Abstract
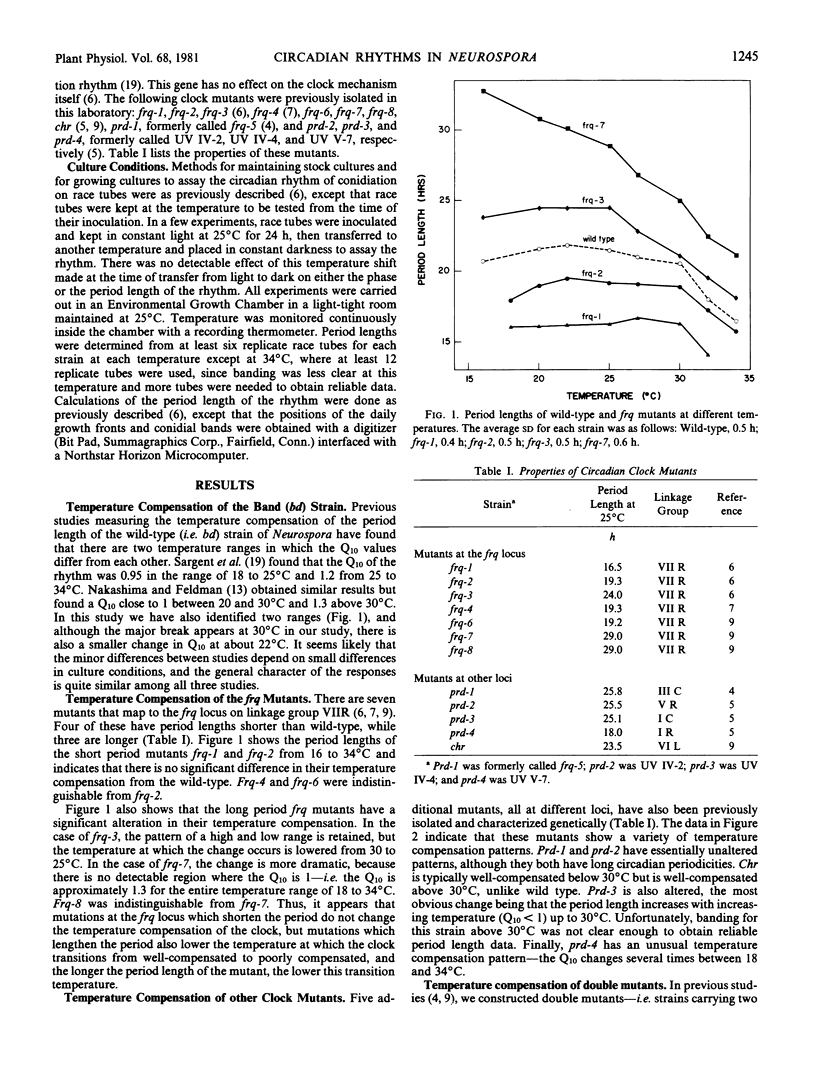
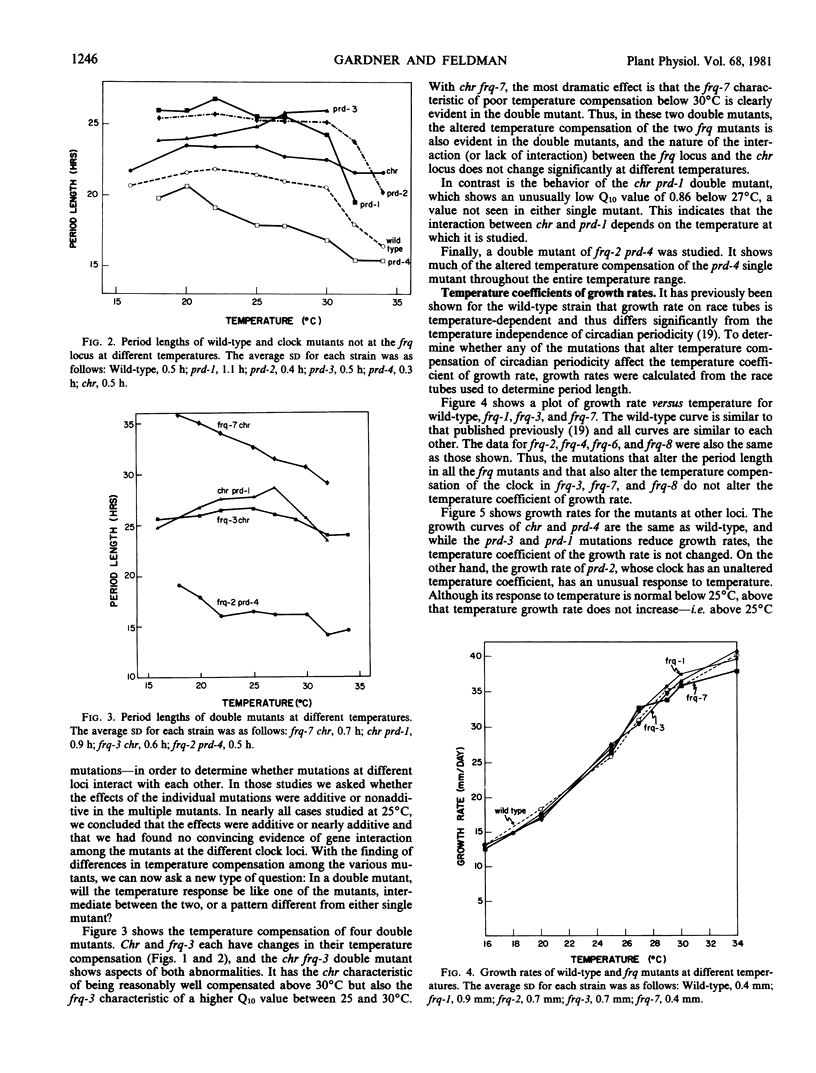
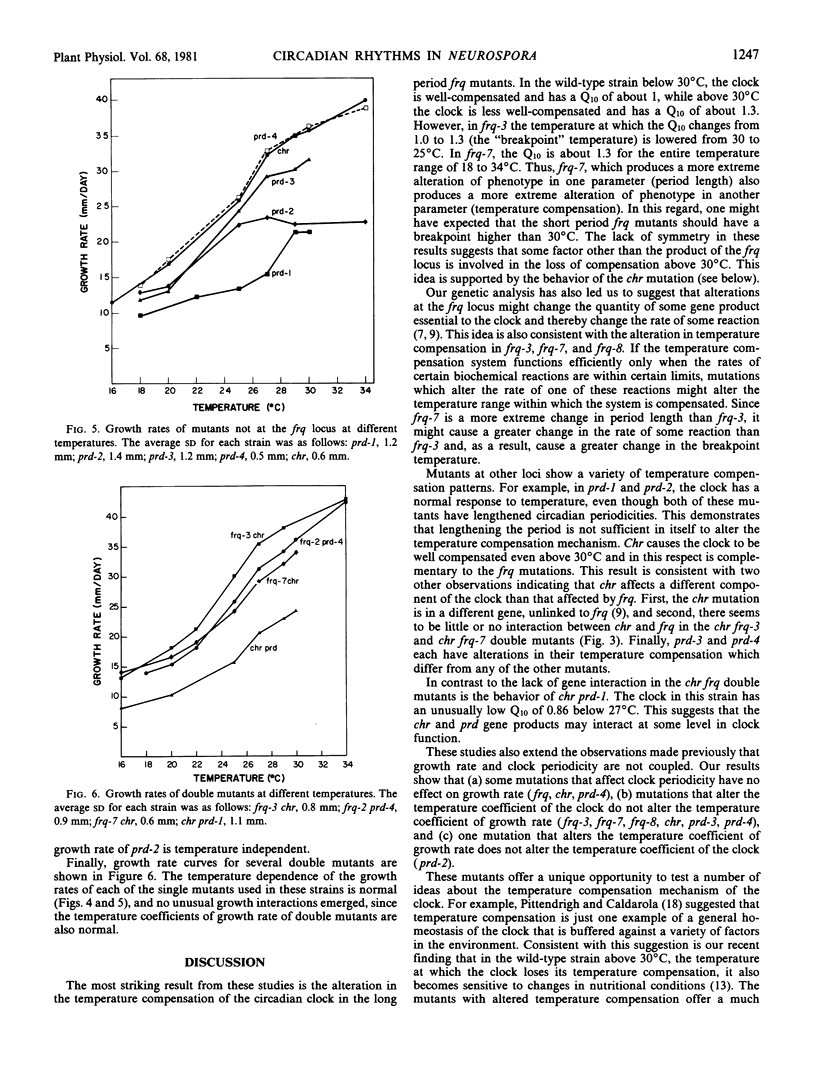
Temperature compensation of circadian period length in 12 clock mutants of Neurospora crassa has been examined at temperatures between 16 and 34°C. In the wild-type strain, below 30°C (the “breakpoint” temperature), the clock is well-compensated (Q10 = 1), while above 30°C, the clock is less well-compensated (Q10 = 1.3). For mutants at the frq locus, mutations that shorten the circadian period length (frq-1, frq-2, frq-4, and frq-6) do not alter this temperature compensation response. In long period frq mutants (frq-3, frq-7, frq-8), however, the breakpoint temperature is lowered, and the longer the period length of the mutants the lower the breakpoint temperature. Long period mutants at other loci exhibit other types of alterations in temperature compensation—e.g. chr is well-compensated even above 30°C, while prd-3 has a Q10 significantly less than 1 below 30°C. Prd-4, a short period mutant, has several breakpoint temperatures. Among four double mutants examined, the only unusual interaction between the individual mutations occurred with chr prd, which had an unusually low Q10 value of 0.86 below 27°C. There was no correlation between circadian period length and growth rate. These strains should be useful tools to test models for the temperature compensation mechanism.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bruce V. G. Mutants of the biological clock in Chlamydomonas reinhardi. Genetics. 1972 Apr;70(4):537–548. doi: 10.1093/genetics/70.4.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce V. G., Pittendrigh C. S. TEMPERATURE INDEPENDENCE IN A UNICELLULAR "CLOCK". Proc Natl Acad Sci U S A. 1956 Sep;42(9):676–682. doi: 10.1073/pnas.42.9.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehret C. F., Trucco E. Molecular models for the circadian clock. I. The chronon concept. J Theor Biol. 1967 May;15(2):240–262. doi: 10.1016/0022-5193(67)90206-8. [DOI] [PubMed] [Google Scholar]
- Feldman J. F., Hoyle M. N. Complementation analysis of linked circadian clock mutants of Neurospora crassa. Genetics. 1976 Jan;82(1):9–17. doi: 10.1093/genetics/82.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman J. F., Hoyle M. N. Isolation of circadian clock mutants of Neurospora crassa. Genetics. 1973 Dec;75(4):605–613. doi: 10.1093/genetics/75.4.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis C. D., Sargent M. L. Effects of temperature perturbations on circadian conidiation in neurospora. Plant Physiol. 1979 Dec;64(6):1000–1004. doi: 10.1104/pp.64.6.1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings J. W., Sweeney B. M. ON THE MECHANISM OF TEMPERATURE INDEPENDENCE IN A BIOLOGICAL CLOCK. Proc Natl Acad Sci U S A. 1957 Sep 15;43(9):804–811. doi: 10.1073/pnas.43.9.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopka R. J., Benzer S. Clock mutants of Drosophila melanogaster. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2112–2116. doi: 10.1073/pnas.68.9.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopka R. J. Genetic dissection of the Drosophila circadian system. Fed Proc. 1979 Nov;38(12):2602–2605. [PubMed] [Google Scholar]
- Njus D., Sulzman F. M., Hastings J. W. Membrane model for the circadian clock. Nature. 1974 Mar 8;248(5444):116–120. doi: 10.1038/248116a0. [DOI] [PubMed] [Google Scholar]
- Pavlidis T., Zimmerman W. F., Osborn J. [A mathematical model for the temperature effects on circadian rhythms]. J Theor Biol. 1968 Feb;18(2):210–221. doi: 10.1016/0022-5193(68)90162-8. [DOI] [PubMed] [Google Scholar]
- Pittendrigh C. S., Caldarola P. C. General homeostasis of the frequency of circadian oscillations. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2697–2701. doi: 10.1073/pnas.70.9.2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittendrigh C. S. ON TEMPERATURE INDEPENDENCE IN THE CLOCK SYSTEM CONTROLLING EMERGENCE TIME IN DROSOPHILA. Proc Natl Acad Sci U S A. 1954 Oct;40(10):1018–1029. doi: 10.1073/pnas.40.10.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SWEENEY B. M., HASTINGS J. W. Effects of temperature upon diurnal rhythms. Cold Spring Harb Symp Quant Biol. 1960;25:87–104. doi: 10.1101/sqb.1960.025.01.009. [DOI] [PubMed] [Google Scholar]
- Sargent M. L., Briggs W. R., Woodward D. O. Circadian nature of a rhythm expressed by an invertaseless strain of Neurospora crassa. Plant Physiol. 1966 Oct;41(8):1343–1349. doi: 10.1104/pp.41.8.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent M. L., Kaltenborn S. H. Effects of medium composition and carbon dioxide on circadian conidiation in neurospora. Plant Physiol. 1972 Jul;50(1):171–175. doi: 10.1104/pp.50.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweiger H. G., Schweiger M. Circadian rhythms in unicellular organisms: an endeavor to explain the molecular mechanism. Int Rev Cytol. 1977;51:315–342. doi: 10.1016/s0074-7696(08)60230-2. [DOI] [PubMed] [Google Scholar]
- Sweeney B. M. A physiological model for circadian rhythms derived from the acetabularia rhythm paradoxes. Int J Chronobiol. 1974;2(1):25–33. [PubMed] [Google Scholar]


