Abstract
Anthropogenic disturbances are detrimental to the functioning and stability of natural ecosystems. Critical ecosystem processes driven by microbial communities are subjected to these disturbances. Here, we examine the stabilizing role of bacterial diversity on community biomass in the presence of abiotic perturbations such as addition of heavy metals, NaCl and warming. Bacterial communities with a diversity gradient of 1–12 species were subjected to the different treatments, and community biomass (OD600) was measured after 24 h. We found that initial species richness and phylogenetic structure impact the biomass of communities. Under abiotic perturbations, the presence of tolerant species in community largely contributed in community biomass production. Bacterial diversity stabilized the biomass across the treatments, and differential response of bacterial species to different perturbations was the key reason behind these effects. The results suggest that biodiversity is crucial for maintaining the stability of ecosystem functioning and acts as ecological insurance under abiotic perturbations. Biodiversity in natural ecosystems may also uphold the ecosystem functioning under anthropogenic disturbance.
Introduction
Natural ecosystems are facing various anthropogenic and climatic disturbances, such as enrichment of atmospheric CO2, deposition of several agricultural and industrial pollutants and rise in temperature. The role of biodiversity is crucial for ecosystem functioning (Hooper et al., 2005; Cardinale et al., 2006; Loreau, 2010; Reich et al., 2012) because diverse communities may use available resources more efficiently and maintain the ecosystem functioning under environmental uncertainty. The importance of biodiversity becomes more evident under environmental fluctuations where it may act as ecological insurance for maintaining the ecosystem functioning (Yachi and Loreau, 1999; Tilman et al., 2006; Loreau and de Mazancourt, 2013). The insurance hypothesis expects a positive effect of biodiversity on ecosystem functioning in a variable environment (Naeem and Li, 1997). Different species respond differently to the environmental conditions, that is, some may tolerate various types of abiotic perturbations and their growth rates may be less affected in comparison with others. Higher diversity provides greater probability of inclusion of tolerant species in the community which may maintain the ecosystem functioning under variable environment (Yachi and Loreau, 1999).
Microbial communities are drivers of major ecosystem processes such as nutrient cycling (van der Heijden et al., 2008), bioremediation (Gilbert et al., 2012) and plant health (Lugtenberg and Kamilova, 2009). These microbial processes are catalysed and supported by several enzymes and other chemicals bound in the biomass or secreted in the environment. Therefore, the microbial biomass accumulation may provide information about ecosystem functioning in these ecosystems. Studying the effects of anthropogenic perturbations on microbial communities may give important information about the biodiversity–ecosystem functioning relationships in fluctuating environments. In earlier studies, the relationship between bacterial diversity and ecosystem functioning was found to be positive (Hodgson et al., 2002; Bell et al., 2005) or negative (Becker et al., 2012). Complementarity among different species (Bell et al., 2005; Venail and Vives, 2013), species identity (Hodgson et al., 2002) or both the mechanisms (Eisenhauer et al., 2013) may be responsible for positive effects of diversity on ecosystem functioning. On the other hand, the prevalence of antagonistic interactions is the main cause behind negative relationships (Becker et al., 2012; Foster and Bell, 2012). The impacts of abiotic stress on biodiversity–ecosystem functioning relationship of microbial systems are generally found negative (Steudel et al., 2012) or positive in some cases (Li et al., 2010). Bacterial diversity has been found to increase the community stability against both biotic and abiotic environmental perturbations (Eisenhauer et al., 2012).
Here, we study the biodiversity–productivity relationship in bacterial communities under different types of abiotic perturbations (addition of heavy metals, NaCl and warming). Specifically, we test the insurance effects of biodiversity in bacterial communities in the presence of abiotic perturbations. Twelve different bacterial species from four distant taxa were taken and assembled in artificial communities across richness levels from 1 to 12 species, and community biomass was recorded after 24 h. We hypothesised that community biomass will increase with increasing the diversity and more diverse communities will be less affected from perturbations than less diverse one.
Materials and methods
Bacterial species and growth conditions
Bacterial strains of different phylogenetic groups were taken from microbial culture collection of CSIR-Central Institute of Medicinal and Aromatic Plants (CSIR-CIMAP), Lucknow, India (Supplementary Table S1). Bacterial species were selected from four phylogenetically distant groups for integrating broad taxonomic range in the experiment. Three species were taken from each group of Bacilli (Bacillus subtilis, Staphylococcus pasteuri, Bacillus megaterium), γ-proteobacteria (Stenotrophomonas sp., Pseudomonas fluorescens, Acinetobacter junii), α-proteobacteria (Ochrobactrum rhizosphaerae, Aurantimonas sp., Agrobacterium sp.) and Actinobacteria (Microbacterium sp., Kocuria marina, Arthrobacter flavus) (see Supplementary Table S1 for more information). Sequences of 16S rRNA gene of these 12 bacteria and three outgroups (Halobacterium, Thermomicrobium, Deinococcua) were aligned using ClustalW to build a Maximum Likelihood phylogenetic tree using MEGA 6 (Supplementary Figure S1). Using the generated tree, the phylogenetic diversity, as the sum of tree-branch lengths connecting the present species together (Faith, 1992) and mean pair wise distance, as the average phylogenetic distance connecting all species present in a community (Webb et al., 2002), was calculated by using R package plicate.
Bacterial cultures were kept in glycerol stocks at −80 °C and grown in nutrient medium (peptic digest of animal tissue 5 g l−1, sodium chloride 5 g l−1, beef extract 1.5 g l−1, yeast extract 1.5 g l−1 each from HiMedia Laboratories, Mumbai, India). Single colony of each bacterial culture was picked, grown overnight in nutrient broth, washed in phosphate-buffered saline and adjusted to an OD600 of 1.0. Bacterial cultures were left for 6 h at room temperature before assembling the communities. Bacterial communities were assembled in culture tubes taking an equal amount of inoculums of each species, present in a particular community, to establish the diversity gradient of 1–12-species richness (Supplementary Table S2). From these assemblages, 50 μl inoculums were inoculated in 950 μl of 25% nutrient broth in 96 deep-well plates (Supplementary Picture S1) making final volume of 1 ml and incubated at 28 °C with shaking at 200 r.p.m.
Bacterial species were assembled in combinations with a diversity gradient (1, 2, 3, 4, 6 and 12) through random samplings from a pool of 12 species as done previously (Bell et al., 2005). Each species was selected only once without replacement at all richness levels. The complete process of constructing a set of experimental units is carried out independently five times at each richness level, except for 1 and 12 (five partitioned species pools) and each of the combination was replicated four times. Thus, a total of 352 experimental units was composed for each treatment (Supplementary Table S2). In addition, un-inoculated controls with four replications were also included in each plate. The final cell density in each microcosm was kept equal, that is, the 12-species assemblage has the same number of cells (1/12 of each strain) as in monocultures.
Treatments of abiotic perturbation were given after 12 h of inoculation at 28 °C. For abiotic perturbations, 100 μl solutions of heavy metals and NaCl were added to the culture, and the same amount of sterile double-distilled water was added into the treatment of warming and control. The final concentration in broth for different stressors was 2.0% for NaCl, 10 p.p.m. heavy-metal solution (10 p.p.m. solution of each in the form of K2CrO4, ZnSO4·7H2O, CuSO4, NiSO4·6H2O and Pb(CH3COO)2·3H2O). For warming treatments, plates were incubated at 38 °C after 12 h. After 24 h of total incubation, 200 μl of culture was taken in fresh microwell plates and growth was recorded as optical density at 600 nm (OD600) as a measure of community biomass with a plate reader (Spectramax Plus, Molecular Devices, Sunnyvale, CA, USA). The tolerance against perturbation was determined on the basis of percent change in growth of monocultures in the absence (control) and the presence of perturbation. The species whose biomass in monoculture was not reduced in the presence of perturbation were assumed to be tolerant. At the end of the experiment, we detected the presence of the inoculated strains from selected samples. In total, 100 μl of culture was spread on nutrient agar plates, and the presence of inoculants was detected on the basis of colour and structure of bacterial colonies.
Statistical analysis
The data of community biomass (OD600) were square root transformed for insuring the normal distribution. The coefficient of variation of each community was calculated on pooled data across the all treatments. The linear and quadratic effects of diversity (species richness, phylogenetic diversity, mean pairwise distance) and presence of tolerant species were calculated through regression. The effects of species richness (linear and fixed factor), absence/presence of individual species and compositions were analysed with general linear models in R version 2.15.1 (http://www.r-project.org) (Bell et al., 2009). For calculating F-statistic, the effects of variables were tested against specific error terms to avoid the problem of pseudoreplication. The effects of species richness were tested against partitioned species pools and the effects of particular species against compositions. Linear model coefficients were used as the measurement of the relative contribution of species identities. The significance of coefficients was tested (whether they are different from zero) after Bonferroni correction of t-test probability (α=0.05/12). Diagnostic plots of residuals versus fitted values revealed no significant heterogeneity of variance and Q-Q plots indicated that assumptions of normality were justified. Cook's distances were examined to test the level of influence of extreme data points. No data point was found influential to change the output of analyses and interpretation of results. For post hoc differentiation between groups the Tukey's test was applied.
Results
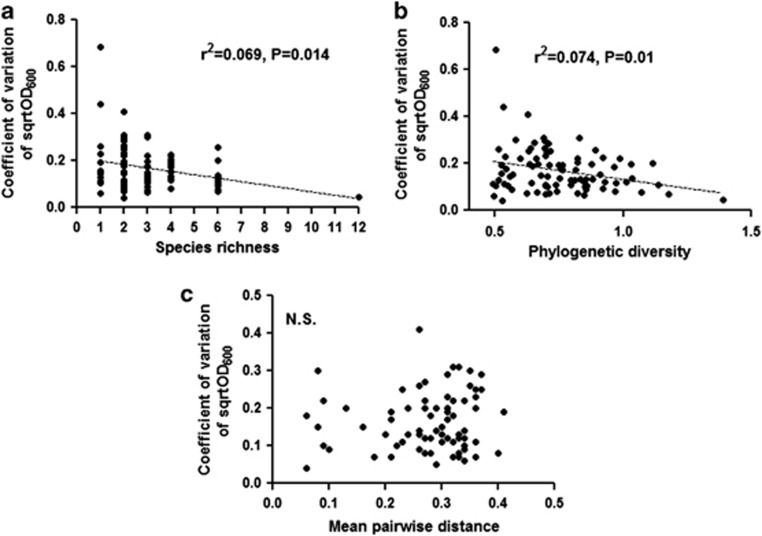
Different treatments of abiotic perturbations differentially affected the biomass of different monocultures (Supplementary Figure S2). The impact of abiotic perturbations was profoundly negative on B. subtilis, S. pasteuri and Stenotrophomonas sp., while other species were found to tolerate at least one type of perturbations. Interestingly, B. megaterium, K. marina and A. flavus were found to tolerate all types of perturbations. The biomass of monocultures was found to vary under different treatments (Supplementary Figure S3). The coefficients of variation of community biomass, across all the treatments, decreased linearly with species richness and phylogenetic diversity (Figure 1), indicating the higher diversity lessens the variations in biomass in the presence of perturbations. However, no correlation between coefficients of variation and mean pairwise distance was found.
Figure 1.
Relationship between coefficient of variation of community biomass (sqrtOD600) and diversity ((a) species richness, (b) phylogenetic diversity and (c) mean pairwise distance). The coefficient of variation of biomass for each community was calculated on pooled data across the all treatments. N.S., not significant.
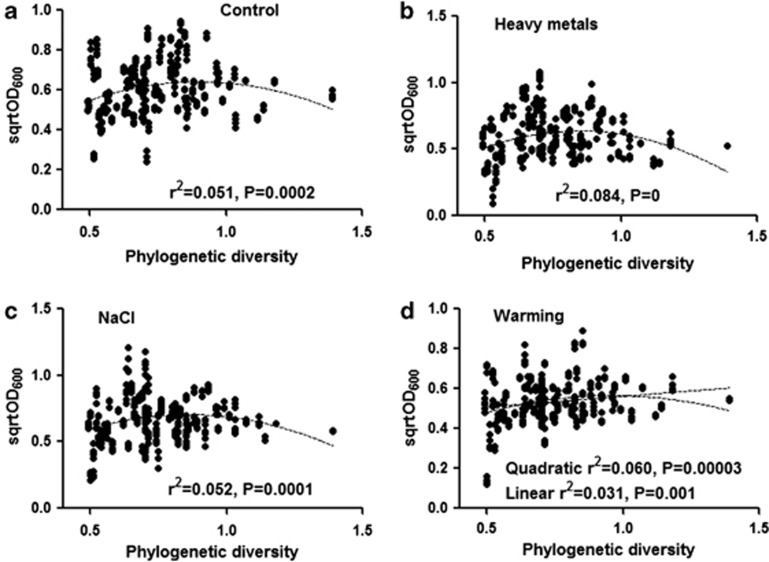
Different measures of diversity (species richness, phylogenetic diversity, mean pairwise distance) affected the biomass of communities (Figure 2, Supplementary Figures S4 and S5); however, in case of heavy metals, the effect of mean pairwise distance was not significant. Effect of the all the diversity measures was best described with a quadratic relationship (Supplementary Table S4). However, a linear relationship was also observed between biomass and diversity (except with mean pairwise distance) in case of warming (Figure 2d and Supplementary Figure S4d), the quadratic relationship being stronger than linear. The phylogenetic diversity was found as the best predictor of community biomass (for the quadratic term, F1330=13.29, P=3.0 × 10−4 for control; F1334=29.67, P=9.9 × 10−8 for heavy metals; F1335=16.43, P=6.2 × 10−5 for NaCl; F1334=10.59, P=2.32 × 10−4 for warming) followed by species richness (for the quadratic term, F1330=10.15, P=1.2 × 10−3 for control; F1334=15.57, P=9.68 × 10−5 for heavy metals; F1335=14.39, P=1.75 × 10−4 for NaCl; F1334=9.56, P=2.15 × 10−3 for warming). The relationship between community biomass and mean pairwise distance (Supplementary Figure S5) was slightly different from those found in the case of species richness or phylogenetic diversity (for quadratic term F1285=19.58, P=1.4 × 10−5 for control; F1289=0.83, P=0.36 for heavy metals; F1287=16.89, P=5.2 × 10−5 for NaCl; F1288=17.99, P=2.3 × 10−5 for warming). It was not significant in case of heavy metals, (Supplementary Figure S5b) and no linear trend was found in warming treatment (Supplementary Figure S5d).
Figure 2.
Effect of phylogenetic diversity on biomass (sqrtOD600) of bacterial communities under different treatments: (a) control, (b) heavy metals, (c) NaCl and (d) warming. For clarity, P-value less than 0.00001 was given as zero.
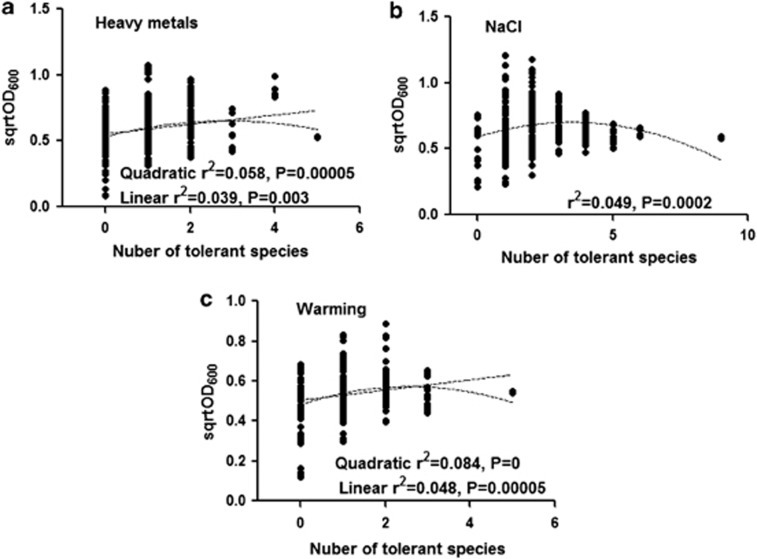
The number of tolerant species present in the community greatly affected the variation in biomass (for the quadratic term, F1334=6.795, P=0.0096 for heavy metals; F1335=16.36, P=6.5 × 10−5 for NaCl; F1334=13.129, P=3.4 × 10−4 for warming and for linear, F1334=13.721, P=2.4 × 10−4 for heavy metals; F1334=17.338, P=4.0 × 10−5 for warming; Figure 3). Community biomass was the highest at intermediate diversity (species richness=4.38–6.55; phylogenetic diversity=2.34–2.45; mean pairwise distance=2.2–2.3, Supplementary Table S3). A unimodal, hump-shaped curve could be established between diversity and biomass (Mitchell-Olds and Shaw test).
Figure 3.
Effect of presence of number of tolerant species on biomass (sqrtOD600) of bacterial communities under different treatments: (a) heavy metals, (b) NaCl and (c) warming. For clarity, P-value less than 0.00001 was given as zero.
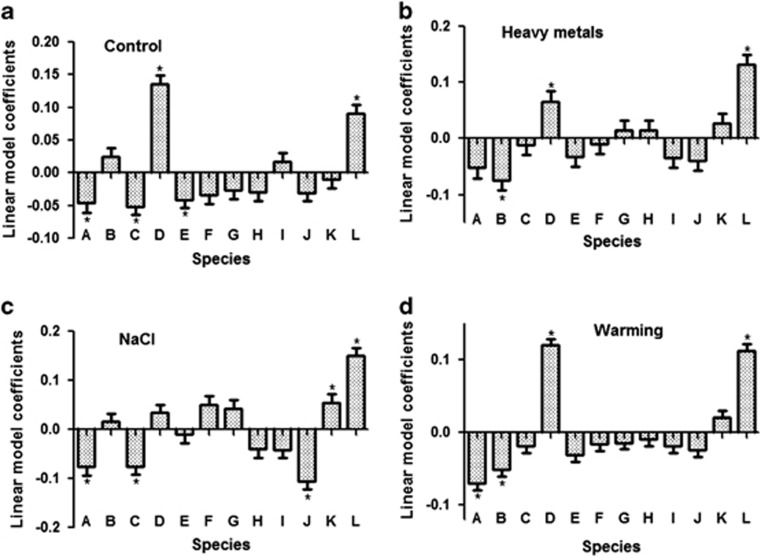
In linear model analyses, the linear effect of species richness was found marginally significant only in case of warming (P=0.058, Table 1); however, fixed effects were significant in case of NaCl (P=0.004) and in warming treatments (P=0.055). The effects of species identity and compositions were consistently significant in all the treatments (Table 1). Standardized linear model coefficients indicate the comparative contributions of species identities on community biomass (Figure 4).The effect of A. flavus was found to be stronger than average in all cases. This indicates, other things being equal, the increase in community biomass in the presence of this species in comparison to an average species effect. The differential contribution of species identities was observed under different types of perturbations. The effects of bacilli remained comparatively negative when significantly different from zero. The effects of species richness and presence of tolerant species remained significant even when both of the factors were fitted after each other (Table 2). The effect of tolerant species increases at higher levels of diversity as indicated from significant interaction between both the factors in case of heavy metals and NaCl.
Table 1. Results of general linear models showing the effects of species richness, species identities and compositions on biomass (sqrtOD600) in bacterial communities.
| Factors | df |
Control |
Heavy metals |
NaCl |
Warming |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SS | F | P | SS | F | P | SS | F | P | SS | F | P | ||
| Sp. richness | |||||||||||||
| Linear | 1, 20 | 0.022 | 0.217 | 0.646 | 0.033 | 0.17 | 0.684 | 0.0374 | 0.396 | 0.536 | 0.098 | 4.055 | 0.058 |
| Fixed | 6, 16 | 0.894 | 2.11 | 0.109 | 1.631 | 1.93 | 0.136 | 1.237 | 5.052 | 0.004 | 0.242 | 2.661 | 0.055 |
| Sp. ID | 12, 76 | 1.994 | 4.078 | 6.8 × 10−5 | 1.983 | 2.221 | 0.037 | 2.99 | 3.86 | 1.3 × 10−4 | 2.05 | 8.52 | 5.6 × 10−10 |
| Compositions | 88, 249 | 3.097 | 73.219 | <2.2 × 10−16 | 5.6559 | 40.753 | <2.2 × 10−16 | 4.9067 | 54.73 | <2.2 × 10−16 | 1.496 | 33.218 | <2.2 × 10−16 |
Abbreviations: df, degrees of freedom; F, F-statistic; P, P-value; Sp. richness, species richness; Sp. ID, species identity; SS, sum of squares.
Figure 4.
Linear model coefficients showing comparative contribution of species effects in community biomass: (a) control, (b) heavy metals, (c) NaCl and (d) warming. Positive values of coefficients show an above-average contribution of species identity to community biomass and negative values show a below-average contribution. Significant coefficients are marked with asterisks. A, Bacillus subtilis; B, Staphylococcus pasteuri; C, Bacillus megaterium; D, Stenotrophomonas sp.; E, Pseudomonas fluorescens; F, Acenetobacter junii; G, Ochrobactrum rhizosphaerae; H, Aurantimonas sp.; I, Agrobacterium sp.; J, Microbacterium sp.; K, Kocuria marina; L, Arthrobacter flavus.
Table 2. Results of general linear models showing the effects of species richness, presence of tolerant species on biomass (sqrtOD600) in bacterial communities.
| Factors |
Heavy metals |
NaCl |
Warming |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| df | SS | F | P | df | SS | F | P | df | SS | F | P | |
| Sp. richness | ||||||||||||
| Fitted first | 5 | 1.66 | 15.33 | 1.5 × 10−13 | 5 | 1.27 | 10.79 | 1.2 × 10−9 | 5 | 0.34 | 6.52 | 8.4 × 10−6 |
| Fitted second | 4 | 1.59 | 18.34 | 1.3 × 10−13 | 4 | 0.67 | 7.08 | 1.7 × 10−5 | 4 | 0.13 | 3.13 | 0.015 |
| Tol. sp. | ||||||||||||
| Fitted first | 4 | 1.01 | 9.27 | 2.8 × 10−8 | 7 | 1.06 | 6.43 | 4.2 × 10−7 | 4 | 0.42 | 9.99 | 1.2 × 10−7 |
| Fitted second | 3 | 0.93 | 10.77 | 3.2 × 10−8 | 6 | 0.46 | 3.23 | 0.004 | 3 | 0.21 | 6.64 | 2.3 × 10−4 |
| Tol. sp *Sp. richness | 6 | 0.54 | 4.43 | 2.5 × 10−4 | 5 | 0.38 | 3.32 | 6.1 × 10−3 | 7 | 0.13 | 1.82 | 0.08 |
Abbreviations: df, degrees of freedom; F, F-statistic; P, P-value; Sp. richness, species richness; SS, sum of squares; Tol. sp., number of tolerant species present.
Discussion
Anthropogenic disturbances affect the ecosystem functioning directly or indirectly through its effects on biodiversity. The main finding of our work is that the diverse communities may tolerate a considerable range of abiotic perturbations and maintain the ecosystem stability. We also found that the abiotic perturbations may alter the relationships between biodiversity and biomass production in some cases. Species identity and initial biodiversity per se both may affect the community biomass in the presence of abiotic perturbations. Biodiversity may enhance the ecosystem stability through affecting temporal (Tilman et al., 2006) and spatial variability (Weigelt et al., 2008), resistance against abiotic perturbations (Mulder et al., 2001) and biotic invasions (Eisenhauer et al., 2013). Different species in diverse communities respond differentially to environmental perturbations and maintain ecosystem functioning (Ives and Carpenter, 2007). In our study, differential response of bacterial species to different agents of perturbation was observed. Under different treatments, the growth of some species was favoured than other. In nature also, the resistance and resilience of microbial communities to perturbations are highly variable (Allison and Martiny, 2008). The higher growths of some species may compensate the negative effects of perturbations on other species and aggregate community performance remains stable across the treatments. The growth of some monocultures was found to be increased under perturbations. Under control, these species may not experience optimum environment and changed conditions through perturbations may be more supportive for their growth. Under abiotic perturbations, these species drive the compensatory dynamics and insurance effects of biodiversity while acting as seed banks under normal conditions (Lennon and Jones, 2011). The decreasing trends in the variation in community biomass, across all the treatments, with increasing species diversity show the importance of biodiversity for maintaining ecosystem functioning under abiotic perturbations. These observations support the importance of biodiversity for stability of ecosystem functioning (McCann, 2000; Ives and Carpenter, 2007) and this study is an addition to the evidences of stabilizing role of diversity in the presence of abiotic perturbations.
Phylogenetic diversity and mean pairwise distance may be considered a proxy for ecological differentiation that may describe the ecosystem functioning better than species richness (Gravel et al., 2012; Venail and Vives, 2013). Similar to earlier studies (Cadotte et al., 2008; Flynn et al., 2011), phylogenetic diversity proved to be the best predictor of ecosystem functioning than other diversity measures. The community biomass was found to be affected with the number of tolerant species present in a community. In the line of insurance hypothesis, diverse communities are more likely to contain some species being able to tolerate the particular stress and maintain the community productivity (Tilman, 1999; Loreau, 2010). The effect of tolerant species was more pronounced at higher levels of species richness than lower one, indicating some type of interactions might be possible between these species. In previous studies also, the selection effect was found to be prevalent under stressful conditions where tolerant species contributed largely to the ecosystem functioning (Boles et al., 2004; Steudel et al., 2012).
Ecosystem functioning was found increasing linearly with species diversity in many studies (Bell et al., 2005; Gravel et al., 2012; Eisenhauer et al., 2013; Venail and Vives, 2013). However, increasing antagonistic interactions also result in negative biodiversity–ecosystem functioning relationships (Jousset et al., 2011; Becker et al., 2012; Foster and Bell, 2012). In our study, the biomass increases with species richness and peaks at intermediate diversity showing positive biodiversity–ecosystem functioning relationship. The decline in community biomass at higher diversity may be due to the increased frequency of pairwise antagonistic interactions, accumulation of toxic metabolites in microcosms. The linear relationship between biodiversity and biomass production in case of warming indicates some relaxation of antagonistic activities. The decline in production of antibiotics with temperature rise, as previously reported by Ritchie (2006), may be one reason behind relaxation in antagonistic activities at higher levels of diversity. Li et al., 2010 also observed that cadmium pollution may trigger positive diversity productivity relationships through the facilitation among algal species. However, the critical examination of these constraints in future experiments may give a better explanation.
The results of present and all these type of studies have limitations in generalisation in natural conditions. Although the diversity of our system was low, we included a broad taxonomic range of bacterial species. Besides, our observations are based on a short-term response of initial diversity to an immediate abiotic perturbation, the competitive relationships among species may, however, change with long term or recurrent exposures leading to the extinction of some species. Studying the long-term effects of abiotic perturbations on ecology and evolution of ecosystems warrants further investigations. In conclusion, our study supports the insurance effects of biodiversity in maintaining ecosystem functioning under abiotic perturbations. Conservation of biodiversity will be helpful to maintain the ecosystem functioning under unpredictable natural and anthropogenic environmental change.
Acknowledgments
We are grateful to Director CSIR-CIMAP for facilities and encouragement and acknowledge the CSIR for the funding of study under network project BSC-203. We are thankful to Dr Shiv Shankar Pandey and Ms. Deepti Barnawal for technical assistance. We thank Mr Rakesh Tiwari and three anonymous reviewers for providing inputs for improving the manuscript.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on The ISME Journal website (http://www.nature.com/ismej)
Supplementary Material
References
- Allison ED, Martiny JBH. Resistance, resilience, and redundancy in microbial communities. Proc Natl Acad Sci USA. 2008;105:11512–11519. doi: 10.1073/pnas.0801925105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker J, Eisenhauer N, Scheu S, Jousset A. Increasing antagonistic interactions cause bacterial communities to collapse at high diversity. Ecol Lett. 2012;15:468–474. doi: 10.1111/j.1461-0248.2012.01759.x. [DOI] [PubMed] [Google Scholar]
- Bell T, Lilley AK, Hector A, Schmid B, King L, Newman JA. A linear model method for biodiversity-ecosystem functioning experiments. Am Nat. 2009;174:836–849. doi: 10.1086/647931. [DOI] [PubMed] [Google Scholar]
- Bell T, Newman JA, Silverman BS, Turner SL, Lilley AK. The contribution of species richness and composition to bacterial services. Nature. 2005;436:1157–1160. doi: 10.1038/nature03891. [DOI] [PubMed] [Google Scholar]
- Boles BR, Thoendel M, Singh PK. Self-generated diversity produces ‘insurance effects' in biofilm communities. Proc Natl Acad Sci USA. 2004;101:16630–16635. doi: 10.1073/pnas.0407460101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadotte MW, Cardinale BJ, Oakley TH. Evolutionary history and the effect of biodiversity on plant productivity. Proc Natl Acad Sci USA. 2008;105:17012–17017. doi: 10.1073/pnas.0805962105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinale BJ, Srivastava DS, Duffy JE, Wright JP, Downing AL, Sankaran M, et al. Effects of biodiversity on the functioning of trophic groups and ecosystems. Nature. 2006;443:989–992. doi: 10.1038/nature05202. [DOI] [PubMed] [Google Scholar]
- Eisenhauer N, Scheu S, Jousset A. Bacterial diversity stabilizes community productivity. PLoS One. 2012;7:e34517. doi: 10.1371/journal.pone.0034517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenhauer N, Schulz W, Scheu S, Jousset A. Niche dimensionality links biodiversity and invisibility of microbial communities. Funct Ecol. 2013;27:282–288. [Google Scholar]
- Faith DP. Conservation evaluation and phylogenetic diversity. Biol Cons. 1992;61:1–10. [Google Scholar]
- Flynn DFB, Mirotchnick N, Jain M, Palmer MI, Naeem S. Functional and phylogenetic diversity as predictors of biodiversity-ecosystem-function relationships. Ecology. 2011;92:1573–1581. doi: 10.1890/10-1245.1. [DOI] [PubMed] [Google Scholar]
- Foster KR, Bell T. Competition, not cooperation, dominates interactions among culturable microbial species. Curr Biol. 2012;22:1845–1850. doi: 10.1016/j.cub.2012.08.005. [DOI] [PubMed] [Google Scholar]
- Gilbert N, Fulthorpe R, Kirkwood AE. Microbial diversity, tolerance, and biodegradation potential of urban wetlands with different input regimes. Can J Microbiol. 2012;58:887–897. doi: 10.1139/w2012-066. [DOI] [PubMed] [Google Scholar]
- Gravel D, Bell T, Barbera C, Combe M, Pommier T, Mouquet N. Phylogenetic constraints on ecosystem functioning. Nat Commun. 2012;3:1117. doi: 10.1038/ncomms2123. [DOI] [PubMed] [Google Scholar]
- Hodgson DJ, Rainey PB, Buckling A. Mechanisms linking diversity, productivity and invasibility in experimental bacterial communities. Proc Natl Acad Sci USA. 2002;269:2277–2283. doi: 10.1098/rspb.2002.2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper DU, Chapin FS, III, Ewel JJ, Hector A, Inchausti P, Lavorel S, et al. Effects of biodiversity on ecosystem functioning: a consensus of current knowledge. Ecol Monogr. 2005;75:3–35. [Google Scholar]
- Ives AR, Carpenter SR. Stability and diversity of ecosystems. Science. 2007;317:58–62. doi: 10.1126/science.1133258. [DOI] [PubMed] [Google Scholar]
- Jousset A, Schulz W, Scheu S, Eisenhauer N. Intraspecific genotypic richness and relatedness predict the invasibility of microbial communities. ISME J. 2011;5:1108–1114. doi: 10.1038/ismej.2011.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennon JT, Jones SE. Microbial seed banks: the ecological and evolutionary implications of dormancy. Nat Rev Microbiol. 2011;9:119–130. doi: 10.1038/nrmicro2504. [DOI] [PubMed] [Google Scholar]
- Li JT, Duan HN, Li SP, Kuang JL, Zeng Y, Shu WS. Cadmium pollution triggers a positive biodiversity–productivity relationship: evidence from a laboratory microcosm experiment. J Appl Ecol. 2010;47:890–898. [Google Scholar]
- Loreau M, de Mazancourt C. Biodiversity and ecosystem stability: a synthesis of underlying mechanisms. Ecol Lett. 2013;16:106–115. doi: 10.1111/ele.12073. [DOI] [PubMed] [Google Scholar]
- Loreau M. Linking biodiversity and ecosystems: towards a unifying ecological theory. Phil Trans R Soc B. 2010;365:49–60. doi: 10.1098/rstb.2009.0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugtenberg B, Kamilova F. Plant-growth-promoting rhizobacteria. Annu Rev Microbiol. 2009;63:541–556. doi: 10.1146/annurev.micro.62.081307.162918. [DOI] [PubMed] [Google Scholar]
- McCann KS. The diversity–stability debate. Nature. 2000;405:228–233. doi: 10.1038/35012234. [DOI] [PubMed] [Google Scholar]
- Mulder CPH, Uliass DD, Doak DF. Physical stress and diversity–productivity relationships: the role of positive interactions. Proc Natl Acad Sci USA. 2001;98:6704–6708. doi: 10.1073/pnas.111055298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naeem S, Li S. Biodiversity enhances ecosystem reliability. Nature. 1997;390:507–509. [Google Scholar]
- Reich PB, Tilman D, Isbell F, Mueller K, Hobbie SE, Flynn DF, et al. Impacts of biodiversity loss escalate through time as redundancy fades. Science. 2012;336:589–592. doi: 10.1126/science.1217909. [DOI] [PubMed] [Google Scholar]
- Ritchie KB. Regulation of microbial populations by coral surface mucus and mucus-associated bacteria. Mar Ecol Prog Ser. 2006;322:1–14. [Google Scholar]
- Steudel B, Hector A, Friedl T, Löfke C, Lorenz M, Wesche M, et al. Biodiversity effects on ecosystem functioning change along environmental stress gradients. Ecol Lett. 2012;15:1397–1405. doi: 10.1111/j.1461-0248.2012.01863.x. [DOI] [PubMed] [Google Scholar]
- Tilman D, Reich PB, Knops JMH. Biodiversity and ecosystem stability in a decade-long grassland experiment. Nature. 2006;441:629–632. doi: 10.1038/nature04742. [DOI] [PubMed] [Google Scholar]
- Tilman D. The ecological consequences of changes in biodiversity: a search for general principles. Ecology. 1999;80:1455–1474. [Google Scholar]
- van der Heijden MG, Bardgett RD, van Straalen NM. The unseen majority: soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol Lett. 2008;11:296–310. doi: 10.1111/j.1461-0248.2007.01139.x. [DOI] [PubMed] [Google Scholar]
- Venail PA, Vives MJ. Positive effects of bacterial diversity on ecosystem functioning driven by complementarity effects in a bioremediation context. PLoS ONE. 2013;8:e72561. doi: 10.1371/journal.pone.0072561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb CO, Ackerly DD, Mcpeek MA, Donoghue MJ. Phylogenies and community ecology. Annu Rev Ecol Syst. 2002;33:475–505. [Google Scholar]
- Weigelt A, Schumacher J, Roscher C, Schmid B. Does biodiversity increase spatial stability in plant community biomass. Ecol Lett. 2008;11:338–347. doi: 10.1111/j.1461-0248.2007.01145.x. [DOI] [PubMed] [Google Scholar]
- Yachi S, Loreau M. Biodiversity and ecosystem functioning in a fluctuating environment: the insurance hypothesis. Proc Natl Acad Sci USA. 1999;96:1463–1468. doi: 10.1073/pnas.96.4.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.