the recommendations in the Guideline for the Care and Use of Laboratory Animals of the National Center for Geriatrics and Gerontology, and the protocol was approved by the Committee on the Ethics of Animal Experiments of the National Center for Geriatrics and Gerontology (Permit Number: 25-16R1). All efforts were made to minimize suffering.
Cell culture, adenoviral infection and fluid shear stress
Primary osteoblastic cells were isolated from new-born mouse calvaria and cultured in α-MEM medium supplemented with 10% fetal bovine serum (FBS) 100 μg/mL streptomycin, and 100 units/mL penicillin as described previously [3]. After washing, GFP-positive cells were sorted using flow cytometry (FACS Aria sorter, BD biosciences). For adenoviral infection, the cultures were exposed to fresh α-MEM containing adeno-LacZ or adeno-Cre virus at a multiplicity of infection of 20-100 for 48 h. The plasmid vectors for making adenoviral preparations (AxCAiLacZ and AxCANCre) were purchased from Takara (Shiga, Japan), and adenovirus was purified before infection using Vivapure AdenoPACK kit (Sartorius, Goettingen, Germany). For the fluid shear stress to primary osteoblasts, a flow in culture medium was generated by shaking culture dishes with a shaker placed in a culture chamber as described [17,18]. Cells were cultured in a-MEM containing 0.3 % FBS for 16 hours before mechanical stimulation.
shRNA delivery by lentiviral transduction
To generate lentiviral particles, 293T cells were transfected with the MISSION shRNA plasmid DNA and MISSION Lentiviral Packaging Mix (Sigma) using X-tremeGENE 9 DNA Transfection Reagent (Roche, CA, USA), and the supernatant was collected from 24 to 48 hours. For infection, calvaria-derived osteoblasts were incubated with lentiviral supernatants for 24 hours followed by selection with puromycin (2.5 μg/ml) for 48 hours. The target sequences were 5′-CCGGGCAGACAGATTCCTTTGTTAACTCGAGTTAACAAAGGAATCTGTCT GCTTTTTG-3′ for YAP #1; 5′ -CCGGCCACCAAGCTAGATAAAGAAACTCGAGTTTCTTTATCTAGCTTGGTG GTTTTTG-3′ for YAP #2.
Gene expression studies
Total RNAs were isolated from cells with RNeasy mini kit (Qiagen, Hilden, Germany) or SuperPrep Cell Lysis Kit (TOYOBO, Osaka, Japan) and bone with TRIzol Reagent (Invitrogen, San Diego, CA). Bone samples were divided into osteoblast- and osteocyte-rich fractions: after bone marrow cells were flushed out with phosphate-buffered saline (PBS), the cells on the endocortical surface were collected as an osteoblast-rich fraction by brushing with an interdental brush in the presence of the TRIzol reagent. The residual bone pieces were crushed in liquid nitrogen to yield an osteocyte-rich fraction [19], Isolated RNAs were reverse transcribed using a High capacity cDNA reverse transcription kit (Applied Biosystems, Foster City, CA, USA). For quantitative RT-PCR, samples were analyzed using PowerSYBR Green PCR master mix and an ABI7300 real time PCR system (Applied Biosystems); the primers used are summarized in Supplemental Table 1. The abundance of each target mRNA was normalized by that of Gapdh mRNA.
Immunoblotting
After calvaria-derived osteoblasts were washed and lysed, cell lysates were boiled in SDS sample buffer and subjected to electrophoresis on 10% SDS-PAGE. Proteins were transferred to PVDF membranes using a semi-dry blotter (Bio-Rad, Hercules, CA) and incubated in blocking solution (3% bovine serum albumin in TBS containing or 5% non-fat dry milk in TBS containing 0.1% Tween 20) for 1 h to reduce nonspecific binding. Membranes were then exposed to primary antibodies overnight at 4 °C, washed three times, and incubated with secondary goat anti-mouse or rabbit IgG horseradish peroxidase-conjugated antibody for 30 min. Membranes were washed extensively, and enhanced chemiluminescence detection assay was performed according to the manufacturer's directions.
Immunofluorescence
Calvaria-derived primary osteoblasts were seeded onto uncoated coverslips and were fixed in a 4% paraformaldehyde in PBS at room temperature for 10 min. The fixed specimens were washed three times in PBS containing 0.01% Triton X-100. For immunostaining, rabbit anti-YAP/TAZ polyclonal antibody diluted at 1:100 in Can Get Signal Immunostain Solution A (TOYOBO) was added with Cy3-conjugated anti-mouse at 1:300 (Molecular Probes, Eugene, OR, USA). Hoechst 33258 was used to visualize nucleus.
Statistical analysis
Data are expressed as the mean ± SD. Statistical analysis was performed using Student's t test and one or two-way ANOVA with Fisher's least significance post-hoc analysis as appropriate for the data set. Values were considered statistically significant at p < 0.05.
Results
In order to study the roles of integrin αv in mechanical response, primary osteoblastic cells with αv integrin (Itgav) flox/flox alleles [11] were isolated from the newborn mouse calvaria, and infected with adeno viral vectors producing Cre recombinase or LacZ as control. As shown in Figure 1A, αv integrin was expressed in primary osteoblasts, and its expression was markedly reduced by infection with adeno-Cre vector at both mRNA and protein levels. In order to impose on osteoblastic cells mechanical stimulation similar to fluid shear stress (FSS), a flow in culture medium was generated by shaking culture dishes according to the previously described method [17,18]; the FSS effect was confirmed by examining the expression of the known mechano-responsive genes, Egr1 and Fos [17,20] (Figure 1B). In cellular response to mechanical stimulation it is known that p130Cas, a substrate of Src kinase, acts as a primary force sensor, transducing force generated by cell extension and thereby priming phosphorylation and activation of downstream signaling molecules [12,21]. Western blot analysis revealed that phosphorylation of tyrosine 165 of p130Cas was stimulated within 20 min following FSS in primary osteoblastic cells, whereas the phosphorylation was markedly inhibited in the absence of integrin αv (Figure 2). In response to the mechanical stimulation, an increase in the stimulatory phosphorylation of c-Src (Y416) and a reciprocal decrease in the inhibitory phosphorylation (Y527) were observed in control cells, while both responses were inhibited in αv-deficient osteoblastic cells (Figure 2). Following phosphorylation of p130Cas, FSS caused a robust induction of JNK phosphorylation in control cells, while this response was delayed in integrin αv-deficient osteoblastic cells (Figure 2). The phosphorylation of p38 and ERK following FSS did not differ between control and integrin αv-deficient osteoblasts (Figure 2 and data not shown).
Figure 1. Osteoblastic cell model for mechanical stimulation.
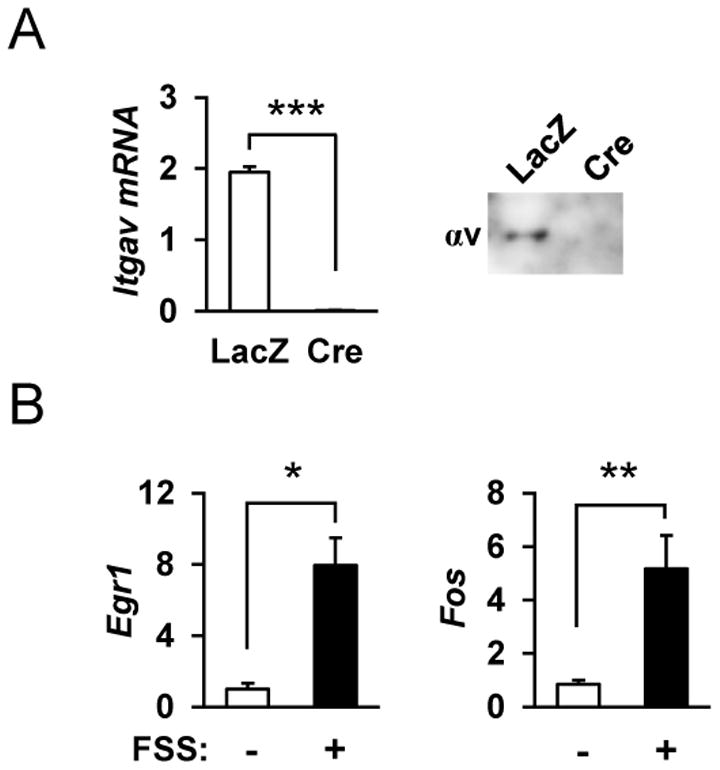
(A) Primary osteoblasts derived from the calvaria of αv flox/flox mice were infected with adeno-LacZ (LacZ) or adeno-Cre (Cre) vectors and, and RNA and protein were extracted for Itgav mRNA and protein expression. ***p<0.001 (B) Calvaria-derived primary osteoblasts respond to fluid shear stress (FSS) with increased expression of Egr1 and Fos. RNA was isolated at 0 and 30 min following FSS, and gene expression was analyzed by qRT-PCR. **p<0.01, *p<0.05 (n=4 each group).
Figure 2. Phosphorylation of p130Cas, c-Src and JNK downstream of integrin αv following mechanical stimulation.
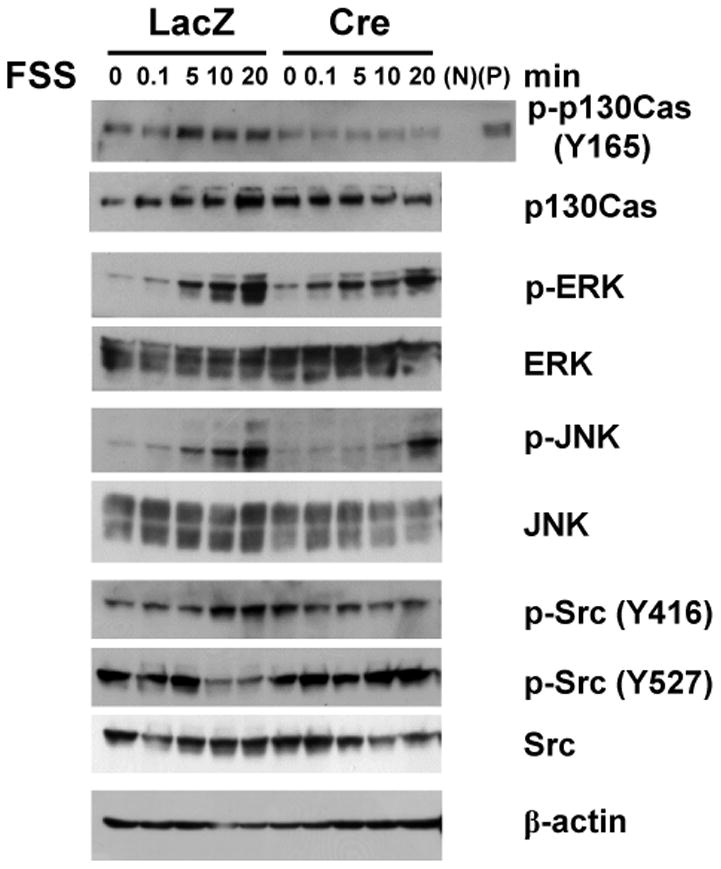
Integrin av-deficient osteoblasts show impaired phosphorylation of p130Cas and JNK in response to FSS. Calvaria-derived primary osteoblasts from αv flox/flox mice were infected with adeno-LacZ (-) or adeno-Cre (+) vectors, and subjected to FSS. Total cellular protein was isolated from control (LacZ) and integrin αv-deficient (Cre) osteoblasts at 0, 0.1, 5, 10, and 20 min following FSS, and immunoblotted with the indicated antibodies. N and P indicate negative and positive controls for phosphorylated p130Cas (p-p130Cas), which represent unattached cells in suspension and phenylarsine oxide-treated attached cells, respectively.
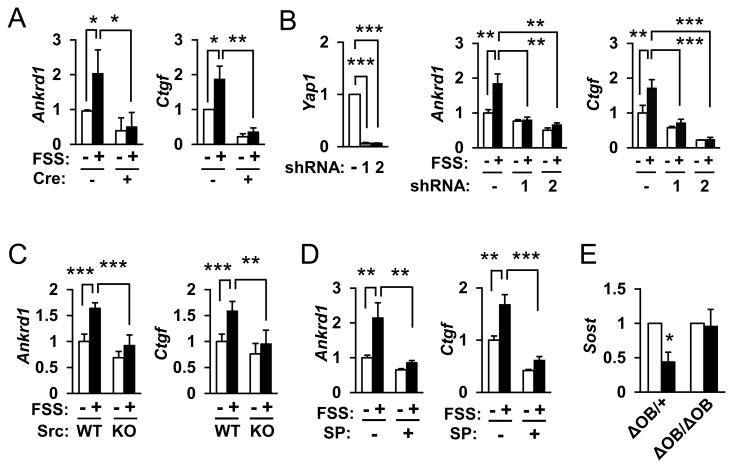
It has recently been reported that the Yorkie-homologues YAP (Yes-associated protein) and TAZ (transcriptional coactivator with PDZ-binding motif) function as nuclear relays of mechanical signals exerted by ECM rigidity and cell shape [22]. Indeed, we found by immunofluorescence that FSS imposed on primary osteoblastic cells enhanced nuclear localization of YAP/TAZ within 30 min (Figure 3A), and that deletion of integrin αv in osteoblastic cells inhibited the nuclear targeting of YAP/TAZ (Figure 3B); the increase in % of cells with predominant nuclear staining in response to FSS was abrogated when integrin αv was deleted with adeno-Cre infection (bar graphs in Figure 3). These results raise the possibility that YAP/TAZ functions downstream of integrin αv in mechanical signaling in osteoblasts. In fact, the expression of Ankrd1 and Ctgf, target genes of YAP/TAZ, was stimulated following FSS in control cells, whereas it was almost completely inhibited in integrin αv-deficient osteoblastic cells (Figure 4A). Knockdown of Yap1 by shRNA inhibited the increases in Ankrdl and Ctgf mRNA expression following FSS (Figure 4B), and Src-deficient osteoblastic cells also exhibited impaired responses in YAP/TAZ target gene responses to FSS (Figure 4C), which can be taken as evidence suggesting that c-Src is involved in the transcriptional activation of YAP/TAZ in response to mechanical stimulation. The stimulation of Ankrd1 and Ctgf expression by FSS was inhibited in the presence of SP600125, a specific inhibitor of JNK (Figure 4D), pointing to an involvement of JNK in FSS response. Taken together, it was suggested that integrin αv plays an important role in the mechanotransduction pathway in osteoblastic cells through a Src-p130Cas-JNK pathway, thereby controlling nuclear localization of YAP/TAZ and their transcription function.
Figure 3. Nuclear localization of YAP/TAZ downstream of integrin αv following mechanical stimulation.
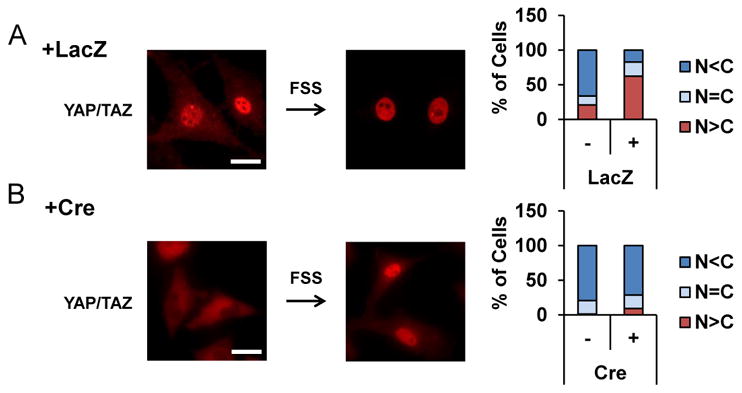
Nuclear localization of YAP/TAZ following FSS in control osteoblasts (A) was impaired in integrin αv -deficient osteoblasts (+Cre in B). YAP/TAZ was visualized at 30 min after FSS by immunofluorescence using an anti-YAP antibody that also recognizes TAZ (Red). Scale bars, 25 μm. (right) % of cells with predominant nuclear (N>C, red) or cytoplasmic (N<C, blue) staining was quantified in more than 100 cells each group.
Figure 4. c-Src, JNK and YAP/TAZ in mechanotransduction pathway downstream of integrin αv.
(A) Impaired expression of YAP/TAZ target genes, Ankrd1 and Ctgf, at 30 min following FSS in integrin αv-deficient osteoblasts (Cre+) by qRT-PCR. **p<0.01, *p<0.05 (n=4 each group). (B) Impaired expression of YAP/TAZ target genes in response to FSS in YAP knockdown osteoblasts. Two shRNAs against Yap1 (1 and 2) were used. - indicates scramble shRNA as negative control. ***p<0.001, **p<0.01 (n=4 each group). (C, D) Involvement of c-Src (C) and JNK (D) in the response of YAP/TAZ target genes to FSS. Primary osteoblasts from Src knockout mice (C) or wild-type osteoblasts treated with a JNK inhibitor, SP600125 (D), were subjected to FSS stimulation, and the expression of Ankrd1 and Ctgf mRNA was quantified. ***p<0.001, **p<0.01 (n=4 for B, n=6 for C, each group). (E) Attenuated response to mechanical loading in mice lacking the Itgav gene in the osteoblast lineage. Quantitative RT-PCR analysis of the relative abundance of Sost mRNA in the ulnae of 18-week-old female mice of the indicated genotypes subjected to mechanical loading on the right forearms (black bars), or without loading on the left forearms (white bars) as control. Data are means ± SD for six mice of each group and are expressed as a percentage of the corresponding control value. *p<0.05.
Finally, in order to examine the role of integrin αv in mechanical stimulation in vivo, we generated mice with specific deletion of the gene in the osteoblast lineage by crossing Itgav flox mice [11] with Osterix-Cre mice [14] and subjected the resultant ΔAOB/ΔAOB mice to mechanical loading on the forearms. When RNAs were prepared separately from osteoblast- and osteocyte-rich fractions of femurs and tibias of wild-type mice [19], the expression of Itgav mRNA was observed in both fractions, and deletion of the Itgav gene with Osterix-Cre resulted in significant decreases in both fractions in vivo (Supplemental Figure 1A). In the light of our experience with the Osterix-Cre mouse capable of deleting a target gene in the osteoblast lineage with more than 80% efficiency [19], it is likely that the apparently only modest reductions in Itgav mRNA levels in the current model is due to the presence in the bone tissues of blood vessels and nerves that are known to express integrin αv abundantly [7,11,13]. In fact, when osterix-positive osteoblastic cells were isolated from the calvaria by FACS using co-expressed GFP as a marker, the expression of Itgav mRNA was markedly reduced in the cells with targeted deletion in osteoblastic cells (ΔAOB/ΔAOB) (Supplemental Figure 1B). Importantly, whereas control (ΔAOB/+) mice showed a significant reduction in Sost expression as expected from the previous studies [16], ΔAOB/ΔAOB mice did not (Figure 4E). Thus, it was suggested that integrin αv in osteoblasts and more likely in osteocytes functions in the mechanical response of bone, at least in terms of Sost expression.
Discussion
Using primary osteoblastic cells and mechanical stimulation in the form of FSS, we have identified in the present study intracellular kinase cascades involving c-Src, p130Cas and JNK as a mechanotransduction pathway. It was proposed that when cells are stretched, p130Cas molecule associated with cytoskeleton is extended so that it is exposed to c-Src and activated through tyrosine phosphorylation [12,21], and together with the current data, it is assumed that integrin αv acts as a bridge between extracellular environment and the cytoskeleton, transmitting an external force imposed on the cell to p130Cas associated with the cytoskeleton. With respect to mechano-signaling pathways in osteoblastic cells, especially in response to FSS, the activation of MAP kinase specifically ERK1/2 has been shown [9,17,23]. In our model with primary osteoblastic cells, we found activation of JNK as well as p38 and ERK1/2 by FSS. However, deletion of integrin αv did not impact p38 or ERK1/2 phosphorylation but substantially inhibited the activation of JNK. Thus, the signaling downstream of integrin αv may be distinct form the reported one involving PKG2, SHPl/2andERKl/2[23].
Importantly, we have found that the mice deficient in integrin αv in osteoblast lineage cells exhibit an impaired skeletal response to loading, at least in terms of Sost gene expression, pointing to the role of integrin av in mechano-sensing in vivo. In the light of the previous observations that Sost expression in osteocytes is regulated by mechanical cues, i.e. decreased by loading [16] and increased under unloading [24], the findings that the response of Sost gene expression to mechanical stimulation was impaired in the absence of integrin αv in the current model can be taken as further evidence for a critical role of integrin αv in the mechanical regulation of osteocyte function and that it is an integral component of the mechano-sensing machinery.
Osteocytes are particularly sensitive to FSS, and integrins have been postulated to function as a mechano-receptor along the cell process by sensing FSS [5]. The peri-cellular space between the osteocyte process and the canalicular wall, which allows for fluid flow and metabolite transport, is estimated to be 50-80 nm, and a recent transmission electron microscopy observation has revealed protrusions from the canalicular wall, which contact the cell membrane of the osteocyte process, where the integrin αvβ3 was identified by immunohistochemistry [25]. Taken together with the current results, it is conceivable that αvβ3 integrin on the osteocyte process is involved in the sensing of a mechanical strain in the form of fluid flow.
YAP and TAZ were identified as transcription factors acting in Hippo pathway [26], and have also been implicated in cell recognition of matrix rigidity and mechanotransduction independently of the Hippo cascade [22]. In the present study we have demonstrated that YAP/TAZ function in osteoblastic cells in the response to mechanical stimulation specifically in the form of FSS. YAP is a transcription coactivator and the TEAD family transcription factors have been identified as mediators of YAP-dependent gene expression [27]. TEAD-binding sites have been shown in the promoter region of the CTGF gene [27], which is used as a readout of YAP/TAZ transcription function in the current study. Physical and functional interactions between YAP/TAZ and Runx2 have also been reported [28,29]; YAP represses the transcription of osteocalcin gene through interaction with Runx2 in osteoblastic ROS17.28 cells [28], while TAZ stimulates osteogenesis from mesenchymal stem cells by activating Runx2 activity and suppressing PPAR γfunction [29]. More recently, YAP/TAZ activities have been shown to be regulated via integrin β1 and to stimulate osteogenesis from mesenchymal stem cells in vivo [30]. In addition, c-Src is required for the activation of YAP and YAP-mediated matrix remodeling by cancer-associated fibroblasts [31], which is consistent with the current findings that c-Src is involved in YAP/TAZ function downstream of αv in responses to FSS. We propose that mechanical stimulation, at least in the form of FSS, is sensed through integrin αv in osteoblast lineage cells and relayed to the stimulation of Src kinase activity and phosphorylation of p130Cas and JNK, culminating in the activation of YAP/TAZ transcription function (Supplemental Figure 2).
Supplementary Material
Acknowledgments
We thank Y Sawada for advice on p130Cas detection. This study was supported by JSPS KAKENHI for Young Investigator (#24790398 to K.K.) and by MEXT KAHENHI for Scientific Research on Innovative Areas (#22118007 to K.I.) from the Ministry of Education, Science of Japan; by a grant from the program Promotion of Fundamental Studies in Health Sciences of the National Institute of Biomedical Innovation (NIBIO) of Japan (#06-31 to K.I. and M.I.); by a grant from Naito Foundation (to K.I.); by a grant from Aichi Health Promotion Foundation (to K.I.); and by a grant for Longevity Sciences from the Ministry of Health, Labor and Welfare of Japan (#H23-12 to K.I. and M.I.).
Footnotes
The authors have no conflict of interest.
References
- 1.Zaidi M. Skeletal remodeling in health and disease. Nat Med. 2007;13:791–801. doi: 10.1038/nm1593. [DOI] [PubMed] [Google Scholar]
- 2.Bonewald LF, Johnson ML. Osteocytes, mechanosensing and Wnt signaling. Bone. 2008;42:606–615. doi: 10.1016/j.bone.2007.12.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tatsumi S, Ishii K, Amizuka N, Li M, Kobayashi T, Kohno K, Ito M, Takeshita S, Ikeda K. Targeted ablation of osteocytes induces osteoporosis with defective mechanotransduction. Cell Metab. 2007;5:464–475. doi: 10.1016/j.cmet.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 4.Bonewald LF. The amazing osteocyte. J Bone Miner Res. 2011;26:229–238. doi: 10.1002/jbmr.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klein-Nulend J, Bakker AD, Bacabac RG, Vatsa A, Weinbaum S. Mechanosensation and transduction in osteocytes. Bone. 2013;54:183–190. doi: 10.1016/j.bone.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 6.Hynes RO. Integrins: bidirectional,allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 7.Katsumi A, Orr AW, Tzima E, Schwartz MA. Integrins in mechanotransduction. J Biol Chem. 2004;279:12001–12004. doi: 10.1074/jbc.R300038200. [DOI] [PubMed] [Google Scholar]
- 8.Weinbaum S, Duan Y, Thi MM, You L. An Integrative Review of Mechanotransduction in Endothelial, Epithelial (Renal) and Dendritic Cells (Osteocytes) Cell Mol Bioeng. 2011;4:510–537. doi: 10.1007/s12195-011-0179-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Plotkin LI, Mathov I, Aguirre JI, Parfitt AM, Manolagas SC, Bellido T. Mechanical stimulation prevents osteocyte apoptosis: requirement of integrins,Src kinases, and ERKs. Am J Physiol Cell Physiol. 2005;289:C633–643. doi: 10.1152/ajpcell.00278.2004. [DOI] [PubMed] [Google Scholar]
- 10.Miyauchi A, Gotoh M, Kamioka H, Notoya K, Sekiya H, Takagi Y, Yoshimoto Y, Ishikawa H, Chihara K, Takano-Yamamoto T, Fujita T, Mikuni-Takagaki Y. AlphaVbeta3 integrin ligands enhance volume-sensitive calcium influx in mechanically stretched osteocytes. J Bone Miner Metab. 2006;24:498–504. doi: 10.1007/s00774-006-0716-x. [DOI] [PubMed] [Google Scholar]
- 11.Lacy-Hulbert A, Smith AM, Tissire H, Barry M, Crowley D, Bronson RT, Roes JT, Savill JS, Hynes RO. Ulcerative colitis and autoimmunity induced by loss of myeloid alphav integrins. Proc Natl Acad Sci U S A. 2007;104:15823–15828. doi: 10.1073/pnas.0707421104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sawada Y, Tamada M, Dubin-Thaler BJ, Cherniavskaya O, Sakai R, Tanaka S, Sheetz MP. Force sensing by mechanical extension of the Src family kinase substrate p130Cas. Cell. 2006;127:1015–1026. doi: 10.1016/j.cell.2006.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCarty JH, Lacy-Hulbert A, Charest A, Bronson RT, Crowley D, Housman D, Savill J, Roes J, Hynes RO. Selective ablation of alphav integrins in the central nervous system leads to cerebral hemorrhage, seizures, axonal degeneration and premature death. Development. 2005;132:165–176. doi: 10.1242/dev.01551. [DOI] [PubMed] [Google Scholar]
- 14.Rodda SJ, McMahon AP. Distinct roles for Hedgehog and canonical Wnt signaling in specification, differentiation and maintenance of osteoblast progenitors. Development. 2006;133:3231–3244. doi: 10.1242/dev.02480. [DOI] [PubMed] [Google Scholar]
- 15.Soriano P, Montgomery C, Geske R, Bradley A. Targeted disruption of the c-src proto-oncogene leads to osteopetrosis in mice. Cell. 1991;64:693–702. doi: 10.1016/0092-8674(91)90499-o. [DOI] [PubMed] [Google Scholar]
- 16.Robling AG, Niziolek PJ, Baldridge LA, Condon KW, Allen MR, Alam I, Mantila SM, Gluhak-Heinrich J, Bellido TM, Harris SE, Turner CH. Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. J Biol Chem. 2008;283:5866–5875. doi: 10.1074/jbc.M705092200. [DOI] [PubMed] [Google Scholar]
- 17.Ogata T. Egr-1 mRNA induction by medium flow involves mRNA stabilization and is enhanced by the p38 inhibitor SB203580 in osteoblast-like cells. Acta Physiol (Oxf) 2008;194:177–188. doi: 10.1111/j.1748-1716.2008.01873.x. [DOI] [PubMed] [Google Scholar]
- 18.Ogawa H, Kozhemyakina E, Hung HH, Grodzinsky AJ, Lassar AB. Mechanical motion promotes expression of Prg4 in articular cartilage via multiple CREB-dependent, fluid flow shear stress-induced signaling pathways. Genes Dev. 2014;28:127–139. doi: 10.1101/gad.231969.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fumoto T, Takeshita S, Ito M, Ikeda K. Physiological Functions of Osteoblast Lineage and T Cell-Derived RANKL in Bone Homeostasis. J Bone Miner Res. 2014;29:830–842. doi: 10.1002/jbmr.2096. [DOI] [PubMed] [Google Scholar]
- 20.Hughes-Fulford M. Signal transduction and mechanical stress. Sci STKE. 2004;2004:RE12. doi: 10.1126/stke.2492004re12. [DOI] [PubMed] [Google Scholar]
- 21.Tamada M, Sheetz MP, Sawada Y. Activation of a signaling cascade by cytoskeleton stretch. Dev Cell. 2004;7:709–718. doi: 10.1016/j.devcel.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 22.Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, Zanconato F, Le Digabel J, Forcato M, Bicciato S, Elvassore N, Piccolo S. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179–183. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- 23.Rangaswami H, Schwappacher R, Marathe N, Zhuang S, Casteel DE, Haas B, Chen Y, Pfeifer A, Kato H, Shattil S, Boss GR, Pilz RB. Cyclic GMP and protein kinase G control a Src-containing mechanosome in osteoblasts. Sci Signal. 2010;3:ra91. doi: 10.1126/scisignal.2001423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin C, Jiang X, Dai Z, Guo X, Weng T, Wang J, Li Y, Feng G, Gao X, He L. Sclerostin mediates bone response to mechanical unloading through antagonizing Wnt/beta-catenin signaling. J Bone Miner Res. 2009;24:1651–1661. doi: 10.1359/jbmr.090411. [DOI] [PubMed] [Google Scholar]
- 25.McNamara LM, Majeska RJ, Weinbaum S, Friedrich V, Schaffler MB. Attachment of osteocyte cell processes to the bone matrix. Anat Rec (Hoboken) 2009;292:355–363. doi: 10.1002/ar.20869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haider G, Johnson RL. Hippo signaling: growth control and beyond. Development. 2011;138:9–22. doi: 10.1242/dev.045500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao B, Ye X, Yu J, Li L, Li W, Li S, Yu J, Lin JD, Wang CY, Chinnaiyan AM, Lai ZC, Guan KL. TEAD mediates YAP-dependent gene induction and growth control. Genes Dev. 2008;22:1962–1971. doi: 10.1101/gad.1664408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zaidi SK, Sullivan AJ, Medina R, Ito Y, van Wijnen AJ, Stein JL, Lian JB, Stein GS. Tyrosine phosphorylation controls Runx2-mediated subnuclear targeting of YAP to repress transcription. EMBO J. 2004;23:790–799. doi: 10.1038/sj.emboj.7600073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hong JH, Hwang ES, McManus MT, Amsterdam A, Tian Y, Kalmukova R, Mueller E, Benjamin T, Spiegelman BM, Sharp PA, Hopkins N, Yaffe MB. TAZ, a transcriptional modulator of mesenchymal stem cell differentiation. Science. 2005;309:1074–1078. doi: 10.1126/science.1110955. [DOI] [PubMed] [Google Scholar]
- 30.Tang Y, Rowe RG, Botvinick EL, Kurup A, Putnam AJ, Seiki M, Weaver VM, Keller ET, Goldstein S, Dai J, Begun D, Saunders T, Weiss SJ. MT1-MMP-Dependent Control of Skeletal Stem Cell Commitment via a beta1-Integrin/YAP/TAZ Signaling Axis. Dev Cell. 2013;25:402–416. doi: 10.1016/j.devcel.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Calvo F, Ege N, Grande-Garcia A, Hooper S, Jenkins RP, Chaudhry SI, Harrington K, Williamson P, Moeendarbary E, Charras G, Sahai E. Mechanotransduction and YAP-dependent matrix remodelling is required for the generation and maintenance of cancer-associated fibroblasts. Nat Cell Biol. 2013;15:637–646. doi: 10.1038/ncb2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.