Abstract
Mammalian DNA polymerase (pol) β is the founding member of a large group of DNA polymerases now termed the X-family. DNA polymerase β has been kinetically, structurally, and biologically well characterized and can serve as a phylogenetic reference. Accordingly, we have performed a phylogenetic analysis to understand the relationship between pol β and other members of the X-family of DNA polymerases. The bacterial X-family DNA polymerases, Saccharomyces cerevisiae pol IV, and four mammalian X-family polymerases appear to be directly related. These enzymes originated from an ancient common ancestor characterized in two Bacillus species. Understanding distinct functions for each of the X-family polymerases, evolving from a common bacterial ancestor is of significant interest in light of the specialized roles of these enzymes in DNA metabolism.
Keywords: DNA polymerase, Evolution, Function, Genomics, Phylogenetic, Structure, X-family
1. Introduction
Phylogenetic analysis is a computational method for quantifying evolutionary changes and relationships between protein sequences from different species over time. This type of analysis contributes to our understanding the functional development of a specific enzyme and the relationship between enzymes within cellular pathways, as well as the origin of enzymatic pathways within a cell. Additionally, evolutionary pathway analysis can provide insight into the origin of a particular disease pathway, as well as the relationship between genes in a model organism, as compared to humans.
DNA polymerases catalyze DNA synthesis during repair, replication, and recombination of DNA, as well as specialized DNA synthesis functions during viral replication and antibody gene maturation. Human cells have at least 16 distinct DNA polymerases, now characterized as members of different groups or “families” (designated A, B, X and Y) based on primary protein sequences and putative structural motifs [1,2]. DNA polymerases are usually multi-domain proteins that include an accessory domain in addition to the polymerase domain [3]. The accessory domain (e.g., proofreading exonuclease) can complement the biological function of the polymerase. The nucleotidyl transferase super-family structural fold consists of a catalytic subdomain with a β-sheet and 2 α-helices [4]. The catalytic subdomain includes three carboxylate side chains that coordinate two divalent metal cations (usually Mg2+) within the polymerase catalytic site.
The human DNA polymerase X-family, or family-X, is comprised of DNA polymerase (pol) β, pol λ, polμ and terminal deoxynucleotidyl transferase (Tdt). Only vertebrates possess members of all four of these family-X DNA polymerases, with plants, fungi and simpler organisms having only one or two family members; in some cases X-family members are not present at all (e.g., Caenorhabditis elegans and Drosophila melanogaster) [5,6]. Tdt has only been identified in vertebrates. Family-X DNA polymerases also include the African swine fever virus pol X, Saccharomyces cerevisiae pol IV and a large and emerging series of recently identified pol X-family members in bacterial systems [7,8].
Human pol β has been kinetically, structurally, and biologically characterized [9], and in the current work serves as a reference for comparison with the bacterial X-family polymerases. Bioinformatic and phylogenetic analyses were used to establish an evolutionary, structural and functional relationship between human pol β and bacterial DNA polymerase X-family members. These in silico studies may facilitate use of bacterial systems as models in understanding DNA transactions in more complex organisms with pol X-family members and/or provide insights into the role of the bacterial enzymes in their native environment.
Aravind and Koonin [10] characterized a broad group of nucleotidyl transfer enzymes that included DNA polymerase X-family members as well as members from related families: archaeal and bacterial CCA-adding enzymes/polyA polymerases, protein nucleotidyltransferases, antibiotic nucleotidyltransferases, and proteobacterial adenylyl cyclases. All of these enzymes transfer a nucleotide to an acceptor hydroxyl group, and their common active site suggested an evolutionary relationship.
Analyses of phylogenetic relationships of X-family members have been reported more recently. Uchiyama et al. [6] suggested that all X-family members evolved from a single pol λ-like gene involved in non-homologous end-joining (NHEJ) and that the other X-family member polymerases arose due to gene duplication of this pol λ-like gene. Similarly, Kodera et al. [11] concluded that since the most basal phylum (e.g., Echinodermata in metazoans) contained three X-family DNA polymerase genes (i.e., βλ, and Tdt/μ-like), it is likely that the eukaryotic pol X-family diverged from a single pol λ-like coelenterate phylum gene. In contrast, the computational analyses presented here suggest that all X-family members evolved from a polymerase nucleotidyl transfer catalytic core protein present in ancient bacterial organisms, and gene duplication and alterations occurred over time providing increasing complexity and organelle differentiation within species. This work was aimed at achieving an understanding of the functional and chronological development of different X-family members, especially in relation to pol β.
DNA polymerase X-family members are present and conserved throughout many of the oldest and most varied forms of life. We applied several established methods for sequence alignment followed by phylogenetic analysis to assess the hypothesis that the various X-family polymerases evolved from a DNA polymerase X present in ancient bacterial species. The analysis was more extensive than that in previously published studies. For example, one such study [12] included only 27 X-family polymerase sequences. With recent advances in genomic DNA sequencing, the present study represents a sampling of more than 100 diverse species’ sequences. Additionally, as crystal structures for many of the polymerase X-family members have been solved, including bacterial representatives such as from Deinococcus radiodurans [13], Thermus thermophilus HB8 [14], and the African swine fever virus (ASFV) pol X [15,16], many structure-function relationships important in phylogenetic considerations of DNA polymerase X-family members could be evaluated.
2. Materials and methods
Several established algorithms were utilized for sequence alignment and analysis of the assembled DNA polymerase X-family phylogenetic tree. For creating the phylogenetic tree, we used the following: Phylome DB v. 3.0 [17], PhyML v 3.0 [18,19], ETE [20], iTOL [21,22], Phylemon 2.0 [23], Archaeopteryx [24], and Tree-Graph2 [25]. MUSCLE v. 3.7 was used as the sequence alignment algorithm [26]. JalView was used for creating visual images of sequence alignments produced by MUSCLE [27].
Phylogenetic analysis was initiated from an alignment of 111 identified X-family DNA polymerase sequences. The resulting phylogenetic tree was developed by beginning with the defined “Phylome” deposited in the PhylomeDB v3.0 [17] for human pol β. PhylomeDB (http://orthology.phylomedb.org), is a database of complete collections of gene phylogenies (phylomes) including a number of model species. Phylome uses orthology prediction on a genomic scale to determine the evolutionary relationship between genes from multiple independent phylogenetic trees. The Phylome DB contains 717 species and provides redundant orthology and paralogy predictions. To the original 63 sequences present in this defined phylogenetic pol β tree in the Phylome DB v. 3.0, were added an additional 48 sequences; 23 identified bacterial DNA X-family polymerases, 5 trypanosomatid sequences, and 20 eukaryote (animal) sequences belonging to the X-family. These sequences were then realigned with the original group defining the “Phylome” using the MUSCLE v. 3.7 sequence alignment algorithm [26] and a new phylogeny tree was generated using PhyML v. 3.0 [18,19]. All the identified pol β sequences analyzed in Asagoshi et al. [5], including bacterial DNA polymerase X sequences, and all the bacterial sequences analyzed by Banos et al. [28] were included in the alignment. Many of these sequences are annotated by GenBank as “hypothetical proteins,” “phosphotransferase domain containing proteins,” and “DNA polymerase X or X-family DNA polymerase” or “predicted to be or similar to DNA polymerase β.”
Eggnog 3.0 [29] was also searched to identify 376 protein members of the COG1796 DNA polymerase IV X-family group or “cog” when searched with the human pol β sequence in 261 species. However, most of these orthologs are based on sequence similarity and polymerase enzymatic function has not been confirmed within these species. KOG2534 includes 198 enzymes in the DNA polymerase X-family category from 86 different species. The DNA polymerase X-family members used in the sequence alignments and subsequent phylogenetic analysis are tabulated in Table 1.
Table 1.
DNA polymerase family X members used for sequence alignments and subsequent phylogenetic analyses.
| Prokaryotic DNA polymerase family X sequences | |
| 1. | Aeromonas hydrophila subsp. hydrophila ATCC 7966 (gb|ABK36337.1|) |
| 2. | Aquifex aeolicus VF5 (ref|NP 213981.1|) |
| 3. | Aurelia sp. 1 sensu (UniProtKB: F7J5W8 (F7J5W8 9CNID)) |
| 4. | Bacillus amyloliquefaciens (UniProtKB: F4EI46 (F4EI46 BACAM)) |
| 5. | Bacillus pumilus SAFR-032 (gb|ABV63179.1|) |
| 6. | Bacillus subtilis subsp. subtilis str. 168 (emb|CAB14819.1|) |
| 7. | Deinococcus radiodurans R1 (ref|NP 294190.1|) |
| 8. | Desulfotomaculum reducens MI-1 (ref|YP 001112987.1|) |
| 9. | Listeria monocytogenes serotype 4b str. F2365 (ref|YP 013839.1|) |
| 10. | Methanosarcina mazei Go1 (ref|NP 633918.1) |
| 11. | Mycobacterium tuberculosis (ref|NP 218373.1) |
| 12. | Salpingoeca rosetta sp. ATCC 50818 (gb|EGD82858.1|) |
| 13. | Sphingobacterium spiritivorum ATCC 33300 (ref|ZP 03968066.1|) |
| 14. | Staphylococcus aureus subsp. aureus JH9 (ref|YP 001246578.1|) |
| 15. | Staphylococcus pseudintermedius str. HKU10-03 (UniProtKB: E8SFK2 STAPH) |
| 16. | Staphylococcus saprophyticus subsp. saprophyticus ATCC 15305 (ref|YP 301742.1|) |
| 17. | Thermoplasma volcanium GSS1 (ref|NP 111375.1|) |
| 18. | Thermus aquaticus (tr|P77987|P77987 THEAQ) |
| 19. | Thermus thermophilus HB8 (ref|YP 144416.1|) |
| 20. | Thiobacillus denitrificans ATCC 25259 (gb|AAZ97399.1|) |
| 21. | Vibrio anguillarum 775 (gb|AEH34679.1|) |
| Eukaryotic DNA polymerase family X sequences | |
| 1. | Acromyrmex echinatior (ant; gb|EGI68014.1|) |
| 2. | Ailuropoda melanoleuca (giant panda; ref|XP 002918406.1|) |
| 3. | Ailurus fulgens (red panda; gb|ADT64454.1|) |
| 4. | Anolis carolinensis (lizard; ref|XP 003228273.1|) |
| 5. | Arabidopsis thaliana (mouse ear cress flowering plant; λ-NCBI GenPept: ADM33939.1 gi:304440990) |
| 6. | Ashbya gossypii (filamentous fungus; UniProtKB: Q757Q1 ASHGO) |
| 7. | Bos taurus (cow; β-ref|NP 001029936.1|, λ-ref|NP 001179488.1|, μ-gb|AAX46342.1|, Tdt-UniProtKB: P06526 TDT BOVIN) |
| 8. | Callithrix jacchus (marmoset; β-ref|XP 002757050.1|) |
| 9. | Candida glabrata (haploid yeast; UniProtKB: Q6FKY2 CANGA) |
| 10. | Canis lupus familiaris (domestic dog; β-NCBI GenPept: AAV66968, λ-ref|XP 861980.1|, Tdt-ref|XP 005637632.1|, μ-RefSeq status WITHDRAWN) |
| 11. | Cricetulus griseus (guinea pig; gb|EGV95023.1|) |
| 12. | Crithidia fasciculate (parasitic protist; gb|AAA68599.2|) |
| 13. | Cryptococcus neoformans var. neoformans B-3501A (encapsulated yeast; ref|XP 777918.1|) |
| 14. | Cryptococcus neoformans var. neoformans JEC21 (encapsulated yeast; ref|XP 568043.1|) |
| 15. | Cryptococcus neoformans var. grubii H99 (fungal pathogen causing fatal meningitis; λ-ref|XP 773693.1|) |
| 16. | Danio rerio (zebrafish; β-ref|NP 001003879|, λ-ref|NP 998408.1|, Tdt-gb|AAI63775.1|) |
| 17. | Dictyostelium discoideum (slime mold; UniProtKB: Q1ZXF2 DICDI) |
| 18. | Equus caballus (horse; β-ref|XP 001489108.2|) |
| 19. | Fusarium graminearum PH-1 (fungal plant pathogen; ref|XP 382072.1|) |
| 20. | Gallus gallus domesticus (chicken; β-ref|XP 001143904|, λ-ref|XP 001232209.2|, Tdt-UniProtKB: P36195 TDT CHICK) |
| 21. | Homo sapiens (human; β-ref|NP 002681.1|, λ-UniProtKB: Q9UGP5 DPOLL HUMAN, μ-UniProtKB: Q9NP87 DPOLM HUMAN, Tdt-UniProtKB: P04053 TDT HUMAN) |
| 22. | Kluyveromyces lactis (yeast; UniProtKB: Q6CX59 KLULA) |
| 23. | Leishmania infantum (parasite; gb|AAF00495.1|) |
| 24. | Leishmania major (parasite; UniProtKB: Q4QI80 LEIMA) |
| 25. | Lepeophtheirus salmonis (sea louse; tr|C1BUF7|C1BUF7 9MAXI) |
| 26. | Loxodonta africana (elephant; ref|XP 003412553.1|) |
| 27. | Macaca mulatta (rhesus monkey; β-ref|XP 001097548.1|, λ-ref|NP 001253835.1|) |
| 28. | Monodelphis domestica (gray short-tailed opossum; β-ref|XP 001373104.1|, λ-ref|XP 001369819.1|, μ-UniProtKB: F6SV89 MONDO, Tdt-UniProtKB:O02789 TDT MONDO) |
| 29. | Mus musculus (mouse; β-ref|NP 035260.1|, λ-UniProtKB: Q9QXE2 DPOLL MOUSE, μ-UniProtKB: Q9JIW4 DPOLM MOUSE, Tdt-UniProtKB: P09838 TDT MOUSE) |
| 30. | Nematostella vectensis (starlet sea anemone; β-ref|XP 001618045.1|) |
| 31. | Neurospora crassa OR74A (bread mold; β-ref|XP 961407.1|, λ-ref|XP 963912.2|) |
| 32. | Ornithorhynchus anatinus (platypus; ref|XP 001509890.2|) |
| 33. | Oryctolagus cuniculus (rabbit; ref|XP 002720811.1|) |
| 34. | Pan troglodytes (chimpanzee; β-ref|XP 001143904|, λ-ref|NP 001267179.1|, Tdt-ref|XP 521569.1|) |
| 35. | Platichthys flesus (European flounder; emb|CAC28866.1|) |
| 36. | Pongo abelii (orangutan; ref|XP 002819090.1|) |
| 37. | Rattus norvegicus (rat; β-ref|NP 058837.2|, λ-UniProtKB: Q5RKI3 DPOLL RAT, μ-ref|NP 001011912.1|, Tdt-gb|EDL94195.1|) |
| 38. | Saccharomyces cerevisiae (budding yeast; UniProtKB: P25615 DPO4 YEAST) |
| 39. | Schizosaccharomyces pombe 972h (fission yeast; ref|NP 592977.1|) |
| 40. | Strongylocentrotus purpuratus (purple sea urchin; ref|XP 787665.2|) |
| 41. | Taeniopygia guttata (zebra finch; ref|XP 002186553.1|) |
| 42. | Takifugu rubripes (Fugu rubripes puffer fish; β-ref|XP 003974975.1|, λ ref|XP 003972614.1|, μ-ref|XP 003965471.1|, Tdt-ref|NP 001027915.1|) |
| 43. | Tetraodon nigrovirdis (green spotted puffer fish; β-ref|XP 003974975.1|, λ-emb|CAG03351.1|, μ-emb|CAG05362.1|, Tdt-emb|CAG10152.1|) |
| 44. | Tribolium castaneum (red flour beetle; dbj|BAK40156.1|) |
| 45. | Trypanosoma brucei (protozoan parasite; gb|AAQ56191.1|) |
| 46. | Trypanosoma cruzi (protozoan parasite; gb|ACQ66108.1|) |
| 47. | Xenopus laevis (frog; sp|O57383|DPOLB XENLA) |
| 48. | Xenopus tropicalis (western clawed frog; β-ref|NP 001006894|, λ-ref|NP 001093716.1|, μ-UniProtKB: Q5FVA7 XENTR) |
| 49. | Xiphophorus maculates (southern platyfish; gb|AAU11318.1|) |
| 50. | Yarrowia lipolytica (non-conventional yeast; λ-ref|XP 502740.1|) |
Gene identifiers are indicated in parenthesizes: ref – RefSeq (NCBI Reference Sequence Database); UniProtKB – The UniProt Knowledgebase (UniProtKB), UniProtKB/Swiss-Prot and UniProtKB/TrEMBL; NCBI GenPept, gb, gi – GenBank; emb, EMBL-EBI – European Nucleotide archive; dbj – DNA Databank of Japan; sp – Swiss-Prot; tr – TrEMBL.
3. Results
3.1. Phylogenetic relationships
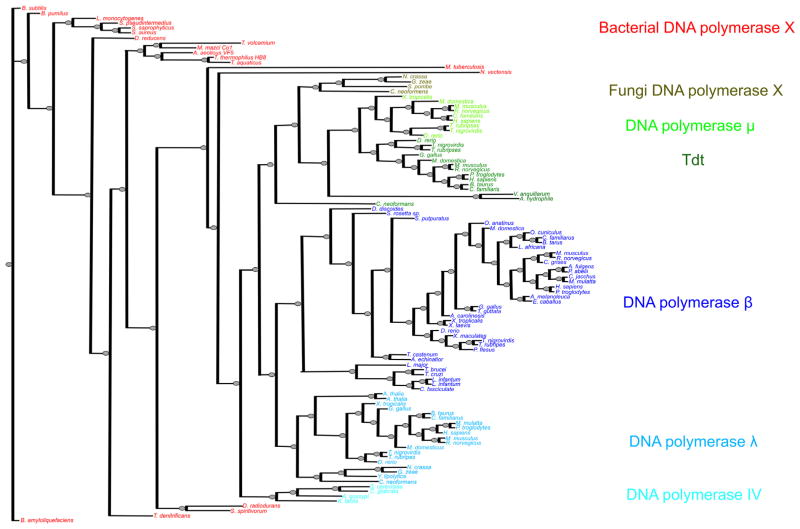
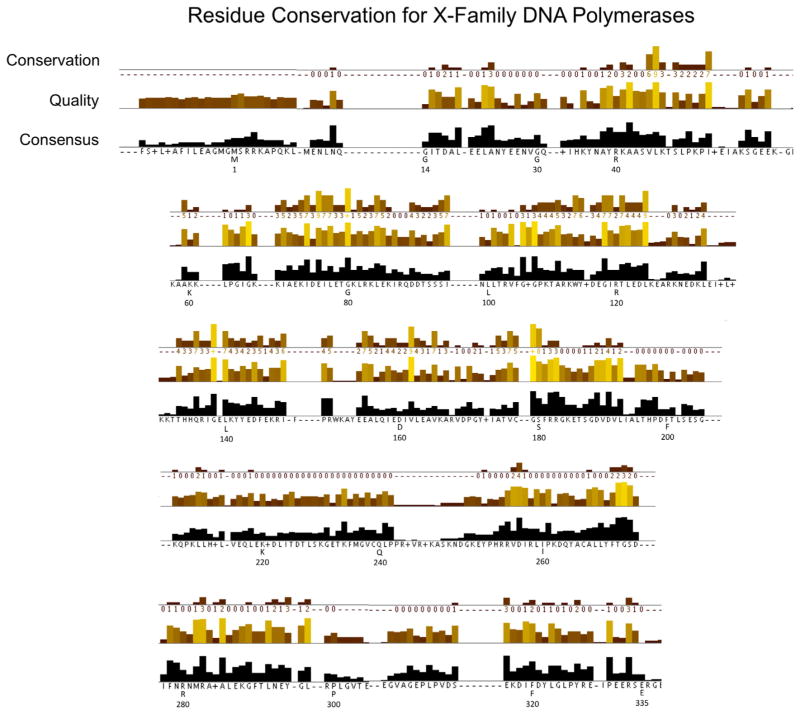
The comprehensive phylogenetic tree developed from the DNA polymerase X-family sequences included in this study is illustrated in Fig. 1. The detailed sequence alignment used to develop this tree is prohibitively large (Supplementary FASTA file). Nevertheless, the quality and consensus of a portion of the alignment is illustrated in Fig. 2. This figure illustrates the sequence conservation exhibited over the human pol β sequence (residues 1–335). Key residues of pol β involved in substrate binding, catalysis, conformational changes, and its deoxyribose phosphate (dRP) lyase activity are tabulated in Table 2.
Fig. 1.
Phylogenetic tree for X-family DNA polymerase sequences. The tree was generated using PhyML v. 3.0 [18,19]. The branches are colored according to general subclasses.
Fig. 2.
Amino acid sequence conservation and analysis corresponding to human pol β. The numbering under the sequence corresponds to that for human pol β, while the letter indicates the consensus sequence at that position. The JalView alignment quality displayed below the conservation columns is a measure of the likelihood of observing mutations at that position in the alignment [27]. Specifically, the quality score is calculated for each column in the alignment by summing, for all mutations, the ratio of the two BLOSUM 62 scores for a mutation pair and each residue’s conserved BLOSUM62 score (which is higher). This value is normalized for each column, and then plotted on a scale from 0 to 1. Conservation is measured as a numerical index reflecting the conservation of chemical properties in the alignment; identical residues score highest with the next most conserved group containing substitutions to amino acids within the same chemical class. Conservation is visualized on the alignment as a histogram giving the score for each column. Conserved columns are indicated by ‘*’ (score of 11 with default amino acid property grouping), and columns with mutations with conserved chemical properties are marked with a ‘+’ (score of 10, indicating all properties are conserved).
Table 2.
DNA polymerase β residues involved in ligand binding/catalysis.
| Residue | Interaction | Referencea |
|---|---|---|
| Catalytic site | ||
| Asp190 | Coordinates two divalent metals | [64] |
| Asp192 | Coordinates two divalent metals | [64] |
| Asp256 | Coordinates catalytic metal | [48,65] |
| dNTP binding | ||
| Ser180 | Coordinates Pγ | [53] |
| Arg183 | Coordinates Pβ | [53,66] |
| Ser188 | H-bonds with Ser180 | [53] |
| Gly189 | Coordinates Pγ | |
| Tyr271 | Sugar discrimination | [52,53,55] |
| Phe272 | Sugar discrimination; subdomain motions | [67] |
| Asp276 | Base van der Waals interactions | [54,68,69] |
| Asn279 | H-bond with the minor groove edge of the base | [52,53,70] |
| Templating (coding) nucleotide | ||
| Lys280 | Base van der Waals interactions | [40,53]} |
| Arg283 | Interactions with the minor groove edge of the base | [52,71] |
| Subdomain motions (open–closed) | ||
| Arg258 | Alters salt bridge | [72] |
| Ile260 | Modulates α-helix M rotation | [72–74] |
| Tyr265 | Modulates α-helix M rotation | [75] |
| Glu295 | Altered H-bonding | [53] |
| Lyase activity (dRP binding pocket) | ||
| Glu26 | Facilitate sugar deprotonation (C2′) | |
| Ser30 | Facilitate sugar deprotonation (C2′) | |
| His34 | Active site pocket | [37] |
| Lys35 | 5′-PO4 binding; sugar ring opening | [37,76] |
| Tyr39 | Stabilize deprotonated Lys72 | [76] |
| Lys41 | Active site pocket | |
| Lys60 | Active site pocket | [37] |
| Lys68 | 5′-PO4 binding | [37,76] |
| Glu71 | Facilitate sugar deprotonation (C2′) | [37] |
| Lys72 | 5′-PO4 binding; Nucleophile involved in C1′ attack | [37,76] |
| Lys84 | Alternative nucleophile | [37,76] |
DNA polymerase β site-directed mutagenesis studies.
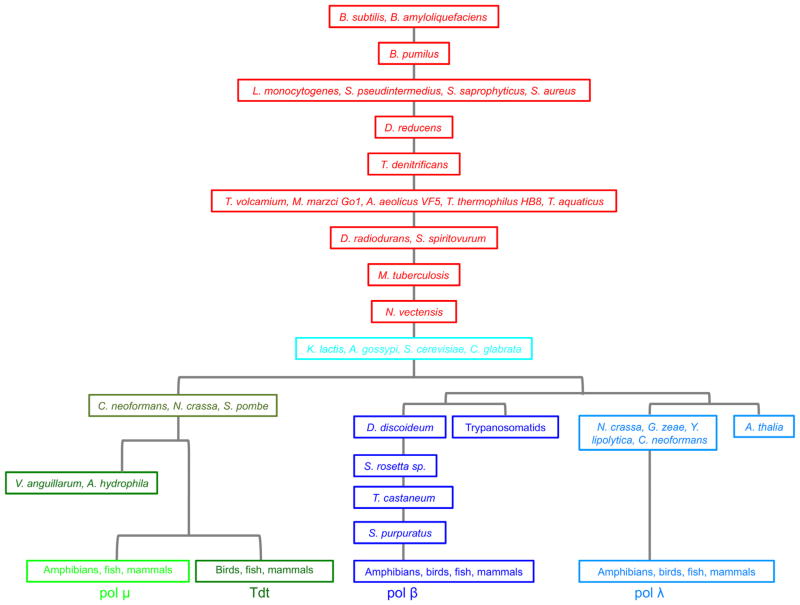
From the standpoint of a broad overview, the bacterial X-family polymerases are the evolutionary ancestors of all family-X polymerases (including the eukaryotic cellular enzymes designated pol β, pol λ pol μ TdT, and pol IV) (Figs. 1 and 3). The ancient bacterial X-family polymerases evolved into an ancestral pol IV from Kluyveromyces lactis and later S. cerevisiae pol IV. After this point, two distinct branches arose: (1) the Tdt and pol μ branch (two gram-negative rod shaped bacteria also have polymerases clustered with this group, as well as several fungi), and the (2) the pol β and λ branch. The pol β branch includes a sub-branch of slime mold and trypanosomatid pol β; and the pol λ sub-branch includes the plant Arabidopsis thaliana pol X and some fungal polymerases.
Fig. 3.
Schematic family tree illustrating the origin and evolution of the X-family DNA polymerases. The most ancient family members are from Bacillus bacterial species.
As illustrated in Figs. 1 and 3, the evolutionary origin of the DNA polymerase X-family members begins with the most ancient pol X from the Bacillus bacterial species, B. subtilis and B. amyloliquefaciens and then B. pumilus. These are gram-positive bacteria with protective endospores permitting the organisms to tolerate extreme environmental conditions. The oldest ancestral Bacillus polymerases X are followed by the Listeria and Staphylococcus pol X that lead to the Desulfotomaculum reducens polymerase and then to the Thermoplasma and Thiobacillus, followed by the thermophilic species, T. thermophilius and T. aquaticus pol X; these are followed by the D. radiodurans, Mycobacterium tuberculosis, and Nematostella vectensis pol X species. Most bacterial pol X-family members are ancestors and do not cluster specifically with either pol β, pol λ, pol μ, or pol IV. There are exceptions, however: Vibrio anguillarum, and Aeromonas hydrophila. V. anguillarum pol X is pol μ/Tdt-like and sequesters with these sequences. These species are members of the bacterial phylum Proteobacteria Class: Gamma Proteobacteria Order: Vibrionales Family: Vibrionaceae. This gram negative curved rod-shaped bacterium with one polar flagellum is an important pathogen of cultured salmonid fish and causes the disease known as vibriosis or red pest of eels. A. hydrophila pol X is also a pol μ/Tdt like-DNA polymerase. It differs in class from the Vibrionales and belongs to the bacteria Proteobacteria: Gammaproteobacteria, Aeromonadales, Aeromonadaceae. Aeromonas bacterium is found in freshwater environments and in brackish water; it is a gram-negative rod that has polar flagella and is a facultative anaerobe.
The pol IV containing fungi species branch evolved from N. vectensis that then broke off into the fungal species group, then pol μ and Tdt followed by a split from the pol λ and pol β groups. In the pol β branch, the trypanosomids form a distinct and earlier pol β sub-group, as do the slime mold, Salpingoeca rosetta sp., Tribolium castaneum, and Strongylocentrotus purpuratus species. The pol λ branch also has an older off-branch comprised of the Arabidopsis and fungi Neurospora crassa, Gibberella zeae, Yarrowia lipolytica, and Cryptococcus neoformans.
3.2. Relationships
3.2.1. X-family polymerases in fungi
These enzymes are divided into two distinctive classes (Fig. 1). One group of fungal species has a pol IV-like DNA polymerase, whereas others have two family-X polymerases, one that clusters with and is similar to mammalian pol λ and another that clusters with mammalian pol μ and Tdt. Plants are represented in this tree by Arabidopsis that only has pol λ.
3.2.2. Functional relationships to S. cerevisiae pol IV
DNA polymerase IV has 5′-dRP lyase activity, an essential activity for single-nucleotide base excision repair (BER), low processivity for DNA synthesis on open template DNA (i.e., not gapped) and can fill short DNA gaps. DNA polymerase IV does not have a proofreading activity and is highly inaccurate. It has the greatest homology with mammalian pol λ, has a BRCT domain, and both pol λ and pol IV contain a large number of positively charged residues in their dRP lyase domain [30]. Schizosaccharomyces pombe pol IV has dRP lyase activity, and properties that are similar to pol β, pol λ and pol μ. DNA polymerases β and λ share 32% sequence identify and pol λ can substitute for pol β during in vitro and in vivo BER [31]. DNA polymerase μ and Tdt share 41% sequence identity. S. pombe pol IV shares the highest sequence similarity to pol μ [31].
3.2.3. X-family polymerases in trypanosomatids
Trypanosomatids have more than one pol β and appear to have evolved a separate and distinct packing and presentation scheme for their pol β-like enzymes compared with other species. These enzymes within some of these parasites are unusual in that they are not nuclear, but instead are mitochondrial. Trypanosomatids have unusual mitochondrial DNA packed in a special structure called kinetoplast DNA (kDNA). The pol β gene has been cloned and characterized from several parasites, including Trypanosoma cruzi, Trypanosoma brucei, Crithidia fasciculata and Leishmania. The Leishmania major pol β, while localized to the nucleus is also involved in kinetoplast mitochondrial DNA replication, BER and translesion DNA synthesis [32]. In the case of T. brucei, two pol β genes have been identified within the mitochondria [33]. In C. fasciculata and the Tryponosoma, pol β with dRP lyase activity has been demonstrated in mitochondria [11,34,35]. Leishmania infantum pol β uses manganese as its divalent cation, prefers gapped DNA substrates with a 5′-phosphate in the gap, and has dRP lyase activity, all features characteristic of mammalian pol β. Leishmania is one of the most primitive eukaryotes, and C. fasciculata and Trypanosoma are related [36]. DNA polymerase β in C. fasciculata mitochondria is error prone in vitro and can only be found when replicating kDNA and is, therefore not thought to be involved in BER. Critical residues for the 5′-dRP lyase activity are conserved between Crithidia and mammalian pol β (Table 3): Lys35, Lys60 and Lys68, important for single-stranded DNA binding; Lys35, important for 5′-phosphate group recognition; and Lys72 and Tyr39, dRP lyase catalytic residues [37,38]. The C. fasciculata pol β-like Schiff base intermediate can be trapped experimentally with NaBH4 reduction, although the enzyme’s dRP lyase activity is lower than the mammalian enzyme.
3.2.4. DNA polymerase β-like enzymes in lower organisms
DNA polymerase β genes from many lower organisms have been cloned and sequenced and the products of these genes are believed to function within BER. These enzymes are highly conserved, and in many species, the pol β proteins are similar in size retaining significant sequence similarity and protein domain structure. Functionally significant amino acid residues involved in catalysis are conserved within species and preserved throughout evolution (Table 2 and Supplementary Table 1). For example, Oncorhynchus masou (cherry salmon), Xiphophorus maculates and Danio rerio (zebrafish) pol β have 337 residues, only a few residues more than human, rodent (335 residues) and frog (334 residues). Zebrafish share 79, 80, and 79% sequence similarity with rat, human, and frog pol β, respectively. Jellyfish (Aurelia sp. 1 As) pol β has also been characterized (335 residues) and found to share 55% sequence identity with the vertebrate pol β and have similar behavior in DNA repair assays as the mammalian enzymes [11]. As an example, the jellyfish enzyme is sensitive to inhibition by dideoxythymidine triphosphate, like mammalian pol β, and demonstrates similar response to several other mammalian pol β inhibitors [11]. However, the X-family polymerases do not share sequence similarity with, and are not related evolutionarily to, Escherichia coli pol I (A-family), pol II (B-family), or pol III (C-family) [39]. Surprisingly, there are no pol X-family members in the genomes of Drosophila or C. elegans [5,6]. Yet as illustrated in Fig. 1, pol β has been found in other ecdysozoans, such as T. castaneum (red flour beetle) and sea anemone (N. vectensis) [11]. To date, no homologs of pol β or Tdt have been identified in fungi, plants and lower fungi, such as slime mold. These organisms have a pol λ-like enzyme only.
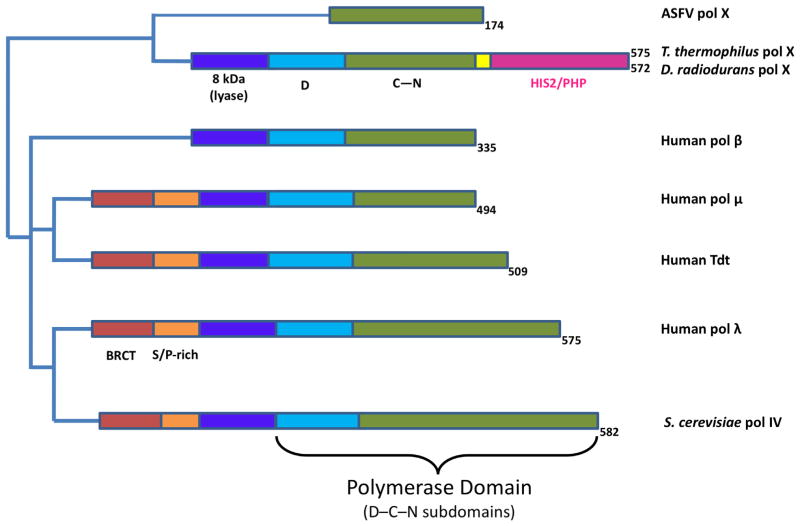
3.3. Domains and enzymatic activities
Although DNA polymerase X-family members only share partial sequence homology, they share a conserved domain organization. Fig. 4 illustrates the domain structure of different X-family members. The polymerase catalytic domain of all X-family members have the common subdomain architecture with DNA binding, catalytic, and dNTP binding subdomains (D-, C-, and N-subdomains, respectively) [40]. Some DNA polymerase X-family members are small enzymes. DNA polymerase β is the smallest mammalian DNA polymerase, while the ASFV pol X is only 20 kDa (174 residues) [41]. Mammalian pol β and pol λ have been shown to have 5′-dRP lyase and polymerase activities required in BER. DNA polymerase λ, pol μ and Tdt also have an amino-terminal BRCT domain, a protein-protein interaction domain involved in NHEJ and possibly other forms of double strand break repair [8]. Importantly, pol μ and Tdt do not have 5′-dRP lyase activity [42]. The X-family members with the lyase activity possess a conserved catalytic lysine nucleophile (pol β, Lys72; pol λ, Lys312) that forms a Schiff base intermediate in the lyase β-elimination reaction mechanism. These enzymes also have a conserved tyrosine residue (pol β, Tyr39; pol λ, Tyr279) suggested to be involved in the lyase catalytic mechanism [39]. This tyrosine residue corresponds to a phenylalanine in family-X members lacking the dRP lyase activity (i.e., pol μ and Tdt). The residues necessary for efficient lyase activity are conserved in the polymerase X-family members of many lower species, e.g., in the 8 kDa domain of the parasitic protist C. fasciculata pol X (Tyr38 and Lys71) and yeast pol IV (Tyr214 and Lys248).
Fig. 4.
Domain organization of selected X-family DNA polymerases. Mammalian DNA polymerase β is composed of two domains that complement its biological role in base excision repair: lyase (purple) and polymerase (light blue and green). The polymerase domain includes three subdomains: catalytic (C, green) and nucleotide binding (N, green) and DNA binding (D, light blue). DNA polymerase μ, Tdt, pol λ and Pol IV have an amino-terminal BRCT domain (light red). The two bacterial enzymes include a carboxyl-terminal PHP/HIS2 domain (pink) connected to the polymerase domain with a linker (yellow). Pol λ includes a serine-proline-rich region (S/P-rich, orange).
B. subtilis pol X has been shown to have DNA synthesis activity, manganese dependent 3′ to 5′ exonuclease, and apurinic/apyrimidinic (AP) endonuclease activities [28]. B. subtilis pol X shares 24% sequence identity with human pol β and requires a template and divalent metal for polymerase activity. DNA polymerase μ and Tdt also have been shown to have template-independent DNA synthesis activity. These template-independent enzymes have a conserved histidine residue (His329, pol μ; His342, Tdt) near the active site that may stabilize the primer terminus in the absence of a complementary templating base (i.e., single-stranded primer terminus). Previously reported sequence alignments identified a conserved Helix-hairpin-Helix motif within bacterial and archeal pol X family members; these Helix-hairpin-Helix motifs were predicted to indicate that these enzymes possessed dRP lyase activity based on the presence of homologous conserved lysine and tyrosine residues observed with enzymes that have this activity, although this activity has not been demonstrated experimentally [28].
Bacillus pol X has 570 residues; residues 1–317 define the polymerase domain, and residues 335–570 define a histidinol phosphatase (PHP) domain unique to bacterial polymerase X-family members (Fig. 4). The enzyme has been shown to possess intrinsic AP endonuclease activity [43]. Other than the polymerase domain, the functions of the bacterial pol X domains are less well established than those of the mammalian polymerases and are still under active investigation. To date, the bacterial pol X enzymes appear to lack a BRCT domain [44]. Several catalytically important residues for DNA synthesis activity have been identified in T. thermophilus pol X [45]. In addition, a PHP domain with intrinsic 3′–5′ exonuclease activity has been identified in this organism. Recently, it has been reported that the T. thermophilus PHP domain has 3′ phosphatase and AP endonuclease activities [45].
3.4. Structural comparisons
The ASFV pol X is the smallest (174 residues) nucleotidyl transfer polymerase identified to date (Figs. 4 and 5). ASFV causes hemorrhagic fever in pigs, and pol X repairs viral DNA damaged as the host fights viral infection. The ASFV pol X has a unique structure involving the polymerase C- and N-subdomains, but without the D-subdomain [15,16]. Despite the lack of a DNA binding subdomain, the enzyme binds DNA with high affinity. The C-subdomain of ASFV pol X is considerably more electropositive than in pol β, even though ASFV pol X shares 55% sequence homology with the carboxyl-terminal half of pol β [41]. The ASFV active site metal coordinating ligands (Asp49, Asp51, and Asp100) are structurally super-imposable with the catalytic aspartate triad of human pol β (Asp190, Asp192, and Asp256). Interestingly, ASFV pol X has a disulfide bond between Cys81 and Cys86 in one solution structure [15], but is reduced in a solution structure determined in a different laboratory [16]. The resultant structures indicate that the N-subdomains are in alternate positions reminiscent of those observed when mammalian pol β binds a nucleoside triphosphate [41]. The significance for this redox activity is not known and has not been observed with the mammalian polymerase. As noted above, ASFV pol X binds DNA intermediates of base excision repair with high affinity, and the enzyme does not have an amino-terminal lyase domain that would facilitate single-nucleotide BER. The enzyme does not interact with the BER proteins AP endonuclease or XRCC1 and does not have a proofreading exonuclease. This polymerase is an example of a minimal functional version of the evolutionary conserved pol β-type core polymerase and is anomalous to other X-family member structures [46].
Fig. 5.
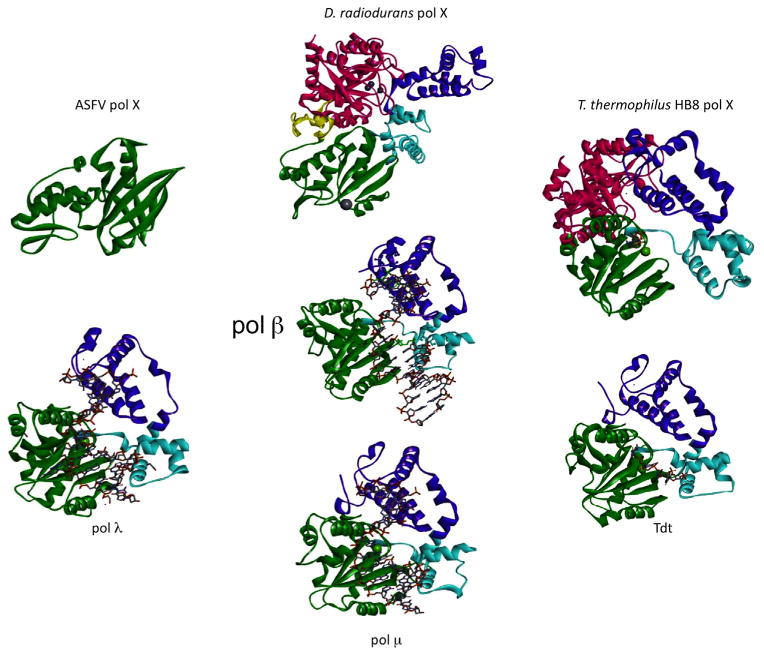
Representative crystal structures of X-family DNA polymerases. The domains/subdomains are colored according to the scheme used in Fig. 4 and are arranged to show similar orientations. The PDB IDs are: human pol β, 2FMS [60]; ASFV pol X, 1JAJ [16]; D. radiodurans pol X, 2W9M [13]; T. thermophilus HB8 pol X, 3AU2 [45]; truncated mouse Tdt, 1KDH [61]; truncated mouse μ, 2IHM [62]; truncated human pol λ, 2BCR [63]. When present, DNA is shown in a stick representation.
Crystal structures of X-family bacterial polymerases from two organisms have been solved (Fig. 5): D. radiodurans, a gram-positive bacterium, and T. thermophilus HB8, a gram-negative eubacterium. The Thermus pol X is atypical in that it binds Mg2+-dNTP prior to binding DNA [45]. Thermus pol X has DNA/RNA polymerase activity and 3′–5′ proofreading exonuclease activity, and structures have been solved of the binary complex with dGTP, template/primer DNA, and with gapped DNA and ddGTP (ternary substrate complex). A pol β-like active site, with 2 metals and aspartate catalytic triad (Asp198, Asp200, and Asp243) is conserved in this bacterial polymerase. Lys263 is essential for Mg2+ binding to dNTP and this lysine residue is conserved in bacterial and archeal pol X enzymes and is also found in pol μ and Tdt. In pol β, however, the corresponding residue is an aspartate. Thermus pol X has Mg2+ and Mn2+-dependent DNA synthesis activities and Mn2+-dependent exonuclease activity [44]. Site-directed mutational studies determined that His344, His374, His468 and Asp529 in the PHP domain were essential residues for the proofreading exonuclease activity, but not polymerase activity [44]. These residues are highly conserved among all bacterial pol X structures [47].
D. radiodurans is highly resistant to ionizing radiation damage. Deletion of the D. radiodurans pol X decreases the rate of DNA double-strand break repair. The polymerase has strong proofreading exonuclease activity stimulated by Mn2+. Its structure displays an extended fold in contrast to the compact structure displayed for the polymerase domain of the mammalian enzymes [13]. Similar to Thermus pol X, it has a carboxyl-terminal PHP domain with a tri-nuclear zinc-binding site. However, the isolated PHP domain does not appear to be functional [13]. The carboxyl-terminal PHP domain isolated from T. thermophilus was shown to have proofreading activity, as well as 3′-phosphatase and AP endonuclease activities that can be utilized during base excision repair [45]. The 3′-phosphatase activity has not been reported in other pol X enzymes. Only prokaryotes have been shown to contain a PHP domain within their X-family DNA polymerase. In higher organisms, a separate and distinct enzyme performs this catalytic function. The PHP domain in Bacillus has AP endonuclease and proofreading exonuclease activities [47].
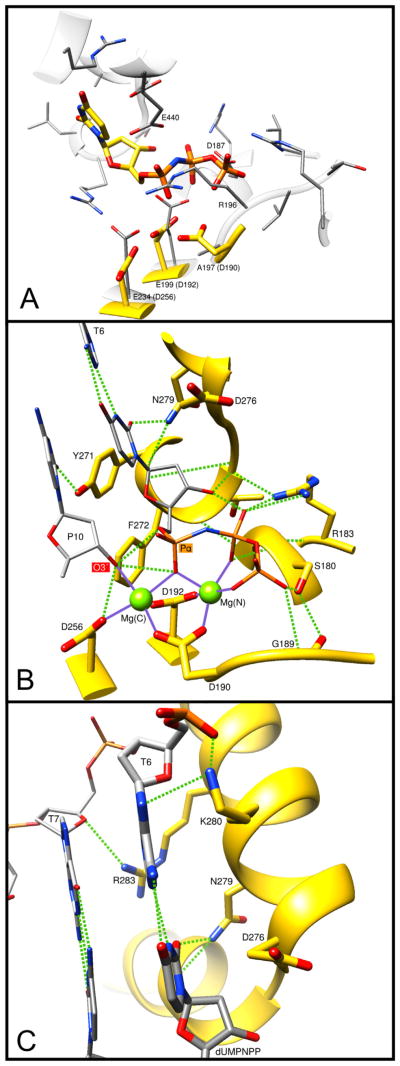
A significant difference between the D. radiodurans enzyme and higher organism pol X-family members is the replacement of the three conserved active site aspartate residues comprising the catalytic triad that binds divalent metals with two glutatmate residues (Fig. 6A). This is the only X-family polymerase, among all of the sequences examined in this study, that has an ‘AAE’ motif for residues corresponding to the ‘DXD’ motif (residues 190–192) in human pol β and conserved in almost all pol X-family members (Supplementary Table 1). The Asp256 residue in human pol β is also a glutatmate in the D. radiodurans enzyme. In addition to binding the catalytic metal, this residue also participates in deprotonation of the primer terminus [48]. The PHP domain is similar to the YcdX E. coli zinc-binding domain, and zinc is found in the D. radiodurans pol X crystal structure. A 5′-dRP lyase and BER activities have been demonstrated in the Deinococcus pol X [49]. This pol X does not have the conserved lysine found in both pol β and λ (Lys72 and Lys312, respectively) that acts as the Schiff base nucleophile for lyase activity. This lysine is Glu72 in Deinococcus pol X. Lys33 of D. radiodurans that corresponds to Lys35 of pol β and Arg275 of pol λ is the only conserved basic residue in this family-X polymerase. In D. radiodurans pol X, Lys64 and Lys67 may be involved in DNA binding and Tyr37 may be involved in 5′-phosphate recognition necessary for DNA gap binding. Tyr37 correspond to Tyr39 in pol β and Tyr279 in pol λ that bind a 5′-phosphate in gapped DNA. The carboxyl-terminal PHP domain, present in many bacterial polymerase X-family members, is not present in mammalian pol X members but has been reported in the structure of E. coli pol IIIa subunit (family-C replicative DNA polymerase) [50]. The D. radiodurans pol X PHP domain superimposes with the Pol IIIa PHP domain, with a RMSD of 2.8 Å (136 Cα), and these PHP domains share only 15% sequence identity [13].
Fig. 6.
Molecular interactions of key DNA polymerase β side-chains. The ternary substrate (DNA/dUMPNPP) complex of human pol β (PDB ID 2FMS) was used to illustrate these interactions. (A) Overlay of the pol β active site (yellow carbons) with that of D. radiodurans pol X (gray carbons, PDB ID 2W9M). Key D. radiodurans residues are indicated and the equivalent pol β residues are given in parentheses. Arg196 (R196) of D. radiodurans appears to occlude the triphosphate binding pocket. In addition, pol β Asp190 (D190) is not conserved; D. radiodurans Ala197 (A197). (B) Key protein interactions with the incoming nucleotide (dUMPNPP). Hydrogen bonds are indicated with green dashed lines and metal coordinations are shown as solid purple lines. The catalytic and nucleotide binding metals are shown as green spheres: Mg(C) and Mg(N), respectively. Protein and substrate carbons are yellow and gray, respectively. The primer terminus (P10) O3′ and the α-phosphate of the incoming nucleotide are indicated. T6 identifies the templating base. The function and conservation of these residues are tabulated in Table 2 and Supplementary Table 1. (C) Key protein interactions with the bases of the nascent base pair. Hydrogen bonds are indicated with green dashed lines. T7 identifies the templating base immediately upstream of the coding templating base T6. The base of the incoming nucleotide (dUMPNPP) is indicated. The function and conservation of these residues are tabulated in Table 2 and Supplementary Table 1.
As is illustrated in Fig. 5 where the structures of various X-family polymerases are compared, the domain orientation in D. radiodurans pol X is different from that of pol β. As noted above, the polymerase nucleotidyl transfer reaction involving two metals bound to three aspartate resides must be different, as these have been replaced with two glutamate residues in D. radiodurans pol X (Fig. 6A). Accordingly, different metal binding sites must exist in this situation.
3.5. Evolutionary conservation of functionally significant residues
Since mammalian pol β has been extensively characterized functionally and structurally [9], it is useful to compare the conservation of functionally significant residues of pol β with those of other family members (Table 2 and Supplementary Table 1).
3.5.1. Active site metal-coordinating aspartates
As noted above, D. radiodurans (PDB ID 2W9M) is unique in that it does not include the active site “DXD” motif (pol β, Asp190 and Asp192). There are only two other X-family polymerases that we have examined that do not have this signature motif conserved, M. tuberculosis and N. vectensis (sea anemone). Fig. 6A illustrates a structural alignment (superposition) of the polymerase domain from human pol β (PDB ID 2FMS) and D. radiodurans pol X (PDB ID 2W9M). The root-mean squared deviations of the superposition of the C- and N-subdomains domains is 1.2 Å (74 Cα). The DXD motif is otherwise conserved in all other bacterial pol X and mammalian pol X sequences examined in this study. Asp256 is highly conserved with the exception of four pol X bacterial species; D. radiodurans, Sphingobacterium spiritivorum, M. tuberculosis, and A. hydrophila. Asparagine replaces this aspartate in one fungal pol λ-like enzyme in N. crassa.
3.5.2. Deoxynucleoside triphosphate binding residues
Arg183 of pol β interacts directly with the triphosphate tail of the incoming nucleotide (Fig. 6B). It is the most conserved residue throughout all X-family members (98%, Supplementary Table 1). Ser180 of pol β also interacts with the triphosphate tail and is conserved in bacterial pol X sequences but is glycine in fungi family-X polymerases, Tdt and pol μ. The backbone of pol β Gly189 interacts with the γ-phosphate of the incoming nucleotide and is a histidine in pol μ and Tdt sequences and a lysine in some bacterial pol X species.
The pol β dNTP binding pocket is formed by Tyr271, Phe272, Asp276, and Asn279 (Fig. 6B). The pol β YFTGSD motif (residues 271–276) is GWTGSK/R in pol μ and Tdt sequences and (Y/H)FTGSK in the bacterial pol X sequences. DNA polymerase β Asn279 is conserved in pol λ (Asn514), but not in pol μ (Gln432) or Tdt (Glu457), but is conserved in the majority of bacterial X-family polymerases. Tyr271 and Phe272 significantly alter their positions and interactions when a nucleotide binds to the binary DNA complex and have been suggested to play important roles in subdomain motions during catalytic cycling [9,51]. Asn279 forms a minor groove hydrogen bond with the base and Asp276 contributes van der Waals interactions with the base of the incoming nucleotide. Alanine substitution for Asn279 weakens nucleotide binding [52,53], but replacing the charged side chain of Asp276 with a hydrophobic side chain increases binding affinity [54]. Tyr271 has also been implicated in substrate specificity during ribo- and deoxyribosugar discrimination [55]. The YFTGSD sequence is highly conserved in all pol β sequences. It is TFTGSK in the trypanosome pol β sequences, YFTGSA in the majority of pol λ sequences, and GWTGSR/K/Q in many pol μ and Tdt sequences. This motif is not conserved in many of fungal species (e.g., S. pombe, Ashbya gossypii, Candida glabrata, N. crassa, G. zeae and K. lactis) and is also not conserved in many of the bacterial pol X sequences. It is HFTGSK in the majority of pol X bacterial sequences, however it diverges significantly in S. spiritivorum, D. radiodurans, M. tuberculosis, N. vectensis, A. hydrophila subsp. hydrophila ATCC 7966 and V. anguillarum 775.
3.5.3. DNA (template) binding residues
Arg283 of pol β plays a critical role in proper positioning of the templating base (Fig. 6C). Alanine substitution for this residue strongly reduces base substitution [52,56] and frameshift fidelity [57]. This is believed to be due to its interactions with the template strand as well as its role in structural transitions during substrate binding. Mutation of this residue destabilizes the closed catalytically active conformation of pol β [52,58]. The loss in fidelity is specifically due to the inability to insert the correct nucleotide [59]. Lys280 forms van der Waals contact with the base of the templating nucleotide. Interestingly, amino acid substitution at this position is strongly dependent on the identity of the templating base as well as the specific side chain at this position. These observations indicate that the interactions provided by substrate binding residues are specific for each base pair [40]. Lys280 aligns with arginine or lysine in most pol β, pol λ, Tdt, and slime mold sequences. It is serine in Monodelphis domestica pol μ (Supplementary Table 1) and valine in all the trypanosome pol β species. It is not conserved in the bacterial pol X, but is generally isoleucine in the prokaryotic enzymes (Supplementary Table 1). DNA polymerase β Arg283 is highly conserved in bacterial pol X enzymes, but is not in S. spiritivorum, D. radiodurans V. anguillarum, or M. tuberculosis (93% overall conservation, Supplementary Table 1).
3.5.4. Lyase domain
The dRP lyase nucleophile Lys72 of pol β that forms a Schiff-base intermediate is highly conserved in all pol β and pol λ species, but is not conserved in Tdt and pol μ (Supplementary Table 1). This lysine appears to be conserved in some bacterial pol X sequences but is valine in several others. Glu26 and Ser30 are not conserved in the bacterial Staphylococcus and D. radiodurans X-family polymerases and Ser30 is not widely conserved even in some mammalian pol β sequences (e.g., Canis lupus familiaris, Bos taurus).
4. Conclusions
Phylogenetic analysis has shown that mammalian X-family DNA polymerases and yeast pol IV share a common origin with bacterial X-family polymerases. These polymerases share a common bacterial ancestor characterized in the Bacillus species designated the evolutionary grandfather of all family-X polymerases. The initial Bacillus X-family polymerases evolutionarily differentiated into branches; one of which became pol μ and Tdt, and a second that includes pol β and pol λ. A third branch includes a plant pol X and fungi pol IV sub-branch. The fungi enzymes are divided into two separate classes one which has a pol IV-like polymerase and another that has two X-family polymerases. Trypanosomatids are unusual and have more than one pol β and share fewer of the highly conserved residues of the X-family. DNA polymerase β is not nuclear and is mitochondrial in some of these trypanosomatid species. Over the millennium of evolution from Bacillus to the present, pol β has retained size, sequence, domain and residue conservation. Bacterial pol X species do have a unique PHP domain not present in other X-family species. The polymerase active site residues that bind the incoming nucleotide are highly conserved with the exception of D. radiodurans, M. tuberculosis and N. vectensis.
Supplementary Material
Acknowledgments
This research was supported by Research Project Numbers Z01-ES050158 and Z01-ES050159 in the Intramural Research Program of the National Institutes of Health, National Institute of Environmental Health Sciences and was in association with the National Institutes of Health Grant 1U19CA105010.
Abbreviations
- AP
apurinic/apyrimidinic
- ASFV
African swine fever virus
- BER
base excision repair
- dNTP
deoxynucleoside triphosphate
- dRP
deoxyribose phosphate
- kDNA
kinetoplast DNA
- NHEJ
non-homologous end-joining
- PHP
polymerase and histidinol phosphatase
- Tdt
terminal deoxynucleotidyl transferase
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.dnarep.2014.07.003.
Footnotes
Conflict of interest
The authors declare that there are no conflicts of interest.
References
- 1.Braithwaite DK, Ito J. Compilation, alignment, and phylogenetic relationships of DNA polymerases. Nucl Acid Res. 1993;21:787–802. doi: 10.1093/nar/21.4.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bebenek K, Kunkel TA. Functions of DNA polymerases. Adv Protein Chem. 2004;69:137–165. doi: 10.1016/S0065-3233(04)69005-X. [DOI] [PubMed] [Google Scholar]
- 3.Wu S, Beard WA, Pedersen LG, Wilson SH. Structural comparison of DNA polymerase architecture suggests a nucleotide gateway to the polymerase active site. Chem Rev. 2014;114:2759–2774. doi: 10.1021/cr3005179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sawaya MR, Pelletier H, Kumar A, Wilson SH, Kraut J. Crystal structure of rat DNA polymerase β: evidence for a common polymerase mechanism. Science. 1994;264:1930–1935. doi: 10.1126/science.7516581. [DOI] [PubMed] [Google Scholar]
- 5.Asagoshi K, Lehmann W, Braithwaite EK, Santana-Santos L, Prasad R, Freedman JH, Van Houten B, Wilson SH. Single-nucleotide base excision repair DNA polymerase activity in C. elegans in the absence of DNA polymerase β. Nucl Acids Res. 2012;40:670–681. doi: 10.1093/nar/gkr727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Uchiyama Y, Takeuchi R, Kodera H, Sakaguchi K. Distribution and roles of X-family DNA polymerases in eukaryotes. Biochimie. 2009;91:165–170. doi: 10.1016/j.biochi.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 7.Lecointe F, Shevelev IV, Bailone A, Sommer S, Hübscher U. Involvement of an X family DNA polymerase in double-stranded break repair in the radioresistant organism Deinococcus radiodurans. Mol Microbiol. 2004;53:1721–1730. doi: 10.1111/j.1365-2958.2004.04233.x. [DOI] [PubMed] [Google Scholar]
- 8.Ramadan K, Shevelev I, Hübscher U. The DNA-polymerase-X family: controllers of DNA quality? Nat Rev Mol Cell Biol. 2004;5:1038–10343. doi: 10.1038/nrm1530. [DOI] [PubMed] [Google Scholar]
- 9.Beard WA, Wilson SH. Structure and mechanism of DNA polymerase β. Chem Rev. 2006;106:361–382. doi: 10.1021/cr0404904. [DOI] [PubMed] [Google Scholar]
- 10.Aravind L, Koonin EV. DNA polymerase β-like nucleotidyltransferase superfamily: identification of three new families, classification and evolutionary history. Nucl Acids Res. 1999;27:1609–1618. doi: 10.1093/nar/27.7.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kodera H, Takeuchi R, Uchiyama Y, Takakusagi Y, Iwabata K, Miwa H, Hanzawa N, Sugawara F, Sakaguchi K. Characterization of marine X-family DNA polymerases and comparative analysis of base excision repair proteins. Biochem Biophys Res Comm. 2011;415:193–199. doi: 10.1016/j.bbrc.2011.10.058. [DOI] [PubMed] [Google Scholar]
- 12.Filée J, Forterre P, Sen-Lin T, Laurent J. Evolution of DNA polymerase families: evidences for multiple gene exchange between cellular and viral proteins. J Mol Evol. 2002;54:763–773. doi: 10.1007/s00239-001-0078-x. [DOI] [PubMed] [Google Scholar]
- 13.Leulliot N, Cladière L, Lecointe F, Durand D, Hübscher U, van Tilbeurgh H. The family X DNA polymerase from Deinococcus radiodurans adopts a non-standard extended conformation. J Biol Chem. 2009;284:11992–11999. doi: 10.1074/jbc.M809342200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin Y, Chen S, Si S, Xie Y. Expression, purification, crystallization and preliminary X-ray crystallographic analysis of the hyperthermophilic nucleotidyltransferase TTHA1015 from Thermus thermophilus HB8. Acta Crystallogr F. 2011;67:782–784. doi: 10.1107/S1744309111017490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Showalter AK, Byeon IJL, Su MI, Tsai MD. Solution structure of a viral DNA polymerase X and evidence for a mutagenic function. Nat Struct Biol. 2001;8:942–946. doi: 10.1038/nsb1101-942. [DOI] [PubMed] [Google Scholar]
- 16.Maciejewski MW, Shin R, Pan B, Marintchev A, Denninger A, Mullen MA, Chen K, Gryk MR, Mullen GP. Solution structure of a viral DNA repair polymerase. Nat Struct Biol. 2001;8:936–941. doi: 10.1038/nsb1101-936. [DOI] [PubMed] [Google Scholar]
- 17.Huerta-Cepas J, Capella-Gutierrez S, Pryszcz LP, Denisov I, Kormes D, Marcet-Houben M, Gabaldón T. PhylomeDB v3.0: an expanding repository of genome-wide collections of trees, alignments and phylogeny-based orthology and paralogy predictions. Nucl Acids Res. 2011;39:D556–D560. doi: 10.1093/nar/gkq1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guindon S, Dufayard J-Fo, Lefort V, Anisimova M, Hordijk W, Gascuel O. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol. 2010;59:307–321. doi: 10.1093/sysbio/syq010. [DOI] [PubMed] [Google Scholar]
- 19.Guindon S, Gascuel O. A Simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol. 2003;52:696–704. doi: 10.1080/10635150390235520. [DOI] [PubMed] [Google Scholar]
- 20.Huerta-Cepas J, Dopazo J, Gabaldon T. ETE: a python environment for tree exploration. BMC Bioinform. 2010;11:24. doi: 10.1186/1471-2105-11-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Letunic I, Bork P. Interactive Tree Of Life (iTOL): an online tool for phylogenetic tree display and annotation. Bioinformatics. 2007;23:127–128. doi: 10.1093/bioinformatics/btl529. [DOI] [PubMed] [Google Scholar]
- 22.Letunic I, Bork P. Interactive Tree Of Life v2: online annotation and display of phylogenetic trees made easy. Nucl Acids Res. 2011;39:W475–W478. doi: 10.1093/nar/gkr201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sánchez R, Serra F, Tárraga J, Medina I, Carbonell J, Pulido L, de María A, Capella-Gutíerrez S, Huerta-Cepas J, Gabaldón T, Dopazo J, Dopazo H. Phylemon 2.0: a suite of web-tools for molecular evolution, phylogenetics, phylogenomics and hypotheses testing. Nucl Acids Res. 2011;39:W470–W474. doi: 10.1093/nar/gkr408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Han M, Zmasek C. phyloXML: XML for evolutionary biology and comparative genomics. BMC Bioinform. 2009;10:356. doi: 10.1186/1471-2105-10-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stöver B, Müller K. TreeGraph 2: combining and visualizing evidence from different phylogenetic analyses. BMC Bioinform. 2010;11:7. doi: 10.1186/1471-2105-11-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucl Acids Res. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Waterhouse AM, Procter JB, Martin DMA, Clamp Ml, Barton GJ. Jalview version 2—a multiple sequence alignment editor and analysis workbench. Bioinformatics. 2009;25:1189–1191. doi: 10.1093/bioinformatics/btp033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baños B, Lázaro JM, Villar L, Salas M, de Vega M. Characterization of a Bacillus subtilis 64-kDa DNA polymerase X potentially Involved in DNA repair. J Mol Biol. 2008;384:1019–1028. doi: 10.1016/j.jmb.2008.09.081. [DOI] [PubMed] [Google Scholar]
- 29.Powell S, Szklarczyk D, Trachana K, Roth A, Kuhn M, Muller J, Arnold R, Rattei T, Letunic I, Doerks T, Jensen LJ, von Mering C, Bork P. eggNOG v3.0: orthologous groups covering 1133 organisms at 41 different taxonomic ranges. Nucl Acids Res. 2012;40:D284–D289. doi: 10.1093/nar/gkr1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bebenek K, Garcia-Diaz M, Patishall SR, Kunkel TA. Biochemical properties of Saccharomyces cerevisiae DNA polymerase IV. J Biol Chem. 2005;280:20051–20058. doi: 10.1074/jbc.M501981200. [DOI] [PubMed] [Google Scholar]
- 31.González-Barrera S, Sánchez A, Ruiz JF, Juáez R, Picher AJ, Terrados G, Andrade P, Blanco L. Characterization of SpPol4, a unique X-family DNA polymerase in Schizosaccharomyces pombe. Nucl Acids Res. 2005;33:4762–4774. doi: 10.1093/nar/gki780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Venegas JA, Åslund L, Solari A. Cloning and characterization of a DNA polymerase β gene from Trypanosoma cruzi. Parasitol Int. 2009;58:187–192. doi: 10.1016/j.parint.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 33.Bruhn DF, Sammartino MP, Klingbeil MM. Three mitochondrial DNA polymerases are essential for kinetoplast DNA replication and survival of bloodstream form Trypanosoma brucei. Eukaryot Cell. 2011;10:734–743. doi: 10.1128/EC.05008-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saxowsky TT, Matsumoto Y, Englund PT. The mitochondrial DNA polymerase β from Crithidia fasciculata has 5′-deoxyribose phosphate (dRP) lyase activity but is deficient in the release of dRP. J Biol Chem. 2002;277:37201–37206. doi: 10.1074/jbc.M206654200. [DOI] [PubMed] [Google Scholar]
- 35.Lopes DdO, Schamber-Reis BLF, Regisda-Silva CG, Rajão MA, DaRocha WD, Macedo AM, Franco GR, Nardelli SC, Schenkman S, Hoffmann J-Sb, Cazaux C, Pena SDJ, Teixeira SMR, Machado CR. Biochemical studies with DNA polymerase β and DNA polymerase β-PAK of Trypanosoma cruzi suggest the involvement of these proteins in mitochondrial DNA maintenance. DNA Repair (Amst) 2008;7:1882–1892. doi: 10.1016/j.dnarep.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 36.Alonso A, Terrados G, Picher AJ, Giraldo R, Blanco L, Larraga V. An intrinsic 5′-deoxyribose-5-phosphate lyase activity in DNA polymerase beta from Leishmania infantum supports a role in DNA repair. DNA Repair (Amst) 2006;5:89–101. doi: 10.1016/j.dnarep.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 37.Prasad R, Beard WA, Chyan JY, Maciejewski MW, Mullen GP, Wilson SH. Functional analysis of the amino-terminal 8-kDa domain of DNA polymerase β as revealed by site-directed mutagenesis: DNA binding and 5′-deoxyribose phosphate lyase activities. J Biol Chem. 1998;273:11121–11126. doi: 10.1074/jbc.273.18.11121. [DOI] [PubMed] [Google Scholar]
- 38.Prasad R, Batra VK, Yang XP, Krahn JM, Pedersen LC, Beard WA, Wilson SH. Structural insight into the DNA polymertase β deoxyribose phosphate lyase mechanism. DNA Repair (Amst) 2005;4:1347–1357. doi: 10.1016/j.dnarep.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 39.Garcia-Diaz M, Bebenek K, Gao G, Pedersen LC, London RE, Kunkel TA. Structure-function studies of DNA polymerase lambda. DNA Repair (Amst) 2005;4:1358–1367. doi: 10.1016/j.dnarep.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 40.Beard WA, Shock DD, Yang XP, DeLauder SF, Wilson SH. Loss of DNA polymerase β stacking interactions with templating purines, but not pyrimidines, alters catalytic efficiency and fidelity. J Biol Chem. 2002;277:8235–8242. doi: 10.1074/jbc.M107286200. [DOI] [PubMed] [Google Scholar]
- 41.Beard WA, Wilson SH. DNA polymerases lose their grip. Nat Struct Biol. 2001;8:915–917. doi: 10.1038/nsb1101-915. [DOI] [PubMed] [Google Scholar]
- 42.Moon AF, Garcia-Diaz M, Batra VK, Beard WA, Bebenek K, Kunkel TA, Wilson SH, Pedersen LC. The X family portrait: structural insights into biological functions of X family polymerases. DNA Repair (Amst) 2007;6:1709–1725. doi: 10.1016/j.dnarep.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baños B, Villar L, Salas M, de Vega M. Intrinsic apurinic/apyrimidinic (AP) endonuclease activity enables Bacillus subtilis DNA polymerase X to recognize, incise, and further repair abasic sites. Proc Natl Acad Sci U S A. 2010;107:19219–19224. doi: 10.1073/pnas.1013603107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nakane S, Nakagawa N, Kuramitsu S, Masui R. Characterization of DNA polymerase X from Thermus thermophilus HB8 reveals the POLXc and PHP domains are both required for 3′–5′ exonuclease activity. Nucl Acids Res. 2009;37:2037–2052. doi: 10.1093/nar/gkp064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nakane S, Ishikawa H, Nakagawa N, Kuramitsu S, Masui R. The structural basis of the kinetic mechanism of a gap-filling X-family DNA polymerase that binds Mg2+-dNTP before binding to DNA. J Mol Biol. 2012;417:179–196. doi: 10.1016/j.jmb.2012.01.025. [DOI] [PubMed] [Google Scholar]
- 46.Oliveros M, Yánez RJ, Salas ML, Salas J, Vinuela E, Blanco L. Characterization of an African swine fever virus 20-kda DNA polymerase involved in DNA repair. J Biol Chem. 1997;272:30899–30910. doi: 10.1074/jbc.272.49.30899. [DOI] [PubMed] [Google Scholar]
- 47.Baños B, Laro JM, Villar L, Salas M, de Vega M. Editing of misaligned 3′-termini by an intrinsic 3′–5′ exonuclease activity residing in the PHP domain of a family X DNA polymerase. Nucl Acids Res. 2008;36:5736–5749. doi: 10.1093/nar/gkn526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Batra VK, Perera L, Lin P, Shock DD, Beard WA, Pedersen LC, Pedersen LG, Wilson SH. Amino acid substitution in the active site of DNA polymerase β explains the energy barrier of the nucleotidyl transfer reaction. J Am Chem Soc. 2013;135:8078–8088. doi: 10.1021/ja403842j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Khairnar NP, Misra HS. DNA polymerase X from Deinococcus radiodurans implicated in bacterial tolerance to DNA damage is characterized as a short patch base excision repair polymerase. Microbiology. 2009;155:3005–3014. doi: 10.1099/mic.0.029223-0. [DOI] [PubMed] [Google Scholar]
- 50.Bailey S, Wing RA, Steitz TA. The structure of T. aquaticus DNA polymerase III is distinct from eukaryotic replicative DNA polymerases. Cell. 2006;126:893–904. doi: 10.1016/j.cell.2006.07.027. [DOI] [PubMed] [Google Scholar]
- 51.Kirby TW, DeRose EF, Beard WA, Wilson SH, London RE. A thymine isostere in the templating position disrupts assembly of the closed DNA polymerase β ternary complex. Biochemistry. 2005;44:15230–15237. doi: 10.1021/bi0511742. [DOI] [PubMed] [Google Scholar]
- 52.Beard WA, Osheroff WP, Prasad R, Sawaya MR, Jaju M, Wood TG, Kraut J, Kunkel TA, Wilson SH. Enzyme-DNA interactions required for efficient nucleotide incorporation and discrimination in human DNA polymerase β. J Biol Chem. 1996;271:12141–12144. doi: 10.1074/jbc.271.21.12141. [DOI] [PubMed] [Google Scholar]
- 53.Kraynov VS, Werneburg BG, Zhong XJ, Lee H, Ahn JW, Tsai MD. DNA polymerase β: analysis of the contributions of tyrosine-271 and asparagine-279 to substrate specificity and fidelity of DNA replication by pre-steady-state kinetics. Biochem J. 1997;323:103–111. doi: 10.1042/bj3230103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vande Berg BJ, Beard WA, Wilson SH. DNA structure and aspartate 276 influence nucleotide binding to human DNA polymerase β: implication for the identity of the rate-limiting conformational change. J Biol Chem. 2001;276:3408–3416. doi: 10.1074/jbc.M002884200. [DOI] [PubMed] [Google Scholar]
- 55.Cavanaugh NA, Beard WA, Batra VK, Perera L, Pedersen LG, Wilson SH. Molecular insights into DNA polymerase deterrents for ribonucleotide insertion. J Biol Chem. 2011;286:31650–31660. doi: 10.1074/jbc.M111.253401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Osheroff WP, Beard WA, Wilson SH, Kunkel TA. Base substitution specificity of DNA polymerase β depends on interactions in the DNA minor groove. J Biol Chem. 1999;274:20749–20752. doi: 10.1074/jbc.274.30.20749. [DOI] [PubMed] [Google Scholar]
- 57.Osheroff WP, Beard WA, Yin S, Wilson SH, Kunkel TA. Minor groove interactions at the DNA polymerase β active site modulate single-base deletion error rates. J Biol Chem. 2000;275:28033–28038. doi: 10.1074/jbc.M003462200. [DOI] [PubMed] [Google Scholar]
- 58.Freudenthal BD, Beard WA, Wilson SH. Structures of dNTP intermediate states during DNA polymerase active site sssembly. Structure. 2012;20:1829–1837. doi: 10.1016/j.str.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Beard WA, Shock DD, Vande Berg BJ, Wilson SH. Efficiency of correct nucleotide insertion governs DNA polymerase fidelity. J Biol Chem. 2002;277:47393–47398. doi: 10.1074/jbc.M210036200. [DOI] [PubMed] [Google Scholar]
- 60.Batra VK, Beard WA, Shock DD, Krahn JM, Pedersen LC, Wilson SH. Magnesium induced assembly of a complete DNA polymerase catalytic complex. Structure. 2006;14:757–766. doi: 10.1016/j.str.2006.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Delarue M, Boulé JB, Lescar J, Expert-Bezancon N, Jourdan N, Sukumar N, Rougeon F, Papanicolaou C. Crystal structures of a template-independent DNA polymerase: murine terminal deoxynucleotidyltransferase. EMBO J. 2002;21:427–439. doi: 10.1093/emboj/21.3.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Moon AF, Garcia-Diaz M, Bebenek K, Davis BJ, Zhong X, Ramsden DA, Kunkel TA, Pedersen LC. Structural insight into the substrate specificity of DNA polymerase μ. Nat Struct Mol Biol. 2007;14:45–53. doi: 10.1038/nsmb1180. [DOI] [PubMed] [Google Scholar]
- 63.Garcia-Diaz M, Bebenek K, Krahn JM, Pedersen LC, Kunkel TA. Structural analysis of strand misalignment during DNA synthesis by a human DNA polymerase. Cell. 2006;124:331–342. doi: 10.1016/j.cell.2005.10.039. [DOI] [PubMed] [Google Scholar]
- 64.Date T, Yamamoto S, Tanihara K, Nishimoto Y, Matsukage A. Aspartic acid residues at positions 190 and 192 of rat polymerase β are involved in primer binding. Biochemistry. 1991;30:5286–5292. doi: 10.1021/bi00235a023. [DOI] [PubMed] [Google Scholar]
- 65.Menge KL, Hostomsky Z, Nodes BR, Hudson GO, Rahmati S, Moomaw EW, Almassy RJ, Hostomska Z. Structure-function analysis of the mammalian DNA polymerase β active site: role of aspartic acid 256, arginine 254, and arginine 258 in nucleotidyl transfer. Biochemistry. 1995;34:15934–15942. doi: 10.1021/bi00049a008. [DOI] [PubMed] [Google Scholar]
- 66.Date T, Yamamoto S, Tanihara K, Nishimoto Y, Liu N, Matsukage A. Site-directed mutagenesis of recombinant rat DNA polymerase β: involvement of arginine-183 in primer recognition. Biochemistry. 1990;29:5027–5034. doi: 10.1021/bi00473a005. [DOI] [PubMed] [Google Scholar]
- 67.Li SX, Vaccaro JA, Sweasy JB. Involvement of phenylalanine 272 of DNA polymerase beta in discriminating between correct and incorrect deoxynucleoside triphosphates. Biochemistry. 1999;38:4800–4808. doi: 10.1021/bi9827058. [DOI] [PubMed] [Google Scholar]
- 68.Lavrik OI, Prasad R, Beard WA, Safronov IV, Dobrikov MI, Srivastava DK, Shishkin GV, Wood TG, Wilson SH. dNTP binding to HIV-1 reverse transcriptase and mammalian DNA polymerase β as revealed by affinity labeling with a photoreactive dNTP analog. J Biol Chem. 1996;271:21891–21897. doi: 10.1074/jbc.271.36.21891. [DOI] [PubMed] [Google Scholar]
- 69.Liu J, Tsai MD. DNA Polymerase β: pre-steady-state kinetic analyses of dATPαS stereoselectivity and alteration of the stereoselectivity by various metal ions and by site-directed mutagenesis. Biochemistry. 2001;40:9014–9022. doi: 10.1021/bi010646j. [DOI] [PubMed] [Google Scholar]
- 70.Miller H, Prasad R, Wilson SH, Johnson F, Grollman AP. 8-OxodGTP incorporation by DNA polymerase β is modified by active-site residue Asn279. Biochemistry. 2000;39:1029–1033. doi: 10.1021/bi991789x. [DOI] [PubMed] [Google Scholar]
- 71.Ahn J, Werneburg BG, Tsai MD. DNA polymerase β: structure-fidelity relationship from pre-steady-state kinetic analyses of all possible correct and incorrect base pairs for wild type and R283A mutant. Biochemistry. 1997;36:1100–1107. doi: 10.1021/bi961653o. [DOI] [PubMed] [Google Scholar]
- 72.Bakhtina M, Roettger MP, Tsai MD. Contribution of the reverse rate of the conformational step to polymerase β fidelity. Biochemistry. 2009;48:3197–3208. doi: 10.1021/bi802119f. [DOI] [PubMed] [Google Scholar]
- 73.Starcevic D, Dalal S, Sweasy J. Hinge residue Ile260 of DNA polymerase β is important for enzyme activity and fidelity. Biochemistry. 2005;44:3775–3784. doi: 10.1021/bi047956x. [DOI] [PubMed] [Google Scholar]
- 74.Roettger MP, Bakhtina M, Tsai MD. Mismatched and matched dNTP incorporation by DNA polymerase β proceed via analogous kinetic pathways. Biochemistry. 2008;47:9718–9727. doi: 10.1021/bi800689d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Opresko PL, Sweasy JB, Eckert KA. The mutator form of polymerase β with amino acid substitution at tyrosine 265 in the hinge region displays an increase in both base substitution and frame shift errors. Biochemistry. 1998;37:2111–2119. doi: 10.1021/bi9722711. [DOI] [PubMed] [Google Scholar]
- 76.Matsumoto Y, Kim K. Excision of deoxyribose phosphate residues by DNA polymerase β during DNA repair. Science. 1995;269:699–702. doi: 10.1126/science.7624801. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.