Abstract
Break-apart fluorescence in situ hybridization (FISH) is the gold standard test for anaplastic lymphoma kinase (ALK) gene rearrangement. However, this methodology often is assumed to be expensive and potentially cost-prohibitive given the low prevalence of ALK-positive non-small cell lung cancer (NSCLC) cases. To more accurately estimate the cost of ALK testing by FISH, we developed a micro-cost model that accounts for all cost elements of the assay, including laboratory reagents, supplies, capital equipment, technical and pathologist labor, and the acquisition cost of the commercial test and associated reagent kits and controls. By applying a set of real-world base-case parameter values, we determined that the cost of a single ALK break-apart FISH test result is $278.01. Sensitivity analysis on the parameters of batch size, testing efficiency, and the cost of the commercial diagnostic testing products revealed that the cost per result is highly sensitive to batch size, but much less so to efficiency or product cost. This implies that ALK testing by FISH will be most cost effective when performed in high-volume centers. Our results indicate that testing cost may not be the primary determinant of crizotinib (Xalkori®) treatment cost effectiveness, and suggest that testing cost is an insufficient reason to limit the use of FISH testing for ALK rearrangement.
Keywords: micro-cost, ALK rearrangement, FISH, cost effectiveness, crizotinib, companion diagnostic
Introduction
Lung cancer is estimated to be the most common cancer worldwide, and is also a leading cause of cancer-related mortality globally, causing an estimated 1.4 million deaths worldwide in 2008.1 Lung cancer is broadly classified into small cell and non-small cell lung cancer (NSCLC), the latter accounting for about 85–90% of the total lung cancer cases. The histopathology of NSCLC is heterogeneous, and the most common subtypes are adenocarcinoma, squamous cell carcinoma, and large cell carcinoma.2
NSCLC is often diagnosed at an advanced stage with a poor prognosis, and for many years, platinum-based chemotherapy has been the mainstay option for treatment of advanced disease. Notably, in the last decade, much progress has been made in delineating different molecular pathways that contribute to pathogenesis of lung cancer.3–5 Targeted therapies that block activation of such molecular pathways, including bevacizumab and gefitinib, have offered significant clinical benefit in combination with first-line chemotherapy.
Genetic aberrations in anaplastic lymphoma kinase (ALK) have been shown to drive tumor formation.6,7 Abnormal activation of the ALK gene is associated with several malignancies.8,9 In ALK-driven NSCLC, ALK is rearranged to fuse with one of several partners to produce a chimeric protein with a constitutively activated tyrosine kinase domain that drives tumor development.7 The incidence of ALK rearrangement in NSCLC varies from 1.4–11.6% as determined by a number of studies conducted in patient cohorts with different ethnic back-grounds.10 While echinoderm microtubule-associated protein-like-4 (EML4) is the predominant fusion partner in NSCLC, other fusion genes such as KIF5B-ALK, KLC1-ALK, TFG-ALK, and HIP1-ALK also have been identified.
Characterization of the role of the ALK-EML4 rearrangement in NSCLC led to clinical testing of crizotinib, a drug with dual inhibitory activity against MET and ALK tyrosine kinase, as well as development of reliable assays for detection of ALK gene rearrangement.11–13 NSCLC patients with ALK rearrangement-positive tumors showed substantial clinical benefit from crizotinib in Phase I/II clinical trials, with an overall response rate of about 50–60%.14 Based on these results, crizotinib received accelerated approval by the US FDA in 2011. Recent crizotinib Phase III trial results showed that patients treated with crizotinib as a second-line therapy demonstrated progression-free survival of 7.7 months vs 3 months relative to chemotherapy alone, as well as improved quality of life; interim analysis, however, did not show any benefit in overall survival.15 Simultaneously with FDA approval of crizotinib, a commercial ALK break-apart fluorescence in situ hybridization (FISH) test (Abbott Molecular) secured FDA approval as a companion diagnostic for the drug. The ALK break-apart FISH assay remains the gold standard to identify patients who are likely to respond to crizotinib treatment.14,16,17
Several other modalities for detecting ALK rearrangement in NSCLC have been explored.18,19 Early studies evaluating immunohistochemistry (IHC) for detection of the ALK fusion protein in NSCLC used an anti-ALK antibody (ALK1) developed to detect ALK fusion proteins in lymphomas, without great success.20,21 However, application of newer antibodies such as D5F3 (Ventana) and 5A4 (Abcam, Cambridge, UK) and more sensitive IHC methodologies have yielded improved results.22–25 D5F3 is labeled for in vitro diagnostic use in the EU and China, but regulatory approval was gained based on concordance with ALK testing by FISH. The direct correlation of D5F3 staining with crizotinib response has not been established (Package Insert, Ventana anti-ALK [D5F3] Rabbit Monoclonal Primary Antibody). The US FDA has not yet approved any anti-ALK antibody for use in predicting crizotinib response. Even with improved antibodies, remaining challenges concerning standardization of IHC protocols and scoring systems have prevented IHC from gaining widespread acceptance as a primary clinical test for ALK rearrangement.19,26,27
Clinical practice guidelines acknowledge variations in the prevalence of ALK rearrangement by patient age, smoking history, and tumor histopathology. Nevertheless, these guidelines generally recommend patient selection for ALK testing only on the basis of histopathology.17 Beyond this primary selection criterion, a number of publications have described potential testing algorithms intended to address the low frequency of ALK rearrangements in NSCLC. Several investigators have noted that ALK rearrangements are very infrequent in tumors with EGFR or KRAS mutations and have suggested foregoing ALK testing in such tumors.28–30 Others have suggested a reflex testing strategy using IHC as a primary test for ALK rearrangement and reflexing positive specimens for confirmation by FISH testing.27,31,32 However, none of these enrichment strategies perfectly predict the absence of ALK rearrangement; therefore, each would leave some potential crizotinib responders unidentified. The magnitude of this potential clinical impact has not been characterized systematically through clinical research, although there has been one recent large-scale study addressing this issue.
Cabillic and collaborators describe the largest published series of consecutive, unenriched NSCLC cases (a total of 3244 from two centers) to be tested in parallel for ALK rearrangement with both FISH and IHC assays.33 They found FISH-positive or IHC-positive results in 4.6% of cases, but surprisingly low concordance (53%) between results of the two tests. They concluded that using IHC testing for population enrichment before FISH is not clinically optimal, and that both FISH and IHC testing should be performed on all NSCLC specimens to maximize detection of candidates for crizotinib treatment.
In such a dual-testing algorithm, the cost of FISH testing becomes very important. For example, Atherly and Camidge have calculated that based on a per-test cost for FISH of $1400, the cost of testing alone (ie, excluding the cost of treatment) per quality-adjusted life year (QALY) gained by screening all advanced NSCLC with ALK FISH is $106,707.34 In such a high-cost testing scenario, targeted crizotinib treatment may not be cost effective.
In this report, we describe a bottom-up “micro-cost” analysis of break-apart FISH testing for ALK rearrangement. Our findings indicate that FISH testing may be considerably less costly than commonly assumed, and further, that concentration of testing in expert centers may additionally reduce cost as it increases the quality of results.
Methods
Micro-costing of a commercial ALK break-apart FISH assay
We programmed a detailed model designed to assess the cost of performing FISH testing for ALK rearrangement and to enable sensitivity analysis of various cost inputs and laboratory practices. The model was based on the manufacturer’s recommended procedure for use of the Vysis ALK Break Apart FISH Probe Kit IVD and the associated CE-marked accessory reagent kit and control slides (all from Abbott Molecular). We assumed that all materials were used in the quantities recommended and all procedure steps were performed as specified.
We analyzed the protocol steps, and for each step, we determined all of the required kit components, other reagents, supplies and disposables, laboratory equipment, capital equipment, and labor (both time and staff level). The steps and resources in the analysis were validated by staff at two independent test-performing laboratories, including the cytogenetics department of the Rennes University Hospital (France) and the Cleveland Clinic (USA). The prices for the ALK Break Apart FISH Probe Kit IVD and the associated reagent kit and controls were from the annual supply contract with INCa laboratories in France as of December 2013, and were provided by the manufacturer. The costs of reagents, supplies and disposables, laboratory equipment, and capital equipment were determined from laboratory supply catalogs and other publicly referenced sources. The cost of capital equipment was amortized per assay based on its expected life, overall usage during that period, and the actual in-use time during a test protocol. The types of labor used in each step and their costs were based on actual practice in the Rennes University Hospital cytogenetics department. The model was programmed in Microsoft Excel, and where necessary, US$ were converted to Euro at an exchange rate of 1.25 to 1. Input parameters are summarized in Table 1.
Table 1.
Input parameters for cost analysis of ALK rearrangement testing by FISH.
| PARAMETER | BASE CASE | SENSITIVITY ANALYSIS RANGE |
|---|---|---|
| Batch size | 8 | 1–20 |
| Testing efficiency | 85% | 100%–80% in 5% increments |
| Vysis ALK Break Apart FISH Probe Kit IVD (20 assays) | $1,350 | INCa Contracted Price +100% to −50% in 25% increments |
| Vysis Paraffin Pretreatment IV and Post-Hybridization Wash Buffer Kit (5 batches of 8 slides each) | $200 | |
| Vysis ProbeChek ALK Negative Control Slides (5 slides) | $230 | |
| Vysis ProbeChek ALK Positive Control Slides (5 slides) | $230 | |
| Pathologist (Annual compensation, 40 hr/week) | $145,500 | Not Tested |
| Laboratory Assistant (Annual compensation, 35 hr/week) | $60,000 | |
| Administrative Assistant (Annual compensation, 35 hr/week) | $60,000 |
Batch size was reflected as appropriate in the calculations for reagent quantities, hands-on labor time, and the number of control slides required. Testing efficiency was calculated as the fraction of tested patient specimens for which an informative answer was obtained. The model was constructed with dynamic architecture, allowing for sensitivity analysis of batch size, kit cost, and testing efficiency on per-result cost. Neither the costs of re-testing non-informative FISH assays nor the costs of any required re-biopsies were included.
Base-case analysis
The base-case analysis used the contracted prices to INCa laboratories for the ALK Break Apart FISH Probe Kit IVD, the associated reagent kit, and the control slides. The base-case analysis also used a batch size of eight and an efficiency of 85%, which, respectively, reflect laboratory practice in the Rennes laboratory and the fraction of FISH non-contributive cases identified by Cabillic et al.33
Sensitivity analyses
Sensitivity analyses were performed on the parameters of batch size, testing efficiency, and diagnostic product cost. Using Visual Basic, macros were programmed in the model to perform sequential cost analysis for specified values of each of the above parameters, with the others fixed at the base-case values. For each analysis, fixed values were set at the base-case number, and the parameter to be tested was varied within the ranges in Table 1. We selected value ranges for the sensitivity analyses intended to represent a range of real-world possibilities. Batch sizes between 1 and 20 were tested to simulate costs that might be experienced by very low- and very high-volume laboratories, respectively. Similarly, efficiencies between 80% and 100% were tested to simulate the independently varying but synergistic effects on test yield of sample quality and laboratory proficiency. In the absence of any commercial rationale to the contrary, the diagnostic kit and control slide costs were varied proportionally, and modeled the cost impact of diagnostic product pricing ranging from a 50% discount to a 100% premium to the INCa contract prices.
Results
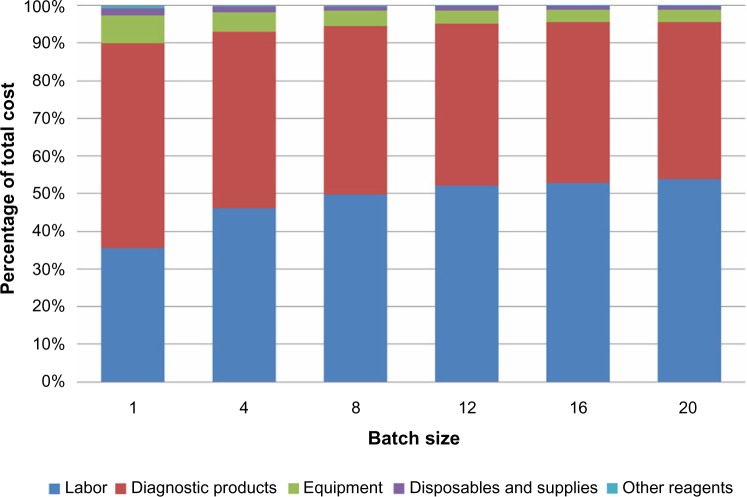
Using the micro-cost model described above, we determined the total testing cost per patient result for ALK rearrangement analysis by break-apart FISH. Using the base-case parameter values (shown in Table 1) as inputs, we determined that the cost of a single ALK break-apart FISH test result is $278.01. The components of this cost are presented in Table 2. In this base-case analysis, labor represents the largest cost, followed by the IVD products. Capital and reusable equipment, disposables and supplies, and other reagents together represent only 5.5% of the total. As shown in Figure 1, the contributions of these cost components to the total change with batch size. When a single specimen is tested, the cost of the diagnostic products dominates at 54.3% of the total, reflecting the need to use a full set of control slides and a certain minimum aliquot of the diagnostic reagent kit. The cost fraction contributed by labor increases with batch size, becoming approximately equivalent to the diagnostic product cost for a batch of four specimens and dominating thereafter, approaching 55% of the total cost per result for a batch of 20.
Table 2.
Component costs of a single test result under base-case parameters.
| COST COMPONENT | AMOUNT | % OF TOTAL |
|---|---|---|
| Labor (all types) | $138.28 | 49.7% |
| Diagnostic Products (test kit, ancillary reagents, controls) | $124.56 | 44.8% |
| Capital and other reusable laboratory equipment | $11.13 | 4.0% |
| Other reagents | $0.59 | 0.2% |
| Disposables and supplies | $3.45 | 1.2% |
| Total | $278.01 | 100% |
Figure 1.
Cost components as a function of batch size.
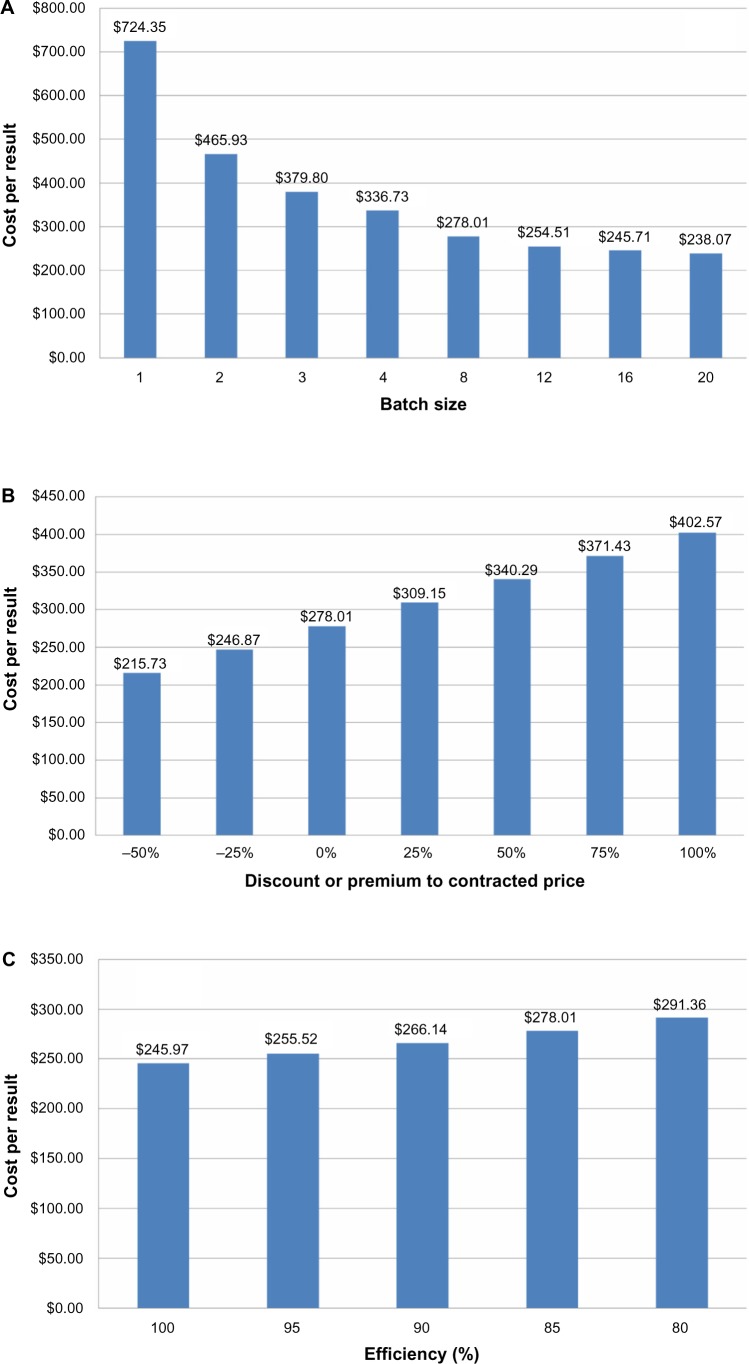
To better understand the cost drivers for ALK testing by FISH, we performed sensitivity testing among the parameters of batch size, testing efficiency, and diagnostic product cost. The results of one-way sensitivity analyses are presented in Figure 2. Per-result cost is highly sensitive to batch size (Fig. 2A), with cost rising most sharply for batches smaller than four. Cost per result is only moderately sensitive to increasing cost of the diagnostic testing products (Fig. 2B) with a direct and approximately linear relationship. Figure 2C shows that cost per result is relatively insensitive to efficiency of testing, with an inverse linear relationship. A two-way sensitivity analysis for batch size and diagnostic product price is presented in Table 3. Testing specimens in a batch of 20 using diagnostic test products at half of the base-case price (−50% relative price) would cost $188 per result, while testing a single specimen (batch of one) at twice the base-case diagnostic product price (+100% relative price) yields a cost per result of $1118.
Figure 2.
One-way sensitivity analyses among the variables of batch size, product cost, and testing efficiency. (A) Per-result cost as a function of batch size, at base-case product cost and efficiency; (B) Per-result cost as a function of product cost, at base-case batch size and efficiency; (C) Per-result cost as a function of efficiency, at base-case product cost and batch size.
Table 3.
Per-result cost at base-case efficiency (85%), as a function of batch size and IVD product cost. IVD product prices are relative to the actual INCa contracted price. Cell colors are scaled from green to red proportionally to the per-test cost. Base-case value is indicated in boldface.
| RELATIVE PRICE | BATCH SIZE | |||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 8 | 12 | 16 | 20 | |
| −50% | $527.58 | $347.70 | $287.74 | $257.76 | $215.73 | $199.76 | $193.24 | $188.16 |
| −25% | $625.96 | $406.82 | $333.77 | $297.24 | $246.87 | $227.14 | $219.48 | $213.11 |
| 0% | $724.35 | $465.93 | $379.80 | $336.73 | $278.01 | $254.51 | $245.71 | $238.07 |
| +25% | $822.73 | $525.05 | $425.83 | $376.21 | $309.15 | $281.89 | $271.94 | $263.03 |
| +50% | $921.11 | $584.17 | $471.86 | $415.70 | $340.29 | $309.27 | $298.17 | $287.98 |
| +75% | $1,019.49 | $643.29 | $517.89 | $455.18 | $371.43 | $336.65 | $324.40 | $312.94 |
| +100% | $1,117.88 | $702.41 | $563.91 | $494.67 | $402.57 | $364.02 | $350.63 | $337.89 |
Discussion
This study uses a micro-costing methodology to determine the cost of identifying ALK rearrangements by break-apart FISH testing using a commercial testing kit labeled for use as a companion diagnostic to crizotinib. With this methodology, we found the cost of a single informative ALK test result to be approximately $278. Performing cost sensitivity analysis on the parameters of batch size, testing efficiency (ie, informative results as a percentage of samples tested), and the cost of the commercial diagnostic testing products revealed that the cost per result is highly sensitive to batch size, but much less so to efficiency or product cost.
Numerous studies examining the performance of enrichment or multi-modal sequential testing algorithms for ALK rearrangement detection assume the unsuitability of FISH testing for primary ALK rearrangement screening. These studies commonly refer to the relatively long turnaround time for FISH testing, the technical difficulty of its performance and interpretation, and its high cost. Generally, however, these studies either do not cite any FISH testing costs or provide a cost estimate without any reference to its source or derivation. In contrast, the cost reported in this study was derived using a bottom-up methodology accounting for all cost elements of the assay, including laboratory reagents, supplies, capital equipment, technical and pathologist labor, and the in-market acquisition cost of the commercial test and associated reagent kits and controls. As such, the cost reported here represents a more accurate depiction of the true costs of ALK testing by FISH than the proxies of billed charges or reimbursed amounts, which can be affected by laboratory billing practices and the budget constraints of payers, respectively.
We are not aware of any previous studies reporting resource-based costing of ALK testing by FISH, though there are several reports of similar analyses for other FISH tests. Barberis et al analyzed the costs of testing for HER2 amplification by FISH using a commercial companion diagnostic testing kit. Using a similar micro-costing approach, they derived a per-test cost for FISH of €177.80 in a batch of 10.35 Baffert et al reported results of micro-costing analysis of FISH used in sarcoma diagnosis. Using prospective and observational data from eight French molecular biology laboratories, they found the mean cost per result of FISH testing to be €318.36 These values range from 20% lower to 43% higher than the cost determined in the current study, a span that is not inconsistent with the combined effects of differences in sample types, specific assay steps, and the skill levels of the respective laboratories.
Recently, both Lee et al and Atherly and Camidge have undertaken health economic analyses of crizotinib therapy for NSCLC directed by companion diagnostic testing. Comparison of their results highlights the importance of test cost in determining overall cost effectiveness. Atherly and Camidge conducted their analysis using billed charges for ALK FISH testing, obtained from a single US university laboratory, of $1400 per test. Using this figure, they found that test cost would dominate over therapy cost at ALK rearrangement frequency rates below approximately 3% to 5% in the screened population. They further calculated that at a marker frequency of 1% and a test cost of $1400, a cost effectiveness threshold of $100,000 per QALY could not be achieved at any drug price.34
In contrast, Lee et al selected the French reimbursement tariff for FISH as the testing cost input for their analysis. At €140 per test ($175 per test at the exchange rate used in this analysis), this amount was one-eighth of the per-test cost used by Atherly and Camidge. Lee et al found in their analysis that overall cost effectiveness of crizotinib treatment was not especially sensitive to test cost and only moderately sensitive to biomarker prevalence, instead responding more acutely to the specificity of the testing strategy and the therapy cost.37
Neither Atherly and Camidge nor Lee et al used the actual cost of FISH testing in their health economic analyses, opting instead for billed charges or a reimbursed amount, respectively. Because of the commercial realities of reimbursement systems, billed charges almost always exceed both reimbursed amounts and actual costs. Therefore, they tend to be poor proxies for health resource costs from either the payer or societal perspectives. Accordingly, we note that the $1400 billed charge amount used by Atherly and Camidge considerably exceeds even the $1118 cost per result calculated by our model for the most unfavorable cost scenario, a batch of one specimen and a diagnostic kit price of twice the actual level. Conversely, using the actual cost of ALK testing by FISH instead of the reimbursed amount to estimate crizotinib cost effectiveness may understate the true cost effectiveness impact of the test. This is because the amount reimbursed for a test often must exceed actual costs in order to provide enough economic incentive to ensure availability. Nevertheless, in situations where the actual cost exceeds the available reimbursement, as may be the case with ALK testing by FISH in France, it is preferable to use actual costs because the economics of reimbursements lower than costs are unsustainable over time.
Our analysis attempts to account for all of the cost inputs for the FISH assay, but nevertheless has several remaining limitations. We developed our labor analysis with input from laboratory personnel well-experienced in performing and interpreting FISH tests. However, less-experienced personnel likely would have specified more time for many steps, resulting in a higher cost per result. In their analysis, Baffert et al reported such a relationship of experience with test cost. Other types of laboratory staff could not adequately perform many tasks performed by the pathologist in our model, but tasks that our model assigns to other staff could be performed by the pathologist. In some under-staffed laboratories, the pathologist may actually perform some or all of these other tasks, which also would lead to a higher cost per result. We calculated that if the pathologist performed all of the assay work identified in our model, using the other base-case inputs the labor cost would increase by approximately a factor of two and the total cost per result by 47%. The actual costs of equipment, supplies, disposables, and reagents may vary from laboratory to laboratory based on contracting for these items, and therefore be different from the costs reflected in our model. However, any positive or negative impact would be minimal, since the aggregate contribution of all such cost elements was found to be less than 6% of the total cost per result. Finally, we did not factor in any costs for re-testing non-informative samples or, if necessary, obtaining a second biopsy. We considered such costs to be dependent on non-laboratory, case-specific factors such as the amount and quality of any remaining fixed tissue, and patient availability for a second biopsy, and therefore difficult to reliably account for in the model.
The results of our sensitivity analyses have several implications for the optimization of ALK rearrangement testing by FISH. We determined that the per-result cost of FISH testing is most sensitive to batch size, with cost per result increasing sharply below a batch size of three to four. This implies that ALK testing by FISH will be most cost effective when performed in high-volume centers where sample flow is sufficient to achieve clinically acceptable turnaround time with at least this batch size. We note that a higher volume of FISH testing also has been reported to correlate with increased quality. Thunnissen et al observed that FISH testing is most reliable when performed and interpreted by well-trained, experienced personnel, and Bartlett et al recommend a minimum of 150 FISH analyses per year to ensure quality in HER2 FISH analysis.32,38 Therefore, performing FISH testing for ALK rearrangement in high-volume centralized laboratories aligns cost minimization with optimized results. This centralized approach already is the operating norm within INCa in France,39,40 and given the availability of rapid intra-country shipping in most developed countries, should be feasible elsewhere as well.
Cabillic et al found that approximately 15% of FISH tests were non-informative in their series of 3244 cases (ie, a testing efficiency of ~85%). They attributed this finding primarily to the lack of standardization of pre-analytical parameters for tissue samples obtained from a wide range of referring sources.33 Our sensitivity analysis of testing efficiency revealed that it has an inverse linear relationship with cost per result, with the result that even improving efficiency to 100% would only reduce the cost per result by approximately 12%. Sample variability within the range of current typical quality is therefore not a primary driver of FISH testing cost. Although efforts to improve the level and consistency of sample preparation quality are nevertheless worthwhile, the cost effectiveness of ALK testing by FISH does not depend on them.
Targeted drug therapy guided by companion diagnostic testing can provide both clinical and economic benefits. Clinical benefits have been well-documented for a variety of targeted therapies, including crizotinib, and can include improved patient outcomes and quality of life and reduction in the incidence of side effects and adverse events.14,41–43 The economic benefits of targeting therapy with companion diagnostics may include avoidance of the costs of ineffective treatment and the quality-adjusted value of additional lifespan gained. Two key factors for realizing economic benefit from targeted therapy are the accuracy of the testing and the cost of the testing itself.44 The economic impact of testing takes on particular importance in crizotinib treatment for ALK-positive NSCLC, where there is low prevalence of patients with the ALK rearrangement and a high cost of the drug therapy directed by the testing result.
Ultimately, the clinical necessity of performing parallel testing for ALK rearrangement with both FISH and IHC, as reported in the Cabillic study, must be determined by assessing clinical response to crizotinib in a large number of patients identified as ALK-positive through the dual-testing strategy. Nevertheless, the results of our micro-cost analysis of ALK FISH testing indicate that testing cost may not be the primary determinant of crizotinib treatment cost effectiveness, and suggest that testing cost is an insufficient reason to limit the use of FISH testing, with the attendant risk of missing NSCLC patients who might benefit from crizotinib therapy.
Footnotes
Author Contributions
Conceived and designed the experiments: DP. Analyzed the data: DP, MABR. Wrote the first draft of the manuscript: DP. Agree with manuscript results and conclusions: DP, MABR. Developed the structure and arguments for the paper: DP. Made critical revisions and approved final version: MABR. Both authors reviewed and approved of the final manuscript.
ACADEMIC EDITOR: William CS Cho, Editor in Chief
FUNDING: This work was supported by Abbott Molecular, Inc. under a contract to Precision for Medicine, Inc. Abbott Molecular provided contracted pricing of the diagnostic test kit products as a data input to the model, but the authors confirm the funder otherwise had no influence over the study design, content of the article, or selection of this journal.
COMPETING INTERESTS: DP is an employee of Precision for Medicine, Inc., which completed this work under a contract with Abbott Molecular, Inc. He has no personal conflicts of interest related to this work. MABR discloses consultation fees from Abbott Molecular during the conduct of the study, and personal fees from Abbott Molecular outside the work presented here, for lectures and communications during Abbott POLARIS workshops.
Paper subject to independent expert blind peer review by minimum of two reviewers. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
REFERENCES
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Travis WD, Colby TV, Corrin B, et al. Histological Typing of Lung and Pleural Tumours. 3rd ed. Berlin: Springer-Verlag; 1999. [Google Scholar]
- 3.Herbst RS, Heymach JV, Lippman SM. Lung cancer. N Engl J Med. 2008;359:1367–80. doi: 10.1056/NEJMra0802714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kulesza P, Ramchandran K, Patel JD. Emerging concepts in the pathology and molecular biology of advanced non-small cell lung cancer. Am J Clin Pathol. 2011;136:228–38. doi: 10.1309/AJCPO66OIRULFNLZ. [DOI] [PubMed] [Google Scholar]
- 5.Reungwetwattana T, Weroha SJ, Molina JR. Oncogenic pathways, molecularly targeted therapies, and highlighted clinical trials in non-small-cell lung cancer (NSCLC) Clin Lung Cancer. 2012;13:252–66. doi: 10.1016/j.cllc.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 6.Rikova K, Guo A, Zeng Q, et al. Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell. 2007;131:1190–203. doi: 10.1016/j.cell.2007.11.025. [DOI] [PubMed] [Google Scholar]
- 7.Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448:561–6. doi: 10.1038/nature05945. [DOI] [PubMed] [Google Scholar]
- 8.Chiarle R, Voena C, Ambrogio C, Piva R, Inghirami G. The anaplastic lymphoma kinase in the pathogenesis of cancer. Nat Rev Cancer. 2008;8:11–23. doi: 10.1038/nrc2291. [DOI] [PubMed] [Google Scholar]
- 9.Webb TR, Slavish J, George RE, et al. Anaplastic lymphoma kinase: role in cancer pathogenesis and small-molecule inhibitor development for therapy. Expert Rev Anticancer Ther. 2009;9:331–56. doi: 10.1586/14737140.9.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perner S, Wagner PL, Demichelis F, et al. EML4-ALK fusion lung cancer: a rare acquired event. Neoplasia. 2008;10:298–302. doi: 10.1593/neo.07878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gerber DE, Minna JD. ALK inhibition for non-small cell lung cancer: from discovery to therapy in record time. Cancer Cell. 2010;18:548–51. doi: 10.1016/j.ccr.2010.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ou SH. Crizotinib: a novel and first-in-class multitargeted tyrosine kinase inhibitor for the treatment of anaplastic lymphoma kinase rearranged non-small cell lung cancer and beyond. Drug Des Devel Ther. 2011;5:471–85. doi: 10.2147/DDDT.S19045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ou SH, Kwak EL, Siwak-Tapp C, et al. Activity of crizotinib (PF02341066), a dual mesenchymal-epithelial transition (MET) and anaplastic lymphoma kinase (ALK) inhibitor, in a non-small cell lung cancer patient with de novo MET amplification. J Thorac Oncol. 2011;6:942–6. doi: 10.1097/JTO.0b013e31821528d3. [DOI] [PubMed] [Google Scholar]
- 14.Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010;363:1693–703. doi: 10.1056/NEJMoa1006448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013;368:2385–94. doi: 10.1056/NEJMoa1214886. [DOI] [PubMed] [Google Scholar]
- 16.Peters S, Adjei AA, Gridelli C, ESMO Guidelines Working Group et al. Metastatic non-small-cell lung cancer (NSCLC): ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2011;23(suppl 7):vii56–64. doi: 10.1093/annonc/mds226. [DOI] [PubMed] [Google Scholar]
- 17.Lindeman NI, Cagle PT, Beasley MB, et al. Molecular testing guideline for selection of lung cancer patients for EGFR and ALK tyrosine kinase inhibitors: guideline from the College of American Pathologists, International Association for the Study of Lung Cancer, and Association for Molecular Pathology. J Thorac Oncol. 2013;8(7):823–59. doi: 10.1097/JTO.0b013e318290868f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shaw AT, Solomon B, Kenudson MM. Crizotinib and testing for ALK. J Natl Compr Canc Netw. 2011;9:1335–41. doi: 10.6004/jnccn.2011.0115. [DOI] [PubMed] [Google Scholar]
- 19.Weickhardt AJ, Aisner DL, Franklin WA, Varella-Garcia M, Doebele RC, Camidge DR. Diagnostic assays for identification of anaplastic lymphoma kinase-positive non-small cell lung cancer. Cancer. 2013;119:1467–77. doi: 10.1002/cncr.27913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martelli MP, Sozzi G, Hernandez L, et al. EML4-ALK rearrangement in non-small cell lung cancer and non-tumor lung tissues. Am J Pathol. 2009;174:661–70. doi: 10.2353/ajpath.2009.080755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodig SJ, Mino-Kenudson M, Dacic S, et al. Unique clinicopathologic features characterize ALK-rearranged lung adenocarcinoma in the western population. Clin Cancer Res. 2009;15:5216–23. doi: 10.1158/1078-0432.CCR-09-0802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mino-Kenudson M, Chirieac LR, Law K, et al. A novel, highly sensitive antibody allows for the routine detection of ALK-rearranged lung adenocarcinomas by standard immunohistochemistry. Clin Cancer Res. 2010;16:1561–71. doi: 10.1158/1078-0432.CCR-09-2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Y, Pan Y, Wang R, et al. ALK-rearranged lung cancer in Chinese: a comprehensive assessment of clinicopathology, IHC, FISH and RT-PCR. PLoS One. 2013;8:e69016. doi: 10.1371/journal.pone.0069016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takeuchi K, Choi YL, Togashi Y, et al. KIF5B-ALK, a novel fusion oncokinase identified by an immunohistochemistry-based diagnostic system for ALK-positive lung cancer. Clin Cancer Res. 2009;15:3143–9. doi: 10.1158/1078-0432.CCR-08-3248. [DOI] [PubMed] [Google Scholar]
- 25.Conklin CM, Craddock KJ, Have C, et al. Immunohistochemistry is a reliable screening tool for identification of ALK rearrangement in non-small-cell lung carcinoma and is antibody dependent. J Thorac Oncol. 2013;8:45–51. doi: 10.1097/JTO.0b013e318274a83e. [DOI] [PubMed] [Google Scholar]
- 26.Selinger CI, Rogers TM, Russell PA, et al. Testing for ALK rearrangement in lung adenocarcinoma; a multicenter comparison of immunohistochemistry and fluorescent in situ hybridization. Mod Pathol. 2013;26(12):1545–53. doi: 10.1038/modpathol.2013.87. [DOI] [PubMed] [Google Scholar]
- 27.Yi ES, Boland JM, Maleszewski JJ, et al. Correlation of IHC and FISH for ALK gene rearrangement in non-small cell lung carcinoma; IHC score algorithm for FISH. J Thorac Oncol. 2011;6:459–65. doi: 10.1097/JTO.0b013e318209edb9. [DOI] [PubMed] [Google Scholar]
- 28.Kobayashi M, Sakakibara T, Inoue A, et al. An effective enrichment strategy for EML4-ALK fusion gene screening in patients with non-small cell lung cancer. Respir Investig. 2014;52(1):49–56. doi: 10.1016/j.resinv.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 29.Takamochi K, Takeuchi K, Hayashi T, Oh S, Suzuki K. A rational diagnostic algorithm for the identification of ALK rearrangement in lung cancer; a comprehensive study of surgically treated Japanese patients. PLoS One. 2013;8:e69794. doi: 10.1371/journal.pone.0069794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koh Y, Kim DW, Kim TM, et al. Clinicopathologic characteristics and outcomes of patients with anaplastic lymphoma kinase-positive advanced pulmonary adenocarcinoma; suggestion for an effective screening strategy for these tumors. J Thorac Oncol. 2011;6(5):905–12. doi: 10.1097/JTO.0b013e3182111461. [DOI] [PubMed] [Google Scholar]
- 31.Paik JH, Choe G, Kim H, et al. Screening of anaplastic lymphoma kinase rearrangement by immunohistochemistry in non-small cell lung cancer; correlation with fluorescence in situ hybridization. J Thorac Oncol. 2011;6(3):466–72. doi: 10.1097/JTO.0b013e31820b82e8. [DOI] [PubMed] [Google Scholar]
- 32.Thunnissen E, Bubendorf L, Dietel M, et al. EML4-ALK testing in non-small cell carcinomas of the lung; a review with recommendations. Virchows Arch. 2012;461(3):245–57. doi: 10.1007/s00428-012-1281-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cabillic F, Gros A, Dugay F, et al. Parallel FISH and immunohistochemical studies of ALK status in 3244 non-small-cell lung cancers reveal major discor-dances. J Thorac Oncol. 2014;9(3):295–306. doi: 10.1097/JTO.0000000000000072. [DOI] [PubMed] [Google Scholar]
- 34.Atherly AJ, Camidge DR. The cost-effectiveness of screening lung cancer patients for targeted drug sensitivity markers. Br J Cancer. 2012;106:1100–6. doi: 10.1038/bjc.2012.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barberis M, Pellegrini C, Cannone M, Arizzi C, Coggi G, Bosari S. Quantitative PCR and HER2 testing in breast cancer; a technical and cost-effectiveness analysis. Am J Clin Pathol. 2008;129:563–70. doi: 10.1309/1AKQDQ057PQT9AKX. [DOI] [PubMed] [Google Scholar]
- 36.Baffert S, Italiano A, Pierron G, et al. Analyse comparative des coûts de techniques de biologie moléculaire dans le diagnostic de sarcomes. Bull Cancer. 2013;100:963–71. doi: 10.1684/bdc.2013.1822. [DOI] [PubMed] [Google Scholar]
- 37.Lee JA, Bubendorf L, Stahel R, Peters S. Testing for anaplastic lymphoma kinase rearrangement to target crizotinib therapy; oncology, pathology and health economic perspectives. Expert Rev Anticancer Ther. 2013;13:625–36. doi: 10.1586/era.13.42. [DOI] [PubMed] [Google Scholar]
- 38.Bartlett JM, Starczynski J, Atkey N, et al. HER2 testing in the UK; recommendations for breast and gastric in-situ hybridisation methods. J Clin Pathol. 2011;64(8):649–53. doi: 10.1136/jcp.2011.089847. [DOI] [PubMed] [Google Scholar]
- 39.Nowak F, Soria JC, Calvo F. Tumour molecular profiling for deciding therapy-the French initiative. Nat Rev Clin Oncol. 2012;9(8):479–86. doi: 10.1038/nrclinonc.2012.42. [DOI] [PubMed] [Google Scholar]
- 40.Andre F, Nowak F, Arnedos M, Lacroix L, Viens P, Calvo F. Biomarker discovery, development, and implementation in France; a report from the French National Cancer Institute and cooperative groups. Clin Cancer Res. 2012;18(6):1555–60. doi: 10.1158/1078-0432.CCR-11-2201. [DOI] [PubMed] [Google Scholar]
- 41.Piccart-Gebhart MJ, Procter M. Leyland-Jones B, Herceptin Adjuvant (HERA) Trial Study Team, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353(16):1659–72. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 42.Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353(16):1673–84. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 43.Sosman JA, Kim KB, Schuchter L, et al. Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib. N Engl J Med. 2012;366(8):707–14. doi: 10.1056/NEJMoa1112302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Elkin EB, Marshall DA, Kulin NA, et al. Economic evaluation of targeted cancer interventions; critical review and recommendations. Genet Med. 2011;13(10):853–60. doi: 10.1097/GIM.0b013e31821f3e64. [DOI] [PMC free article] [PubMed] [Google Scholar]