Abstract
This study aimed to investigate the pharmacological effect of caffeine on functional connectivity measured by resting-state blood oxygenation level-dependent (BOLD) MRI in the motor cortex, visual cortex and default mode network (DMN). The protocols and procedures of the study were reviewed and approved by the Institutional Review Board of our institution. On a 3-T clinical MR system, 20 healthy volunteers underwent imaging before and after oral ingestion of a 200-mg over-the-counter caffeine pill (data from three individuals were excluded from further analysis because of excessive motion). The demographics of the remaining participants were as follows: female/male, 8/9; age, 21–35 years; non-habitual caffeine consumers over the past 6 months. Functional connectivity was calculated using the general linear model, assessed in terms of connected area (voxels) and statistical significance (Student t-values), and correlated with changes in regional cerebral blood flow as measured by arterial spin labeling MRI. Per-subject data analysis showed that caffeine decreased functional connectivity in the motor/visual cortices, but its effects on DMN varied among subjects. Correlation analysis of the changes in functional connectivity and regional blood flow suggested that the effect of caffeine on BOLD functional connectivity was predominantly neural (motor/visual cortices) and partly vascular (DMN). Group analysis showed that, after caffeine ingestion, DMN involved more attentional networks, and more extrastriate areas were integrated into the functional connectivity of the visual cortex, which may be associated with the known pharmacological effect of caffeine in elevating alertness. Caffeine consumption should thus be considered in the experimental design and data interpretation of functional connectivity studies using resting-state BOLD MRI.
Keywords: functional connectivity, caffeine, MRI
INTRODUCTION
Blood oxygenation level-dependent (BOLD) MRI is arguably one of the most popular tools in current neuroscience research. BOLD MRI measures the hemodynamic responses that accompany the neuronal activities associated with predefined tasks 1,2. BOLD signals are believed to arise because of neurovascular coupling mediated by astrocytes 3, and are believed to reflect the local concentrations of deoxygenated hemoglobin, which is a composite product of the blood flow, blood volume and oxygen consumption rate 4,5. Interestingly, even in the absence of explicit sensory stimulation and task performance, BOLD signals have also been found to exhibit temporal correlation among cerebral areas that are anatomically separate, but functionally related 6,7. Such correlated signal fluctuations, predominantly revealed at frequencies between 0.01 and 0.1 Hz, have been viewed as a manifestation of cerebral functional connectivity. Several studies 8–10 have conducted experiments to demonstrate that the connectivity measured by task-free or resting-state BOLD MRI is neural (i.e. secondary and related to neuronal activity), whereas Biswal et al. 11 and others 12,13 have reported a non-neural vascular component in measurements.
Caffeine is a methylxanthine drug widely used as a psychostimulant, and is present in various foods and drinks. Caffeine has been suggested to affect the central nervous system by being an adenosine antagonist, resulting in neuronal hyperexcitability (increased firing) and vasoconstriction via the adenosine A1 and A2A receptors, respectively 14. Mulderink et al. 15 proposed the use of caffeine to boost task-induced BOLD signals by demonstrating that the BOLD response increased by 37% in the motor area and 26% in the visual area after ingestion of a 200-mg caffeine pill. These changes are presumably because the decline in blood flow decreases the BOLD baseline, thus yielding a larger capacity of the BOLD response to activation. The complex relationship between dose and neuronal activity/metabolism, however, makes data interpretation difficult, and has led to a debate on the role played by caffeine in measurement variability 16,17. More recently, diminished BOLD functional connectivity was found in the motor cortex 18 and other template-defined anatomical regions 19 after caffeine ingestion. Wong et al. 20 further reported that the resting-state BOLD global signal correlated with vigilance measures recorded using simultaneous electroencephalography. From the signal processing perspective, these studies suggest that caffeine-induced reduction in the measured BOLD connectivity is neural. However, a notable difference still exists between the connectivity measured by electrophysiology and that measured by resting-state BOLD MRI 19. In addition, the link between the influence of caffeine on the resting-state BOLD signal and increased alertness/attention remains unclear.
We hypothesized that caffeine-induced changes in BOLD connectivity, except those accountable with signal processing and regression, might be anatomically heterogeneous. We measured BOLD connectivity in the motor cortex, visual cortex and default mode network (DMN) 21, both before and after the oral ingestion of a 200-mg caffeine pill. The motor/visual cortices and DMN were selected because they are well-known task-positive and task-negative networks, respectively. These networks have been commonly studied targets in the literature on BOLD MRI. The caffeine-induced connectivity change was then correlated with regional cerebral blood flow (CBF), as measured by a non-invasive MRI technique called arterial spin labeling (ASL) 22,23. We demonstrated that caffeine decreased functional connectivity in the motor/visual cortices, whereas the changes in DMN varied across individuals. Group analysis revealed that, after caffeine ingestion, DMN involved more areas concerning externally directed attention. ASL data further suggested that the functional connectivity measured by resting-state BOLD MRI was predominantly associated with neuronal activity in the motor and visual areas, and was partly contributed to by regional blood flow in the medial prefrontal cortex, which is a part of DMN.
MATERIALS AND METHODS
MRI
The Research Ethics Committee at the National Taiwan University Hospital approved this study. All study procedures conformed with The Code of Ethics of the World Medical Association (Declaration of Helsinki) 24. MRI was performed on a 3-T whole-body scanner (Tim Trio, Siemens, Erlangen, Germany) using the body coil to transmit radiofrequency pulses and a 12-channel phased-array head coil to receive signals. The experiment comprised two identical sessions (pre-dose and post-dose), each including a scout, T1-weighted, three-dimensional, magnetization-prepared rapid gradient-echo anatomical imaging sequence [TR/TE/TI, 2530/3/1100 ms; flip angle, 7°; voxel size, 1 × 1 × 1 mm3; generalized autocalibrating partially parallel acquisition (GRAPPA) acceleration factor, 2], task-based BOLD imaging sequence, resting-state ASL and BOLD imaging. For ASL and BOLD imaging, 19 axial slices were prescribed, with the center slice at the level of the corpus callosum (slice thickness, 5 mm; interslice gap, 1 mm; in-plane matrix, 64 × 64; field of view, 20 cm; single-shot, gradient-echo, echo-planar readout). In particular, the following settings were used for task-based and resting-state BOLD imaging: TR = 2 s; TE = 30 ms; measurements, 170 and 240, respectively. For ASL imaging: TR = 4 s; TE = 18 ms; measurements, 60 (i.e. 30 pairs of tag and control images). A pseudocontinuous version of ASL 25,26 was adopted with a tagging duration of 1 s and post-labeling delay of 1.2 s. Calibration and reference scans were obtained for the correction of the tagging efficiency and coil sensitivity 27, as well as for flow quantification 23.
Subjects
Twenty healthy volunteers (10 women and 10 men; age, 21–35 years) were recruited. All subjects provided written informed consent, before which they were informed of their participation in a single-blind caffeine/placebo experiment. Three were excluded from data analysis because of excessive motion (a man aged 28 years and two women aged 22 and 30 years). The subjects were instructed to abstain from caffeine intake 24 h before the experiment. All participants self-reported to be right-handed, and none had been habitual caffeine consumers within the last 6 months (<300 mg per week). The subjects were given a headphone to attenuate the acoustic noise from the scanner. Foam pads were wedged between the subject's head and the coil for stabilization. During the resting-state BOLD and ASL imaging, the subjects were asked to keep their eyes open and to avoid cognitive activities. After the pre-dose session, the subjects were brought out of the scanner, requested to take a 200-mg over-the-counter caffeine pill and allowed to rest outside the scanner for 30 min. They were then repositioned for the post-dose session. The post-dose functional imaging started approximately 45 min after oral ingestion to permit appropriate absorption and distribution of caffeine 28,29. No adverse effects were reported during and after the experiment.
As a control arm, 10 subjects were asked to repeat the above experiment with the same procedure (on a different day), except that they were all given placebo.
Task paradigm and physiological signal recording
The subjects were instructed via the intercom to use their right hand to tap the thumb against the fingers in a sequential order at a self-paced speed. For visual stimulation, an 8-Hz, whole-field, black/white flashing checkerboard was projected onto a screen and viewed by the subject via a mirror attached to the head coil. A block design was adopted to include five 20-s ‘on’ epochs interleaved with ‘off’ epochs of varied durations (20–60 s), such that the motor and visual tasks were semi-random to each other. For the prospective recording of the physiological signals, a pulse oximeter was clipped to the subjects' left index finger, and a pneumatic respiratory belt was placed on their upper abdomen. Transistor–transistor logic pulse data from the scanner were also recorded for subsequent identification of the cardiac and respiratory phases at which the images were acquired.
Data analysis
Complex data were reconstructed online into magnitude images and then exported to a laptop computer for offline processing. All echo-planar images underwent realignment series by series for motion correction. Physiological noise, including cardiac pulsations and respiratory motion, was removed from the resting-state BOLD images following the RETROICOR algorithm 30. For the ASL series, the tag and control images were separately realigned and averaged, followed by co-registration to the series mean. Perfusion-weighted images were generated by subtraction and then converted to quantitative perfusion maps in units of mL/100 mL/min after tagging efficiency calibration and coil sensitivity correction.
Gray matter masks were created to include the voxels classified as gray matter at a probability level of 0.90 in both the high-resolution anatomical images and the reference images for flow calculation. For per-subject analysis, all images (including the masks) were co-registered to the mean echo-planar images of the two sessions. To detect activated areas, a boxcar function of the experimental paradigm convolved with a hemodynamic response function was fed to the general linear model, with the criteria of family-wise corrected p of ≤0.05 and cluster size of ≥3 voxels. Seeds were determined for the motor and visual cortices as the voxels detected in both sessions and contained in the gray matter mask. For DMN, the posterior cingulate cortex (PCC) was manually defined on the gray matter mask in reference to the anatomical landmarks and used as the seed. Functional connectivity was calculated using the general linear model with regressors of the seed, global signal and signal fluctuations estimated in the lateral ventricles. Voxels were deemed to be connected if all the following criteria were met: family-wise corrected p of ≤0.05, cluster size of ≥3 voxels and contained in the gray matter mask. To avoid bias in the selection of regions of interest, the intersession comparison of functional connectivity was performed on three contiguous slices that included the greatest number of connected voxels identified by both sessions. For group analysis, the above results were normalized to the Montreal Neurological Institute templates. The threshold of cluster size was set to 20 to account for the change in voxel dimension after spatial normalization (3.1 × 3.1 × 5 mm3 versus 2 × 2 × 2 mm3).
All data analyses were performed using Statistical Parametric Mapping software (http://www.fil.ion.ucl.ac.uk/spm/) and homemade programs under the environment of MATLAB (http://www.mathworks.com).
Computer simulation of signal-to-noise ratio (SNR) effect
We used the deoxyhemoglobin dilution model 31, which was initially proposed for task-induced BOLD signals, to relate the resting-state signal fluctuations (ΔBOLD) to CBF and the cerebral metabolic rate of oxygen (CMRO2) as follows:
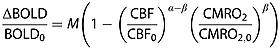 |
1 |
where subscript ‘0’ denotes the baseline value, α is a constant related to the passive dependence of the blood volume on blood flow, with an approximate value of 0.38, β is a constant with a value between 1 and 2 depending on the average blood volume in tissue, and M is a constant determined by the field strength, echo time, baseline cerebral blood volume and baseline concentration of deoxyhemoglobin in venous blood.
According to previous studies in human visual cortex, the baseline concentration of oxyhemoglobin ([HbO2]) is 26 µm 32 and fluctuates with an amplitude of 1 µm 33, which leads to ∼4% fluctuations in CMRO2. If [HbO2] is partly related to perfusion, the fluctuation amplitude of CBF should be at the same order as that of CMRO2. Alternatively, CBF fluctuation can be estimated from ASL-based functional MRI studies. In Yang et al. 34, the ASL signal fluctuation is ∼10% in the baseline and task-off periods. With no further information, one can assume that physiological fluctuation and non-physiological fluctuation have equal contributions. That is, the fluctuation of baseline CBF is no larger than 10%. Therefore, by assuming that the baseline fluctuations are small (e.g. <10%) for CBF and CMRO2, Equation [1] can be modified to approximately account for the pharmacological effect of caffeine by including a constant k1 for the neural effect and a constant k2 for the vascular effect:
| 2 |
Presumably, the functional connectivity measured by the resting-state BOLD MRI is neural [i.e. CBF = CBF0 at all times in Equation [1]; vascular source of fluctuation is time independent and equal to zero in Equation [2]]. When noise is absent, the derived connectivity will be independent of the caffeine ingestion, as BOLD signals are associated with neural fluctuations through linear transformation. The correlation between two time series, on the basis of which functional connectivity is computed, does not change after these time series undergo separate linear transformations.
To estimate the effect of SNR on the computed functional connectivity, numerical simulation was performed on the basis of Equation [1], with β assumed to be 1.5. Resting-state fluctuation was presumed to be Gaussian and of 1% amplitude, whereas caffeine was assumed to double the fluctuation (240 time points, in accordance with 240 measurements in our resting-state BOLD imaging). Background noise was assumed to be Gaussian, with the SNR varying from 0.1 to 10 in steps of 0.1. Noise was added to the fluctuation to create two time series, from which the correlation coefficient was calculated. One hundred random samples were generated for each degree of SNR, and the correlation coefficient was calculated for each sample.
RESULTS
Per-subject analysis of the effect of caffeine on functional connectivity
Figure 1 shows the Bland–Altman plots (pre-dose values minus post-dose values versus average, n = 17). As aforementioned, all subjects self-reported to be right-handed and had performed finger-tapping only with their right hand to extract the seed for the calculation of functional connectivity in the motor area. After caffeine ingestion, the connected area diminished in the motor and visual cortices (p < 0.01 in both areas, Wilcoxon signed rank test) in most subjects (15 and 16 of 17 cases, respectively), whereas the area change in DMN was variable across subjects (10 of 17 exhibited a decrease). Chi-squared test further indicated the proportion differences between the motor/visual cortices and DMN (likelihood ratio, 7.53; asymptotic p = 0.02; Fisher's exact p = 0.05). Among the voxels revealed to be connected in both sessions, the decrease in connectivity in the motor and visual cortices also manifested in terms of statistical significance (i.e. Student t-values), as shown in Fig. 2 (Bland–Altman plots, pre-dose values minus post-dose values versus average, n = 17). Again, no consistent change in t values in DMN was observed between sessions. Interestingly, in DMN, we noted that the intersession change in the t value was correlated with the intersession change in regional CBF (Pearson's correlation coefficient, 0.25; p < 10−6). In contrast, no correlation was observed between connectivity and regional blood flow in the motor and visual areas (see Table 1). Overall, 200 mg of caffeine decreased the gray matter perfusion by 24% ± 7%, which is in reasonable agreement with previous reports 35,36. Meanwhile, in the placebo data, no significant intersession changes were noted in the blood flow and the number of connected voxels.
Figure 1.
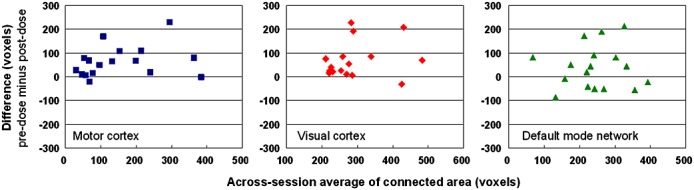
Intersession comparison of functional connectivity by area (voxels) for each subject. Left to right: motor cortex (blue squares), visual cortex (red diamonds) and default mode network (green triangles). Each subplot has 17 data points that represent the 17 subjects included in this study.
Figure 2.
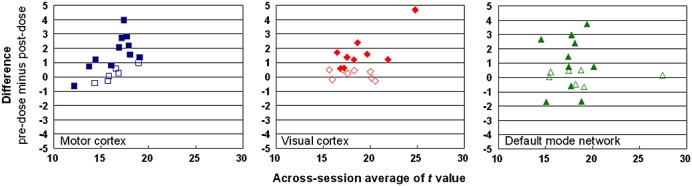
Intersession comparison of functional connectivity by statistical significance (t values) for each subject. Left to right: motor cortex (blue squares), visual cortex (red diamonds) and the default mode network (green triangles). Each subplot has 17 data points that represent the 17 subjects included in this study. For each subject and for each of the three anatomical areas investigated, the t value difference between sessions was inspected within the voxels revealed to be connected both before and after caffeine ingestion using the paired Student's t-test (significance level = 0.05 after Bonferroni correction for multiple comparisons). The subjects in whom a significant difference was not found are denoted with open symbols.
Table 1.
Results of correlation analysis of caffeine-induced changes in functional connectivity and regional blood flow
| Motor area | Visual area | DMN | |
|---|---|---|---|
| Pearson correlation coefficient | 0.05 | 0.01 | 0.25* |
DMN, default mode network.
p < 0.05.
Group analysis of the effect of caffeine on functional connectivity
Figures 3–5 display the group functional connectivity of the motor cortex, visual cortex and DMN, respectively. Caffeine seemed to cause slight decreases to the connection between the visual cortex and the lateral geniculate nucleus (LGN), whilst increasing the integration of extrastriate visual areas (uncorrected p < 10−5). In the post-dose session, DMN decreased in the anterior medial prefrontal cortex, but increased slightly in the lateral parietal cortex (uncorrected p < 10−5). Figure 6 shows maps of the intersession blood flow difference (pre-dose values minus post-dose values) and the average blood flow (only the slices displayed in Figs 5 are shown). A significant decline in blood flow was also detected in the medial prefrontal cortex. Of note, because blood flow decreased globally after caffeine ingestion, the significance level in Fig. 6 was increased to highlight the areas of most significant changes.
Figure 3.
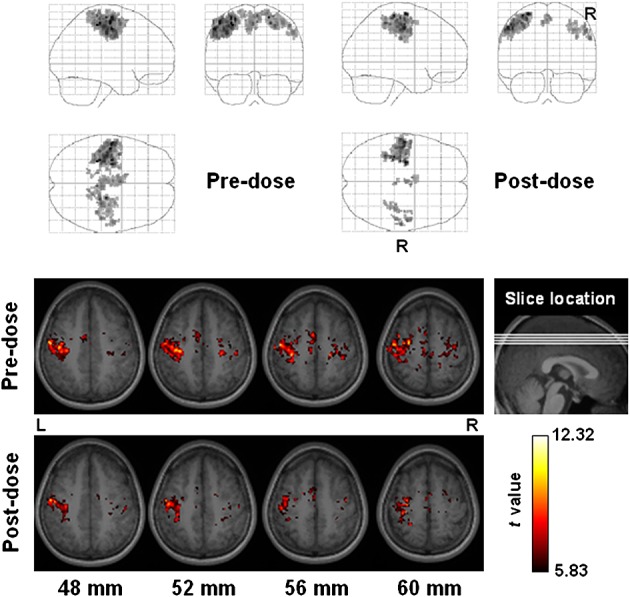
Functional connectivity of the motor cortex among all subjects (n = 17). All subjects self-reported to be right-handed and had performed finger-tapping only with their right hand to extract the seed for the calculation of the functional connectivity. Top panel: projection viewed from right (sagittal), behind (coronal, right side on the right) and above (axial, right side at bottom). Bottom panel: sectional axial view in neurological convention. Slice location is marked in units of millimeters.
Figure 5.
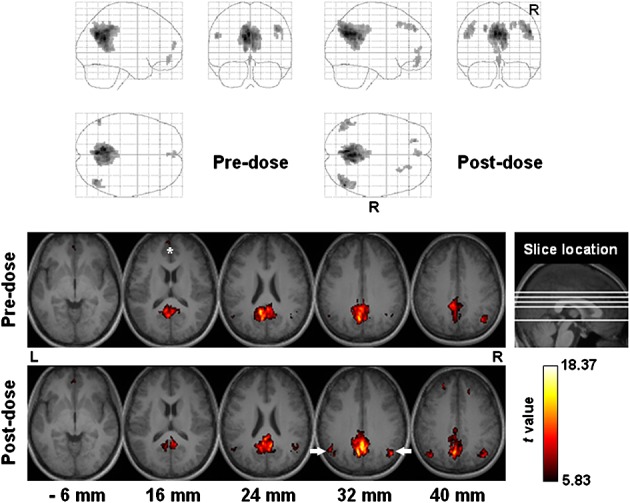
Functional connectivity of the default mode network among all subjects (n = 17). Top panel: projection viewed from right (sagittal), behind (coronal, right side on the right) and above (axial, right side at bottom). Bottom panel: sectional axial view in neurological convention. Slice location is marked in units of millimeters. The asterisk and arrows indicate the medial prefrontal cortex and lateral parietal cortex, respectively.
Figure 6.
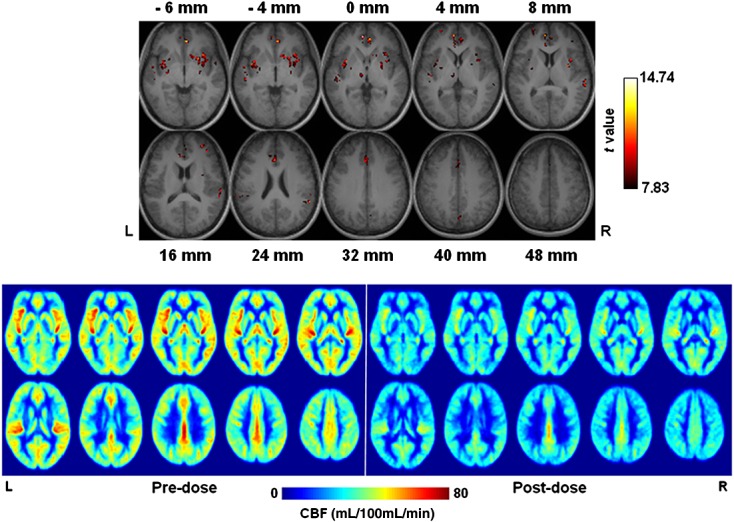
Difference in cerebral blood flow (CBF) between sessions (pre-dose values minus post-dose values), overlaid on anatomical images (top panel). The slices displayed in Figs 5 are shown here, except for those corresponding to 52, 56 and 60 mm, where no significant flow difference was found. The bottom panel shows the group average CBF (n = 17).
Figure 4.
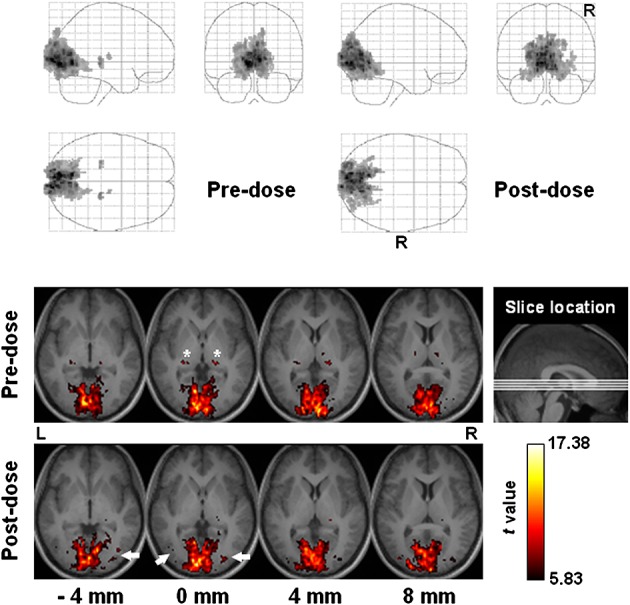
Functional connectivity of the visual cortex among all subjects (n = 17). Top panel: projection viewed from right (sagittal), behind (coronal, right side on the right) and above (axial, right side at bottom). Bottom panel: sectional axial view in neurological convention. Slice location is marked in units of millimeters. Asterisks and arrows indicate the lateral geniculate nuclei and the extrastriate visual areas, respectively.
SNR effect on the calculation of functional connectivity
Figure 7 shows the result of the computer simulation of the effect of SNR on the correlation coefficient on the basis of which the BOLD connectivity was determined. The correlation coefficient was more sensitive to SNR when SNR was low. According to the range of t values obtained in our experiment (see dotted lines), 200 mg of caffeine decreased SNR by approximately 1.
Figure 7.
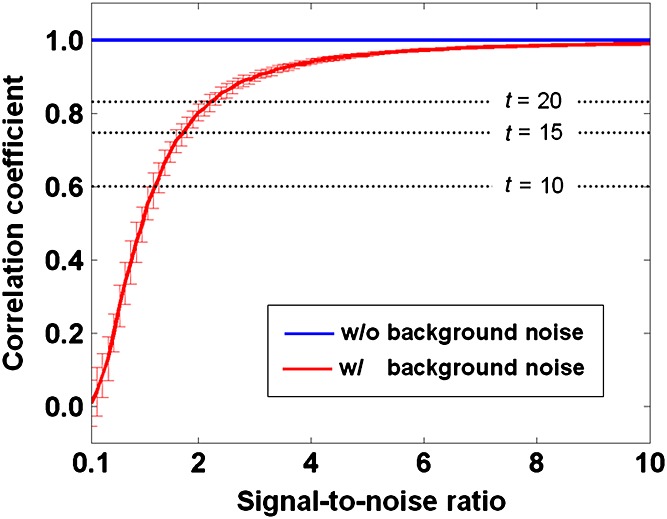
Simulated effect of the signal-to-noise ratio (SNR) on the calculation of the functional connectivity. Resting-state fluctuation was presumed to be Gaussian and 1% in amplitude, whereas caffeine was assumed to double the fluctuation. Background noise was assumed to be Gaussian, with the SNR varied from 0.1 to 10 in steps of 0.1. One hundred random samples were generated for each degree of SNR. The thick red curve and error bars indicate the mean and standard deviation, respectively, of the computed correlation coefficients in the presence of noise. The thick blue line shows the mean correlation coefficient computed without noise (standard deviation is too small to be included in the plot). The dotted lines indicate the t values corresponding to the correlation coefficients after correction for family-wise errors. The t values are shown in reference to Fig. 2.
DISCUSSION
Pharmacological effect of caffeine on functional connectivity is area dependent
Caffeine decreased the measured connectivity in the motor and visual cortices (left and middle panels, respectively, in Figs 1 and 2), whereas, in DMN, the caffeine-induced change in connectivity varied across subjects (right panel in Figs 1 and 2). One possible cause of this difference may be the spatial distribution of adenosine receptors. Caffeine has been documented to increase neurotransmission and decrease CBF by antagonizing adenosine A1 and A2A receptors, respectively 14. The A2A receptors exhibit a higher distribution in the striatum and PCC, a cortical hub 37 that served as the seed to extract DMN in our study, than in the occipital cortex (where the visual cortex is located) and the frontal/parietal cortices (where the motor cortex is located) 38. In contrast, the distribution of A1 receptors is greater in the occipital cortex than in PCC and the sensorimotor cortex 39,40. The high expression of A2A receptors in PCC may result in an enhanced vascular effect on the BOLD MRI measurement of DMN. Aside from the more prominent myogenic fluctuations in the presence of vasoconstriction, decreased baseline blood flow is known to increase the range of fluctuations in the BOLD signal and may also influence the connectivity measurement. Our data reveal that caffeine decreased the connectivity of DMN in the medial prefrontal cortex (Fig. 5), where blood flow decreased after caffeine ingestion at a higher significance level than in the motor and visual cortices (Fig. 6). In addition, caffeine-induced changes in functional connectivity and blood flow were correlated in DMN (Table 1). In contrast, no correlation was observed between functional connectivity and blood flow in the motor and visual cortices after caffeine intake, suggesting that vascular fluctuations in these two regions have a minor effect compared with the neural effect. A recent study 41 has shown that caffeine has an anatomically differential effect on brain responses to tasks. Although the study was based on external tasks, it provided evidence that caffeine's effect on BOLD MRI is area dependent. It is worth mentioning that, in accordance with previous studies 41,42, the neural effect in our context means that the observed signal fluctuations are related to, or driven by, neuronal activity, as opposed to the vascular effect that includes vasomotor fluctuations unrelated to neuronal activity.
Caffeine recruits more areas responsible for externally directed attention in DMN
Recently, Leech et al. 43 have demonstrated the functional dissociation between the ventral and dorsal parts of PCC, echoing the previously reported heterogeneous cytoarchitectonics 44. Briefly, the ventral PCC showed decreased integration within DMN and less anticorrelation with the cognitive control network as the difficulty of a working memory task increased. The opposite pattern was observed in the dorsal PCC, where resting-state functional connectivity was found with both DMN and attentional networks. Thus, the authors proposed that the ventral PCC was involved in internally directed thought, whereas the dorsal PCC was involved in the management of externally directed attention. By comparing the functional connectivity measured before and after caffeine intake, our study presents experimental data that indirectly support the work of Leech et al. In the post-dose session, DMN was observed to have greater connectivity with the lateral parietal cortex (a part of the attentional network), whereas the connected PCC shifted slightly to the dorsal part after caffeine ingestion (Fig. 5). The post-dose DMN involved more areas concerning externally directed attention, which might be associated with the known pharmacological effect of caffeine in elevating alertness and the ability to respond to events occurring at unexpected times or in unknown places.
Caffeine alters the integration of relay and attention-associated areas in the functional connectivity of the visual cortex
In the human visual system, the primary visual cortex (V1) receives most visual inputs relayed by LGN, whereas extrastriate areas, such as V2, V4 and V5 (also known as the middle temporal visual area), have been shown to be modulated by attention 45,46. Our data reveal that caffeine decreased the integration of LGN, whilst recruiting more extrastriate visual areas in the functional connectivity of the visual cortex. Given that the subjects were instructed to keep their eyes open during resting-state BOLD imaging, such a change in functional connectivity suggests increased association of visual attention and adjusted configuration for faster and higher order processing of visual signals. Meanwhile, LGN was less connected presumably to avoid competition for computational resources, as the signal transmission from LGN to V1 can largely rely on the optical radiation (anatomical connectivity supported by fiber tracts).
Functional connectivity measured by BOLD MRI is mainly neural, but has a measurable vascular component in DMN
In the motor and visual cortices, diminished functional connectivity was observed (in terms of voxel number as well as statistical significance) in most subjects after caffeine ingestion in the absence of significant blood flow decrease and correlation, which suggests that neuronal activity was the dominant, if not the only, source of the connectivity measured by resting-state BOLD MRI. Meanwhile, in DMN, caffeine caused connectivity changes correlated with regional blood flow change (Pearson's correlation coefficient, 0.25; p < 10−6), which indicates the vascular constituent in the measured functional connectivity. Notably, blood flow change could be secondary to the neuronal activity change caused by caffeine. Although previous task-based BOLD MRI studies have shown that caffeine induces uncoupling between CBF and CMRO2 16,17, whether neurovascular coupling is established at rest or maintained to the same extent as in the presence of explicit task performance remains unclear.
SNR may play a role in BOLD MRI measurement of functional connectivity
As shown in Fig. 7, the correlation coefficient was more sensitive to SNR when SNR was low. According to the range of t values obtained in our experiment, 200 mg of caffeine decreases SNR by approximately 1. Such a drop in SNR is critical in resting-state BOLD MRI, but minor for task-based BOLD MRI, in which SNR is usually well above 2. Given that caffeine increases neuronal activities, the elevated ‘background noise’ from neighboring neurons that are not connected with the seed of interest may bring down the computed correlation and its statistical significance. It should be noted that Equation [2] cannot interpret coupled neural/vascular effects, but can serve as a first approximation, as long as neural and vascular fluctuations are not entirely coupled.
Comparison with related studies
Several studies have previously investigated the effect of caffeine on the resting-state BOLD signal and functional connectivity. By comparing with the connectivity derived from source-localized magnetoencephalography, Tal et al. 19 showed a global reduction in BOLD connectivity in support of the neural basis. Differences remained between the magnetoencephalographic connectivity and the BOLD connectivity, suggesting the effect of other factors in BOLD connectivity (e.g. vascular effect). In this sense, our finding is in agreement with the hypothesis that the neural effect serves a major function in the motor/visual areas (no measurable vascular effect), whereas, in DMN, the vascular effect is modestly measurable. Another study from the same group 47 reported that caffeine-induced connectivity change is present in the eyes-closed condition, but not in the eyes-open condition, mainly because of global signal regression. Our study was performed in the eyes-open condition, and decreased connectivity was still observed in the motor/visual areas, together with varied connectivity change in DMN across subjects. We currently have no explanation for this discrepancy. However, caffeine has been documented to decrease resting-state electroencephalography activities in both alpha and beta bands 48,49, and this phenomenon does not seem to be particularly related to the status of the eyes. We also used global signal regression which, according to Wong et al. 47, tends to shift positive correlation towards the negative direction. This condition might mean that we lost some statistical power, but we could not explain why the changes were located at attention-associated areas (e.g. middle temporal visual area, lateral parietal cortex). The studies cited for comparison have investigated the effect of caffeine from the perspective of signal processing and regression. Although these findings help in the identification of the global source and characteristics of the resting-state BOLD signal, caffeine has a known effect on human behavior and is mostly consumed as a psychostimulant for the eyes-open condition. Thus, the effect of caffeine on BOLD connectivity, if measurable, should have anatomical heterogeneity to some extent. Our data suggest that the detection of the differential effect with resting-state BOLD MRI is possible.
Notably, several BOLD connectivity studies 19,47 have reported results using the connectivity matrix, which is the normalized covariance matrix of the regions of interest (usually on a region-wise, not voxel-wise, basis). The method provides a compact display of the correlation between a large number of regions, although caution should be used to deal with the inflated type 1 error rate.
Technical limitations
A few technical limitations should be noted in our study. First, we used a fixed caffeine dose (200 mg) and the same time period (about 45 min) for the absorption/distribution in all the subjects. Dose dependence has been reported for task-based BOLD MRI 50, thus requiring further investigation for resting-state BOLD MRI. Second, the plasma caffeine level was not obtained in our study. We recruited subjects who had not been habitual caffeine consumers over the past 6 months and asked them to abstain from caffeine intake 24 h before the experiment. Nonetheless, the effective dose and caffeine tolerance are known to vary from person to person, and these factors may have introduced variance in our measurement. Third, the effect of global signal regression was not assessed. Global signal regression has been employed in many studies to reduce spurious correlation between voxels 51,52, but at the same time has the tendency to globally decrease positive correlation 47 and thus measurement sensitivity. As we used global signal regression for both sessions, the intersession difference observed in our study is still valid. The potential issue is that, with the decreased sensitivity, there could be other areas that are affected by caffeine intake, but were not detected.
Acknowledgments
This work was supported by the National Science Council of Taiwan (grants 103-2420-H-002-006-MY2 and 102-2221-E-002-219).
Glossary
- ASL
arterial spin labeling
- BOLD
blood oxygenation level-dependent
- CBF
cerebral blood flow
- CMRO2
cerebral metabolic rate of oxygen
- DMN
default mode network
- LGN
lateral geniculate nucleus
- PCC
posterior cingulate cortex
- SNR
signal-to-noise ratio.
REFERENCES
- 1.Ogawa S, Tank DW, Menon R, Ellermann J, Kim S-G, Merkle H, Ugurbil K. Intrinsic signal changes accompanying sensory stimulation: functional brain mapping with magnetic resonance imaging. Proc. Natl. Acad. Sci. U. S. A. 1992;89:5951–5955. doi: 10.1073/pnas.89.13.5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kwong KK, Belliveau JW, Chesler DA, Goldberg IE, Weisskoff RM, Poncelet BP, Kennedy DN, Hoppel BE, Cohen MS, Turner R. Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation. Proc. Natl. Acad. Sci. U. S. A. 1992;89:5675–5679. doi: 10.1073/pnas.89.12.5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haydon PG, Carmignoto G. Astrocyte control of synaptic transmission and neurovascular coupling. Physiol. Rev. 2006;86(3):1009–1031. doi: 10.1152/physrev.00049.2005. [DOI] [PubMed] [Google Scholar]
- 4.Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412(6843):150–157. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- 5.Buxton RB. Dynamic models of BOLD contrast. Neuroimage. 2012;62(2):953–961. doi: 10.1016/j.neuroimage.2012.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biswal B, Yetkin FZ, Haughhton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar imaging. Magn. Reson. Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 7.Lowe MJ, Mock BJ, Sorenson JA. Functional connectivity in single and multislice echoplanar imaging using resting-state fluctuations. Neuroimage. 1998;7(2):119–132. doi: 10.1006/nimg.1997.0315. [DOI] [PubMed] [Google Scholar]
- 8.Kruger G, Glover GH. Physiological noise in oxygenation-sensitive magnetic resonance imaging. Magn. Reson. Med. 2001;46(4):631–637. doi: 10.1002/mrm.1240. [DOI] [PubMed] [Google Scholar]
- 9.He BJ, Snyder AZ, Vincent JL, Epstein A, Shulman GL, Corbetta M. Breakdown of functional connectivity in frontoparietal networks underlies behavioral deficits in spatial neglect. Neuron. 2007;53(6):905–918. doi: 10.1016/j.neuron.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 10.Brookes MJ, Woolrich M, Luckhoo H, Price D, Hale JR, Stephenson MC, Barnes GR, Smith SM, Morris PG. Investigating the electrophysiological basis of resting state networks using magnetoencephalography. Proc. Natl. Acad. Sci. U. S. A. 2011;108(40):16 783–16 788. doi: 10.1073/pnas.1112685108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Biswal BB, Van Kylen J, Hyde JS. Simultaneous assessment of flow and BOLD signals in resting-state functional connectivity maps. NMR Biomed. 1997;10(4–5):165–170. doi: 10.1002/(sici)1099-1492(199706/08)10:4/5<165::aid-nbm454>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 12.Obrig H, Neufang M, Wenzel R, Kohl M, Steinbrink J, Einhaupl K, Villringer A. Spontaneous low frequency oscillations of cerebral hemodynamics and metabolism in human adults. Neuroimage. 2000;12(6):623–639. doi: 10.1006/nimg.2000.0657. [DOI] [PubMed] [Google Scholar]
- 13.Mitra PP, Ogawa S, Hu X, Ugurbil K. The nature of spatiotemporal changes in cerebral hemodynamics as manifested in functional magnetic resonance imaging. Magn. Reson. Med. 1997;37(4):511–518. doi: 10.1002/mrm.1910370407. [DOI] [PubMed] [Google Scholar]
- 14.Tarter RE, Ammerman RT, Ott PJ. Handbook of Substance Abuse: Neurobehavioral Pharmacology. New York: Plenum Press; 1998. [Google Scholar]
- 15.Mulderink TA, Gitelman DR, Mesulam MM, Parrish TB. On the use of caffeine as a contrast booster for BOLD fMRI studies. Neuroimage. 2002;15(1):37–44. doi: 10.1006/nimg.2001.0973. [DOI] [PubMed] [Google Scholar]
- 16.Perthen JE, Lansing AE, Liau J, Liu TT, Buxton RB. Caffeine-induced uncoupling of cerebral blood flow and oxygen metabolism: a calibrated BOLD fMRI study. Neuroimage. 2008;40(1):237–247. doi: 10.1016/j.neuroimage.2007.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen Y, Parrish TB. Caffeine's effects on cerebrovascular reactivity and coupling between cerebral blood flow and oxygen metabolism. Neuroimage. 2009;44(3):647–652. doi: 10.1016/j.neuroimage.2008.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rack-Gomer AL, Liau J, Liu TT. Caffeine reduces resting-state BOLD functional connectivity in the motor cortex. Neuroimage. 2009;46(1):56–63. doi: 10.1016/j.neuroimage.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tal O, Diwakar M, Wong CW, Olafsson V, Lee R, Huang MX, Liu TT. Caffeine-induced global reductions in resting-state BOLD connectivity reflect widespread decreases in MEG connectivity. Front. Hum. Neurosci. 2013;7:63. doi: 10.3389/fnhum.2013.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wong CW, Olafsson V, Tal O, Liu TT. The amplitude of the resting-state fMRI global signal is related to EEG vigilance measures. Neuroimage. 2013;83:983–990. doi: 10.1016/j.neuroimage.2013.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc. Natl. Acad. Sci. U. S. A. 2001;98(2):676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Detre JA, Zhang W, Roberts DA, Silva AC, Williams DS, Grandis DJ, Koretsky AP, Leigh JS. Tissue specific perfusion imaging using arterial spin labeling. NMR Biomed. 1994;7(1–2):75–82. doi: 10.1002/nbm.1940070112. [DOI] [PubMed] [Google Scholar]
- 23.Wu WC, St Lawrence KS, Licht DJ, Wang DJ. Quantification issues in arterial spin labeling perfusion magnetic resonance imaging. Top. Magn. Reson. Imaging. 2010;21(2):65–73. doi: 10.1097/RMR.0b013e31821e570a. [DOI] [PubMed] [Google Scholar]
- 24.World Medical Association. Human experimentation: Code of Ethics of the World Medical Association. Br. Med. J. 1964;2:177. doi: 10.1136/bmj.2.5402.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garcia DM, de Bazelaire C, Alsop D. Pseudo-continuous flow driven adiabatic inversion for arterial spin labeling. Proc. Int. Soc. Magn. Reson. Med. 2005;13:37. doi: 10.1002/mrm.21790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu WC, Fernandez-Seara M, Detre JA, Wehrli FW, Wang J. A theoretical and experimental investigation of the tagging efficiency of pseudocontinuous arterial spin labeling. Magn. Reson. Med. 2007;58(5):1020–1027. doi: 10.1002/mrm.21403. [DOI] [PubMed] [Google Scholar]
- 27.Wu WC, Jiang SF, Yang SC, Lien SH. Pseudocontinuous arterial spin labeling perfusion magnetic resonance imaging – a normative study of reproducibility in the human brain. Neuroimage. 2011;56(3):1244–1250. doi: 10.1016/j.neuroimage.2011.02.080. [DOI] [PubMed] [Google Scholar]
- 28.Liguori A, Hughes JR, Grass JA. Absorption and subjective effects of caffeine from coffee, cola and capsules. Pharmacol. Biochem. Behav. 1997;58(3):721–726. doi: 10.1016/s0091-3057(97)00003-8. [DOI] [PubMed] [Google Scholar]
- 29.Fredholm BB, Battig K, Holmen J, Nehlig A, Zvartau EE. Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol. Rev. 1999;51(1):83–133. [PubMed] [Google Scholar]
- 30.Glover GH, Li TQ, Ress D. Image-based method for retrospective correction of physiological motion effects in fMRI: RETROICOR. Magn. Reson. Med. 2000;44(1):162–167. doi: 10.1002/1522-2594(200007)44:1<162::aid-mrm23>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 31.Hoge RD, Atkinson J, Gill B, Crelier GR, Marrett S, Pike GB. Investigation of BOLD signal dependence on cerebral blood flow and oxygen consumption: the deoxyhemoglobin dilution model. Magn. Reson. Med. 1999;42(5):849–863. doi: 10.1002/(sici)1522-2594(199911)42:5<849::aid-mrm4>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 32.McIntosh MA, Shahani U, Boulton RG, McCulloch DL. Absolute quantification of oxygenated hemoglobin within the visual cortex with functional near infrared spectroscopy (fNIRS) Invest. Ophthalmol. Vis. Sci. 2010;51(9):4856–4860. doi: 10.1167/iovs.09-4940. [DOI] [PubMed] [Google Scholar]
- 33.Kato T, Kamei A, Takashima S, Ozaki T. Human visual cortical function during photic stimulation monitoring by means of near-infrared spectroscopy. J. Cerebr. Blood Flow Metab. 1993;13(3):516–520. doi: 10.1038/jcbfm.1993.66. [DOI] [PubMed] [Google Scholar]
- 34.Yang Y, Gu H, Stein EA. Simultaneous MRI acquisition of blood volume, blood flow, and blood oxygenation information during brain activation. Magn. Reson. Med. 2004;52(6):1407–1417. doi: 10.1002/mrm.20302. [DOI] [PubMed] [Google Scholar]
- 35.Cameron OG, Modell JG, Hariharan M. Caffeine and human cerebral blood flow: a positron emission tomography study. Life Sci. 1990;47(13):1141–1146. doi: 10.1016/0024-3205(90)90174-p. [DOI] [PubMed] [Google Scholar]
- 36.Liu TT, Behzadi Y, Restom K, Uludag K, Lu K, Buracas GT, Dubowitz DJ, Buxton RB. Caffeine alters the temporal dynamics of the visual BOLD response. Neuroimage. 2004;23(4):1402–1413. doi: 10.1016/j.neuroimage.2004.07.061. [DOI] [PubMed] [Google Scholar]
- 37.Hagmann P, Cammoun L, Gigandet X, Meuli R, Honey CJ, Wedeen VJ, Sporns O. Mapping the structural core of human cerebral cortex. PLoS Biol. 2008;6(7):e159. doi: 10.1371/journal.pbio.0060159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mishina M, Ishiwata K, Kimura Y, Naganawa M, Oda K, Kobayashi S, Katayama Y, Ishii K. Evaluation of distribution of adenosine A2A receptors in normal human brain measured with [11C]TMSX PET. Synapse. 2007;61(9):778–784. doi: 10.1002/syn.20423. [DOI] [PubMed] [Google Scholar]
- 39.Fukumitsu N, Ishii K, Kimura Y, Oda K, Sasaki T, Mori Y, Ishiwata K. Adenosine A1 receptor mapping of the human brain by PET with 8-dicyclopropylmethyl-1-11C-methyl-3-propylxanthine. J. Nucl. Med. 2005;46(1):32–37. [PubMed] [Google Scholar]
- 40.Fastbom J, Pazos A, Probst A, Palacios JM. Adenosine A1 receptors in the human brain: a quantitative autoradiographic study. Neuroscience. 1987;22(3):827–839. doi: 10.1016/0306-4522(87)92962-9. [DOI] [PubMed] [Google Scholar]
- 41.Diukova A, Ware J, Smith JE, Evans CJ, Murphy K, Rogers PJ, Wise RG. Separating neural and vascular effects of caffeine using simultaneous EEG-FMRI: differential effects of caffeine on cognitive and sensorimotor brain responses. Neuroimage. 2012;62(1):239–249. doi: 10.1016/j.neuroimage.2012.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? Neuroimage. 2009;44(3):893–905. doi: 10.1016/j.neuroimage.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leech R, Kamourieh S, Beckmann CF, Sharp DJ. Fractionating the default mode network: distinct contributions of the ventral and dorsal posterior cingulate cortex to cognitive control. J. Neurosci. 2011;31(9):3217–3224. doi: 10.1523/JNEUROSCI.5626-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vogt BA, Vogt L, Laureys S. Cytology and functionally correlated circuits of human posterior cingulate areas. Neuroimage. 2006;29(2):452–466. doi: 10.1016/j.neuroimage.2005.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Treue S, Maunsell JH. Attentional modulation of visual motion processing in cortical areas MT and MST. Nature. 1996;382(6591):539–541. doi: 10.1038/382539a0. [DOI] [PubMed] [Google Scholar]
- 46.Luck SJ, Chelazzi L, Hillyard SA, Desimone R. Neural mechanisms of spatial selective attention in areas V1, V2, and V4 of macaque visual cortex. J. Neurophysiol. 1997;77(1):24–42. doi: 10.1152/jn.1997.77.1.24. [DOI] [PubMed] [Google Scholar]
- 47.Wong CW, Olafsson V, Tal O, Liu TT. Anti-correlated networks, global signal regression, and the effects of caffeine in resting-state functional MRI. Neuroimage. 2012;63(1):356–364. doi: 10.1016/j.neuroimage.2012.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dimpfel W, Schober F, Spuler M. The influence of caffeine on human EEG under resting conditions and during mental loads. Clin. Invest. 1993;71(3):197–207. doi: 10.1007/BF00180102. [DOI] [PubMed] [Google Scholar]
- 49.Siepmann M, Kirch W. Effects of caffeine on topographic quantitative EEG. Neuropsychobiology. 2002;45(3):161–166. doi: 10.1159/000054958. [DOI] [PubMed] [Google Scholar]
- 50.Chen Y, Parrish TB. Caffeine dose effect on activation-induced BOLD and CBF responses. Neuroimage. 2009;46(3):577–583. doi: 10.1016/j.neuroimage.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fox MD, Zhang D, Snyder AZ, Raichle ME. The global signal and observed anticorrelated resting state brain networks. J. Neurophysiol. 2009;101(6):3270–3283. doi: 10.1152/jn.90777.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Aguirre GK, Zarahn E, D'Esposito M. Empirical analyses of BOLD fMRI statistics. II. Spatially smoothed data collected under null-hypothesis and experimental conditions. Neuroimage. 1997;5(3):199–212. doi: 10.1006/nimg.1997.0264. [DOI] [PubMed] [Google Scholar]


