Abstract
Tetrabromobisphenol A (TBBPA) is a widely used flame retardant. Despite the presence of TBBPA in gestational tissues and the importance of proper regulation of inflammatory networks for successful pregnancy, there is no prior study on the effects of TBBPA on inflammatory responses in gestational tissues. The present study aimed to investigate TBBPA activation of inflammatory pathways, specifically cytokine and prostaglandin production, in the human first trimester placental cell line HTR-8/SVneo. TBBPA enhanced release of interleukin (IL)-6, IL-8, and prostaglandin E2 (PGE2), and suppressed TGF-β release in HTR-8/SVneo cells. The lowest effective concentration was 10 μM TBBPA. A commercial immune response PCR array revealed increased expression of genes involved in inflammatory pathways stimulated by TBBPA in HTR-8/SVneo cells. Because proper regulation of inflammatory mediators in the gestational compartment is necessary for normal placental development and successful pregnancy, further investigation on the impact of TBBPA-stimulated responses on trophoblast function is warranted.
Keywords: Tetrabromobisphenol A (TBBPA), HTR-8/SVneo cells, human extravillous trophoblasts, cytokines, prostaglandins, placenta, brominated flame retardant
1. Introduction
Tetrabromobisphenol A (TBBPA) is the most widely used brominated flame retardant (BFRs) in the world representing about 60% of the total brominated flame retardant market [1]. Although TBBPA is mainly used as a reactive flame retardant, approximately 10% is used as an additive flame retardant, facilitating release of TBBPA into the environment [2, 3]. TBBPA has been detected in the air [4], dust [5], soil, sediment [6], and food [7]. TBBPA has also been found in human breast milk [7, 8], adipose tissue, serum [8], and gestational compartments such as umbilical cord serum [8]. Because of TBBPA’s environmental persistence and toxicity, it is on the 4th Priority List of the European Union (EU) Existing Substance Regulation [9]. Moreover, TBBPA is considered to be a “persistent, bioaccumulative and toxic (PBT)” chemical under Washington State’s PBT rule [10] and is a chemical of high concern to children by the Washington State Department of Health in the Children’s Safe Product Act [11]. TBBPA exhibits neurodevelopmental, hepatic, renal, immunological and thyroid toxicities in animal and in vitro studies [12–16]. Despite the presence of TBBPA in gestational compartments [8], the impact of TBBPA on pregnancy is poorly understood.
To date, no epidemiological or experimental data on the effects of TBBPA during human pregnancy are available. However, a few animal studies suggest reproductive toxicity of TBBPA. For example, in one study, exposure of adult zebra fish to TBBPA resulted in decreased egg production and increased premature oocytes [2]. In the same study, exposure of eggs to TBBPA reduced hatching, increased post-hatching mortality, and increased caudal and cranial malformation of embryos and pericardial fluid accumulation, indicating decreased reproductive success in zebra fish [2]. Oral administration of TBBPA as its formulated product, Saytex 111, to pregnant rats resulted in reduced fetal weight, increased malformations, and increased fetal death [17]. In another study, rats orally exposed to TBBPA prior to mating and during mating, pregnancy, and lactation resulted in increased weight of the testis and pituitary gland in males, delayed sexual development in females, and decreased pup mortality [9]. These data suggest potential toxicity of TBBPA exposure during pregnancy, calling for studies about TBBPA effects on human gestational cells and tissues.
Inflammatory mediators, such as cytokines and prostaglandins, not only play a role in innate immune response as a part of host-defense mechanisms, but also are considered to be key components in reproductive processes including the establishment and maintenance of pregnancy, and the initiation of labor [18–26]. Improper regulation of the inflammatory networks may lead to adverse pregnancy outcomes such as miscarriage, preeclampsia, intrauterine growth restriction and preterm labor [27, 28]. For example, increased levels of cytokines, prostaglandins, adhesion molecules, C-reactive protein in cervical fluid, amniotic fluid, and maternal serum have been linked to the pathophysiology of preterm birth, preeclampsia, and intrauterine growth restriction [29–34]. Through pathologic activation of pro-inflammatory pathways, pregnancies complicated with bacterial vaginosis [35, 36] or intrauterine infection [37, 38] have been associated with increased risk of preterm birth. Such findings suggest that inflammation occurring at the maternal–fetal interface during pregnancy could contribute to adverse obstetrical outcomes.
Studies conducted with immune cells in vitro examined the effects of TBBPA on inflammatory or innate immune responses. In murine RAW 264.7 macrophages, TBBPA induced cyclooxygenase (COX)-2 and pro-inflammatory cytokine expression through activation of Akt/MAPK/NF-κB/AP-1 signaling, and also enhanced production of the COX-2 metabolite prostaglandin (PG) E2 [39]. In addition, TBBPA had an immunosuppressive effect on human natural killer cells by decreasing their lytic function [40]. Moreover, exposure of human neutrophil granulocytes to TBBPA led to an activation of the NADPH oxidase by an ERK 1/2 stimulated pathway [13]. Although these data suggest that TBBPA may play a role in the activation of inflammatory pathways and modulating innate responses, to the best of our knowledge, there are no reports on the effects of TBBPA on gestational cells or tissues. Given the detection of TBBPA in the gestational compartment and the importance of proper regulation of inflammatory networks for successful pregnancy, a study on the effects of TBBPA on inflammatory responses in gestational tissues is warranted. The purpose of this study is to investigate the effect of TBBPA on the activation of inflammatory pathways, specifically cytokine and prostaglandin production in the human first trimester placental cell line HTR-8/SVneo.
2. Materials and Methods
2.1. Chemicals and assay kits
TBBPA (97% purity), dimethyl sulfoxide (DMSO), indomethacin, and NS-398 were purchased from Sigma Aldrich (St. Louis, MO, USA). TBBPA was prepared in DMSO as a 50 mM stock solution. RPMI medium 1640, fetal bovine serum (FBS), OptiMem 1 reduced-serum medium, Hank’s balanced salt solution (HBSS), 0.25% trypsin/EDTA solution and penicillin/streptomycin (P/S) were purchased from Invitrogen Life Technologies (Carlsbad, CA, USA).
2.2. Cell Culture and treatment
The human first trimester extravillous trophoblast cell line HTR-8/SVneo was kindly provided by Dr. Charles S. Graham (Queen’s University, Kingston, ON, Canada) [41]. Cells between passages 71 and 84 were cultured in RPMI 1640 medium supplemented with 10% FBS and 1% penicillin/streptomycin at 37°C in a 5% CO2 humidified atmosphere. Cells were grown to 70–90% confluence before treatment. From solutions of 5, 10, 20 and 50 mM TBBPA in DMSO, exposure media containing 5, 10, 20 and 50 μM TBBPA were made in OptiMem 1 containing 1% FBS and 1% P/S immediately prior to initiating the experiment. Exposure concentrations were selected based on preliminary experiments and are comparable to concentrations used in other published in vitro studies [42–44]. The final concentration of DMSO in medium was 0.1 % (v/v).
2.3. Cytotoxicity and cell viability assays
Cells were seeded in a white 96-well plate at a density of 1 × 104 cells per well and incubated for 24 h at 37 °C. Cells were exposed to DMSO (0.1% v/v, solvent control) or TBBPA (5, 10, 20 or 50 μM), and then incubated for 8, 16, or 24 h. After treatment with TBBPA, TBBPA-induced toxicity was measured using the CyQuant Direct Cell Proliferation Assay (Invitrogen), following the manufacturer’s protocols. The assay is based on a cell-permeant DNA-binding dye in combination with a background suppression reagent. The DNA-binding dye is a live cell-permeable reagent while the suppression dye is designed to selectively penetrate only the compromised membranes of dying cells, suppressing the fluorescence of the DNA binding dye. Therefore, the decrease in fluorescence is directly proportional to the degree of TBBPA-induced toxicity. Cell viability was quantified using the Cell Titer Glo Luminescent Cell Viability Assay (Promega; Madison, WI), following the manufacture’s protocols. The luminescence is directly proportional to intracellular ATP production in cells.
2.4. Measurement of cytokine release
HTR-8/SVneo cells were seeded at a density of 5 × 104 cells per well in a 24-well plate and cultured for 24 h at 37 °C. Cells were washed with OptiMem 1 containing 1% FBS and 1% P/S twice and acclimated with the medium for 1 h at 37 °C. After incubation with 5, 10, or 20 μM TBBPA for 4, 8, 16, or 24 h, culture medium was collected and centrifuged to remove any residual cell lysates. The concentration of interleukin (IL)-6, IL-8, and transforming growth factor-beta (TGF-β) in the supernatant was measured by sandwich enzyme-linked immunosorbent assay (ELISA) following the manufacturer’s protocols (R & D systems; Minneapolis) and expressed as pg/ml.
2.5. Measurement of prostaglandin production
Cells were prepared and treated as previously described in the preceding section on measurement of cytokine release. To determine if TBBPA-stimulated PGE2 release is dependent upon the activity of cyclooxygenase (COX), cells were treated with TBBPA in the absence and presence of 10 μM indomethacin (COX-1 and COX-2 inhibitor) or 10 μM NS-398 (COX-2 specific inhibitor). After 24-h incubation with TBBPA, the culture medium was removed and cells were washed with HBSS once. Then, cells were incubated with 2.5 μM arachidonic acid in HBSS (without TBBPA) for 4 h at 37 °C. After 4-h incubation with arachidonic acid, the concentration of PGE2 in the medium was measured by ELISA following the manufacturer’s procotols (Cayman Chemical). Concentrations of PGE2 were expressed as pg/ml.
2.6. PCR array and qRT-PCR validation
Changes in mRNA expression of 84 target genes by TBBPA treatment of HTR-8/SVneo cells were quantified using the Innate and Adaptive Immune Responses PCR Array (Qiagen; Valencia, CA). This commercial array includes 84 preselected genes involved in host response to bacterial infection and sepsis. We chose this pathway-specific array to assess TBBPA-stimulated activation of inflammatory responses, many of which have been associated with adverse birth outcomes. Cells were treated with DMSO (solvent control) or TBBPA (5 or 10 μM) for 24 h. After 24 h of exposure, cell lysates were collected and homogenized using QIA shredder (Qiagen; Valencia, CA). Total RNA was extracted from homogenized lysates using RNeasy mini plus kit (Qiagen; Valencia, CA) and cDNA was synthesized from 1 μg of total RNA using RT2 First Strand Kit (Qiagen; Valencia, CA) following the manufacturer’s protocols. For the array, cDNA from the solvent control and TBBPA treatment groups were analyzed using the Applied Biosystems 7900HT Sequence Detection System following the Qiagen recommended protocol. Fold changes were calculated from ΔCT values (gene of interest CT values − average of all housekeeping gene CT values) using the ΔΔCT method. Mean ΔCT values were compared between groups using paired t-tests from the Limma package of Bioconductor [45]. With qRT-PCR, we validated the findings of the array for those genes with significant mRNA expression changes that were approximately two-fold or more with 10 μM TBBPA treatment: IL-6, lipopolysaccharide binding protein (LBP), peptidoglycan recognition protein 1 (PGLYRP1), toll-like receptor 1 (TLR1), triggering receptor expressed on myeloid cells 1 (TREM1), IL-1 family, member 7 zeta (IL1F7), and heme oxygenase 1 (HMOX1). In addition to the latter 7 genes, mRNA expression of IL8, TGFB1, and prostaglandin-endoperoxide synthase 2 (PTGS2; encodes for COX-2) were also analyzed. qRT-PCR reactions were prepared with Qiagen SYBR Green mastermix and primers, and run on a Bio-Rad CFX96 Real time C1000 thermal cycler following the manufacturer’s recommended protocols. The mRNA levels of each gene of interest were normalized to β-2-micoglobulin mRNA levels and presented as fold change compared to solvent controls.
2.7. Statistical analysis
Statistical analysis was performed with Sigma Plot 11.0 software (Systat Software Inc., San Jose, CA, USA). After determining acceptable homogeneity of variance and normality (P<0.05), data were analyzed either by one-way analysis of variance (ANOVA) or repeated measured two-way ANOVA. When significant effects were detected, the ANOVA was followed by Tukey post-hoc comparison of means. A P <0.05 was considered statistically different. Data were expressed as means ± SEM. All experiments were performed at least in triplicate and repeated three or more times.
3. Results
3.1. Cytotoxicity and cell viability
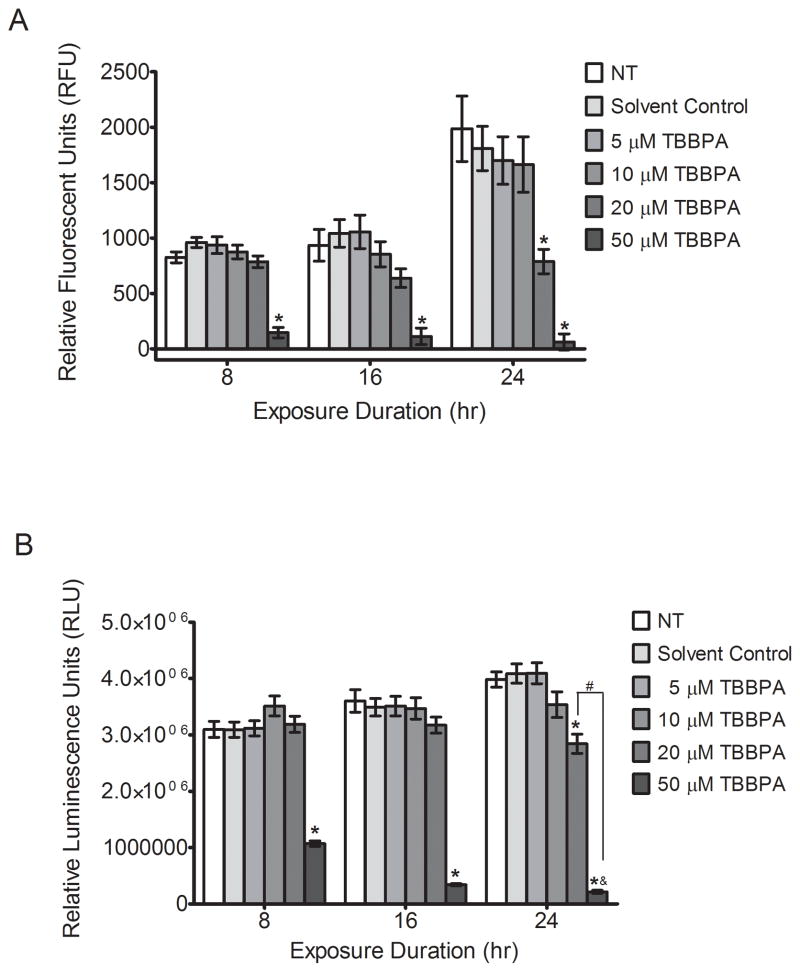
Treatment with 50 μM TBBPA for 8, 16, and 24 h markedly increased cytotoxicity in HTR-8/SVneo cells, as indicated by decreased fluorescence with the Invitrogen CyQuant Direct Cell Proliferation Assay (P<0.05, Figure 1A). Concentrations of 5 and 10 μM TBBPA did not elicit cytotoxicity at any timepoint, and 20 μM TBBPA was cytotoxic only after 24 h of exposure (P<0.05, Figure 1A). Likewise, treatment with 50 μM TBBPA decreased intracellular ATP levels by 65%, 90%, and 95% at 8, 16 and 24 h, respectively (P<0.05, Figure 1B), and exposure to 20 μM TBBPA for 24 h decreased ATP levels by 30% (P<0.05, Figure 1B). Considering these results, 50 μM TBBPA was excluded from subsequent experiments.
Figure 1. TBBPA effects on cytotoxicity and cell viability in HTR-8/SVneo cells.
HTR-8/SVneo cells were non-treated control (NT), or were treated with DMSO (0.1% v/v, solvent control) or TBBPA for 8, 16, or 24 h, then cytotoxicity and cell viability were measured. (A) TBBPA-induced cytotoxicity was measured using a cell-permeant DNA-binding dye in combination with a background suppression reagent (Invitrogen CyQuant Direct Cell Proliferation Assay): the decrease in fluorescence is directly proportional to the degree of TBBPA-induced toxicity. (B) Cell viability was quantified using a luminescence-based assay measuring intracellular ATP: the luminescence is directly proportional to intracellular ATP production in cells. Bars represent the means of 3 independent experiments containing 5 replicates each ±SE. *P<0.05, significantly different compared to solvent control within same time point. #P<0.05, significantly different from each other. &P<0.05, compared to 50 μM TBBPA at 8 or 16 h.
3.2. TBBPA effects on cytokine production
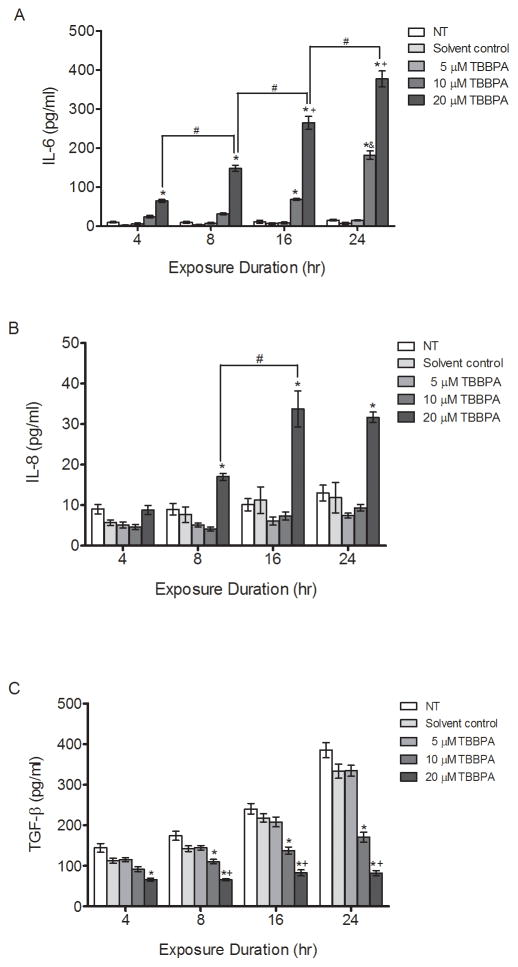
Because cytokines play critical roles in pregnancy and aberrant production has been associated with adverse pregnancy outcomes such as preeclampsia and preterm birth [18–20], we investigated the effect of TBBPA on IL-6, IL-8 and TGF-β production in HTR-8/SVneo cells. TBBPA treatment stimulated concentration-dependent and time-dependent increases in IL-6 (P<0.05, Figure 2A). Treatment with 20 μM TBBPA stimulated IL-6 release significantly compared to the solvent control at 4 and 8 h (P<0.05, Figure 2A). Treatment with 10 μM and 20 μM TBBPA increased IL-6 release significantly compared to the solvent control at 16 and 24 h in a concentration-dependent manner (P<0.05, Figure 2A). IL-6 increases were 25.7-fold and 53.2-fold after 24-h exposure to 10 and 20 μM TBBPA, respectively, compared to controls at the same time point (Figure 2A). Treatment with 10 μM TBBPA at 24 h increased IL-6 release compared to treatment with 10 μM TBBPA at 16 h, showing a time-dependent response.
Figure 2. TBBPA effects on cytokine production in HTR-8/SVneo cells.
HTR-8/SVneo cells were non-treated control (NT), or were exposed to DMSO (0.1% v/v, solvent control) or TBBPA for 4, 8, 16 or 24 h, and then culture medium concentrations of (A) IL-6, (B) IL-8, and (C) TGF-β were quantified by EIA. Bars represent the means ± SE of 4 independent experiments containing 3 replicates each. *P<0.05, significantly different compared to solvent control within same time point. #P<0.05, significantly different from each other. &P<0.05, significantly different compared to 20 μM TBBPA at 4, 8, and16 h. +P<0.05, significantly different compared to 10 μM TBBPA within same time point.
Likewise, treatment with 20 μM TBBPA resulted in a time-dependent increase of IL-6 release at 4, 8, 16, and 24 h (P<0.05, Figure 2A). IL-6 concentrations did not change significantly over the 24-h exposure period in non-treated and solvent control cell cultures, nor in cultures treated with 5 μM TBBPA. Statistically significant increases in IL-8 concentrations were observed at 8, 16 and 24 h compared with solvent controls at each time point, but only with 20 μM TBBPA treatment (P<0.05, Figure 2B). IL-8 increases were 2.2-fold, 3-fold, and 2.7-fold after exposure to 20 μM TBBPA at 8, 16, and 24 h, respectively, compared to controls at the same time point. Time-dependent increases in IL-8 release were observed with 20 μM TBBPA comparing 8 and 16 h (P<0.05, Figure 2B). On the other hand, TGF-β declined with 20 μM TBBPA treatment after 4 h compared to the solvent control at the same point (P<0.05, Figure 2C). Moreover, 10 μM and 20 μM TBBPA reduced TGF-β release after 8, 16 and 24 h compared to the solvent control at the same time point and in a concentration-dependent manner (P<0.05, Figure 2C).
3.3. TBBPA effects on prostaglandin production
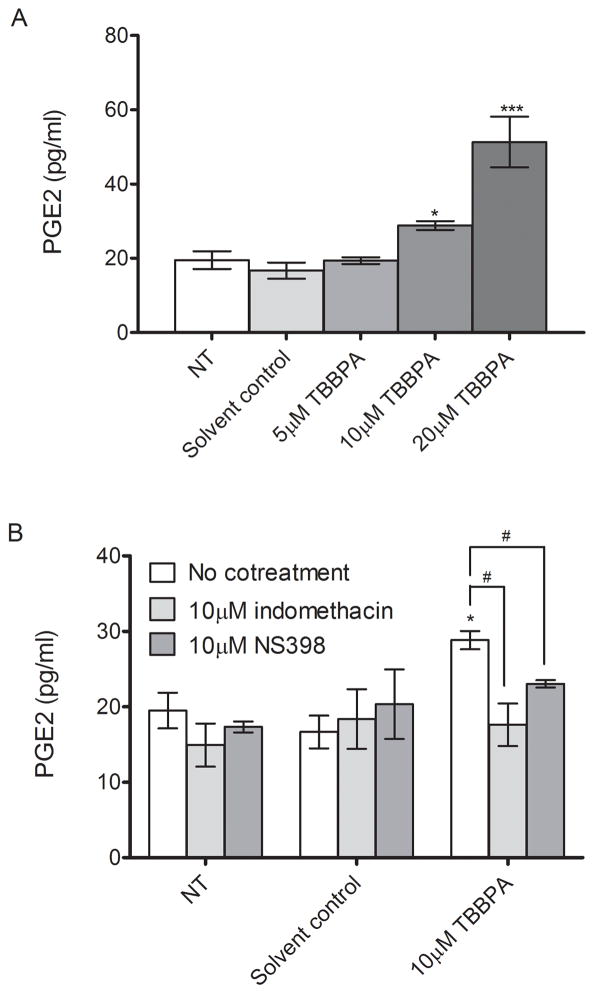
Treatment with 10 and 20 μM induced significant increases in PGE2 release by 1.7-fold and 3-fold, respectively, compared to the solvent control from HTR-8/SVneo cells (P<0.05, Figure 3A). Cotreatment with indomethacin, a COX inhibitor, or NS-398, a COX-2 specific inhibitor, resulted in 40% and 20% reduction in TBBPA-stimulated PGE2 release to the levels comparable to the solvent control (P<0.05, Figure 3B), indicating that TBBPA-induced PGE2 release is dependent on COX activity. Notably, NS-398-mediated PGE2 decrease was comparable to indomethacin-mediated PGE2 decrease, suggesting that TBBPA-stimulated PGE2 production is mainly dependent on COX-2 activity.
Figure 3. TBBPA effects on prostaglandin (PG) E2 production in HTR-8/SVneo cells.
HTR-8/SVneo cells were non-treated control (NT), or were treated with solvent control (DMSO, 0.1% v/v) or TBBPA for 24 h in the absence or presence of the COX inhibitor indomethacin or the COX-2 specific inhibitor NS 398. The levels of PGE2 in the culture media were quantified by EIA. A) TBBPA effects on PGE2 production. B) Suppression of TBBPA-stimulated PGE2 release by treatment with cyclooxygenase (COX) inhibitors. Bars represent the means of 3 independent experiments containing 3 replicates each ±SE. *P<0.05, significantly different compared to solvent control. #P<0.05, significantly different from each other.
3.4. PCR array results and validation
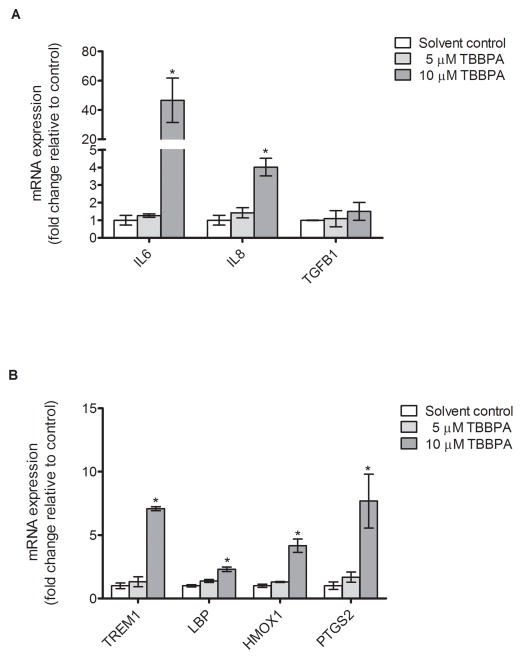
The Innate and Adaptive Immune Responses PCR Array identified four genes with mRNA expression significantly increased two-fold or more by 10 μM TBBPA treatment compared to solvent control: HMOX1, IL6, LBP, and TREM1. The fold change and P-value for each gene is shown in Table 1 (for complete PCR array data, see Supplemental material, Table 1). The clustergram provides a graphical representation of fold expression compared to solvent control with hierarchical clustering indicating genes with similar expression patterns grouped together and connected by dendrograms. Changes in mRNA expression of the array-identified genes were then validated by qRT-PCR, shown in Figure 5. Consistent with the array results, treatment with 10μM TBBPA for 24 h significantly increased mRNA expression of IL6, TREM1, LBP, and HMOX1: the increases detected by qRT-PCR were 46.6-fold, 7.1-fold, 2.3-fold, and 4.2-fold, respectively (P<0.05, Figure 5). The magnitudes of change detected with qRT-PCR differed from those observed with the array, likely a result of array conditions optimized for broad detection of multiple genes whereas the qRT-PCR conditions were optimized for each gene [46]. The mRNA expression of IL8, which was not a gene included in the PCR array, increased by 4.0-fold with 10 μM TBBPA treatment after 24-h incubation as assessed by qRT-PCR (P<0.05, Figure 5A), conforming to increased IL-8 protein levels by 20 μM TBBPA treatment (Figure 2B). The mRNA expression of PTGS2 increased by 7.7-fold as measured by qRT-PCR (P<0.05, Figure 5B), although significant change of expression of this gene was not detected in the PCR array. Although the PCR array showed significant downregulation by BDE-47 treatment of mRNA expression of IL1F7, TLR1 and PGLYRP1 (Supplemental Data, Table 1), these changes were not validated by qRT-PCR with either 5μM or 10μM TBBPA treatment (data not shown), possibly related to low expression of the genes as indicated by high Ct values (> 30) for the qRT-PCR assay [47, 48].
Table 1.
Fold change of selected genes from Innate and Adaptive Immune Responses PCR Array1
| PCR Array | qRT-PCR Validation | ||||
|---|---|---|---|---|---|
| Gene Name | Symbol | Fold changea | P-value | Fold change (± SE)a | P-value |
| Heme oxygenase (decycling) 1 | HMOX1 | 2.9 | 0.01 | 4.159 ± 0.524 | <0.001 |
| Interleukin 6 (interferon, beta 2) | IL6 | 9.7 | 2.2 E-5 | 46.555 ± 15.197 | 0.016 |
| Lipopolysaccharide binding protein | LBP | 3.5 | 0.018 | 2.301 ± 0.171 | 0.001 |
| Triggering receptor expressed on myeloid cells 1 | TREM1 | 115.2 | 0.0009 | 7.079 ±0.162 | <0.001 |
| Prostaglandin-endoperoxide synthase 2b | PTGS2 | −1.2 | 0.59 | 7.677 ± 2.128 | 0.018 |
HTR-8 cells were treated with DMSO (0.1 % v/v; solvent control) or 10 μM TBBPA for 24 h. The Innate and Adaptive Immune Responses PCR Array (SABiosciences; Valencia, CA) was used to explore changes in gene expression as described in the “Materials and Methods” section. Fold changes and p-values are shown for the genes with significant changes compared to the solvent control.
Mean fold change relative to the solvent control. PCR Array: n= 4 experiments, qRT-PCR Validation: n=3 experiments. Each experiment was performed in triplicate.
Although the change was not statistically significant in the array, gene expression was assessed by qRT-PCR because of this gene’s pertinence to human pregnancy.
Figure 5. TBBPA effects on HTR-8/SVneo mRNA expression of genes previously identified with a PCR array.
A) TBBPA effects on mRNA expression of genes for IL-6, IL-8, TGF-β and IL-1F7 after 24-h exposure. B) TBBPA effects on mRNA expression of genes for TREM1, LBP, PGLYRP1, TLR1, HMOX1, and PTGS2 after 24-h exposure. The mRNA expression was quantified by qRT-PCR. Bars represent means ± SE (n=3 experiments, each with 3 replicates). *P<0.05, significant compared to solvent control.
4. Discussion
TBBPA is the most widely used brominated flame retardant in consumer products with a global annual demand of about 200000 metric tons [49]. Due to widespread use and bioaccumulation of TBBPA, the levels of TBBPA in human serum samples have been increasing [50]. Despite its presence in human gestational compartments [8] and the importance of proper regulation of inflammatory pathways for successful pregnancy [27, 28], there are no previous reports of TBBPA-stimulated effects on inflammatory pathways in human first trimester placental cells. The objective of the current study was to investigate TBBPA-stimulated inflammatory responses in human placental cells. Our findings demonstrate that TBBPA treatment increases proinflammatory IL-6 and IL-8 and decreased anti-inflammatory TGF-β release. In addition, TBBPA treatment stimulated PGE2 release and resulted in stimulated expression of inflammatory genes such as IL6, IL8, PTGS2, TREM1, LBP, and HMOX1.
4.1. TBBPA stimulates inflammatory responses in human first trimester trophoblasts
To our knowledge, this is the first study to report TBBPA-stimulated inflammatory responses in human placental cells. The present study clearly showed that TBBPA induced secretion and mRNA expression of proinflammatory IL-6 and IL-8 in the human first trimester trophoblast cell line HTR-8/SVneo, while reducing release of the anti-inflammatory cytokine TGF-β. Interestingly, mRNA expression of TGF-β did not change significantly with TBBPA treatment, suggesting that the decrease in TGF-β secretion may be regulated through post-transcriptional mechanisms. TBBPA treatment also resulted in an increased release of PGE2 into the culture medium. Suppression of PGE2 release by co-treatment with COX inhibitors shows that TBBPA-induced PGE2 production is dependent on COX activity. Because treatment with NS-398, a COX-2-specific inhibitor, was sufficient to suppress TBBPA-stimulated PGE2 release, it is suggested that TBBPA-mediated PGE2 production is mainly dependent on COX-2 activity. This is consistent with our finding that TBBPA stimulated increased mRNA expression of PTGS-2, the gene for COX-2.
The current findings are consistent with a previous study showing that in vitro TBBPA exposure of murine RAW 264.7 macrophages induced secretion and mRNA expression of pro-inflammatory cytokines, such as TNF-α, IL-6 and IL-1β, as well as the release of PGE2 with increased expression of COX-2 [39]. Moreover, the latter study reported that NF-κB mediated the TBBPA-induced effects on proinflammatory cytokine and COX-2 expression in murine RAW 264.7 macrophages [39]. Although we did not assess TBBPA-induced NF-κB activation, we suggest that NF-κB may be involved in TBBPA-stimulated cytokine release and COX-2 induction in HTR-8/SVneo cells, also, as reported previously in RAW 264.7 macrophages [39].
4.2. Inappropriate activation of inflammatory pathways within the gestational compartment has been linked to adverse obstetrical outcomes originated from abnormal placentation
Dysregulation of inflammatory mediators within the gestational compartment has been linked to adverse birth outcomes such as intrauterine growth restriction, preeclampsia and preterm birth [51–55]. For example, high levels of IL-6, IL-8 and PGE2 in the cervicovaginal fluid and amnionic fluid of pregnant women were associated with increased risk for preterm birth [33, 56–58]. In addition, Chegini et al. reported that myometrium from women who had unsuccessful labor induction expressed higher levels of TGF-β 1 mRNA than those with preterm labor or without labor. On the other hand, TGF-β receptor type II expression was significantly lower in myometrium from preterm labor compared with those from unsuccessful labor induction or without labor. These findings suggest that TGF-β and TGF-β receptors may play a role in mechanisms involving normal and preterm labor [59]. Moreover, PTGS2 mRNA levels were approximately seven times higher in chorion laeve from spontaneous preterm extraembryonic membranes compared to non-laboring tissues of equivalent gestational age [60], an increase comparable to the 7.7-fold increase that we observed with TBBPA in the present study. Although the etiology of inflammation-related adverse birth outcomes is not fully understood, it has been suggested that inflammation within the gestational compartment may lead to impaired trophoblast cellular function, contributing to the placental dysfunction seen in pregnancy-related disorders [61].
The possible link between placental dysfunction and inflammation have been implicated in previous studies showing that women who delivered preterm had higher rates of placental ischemia and abnormal placentation than controls [51, 62], with high levels of IL-6 and IL-8 in cervical fluid, amniotic fluid and maternal serum [34]. Migration and invasion of extravillous trophoblast into the spiral arteries are critical events during placentation [63–65], and dysruption of these processes is associated with abnormal placentation [66, 67]. In in vitro studies, LPS reduced invasion of HTR-8/SVneo cells with increased production of IL-8 and IL-6 [61]. IL-6 also has been shown to increase migration and invasion in HTR-8/SVneo cells [68, 69] while inhibition of endogenous IL-6 in JEG-3 choriocarcinoma cells inhibited migration and invasion [70]. In addition, In vitro studies showed that TGFβ1 inhibits cytotrophoblast cell migration and invasiveness potentially by the upregulation of the endogenous tissue inhibitors of MMPs (TIMP)-1 and 2 [71, 72]. The roles of PGE2 in trophoblast cellular function have been implicated although the findings have been controversial. For example, PGE2 promoted migration of HTR-8/SVneo cells [73, 74] with suppressed migration by COX-2 inhibition. On the other hand, Biondi et al. [75] showed that PGE2 suppressed the proliferation and migration of HTR-8/SVneo cells. Despite some inconsistencies, these reports implicate that inflammatory mediators including IL-6, IL-8 and PGE2 may play a critical role in regulating trophoblast cellular function during placentation. The present study showed that exposure to TBBPA stimulated production of proinflammatory IL-6, IL-8, and PGE2 with increased COX-2 mRNA expression in HTR-8/SVneo cells, suggesting that TBBPA could potentially impair normal trophoblast cellular function and invasion. Further investigation of the effects of TBBPA on important trophoblast functions such as invasion and migration will be needed to confirm our hypothesis on impaired trophoblast cellular functions from TBBPA-mediated inflammation.
4.3. Genes differentially expressed with TBBPA exposure
A screen of innate and adaptive immune response genes was conducted with a commercial PCR array to define changes in expression of inflammatory genes by TBBPA treatment of human placental cells. The array results were validated and supplemented with qRT-PCR assay of targeted genes. Our study clearly showed that exposure to TBBPA activated inflammatory pathways in human placental cells. We identified six genes differentially expressed compared to the solvent control: IL6, IL8, PTGS2, TREM1, LBP, and HMOX1. The increased mRNA expression of IL6, IL8, PTGS2 was in accordance with the stimulated secretion of IL-6, IL-8, and PGE2. The present study is the first to show that exposure of human placental cells to TBBPA stimulated gene expression of TREM1and LBP. Few studies have reported the involvement of TREM1 and LBP in inflammatory responses at gestational compartments. TREM1 is normally found in amniotic fluid and its levels are elevated with intra-amniotic infection and elevated cytokine production [76]. Its mRNA expression increases in myometrial and cervical tissue after term labor [77]. LBP encodes for a protein that binds LPS and plays a critical role in activating acute-phase response to LPS. LBP was detected in amniotic fluid and fetal cord blood from women at term, and elevated amniotic fluid LBP levels were associated with increased amniotic fluid cytokine concentrations (TNF-α, IL-6 and IL-8), chorioamnionitis, and labor [78], suggesting that LBP may mediate intrauterine inflammatory responses. Furthermore, it is reported that TREM1 and LBP amplify and stimulate release of pro-inflammatory chemokines and cytokines in monocytes or neutrophils [79–82]. Although the roles of TREM1and LBP in placental cells are not clear, we speculate that increased expression of these genes synergistically activates inflammatory pathways in HTR-8/SVneo cells exposed to TBBPA, inducing production of pro-inflammatory cytokine IL-6 and IL-8. However, further study is warranted to investigate how expression of TREM1and LBP is linked to toxicant-mediated inflammation in human placental cells.
Notably, the present study found that TBBPA stimulated mRNA expression of HMOX1, the gene for heme oxygenase (HO)-1, an antioxidant and anti-inflammatory enzyme [30]. Several lines of evidence suggest that HO-1 is a key regulator during pregnancy [31]. For example, induction of HO-1 caused a significant attenuation of TNF-α-mediated cellular damage in placental villous explants [83]. Moreover, placentas from human pathologic pregnancies including preeclampsia, spontaneous abortion, choriocarcinoma, and hydatidiform mole express lower levels of HO-1 compared with normal pregnancies [83, 84]. In contrast, decidual expression and maternal serum levels of preeclamptic women are elevated [85]. Despite inconsistencies in previous reports, a role for HO-1 in of adverse pregnancy outcomes is suggested. We offer that induction of HMOX1 may counteract TBBPA-induced inflammatory responses to protect cells from further damage. Although speculative, this proposed role of HO-1 in toxicant-induced inflammatory responses in gestational tissues suggests that HO-1 could be a potential therapeutic target for future research to prevent adverse birth outcomes.
Although the present study reports new findings in TBBPA-stimulated inflammatory responses in human placental cells, there are limitations to be addressed. First, the responses observed in HTR-8/SVneo cells in the present study may not be necessarily applied to primary extravillous trophoblast cells. The HTR-8/SVneo cell line is known to have a similar phenotype compared to its primary counterparts [41, 69, 75], retaining migratory capability and expressing specific placental trophoblast markers [74, 86]. However, it is reported that these cells have different gene expression and gene methylation profiles compared to primary extravillous trophoblast cells [87, 88]. Moreover, results from an in vitro study may not directly translate to the in vivo situation because trophoblast invasion is a complex process involving various cytokines, integrins, and adhesion and proteolytic molecules [61] other than IL-6, IL-8 and PGE2, which were the focus of in the current study. For these reasons, future studies should explore the mechanisms of TBBPA-stimulated effects using primary cells from the placenta or using extraembryonic membranes. Another limitation is that TBBPA concentrations used in this study are higher than what is detected in human samples, although they are comparable to the concentrations in other in vitro studies [42–44]. Limited studies reported that levels of TBBPA in human breast milk (0.06–37.34 ng/g lipid), maternal serum (0.23–93.22 ng/g lipid), and cord blood (2.09–649.45 ng/g lipid) are up to a few nM concentrations [8, 89]. However, TBBPA has been found to be accumulative in biota [90, 91] and the levels of brominated flame retardant are rapidly increasing both in human and environmental samples [13]. Moreover, we are continuously exposed to other environmental contaminants with similar chemical and toxicological properties such as PCBs and dioxins [13]. Some studies also show that environmental contaminants may have an additive or synergistic effects when combined [92, 93]. Additional studies in our laboratory on the effect of chronic exposure of TBBPA at lower concentrations will lead us toward a better understanding of the mechanisms and relevant risks associated with TBBPA exposures in gestational compartments.
4.4. Conclusion
In summary, this is the first study to show that TBBPA, a widely used flame retardant chemical found in human tissues, activates proinflammatory responses in human first trimester trophoblasts. Our results provide evidence of dysregulated production of IL-6, IL-8, TGF-β and PGE2, and increased expression of genes involved in inflammatory pathways stimulated by TBBPA in human placental cells. Because proper regulation of inflammatory mediators in the gestational compartment is necessary for normal placental development and successful pregnancy, further investigation on the impact of TBBPA-stimulated responses on trophoblast function is warranted.
Supplementary Material
Figure 4. Hierarchical clustergram summarizing results from the Innate and Adaptive Immune Responses PCR array.
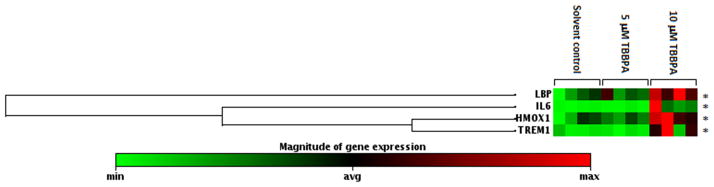
HTR-8/SVneo cells were treated with DMSO (0.1% v/v, solvent control) or TBBPA (5 or 10 μM), and then mRNA was isolated for the Innate and Adaptive Immune Responses PCR array. Each row represents mRNA expression of a particular gene. Each treatment group (DMSO, 5 or 10 μM TBBPA) contains 4 columns, and each column represents an independent experiment on a different day. Each experiment was conducted in triplicate, and extracted RNA from the triplicates were pooled together for the PCR array analysis. *P < 0.05, significantly different from the solvent control with 10 μM TBBPA treatment. n.s., Not significantly different compared to the solvent control.
Highlights.
A brominated flame retardant activated proinflammatory response in placental cells.
TBBPA stimulated cell release of the pro-inflammatory cytokines IL-6 and IL-8.
TBBPA suppressed cell release of the anti-inflammatory cytokine TGF-β.
TBBPA enhanced cyclooxygenase-2 (COX-2) mRNA expression and PGE2 production.
We identified 7 genes differentially expressed with TBBPA exposure.
Acknowledgments
We thank the University of Michigan’s Immunology Core for its assistance with cytokine ELISA analysis. This work was supported by a research grant to RL-C (R01 ES014860), a project in the Superfund Research Program PROTECT Center to RL-C (P42 ES017198), fellowships from an Institutional NRSA to CK and PWK (T32 ES007062), and the Center for Lifestage Exposure and Adult Disease (P30 ES017885) from the National Institute of Environmental Health Sciences (NIEHS), National Institutes of Health (NIH).
Footnotes
Conflict of interest statement
The authors declare that there are no conflicts of interest.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIEHS or NIH.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Morose G. An Overview of Alternatives to Tetrabromobisphenol A (TBBPA) and Hexabromocyclododecane (HBCD) Lowell Center for Sustainable Production, University of Massachusetts Lowell; 2006. [Google Scholar]
- 2.Kuiper RV, van den Brandhof EJ, Leonards PE, van der Ven LT, Wester PW, Vos JG. Toxicity of tetrabromobisphenol A (TBBPA) in zebrafish (Danio rerio) in a partial life-cycle test. Arch Toxicol. 2007;81:1–9. doi: 10.1007/s00204-006-0117-x. [DOI] [PubMed] [Google Scholar]
- 3.EFSA. Scientific Opinion on Tetrabromobisphenol A (TBBPA) and its derivatives in food. European Food Safety Authority Journal. 2011;9:1–61. [Google Scholar]
- 4.Sjodin A, Carlsson H, Thuresson K, Sjolin S, Bergman A, Ostman C. Flame retardants in indoor air at an electronics recycling plant and at other work environments. Environ Sci Technol. 2001;35:448–54. doi: 10.1021/es000077n. [DOI] [PubMed] [Google Scholar]
- 5.D’Hollander W, Roosens L, Covaci A, Cornelis C, Reynders H, Campenhout KV, et al. Brominated flame retardants and perfluorinated compounds in indoor dust from homes and offices in Flanders, Belgium. Chemosphere. 2010;81:478–87. doi: 10.1016/j.chemosphere.2010.07.043. [DOI] [PubMed] [Google Scholar]
- 6.Luo XJ, Zhang XL, Chen SJ, Mai BX. Free and bound polybrominated diphenyl ethers and tetrabromobisphenol A in freshwater sediments. Mar Pollut Bull. 2010;60:718–24. doi: 10.1016/j.marpolbul.2009.11.023. [DOI] [PubMed] [Google Scholar]
- 7.Shi ZX, Wu YN, Li JG, Zhao YF, Feng JF. Dietary exposure assessment of Chinese adults and nursing infants to tetrabromobisphenol-A and hexabromocyclododecanes: occurrence measurements in foods and human milk. Environ Sci Technol. 2009;43:4314–9. doi: 10.1021/es8035626. [DOI] [PubMed] [Google Scholar]
- 8.Cariou R, Antignac JP, Zalko D, Berrebi A, Cravedi JP, Maume D, et al. Exposure assessment of French women and their newborns to tetrabromobisphenol-A: occurrence measurements in maternal adipose tissue, serum, breast milk and cord serum. Chemosphere. 2008;73:1036–41. doi: 10.1016/j.chemosphere.2008.07.084. [DOI] [PubMed] [Google Scholar]
- 9.Van der Ven LT, Van de Kuil T, Verhoef A, Verwer CM, Lilienthal H, Leonards PE, et al. Endocrine effects of tetrabromobisphenol-A (TBBPA) in Wistar rats as tested in a one-generation reproduction study and a subacute toxicity study. Toxicology. 2008;245:76–89. doi: 10.1016/j.tox.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 10.Washington State Department of Ecology; Ecology WSDo, editor. Summary of Technical Background Information for the Proposed PBT List. 2005. [Google Scholar]
- 11.Washington State Department of Health; Act WSDoHftCsSP, editor. Chemicals of High Concern to Children. 2011. [Google Scholar]
- 12.Saegusa Y, Fujimoto H, Woo GH, Ohishi T, Wang L, Mitsumori K, et al. Transient aberration of neuronal development in the hippocampal dentate gyrus after developmental exposure to brominated flame retardants in rats. Arch Toxicol. 2012;86:1431–42. doi: 10.1007/s00204-012-0824-4. [DOI] [PubMed] [Google Scholar]
- 13.Reistad T, Mariussen E, Fonnum F. The effect of a brominated flame retardant, tetrabromobisphenol-A, on free radical formation in human neutrophil granulocytes: the involvement of the MAP kinase pathway and protein kinase C. Toxicol Sci. 2005;83:89–100. doi: 10.1093/toxsci/kfh298. [DOI] [PubMed] [Google Scholar]
- 14.Kitamura S, Jinno N, Ohta S, Kuroki H, Fujimoto N. Thyroid hormonal activity of the flame retardants tetrabromobisphenol A and tetrachlorobisphenol A. Biochem Biophys Res Commun. 2002;293:554–9. doi: 10.1016/S0006-291X(02)00262-0. [DOI] [PubMed] [Google Scholar]
- 15.Fukuda N, Ito Y, Yamaguchi M, Mitumori K, Koizumi M, Hasegawa R, et al. Unexpected nephrotoxicity induced by tetrabromobisphenol A in newborn rats. Toxicol Lett. 2004;150:145–55. doi: 10.1016/j.toxlet.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 16.Szymanska JA, Piotrowski JK, Frydrych B. Hepatotoxicity of tetrabromobisphenol-A: effects of repeated dosage in rats. Toxicology. 2000;142:87–95. doi: 10.1016/s0300-483x(99)00108-0. [DOI] [PubMed] [Google Scholar]
- 17.WHO; Organization) IPoCSWH, editor. Environmental Health Criteria 172: Tetrabromobisphenol A and Derivatives. Geneva, Switzerland: 1995. [Google Scholar]
- 18.Orsi NM. Cytokine networks in the establishment and maintenance of pregnancy. Hum Fertil (Camb) 2008;11:222–30. doi: 10.1080/14647270802206879. [DOI] [PubMed] [Google Scholar]
- 19.Bowen JM, Chamley L, Keelan JA, Mitchell MD. Cytokines of the placenta and extra-placental membranes: roles and regulation during human pregnancy and parturition. Placenta. 2002;23:257–73. doi: 10.1053/plac.2001.0782. [DOI] [PubMed] [Google Scholar]
- 20.Goldenberg RL, Andrews WW. Intrauterine infection and why preterm prevention programs have failed. Am J Public Health. 1996;86:781–3. doi: 10.2105/ajph.86.6.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mitchell MD, Romero RJ, Edwin SS, Trautman MS. Prostaglandins and parturition. Reprod Fertil Dev. 1995;7:623–32. doi: 10.1071/rd9950623. [DOI] [PubMed] [Google Scholar]
- 22.Gibb W. The role of prostaglandins in human parturition. Ann Med. 1998;30:235–41. doi: 10.3109/07853899809005850. [DOI] [PubMed] [Google Scholar]
- 23.Kniss DA. Cyclooxygenases in reproductive medicine and biology. Journal of the Society for Gynecologic Investigation. 1999;6:285–92. doi: 10.1016/s1071-5576(99)00034-9. [DOI] [PubMed] [Google Scholar]
- 24.Gomez R, Romero R, Edwin SS, David C. Pathogenesis of preterm labor and preterm premature rupture of membranes associated with intraamniotic infection. Infect Dis Clin North Am. 1997;11:135–76. doi: 10.1016/s0891-5520(05)70347-0. [DOI] [PubMed] [Google Scholar]
- 25.Hansen WR, Keelan JA, Skinner SJ, Mitchell MD. Key enzymes of prostaglandin biosynthesis and metabolism. Coordinate regulation of expression by cytokines in gestational tissues: a review. Prostaglandins Other Lipid Mediat. 1999;57:243–57. doi: 10.1016/s0090-6980(99)00008-8. [DOI] [PubMed] [Google Scholar]
- 26.Khan AH, Carson RJ, Nelson SM. Prostaglandins in labor--a translational approach. Front Biosci. 2008;13:5794–809. doi: 10.2741/3117. [DOI] [PubMed] [Google Scholar]
- 27.Tjoa ML, Oudejans CB, van Vugt JM, Blankenstein MA, van Wijk IJ. Markers for presymptomatic prediction of preeclampsia and intrauterine growth restriction. Hypertens Pregnancy. 2004;23:171–89. doi: 10.1081/PRG-120028292. [DOI] [PubMed] [Google Scholar]
- 28.Orsi NM, Tribe RM. Cytokine networks and the regulation of uterine function in pregnancy and parturition. J Neuroendocrinol. 2008;20:462–9. doi: 10.1111/j.1365-2826.2008.01668.x. [DOI] [PubMed] [Google Scholar]
- 29.Lyall F, Greer IA, Boswell F, Macara LM, Walker JJ, Kingdom JC. The cell adhesion molecule, VCAM-1, is selectively elevated in serum in pre-eclampsia: does this indicate the mechanism of leucocyte activation? Br J Obstet Gynaecol. 1994;101:485–7. doi: 10.1111/j.1471-0528.1994.tb13146.x. [DOI] [PubMed] [Google Scholar]
- 30.Tjoa ML, van Vugt JM, Go AT, Blankenstein MA, Oudejans CB, van Wijk IJ. Elevated C-reactive protein levels during first trimester of pregnancy are indicative of preeclampsia and intrauterine growth restriction. J Reprod Immunol. 2003;59:29–37. doi: 10.1016/s0165-0378(02)00085-2. [DOI] [PubMed] [Google Scholar]
- 31.Vince GS, Starkey PM, Austgulen R, Kwiatkowski D, Redman CW. Interleukin-6, tumour necrosis factor and soluble tumour necrosis factor receptors in women with pre-eclampsia. Br J Obstet Gynaecol. 1995;102:20–5. doi: 10.1111/j.1471-0528.1995.tb09020.x. [DOI] [PubMed] [Google Scholar]
- 32.Cox S, King M, Casey M, MacDonald P. Interleukin-1 beta, -1 alpha, and -6 and prostaglandins in vaginal/cervical fluids of pregnant women before and during labor. J Clin Endocrinol Metab. 1993;77:805–15. doi: 10.1210/jcem.77.3.8370702. [DOI] [PubMed] [Google Scholar]
- 33.Romero R, Espinoza J, Chaiworapongsa T, Kalache K. Infection and prematurity and the role of preventive strategies. Semin Neonatol. 2002;7:259–74. doi: 10.1016/s1084-2756(02)90121-1. [DOI] [PubMed] [Google Scholar]
- 34.Goldenberg RL, Goepfert AR, Ramsey PS. Biochemical markers for the prediction of preterm birth. American journal of obstetrics and gynecology. 2005;192:S36–46. doi: 10.1016/j.ajog.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 35.Leitich H, Bodner-Adler B, Brunbauer M, Kaider A, Egarter C, Husslein P. Bacterial vaginosis as a risk factor for preterm delivery: a meta-analysis. Am J Obstet Gynecol. 2003;189:139–47. doi: 10.1067/mob.2003.339. [DOI] [PubMed] [Google Scholar]
- 36.Flynn CA, Helwig AL, Meurer LN. Bacterial vaginosis in pregnancy and the risk of prematurity: a meta-analysis. J Fam Pract. 1999;48:885–92. [PubMed] [Google Scholar]
- 37.Romero R, Gomez R, Chaiworapongsa T, Conoscenti G, Kim JC, Kim YM. The role of infection in preterm labour and delivery. Paediatr Perinat Epidemiol. 2001;15 (Suppl 2):41–56. doi: 10.1046/j.1365-3016.2001.00007.x. [DOI] [PubMed] [Google Scholar]
- 38.Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371:75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Han EH, Park JH, Kang KW, Jeong TC, Kim HS, Jeong HG. Risk assessment of tetrabromobisphenol A on cyclooxygenase-2 expression via MAP kinase/NF-kappaB/AP-1 signaling pathways in murine macrophages. J Toxicol Environ Health A. 2009;72:1431–8. doi: 10.1080/15287390903212873. [DOI] [PubMed] [Google Scholar]
- 40.Kibakaya EC, Stephen K, Whalen MM. Tetrabromobisphenol A has immunosuppressive effects on human natural killer cells. J Immunotoxicol. 2009;6:285–92. doi: 10.3109/15476910903258260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Graham CH, Hawley TS, Hawley RG, MacDougall JR, Kerbel RS, Khoo N, et al. Establishment and characterization of first trimester human trophoblast cells with extended lifespan. Experimental cell research. 1993;206:204–11. doi: 10.1006/excr.1993.1139. [DOI] [PubMed] [Google Scholar]
- 42.Reistad T, Mariussen E, Ring A, Fonnum F. In vitro toxicity of tetrabromobisphenol-A on cerebellar granule cells: cell death, free radical formation, calcium influx and extracellular glutamate. Toxicological sciences : an official journal of the Society of Toxicology. 2007;96:268–78. doi: 10.1093/toxsci/kfl198. [DOI] [PubMed] [Google Scholar]
- 43.Reistad T, Mariussen E, Fonnum F. The effect of a brominated flame retardant, tetrabromobisphenol-A, on free radical formation in human neutrophil granulocytes: the involvement of the MAP kinase pathway and protein kinase C. Toxicological sciences : an official journal of the Society of Toxicology. 2005;83:89–100. doi: 10.1093/toxsci/kfh298. [DOI] [PubMed] [Google Scholar]
- 44.Hendriks HS, van Kleef RG, van den Berg M, Westerink RH. Multiple novel modes of action involved in the in vitro neurotoxic effects of tetrabromobisphenol-A. Toxicological sciences : an official journal of the Society of Toxicology. 2012;128:235–46. doi: 10.1093/toxsci/kfs136. [DOI] [PubMed] [Google Scholar]
- 45.Smyth GK. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol. 2004;3:Article 3. doi: 10.2202/1544-6115.1027. [DOI] [PubMed] [Google Scholar]
- 46.Rajeevan MS, Vernon SD, Taysavang N, Unger ER. Validation of array-based gene expression profiles by real-time (kinetic) RT-PCR. The Journal of molecular diagnostics : JMD. 2001;3:26–31. doi: 10.1016/S1525-1578(10)60646-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brooks EM, Sheflin LG, Spaulding SW. Secondary structure in the 3′ UTR of EGF and the choice of reverse transcriptases affect the detection of message diversity by RT-PCR. BioTechniques. 1995;19:806–12. 14–5. [PubMed] [Google Scholar]
- 48.Gerard GF, Fox DK, Nathan M, D’Alessio JM. Reverse transcriptase. The use of cloned Moloney murine leukemia virus reverse transcriptase to synthesize DNA from RNA. Molecular biotechnology. 1997;8:61–77. doi: 10.1007/BF02762340. [DOI] [PubMed] [Google Scholar]
- 49.Alaee M, Arias P, Sjodin A, Bergman A. An overview of commercially used brominated flame retardants, their applications, their use patterns in different countries/regions and possible modes of release. Environ Int. 2003;29:683–9. doi: 10.1016/S0160-4120(03)00121-1. [DOI] [PubMed] [Google Scholar]
- 50.Thomsen C, Lundanes E, Becher G. Brominated flame retardants in archived serum samples from Norway: a study on temporal trends and the role of age. Environmental science & technology. 2002;36:1414–8. doi: 10.1021/es0102282. [DOI] [PubMed] [Google Scholar]
- 51.Germain AM, Carvajal J, Sanchez M, Valenzuela GJ, Tsunekawa H, Chuaqui B. Preterm labor: placental pathology and clinical correlation. Obstet Gynecol. 1999;94:284–9. doi: 10.1016/s0029-7844(99)00324-5. [DOI] [PubMed] [Google Scholar]
- 52.Kim YM, Chaiworapongsa T, Gomez R, Bujold E, Yoon BH, Rotmensch S, et al. Failure of physiologic transformation of the spiral arteries in the placental bed in preterm premature rupture of membranes. American journal of obstetrics and gynecology. 2002;187:1137–42. doi: 10.1067/mob.2002.127720. [DOI] [PubMed] [Google Scholar]
- 53.Ness RB, Sibai BM. Shared and disparate components of the pathophysiologies of fetal growth restriction and preeclampsia. American journal of obstetrics and gynecology. 2006;195:40–9. doi: 10.1016/j.ajog.2005.07.049. [DOI] [PubMed] [Google Scholar]
- 54.Riewe SD, Mans JJ, Hirano T, Katz J, Shiverick KT, Brown TA, et al. Human trophoblast responses to Porphyromonas gingivalis infection. Molecular oral microbiology. 2010;25:252–9. doi: 10.1111/j.2041-1014.2010.00573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gomez LM, Ma Y, Ho C, McGrath CM, Nelson DB, Parry S. Placental infection with human papillomavirus is associated with spontaneous preterm delivery. Hum Reprod. 2008;23:709–15. doi: 10.1093/humrep/dem404. [DOI] [PubMed] [Google Scholar]
- 56.Goepfert AR, Goldenberg RL, Andrews WW, Hauth JC, Mercer B, Iams J, et al. The Preterm Prediction Study: association between cervical interleukin 6 concentration and spontaneous preterm birth. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Am J Obstet Gynecol. 2001;184:483–8. doi: 10.1067/mob.2001.109653. [DOI] [PubMed] [Google Scholar]
- 57.Wenstrom KD, Andrews WW, Tamura T, DuBard MB, Johnston KE, Hemstreet GP. Elevated amniotic fluid interleukin-6 levels at genetic amniocentesis predict subsequent pregnancy loss. Am J Obstet Gynecol. 1996;175:830–3. doi: 10.1016/s0002-9378(96)80007-x. [DOI] [PubMed] [Google Scholar]
- 58.Dortbudak O, Eberhardt R, Ulm M, Persson GR. Periodontitis, a marker of risk in pregnancy for preterm birth. J Clin Periodontol. 2005;32:45–52. doi: 10.1111/j.1600-051X.2004.00630.x. [DOI] [PubMed] [Google Scholar]
- 59.Chegini N, Ma C, Davis J, Duff P, Rosa C. Differential expression of transforming growth factor-beta 1 and transforming growth factor-beta receptors in myometrium of women with failed induction of labor, no labor, and preterm labor. J Soc Gynecol Investig. 1999;6:258–63. doi: 10.1016/s1071-5576(99)00029-5. [DOI] [PubMed] [Google Scholar]
- 60.Mijovic JE, Zakar T, Nairn TK, Olson DM. Prostaglandin endoperoxide H synthase (PGHS) activity and PGHS-1 and -2 messenger ribonucleic acid abundance in human chorion throughout gestation and with preterm labor. J Clin Endocrinol Metab. 1998;83:1358–67. doi: 10.1210/jcem.83.4.4692. [DOI] [PubMed] [Google Scholar]
- 61.Anton L, Brown AG, Parry S, Elovitz MA. Lipopolysaccharide induces cytokine production and decreases extravillous trophoblast invasion through a mitogen-activated protein kinase-mediated pathway: possible mechanisms of first trimester placental dysfunction. Human reproduction. 2012;27:61–72. doi: 10.1093/humrep/der362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim YM, Bujold E, Chaiworapongsa T, Gomez R, Yoon BH, Thaler HT, et al. Failure of physiologic transformation of the spiral arteries in patients with preterm labor and intact membranes. American journal of obstetrics and gynecology. 2003;189:1063–9. doi: 10.1067/s0002-9378(03)00838-x. [DOI] [PubMed] [Google Scholar]
- 63.Brosens I, Robertson WB, Dixon HG. The physiological response of the vessels of the placental bed to normal pregnancy. The Journal of pathology and bacteriology. 1967;93:569–79. doi: 10.1002/path.1700930218. [DOI] [PubMed] [Google Scholar]
- 64.Pijnenborg R, Bland JM, Robertson WB, Brosens I. Uteroplacental arterial changes related to interstitial trophoblast migration in early human pregnancy. Placenta. 1983;4:397–413. doi: 10.1016/s0143-4004(83)80043-5. [DOI] [PubMed] [Google Scholar]
- 65.Pijnenborg R, Dixon G, Robertson WB, Brosens I. Trophoblastic invasion of human decidua from 8 to 18 weeks of pregnancy. Placenta. 1980;1:3–19. doi: 10.1016/s0143-4004(80)80012-9. [DOI] [PubMed] [Google Scholar]
- 66.Zhou Y, Damsky CH, Fisher SJ. Preeclampsia is associated with failure of human cytotrophoblasts to mimic a vascular adhesion phenotype. One cause of defective endovascular invasion in this syndrome? The Journal of clinical investigation. 1997;99:2152–64. doi: 10.1172/JCI119388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhou Y, Fisher SJ, Janatpour M, Genbacev O, Dejana E, Wheelock M, et al. Human cytotrophoblasts adopt a vascular phenotype as they differentiate. A strategy for successful endovascular invasion? The Journal of clinical investigation. 1997;99:2139–51. doi: 10.1172/JCI119387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jovanovic M, Vicovac L. Interleukin-6 stimulates cell migration, invasion and integrin expression in HTR-8/SVneo cell line. Placenta. 2009;30:320–8. doi: 10.1016/j.placenta.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 69.Jovanovic M, Stefanoska I, Radojcic L, Vicovac L. Interleukin-8 (CXCL8) stimulates trophoblast cell migration and invasion by increasing levels of matrix metalloproteinase (MMP)2 and MMP9 and integrins alpha5 and beta1. Reproduction. 2010;139:789–98. doi: 10.1530/REP-09-0341. [DOI] [PubMed] [Google Scholar]
- 70.Dubinsky V, Poehlmann TG, Suman P, Gentile T, Markert UR, Gutierrez G. Role of regulatory and angiogenic cytokines in invasion of trophoblastic cells. Am J Reprod Immunol. 2010;63:193–9. doi: 10.1111/j.1600-0897.2009.00778.x. [DOI] [PubMed] [Google Scholar]
- 71.Graham CH, Lala PK. Mechanisms of placental invasion of the uterus and their control. Biochem Cell Biol. 1992;70:867–74. doi: 10.1139/o92-135. [DOI] [PubMed] [Google Scholar]
- 72.Karmakar S, Das C. Regulation of trophoblast invasion by IL-1beta and TGF-beta1. Am J Reprod Immunol. 2002;48:210–9. doi: 10.1034/j.1600-0897.2002.01151.x. [DOI] [PubMed] [Google Scholar]
- 73.Horita H, Kuroda E, Hachisuga T, Kashimura M, Yamashita U. Induction of prostaglandin E2 production by leukemia inhibitory factor promotes migration of first trimester extravillous trophoblast cell line, HTR-8/SVneo. Human reproduction. 2007;22:1801–9. doi: 10.1093/humrep/dem125. [DOI] [PubMed] [Google Scholar]
- 74.Nicola C, Timoshenko AV, Dixon SJ, Lala PK, Chakraborty C. EP1 receptor-mediated migration of the first trimester human extravillous trophoblast: the role of intracellular calcium and calpain. J Clin Endocrinol Metab. 2005;90:4736–46. doi: 10.1210/jc.2005-0413. [DOI] [PubMed] [Google Scholar]
- 75.Biondi C, Ferretti ME, Pavan B, Lunghi L, Gravina B, Nicoloso MS, et al. Prostaglandin E2 inhibits proliferation and migration of HTR-8/SVneo cells, a human trophoblast-derived cell line. Placenta. 2006;27:592–601. doi: 10.1016/j.placenta.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 76.Kusanovic JP, Romero R, Chaiworapongsa T, Mittal P, Mazaki-Tovi S, Vaisbuch E, et al. Amniotic fluid sTREM-1 in normal pregnancy, spontaneous parturition at term and preterm, and intra-amniotic infection/inflammation. J Matern Fetal Neonatal Med. 2010;23:34–47. doi: 10.3109/14767050903009248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Youssef RE, Ledingham MA, Bollapragada SS, O’Gorman N, Jordan F, Young A, et al. The role of toll-like receptors (TLR-2 and -4) and triggering receptor expressed on myeloid cells 1 (TREM-1) in human term and preterm labor. Reprod Sci. 2009;16:843–56. doi: 10.1177/1933719109336621. [DOI] [PubMed] [Google Scholar]
- 78.Roos T, Martin TR, Ruzinski JT, Leturcq DJ, Hillier SL, Patton DL, et al. Lipopolysaccharide binding protein and soluble CD14 receptor protein in amniotic fluid and cord blood in patients at term. Am J Obstet Gynecol. 1997;177:1230–7. doi: 10.1016/s0002-9378(97)70044-9. [DOI] [PubMed] [Google Scholar]
- 79.Bouchon A, Dietrich J, Colonna M. Cutting edge: inflammatory responses can be triggered by TREM-1, a novel receptor expressed on neutrophils and monocytes. J Immunol. 2000;164:4991–5. doi: 10.4049/jimmunol.164.10.4991. [DOI] [PubMed] [Google Scholar]
- 80.Bouchon A, Facchetti F, Weigand MA, Colonna M. TREM-1 amplifies inflammation and is a crucial mediator of septic shock. Nature. 2001;410:1103–7. doi: 10.1038/35074114. [DOI] [PubMed] [Google Scholar]
- 81.Fenton MJ, Golenbock DT. LPS-binding proteins and receptors. J Leukoc Biol. 1998;64:25–32. doi: 10.1002/jlb.64.1.25. [DOI] [PubMed] [Google Scholar]
- 82.Gutsmann T, Muller M, Carroll SF, MacKenzie RC, Wiese A, Seydel U. Dual role of lipopolysaccharide (LPS)-binding protein in neutralization of LPS and enhancement of LPS-induced activation of mononuclear cells. Infect Immun. 2001;69:6942–50. doi: 10.1128/IAI.69.11.6942-6950.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ahmed A, Rahman M, Zhang X, Acevedo CH, Nijjar S, Rushton I, et al. Induction of placental heme oxygenase-1 is protective against TNFalpha-induced cytotoxicity and promotes vessel relaxation. Mol Med. 2000;6:391–409. [PMC free article] [PubMed] [Google Scholar]
- 84.Zenclussen AC, Lim E, Knoeller S, Knackstedt M, Hertwig K, Hagen E, et al. Heme oxygenases in pregnancy II: HO-2 is downregulated in human pathologic pregnancies. American journal of reproductive immunology. 2003;50:66–76. doi: 10.1034/j.1600-0897.2003.00047.x. [DOI] [PubMed] [Google Scholar]
- 85.Eide IP, Isaksen CV, Salvesen KA, Langaas M, Schonberg SA, Austgulen R. Decidual expression and maternal serum levels of heme oxygenase 1 are increased in pre-eclampsia. Acta Obstet Gynecol Scand. 2008;87:272–9. doi: 10.1080/00016340701763015. [DOI] [PubMed] [Google Scholar]
- 86.Khan GA, Girish GV, Lala N, Di Guglielmo GM, Lala PK. Decorin is a novel VEGFR-2-binding antagonist for the human extravillous trophoblast. Mol Endocrinol. 2011;25:1431–43. doi: 10.1210/me.2010-0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bilban M, Tauber S, Haslinger P, Pollheimer J, Saleh L, Pehamberger H, et al. Trophoblast invasion: assessment of cellular models using gene expression signatures. Placenta. 2010;31:989–96. doi: 10.1016/j.placenta.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 88.Novakovic B, Gordon L, Wong NC, Moffett A, Manuelpillai U, Craig JM, et al. Wide-ranging DNA methylation differences of primary trophoblast cell populations and derived cell lines: implications and opportunities for understanding trophoblast function. Mol Hum Reprod. 2011;17:344–53. doi: 10.1093/molehr/gar005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Thomsen C, Lundanes E, Becher G. Brominated flame retardants in plasma samples from three different occupational groups in Norway. J Environ Monit. 2001;3:366–70. doi: 10.1039/b104304h. [DOI] [PubMed] [Google Scholar]
- 90.Berger U, Herzke D, Sandanger TM. Two trace analytical methods for determination of hydroxylated PCBs and other halogenated phenolic compounds in eggs from Norwegian birds of prey. Anal Chem. 2004;76:441–52. doi: 10.1021/ac0348672. [DOI] [PubMed] [Google Scholar]
- 91.Sun Y, Guo H, Yu H, Wang X, Wu J, Xue Y. Bioaccumulation and physiological effects of tetrabromobisphenol A in coontail Ceratophyllum demersum L. Chemosphere. 2008;70:1787–95. doi: 10.1016/j.chemosphere.2007.08.033. [DOI] [PubMed] [Google Scholar]
- 92.Fischer C, Fredriksson A, Eriksson P. Neonatal co-exposure to low doses of an ortho-PCB (PCB 153) and methyl mercury exacerbate defective developmental neurobehavior in mice. Toxicology. 2008;244:157–65. doi: 10.1016/j.tox.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 93.Bemis JC, Seegal RF. Polychlorinated biphenyls and methylmercury act synergistically to reduce rat brain dopamine content in vitro. Environ Health Perspect. 1999;107:879–85. doi: 10.1289/ehp.99107879. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.