Abstract
Objective
Cervical and vaginal cancers have virally-mediated or mutated defects in DNA damage repair responses, making these cancers sensible targets for ribonucleotide reductase inhibition during radiochemotherapy.
Methods
We conducted a phase II study evaluating 3x weekly 2-hour intravenous 3-aminopyridine-2-carboxaldehyde thiosemicarbazone (3-AP, 25 mg/m2) co-administered with 1x weekly intravenous cisplatin (40 mg/m2) and daily pelvic radiation (45 Gy) in women with stage IB2-IVB cervical (n = 22) or stage II-IV vaginal (n = 3) cancers. Brachytherapy followed (40 Gy). Toxicity was monitored by common terminology criteria for adverse events (version 3.0). The primary end point of response was assessed by 3-month posttherapy 2-[18F] fluoro-2-deoxy-D-glucose positron emission tomography (PET/CT) and clinical examination.
Results
3-AP radiochemotherapy achieved clinical responses in 24 (96% [95% confidence interval: 80-99%]) of 25 patients (median follow-up 20 months, range 2-35 months). 23 (96% [95% confidence interval: 80-99%]) of 24 patients had 3-month posttherapy PET/CT scans that recorded metabolic activity in the cervix or vagina equal or less than that of the cardiac blood pool, suggesting complete metabolic responses. The most frequent 3-AP radiochemotherapy-related adverse events included fatigue, nausea, diarrhea, and reversible hematological and electrolyte abnormalities.
Conclusions
The addition of 3-AP to cisplatin radiochemotherapy was tolerable and produced high rates of clinical and metabolic responses in women with cervical and vaginal cancers. Future randomized phase II and III clinical trials of 3-AP radiochemotherapy are warranted.
Keywords: Triapine, cervical cancer, ribonucleotide reductase, radiosensitization
Introduction
Advanced stage cervical and vaginal cancers – which are malignancies invading pelvic tissues and organs rendering them not amenable to radical hysterectomy – are aggressive malignancies marked by higher rates of metastases and poorer disease-specific survival [1] than organ-confined cancers [2]. For instance, patients with advanced stage IB2-IIB cervical cancers treated weekly with cisplatin co-administered with daily radiation have lower rates of complete pathological response (68%) [3] than patients with organ-confined (< 6 cm) cervical cancer (90%) [4]. Metabolic responses to cisplatin radiochemotherapy, as assessed by 2-[18F]fluoro-2-deoxy-D-glucose positron emission tomography, are noted to be higher at a rate of 84% [5].Cervical and vaginal cancers incompletely responsive to standard-of-care cisplatin radiochemotherapy foreshadow a very poor prognosis, with median patient survival under two years [4-7].
Mechanisms that account for these observations are many, but 17-fold elevations in ribonucleotide reductase proteins after irradiation have been found in cervical cancer cells [8]. Functional ribonucleotide reductase enzyme consists of a heterotetramer of either M1/M2 or M1/M2b (a.k.a., p53R2) [9]. Ribonucleotide reductase exchanges hydrogen for a hydroxyl on ribose, creating a deoxynucleotide diphosphate (dNDP) that can be used as a building block for DNA [10]. Elevated ribonucleotide reductase subunits are associated with less antitumor response to standard-of-care cisplatin radiochemotherapy [11], perhaps vitally-related to disruptions in virally-inactivated or mutated p53 [12-15]. In clinical studies, combinations of radiochemotherapy and ribonucleotide reductase inhibitors (e.g., hydroxyurea, 5-FU, and gemcitabine) have been met with clinical benefit and improved cancer survival [16-19].
Pharmacological inhibition of ribonucleotide reductase by 3-aminopyridine-2-carboxaldehyde thiosemicarbazone (3-AP) lowers enzymatic activity, protracts the repair of radiochemotherapy damaged DNA, and invokes cell death [12-15]. We conducted a phase I study of 3-AP radiochemotherapy in women with cervical cancer [20] and established a recommended phase II dose. The prior phase I study demonstrated a very high cervical cancer response rate. In the present phase II study, we further investigated the safety and efficacy of three-times weekly 3-AP in combination with once weekly cisplatin and daily pelvic radiation in women with advanced stage cervical and vaginal cancers.
Methods
Patients
Patient demographic and tumor variables are listed in Table 1. Inclusion criteria were an age of 18 years or older and untreated stage IB2-IVB cervical or stage II-IV vaginal cancers of squamous, adenosquamous, or adenocarcinoma histopathology. Other inclusion criteria were Gynecologic Oncology Group performance status score 0, 1, or 2 and adequate bone marrow, hepatic, and renal function. Patients were excluded if they had known brain metastases or uncontrolled medical co-morbidities including symptomatic cardiac and/or pulmonary disease. Known glucose-6-phosphate dehydrogenase deficiency was also an exclusion because the methemoglobinemia antidote, methylene blue, may invoke hemolysis in these patients [21]. All patients provided written informed consent before enrollment.
Table 1.
Baseline Characteristics of the Study Patients
| Characteristic | |||
|---|---|---|---|
| Female sex — no. (%) | 25 (100) | ||
| Age — year | |||
| Median | 54 | ||
| Range | 35-75 | ||
| Race — no. (%)* | |||
| White | 17 (68) | ||
| Black or African ancestry | 6 (24) | ||
| Hispanic | 2 (8) | ||
| GOG performance status — no. (%)† | |||
| 0 | 23 (92) | ||
| 1 | 2 (8) | ||
| Histopathology — no. (%) | |||
| Squamous cell carcinoma | 23 (92) | ||
| Adenosquamous carcinoma | 1 (4) | ||
| Adenocarcinoma | 1 (4) | ||
| Tumor Grade — no. (%) | |||
| Grade 2 | 13 (52) | ||
| Grade 3 | 12 (48) | ||
| Cervical Stromal Invasion — no. (%) | |||
| Less than 2/3rds invasion | 3 (12) | ||
| 2/3rds or greater invasion | 22 (88) | ||
| Lymphovascular invasion | |||
| Absent | 7 (28) | ||
| Present | 18 (72) | ||
| International Federation of Gynecology and Obstetrics (FIGO) Stage | |||
| Cervix | IB2 node negative | 1 (4) | |
| IB2 node-positive | 4 (16) | ||
| IIA | 4 (16) | ||
| IIB | 1 (4) | ||
| IIIB | 10 (40) | ||
| IVB | 2 (8) | ||
| Vagina | II | 2 (8) | |
| IVA | 1 (4) |
Race was self-reported.
The Gynecologic Oncology Group (GOG) performance status reflects individual daily living activities on a scale of 0 (fully active with symptoms) to 5 (dead).
no. = number
Study Design
This single-arm phase IIb clinical trial was approved by the National Cancer Institute Cancer Therapy Evaluation Program (NCI-CTEP; Bethesda, MD) and the University Hospitals of Cleveland and Case Western Reserve University Institutional Review Board (Cleveland, OH). The trial complied with the provisions of the Case Comprehensive Cancer Center Data Safety Monitoring Board (Cleveland, OH). 26 women were recruited from August, 2009, through November, 2011, and as done previously [20], included women with either cervical or vaginal cancer given the anticipated similarity in treatment response [22]. The primary end point was 3-month posttherapy 2-[18F]fluoro-2-deoxy-D-glucose positron emission tomography (18F-FDG PET/CT) complete plus partial metabolic response in all pretherapy sites of disease. Using established guidelines [23, 24], a complete metabolic response was defined as absence of abnormal 18F-FDG uptake (i.e., calibrated measure of radiotracer activity falling within the background range of the radiotracer in the cardiac blood pool). A partial metabolic response reflected a 15% to 25% reduction in tumor 18F-FDG uptake. Secondary end points were safety and tolerability of 3-AP radiochemotherapy, as well as, the rate of clinical response. Progression-free survival was defined as the time from enrollment to confirmation of disease progression or cancer-related death.
Treatment
For a five day treatment cycle, patients received day 1 to 5 four-field box pelvic radiation (1.8 Gy per fraction), day 2 intravenous cisplatin (40 mg/m2; 70 mg maximum) over 90 minutes, and day 1, 3, 5 intravenous 3-AP (25 mg/m2; 50 mg maximum) administered over 120 minutes, as before [20]. Four-field box anteroposterior-posteroanterior (AP/PA) field borders were the L4-L5 interspace superiorly, 2 cm wider than the pelvic brim laterally, and the inferior aspect of the ischial tuberosities ensuring that the field edge was at least 3 cm inferior to disease. Lateral field borders matched the AP/PA superior and inferior borders, were 1.5 cm anterior to the pubic symphysis, and included the entire sacrum. Extended para-aortic radiation fields were not permitted. Each cycle was repeated for five weeks (45 Gy, x5 cisplatin, x15 3-AP). An anteroposterior-posteroanterior parametrial radiation boost (5.4 Gy) was administered to all in the sixth treatment week. Women underwent any ‘make-up’ chemotherapy infusions in the sixth week (e.g., cancer center closure for holiday or patient missed appointment; 7 [28%] of 25 patients). Image-guided 40 Gy low-dose-rate (23 [92%] of 25 patients) or 30 Gy high-dose-rate (6 Gy x 5 fractions; 2 [8%] of 25 patients) brachytherapy was prescribed to point A and followed pelvic radiation.
Assessment
Metabolic tumor response was based on nuclear medicine radiologist and radiation oncologist assessment of primary disease and assigned by means of baseline and 3-month 18F-FDG PET/CT [23, 24]. Also, a ratio of 3-month standard uptake value (SUV) to baseline SUV was computed, with a ratio of 0.33 or less indicating complete metabolic response [5]. Tumor measurement criteria according to the modified Response Evaluation Criteria in Solid Tumors (RECIST version 1.0) were applied at the time of brachytherapy, 1-month and 3-month follow-up by treating radiation and gynecologic oncologists for clinical response and to establish disease progression [25].
Safety was assessed with physical examinations and standard clinical hematologic and blood chemical tests weekly and up to 30 days after the last dose of 3-AP was administered. Adverse event grades were defined according to Common Terminology Criteria for Adverse Events (CTCAE version 3.0). Recommended patient follow-up was at 1-month posttherapy, every 3 months in the first year posttherapy, and every 6 months thereafter.
Statistical Analysis
The primary goal of this trial was to establish for the first time in cervical cancer clinical trials a rate of metabolic response by 3-month 18F-FDG PET/CT. Assuming that the observed response rate of standard-of-care cisplatin radiochemotherapy was at least 68% [3], we calculated that a sample size of 25 patients ensured an 85% power (beta is 0.15) to test the phase II hypothesis of Ho: 3-month 18F-FDG PET/CT is 70% versus Ha: 3-month 18F-FDG PET/CT is 90%. A Simon MinMax two stage clinical trial design was used, assuming a 10% dropout rate and an early stopping rule that if ≤ 11 if the first 16 patients have a clinical response rate less than 70% the study was to be terminated [26]. Progression-free survival estimates were determined univariately by the method of Kaplan and Meier [27]. Any log-rank P values and confidence intervals were two-sided and followed intention-to-treat principles (SPSS 18.0, SPSS Inc., Chicago, IL). Adverse events were tabulated according to CTCAE version 3.0 categorization and terms. Confidence intervals (CI) for single proportions were calculated without continuity correction [28].
Results
Patients
Between August 2, 2009 and November 21, 2011, 26 patients were assigned 3-AP radiochemotherapy treatment (Table 1). One patient was enrolled but underwent no therapy; this patient was excluded from all analyses. Of the remaining 25 study patients, 16 patients had metastatic nodal disease and two patients had metastatic visceral disease at diagnosis (i.e., stage IVB). 24 (96%) of 25 women underwent all planned radiation therapy and brachytherapy, achieving a radiation dose of 85 Gy to cervical or vaginal cancer disease over a median 52 days (range 44 — 72 days). One patient with pre-existing portal hypertension died in the period between 3-AP radiochemotherapy and brachytherapy due to an iatrogenic Mallory-Weiss tear, and as such, the patient did not undergo the primary end point 3-month 18F-FDG PET/CT. A total of 118 (94%) of 125 planned cisplatin infusions and 358 (95%) of 375 planned 3-AP infusions were administered to the 25 patients. As of the date of data cutoff, June 1, 2012, 21 patients remain alive and have median follow-up of 20 months (range 7-35 months).
Safety and Tolerability
Table 2 catalogues the most common adverse events. The most frequent adverse events attributed to 3-AP radiochemotherapy included reversible grade 2 or higher diarrhea (24%), grade 2 or higher electrolyte imbalance (24%), and grade 2 fatigue (24%). In our study, grade 3 or higher leucopenias (20%), thrombocytopenias (12%), or anemias (8%) were relatively infrequent and reversible. To put this clinical trial’s toxicity profile in perspective, a clinical trial of 176 women randomly allocated to once weekly cisplatin and daily pelvic radiation reported a 40% rate of grades 2-4 gastrointestinal symptoms, 10% rate of grades 2-4 electrolyte imbalances, and 3% rate of grade 2-4 fatigue, while rates of leukopenia, thrombocytopenia, or other hematologic effects were 49%, 6%, and 42%, respectively [29].
Table 2.
3-AP radiochemotherapy-related worst grade toxicities (n = 25)
| Adverse event | Grade (no. patients, %) | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| Cardiovascular | ||||||
| Atrial fibrillation | 0 | 1 (4) | 0 | 0 | 0 | |
| Constitutional and Blood | ||||||
| Anemia | 5 (20) | 6 (24) | 1 (4) | 1 (4)* | 0 | |
| Fatigue | 5 (20) | 6 (24) | 0 | 0 | 0 | |
| Hypotension | 0 | 1 (4) | 0 | 0 | 0 | |
| Thromboembolism | 0 | 1 (4) | 2 (8) | 0 | 0 | |
| Gastrointestinal | ||||||
| Nausea | 12 (46) | 1 (4) | 2 (8) | 0 | 0 | |
| Emesis | 3 (12) | 2 (8) | 0 | 0 | 0 | |
| Diarrhea | 6 (24) | 5 (20) | 1 (4) | 0 | 0 | |
| Constipation | 2 (8) | 1 (4) | 0 | 0 | 0 | |
| Enterocolitis | 0 | 0 | 1 (4) | 0 | 0 | |
| Fistula – rectovaginal | 0 | 0 | 1 (4) | 0 | 0 | |
| Mallory-Weiss Tear | 0 | 0 | 1 (4)* | 0 | 0 | |
| Gastrointestinal hemorrhage | 0 | 0 | 0 | 0 | 1 (4)* | |
| Genitourinary | ||||||
| Dysuria | 3 (12) | 1 (4) | 0 | 0 | 0 | |
| Infection | ||||||
| Febrile Neutropenia | 0 | 0 | 1 (4) | 0 | 0 | |
| Metabolic / Laboratory | ||||||
| Hypernatremia | 1 (4) | 0 | 1 (4) | 0 | 0 | |
| Hypoalbuminemia | 0 | 1 (4) | 0 | 0 | 0 | |
| Hypocalcemia | 0 | 1 (4) | 0 | 0 | 0 | |
| Hypochloridemia | 1 (4) | 0 | 0 | 0 | 0 | |
| Hypokalemia | 6 (24) | 0 | 1 (4) | 3 (12) | 0 | |
| Hypomagnesemia | 0 | 1 (4) | 0 | 0 | 0 | |
| Hyponatremia | 0 | 0 | 1 (4) | 0 | 0 | |
| International normalized ratio (INR) of prothrombin time elevated |
1 (4) | 0 | 0 | 0 | 0 | |
| Liver dysfunction – clinical | 1 (4) | 1 (4) | 1 (4) | 0 | 0 | |
| Neutropenia | 2 (8) | 3 (12) | 4 (16) | 1 (4) | 0 | |
| Thrombocytopenia | 11 (44) | 5 (20) | 3 (12) | 0 | 0 | |
| Neurology | ||||||
| Hemorrhagic stroke | 0 | 0 | 0 | 1 (4)† | 0 | |
| Pain | ||||||
| Abdominopelvic pain | 1 (4) | 1 (4) | 1 (4) | 0 | 0 | |
| Arthralgia / Myalgia | 3 (12) | 0 | 0 | 0 | 0 | |
Adverse event attributed to iatrogenic Mallory-Weiss tear in a single patient with pre-existing portal hypertension.
Occurred after 3-AP radiochemotherapy, but within 30 days posttherapy.
no. = number
3-AP induces methemoglobinemia by impairing recycling of methemoglobin to hemoglobin in erythrocytes [21]. In our study utilizing a dose of 25 mg/m2, 3-AP gave rise to no symptomatic elevated methemoglobin level, although methemoglobin proportion was not tested specifically unless symptomatic dyspnea was witnessed.
Other adverse events occurred infrequently (Table 2). Three lower extremity deep venous thromboses were encountered during the study period, with one event prompting the treating investigator to stop 3-AP infusions (n=7 delivered) midway through the radiation course. One (4%) patient suffered an ischemic stroke without lasting deficit within 30 days after study drug treatment. One death occurred during the trial period unrelated to 3-AP radiochemotherapy and attributed to an iatrogenic Mallory-Weiss tear leading to uncontrolled hemorrhage. Late greater than 30-day toxicities included vaginitis in three (9%) patients, grade 2 abdominopelvic pain in three (9%) patients, a rectovaginal fistula requiring hyperbaric oxygen therapy in one (4%) patient (10 months after treatment), and sigmoid colitis requiring colonic diversion surgery in one (4%) patient (22 months after treatment).
Efficacy
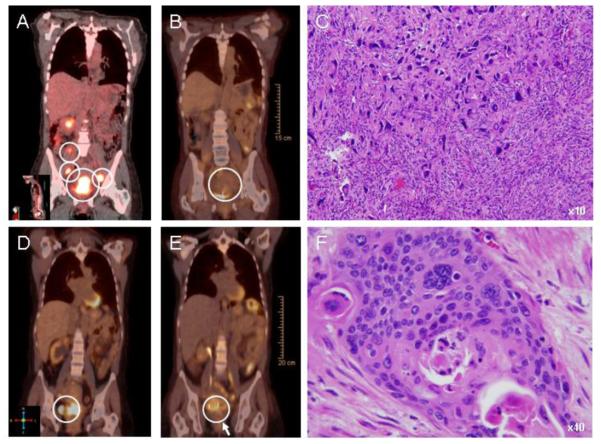
The 3-month 18F-FDG PET/CT response rate of the primary tumor following NCI-CTEP guidelines was 100% (24 of 24 patients) in whom the study was conducted. Median pretherapy SUV in the cervix or vagina was 14.6 (25-75% quartile: 12.1 — 17.7); the corresponding median 3-month posttherapy SUV was 2.6 (25-75% quartile: 2.0 — 3.4). Applying the more stringent criteria utilized at our institution of a posttherapy-to-pretherapy 18F-FDG PET/CT ratio less than 0.33 [5], the complete metabolic response rate of the primary tumor was 96% (23 of 24 [95% CI: 80-99%], Fig. 1). The median SUV ratio was 0.19 (25-75% quartile: 0.12 — 0.24). The one patient who did not achieve a complete metabolic response by our institutional criteria developed the only in-field local disease relapse 10 months posttherapy (Fig. 2). Two patients initiated trial therapy with known metastatic disease (one abdominal and one chest visceral site each). The pretherapy SUVs were 4.3 for the abdominal site and 9.6 for the chest site. Reflecting cisplatin-3-AP treatment effect only, the posttherapy SUVs were 1.8 and 5.2, respectively.
Figure 1.
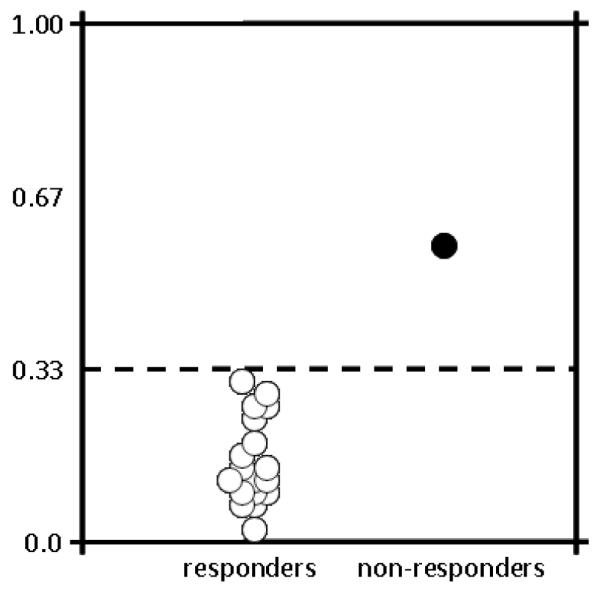
18F-FDG PET/CT ratio of standard uptake value (SUV) posttherapy-to-pretherapy. A ratio of 0.33 or less (dotted line) significantly discriminates responders and non-responders to radiochemotherapy [5]. The single patient whose ratio did not cross the 0.33 threshold developed relapse at 10 months posttherapy.
Figure 2.
Representative cervical cancer 18F-FDG PET/CT images are shown for baseline and for 3 months after 3-AP radiochemotherapy in a node positive complete metabolic responder [SUV ratio 0.32] (A-B) and in a node-positive incomplete responder [SUV ratio 0.56] (D-E). White circles identify primary and nodal disease (A-B, D-E). Posttherapy biopsy of the uterine cervix showed markedly distorted, enlarged, hyperchromatic and vesicular nuclear changes in keeping with 3-AP radiochemotherapy effect in the partial responder (C). Posttherapy surgical specimens obtained at disease recurrence displayed densely hyalinized tissue with necrosis and cell nests of squamous cancer (F). Cervical cancer cells are stained with hematoxalin and eosin.
The mean treated tumor size was 6.2 cm (standard deviation: 2.0 cm), with 15 patients having pretherapy tumors measuring 6 cm or greater. Clinical responses (i.e., no clinically-apparent or measurable cervical or vaginal tumor) were recorded in 21 (84% [95% CI: 65-94%]) of 25 patients at the end of 3-AP, cisplatin, and external beam radiation (45 Gy). 23 (96% [95% CI: 80-99%]) of 24 patients had clinical responses of their cervical and vaginal cancers at the 1-month assessment, and again at the 3-month evaluation. The one patient dying during the trial period before brachytherapy had a clinical response before the fatal iatrogenic Mallory-Weiss tear.
One local pelvic relapse and four extrapelvic relapses have been observed through a median 20 months of follow-up. Interventions for recurrent disease included surgery in three patients and palliative radiation for two patients. An 18-month estimate for control of pelvic disease was 95% (confidence interval [CI]: 90% — 100%). Before therapy, 16 of 25 patients had 18F-FDG PET/CT metabolically-avid pelvic or lower (L4-L5) para-aortic lymphadenopathy. Four (25%) of these 16 node-positive patients have had one pulmonary site and three para-aortic nodal sites of disease relapse.
The progression-free survival rate at 18 months was 67% (95% CI: 56% — 78%). For the five women whose disease relapsed, the time interval to disease progression was 5, 8, 10, 13, and 24 months. Of note, this progression-free survival analysis includes both patients with visceral metastatic disease at diagnosis having eventual progression of disease at 5 months and 13 months respectively. To put this clinical trial’s rate of progression-free survival in context, a contemporary cisplatin-radiation combination treating a similar at-risk cervical cancer patients achieved a progression-free survival estimate, as assessed by 18F-FDG PET/CT, at 18 months of 25% [7].
Discussion
This phase II trial resulted worthwhile rates of 18F-FDG PET/CT complete metabolic response, clinical response, and progression-free survival when 3-AP was added3-AP to cisplatin radiochemotherapy in women with advanced stage cervical and vaginal cancers.
The rate of 3-month 18F-FDG PET/CT overall metabolic response was selected as a primary end point for this study, rather than the usual phase II efficacy end point of overall objective rate of response, on the basis that residual metabolic activity may be an earlier indicator of posttherapy persistent disease. Whole body 18F-FDG PET/CT imaging took advantage of evaluating simultaneously a radiation-cisplatin-3-AP effect on primary cervical and vaginal cancer, and also of assessing a cisplatin-3AP effect on known or occult metastatic disease. NCI-CTEP guidelines for 18F-FDG PET/CT response are particularly useful for assessing antitumor effect of chemotherapies, but those criteria do not account for the substantial reduction in cancer cell number after radiation. Our data fits this expectation as a 100% metabolic complete plus partial response rate arose when we applied NCI-CTEP guidelines. To improve the discriminatory power of 18F-FDG PET/CT when radiation is part of a therapeutic program, we introduced a new, stricter criteria of a posttherapy-to-pretherapy SUV ratio to be 0.33 or less [5]. Although the number of women treated with 3-AP radiochemotherapy is small in this study, we observed that the only patient who had disease recurrence in the pelvis did not cross the 0.33 threshold (Figs. 1 and 2). When cervical and vaginal cancer radiochemotherapy clinical trials employ 18F-FDG PET/CT imaging as an indicator of therapeutic response, a clinically meaningful SUV ratio of 0.33 might better assess tumor responses and antitumor activity of biologic agents.
In our study, we studied 3-AP administered three times weekly during cisplatin radiochemotherapy because of 3-AP’s short 2-hour half-life [20]. Pre-clinical [12-15] studies indicated that 3-AP needed to be administered in close proximity to a dose of radiation to take advantage of synergy among the therapies. Damage done by radiation appears instantaneously after exposure, peaks at one hour after dosing, increases ribonucleotide reductase activity, and resolves in four hours [12, 30]. By infusing 3-AP immediately after a radiation dose, optimal inhibition of ribonucleotide reductase occurs when dNDP demand is highest, protracting DNA repair. Radiation-induced DNA damage, lethal to cells, is accentuated by 3-AP when timed in this way [12, 30]. Conversely, cisplatin damage in the form of DNA adducts peaks at 6 hours after exposure, increases ribonucleotide reductase activity, and lasts 24 hours or more [30, 31]. When 3-AP is administered before and after cisplatin, cisplatin-related DNA damage persists and increases cell death [32]. As a particular highlight of this clinical trial, most (20 [83%] of 24) patients achieved a clinical response after 3-AP radiochemotherapy (45 Gy radiation dose) prior to their brachytherapy. This 3-AP radiochemotherapy radiation dose that achieves clinical response is lower than the typical 80 Gy for standard radiochemotherapy. More data are needed to provide an interpretive context for this provocative radiation-drug finding. However, enthusiasm for novel radiation-drug regimens that are highly effective should be tempered by advances in better radiation quality, such as that of image-guided brachytherapy which achieves comparable responses in 90 percent or more of treated women [33, 34].
3-AP radiochemotherapy was reasonably safe. Our rates of grade 3 or higher gastrointestinal (25%) and reversible hematological (36%) toxicity are in line with gastrointestinal (40%) and hematological (49%) toxicity seen after cisplatin radiochemotherapy [29]. We attribute this finding both to volume-directed radiation planning tuned to cancer targets while avoiding normal tissues and to the relatively quick recycling of ribonucleotide reductase in normal cells. But, other factors may be in play. We also did not observe symptomatic dyspnea or methemoglobinemia, both of which are recognized sequelae of 3-AP inhibition of cytochrome b5 reductase in red blood cells [21]. The incidence of late greater than 30-day toxicities was low (Table 2), with the caveat that long-term clinical follow-up is short. Collectively, the high clinical activity without undue severe short-term adverse events has promoted 3-AP among a select few anticancer agents being considered for the next randomized phase II / III radiochemotherapy clinical trials for women with cervical or vaginal cancers.
Limitations of this phase II clinical trial include small sample size and potential bias in clinical and radiographic surveillance to detect events that would alter estimates of progression-free survival. More mature follow-up is needed to further assess overall survival. Logistically, intravenous 3-AP is administered on three days and a cisplatin infusion occurs on a fourth day of each cycle. This is repeated five times to complete a course of radiochemotherapy. Compliance to such a regimen may be difficult in rural communities [35, 36] and in elderly populations [36, 37]. An oral 3-AP formulation has begun clinical development [38]. As such and to overcome compliance barriers, our research team and NCI-CTEP are considering a phase I clinical trial of daily oral 3-AP during cisplatin radiochemotherapy in women with cervical or vaginal cancers.
In conclusion, this phase II study provides evidence that ribonucleotide reductase inhibition by 3-AP during cisplatin radiochemotherapy provides clinical benefit with a favorable safety profile in women with advanced stage cervical and vaginal cancer. Trials are being planned that are adequately powered to study the predictive significance of 18F-FDG PET/CT and of ribonucleotide reductase biomarkers upon overall and progression-free survivals, such as the randomized phase II 3-AP radiochemotherapy trial (National Cancer Institute protocol #9434).
Highlights.
We report a phase II 3-AP radiochemotherapy trial for cervix/vaginal cancer.
For the first time, 3-month 18F-FDG PET/CT assessed metabolic complete response.
Reduction in 18F-FDG SUV by > 66% identified 23(96%) metabolic complete responders.
Acknowledgements
We would like to thank all of the patients and allied personnel that participated in this clinical trial.
Role of the Funding Source This clinical trial was supported in part by NIH grants U01 CA62502 and P30 CA43703-17. The funding source had no involvement in data collection or data interpretation for this clinical trial.
SOURCES OF SUPPORT: Supported in part by NIH grants U01 CA62502 and P30 CA43703-17.
Footnotes
Contributors CK, SW, NF, and AD contributed to the design, implementation, data collection on the clinical trial as well as drafted this manuscript. CK and TR performed statistical analyses in this manuscript. RD, KZ, KR RA, RR, PF contributed to data collection and analysis in this manuscript as well as drafted this manuscript.
Conflicts of Interest There are no potential conflicts of interest among the authors. This manuscript has been seen, read, and agreed upon in its content by all designated authors. This manuscript has not been submitted or published elsewhere.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CLINICAL TRIAL REGISTRY: NCT00941070 (clinical trials.gov)
References
- [1].Rose P, Ali S, Watkins E, Thigpen J, Deppe G, Clark-Pearson D, et al. Long-term follow-up of a randomized trial comparing concurrent single agent cisplatin, cisplatin-based combination chemotherapy, or hydroxyurea during pelvic irradiation for locally advanced cervical cancer: A Gynecologic Oncology Group Study. J Clin Oncol. 2007;25:2804–10. doi: 10.1200/JCO.2006.09.4532. [DOI] [PubMed] [Google Scholar]
- [2].Stehman F, Ali S, Keys H, Muderspach L, Chafe W, Gallup D, et al. Radiation therapy with or without weekly cisplatin for bulky stage 1B cervical carcinoma: follow-up of a Gynecologic Oncology Group trial. Am J Obstet Gynecol. 2007;197:503.e1–.e6. doi: 10.1016/j.ajog.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Candelaria M, Chanona-Vilchis J, Cetina L, Flores-Estrada D, López-Graniel C, González-Enciso A, et al. Prognostic significance of pathological response after neoadjuvant chemotherapy or chemoradiation for locally advanced cervical carcinoma. Int Semin Surg Oncol. 2006;3:3–1. doi: 10.1186/1477-7800-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kunos C, Ali S, Abdul-Karim F, Stehman F, Waggoner S. Posttherapy residual disease associates with long-term survival after chemoradiation for bulky stage 1B cervical carcinoma: a Gynecologic Oncology Group Study. Am J Obstet Gynecol. 2010;203:351.e1–8. doi: 10.1016/j.ajog.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kunos C, Radivoyevitch T, Abdul-Karim F, Faulhaber P. 18F-fluoro-2-deoxy-d-glucose positron emission tomography standard uptake value as an indicator of cervical cancer chemoradiation therapeutic response. Int J Gynecol Cancer. 2011;21:1117–23. doi: 10.1097/IGC.0b013e31821dc8b5. [DOI] [PubMed] [Google Scholar]
- [6].Samant R, Lau B, E C, Le T, Tam T. Primary vaginal cancer treated with concurrent chemoradiation using Cis-platinum. Int J Radiat Oncol Biol Phys. 2007;69:746–50. doi: 10.1016/j.ijrobp.2007.04.015. [DOI] [PubMed] [Google Scholar]
- [7].Schwartz J, Grigsby P, Dehdashti F, Delbeke D. The role of 18F-FDG PET in assessing therapy response in cancer of the cervix and ovaries. J Nucl Med. 2009;50:64S–73S. doi: 10.2967/jnumed.108.057257. [DOI] [PubMed] [Google Scholar]
- [8].Kuo M-L, Kinsella T. Expression of ribonucleotide reductase after ionizing radiation in human cervical carcinoma cells. Cancer Res. 1998;58:2245–52. [PubMed] [Google Scholar]
- [9].Tanaka H, Arakawa H, Yamaguchi T, Shiraishi K, Fukuda S, Matsui K, et al. A ribonucleotide reductase gene involved in a p53-dependent cell-cycle checkpoint for DNA damage. Nature. 2000;404:42–9. doi: 10.1038/35003506. [DOI] [PubMed] [Google Scholar]
- [10].Hakansson P, Hofer A, Thelander L. Regulation of mammalian ribonucleotide reduction and dNTP pools after DNA damage and in resting cells. J Biol Chem. 2006;281:7834–41. doi: 10.1074/jbc.M512894200. [DOI] [PubMed] [Google Scholar]
- [11].Weidhaas J, Li S, Winter K, Ryu J, Jhingran A, Miller B, et al. Changes in gene expression predicting local control in cervical cancer: results from Radiation Therapy Oncology Group 0128. Clin Cancer Res. 2009;15:4199–206. doi: 10.1158/1078-0432.CCR-08-2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kunos C, Chiu S, Pink J, Kinsella T. Modulating radiation resistance by inhibiting ribonucleotide reductase in cancers with virally or mutationally silenced p53 protein. Radiation Res. 2009;172:666–76. doi: 10.1667/RR1858.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kunos C, Radivoyevitch T, Pink J, Chiu S, Stefan T, Jaccobberger J, et al. Ribonucleotide Reductase Inhibition Enhances Chemoradiosensitivity of Human Cervical Cancers. Radiation Res. 2010;174:574–81. doi: 10.1667/RR2273.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kunos C, Colussi V, Pink J, Radivoyevitch T, Oleinick N. Radiosensitization of human cervical cancer cells by inhibiting ribonucleotide reductase: enhanced radiation response at low dose rates. Int J Radiat Oncol Biol Phys. 2011;80:1198–204. doi: 10.1016/j.ijrobp.2011.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kunos C, Ferris G, Pyatka N, Pink J, Radivoyevitch T. Deoxynucleoside salvage facilitates DNA repair during ribonucleotide reductase blockade in human cervical cancers. Radiat Res. 2011;176:425–33. doi: 10.1667/rr2556.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hreshchyshyn MM, Aron BS, Boronow RC, Franklin EW, 3rd, Shingleton HM, Blessing JA. Hydroxyurea or placebo combined with radiation to treat stages IIIB and IV cervical cancer confined to the pelvis. International Journal of Radiation Oncology, Biology, Physics. 1979;5:317–22. doi: 10.1016/0360-3016(79)91209-4. [DOI] [PubMed] [Google Scholar]
- [17].Eifel PJ, Winter K, Morris M, Levenback C, Grigsby PW, Cooper J, et al. Pelvic irradiation with concurrent chemotherapy versus pelvic and para-aortic irradiation for high-risk cervical cancer: an update of radiation therapy oncology group trial (RTOG) 90-01. J Clin Oncol. 2004;22:872–80. doi: 10.1200/JCO.2004.07.197. [DOI] [PubMed] [Google Scholar]
- [18].Whitney CW, Sause W, Bundy BN, Malfetano JH, Hannigan EV, Fowler WC, Jr., et al. Randomized comparison of fluorouracil plus cisplatin versus hydroxyurea as an adjunct to radiation therapy in stage IIB-IVA carcinoma of the cervix with negative para-aortic lymph nodes: a Gynecologic Oncology Group and Southwest Oncology Group study. Journal of Clinical Oncology. 1999;17:1339–48. doi: 10.1200/JCO.1999.17.5.1339. [DOI] [PubMed] [Google Scholar]
- [19].Duenas-Gonzalez A, Zarba J, Patel F, Alcedo J, Beslija F, Casanova L, et al. A phase III, open-label, randomized study comparing concurrent gemcitabine plus cisplatin and radiation followed by adjuvant gemcitabine and cisplatin versus concurrent cisplatin and radiation in patients with stage IIB to IVA carcinoma of the cervix. J Clin Oncol. 2011;29:1678–85. doi: 10.1200/JCO.2009.25.9663. [DOI] [PubMed] [Google Scholar]
- [20].Kunos C, Waggoner S, Von Gruenigen V, Eldermire E, Pink J, Dowlati A, et al. Phase I trial of intravenous 3-aminopyridine-2-carboxaldehyde thiosemicarbazone (3-AP, NSC #663249) in combination with pelvic radiation therapy and weekly cisplatin chemotherapy for locally advanced cervical cancer. Clin Cancer Res. 2010;16:1298–306. doi: 10.1158/1078-0432.CCR-09-2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kunos C, Radivoyevitch T, Ingalls S, Hoppel C. Management of 3-aminopyridine-2-carboxaldehyde thiosemicarbazone induced methemoglobinemia. Future Oncol. 2012;8:145–50. doi: 10.2217/fon.11.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kunos C. Therapeutic mechanisms of treatment in cervical and vaginal cancer. Oncology & Hematology Review. 2012;8:55–60. [PMC free article] [PubMed] [Google Scholar]
- [23].Shankar L, Hoffman J, Bacharach S, Graham M, Karp J, Lammertsma A, et al. Consensus recommendations for the use of 18F-FDG PET as an indicator of therapeutic response in patients in National Cancer Institute Trials. J Nucl Med. 2006;47:1059–66. [PubMed] [Google Scholar]
- [24].Young H, Baum R, Cremerius U, Herholz K, Hoekstra O, Lammertsma A, et al. Measurement of clinical and subclinical tumor response using 18F-fluorodeoxyglucose and positron emission tomography: review and 1999 EORTC recommendations. Eur J Cancer. 1999;35:1771–82. doi: 10.1016/s0959-8049(99)00229-4. [DOI] [PubMed] [Google Scholar]
- [25].Therasse P, Arbuck S, Eisenhauer E, et al. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- [26].Simon R, Freidlin B, Rubinstein L, Arbuck S, Collins J, Christian M. Accelerated titration designs for phase 1 clinical trials in oncology. J Natl Cancer Instut. 1997;89:1138–47. doi: 10.1093/jnci/89.15.1138. [DOI] [PubMed] [Google Scholar]
- [27].Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81. [Google Scholar]
- [28].Newcombe R. Two-sided confidence intervals for the single proportion: Comparison of seven methods. Statistics Med. 1998;17:857–72. doi: 10.1002/(sici)1097-0258(19980430)17:8<857::aid-sim777>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- [29].Rose PG, Bundy BN, Watkins EB, Thigpen JT, Deppe G, Maiman MA, et al. Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. N Engl J Med. 1999;340:1144–53. doi: 10.1056/NEJM199904153401502. [DOI] [PubMed] [Google Scholar]
- [30].Kunos C, Radivoyevitch T, Pink J, Chiu S, Stefan T, Jacobberger J, et al. Ribonucleotide reductase inhibition enhances chemoradiosensitivity of human cervical cancers. Radiation Res. 2010;174:574–81. doi: 10.1667/RR2273.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Olive P, Banath J. Kinetics of H2AX phosphorylation after exposure to cisplatin. Cytometry Part B. 2009;76B:79–90. doi: 10.1002/cyto.b.20450. [DOI] [PubMed] [Google Scholar]
- [32].Kunos C, Radivoyevitch T, Abdul-Karim F, Fanning J, Abulafia O, Bonebrake A, et al. Ribonucleotide reductase inhibition restores platinum-sensitivity in platinum-resistant ovarian cancer: a Gynecologic Oncology Group study. J Transl Med. 2012;10:79.1–10. doi: 10.1186/1479-5876-10-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Pötter R, Georg P, Dimopoulos J, Grimm M, Berger D, Nesvacil N, et al. Clinical outcome of protocol based image (MRI) guided adaptive brachytherapy combined with 3D conformal radiotherapy with or without chemotherapy in patients with locally advanced cervical cancer. Radiother Oncol. 2011;100:116–23. doi: 10.1016/j.radonc.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Dimopoulos J, Schmid M, Fidarova E, Berger D, Kirisits C, Pötter R. Treatment of locally advanced vaginal cancer with radiochemotherapy and magnetic resonance image-guided adaptive brachytherapy: dose-volume parameters and first clinical results. Int J Radiat Oncol Biol Phys. 2012;82:1880–8. doi: 10.1016/j.ijrobp.2011.03.049. [DOI] [PubMed] [Google Scholar]
- [35].Kunos C, Ferris G, Waggoner S. Implementing chemoradiation treatment for patients with cervical cancer in a comprehensive cancer center community oncology practice. Commun Oncol. 2010;7:446–50. [PMC free article] [PubMed] [Google Scholar]
- [36].Kunos C, Gibbons H, Simpkins F, Waggoner S. Chemotherapy administration during pelvic radiation for cervical cancer patients aged ≥55 years in the SEER-Medicare population. J Oncol. 2008;2008:1–7. doi: 10.1155/2008/931532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Kunos C, Tian C, Waggoner S, Rose P, Lanciano R. Retrospective Analysis of Concomitant Cisplatin during Radiation in Patients aged ≥55 years for Treatment of Advanced Cervical Cancer: A Gynecologic Oncology Group Study. Int J Gyn Oncol. 2009;19:1258–63. doi: 10.1111/IGC.0b013e3181b33ace. [DOI] [PubMed] [Google Scholar]
- [38].Chao J, Synold T, Morgan R, Jr., Kunos C, Longmate J, Lenz H-J, et al. A phase I and pharmacokinetic study of oral 3-aminopyridine-2-carboxaldehyde thiosemicarbazone (3-AP, NSC #663249) in the treatment of advanced stage solid cancers – A California Cancer Consortium Study. Cancer Chemother Pharmacol. 2012;69:835–43. doi: 10.1007/s00280-011-1779-5. [DOI] [PMC free article] [PubMed] [Google Scholar]