Abstract
The objective of the current article was to evaluate 2-[18F]fluoro-2-deoxy-D-glucose 18F-FDG) as measured by positron emission tomography for delineation of abdominopelvic gross tumor volumes (GTV) for stereotactic body radiosurgery treatment (SBRT) of metastatic gynecologic cancers. A retrospective review of SBRT was conducted in 27 women with stage IV gynecologic cancers recurring in para-aortic lymph nodes. Robotic SBRT involved 2400 cGy in 3 consecutive 800 cGy daily fractions prescribed to a 3.0 mm expanded planning tumor volume (PTV) defined by both CT-based and 18F-FDG-based GTVs. In this study, 18F-FDG-based GTVs led to significantly larger PTVs in all 27 women, than if they had been based on CT GTVs alone (P < 0.001). Enlarged PTVs may have resulted from the breathing-induced target motion during the time of 18F-FDG image acquisition smearing 18F-FDG signal over a greater anatomic dimension. Ultimately, SBRT-target local control, based on the RECIST 1.1 criteria, was 96% (26 of 27), and associated with minor reversible toxicity. The use of 18F-FDG to define SBRT target volumes warrants further interrogation in SBRT clinical trials.
Introduction
This article explores the utility of 2-[18F] fluoro-2-deoxy-D-glucose (18F-FDG) for delineation of stereotactic body radiosurgery cancer targets when treating abdominopelvic sites of metastatic gynecologic cancers. Many radiation oncologists have adopted either side-by-side comparisons, image overlays, or direct fusion of positron emission tomography (PET) and computed tomography (CT) images for the purpose of radiation treatment planning. The radioactive tracer 18F-FDG, a sugar analogue taken up and sequestered in cells by the intracellular enzyme hexokinase, helps spot or make more clear cancer targets [1–5]. Treating radiation oncologists often regard 18F-FDG uptake by cancer cells as a marker of cancer cell viability, and subsequently focus radiation dose on ‘18F-FDG-viable’ cancer targets in an effort to improve therapeutic gain. Physician contours for cancer targets, and thus, the intended radiotherapeutic clinical target volumes, are influenced by threshold settings and by respiration-driven motion recorded on fused 18F-FDG PET and CT images [6]. As radiation therapy devices become more accurate and more precise in their delivery of radiation, accurate anatomic and metabolic delineation of cancer targets becomes imperative.
Stereotactic body radiosurgery has an emerging role in the management of persistent or recurrent gynecologic malignancies [7,8]. A frameless, robotic Cyberknife® stereotactic body radiosurgery system (Accuray, Sunnyvale, CA) is but one example of a radiation delivery system where sub-millimeter accuracy and precision of ablative radiation dose can be applied in abdominopelvic recurrences of gynecologic malignancies [9,10]. While early clinical success has been documented with this radiosurgical technique [11,12], contouring guidelines for the use of 18F-FDG PET image overlays paired with anatomical CT images have not been fully explored. This retrospective study offers initial guidelines for radiosurgical target contouring using overlaid 18F-FDG PET and CT images among women with abdominopelvic para-aortic lymph node recurrences of gynecologic cancers.
Methods and Materials
Patients
Institutional review board approval was granted for study prior to review of stereotactic body radiosurgery datasets. All women provided informed consent for stereotactic body radiation. For this article, inclusion criteria were female sex, an age of 18 years or older, and a diagnosis of metastatic stage IV gynecological cancer occurring in an abdominopelvic para-aortic lymph node site. In our stereotactic body radiosurgery program, 27 women met these criteria at retrospective study selection among 74 women treated between 2007 and 2011 [Figure 1]. Table 1 lists patient demographics, gynecologic cancer type, number of sites to be treated by radiosurgery, and administered prior therapies.
Figure 1.
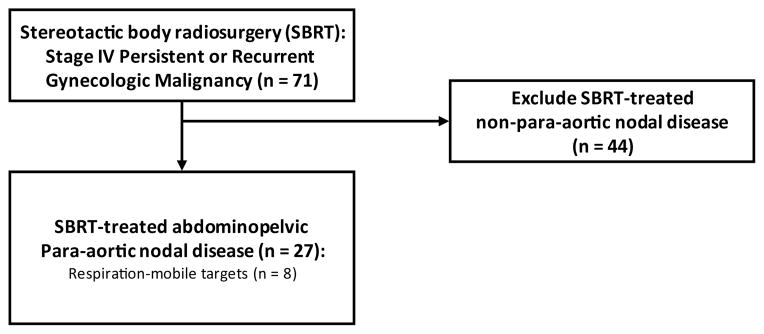
Retrospective study patient selection flow diagram.
Table 1.
Baseline study patient parameters
| Characteristic | |
|---|---|
| Female sex — no. (%) | 27 (100) |
|
| |
| Age — year | |
| Median | 65 |
| Range | 27–80 |
|
| |
| Race — no. (%)* | |
|
| |
| White | 22 (81) |
| Black or African ancestry | 3 (11) |
| Hispanic | 2 (7) |
|
| |
| GOG performance status — no. (%)† | |
|
| |
| 0 | 19 (70) |
| 1 | 5 (19) |
| 2 | 3 (11) |
| No. of metastases to be treated by radiosurgery — no. (%) | |
| 1 | 7 (26) |
| 2 | 8 (30) |
| 3 | 10 (37) |
| 4 | 2 (7) |
| ≥ 4 | 0 (0) |
|
| |
| Histopathology — no. (%) | |
|
| |
| Cervix/vagina squamous cell carcinoma | 4 (15) |
| Endometrial adenocarcinoma | 7 (26) |
| Ovarian adenocarcinoma | 15 (56) |
| Vulvar squamous cell carcinoma | 1 (4) |
|
| |
| Prior radiation — no. (%) | 13 (48) |
|
| |
| Inclusive of radiosurgery sit e | 9 (33) |
| > 5000 cGy delivered pretherapy to radio surgery sit e | 6 (22) |
|
| |
| Prior chemotherapy — no. (%) | 27 (100) |
|
| |
| No. of prior courses of chemotherapy for metastases — no. (%) | |
| 0 | 1 (4) |
| 1 | 17 (63) |
| 2 | 8 (30) |
| 3 | 1 (4) |
| Platinum-containing regimen — no. (%) | 27 (100) |
| Taxane-containing regimen — no. (%) | 21 (78) |
| Anthracycline-containing regimen — no. (%) | 6 (22) |
| Topotecan-containing regimen — no. (%) | 3 (11) |
| Gemcitabine-containing regimen — no. (%) | 5 (19) |
Race was self-reported.
The Gynecologic Oncology Group (GOG) performance status reflects individual daily living activities on a scale of 0 (fully active with symptoms) to 5 (dead).
Stereotactic body radiosurgery 18F-FDG PET and CT simulation
Women either had three or more 1.6 × 3 mm soft tissue gold seed fiducials implanted within 4 cm to 6 cm of the radiosurgical targets under anesthesia or had bony landmarks of the spine identified for radiosurgical targeting [8]. Women were scanned on a flat tabletop either with two-pin knee sponge tabletop registry or with evacuated vacuum-bag immobilization for reproducible body alignment. For target motion due to quiet breathing, women wore a firmly-conforming vest with light-emitting diodes tracked by in-room motion-detecting cameras. If respiration was anticipated clinically to move the target, the Synchrony Motion Tracking system (MTS) was selected and was utilized during delivery of radiation.
Women rested comfortably in a head-first treatment position with their arms across their chest for a mid-thorax to mid-thigh non-contrasted contiguous helical CT high-resolution scan (1 to 1 pitch, voxel 0.98 × 0.98 × 1.0 mm, voltage 120 kVp, 450 mAs; 64-slice SOMATOM Sensation scanner, Siemens Medical Solutions). CT images were acquired during quiet breathing. Afterwards, women had 18F-FDG PET images acquired of the torso from the orbitomeatal line to the upper thighs in the head-first supine treatment position (voxel 4.0 × 4.0 × 4.0 mm; Phillips Gemini TF, Phillips Healthcare). Emission 18F-FDG PET images occurred 60 minutes (range: 55 minutes – 74 minutes) after intravenous administration of a median 11 mCi (range: 10.2 mCi – 14.9 mCi) of 18F-FDG. Oral hydration was provided; post-void images of the pelvis were acquired. Contemporaneously, low dose non contrast CT images were acquired over the torso (5mm intervals, voltage 120 kVp, 60–90 mAs with dose modulation). 18F-FDG PET images were reconstructed with and without CT attenuation correction (AC) following institutional protocol [13]. Importation, digital overlay, and co-registration of high resolution CT and AC 18F-FDG PET images were done by a certified medical physicist in the MultiPlan 3.5.2 Treatment Planning System (Accuray).
Clinical target definitions
CT-based abdominopelvic gross tumor volumes (CT-GTV) was contoured with abdomen window settings (1,200 and 0), and then, agreed by both a treating radiation oncologist and gynecologic oncologist. 18F-FDG PET gross tumor volume (PET-GTV), with a user-defined PET setting threshold of about 40% of the standard uptake value maximum selected on the basis of single standardized uptake value threshold approaches in lung cancer[6], was delineated for each CT-GTV image using co-registered axial, coronal, and sagittal images. Ellipsoid PET-GTV regions of interest were agreed upon after independent review by both the treating radiation oncologist and gynecologic oncologist. Inverse treatment planning was done by a certified medical dosimetrist utilizing typically a 3.0 mm margin encompassing the PET-GTV to create a radiosurgical planning tumor volume (PTV) [12]. Nearby normal tissue structures such as the small bowel, rectum, bladder, liver, kidneys, lungs, bilateral proximal femurs, vagina, and sacral nerve roots were contoured by the radiation oncologist, medical physicist, or certified medical dosimetrist. For this article, volume calculations in cubic centimeters for CT-GTV, PET-GTV and PTV were determined by dosimetric convention. Longest sum diameter of lymph node short axis for radiosurgical targets were recorded following guidelines of RECIST version 1.1 (complete response [CR] = reduction of target lymph node < 10 mm; partial response [PR] = persistence of lymph node(s) equating to non-CR and non-progressive disease). Descriptive and graphical statistics were computed using statistical software (SPSS 18.0, Chicago, IL).
Treatment Planning, Radiation Delivery, and Follow-up
Based on prior clinical experience [11,12], a radiation prescription dose of 800 cGy per fraction totaling 2400 cGy (prescribed to the 70% isodose line) was employed for three consecutive daily fractions of stereotactic body radiosurgery. The Cyberknife 6 MV SBRT linear accelerator was collimated either by using one of 12 fixed tungsten circular collimators or by using a tungsten-copper alloy segmented hexagon IRIS collimator [14]. Treatment parameters are listed in Table 2. Women were seen at one and then every three months after therapy for toxicity assessments. Common toxicity criteria for adverse events (version 4.0) were retrospectively abstracted from self-reported treatment-related toxicity at one- and three-month visits. Restaging CT imaging was obtained at one- and three-months after therapy, and then at investigator discretion.
Table 2.
Stereotactic body radio surgery treatment parameters
| Parameter | |
|---|---|
| Median prescription dose (dose x fraction) | 2400 cGy (800 cGy x 3) |
| Median prescription isodose (25%–75% quartile) | 70% (70% – 75%) |
| Radio surgical tracking | |
| By gold seed fiducials - no. (%) | 16 (59%) |
| By bony spine landmarks - no. (%) | 11 (41%) |
| Radio surgical beam collimation | |
| By fixed collimator - no. (size range) | 13 (10 mm – 50 mm) |
| By IRIS - no. (size range) | 14 (5 mm – 60 mm) |
| Synchrony motion tracking - no. (%) | 8 (30%) |
| Median CT-GTV (25%–75% quartile) | 10.7 cm3 (5.4 cm3 – 19.4 cm3) |
| Median 18F-FDG-GTV (25%–75% quartile) | 25.7 cm3 (12.9 cm3 – 46.6 cm3) |
| Median planning tumor volume (25%–75% quartile) | 52.2 cm3 (23.1 cm3 – 74.6 cm3) |
GTV= gross tumor volume
Results
27 women had stage IV gynecologic cancers with individual or a single conglomerate of multiple para-aortic lymph node metastases measurable on CT and 18F-FDG PET images. Median clinical follow-up was 8 months (25%–75% quartile: 5 months – 13 months). Eight (30%) of 27 women have died from non-radiosurgical target gynecologic cancer disease progression during clinical follow-up.
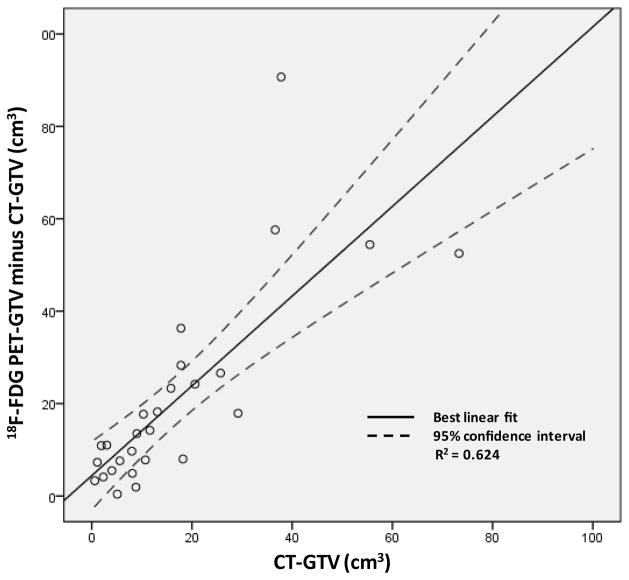
The median pretherapy sum of longest CT-based diameter of radiosurgical targets was 36 cm (25%–75% quartile: 25 cm – 63 cm). The mean pretherapy 18F-FDG PET maximum standard uptake value was 11.9 (standard deviation = 4.1). For 18F-FDG PET acquisitions, the reference mediastinal blood pool standard uptake value ranged between 1.4 and 2.5. Median pretherapy CT-GTV, PET-GTV, and PTV volumes have been listed in Table 2. The PET-GTV overestimated gross target volume in all 27 women. The median increase in gross target volume determined by 18F-FDG PET was 13.5 cm3 (25%–75% quartile: 7.4 cm3 – 23.8 cm3, P < 0.001). A positive linear relationship between the PET-GTV minus CT-GTV versus CT-GTV was found (R2 = 0.624, P < 0.001; Figure 2); PET-GTV minus CT-GTV versus CT-GTV data implicate that PET-GTV doubles the size of the CT-GTV.
Figure 2.
Plotted are differences in 18F-FDG PET-GTV (40% threshold) and CT-GTV contour volumes versus the CT-GTV contour volumes for abdominopelvic para-aortic lymph node targets.
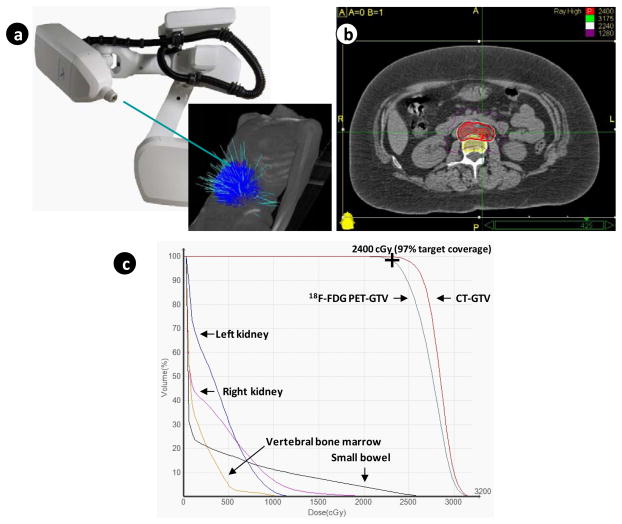
Multiple individual radiation beams were used to deliver stereotactic body radiation, with beams converging on single or multiple closely-associated clinical abdominopelvic cancer targets (Figure 3, Table 2). To provide coverage of the PTV with 2400 cGy, a median isodose line contour of 70% (25%–75% quartile: 70% – 75%) was used as the prescription line. 1- and 3-month radiosurgical target response rates (CR + PR) were 96% (26 of 27) and 96% (26 of 27), respectively. One patient has had stable para-aortic lymph node short-axis dimension throughout her follow-up. Post-therapy 18F-FDG PET images for standard uptake value response were not acquired. Non-target disease progression occurred in 13 (48%) of the 27 women. Within 30 days after radiosurgery treatment, eight (30%) had self-reported grade 2 fatigue after radiosurgery and two (7%) had a grade 2 increase of 4–6 stools per day over baseline.
Figure 3.
(A) Depicted are 103 treatment beams employed by the Cyberknife radiosurgery accelerator during targeting of right-sided and left-sided para-aortic lymph nodes. (B) Depicted is an axial projection of radiosurgical treatment, with the 2400 cGy prescription isodose line highlighted in red covering the nodal planning target volume shaded in red. The green cross-hairs designate the maximum dose point within targeted para-aortic lymph nodes. (C) Plotted are the dose-volume histograms for the para-aortic lymph node target (18F-FDG PET-GTV in maroon and CT-GTV in aqua blue), vertebral bone marrow (gold), small bowel (purple), left kidney (dark blue), and right kidney (magenta).
Discussion
This retrospective study showed that 18F-FDG PET-based contours were significantly larger than CT-based contours among women with metastatic abdominopelvic gynecologic cancer. Irradiated planning tumor volumes were sterilized at a rate of 96% in this series with minimal morbidity.
In this series of women with recurrent gynecologic cancers in para-aortic lymph nodes, both CT-based and 18F-FDG PET-based contours contributed data used to generate the radiosurgical planning tumor volume. Use of both imaging sets was chosen by the investigators based on the hypothesis that 18F-FDG PET images acquired during quiet breathing in part may reflect the relative inspiratory-expiratory paths of abdominopelvic targets during respiration. Given the overall accuracy and sub-millimeter precision of robotic stereotactic body radiosurgery, the investigators desired to have as much information regarding target movement during radiosurgical planning for ablative dose delivery. It is conceivable that abdominopelvic targets not fixed to the abdominal cavity could have moved and could have resided outside the treatment path of such narrowly-collimated radiation beams used in robotic stereotactic body radiosurgery. While the investigators recognize that the Cyberknife’s capability for monitoring and correcting for intrafraction target motion lowers the hazard of missing a radiosurgical target, the extent of tumor motion may not be captured fully on static CT-based imaging resulting in an actual planning tumor volume that may not entirely encompass moving targets in real-time. The investigators speculated that overall respiratory motion and time spent by abdominopelvic tumor targets in different portions of the breathing cycle contribute to 18F-FDG PET uptake fluctuations, as others have [6]. Certainly, an acknowledged limitation of 18F-FDG PET-based contouring for radiation planning is the subjective nature of user-defined image threshold settings. Although a 40% threshold was used consistently in our study, other threshold values could just as well serve to adequately delineate tumor volumes. In previous study of stereotactic body radiosurgery for recurrent vulvar cancers without 18F-FDG PET-based contouring [12], ablative radiation dose initially sterilized clinical and CT identified cancer targets in all three women studied. And yet, follow-up revealed disease progression in immediately adjacent tissue not targeted by radiosurgery in all three women. By contrast, the high therapeutic response rate (96%) presented here on larger 18F-FDG PET-based and CT-based contours attests to greater accounting of both nearby occult disease and target motion due to respiration. Notwithstanding these results, further research is needed before drawing broad conclusions regarding 18F-FDG PET and a percentage threshold method for radiation planning.
This article has limitations of small sample size; user-defined 18F-FDG PET threshold settings, potential investigator bias in retrospective assignment of toxicity criteria; and limited overall follow-up. Accrual to a 50 patient prospective robotic stereotactic body radiosurgery trial for women with metastatic gynecologic malignancies using more rigorous 18F-FDG PET guidelines has been completed in an effort to report safety, tolerability, and outcomes measures. Mature results are eagerly awaited [15].
As stereotactic body radiosurgery for abdominopelvic sites of metastatic gynecologic cancers becomes more mainstream, accumulated 18F-FDG PET and CT data will better guide treating physician target contouring. Future research directions by our group will study better radiosurgical target delineation, as modified by the extent of respiratory motion, by four-dimensional (4D)-CT scanners or by single 18F-FDG PET-magnetic resonance imaging (PET/MRI) scanners.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- 1.Kunos C, Radivoyevitch T, Abdul-Karim FW, Faulhaber P. 18F-fluoro-2-deoxy-d-glucose positron emission tomography standard uptake value as an indicator of cervical cancer chemoradiation therapeutic response. Int J Gynecol Cancer. 2011;21:1117–1123. doi: 10.1097/IGC.0b013e31821dc8b5. [DOI] [PubMed] [Google Scholar]
- 2.Rose PG, Adler LP, Rodriguez M, Faulhaber PF, Abdul-Karim FW, et al. Positron emission tomography for evaluating para-aortic nodal metastasis in locally advanced cervical cancer before surgical staging: a surgicopathologic study. J Clin Oncol. 1999;17:41–45. doi: 10.1200/JCO.1999.17.1.41. [DOI] [PubMed] [Google Scholar]
- 3.Grigsby PW, Siegel BA, Dehdashti F. Lymph node staging by positron emission tomography in patients with carcinoma of the cervix. J Clin Oncol. 2001;19:3745–3749. doi: 10.1200/JCO.2001.19.17.3745. [DOI] [PubMed] [Google Scholar]
- 4.Esthappan J, Mutic S, Malyapa RS, Grigsby PW, Zoberi I, et al. Treatment planning guidelines regarding the use of CT/PET-guided IMRT for cervical carcinoma with positive paraaortic lymph nodes. Int J Radiat Oncol Biol Phys. 2004;58:1289–1297. doi: 10.1016/j.ijrobp.2003.09.074. [DOI] [PubMed] [Google Scholar]
- 5.Horowitz NS, Dehdashti F, Herzog TJ, Rader JS, Powell MA, et al. Prospective evaluation of FDG-PET for detecting pelvic and para-aortic lymph node metastasis in uterine corpus cancer. Gynecol Oncol. 95:546–551. doi: 10.1016/j.ygyno.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 6.Biehl KJ, Kong FM, Dehdashti F, Jin JY, Mutic S, et al. 18F-FDG PET definition of gross tumor volume for radiotherapy of non-small cell lung cancer: Is a single standardized uptake value threshold approach appropriate? J Nucl Med. 2006;47:1808–1812. [PubMed] [Google Scholar]
- 7.Mayr NA, Huang Z, Sohn JW, Lo SS, Teh BS, et al. Emerging application of stereotactic body radiation therapy for gynecologic malignancies. Expert Rev Anticancer Ther. 11:1069–1075. doi: 10.1586/era.11.81. [DOI] [PubMed] [Google Scholar]
- 8.Kunos C, Chen W, DeBernardo R, Waggoner S, Brindle J, et al. Stereotactic body radiosurgery in gynecologic carcinomas. Technol Cancer Res Treat. 8:393–400. doi: 10.1177/153303460900800510. [DOI] [PubMed] [Google Scholar]
- 9.Kilby W, Dooley JR, Kuduvalli G, Sayeh S, Maurer CR., Jr The Cyberknife robotic radiosurgery system in. Technol Cancer Res Treat. 2010;9:433–452. doi: 10.1177/153303461000900502. [DOI] [PubMed] [Google Scholar]
- 10.Wilcox EE, Daskalov GM. Evaluation of GAFCHROMIC EBT film for Cyberknife dosimetry. Med Phys. 2007;34:1967–1974. doi: 10.1118/1.2734384. [DOI] [PubMed] [Google Scholar]
- 11.Kunos C, Chen W, DeBernardo R, Waggoner S, Brindle J, et al. Stereotactic body radiosurgery for pelvic relapse of gynecologic malignancies. Technol Cancer Res Treat. 2009;8:393–400. doi: 10.1177/153303460900800510. [DOI] [PubMed] [Google Scholar]
- 12.Kunos C, von Gruenigen V, Waggoner S, Brindle J, Zhang Y, et al. Cyberknife radiosurgery for squamous cell carcinoma of the vulva after prior pelvic radiation therapy. Technol Cancer Res Treat. 2008;7:375–380. doi: 10.1177/153303460800700504. [DOI] [PubMed] [Google Scholar]
- 13.Spring-Robinson C, Chandramouli V, Schumann WC, Faulhaber PF, Wang Y, et al. Uptake of 18F-labeled 6-fluro-6-deoxy-D-glucoseby skeletal muscle is responsive to insulin stimulation. J Nucl Med. 2009;50:912–919. doi: 10.2967/jnumed.109.062687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Echner GG, Kilby W, Lee M, Earnst E, Sayeh S, et al. The design, physical properties and clinical utility of an iris collimator for robotic radiosurgery. Phys Med Biol. 2009;54:5359–5380. doi: 10.1088/0031-9155/54/18/001. [DOI] [PubMed] [Google Scholar]
- 15.Mayr Nina A, Zhibin Huang, Sohn Jason W, Lo Simon S, The Bin S, et al. A prospective phase 2 evaluation of stereotactic body radiosurgery for gynecologic malignancies. Expert Review of Anticancer Therapy. 2011;7:1071–1077. doi: 10.1586/era.11.81. [DOI] [PubMed] [Google Scholar]