Abstract
Compensatory counter-rotations of the eyes provoked by head turns are commonly attributed to the vestibulo-ocular reflex (VOR). A recent study in guinea pigs demonstrates, however, that this assumption is not always valid. During voluntary head turns, guinea pigs make highly accurate compensatory eye movements that occur with zero or even negative latencies with respect to the onset of the provoking head movements. Furthermore, the anticipatory eye movements occur in animals with bilateral peripheral vestibular lesions, thus confirming that they have an extra vestibular origin. This discovery suggests the possibility that anticipatory responses might also occur in other species including humans and non-human primates, but have been overlooked and mistakenly identified as being produced by the VOR. This review will compare primate and guinea pig vestibular physiology in light of these new findings. A unified model of vestibular and cerebellar pathways will be presented that is consistent with current data in primates and guinea pigs. The model is capable of accurately simulating compensatory eye movements to active head turns (anticipatory responses) and to passive head perturbations (VOR induced eye movements) in guinea pigs and in human subjects who use coordinated eye and head movements to shift gaze direction in space.
Anticipatory responses provide new evidence and opportunities to study the role of extra vestibular signals in motor control and sensory-motor transformations. Exercises that employ voluntary head turns are frequently used to improve visual stability in patients with vestibular hypofunction. Thus, a deeper understanding of the origin and physiology of anticipatory responses could suggest new translational approaches to rehabilitative training of patients with bilateral vestibular loss.
Keywords: Vestibulo-ocular reflex, VOR, efference copy, motor control, sensory-motor transformations, cerebellum
Introduction
Walls (1962) wrote, “the ancient and original function of the eye muscles was not really to move the eye but rather to hold it still with respect to the environment”. He added “if the original control of the eye muscles was from the labyrinths, then one may say that the resulting movements of the globes must have been the very raison d’être of the muscles themselves … to impart appropriate movements of the eyes as dictated by head movements”. Ironically, according to his viewpoint, the goal of eye movements is to hold the globes, and hence the retinas, stationary in space during head movements.
The purpose of maintaining retinal stability in man, as achieved by the vestibulo-ocular reflex (VOR), is prevention of motion-provoked visual blur, which is commonly experienced by patients with vestibular hypofunction (Leigh and Zee, 2006). A physician, John Crawford, experienced an acute ototoxic lesion of his vestibular system and, with his head stabilized in bed, described how “… the pulse beat in my head became a perceptible motion, disturbing my equilibrium” (JC, 1952). This interpretation is clearly valid for primates that have high visual acuity created by a dense concentration of photoreceptors in the fovea (Mollon, 1982). However, many other species such as guinea pigs have fewer photoreceptors spread across their retinas and poorer visual acuity than primates (Buttery et al., 1991). For these species, Land (1999) argued that visual blur caused by motion would be less of a problem because the lower density of photoreceptors would restrict blur to higher speeds of head movement. Instead of preserving acuity, Land suggested that retinal stability is essential to an afoveate animal’s ability to detect motion. Indeed, if image stability were perfect, then the visual scene would dissolve and only that which moves would be seen: “It simplifies the world enormously if the ‘AC-coupled’ nature of the early visual process can be used to restrict what can be detected to just those things that are of vital importance: those that move” (Land, 1999). An important corollary to this idea is that an animal must stabilize its eyes in space during voluntary head movements in order to distinguish image motion that is not produced by self-motion (Walls, 1962).
This review compares the VOR in man and non-human primates to that of an afoveate mammal, the guinea pig. Although the functional aspects and physiology may appear to be quite different in these two species, this review will argue that the underlying anatomy and neural signal processing is fundamentally similar and that understanding how the guinea pig stabilizes its eyes in space has significant value for understanding human vestibular physiology and for treatment of patients with profound vestibular loss such as that experienced by John Crawford.
The vestibulo-ocular reflex
The vestibulo-ocular reflex has been studied intensively in multiple species. The basic pathway underlying the reflex is remarkably simple: a 3-neuron arc that links receptors and primary neurons located in the inner ear to the extraocular muscles of the eye (Lorente de No, 1933). Numerous studies over the past 50 years have characterized the connectivity and discharge properties of the peripheral neurons in the inner ear (Goldberg and Fernandez, 1971c; Goldberg and Fernandez, 1971b; Goldberg and Fernandez, 1971a); secondary vestibular neurons in the vestibular nuclei (Precht and Shimazu, 1965; Precht and Baker, 1972; Fuchs and Kimm, 1975a; Buttner and Waespe, 1981; Tomlinson and Robinson, 1984; McCrea et al., 1987; Scudder and Fuchs, 1992; Stahl and Simpson, 1995; Ris et al., 1995a; Phillips et al., 1996; Serafin et al., 1999; Gdowski and McCrea, 1999; Roy and Cullen, 2001; Cullen and Roy, 2004; Roy and Cullen, 2004; Beraneck and Cullen, 2007), and plasticity and motor learning within the VOR pathways (Gonshor and Jones, 1976; Lisberger et al., 1994; Lisberger, 1994; Miles and Lisberger, 1981a; Sadeghi et al., 2010; Sadeghi et al., 2011). Measurement of eye movements produced by the VOR is an essential component of contemporary clinical tests of vestibular function, largely based on methods established by Barany for which he received the Nobel Prize in 1914 (for an example of Barany’s teachings translated into English, see Ibershoff and Copeland, 1910; for a review of clinical applications, see Leigh and Zee, 2006; Baloh and Halmagyi, 1996). Eye and head movement exercises that provoke the VOR are basic techniques that can improve retinal stability in patients with vestibular hypofunction (Herdman et al., 2007; Schubert et al., 2008; Scherer et al., 2008; Boyer et al., 2008).
More recently, neurophysiological studies of the VOR have focused on the possible roles played by efference copy or proprioceptive signals in producing or modulating ocular responses to head movements. Efference copy, as originally described by von Holst and Mittelstaedt (1950) and the concept that the brain uses internal models of sensory organs are essential features of many motor control hypotheses (Merfeld et al., 1999; Angelaki et al., 2004; Shadmehr et al., 2010; Laurens and Angelaki, 2011). Sadeghi et al. (2011) have described the influence of proprioceptive inputs from neck receptors onto secondary vestibular neurons in the VOR pathway in animals recovering from acute unilateral vestibular loss. Cullen and her colleagues have also described vestibular neurons whose discharge patterns may reflect cancellation of reafferent signals resulting from active head movements by internal signals based on efference copy or proprioception (Cullen et al., 1991; Roy and Cullen, 2001; Roy and Cullen, 2002; Cullen and Roy, 2004; Roy and Cullen, 2004; Cullen et al., 2011). Roy and Cullen described modulation of secondary vestibular neuron activity that apparently was related to efference copy in a task that excluded neck proprioception (Roy and Cullen, 2001; Roy and Cullen, 2002). In guinea pigs, compensatory eye movements have been reported, which anticipate voluntary head movements and which are likely to be produced either by proprioception originating in the neck or an efference copy of voluntary head movement (Shanidze et al., 2010a; King and Shanidze, 2011).
Eye/head coordination in guinea pigs
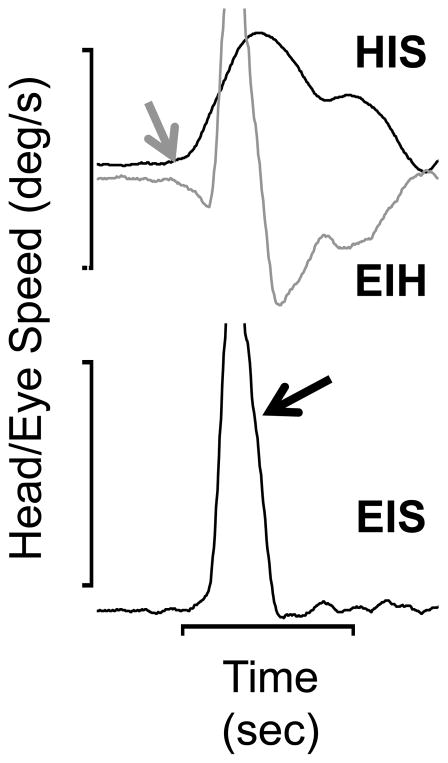
Guinea pigs do not have a fovea and experience relatively poor visual acuity (at a maximum, 2.7 cycles/deg along the visual streak, Buttery et al., 1991). They make very few, if any, voluntary saccades (Escudero et al., 1993; Shanidze et al., 2010b). They do, however, exhibit a typical pattern of voluntary gaze shifts that closely resemble the predictive gaze shifts described by Bizzi et al. in non-human primates (1972). Figure 1 illustrates a typical voluntary gaze shift made by a guinea pig (modified from Figure 2 in King and Shanidze, 2011). Typically, the head (gray arrow, trace labeled HIS) initiates the gaze shift. Eye position in space (EIS) during the head movement is stabilized by compensatory counter-rotations of the eyes within the orbits (EIH). About 75 msec after the onset of the head movement, the animal makes a rapid eye movement in the same direction as the head movement (black arrow, anti-compensatory), which is followed by further ocular counter-rotation. It was initially assumed the compensatory eye movements were caused by the VOR, but in 3 animals careful measurements of latencies with respect to the head movements using cross-correlation showed that the compensatory eye movements occurred with a mean latency of −0.2 ± 0.27 msec (in the example of Figure 1, the latency was −1.0 msec, King and Shanidze, 2011). For comparison, the mean latency of the passively evoked VOR in these animals was 6.9 msec (Shanidze et al., 2010b). The negative or zero latency of the compensatory eye movements strongly suggests that they have an extra vestibular origin. Further evidence for this hypothesis was obtained from guinea pigs with chemically induced bilateral lesions of the labyrinth that eliminated most, or all of their vestibular hair cells (Shanidze, 2010b). The lesioned animals had no responses to passive rotations at accelerations comparable to those produced by voluntary head movements. However, within a week of the ototoxic insult, lesioned animals recovered the ability to make zero latency compensatory eye movements in association with voluntary head movements (mean latency −0.001 ± 0.001 msec). Another distinguishing characteristic of the anticipatory eye movements was their accuracy. In intact animals, the ratio of anticipatory eye speed to head speed was 0.81 ± 0.23, significantly larger than the gain of the VOR in response to passive head movements in the same animals (0.46 ± 0.001).
Figure 1.
An example of a guinea pig’s voluntary gaze shift (modified from King & Shanidze, 2011). The head movement (HIS, gray arrow) initiates the gaze shift. Note the oppositely directed compensatory eye movement (EIH, gray trace) that anticipated the head movement one millisecond. About 75 msec after the onset of the head movement, a rapid eye movement (quick phase, black arrow), occurs that re-centers the eye in the orbit. Except during the occurrence of the rapid eye movement, eye speed in space or gaze (EIS, lowermost trace) is near zero throughout the head movement. Calibrations are 100 deg/s (speed) and 0.2 sec.
Since the latency of the eye movements with respect to head movement is essentially zero msec, we called them anticipatory (Klam and Graf (2006) used similar terminology to describe neuronal activity in macaque intraparietal cortex that anticipated active head movements). Since anticipatory responses are present in animals with total vestibular loss, they must have an extra vestibular origin, either proprioceptive feedback from cervical receptors or efference copy of head movement. We believe efference copy is the more likely explanation for three reasons. First, anticipatory responses occur only in association with active head movements; proprioceptive feedback would be present with either passive or active head movements. Second, Sadeghi et al. (2011) reported that neck proprioception could modulate the discharge of secondary neurons in unilaterally labyrinthectomized, but not intact monkeys. Third, our data show that proprioceptive feedback must be gated during passive perturbations of the head in bilaterally lesioned animals since there was no compensatory response. These assertions assume that proprioceptive feedback produced by passive rotation of the body under the head is similar to feedback produced during active head movement. This assumption, however, may not be valid since fusimotor activity during voluntary movements may bias proprioceptive feedback (Dmitriou and Edin 2010). Although anticipatory responses are a novel finding, it is reasonable to hypothesize that humans and non-human primates must also be capable of producing them during active head movements.
Eye/head coordination in humans and non-human primates
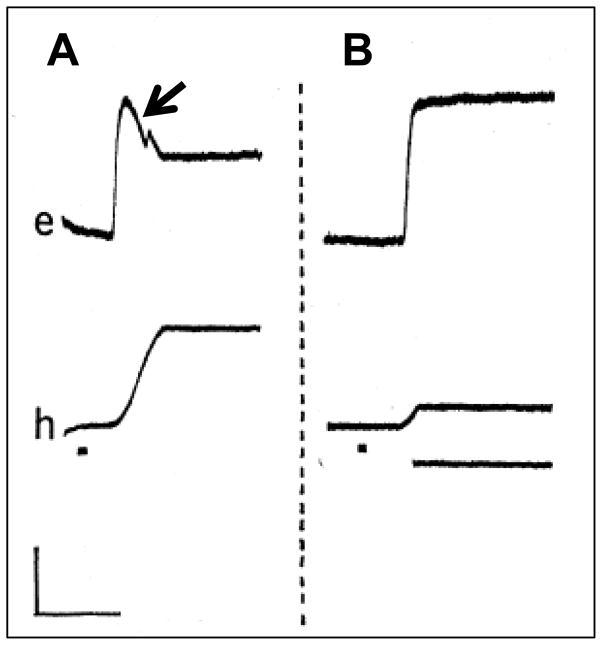
In order to see with maximum acuity, primates must aim their eyes so as to place the object of regard onto the fovea. If the object (e.g., another animal or food) is in their peripheral vision, then a saccadic eye movement followed by a head movement is the typical pattern (Figure 2A, from Bizzi et al., 1971a). Although the eye movement typically preceded the head movement by 20–40 msec, Bizzi et al. recorded activity with extraocular and neck EMG electrodes in agonist neck muscles 20 msec prior to the onset of activity in agonist extraocular muscles. Thus the sequence of neural innervation did not match the sequence of behavior. The saccadic eye movement acquired the target shortly after the onset of the head movement and evoked compensatory counter-rotations of the eyes as the head rotated toward the target (black arrow, Figure 2A). By using a brake that could unexpectedly stop the head movement (Figure 2B), Bizzi et al. (1971) showed that the compensatory counter-rotation was dependent on movement of the head and must result either from the VOR or a combination of vestibular and proprioceptive inputs. Bizzi called this pattern of eye-head coordination “triggered” gaze shifts. For triggered gaze shifts, the temporal sequence of eye and head movements is opposite that which we observed in guinea pigs (Figure 1).
Figure 2.
Examples of non-human primate gaze shifts “triggered” by the appearance of a visual target (from Bizzi, 1971). A. For triggered gaze shifts, a saccadic eye movement (upper trace) precedes the head movement (lower trace). The arrow points to a compensatory eye movement that follows the saccade. B. A brake (application indicated by lowermost solid line) unexpectedly interrupts the head movement component of a triggered gaze shift so there is no compensatory eye movement. In both panels the trace labeled e is eye position and the trace labeled h is head position. The dot beneath the head position trace indicates the appearance of the visible target. Calibration is 500 msec and 15 deg.
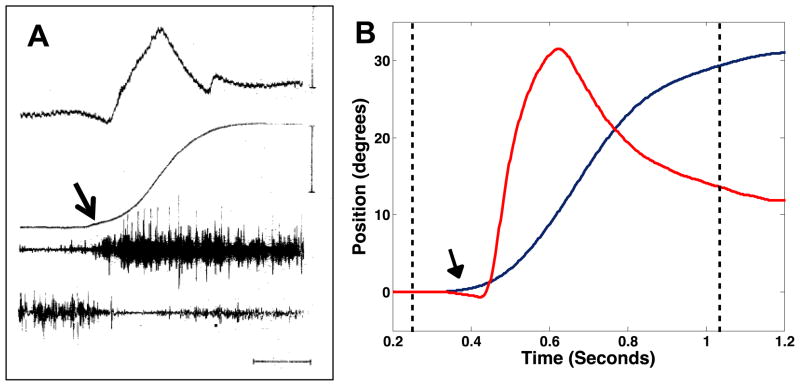
Triggered gaze shifts are typical of primates trained to respond to visual targets at randomized locations and presented at random times. Bizzi and colleagues described a different sequence of coordination when the targets were presented at predictable times and locations. For predictive targets, the gaze shift preceded the appearance of the target and the head movement preceded the eye movement Figure 3A is an example of a predictive gaze shift (modified from Figure 1, Bizzi et al. 1972). Eye position is effectively stabilized in space during most of the gaze shift because of compensatory eye movements which are interrupted by rapid eye movements that re-center the eye in the orbit (similar to the quick phase eye movements of guinea pigs: compare Figure 3A to Escudero et al., 1993, Figure 1 and Shanidze et al., 2010b, Figure 1. Bizzi et al. also distinguished predictive from triggered gaze shifts by differences in the patterns of neck EMG activation, which implied a difference in the neural substrates that produce the movements. The triggered pattern of coordination (Figure 2) is reflexively evoked by visual targets presented to an animal trained to make gaze shifts in exchange for juice rewards. The predictive pattern (Figure 3) is initiated voluntarily when an animal anticipates a target will appear. Humans also produce predictive gaze shifts. Figure 3B shows an example obtained when a human subject made gaze shifts to a target predictable in time and in location (Taylor and King, unpublished observations). Note that the gaze shift occurred after the fixation light was extinguished (first dashed line) and before the target appeared (second dashed line). The apparent similarity of non-human primate and human predictive gaze shifts (Figure 3) with guinea pigs’ gaze shifts (Figure 1) suggests that this pattern of eye and head coordination might reflect a common neurophysiological substrate. The similarity also suggests that compensatory eye movements during predictive gaze shifts in primates might also be anticipatory. Measuring the latency of compensatory eye movements during “predictive” gaze shifts could test this hypothesis.
Figure 3.
Examples of non-human primate (panel A from Bizzi, et al. 1972) and human subject (panel B, Taylor and King, unpublished results) “predictive” gaze shifts. In A, The traces from top to bottom are eye position, head position, and right and left splenii capitus EMG. In B, the red trace is eye position in the orbit and the blue trace is head position in space. In both panels, the arrows point to the onset of the head movement, which precedes the rapid eye movement. In A, the calibrations are 200 msec, 30 deg eye position and 40 deg, head position.
Another study from Bizzi’s laboratory in 1973 supports the notion that primates can produce efference copy driven anticipatory responses (Dichgans et al., 1973). They showed that a bilaterally labyrinthectomized monkey whose C1–C6 dorsal roots were sectioned (to eliminate cervical proprioception) was able make accurate compensatory eye movements during triggered gaze shifts. These responses appeared spontaneously within 10 days of the lesion and were 90% accurate within one month (Dichgans et al., 1973). Significantly, if the head were unexpectedly prevented from moving immediately after the appearance of the visual target, the animal executed compensatory eye movements in the absence of a head movement (Figure 7C, Dichgans et al., 1973). The compensatory responses were interpreted as learned behaviors produced by central commands linked to the planned (but blocked) head movements. Kasai and Zee (1978) reported similar phenomena in 3 human subjects that had lost vestibular function as a result of childhood meningitis. They noted that gaze stability was markedly improved during gaze shifts to a predictable target location and they observed “preprogrammed” compensatory responses during trials in which the subjects’ head movements were partially blocked (Kasai and Zee, 1978). In agreement with the data from Bizzi’s laboratory, they regarded the compensatory responses as an adaptive or learned behavior. Alternatively, in light of the guinea pigs’ data, they might have been anticipatory responses that replaced the VOR.
Guinea pig and primate vestibular neurons
Although the data are limited, there are a few electrophysiological studies of vestibular neuron activity in alert and intact guinea pigs that may be compared to studies in non-human primates. Ris et al. (Ris et al., 1995b) recorded 159 neurons in the vestibular nuclei of unanaesthetized guinea pigs. Of this group, 103 neurons were secondary vestibular neurons based on electrical stimulation of the VIIIth nerve. Seventy-nine percent (81 cells) were classified as type I neurons because they exhibited increased activity with ipsilateral passive head rotations. Seventeen percent of the secondary neurons exhibited eye position related activity and 15% exhibited either pauses of activity or bursts of activity with rapid eye movements in one or more directions. Unfortunately, the eye movement responses of these cells were not described in sufficient detail to compare them directly to descriptions of vestibular neurons recorded in non-human primates. However, in a recent study of the afoveate mouse VOR, Beraneck and Cullen (2007) identified 2 populations of secondary vestibular neurons. The first group of cells did not have eye movement sensitivity and were similar to primate vestibular only (VO) cells (Scudder and Fuchs, 1992; Cullen and McCrea, 1993; Fuchs and Kimm, 1975b). It is noteworthy that none of the “vestibular” (VO) units encountered by Scudder and Fuchs (1992) were shown to project to extraocular muscles based on spike-triggered averaging of extraocular EMG activity.
In primates VO neurons are believed to project into descending spinal, ascending vestibulo-thalamic, and vestibulo-cerebellar pathways (McCrea et al., 1999). In squirrel monkeys and macaques, VO neurons exhibited weaker responses to head rotation during active movements than during passive whole body rotations that did not produce head on body motion (Boyle et al., 1996; McCrea et al., 1999; Gdowski et al., 2000; Phillips et al., 1996; Cullen et al., 2001; Roy and Cullen, 2001). The attenuation of vestibular sensitivity could have been caused either by neck proprioception or an efference copy of the active head movement. In squirrel monkeys, passive rotation of the body under a stationary head (a stimulus that excites proprioceptive afferents but not vestibular afferents) does attenuate VO neuronal activity (Gdowski et al., 2001). The same stimulation is, however, ineffective in the rhesus monkey (Roy and Cullen, 2001) suggesting a species difference. Roy and Cullen (2001) also employed a unique paradigm whereby the monkey could “steer” his motion in space (without turning his head relative to his body) and were able to show that an efference copy related to the steered turn could attenuate VO neuron activity. Regardless of the mechanism, the evident conclusion is that VO neurons encode passive perturbations of the head (“exafference”) rather than the motion produced by voluntary movement (“reafference”). Preliminary data show that guinea pigs’ VO neurons may be similar to primates’ VO neurons; they encode passive but not active head rotation (Haggerty and King, unpublished observations).
In primates, position-vestibular-pause (PVP) neurons are secondary vestibular neurons in the VOR pathway (Scudder and Fuchs, 1992). These cells encode signals related to head velocity, eye velocity, eye position and rapid eye movements. Guinea pigs do not make voluntary eye movements. Thus, eye velocity is either produced by a vestibular quick phase, an optokinetic stimulus (Marlinsky and Kroller, 2000) or a response to head movement. In this animal, the most likely candidates for VOR interneurons are the secondary vestibular neurons described by Ris et al. (1995) that respond to head rotation, changes in eye position and quick phase eye movements. In agreement with Beraneck and Cullen’s terminology for secondary VOR neurons in the mouse, we call these “eye sensitivity” (ES) neurons. This population may include secondary vestibular neurons that also receive inhibitory inputs from the cerebellar flocculus (Babalian and Vidal, 2000). In monkeys, neurons that receive flocculus inhibition are called eye-head (EH), flocculus target neurons (FTNs), or gaze neurons because they encode head and eye velocity in the same direction. The discharge pattern of FTNs in guinea pigs is not known but may be different from primate FTNs since guinea pigs do not produce eye velocity independently of head velocity or optokinetic stimulation.
A unified model for anticipatory eye movements
It is likely that the physiology and neural circuitry underlying the VOR and anticipatory eye movements in guinea pigs is similar to that of primates. The 3-neuron arc that is the basis of the VOR is identical in guinea pigs and primates as is the functional goal of stabilizing eye position in space. It is also likely that VO neurons perform similar functions in both species because of the similarity of their discharge patterns and the apparent encoding of signals produced by passive, but not active, head movements. Although anticipatory eye movements have not yet been identified in primates, there is evidence that humans with vestibular hypofunction have better visual acuity when they actively move their heads (Herdman et al., 2001; Schubert et al., 2008) and produce shorter latency compensatory responses during active head movements (Della Santina et al., 2002). An ability to preprogram or use efference copy of planned head movements was directly demonstrated in monkeys with bilateral vestibular lesions (Dichgans et al., 1973) and in labyrinthine deficient humans (Kasai and Zee, 1978). Furthermore, when monkeys or humans make predictive gaze shifts, they employ a strategy of eye head coordination that is identical to that of guinea pigs (Bizzi et al., 1972 ,Taylor and King, unpublished observations).
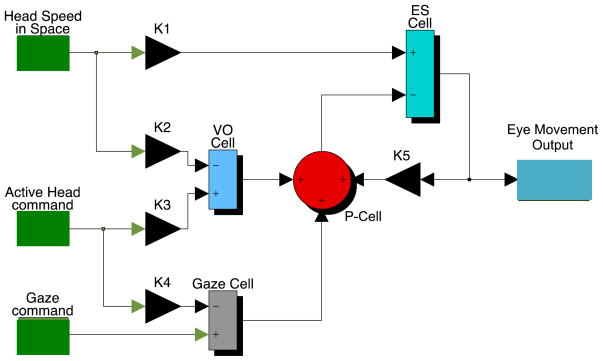
Figure 4 is a model showing how vestibular circuitry common to both guinea pigs and primates can produce a VOR to compensate for passive perturbation of the head in space and an anticipatory response to compensate for voluntary head movement. The basic structure of this model is similar to models proposed by Cullen and her colleagues (see Cullen, 2012 for a recent review) and incorporates key concepts expressed earlier by von Holst and Mittlestaedt (1950). In the model, the blue summing junction corresponding to a secondary neuron in the direct VOR pathway (ES cell) is likely to represent a heterogeneous group of cell types that convey head velocity and eye position signals to oculomotoneurons. Purkinje cells in the flocculus (P-cells) are hypothesized to inhibit at least some secondary vestibular neurons (FTNs). Cells not inhibited by the flocculus would be the guinea pig’s equivalent to the primate’s PVP cell. Consistent with known physiology, VO neurons are assumed in the model to encode a signal related to exafferent (passive) sensory inflow; their output is the difference between an expected reafferent signal produced by an active head movement and the sensory consequence of that movement (in the complete model used for simulation, the active head command is filtered by an internal model of the semicircular canals). We assume that a subset of VO neurons project to the cerebellar flocculus. During passive head movements, the head velocity signal in the direct VOR pathway is combined with a head velocity signal in the VO/P-cell pathway. This cerebellar side loop could act to calibrate the VOR (Miles and Lisberger, 1981a). The notion that VO neurons attenuate reafferent signals during voluntary head movements is consistent with previously reported data from Cullen’s laboratory and our preliminary findings in guinea pigs; what is novel is the proposed role of these neurons in attenuating reafference during anticipatory eye movements by way of their hypothesized projection to the flocculus. In primates, gerbils and rats, there are substantial projections to the flocculus from regions of the vestibular nuclei that contain VO neurons (Langer 1985; Kaufman 1996).
Figure 4.
Simulink model of the VOR and anticipatory eye movement circuit. The black triangles represent synaptic weights used to optimize the simulated response. In the guinea pig simulations, K1=0.73, K2=K3=0.25; K5=0.5 and K4=0.75. The green boxes to the left represent inputs to the model. Head speed in space is an actual data segment of recorded head velocity. In the model, this input is low pass filtered to simulate a single pole semicircular canal transfer function (time constant = 4 seconds). The active head command is assumed to be a data segment of active head velocity. This signal is also low pass filtered to simulate an internal model of the semicircular canal (4 second time constant). The active head command is also used as an efference copy of the gaze command when the model simulates an anticipatory response. The blue summing junctions represent VO or ES vestibular neurons; the red summing junction represents a flocculus Purkinje cell and the gray box represents a “gaze” cell that could be located in cerebral cortex or in the cerebellum. The simulated output of the model is eye velocity. Note that eye velocity is also fed back via K5 to the P-cell.
An important aspect of the model is its direct linkage to primate models of how Purkinje cells in the flocculus control gaze (reviewed in Miles and Lisberger, 1981b). As emphasized by Cullen (Cullen and Roy, 2004; Cullen et al., 2011; Cullen, 2012) the key to understanding how the brain encodes gaze is to distinguish gaze shifts from gaze stabilizing behaviors. In guinea pigs, it is assumed that there are no voluntary eye movements (Shanidze et al., 2010b); all gaze shifts must be accomplished with the head alone (whole body turns are excluded for simplicity, but they could readily be incorporated into the model). Thus, the gaze command in the model (lowermost green box) is either a signal to stabilize gaze with an anticipatory eye movement or a signal to facilitate a change in gaze direction by prevention of a compensatory eye movement. In order to stabilize gaze with an anticipatory eye movement, the gaze command is assumed in the model to be zero (i.e., zero gaze shift). Thus the active head command (assumed to be identical to an actual voluntary head movement) is relayed through K4, the gaze cell (gray summing junction) and the P-cell (red circular summing junction) to a subpopulation of ES neurons in the direct VOR pathway. The same active head command is also used to cancel the reafference at the level of the VO-neuron. Alternatively, if the desire is to change gaze direction by moving head and eyes together in space, the active head command is cancelled at the level of the gaze cell by a gaze command assumed to be identical to the active head command. As a result, the gaze cell output is zero. Thus, no signal is relayed to the P-cell and reafference is cancelled at the level of the VO cell.
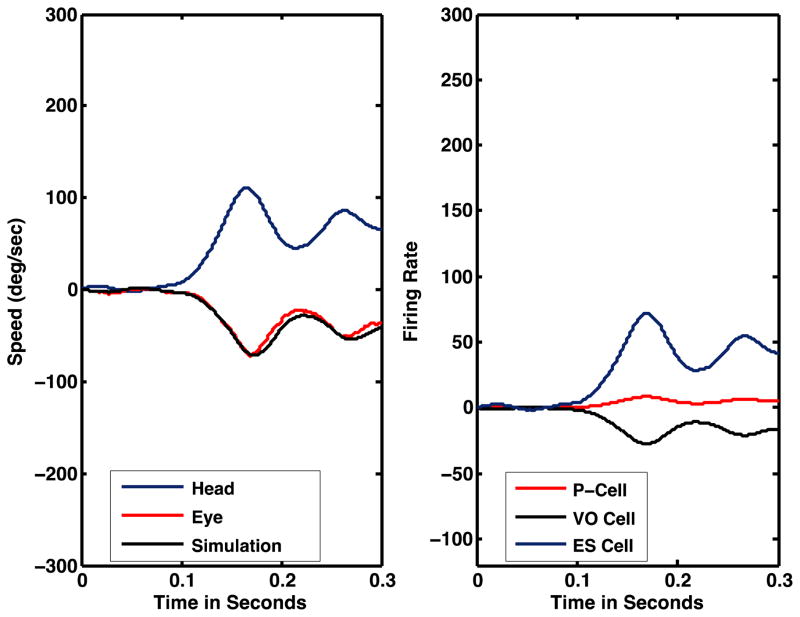
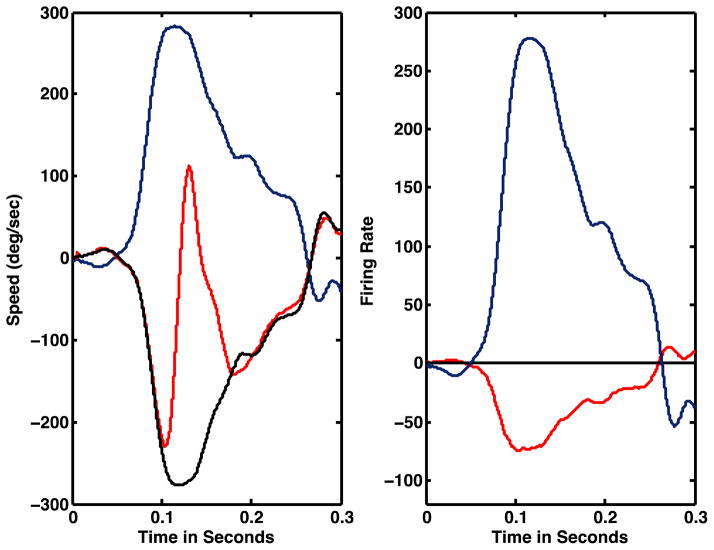
Figure 5 shows the model’s simulated eye movement response to a passive head rotation (black trace, 5A) and the simulated neural discharges (5B) of VO cells (dashed blue trace), ES cells (black trace) and P-cells (red trace). The model accurately simulates actual behavior (compare the black trace of simulated eye speed to the red trace of actual eye speed) during the initial portion of the VOR. The simulated neuronal activity suggests that (1) VO neuronal activity should encode the speed of passive head rotation; (2) P-cell modulation will be weak (passive VOR); and (3) the activity of ES neurons will encode eye speed.
Figure 5.
A. Comparison of the model’s simulated eye speed (black trace) and actual eye speed (red trace) produced by the passive VOR. The blue trace is actual head in space speed. The eye and head movement data are from a guinea pig subjected to whole body angular rotation (body speed not shown). B. Simulated neural activity. During the passive VOR, eye speed is a scaled and inverted replica of head speed. The firing rates of ES (blue trace) and VO (black trace) cells are proportional to head/eye velocity. The P-cell’s firing rate is only slightly modulated.
Without changing any of the parameters used to simulate passive head rotations, the model accurately simulates a guinea pig’s anticipatory response to voluntary head movements (Figure 6, note the quick phase is not simulated by the model). During anticipatory responses, VO modulation is attenuated to zero (dashed blue line) but the ES cell still encodes head velocity. P-cell activity is greater than during the passive VOR and results from a summation of efference copy of the head command and eye velocity feedback that calibrates the cell’s discharge so as to produce an accurate compensatory response in the target ES/FTN neuron. For convenience, the model does not distinguish secondary vestibular neurons that receive flocculus inhibition ( FTNs, Babalian and Vidal, 2000) and those (ES) that are not inhibited by the cerebellum. We would expect the latter cells to exhibit similar head velocity sensitivity during active and passive movements. The model, however, does predict that FTNs will have greater sensitivity to active than to passive head movements.
Figure 6.
A. Comparison of the model simulated eye speed (black trace) and actual eye speed (red trace) produced by an anticipatory response during a voluntary head movement (blue trace). The eye and head movement data are from a voluntary head movement made by a guinea pig. B. Simulated neural activity. Similar to the passive VOR (Figure 5), eye speed is a scaled and inverted replica of head speed and the simulated ES cell’s firing rate is proportional to head/eye velocity. Unlike the passive VOR, the VO cell’s firing rate (black trace) is not modulated, but the P-cell’s firing rate (red trace) is significantly modulated, during an active head movement.
The signal flow in the model (Figure 4) is consistent with non-human primate data. With minor changes in a subset of parameters, it can effectively simulate primate as well as guinea pig behavior (King, unpublished observations). In primates, the key distinction is that a gaze shift command is the sum of a head movement and an eye movement command. During simulation of a primate gaze shift, the active head movement command is still assumed to cancel the head movement component of the gaze command. However, there is either a saccadic eye movement or a smooth pursuit command, which may drive the eyes via brainstem pathways for saccades or the flocculus P-cell and FTN pathway in the case of smooth pursuit.
Conclusions
The primate’s eye evolved a fovea and with it the necessity of developing novel eye movement behaviors to voluntarily aim the fovea at objects of interest in space and to stabilize images of those objects on the fovea. Despite the obvious differences in neuronal organization related to the evolution of voluntary eye movements, the underlying vestibular and cerebellar circuits that control vestibulo-oculomotor behavior are essentially identical in foveate and afoveate mammals (specifically the 3-neuron VOR pathway and the cerebellar circuit linking flocculus Purkinje cells and secondary vestibular neurons, Figure 4). It is not surprising, therefore, that the circuit model depicted in Figure 4 can accurately simulate either primate or guinea pig gaze shift behavior. What is surprising is the apparent lack of a robust anticipatory response in primates similar to that reported in guinea pigs (Shanidze et al., 2010a; King and Shanidze, 2011). Clearly the capability to produce these responses is inherent in the circuitry as they occur in lesioned animals (Dichgans et al., 1973), in humans with vestibular hypofunction (Kasai and Zee, 1978) and by the findings of Della Santina (2002) and Herdman et al. (2001). These data and the discovery of anticipatory responses in intact guinea pigs suggests that primates might also make anticipatory responses (e.g., to predictable targets) but that they have been misidentified as having been produced by the VOR. Alternatively, the development of smooth pursuit eye movements and the resultant voluntary behaviors that coevolved may have supplanted the anticipatory responses in primates. For example, the need to minimize retinal blur by stabilizing foveal images could have placed a premium on using voluntary saccades, smooth pursuit and fusional vergence to maximize visual acuity. Primates also evolved translational vestibulo-ocular reflexes (TVOR) that are dependent on the distance of the eyes from the object whose image is to be stabilized. If anticipatory responses stabilized images solely against angular head movement (and regardless of the target’s distance from the eye), then head translation could produce image slip of near objects. This outcome would be undesirable for a guinea pig since any image slip would degrade the animal’s ability to detect motion in the environment. Interestingly, there is no evidence that the guinea pig has the ability to minimize translational motion except by maintaining a stationary head posture in space (“freezing”). This reasoning suggests that anticipatory responses might specifically stabilize the eyes during rotational head movement. If so, then they should not conflict with the TVOR in primates, which would stabilize the point of fixation against translation of the head. If this argument is correct, then there seems to be no inherent reason why anticipatory responses should not occur in humans and specifically in subjects with vestibular hypofunction that produces visual blur and oscillopsia. Further studies in guinea pigs and primates that address these questions are necessary to provide insights for how therapists might train and encourage development of anticipatory responses in patients with vestibular hypofunction.
Acknowledgments
Natela Shanidze, Ph.D. performed the research related to guinea pig behavior, summarized by this review, as part of her Doctoral Dissertation (2011) and provided editorial comments on this manuscript. Alyssa Taylor performed the studies of human gaze shifts in the author’s laboratory. I also want to recognize the many valuable contributions of Jonie Dye and the technical contributions of Chris Ellinger and Dwayne Vaillencourt. The research was supported by National Institutes of Health grants P30 NDC005188, R21 DC008607, and T32 DC000011.
References
- Angelaki DE, Shaikh AG, Green AM, Dickman JD. Neurons compute internal models of the physical laws of motion. Nature. 2004;430:560–564. doi: 10.1038/nature02754. [DOI] [PubMed] [Google Scholar]
- Babalian AL, Vidal PP. Floccular modulation of vestibuloocular pathways and cerebellum-related plasticity: An in vitro whole brain study. J Neurophysiol. 2000;84:2514–2528. doi: 10.1152/jn.2000.84.5.2514. [DOI] [PubMed] [Google Scholar]
- Baloh RW, Halmagyi GM. Disorders of the vestibular system. New York: Oxford University Press; 1996. [Google Scholar]
- Beraneck M, Cullen KE. Activity of vestibular nuclei neurons during vestibular and optokinetic stimulation in the alert mouse. J Neurophysiol. 2007;98:1549–1565. doi: 10.1152/jn.00590.2007. [DOI] [PubMed] [Google Scholar]
- Bizzi E, Kalil RE, Morasso P. Two modes of active eye-head coordination in monkeys. Brain Res. 1972;40:45–48. doi: 10.1016/0006-8993(72)90104-7. [DOI] [PubMed] [Google Scholar]
- Bizzi E, Kalil RE, Tagliasco V. Eye-Head Coordination in Monkeys: Evidence for Centrally Patterned Organization. Science. 1971a;173:452–454. doi: 10.1126/science.173.3995.452. [DOI] [PubMed] [Google Scholar]
- Boyer FC, Percebois-Macadré L, Regrain E, Lévêque M, Taïar R, Seidermann L, Belassian G, Chays A. Vestibular rehabilitation therapy. Neurophysiologie Clinique/Clinical Neurophysiology. 2008;38:479–487. doi: 10.1016/j.neucli.2008.09.011. [DOI] [PubMed] [Google Scholar]
- Boyle R, Belton T, McCrea RA. Responses of identified vestibulospinal neurons to voluntary eye and head movements in the squirrel monkey. Ann N Y Acad Sci. 1996;781:244–263. doi: 10.1111/j.1749-6632.1996.tb15704.x. [DOI] [PubMed] [Google Scholar]
- Buttery RG, Hinrichsen CF, Weller WL, Haight JR. How thick should a retina be? A comparative study of mammalian species with and without intraretinal vasculature. Vision Res. 1991;31:169–187. doi: 10.1016/0042-6989(91)90110-q. [DOI] [PubMed] [Google Scholar]
- Buttner U, Waespe W. Vestibular nerve activity in the alert monkey during vestibular and optokinetic nystagmus. Experimental Brain Research. 1981;41:310–315. doi: 10.1007/BF00238888. [DOI] [PubMed] [Google Scholar]
- Cullen KE. The vestibular system: multimodal integration and encoding of self- motion for motor control. Trends Neurosci. 2012;35:185–196. doi: 10.1016/j.tins.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen KE, Belton T, McCrea RA. A non-visual mechanism for voluntary cancellation of the vestibulo- ocular reflex. Experimental Brain Research. 1991;83:237–252. doi: 10.1007/BF00231150. [DOI] [PubMed] [Google Scholar]
- Cullen KE, Brooks JX, Jamali M, Carriot J, Massot C. Internal models of self-motion: computations that suppress vestibular reafference in early vestibular processing. Exp Brain Res. 2011;210:377–388. doi: 10.1007/s00221-011-2555-9. [DOI] [PubMed] [Google Scholar]
- Cullen KE, McCrea RA. Firing behavior of brain stem neurons during voluntary cancellation of the horizontal vestibuloocular reflex. I. Secondary vestibular neurons. J Neurophysiol. 1993;70:828–843. doi: 10.1152/jn.1993.70.2.828. [DOI] [PubMed] [Google Scholar]
- Cullen KE, Roy JE. Signal processing in the vestibular system during active versus passive head movements. J Neurophysiol. 2004;91:1919–1933. doi: 10.1152/jn.00988.2003. [DOI] [PubMed] [Google Scholar]
- Cullen KE, Roy JE, Sylvestre PA. Signal processing by vestibular nuclei neurons is dependent on the current behavioral goal. Ann N Y Acad Sci. 2001;942:345–363. doi: 10.1111/j.1749-6632.2001.tb03759.x. [DOI] [PubMed] [Google Scholar]
- Della Santina CC, Cremer PD, Carey JP, Minor LB. Comparison of head thrust test with head autorotation test reveals that the vestibulo-ocular reflex is enhanced during voluntary head movements. Arch Otolaryngol Head Neck Surg. 2002;128:1044–1054. doi: 10.1001/archotol.128.9.1044. [DOI] [PubMed] [Google Scholar]
- Dichgans J, Bizzi E, Morasso P, Tagliasco V. Mechanisms underlying recovery of eye-head coordination following bilateral labyrinthectomy in monkeys. Experimental Brain Research. 1973;18:548–562. doi: 10.1007/BF00234137. [DOI] [PubMed] [Google Scholar]
- Dimitriou M, Edin BB. Human muscle spindles act as forward sensory models. Curr Biol. 2010;20:1763–1767. doi: 10.1016/j.cub.2010.08.049. [DOI] [PubMed] [Google Scholar]
- Escudero M, de Waele C, Vibert N, Berthoz A, Vidal PP. Saccadic eye movements and the horizontal vestibulo-ocular and vestibulo-collic reflexes in the intact guinea-pig. Exp Brain Res. 1993;97:254–262. doi: 10.1007/BF00228694. [DOI] [PubMed] [Google Scholar]
- Fuchs AF, Kimm J. Unit activity in vestibular nucleus of the alert monkey during horizontal angular acceleration and eye movement. J Neurophysiol. 1975a;38:1140–1161. doi: 10.1152/jn.1975.38.5.1140. [DOI] [PubMed] [Google Scholar]
- Gdowski GT, Belton T, McCrea RA. The neurophysiological substrate for the cervico-ocular reflex in the squirrel monkey. Experimental Brain Research. 2001;140:253–264. doi: 10.1007/s002210100776. [DOI] [PubMed] [Google Scholar]
- Gdowski GT, Boyle R, McCrea RA. Sensory processing in the vestibular nuclei during active head movements. Arch Ital Biol. 2000;138:15. [PubMed] [Google Scholar]
- Gdowski GT, McCrea RA. Integration of vestibular and head movement signals in the vestibular nuclei during whole-body rotation. J Neurophysiol. 1999;82:436–449. doi: 10.1152/jn.1999.82.1.436. [DOI] [PubMed] [Google Scholar]
- Goldberg JM, Fernandez C. Physiology of peripheral neurons innervating semicircular canals of the squirrel monkey. 3. Variations among units in their discharge properties. J Neurophysiol. 1971a;34:676–684. doi: 10.1152/jn.1971.34.4.676. [DOI] [PubMed] [Google Scholar]
- Goldberg JM, Fernandez C. Physiology of peripheral neurons innervating semicircular canals of the squirrel monkey. I. Resting discharge and response to constant angular accelerations. J Neurophysiol. 1971b;34:635–660. doi: 10.1152/jn.1971.34.4.635. [DOI] [PubMed] [Google Scholar]
- Goldberg JM, Fernandez C. Physiology of peripheral neurons innervating semicircular canals of the squirrel monkey. II. Response to sinusoidal stimulation and dynamics of peripheral vestibular system. J Neurophysiol. 1971c;34:661–675. doi: 10.1152/jn.1971.34.4.661. [DOI] [PubMed] [Google Scholar]
- Gonshor A, Jones GM. Short-term adaptive changes in the human vestibulo-ocular reflex arc. J Physiol. 1976;256:361–379. doi: 10.1113/jphysiol.1976.sp011329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herdman SJ, Hall CD, Schubert MC, Das VE, Tusa RJ. Recovery of dynamic visual acuity in bilateral vestibular hypofunction. Arch Otolaryngol Head Neck Surg. 2007;133:383–389. doi: 10.1001/archotol.133.4.383. [DOI] [PubMed] [Google Scholar]
- Herdman SJ, Schubert MC, Tusa RJ. Role of central preprogramming in dynamic visual acuity with vestibular loss. Arch Otolaryngol Head Neck Surg. 2001;127:1205–1210. doi: 10.1001/archotol.127.10.1205. [DOI] [PubMed] [Google Scholar]
- Ibershoff AE, Copeland RS. Physiology and pathology of the semicircular canals. New York: Paul B. Hoeber; 1910. [Google Scholar]
- JC Living without a balancing mechanism. New England Journal of Medicine. 1952;246:458–460. doi: 10.1056/NEJM195203202461207. [DOI] [PubMed] [Google Scholar]
- Kasai T, Zee DS. Eye-head coordination in labyrinthine-defective human beings. Brain Res. 1978;144:123–141. doi: 10.1016/0006-8993(78)90439-0. [DOI] [PubMed] [Google Scholar]
- Kaufman GD, Mustari MJ, Miselis RR, Perachio AA. Transneuronal pathways to the vestibulocerebellum. J Comp Neurol. 1996;370:501–523. doi: 10.1002/(SICI)1096-9861(19960708)370:4<501::AID-CNE7>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- King WM, Shanidze N. Anticipatory eye movements stabilize gaze during self- generated head movements. Ann N Y Acad Sci. 2011;1233:219–225. doi: 10.1111/j.1749-6632.2011.06165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klam F, Graf W. Discrimination between active and passive head movements by macaque ventral and medial intraparietal cortex neurons. J Physiol (Lond) 2006;574:367–386. doi: 10.1113/jphysiol.2005.103697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land MF. Motion and vision: why animals move their eyes. J Comp Physiol A. 1999;185:341–352. doi: 10.1007/s003590050393. [DOI] [PubMed] [Google Scholar]
- Langer T, Fuchs AF, Chubb MC, Scudder CA, Lisberger SG. Flocculus efferents in rhesus macaque as revealed by autoradiography and horseradish peroxidase. J Comp Neurol. 1985;235:26–37. doi: 10.1002/cne.902350103. [DOI] [PubMed] [Google Scholar]
- Laurens J, Angelaki DE. The functional significance of velocity storage and its dependence on gravity. Exp Brain Res. 2011;210:407–422. doi: 10.1007/s00221-011-2568-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leigh RJ, Zee DS. The Neurology of Eye Movements. New York: Oxford University Press; 2006. [Google Scholar]
- Lisberger SG. Neural basis for motor learning in the vestibuloocular reflex of primates. III. Computational and behavioral analysis of the sites of learning. J Neurophysiol. 1994;72:974–998. doi: 10.1152/jn.1994.72.2.974. [DOI] [PubMed] [Google Scholar]
- Lisberger SG, Pavelko TA, Broussard DM. Neural basis for motor learning in the vestibuloocular reflex of primates. I. Changes in the responses of brain stem neurons. J Neurophysiol. 1994;72:928–953. doi: 10.1152/jn.1994.72.2.928. [DOI] [PubMed] [Google Scholar]
- Lorente de No R. Vestibulo-ocular reflex arc. Archives of Neurological Psychiatry. 1933;30:245–291. [Google Scholar]
- Marlinsky VV, Kroller J. Optokinetic eye movements elicited by an apparently moving visual pattern in guinea pigs. Experimental Brain Research. 2000;131:350–358. doi: 10.1007/s002219900308. [DOI] [PubMed] [Google Scholar]
- McCrea RA, Gdowski GT, Boyle R, Belton T. Firing behavior of vestibular neurons during active and passive head movements: vestibulo-spinal and other non-eye-movement related neurons. J Neurophysiol. 1999;82:416. doi: 10.1152/jn.1999.82.1.416. [DOI] [PubMed] [Google Scholar]
- McCrea RA, Strassman A, May E, Highstein SM. Anatomical and physiological characteristics of vestibular neurons mediating the horizontal vestibulo-ocular reflex of the squirrel monkey. Journal of Comparative Neurology. 1987;264:547–570. doi: 10.1002/cne.902640408. [DOI] [PubMed] [Google Scholar]
- Merfeld DM, Zupan L, Peterka RJIPRJ. Humans use internal models to estimate gravity and linear acceleration. Nature. 1999;398:615–618. doi: 10.1038/19303. [DOI] [PubMed] [Google Scholar]
- Miles FA, Lisberger SG. Plasticity in the vestibulo-ocular reflex: a new hypothesis. Annu Rev Neurosci. 1981a;4:273–299. doi: 10.1146/annurev.ne.04.030181.001421. [DOI] [PubMed] [Google Scholar]
- Mollon JD. The Senses. Cambridge [Cambridgeshire] ; New York: Cambridge University Press; 1982. [Google Scholar]
- Phillips JO, Ling I, Siebold C, Fuchs AF. Behavior of primate vestibulo-ocular reflex neurons and vestibular neurons during head-free gaze shifts. Ann N Y Acad Sci. 1996;781:276–291. doi: 10.1111/j.1749-6632.1996.tb15706.x. [DOI] [PubMed] [Google Scholar]
- Precht W, Baker R. Synaptic organization of the vestibulo-trochlear pathway. Experimental Brain Research. 1972;14:158–184. doi: 10.1007/BF00234797. [DOI] [PubMed] [Google Scholar]
- Precht W, Shimazu H. Functional connections of tonic and kinetic vestibular neurons with primary vestibular afferents. J Neurophysiol. 1965;28:1014–1028. doi: 10.1152/jn.1965.28.6.1014. [DOI] [PubMed] [Google Scholar]
- Ris L, de Waele C, Serafin M, Vidal PP, Godaux E. Neuronal activity in the ipsilateral vestibular nucleus following unilateral labyrinthectomy in the alert guinea pig. J Neurophysiol. 1995a;74:2087–2099. doi: 10.1152/jn.1995.74.5.2087. [DOI] [PubMed] [Google Scholar]
- Roy JE, Cullen KE. Selective processing of vestibular reafference during self- generated head motion. Journal of Neuroscience. 2001;21:2131–2142. doi: 10.1523/JNEUROSCI.21-06-02131.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy JE, Cullen KE. Vestibuloocular reflex signal modulation during voluntary and passive head movements. J Neurophysiol. 2002;87:2337–2357. doi: 10.1152/jn.2002.87.5.2337. [DOI] [PubMed] [Google Scholar]
- Roy JE, Cullen KE. Dissociating self-generated from passively applied head motion: neural mechanisms in the vestibular nuclei. J Neurosci. 2004;24:2102–2111. doi: 10.1523/JNEUROSCI.3988-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeghi SG, Minor LB, Cullen KE. Neural correlates of motor learning in the vestibulo-ocular reflex: dynamic regulation of multimodal integration in the macaque vestibular system. J Neurosci. 2010;30:10158–10168. doi: 10.1523/JNEUROSCI.1368-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeghi SG, Minor LB, Cullen KE. Multimodal integration after unilateral labyrinthine lesion: single vestibular nuclei neuron responses and implications for postural compensation. J Neurophysiol. 2011;105:661–673. doi: 10.1152/jn.00788.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer M, Migliaccio AA, Schubert MC. Effect of vestibular rehabilitation on passive dynamic visual acuity. J Vestib Res. 2008;18:147–157. [PMC free article] [PubMed] [Google Scholar]
- Schubert MC, Migliaccio AA, Clendaniel RA, Allak A, Carey JP. Mechanism of dynamic visual acuity recovery with vestibular rehabilitation. Arch Phys Med Rehabil. 2008;89:500–507. doi: 10.1016/j.apmr.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scudder CA, Fuchs AF. Physiological and behavioral identification of vestibular nucleus neurons mediating the horizontal vestibuloocular reflex in trained rhesus monkeys. J Neurophysiol. 1992;68:244–264. doi: 10.1152/jn.1992.68.1.244. [DOI] [PubMed] [Google Scholar]
- Serafin M, Ris L, Bernard P, Muhlethaler M, Godaux E, Vidal PP. Neuronal correlates of vestibulo-ocular reflex adaptation in the alert guinea-pig. Eur J Neurosci. 1999;11:1827–1830. doi: 10.1046/j.1460-9568.1999.00572.x. [DOI] [PubMed] [Google Scholar]
- Shadmehr R, Smith MA, Krakauer JW. Error Correction, Sensory Prediction, and Adaptation in Motor Control. Annu Rev Neurosci. 2010;33:89–108. doi: 10.1146/annurev-neuro-060909-153135. [DOI] [PubMed] [Google Scholar]
- Shanidze N, Kim AH, Loewenstein S, Raphael Y, King WM. Eye-head coordination in the guinea pig II. Responses to self-generated (voluntary) head movements. Exp Brain Res. 2010a;205:445–454. doi: 10.1007/s00221-010-2375-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanidze N, Kim AH, Raphael Y, King WM. Eye-head coordination in the guinea pig I. Responses to passive whole-body rotations. Exp Brain Res. 2010b;205:395–404. doi: 10.1007/s00221-010-2374-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl JS, Simpson JI. Dynamics of rabbit vestibular nucleus neurons and the influence of the flocculus. J Neurophysiol. 1995;73:1396–413. doi: 10.1152/jn.1995.73.4.1396. [DOI] [PubMed] [Google Scholar]
- Tomlinson RD, Robinson DA. Signals in vestibular nucleus mediating vertical eye movements in the monkey. J Neurophysiol. 1984;51:1121–1136. doi: 10.1152/jn.1984.51.6.1121. [DOI] [PubMed] [Google Scholar]
- von Holst E, Mittelstaedt HM. Das Reafferenzprinzip. Naturwissenschaften. 1950;20:464–476. [Google Scholar]
- Walls GL. The evolutionary history of eye movements. Vision Res. 1962;2:69–80. [Google Scholar]