Abstract
In the rodent trigeminal pathway, trigeminal axons invade the developing whisker pad from a caudal to rostral direction. We investigated directional specificity of embryonic day (E). 15 rat trigeminal axons within this peripheral target field using explant cocultures. E15 trigeminal axons readily grow into the same age whisker pad explants and form follicle-related patterns along a caudal to rostral direction. They also can grow into this target from its lateral aspects. In contrast, they are unable to invade the whisker pad from the rostral (nasal). pole. We did not find any correlation between the distribution of extracellular matrix molecules and trigeminal axon growth preferences. We also examined age-related changes in trigeminal axon responsiveness to directional cues. E19 trigeminal axons readily grew into E15 whisker pad explants from either the caudal or the rostral pole. These results suggest the presence of growth permissive and repulsive cues that guide sensory axons in the whisker pad. Furthermore, trigeminal axons lose their responsiveness to growth inhibitory cues at later stages of development.
Keywords: Infraorbital nerve, Whisker follicle, Chondroitin sulfate proteoglycan, Fibronectin, DiI
The whisker pad, with five rows of mystacial vibrissae and sinus hair follicles, is a major peripheral target for trigeminal ganglion (TG). cells in rodents. The infraorbital branch (IO). of the trigeminal nerve provides the sensory innervation to this target. In the rat, IO axons reach the maxillary process around embryonic day (E). 12 [9,10]. These axons first form a tightly fasciculated bundle within the infraorbital foramen, and then fan out into the presumptive whisker pad from a caudal to rostral direction. Thus, blades of whisker row nerves display a centrifugal dispersion from their point of entry into the whisker pad. By E15, deep and superficial follicular nerves have left their parent fascicles, and formed dense, cup-shaped plexuses associated with the sensory receptors of the emerging whisker and sinus hair follicles. The nature of guidance cues underlying directional specificity and patterning of sensory axons in the whisker pad is not known. In the present study, we investigated this issue in explant cocultures of the TG and whisker pad. Many facets of axon–target interactions can be captured in such organ-otypic cultures [11–13,31].
E15 and E19 embryos were harvested from timed-pregnant (day of sperm positivitys = E0). Sprague–Dawley rats (Taconic Farms). Tissue dissection and coculture procedures were previously described in detail [11,12,31]. In one series of experiments, E15 TG explants were placed abutting the caudal (temporal), dorsal/ventral, or rostral (nasal) poles of the same age whisker pad explants (n = 25, each condition). In another series of experiments, E19 TG ex-plants were placed either along the caudal (n = 16) or the rostral (n = 24) edges of E15 whisker pads. All cultures were grown in serum-free medium [11] for 5 days, after which they were fixed with 4% buffered paraformaldehyde. Trigeminal axon growth was visualized by labeling the ganglion with similar amounts of the lipophilic tracer DiI (Molecular Probes). Analyses were performed following photoconversion of the fluorescent label [26.]. We also fixed E15 embryos (n = 4), and labeled the TG with DiI. The whisker pads from these embryos were later sectioned either in the tangential or transverse plane on a vibratome, and DiI-labeled axon trajectories were analyzed following photoconversion. Low and high power photomi- crographs were taken with a digital camera, transferred to a computer, and the images were optimized using the Adobe Photoshop program.
In a separate series of experiments, E15 isochronic cocultures were processed for immunohistochemistry, and counterstained with the nuclear stain bisbenzimide. We used anti-chondroitin sulfate proteoglycan (CSPG, mouse IgM isotype, Sigma), anti-fibronectin (mouse IgM, Sigma), and anti-neurofilament (C-terminus, Chemicon) antibodies to visualize the distribution of TG axons in relation to extracellular matrix (ECM) protein expression patterns and nucleoarchitecture of the whisker pad explants maintained in culture.
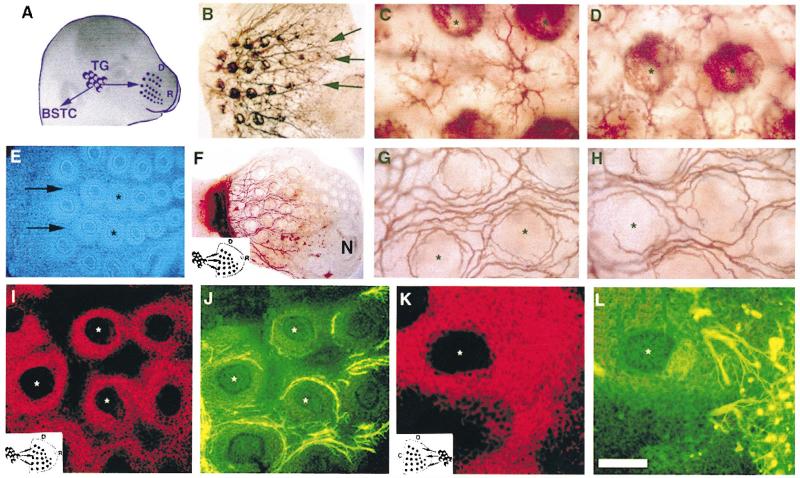
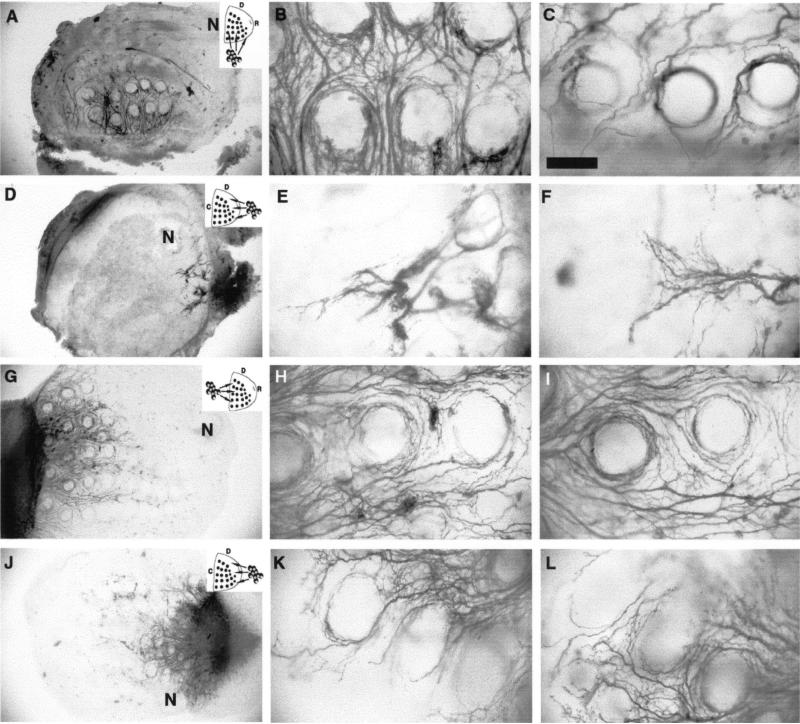
Trigeminal sensory axons arrive at their central and peripheral targets by E12 in the rat [9,10]. Central axons first elongate along the trigeminal tract, and later emit radial collaterals into the brainstem trigeminal nuclear complex around E17. IO axons develop into whisker row nerves as they invade their peripheral target from a caudal to rostral direction between E13 and E15. Five curvilinear rows of whisker follicles, along the rostrocaudal (RC). axis, become distinguishable by E15 [10], and dense, cup-shaped terminal plexuses envelope the external root sheaths of the developing follicles at this time (Fig. 1B–D). In explant cocultures, follicular organization and the patterned array of follicles were maintained for several days (Fig. 1E). When E15 TG explants were cocultured abutting the caudal edge of the whisker pad, TG axons grew in fascicles between follicle rows and formed circumfollicular plexuses (Fig. 1F–H). In such cultures the patterning of TG axons was similar to that seen in E15 normal embryos. However, due to flattening of the tissue in culture, the three-dimensional organization of follicle innervation was compressed (compare Fig. 1C, D with G, H). In addition, the density of innervation was less than that seen in normal embryos (compare Fig. 1B with F). This was most likely due to inadvertent damage to the ganglia during the dissection process and consequent cell death or loss. Regardless, a robust innervation pattern was evident in cocultures. In cases where TG explants were placed next to the dorsal or ventral (lateral) edges of the whisker pad, TG axon growth was still considerable and circular arrangement of axon terminals could be seen around the follicles (Fig 2A–C). Thick axon fascicles grew into the whisker pad along a route perpendicular to their normal path, but they appeared entangled between the follicle rows (Fig. 2B, C). In strik ing contrast to TG axon growth from caudal or lateral edges of the whisker pad, TG axons showed limited growth and hardly any patterning when they were placed next to the rostral pole of the target (Fig. 2D–F). Some axon fascicles grew in for short distances, and then appeared to be “deflected” by an invisible barrier. Under all conditions the overall size of the ganglia, and whisker pad explants remained similar.
Fig. 1.
Trigeminal axon growth specificity along the caudal to rostral axis of the whisker pad. (A). The normal positioning and peripheral projection routes of trigeminal axons in the embryo. (B). Photomicrograph of a tangential section through an E15 whisker pad labeled with DiI placed in the TG. Note the whisker row nerves (arrows). (C, D) High power photomicrographs illustrating the normal innervation pattern between whisker follicles (C) and within the follicles (D) in an E15 embryo. (E) Bisbenzimide stained section through an E15 whisker pad explant maintained in vitro. Caudal is to the left and rostral is to the right. Note that whisker row arrangements (arrows) and follicular organization (asterisks) remain largely uncompromised. (F) Low power photomicrograph of a DiI-labeled TG–whisker pad coculture with the ganglion placed next to the caudal edge of the whisker pad explant (schematized in the inset, D: dorsal, R: rostral, N: nasal opening). (G–H) High power views of axon growth between and around the follicles in cocultures such as that shown in F. (I–J) CSPG labeling and TG axon growth in the whisker pad with a caudally placed TG explant. Double immunostained section for CSPG and neurofilament antibodies. The same section and region was photographed using rhodamine and FITC filter sets, respectively. Asterisks mark the same follicle in each section. (K–L) Double immunostained section for CSPG and neurofilament antibodies from a case where the TG was placed next to the rostral pole of the whisker pad explant (asterisks mark the same follicle). Note that in this case TG axons are not present in CSPG-rich zones. Scale bar = 100 μm (C, D, G–L), 200 μm (E), and 500 μm (B, F).
Fig. 2.
E15 TG axon growth into whisker pad explants from different poles of the target and the behavior of E19 TG axons. Insets indicate the arrangement of cocultures, D: dorsal, C: caudal, R: rostral. (A). Low power photomicrograph illustrating a case where an E15 TG explant was placed along the ventral edge of the same age whisker pad explant. (B–C) High power views of TG axons coming in to the whisker pad explants at a 90° angle to their normal route, from a case like that shown in A. Note the dense reticular network of axon bundles between whisker follicle rows. (D) Low power photomicrograph of a case where the E15 TG explant was placed next to the rostral pole of an E15 whisker pad; axon growth is strikingly minimal. (E–F) High power photomicrographs of TG axons attempting to invade the whisker pad from its rostral pole. Note that axonal profiles appear to be deflected. (G) Low power photomicrograph of an E19 TG–E15 whisker pad coculture. Older trigeminal axons grow into this younger target with ease and pattern around the whisker follicles. (H–I) High power photomicrographs showing E19 TG axons around E15 whisker follicles. patterning of TG axons is clearly evident. N = nasal opening. Scale bar = 200 μm (A, D, G, J), and 100 μm (B, C, E, F, H, I, K, L).
We measured the RC and dorsoventral (DV) axes of each whisker pad explant, and these dimensions were 1.9–2.1 and 2.2–2.9 mm, respectively. Next we used a simple quantification method for axon growth, and measured the RC and DV extents of axons in each case. The mean RC extent was 1.3 mm in caudal TG cultures, 0.9 mm in lateral TG cultures, and 0.5 mm in rostral TG cultures. The mean DV extent was, in the same order, 1.7, 0.5 and 0.5 mm. While these are crude measures of axon growth analyses, they did confirm the observed morphological differences. We also noted that when E15 whisker pad explants were bisected along the dorsoventral axis and the TG was placed in the middle of this plane, axon growth was predominantly directed to the caudal half of the whisker pad [18].
Next, we examined the distribution of the ECM molecules CSPG and fibronectin in relation to directional preferences of axons in the whisker pad explants. Both of these molecules were expressed in similar regions of the E15 whisker pad. However, TG axon growth zones did not correlate with the patterned distribution of ECM molecules (Fig. 1I–L). When TG axons entered the whisker pad explants from a caudal to rostral direction, axon fascicles and terminal plexuses around the follicles were located within the CSPG or fibronectin-rich zones (Fig. 1I, J). In contrast, fibronectin or CSPG-rich areas immediately adjacent to the rostrally placed TG explants were devoid of axons (Fig. 1K, L). Similar observations were made following immunostaining for other ECM molecules such as, laminin, tenascin C and collagen IV (data not shown).
To investigate the possibility that TG axons might be responsive to putative, rostrally positioned inhibitory cues during a narrow window in development, we cocultured E19 TG explants with E15 whisker pads. In the developmental history of the trigeminal pathway, E19 is a time period at which most connections have formed, and in the brainstem trigeminal nuclei, whisker-specific patterns (“barrelettes”) are emerging [3,10]. In these heterochronic cocultures, TG axons grew into E15 whisker pad explants equally well from either the caudal (Fig. 2G–I) or rostral fronts (Fig. 2J–L). In such cultures, the mean RC extent of axons for each culture type was 1.2 mm and DV extent was 1.5 mm. Patterned arrangement of axons around the follicles was also seen in both types of cocultures.
The role of a variety of target-derived molecular cues that direct axon growth patterns have been underscored in many sensory systems. These include ECM molecules, semaphorins, ephrins, netrins, neurotrophins and their receptors [15,22–24,29,30,34]. The explant cocultures of the TG and the whisker pad, provide an excellent in vitro model to assay the role of molecular cues that channel incoming sensory axons into specific pathways and lead to their patterning. Previously, we reported that in explant cocultures of the TG or dorsal root ganglia (DRG) sensory axons can innervate native and foreign cutaneous targets and form target-specific patterning in each, but avoid noncutaneous targets when these foreign tissues express axon growth-inhibitory or repellent cues [31]. As an attempt to elucidate the nature of these cues, we examined the distribution of ECM molecules. These molecules are reasonable suspects because of their role in providing axon growth-permissive or inhibitory substrates in vitro [2,4,16,17,19,24,27,28]. In our study, their distribution did not correlate with the direction and density of axon growth. While these negative findings do not rule out the relative contribution of ECM molecules in directing trigeminal axon growth, they point out that other molecular signals must be involved in this process.
Recently, semaphorins, a large family of secreted and transmembrane proteins [22,34] have been implicated in patterning of peripheral sensory axon projections. Studies on the sensory axon projection phenotypes of semaIII or its receptor neuropilin-1 null mutant mice revealed that in the absence of semaIII function, peripheral projections of TG and DRG axons are disarrayed [1,21,32]. We do not know if semaIII (or other members of this family) expression patterns in the whisker pad could account for directional preferences of TG axon growth. It would be interesting to see if whisker pad explants from semaIII knockout mice would allow early TG axons to grow into this target freely from its rostral aspect.
Another family of molecules, neurotrophins, are produced and expressed in a patterned fashion within the whisker pad of developing rodents [5–8,14,20,33]. These molecules play an essential role in survival of TG cells during normal development, and it is possible that TG axons advance within the whisker pad explants along gradients of neurotrophin expression. Relative access to neurotrophin wells within the whisker pad from caudal vs. rostral approaches by TG axons may explain the observed differences. Recent studies by Rice and his colleagues indicate developmental gradients in the expression patterns of truncated forms of neurotrophin receptors around whisker follicles ([25]; F. Rice, personal communicationx]. The coculture model presented here is an ideal in vitro system to examine instructive or permissive roles of neurotrophin and trk receptor gradients in directional sensitivity of sensory axons.
Finally, our experiments with E19 axons growing into E15 whisker pad explants bear upon an important problem: spatiotemporal regulation of axon guidance cues in targets vs. temporal regulation of receptors for these cues in developing axons. Our results clearly indicate that E19 ganglion cells are not responsive to “inhibitory” cues positioned along the rostral portions of the E15 whisker pads. This suggests that there are developmental changes in the responsiveness (i.e., “receptor expression”) to axon growth-repellent cues within the whisker pad. We were not able to perform the reverse experiments where one would coculture E15 TG explants with much older whisker pad explants. After E16, the whisker pad becomes considerably thick, and it is not possible to obtain a viable explant or a slice with organotypic characteristics. If E15 axons could grow from the rostral pole of the whisker pad, this would suggest that target tissue also temporally regulates expression of growth permissive and growth inhibitory cues along specific axes. Overall, the results presented here demonstrate the presence of target-derived cues that convey positional information to incoming sensory axons and traffic them into specific routes and compartments. At this stage of development, sensory axons are highly responsive to these signals but lose their responsiveness after pathways and terminal connections are laid down.
Acknowledgements
We thank Dr. E. Ulupinar for her expert contributions to several of the experiments reported here and discussions of these results, and Dr. F. Rice for his helpful discussions of these experiments. Research supported by NIH NS32195 (to R.S.E.).
References
- 1.Behar O, Golden JA, Mashimo H, Schoen FJ, Fishman MC. Semaphorin III is needed for normal patterning and growth of nerves, bones and heart. Nature. 1996;383:525–528. doi: 10.1038/383525a0. [DOI] [PubMed] [Google Scholar]
- 2.Chiquet M, Wehrle-Haller B. Tenascin-C in peripheral nerve morphogenesis. Perspect. Dev. Neurobiol. 1994;2:67–74. [PubMed] [Google Scholar]
- 3.Chiaia NL, Bennett-Clarke CA, Eck M, White FA, Crissman RS, Rhoades RW. Evidence for prenatal competition among the central arbors of trigeminal primary afferent neurons. J. Neurosci. 1992;12:62–76. doi: 10.1523/JNEUROSCI.12-01-00062.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen J, Burne JF, McKinlay C, Winter J. The role of laminin and the laminin/fibronectin receptor complex in the outgrowth of retinal ganglion cell axons. Exp. Biol. 1987;122:407–418. doi: 10.1016/0012-1606(87)90305-8. [DOI] [PubMed] [Google Scholar]
- 5.Davies AM, Bandtlow C, Heumann R, Korsching S, Rohrer H, Thoenen H. Timing and site of nerve growth factor synthesis in developing skin in relation to innervation and expression of the receptor. Nature. 1987;326:353–358. doi: 10.1038/326353a0. [DOI] [PubMed] [Google Scholar]
- 6.Ernfors P, Wetmore C, Olson L, Persson H. Identification of cells in rat brain and peripheral tissues expressing mRNA for members of the nerve growth factor family. Neuron. 1990;5:511–526. doi: 10.1016/0896-6273(90)90090-3. [DOI] [PubMed] [Google Scholar]
- 7.Ernfors P, Merlio J-P, Persson H. Cells expressing the mRNA for neurotrophins and their receptors in embryonic rat development. Eur. J. Neurosci. 1992;4:1140–1158. doi: 10.1111/j.1460-9568.1992.tb00141.x. [DOI] [PubMed] [Google Scholar]
- 8.Ernfors P, Lee KF, Jaenisch R. Mice lacking brain-derived neurotrophic factor develop with sensory deficits. Nature. 1994;368:147–150. doi: 10.1038/368147a0. [DOI] [PubMed] [Google Scholar]
- 9.Erzurumlu RS, Killackey HP. Development of order in the rat trigeminal system. J. Comp. Neurol. 1983;213:365–380. doi: 10.1002/cne.902130402. [DOI] [PubMed] [Google Scholar]
- 10.Erzurumlu RS, Jhaveri S. Trigeminal ganglion cell processes are spatially ordered prior to the differentiation of the vibrissa pad. J. Neurosci. 1992;12:3946–3955. doi: 10.1523/JNEUROSCI.12-10-03946.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Erzurumlu RS, Jhaveri S, Takahashi H, McKay RDG. Target-derived influences on axon growth modes in explant cocultures of trigeminal neurons. Proc. Natl. Acad. Sci. U.S.A. 1993;90:7235–7239. doi: 10.1073/pnas.90.15.7235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Erzurumlu RS, McKay RDG, Jhaveri S. Morphological specification of trigeminal neurites depends on target fields. Dev. Brain Res. 1994;83:132–137. doi: 10.1016/0165-3806(94)90187-2. [DOI] [PubMed] [Google Scholar]
- 13.Erzurumlu RS, Jhaveri S. Target influences on the morphology of trigeminal axons. Exp. Neurol. 1995;135:1–16. doi: 10.1006/exnr.1995.1061. [DOI] [PubMed] [Google Scholar]
- 14.Fariñas I, Jones KR, Backus C, Wang XY, Reichardt LF. Severe sensory and sympathetic deficits in mice lacking neurotrophin-3. Nature. 1994;369:658–661. doi: 10.1038/369658a0. [DOI] [PubMed] [Google Scholar]
- 15.Flanagan JG, Vanderhaeghen P. The ephrins and Eph receptors in neural development. Annu. Rev. Neurosci. 1998;21:309–345. doi: 10.1146/annurev.neuro.21.1.309. [DOI] [PubMed] [Google Scholar]
- 16.Fundin BT, Arvidsson J, Aldskohius H, Johansson O, Rie SN, Rice FL. Comprehensive immunofluorescence and lectin binding analysis on intervibrissal fur innervation in the mystacial pad of the rat. J. Comp. Neurol. 1997;385:185–206. doi: 10.1002/(sici)1096-9861(19970825)385:2<185::aid-cne2>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 17.Grumet M, Milev P, Sakurai T, Karthikeyan L, Bourdon M, Margolis RK, Margolis RU. Interactions with tenascin and differential effects on cell adhesion of neurocan and phosphocan, two major chondroitin sulfate proteoglycans of nervous tissue. J. Biol. Chem. 1994;269:12142–12146. [PubMed] [Google Scholar]
- 18.Haeberle A, Ulupinar E, Erzurumlu R. ECM molecules and neurotrophins in directional growth and positioning of trigeminal axons in the embryonic whisker pad. Soc. Neurosci. Abstr. 1998;24:117.11. [Google Scholar]
- 19.Hantaz-Ambroise D, Vigny M, Koenig J. Heparan sulfate proteoglycan and laminin mediate two different types of neurite outgrowth. J. Neurosci. 1987;7:2293–2304. [PMC free article] [PubMed] [Google Scholar]
- 20.Ibáñez CF, Ernfors P, Timmusk T, Ip NY, Arenas E, Yancopoulos GD, Persson H. Neurotrophin-4 is a target-derived neurotrophic factor for neurons of the trigeminal ganglion. Development. 1993;117:1345–1353. doi: 10.1242/dev.117.4.1345. [DOI] [PubMed] [Google Scholar]
- 21.Kitsukawa T, Shimizu M, Sanbo M, Hirata T, Taniguchi M, Bekku Y, Yagi T, Fujisawa H. Neuropilin–semaphorin III/D-mediated chemorepulsive signals play a crucial role in peripheral nerve projection in mice. Neuron. 1997;19:995–1005. doi: 10.1016/s0896-6273(00)80392-x. [DOI] [PubMed] [Google Scholar]
- 22.Kolodkin AL, Ginty DD. Steering clear of semaphorins: neuropilins sound the retreat. Neuron. 1997;19:1159–1162. doi: 10.1016/s0896-6273(00)80408-0. [DOI] [PubMed] [Google Scholar]
- 23.Lander AD. Proteoglycans in the nervous system. Curr. Opin. Neurobiol. 1993;3:716–723. doi: 10.1016/0959-4388(93)90143-m. [DOI] [PubMed] [Google Scholar]
- 24.Margolis RU, Margolis RK. Chondroitin sulfate proteoglycans as mediators of axon growth and pathfinding. Cell Tissue Res. 1997;290:343–348. doi: 10.1007/s004410050939. [DOI] [PubMed] [Google Scholar]
- 25.Rice FL, Casadermunt E, Cronk KM, Strominger NS, Barde Y-A. Neurotrophin receptor interacting factor is selectively involved in the survival of subsets of unmyelinated cutaneous innervation. Soc. Neurosci. Abstr. 1998;24:25.13. [Google Scholar]
- 26.Sandell JH, Masland RH. Photoconversion of some fluorescent markers to diaminobenzidine reaction product. Cytochemistry. 1988;36:555–559. doi: 10.1177/36.5.3356898. [DOI] [PubMed] [Google Scholar]
- 27.Sanes JR. Extracellular matrix molecules that influence neural development. Annu. Rev. Neurosci. 1989;12:491–516. doi: 10.1146/annurev.ne.12.030189.002423. [DOI] [PubMed] [Google Scholar]
- 28.Shepard AM, Hamilton SK, Pearlman AL. Changes in the distribution of extracellular matrix components accompany early morphogenetic events of mammalian cortical development. J. Neurosci. 1991;11:3928–3942. doi: 10.1523/JNEUROSCI.11-12-03928.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Snider WD. Functions of the neurotrophins during nervous system development: what the knockouts are teaching us. Cell. 1994;77:627–638. doi: 10.1016/0092-8674(94)90048-5. [DOI] [PubMed] [Google Scholar]
- 30.Tessier-Lavigne M, Goodman CS. The molecular biology of axon guidance. Science. 1996;274:1123–1133. doi: 10.1126/science.274.5290.1123. [DOI] [PubMed] [Google Scholar]
- 31.Ulupinar E, Erzurumlu RS. Peripheral target-specific influences on embryonic neurite growth vigor and patterns. J. Comp. Neurol. 1998;399:427–439. doi: 10.1002/(sici)1096-9861(19981005)399:4<427::aid-cne1>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 32.Ulupinar E, Datwani A, Behar O, Erzurumlu R. Role of semaphorin III in the developing rodent trigeminal system. Mol. Cell. Neurosci. 1999;13:281–292. doi: 10.1006/mcne.1999.0747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilkinson GA, Fariñas I, Backus C, Yoshida CK, Reichardt LF. Neurotrophin-3 is a survival factor in vivo for early mouse trigeminal neurons. J. Neurosci. 1996;16:7661–7669. doi: 10.1523/JNEUROSCI.16-23-07661.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wright DE, White FA, Gerfen RW, Silos-Santiago I, Snider WD. The guidance molecule semaphorin III is expressed in regions of spinal chord and periphery avoided by growing sensory axons. J. Comp. Neurol. 1995;361:321–333. doi: 10.1002/cne.903610209. [DOI] [PubMed] [Google Scholar]