Abstract
Background and Purpose
Although arteriopathies are the most common cause of childhood arterial ischemic stroke (AIS), and the strongest predictor of recurrent stroke, they are difficult to diagnose. We studied the role of clinical data and follow-up imaging in diagnosing cerebral and cervical arteriopathy in children with AIS.
Methods
VIPS, an international prospective study, enrolled 355 cases of AIS (age 29d-18y) at 39 centers. A neuroradiologist and stroke neurologist independently reviewed vascular imaging of the brain (mandatory for inclusion) and neck to establish a diagnosis of arteriopathy (definite, possible, or absent) in 3 steps: (1) baseline imaging alone; (2) plus clinical data; (3) plus follow-up imaging. A 4-person committee, including a second neuroradiologist and stroke neurologist, adjudicated disagreements. Using the final diagnosis as the gold standard, we calculated the sensitivity and specificity of each step.
Results
Cases were median 7.6 years of age (IQR 2.8, 14); 56% male. The majority (52%) were previously healthy; 41% had follow-up vascular imaging. Only 56 (16%) required adjudication. The gold standard diagnosis was definite arteriopathy in 127 (36%), possible in 34 (9.6%), and absent in 194 (55%). Sensitivity was 79% at Step 1, 90% at Step 2, and 94% at Step 3; specificity was high throughout (99%, 100%, 100%), as was agreement between reviewers (Kappa 0.77, 0.81, 0.78).
Conclusions
Clinical data and follow-up imaging help, yet uncertainty in the diagnosis of childhood arteriopathy remains. This presents a challenge to better understanding the mechanisms underlying these arteriopathies and designing strategies for prevention of childhood AIS.
Keywords: ischemic stroke, arteriopathy, vasculopathy, children, pediatric
Introduction
Stroke is among the top ten causes of death in childhood.1 Population-based estimates of the annual incidence of childhood stroke range from 4.6 to 13 per 100 000 children.2–4 Traditional adult arterial ischemic stroke (AIS) risk factors such as hypertension, diabetes, smoking, and hypercholesterolemia are uncommon in children. Instead, pediatric AIS risk factors include arteriopathy, congenital heart disease, sickle cell disease, and hematologic abnormalities, among others.5–7 Childhood arteriopathies are increasingly recognized as a prevalent cause of childhood AIS, a strong predictor of recurrence and a predictor of poor short-term outcome.8–11 Prior estimates of the prevalence of arteriopathy range from 18% to 64% of pediatric AIS cases.8, 12–15 This wide range likely reflects differences in imaging modalities, classification, and study populations, but primarily the fact that childhood arteriopathies are difficult to diagnose. Challenges to diagnosis include lack of standardized diagnostic criteria, technical limitations of imaging studies, reliance on MRA over conventional angiography, and heterogeneity of childhood arteriopathies.
In the prospective, international, NIH-funded “Vascular effects of Infection in Pediatric Stroke” (VIPS) study, we enrolled 355 cases of childhood AIS, and collected extensive clinical data and imaging studies for central review by study investigators. We sought to determine the role of clinical data, and baseline and follow-up imaging, in diagnosing the presence of a childhood arteriopathy.
Material and Methods
Study Design
The VIPS study built on the existing infrastructure of the International Pediatric Stroke Study (IPSS).10 VIPS prospectively enrolled patients at 37 sites: 21 in the US, 6 in Canada, 5 in Europe (UK, France Serbia), 3 in Asia (Philippines, China, Hong Kong), and 1 each in Australia and South America (Chile). Ethics committee approvals were obtained at all participating sites. Details of VIPS methods have been published.16 Criteria for site participation included MRI scanner with minimum magnet strength of 1.5 Tesla, MRI slice thickness of 5 mm or less, and ability to provide DICOM imaging data. With parents’ or guardians’ consent, sites enrolled pediatric patients aged 29 days through 18 years with AIS and collected extensive clinical histories, biological samples, and standardized brain and cerebrovascular imaging studies. All imaging was performed on a clinical basis. VIPS cases were initially confirmed by the local investigator using clinical and imaging diagnostic criteria for AIS: (1) a focal neurological deficit of acute onset or a seizure; and (2) a CT or MRI showing a focal brain infarct conforming to an established arterial territory in a location and of a maturity consistent with the neurological signs and symptoms. The clinical and imaging data were then subjected to a centralized review and case confirmation process, described below.
Brain and Vascular Imaging
All clinically obtained brain and vascular imaging were collected for central review. The minimum neuroimaging protocol for patient inclusion in VIPS consisted of the following brain MRI sequences: axial diffusion-weighted images (DWI), axial T2-weighted images, axial or coronal fluid-attenuated inversion recovery (FLAIR) images, and magnetic resonance angiography (MRA) of the brain. Conventional angiography and CT angiography (CTA) were also accepted in lieu of MRA. MRA of the neck was collected when performed. Participants were followed prospectively for a minimum of 1 year, and all follow-up imaging was collected during that time.
Case Confirmation
A study neuroradiologist (MW) and pediatric stroke neurologist (HJF) centrally reviewed baseline brain MRI scans and clinical data to confirm that each case met clinical and imaging criteria for AIS. Disagreements were resolved by a second neuroradiologist (AJB).
Initial Descriptive Imaging Review
Descriptive imaging review was performed centrally by two study neuroradiologists; disagreements were resolved by discussion including a third neuroradiologist. In their review of brain parenchymal imaging, the radiologists described infarct size (using ABC/2),17 location, acuity, and associated hemorrhage. For the vascular imaging review (Figure 1), the neuroradiologists initially classified the vascular imaging as normal or abnormal, and then completely described the vascular findings, including type of abnormality (hypoplasia, irregularity, banding, stenosis, intimal flap, mural hematoma, ectasia, fusiform aneurysm/pseudoaneurysm, and occlusion), vascular segments affected and degree of collateral flow. This was done for both baseline and all follow-up vascular imaging.
Figure 1.
Flow diagram demonstrating how 355 children with arterial ischemic stroke (AIS) received a primary diagnosis of definite, possible, or no arteriopathy.
Arteriopathy Review and Classification
Images from VIPS patients showing vascular abnormalities during the initial vascular imaging review underwent a subsequent arteriopathy review process (Figure 1) that incorporated clinical data. A pediatric stroke neurologist (HJF) and neuroradiologist (MW) independently performed this review in three successive steps: Step 1, review of baseline imaging studies (and their centralized interpretation); Step 2, reevaluation with addition of clinical information; Step 3, reevaluation with addition of follow-up imaging when available.
At each step, the reviewers classified the “primary diagnosis”: no arteriopathy, possible arteriopathy or definite arteriopathy. Arteriopathy was defined as the imaging appearance of an in situ arterial abnormality (stenosis, irregularity, occlusion, banding, pseudoaneurysm, dissection flap) not attributable to an exogenous thrombus (e.g., cardioembolism) and not considered a normal developmental variant. Patients with an isolated arterial occlusion could be classified as having no arteriopathy (high certainty of occlusion due to thrombus), possible arteriopathy (etiology of occlusion unclear), or definite arteriopathy (high certainty of occlusion due to arteriopathy). We used features of both vascular and parenchymal imaging and clinical history (in Steps 2 and 3) to distinguish between an occlusion due to arteriopathy versus an occlusion due to thrombus. Features favoring thrombus (no arteriopathy) included: abrupt (as opposed to tapering) vessel occlusion, multiple arterial occlusions in a vascular tree (suggestive of an embolus that fragmented and resulted in multiple occlusions), multiple infarcts in a pattern suggestive of cardioembolism, clinical history suggesting high risk of cardioembolism (e.g., cardiac thrombus visualized on echocardiogram), and rapid resolution of occlusion on follow-up imaging. Features favoring arteriopathy included a pattern of vascular changes suggestive of moyamoya (distal internal carotid artery occlusion with lenticulostriate collaterals), clinical history of a disorder associated with moyamoya (e.g., sickle cell disease, trisomy 21), and changes suggestive of dissection (e.g., dissection flap or tapering occlusion), especially with a history of severe head or neck injury. If cause of the occlusion was unclear, the reviewers classified these as “possible arteriopathy.”
At each step, for patients with possible and definite arteriopathy, the reviewers also attempted to establish a “secondary diagnosis” by classifying the arteriopathies into subtypes: arterial dissection, transient cerebral arteriopathy (TCA), primary and secondary moyamoya, genetic or syndromic arteriopathies such as PHACE syndrome,18, 19 primary and secondary vasculitis, fibromuscular dysplasia, iatrogenic, and others. If a single diagnosis could not be made with high certainty, they created a differential diagnosis. The reviewers used pre-established definitions for childhood arteriopathies.20 TCA referred very specifically to a focal cerebral arteriopathy involving the distal internal carotid artery and/or its proximal branches, presumed inflammatory, with a stereotyped, monophasic natural history characterized by frequent early progression (over days to weeks), plateau with nonprogression by 6 months, and subsequent improvement in some with complete resolution in a minority.20, 21 Focal cerebral arteriopathy of childhood (FCA) is a broader label coined to describe intracranial anterior circulation pathology in children at the time of AIS, when TCA may be suspected but cannot be diagnosed with certainty due to lack of follow-up imaging.9 FCA has its own differential diagnosis (including TCA and intracranial dissection); hence, we did not include it as an option for secondary diagnosis.
At any step, if the reviewers disagreed on the primary diagnosis, or had no overlap in their differentials for the secondary diagnosis, the cases were adjudicated by a four-person committee, including a second neuroradiologist (AJB) and a second pediatric neurologist (GDV). The adjudication became the “gold standard” for those cases. If no disagreement, the gold standard was the Step 3 interpretation when follow-up imaging was available, and the Step 2 interpretation when no follow-up imaging was available.
Statistical Analysis
Characteristics were compared across the three arteriopathy groups (definite, possible, and none) using the non-parametric Kruskal-Wallis test for continuous variables, and the chi-square test for categorical variables. When the latter contained cells of fewer than five observations, p-values were calculated using Fisher’s exact test. For each of the three arteriopathy review steps, we calculated the sensitivity, specificity, positive predictive value and negative predictive value of that step’s primary diagnosis (definite arteriopathy as a binary variable) using the gold standard primary diagnosis described above. Individual reviewer diagnoses at each step were also compared to each other in order to assess reviewer agreement (independent of “gold standard” diagnosis). Interobserver agreement at each of the three steps was represented utilizing simple Kappa statistics and their confidence intervals. All analyses were done using Stata v12 (Stata Corp., College Station, TX).
Results
Between 1/2010 and 3/2014, VIPS prospectively enrolled 387 pediatric patients. Of these, 355 (92%) were centrally confirmed as meeting study criteria for AIS. Demographics, presentation, and co-morbidities are shown in Table 1; 184 (52%) were previously healthy (no chronic or acute illness prior to their stroke diagnosis).
Table 1.
Demographics and clinical characteristics of 355 childhood arterial ischemic stroke cases
| n | (%) | |
|---|---|---|
| Demographics | ||
| Age in years, median (IQR) | 7.6 | (2.8, 14.3) |
| Male gender | 199 | (56.1) |
| Race | ||
| White | 230 | (64.8) |
| Black | 39 | (11.0) |
| Indian/South Asian | 26 | (7.3) |
| East Asian | 9 | (2.5) |
| First Nations/Aboriginal | 4 | (1.1) |
| Middle Eastern | 3 | (0.8) |
| Pacific Islander | 1 | (0.3) |
| Mixed or other | 38 | (10.7) |
| Unknown | 5 | (1.4) |
| Ethnicity | ||
| Non-Hispanic | 289 | (81.4) |
| Hispanic | 47 | (13.2) |
| Mixed or other | 19 | (5.4) |
| Country | ||
| USA | 223 | (62.8) |
| Canada | 60 | (16.9) |
| Australia | 16 | (4.5) |
| Philippines | 16 | (4.5) |
| Chile | 14 | (3.9) |
| United Kingdom | 11 | (3.1) |
| France | 6 | (1.7) |
| Serbia | 5 | (1.4) |
| China | 4 | (1.1) |
| Stroke presentation | ||
| Focal signs | ||
| Hemiparesis | 285 | (80.3) |
| Dysarthria | 97 | (27.3) |
| Aphasia | 79 | (22.3) |
| Ataxia | 71 | (20.0) |
| Visual field deficit | 46 | (13.0) |
| Non-focal signs | ||
| Headache | 126 | (35.5) |
| Decreased level of consciousness | 102 | (28.7) |
| Nausea/vomiting | 85 | (23.9) |
| Seizures at presentation | 84 | (23.7) |
| Vertigo | 40 | (11.3) |
| Diplopia | 12 | (3.4) |
| Papilledema | 4 | (1.1) |
| Risk factors or co-morbidities (not mutually exclusive) | ||
| Cardiac disease | 107 | (30.1) |
| Congenital heart disease | 64 | (18.0) |
| Acquired heart disease | 21 | (5.9) |
| Isolated patent foramen ovale | 21 | (5.9) |
| Stroke at cardiac surgery (<72 hours) | 10 | (2.8) |
| Other cardiac disease | 41 | (11.5) |
| Other chronic disorders | ||
| Sickle cell anemia | 13 | (3.7) |
| Downs syndrome | 11 | (3.1) |
| Other genetic syndrome | 16 | (4.5) |
| Migraine | 12 | (3.4) |
| Prothrombotic state | 10 | (2.8) |
| Oral contraceptives (females only) | 10 | (6.4) |
| Indwelling catheter | 9 | (2.5) |
| Iron deficiency anemia | 6 | (1.7) |
| Brain tumor | 5 | (1.4) |
| Benign | 2 | (0.6) |
| Malignant | 3 | (0.8) |
| Aneurysm | 3 | (0.8) |
| PHACES syndrome/hemangioma | 3 | (0.8) |
| Hematologic malignancy | 2 | (0.6) |
| L-asparaginase therapy | 2 | (0.6) |
| Connective tissue disease | 2 | (0.6) |
| Ventriculoperitoneal shunt | 1 | (0.3) |
| Acute systemic illness | ||
| Fever lasting >48 hours | 44 | (12.4) |
| Systemic sepsis or bacteremia | 20 | (5.6) |
| Dehydration | 18 | (5.1) |
| Shock | 9 | (2.5) |
| Viral gastroenteritis | 2 | (0.6) |
Arteriopathy Review
In 116 (32.7%) patients, there were no abnormalities on the initial vascular imaging review, and the arteriopathy review was not performed (Figure 1). The other 239 underwent arteriopathy review; full committee adjudication was performed in 56 cases (23% of 239). The source of the gold standard arteriopathy diagnosis is shown in Table 2. Overall, 127 patients (36%) received a gold standard diagnosis of definite arteriopathy, 34 (9.6%) possible arteriopathy, and 194 (55%) no arteriopathy (Table 2). Of the 184 previously healthy children, 42% had a definite arteriopathy, 13% possible arteriopathy, and 45% no arteriopathy, compared to 29%, 6%, and 65%, respectively, for the 171 non-healthy children (p=0.0005). Of the 127 with definite arteriopathy, 109 (86%) received a single secondary diagnosis, while 18 (14%) could not be further classified with certainty. Demographics were similar amongst patients with no, possible and definite arteriopathy, although the Filipino site had a high proportion of cases with definite arteriopathy related to cases of secondary vasculitis due to tuberculosis meningitis (Supplemental Table I).
Table 2.
"Gold standard" arteriopathy diagnosis in 355 childhood arterial ischemic stroke cases
| n | (%) | ||
|---|---|---|---|
| Source of "gold standard" arteriopathy classification | |||
| Vascular imaging review (normal, no arteriopathy review) | 116 | (32.7) | |
| Arteriopathy diagnosis review | 239 | (67.3) | |
| Baseline with clinical findings (Step 2) | 94 | (26.5) | |
| Follow-up (Step 3) | 89 | (25.1) | |
| Adjudication | 56 | (15.8) | |
| Definite arteriopathy | 127 | (35.8) | |
| Secondary diagnosis classified with high certainty* | 109 | (30.7) | |
| Arterial dissection | 26 | (7.3) | |
| Transient cerebral arteriopathy | 25 | (7.0) | |
| Primary moyamoya | 17 | (4.8) | |
| Secondary moyamoya | 17 | (4.8) | |
| PHACES | 2 | (0.6) | |
| Genetic arteriopathy | 4 | (1.1) | |
| Primary vasculitis | 0 | (0) | |
| Secondary vasculitis (including meningitis) | 15 | (4.2) | |
| Iatrogenic | 1 | (0.3) | |
| Fibromuscular dysplasia | 2 | (0.6) | |
| Secondary diagnosis not further classified | 18 | (5.1) | |
| Differential diagnosis includes:** | |||
| Transient cerebral arteriopathy | 9 | (2.5) | |
| Arterial dissection | 11 | (3.1) | |
| Primary moyamoya | 4 | (1.1) | |
| Secondary moyamoya | 0 | (0) | |
| Genetic arteriopathy | 1 | (0.3) | |
| Primary vasculitis | 2 | (0.6) | |
| Secondary vasculitis (including meningitis) | 4 | (1.1) | |
| Iatrogenic | 0 | (0) | |
| Possible arteriopathy | 34 | (9.6) | |
| Differential diagnosis includes:** | |||
| Transient cerebral arteriopathy | 9 | (2.5) | |
| Arterial dissection | 27 | (7.6) | |
| Primary moyamoya | 1 | (0.3) | |
| Secondary moyamoya | 0 | (0) | |
| Genetic arteriopathy | 0 | (0) | |
| Primary vasculitis | 1 | (0.3) | |
| Secondary vasculitis (including meningitis) | 7 | (2.0) | |
| Iatrogenic | 0 | (0) | |
| Embolic (not arteriopathy) | 33 | (9.3) | |
| No arteriopathy | 194 | (54.6) | |
| No abnormalities on vascular imaging (no arteriopathy review) | 116 | (32.7) | |
| Arteriopathy review | 78 | (22.0) | |
| Classified as no arteriopathy, no embolus | 1 | (0.3) | |
| Classified as no arteriopathy, low likelihood embolic | 2 | (0.6) | |
| Classified as isolated occlusion, high likelihood embolic | 75 | (21.1) | |
Sub-categories are mutually exclusive
Sub-categories are not mutually exclusive
Interobserver agreement on the primary diagnosis yielded a Kappa of 0.77 (95% CI, 0.70, 0.84) at Step 1. Sensitivity of the Step 1 primary diagnosis (for the gold standard primary diagnosis) was 79%, and specificity was 99%. The positive predictive value (PPV) of the Step 1 primary diagnosis was 97%, and the negative predictive value (NPV) was 89%. When clinical data were added (Step 2), interobserver agreement yielded a Kappa coefficient of 0.81 (95% CI, 0.74, 0.87) and sensitivity and specificity increased to 90% and 100%, respectively. PPV rose to 100% and NPV to 95%. In the 110 patients with follow-up imaging (Step 3), the Kappa coefficient for interobserver agreement was 0.78 (95% CI 0.66, 0.90). Sensitivity for the Step 3 primary diagnosis rose to 94%, with a specificity of 100%. PPV remained at 100%, while NPV dropped slightly to 91%.
Imaging Work-Up and Infarct Characteristics
The imaging studies performed were similar across children stratified by primary arteriopathy diagnosis, except that those with no arteriopathy were less likely to have conventional angiograms and follow-up vascular imaging (Supplemental Table II). Infarcts in the territory of the middle cerebral artery were the most common location in each stratum. Infarct volume was greatest in those with definite arteriopathy (p=0.009; Table 3).
Table 3.
Infarct characteristics, and vascular imaging findings in 355 childhood arterial ischemic stroke cases, stratified by primary arteriopathy diagnosis Infarct Characteristics at Baseline
| Arteriopathy | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Total N=355 |
Definite N=127 |
Possible N=34 |
No N=194 |
||||||
| n | (%) | n | (%) | n | (%) | n | (%) | P-value | |
| Infarct Characteristics at Baseline | |||||||||
| Location | |||||||||
| Vascular distribution of infarction | |||||||||
| Superficial MCA | 214 | (60.3) | 87 | (68.5) | 15 | (44.1) | 112 | (57.7) | 0.02 |
| Lenticulostriate | 144 | (40.6) | 59 | (46.5) | 11 | (32.4) | 74 | (38.1) | 0.20 |
| PCA | 69 | (19.4) | 15 | (11.8) | 7 | (20.6) | 47 | (24.2) | 0.02 |
| PICA | 37 | (10.4) | 13 | (10.2) | 6 | (17.6) | 18 | (9.3) | 0.34 |
| ACA | 35 | (9.9) | 17 | (13.4) | 3 | (8.8) | 15 | (7.7) | 0.23* |
| SCA | 27 | (7.6) | 8 | (6.3) | 5 | (14.7) | 14 | (7.2) | 0.25 |
| Anterior choroidal | 25 | (7.0) | 8 | (6.3) | 2 | (5.9) | 15 | (7.7) | 0.91 |
| Basilar | 23 | (6.5) | 3 | (2.4) | 3 | (8.8) | 17 | (8.8) | 0.04* |
| AICA | 8 | (2.3) | 1 | (0.8) | 4 | (11.8) | 3 | (1.5) | 0.005* |
| Other | 10 | (2.8) | 5 | (3.9) | 0 | (0.0) | 5 | (2.6) | 0.63* |
| Infarct side | 0.12 | ||||||||
| Left | 121 | (34.1) | 46 | (36.2) | 17 | (50.0) | 58 | (29.9) | |
| Right | 139 | (39.2) | 52 | (40.9) | 10 | (29.4) | 77 | (39.7) | |
| Bilateral | 93 | (26.2) | 29 | (22.8) | 6 | (17.6) | 58 | (29.9) | |
| Volume of largest infarct, cm3 | |||||||||
| Median | 18 | 31 | 19 | 11 | 0.009 | ||||
| (IQR) | (3.2, 69.1) | (5.8, 96.6) | (4.7, 62.1) | (2.7, 51.9) | |||||
| Vascular Imaging Findings | |||||||||
| No pathologic findings | 118 | 33.2 | |||||||
| Pathologic finding | |||||||||
| Occlusion | 164 | (46.2) | 74 | (58.3) | 25 | (73.5) | 65 | (33.5) | 0.0007 |
| Stenosis | 117 | (33.0) | 87 | (68.5) | 11 | (32.4) | 19 | (9.8) | <0.0001 |
| Irregularity | 87 | (24.5) | 65 | (51.2) | 10 | (29.4) | 12 | (6.2) | <0.0001 |
| Ectasia or fusiform (pseudo)aneurysm | 9 | (2.5) | 8 | (6.3) | 0 | (0.0) | 1 | (0.5) | 0.02* |
| Banding | 8 | (2.3) | 8 | (6.3) | 0 | (0.0) | 0 | (0.0) | 0.006* |
| Intimal flap or mural hematoma | 3 | (0.8) | 3 | (2.4) | 0 | (0.0) | 0 | (0.0) | 0.15* |
| Pathologically affected vessels | |||||||||
| Arterial segments affected | <0.0001 | ||||||||
| One | 64 | (18.0) | 18 | (14.2) | 16 | (47.1) | 30 | (15.5) | |
| More than one | 173 | (48.7) | 109 | (85.8) | 18 | (52.9) | 46 | (23.7) | |
| Vascular territories affected** | <0.0001* | ||||||||
| One | 162 | (45.6) | 72 | (56.7) | 28 | (82.4) | 62 | (32.0) | |
| Two | 56 | (15.8) | 41 | (32.3) | 5 | (14.7) | 10 | (5.2) | |
| Three | 6 | (1.7) | 3 | (2.4) | 0 | (0.0) | 3 | (1.5) | |
| Four | 13 | (3.7) | 11 | (8.7) | 1 | (2.9) | 1 | (0.5) | |
| Sides affected | <0.0001 | ||||||||
| Unilateral | 165 | (46.5) | 73 | (57.5) | 29 | (85.3) | 63 | (32.5) | |
| Bilateral | 72 | (20.3) | 54 | (42.5) | 5 | (14.7) | 13 | (6.7) | |
| Segments affected | |||||||||
| Proximal MCA (M1) | 153 | (43.1) | 101 | (79.5) | 12 | (35.3) | 40 | (20.6) | <0.0001 |
| Supraclinoid ICA | 103 | (29.0) | 82 | (64.6) | 5 | (14.7) | 16 | (8.2) | <0.0001 |
| Vertebrobasilar | 68 | (19.2) | 34 | (26.8) | 14 | (41.2) | 20 | (10.3) | 0.2 |
| Cervical arteries | 56 | (15.8) | 34 | (26.8) | 11 | (32.4) | 11 | (5.7) | 0.05 |
| Evolution of vascular imaging abnormality | <0.0001* | ||||||||
| Stable | 27 | (7.6) | 16 | (12.6) | 7 | (20.6) | 4 | (2.1) | |
| Improves or resolves | 45 | (12.7) | 20 | (15.7) | 6 | (17.6) | 19 | (9.8) | |
| Progresses | 27 | (7.6) | 22 | (17.3) | 1 | (2.9) | 4 | (2.1) | |
| Progresses then improves or resolves | 14 | (3.9) | 13 | (10.2) | 1 | (2.9) | 0 | (0.0) | |
| No follow-up imaging | 208 | (58.6) | 59 | (46.5) | 19 | (55.9) | 130 | (67.0) | 0.001 |
Fisher's exact
right anterior, left anterior, right posterior, left posterior circulations
ICA=internal cerebral artery; MCA=middle cerebral artery; ACA=anterior cerebral artery; PCA=posterior cerebral artery; SCA=superior cerebellar artery; AICA=anterior inferior cerebellar artery; PICA=posterior inferior cerebellar artery
Vascular Imaging Findings
Arterial banding, intimal flap, and intramural hematoma were seen exclusively in patients with definite arteriopathy as these imaging features were used to diagnose arteriopathy (Table 3). One child in the “no arteriopathy” group had a mycotic aneurysm: because the stroke was due to underlying endocarditis, and unrelated to the aneurysm, it was considered cardioembolic. Vascular irregularity and stenosis were observed more often in patients with arteriopathy, but were also seen in patients without arteriopathy and attributed to recanalizing thrombus (such as from cardioembolism). Occlusion was a nonspecific finding that was observed frequently in all subgroups, but most commonly in the possible arteriopathy subgroup, reflecting uncertainty regarding whether the occlusion was due to arterial disease or embolism. When more than one arterial segment was affected, or infarcts were present in more than one vascular territory, definite arteriopathy was more likely. The diagnosis of definite arteriopathy was associated with a higher proportion of vascular imaging findings progressing over time, or progressing then improving.
Discussion
In a large prospective, multicenter, international study, we found that arteriopathy is a common cause of childhood AIS, present in up to 45% of cases (including those with a “possible” arteriopathy). More than half the cases were previously healthy children at the time of their stroke; among them, arteriopathy was present in up to 58%. In our stepwise review process, reviewer agreement was high at each step, and clinical data and follow-up imaging increased the sensitivity for the final “gold standard” primary diagnosis of definite arteriopathy. However, 10% of all cases could not be definitively classified as having, or not having, arteriopathy—suggesting that even with central review by a panel of pediatric stroke investigators, considerable uncertainty remains around childhood arteriopathy diagnosis.
Arteriopathies are important because they are not only a prevalent cause of childhood AIS, but the strongest predictor of recurrence. In 667 cases enrolled at 30 sites in the IPSS (2003–2007), 53% were classified as having an arteriopathy by the site investigators.9 In a population-based study of Californian children enrolled in a managed care plan (1993–2004), 52 children had vascular imaging after AIS: 22 (42%) had non-occlusive abnormalities of cerebral or cervical arteries and another 6 (12%) had large-vessel occlusion.8 While there were no recurrences among 24 children with normal vascular imaging, the 5-year cumulative recurrence rate among those with abnormalities was 66% (p<0.001). A prospective German study of 301 childhood AIS cases (1995–2000) similarly found that arteriopathy was the strongest predictor of recurrence (OR 3.9, 95% CI 1.4, 10.6).13 Hence, accurate diagnosis of childhood arteriopathy is important both for understanding an individual child’s risk of recurrent stroke and for the design of secondary stroke prevention trials.
The results of VIPS also demonstrate that childhood arteriopathies remain difficult to diagnose. Standard vascular imaging is currently limited to “lumenology”—imaging of the arterial lumen, and not the wall of the artery—making it difficult to distinguish a diseased artery from thrombus in a vessel. Imaging features that were particularly suggestive of definite arteriopathy included arterial banding, intimal flap, intramural hematoma, ectasia/aneurysm, and pseudoaneurysm. Vascular irregularity and stenosis were less specific. Occlusion was nonspecific, common in all groups, and most common in the “possible arteriopathy” group, reflecting uncertainty as to whether the artery was diseased, or occluded by thrombus. In VIPS, we observed cases with arterial stenosis on baseline imaging highly suggestive of arteriopathy, yet a clinical history of cardiac thrombus suggested partially recanalized thrombus (Figure 2). The addition of clinical data increased the sensitivity of the primary diagnosis of arteriopathy (from 79% to 90%), but must be applied with caution as cardiac disease can be coincident with arteriopathy in childhood: Down syndrome, for example, is associated with congenital heart defects, moyamoya syndrome, and arterial dissection due to cervical instability.22–24 Follow-up vascular imaging was important, increasing the sensitivity to 94%. Rapid resolution of stenosis pointed towards a resolving thrombus, while a persistent, or even progressive, stenosis suggested arteriopathy. However, rapid diagnosis of arteriopathy is important if we hope to develop interventions to prevent recurrent stroke in children, most of which occur days to weeks after first AIS.8, 13, 25 Improved imaging techniques are needed. Arterial wall imaging, which allows the detection of blood products and enhancement in the vessel walls, may prove to be of benefit to children with AIS by allowing early arteriopathy diagnosis.26, 27
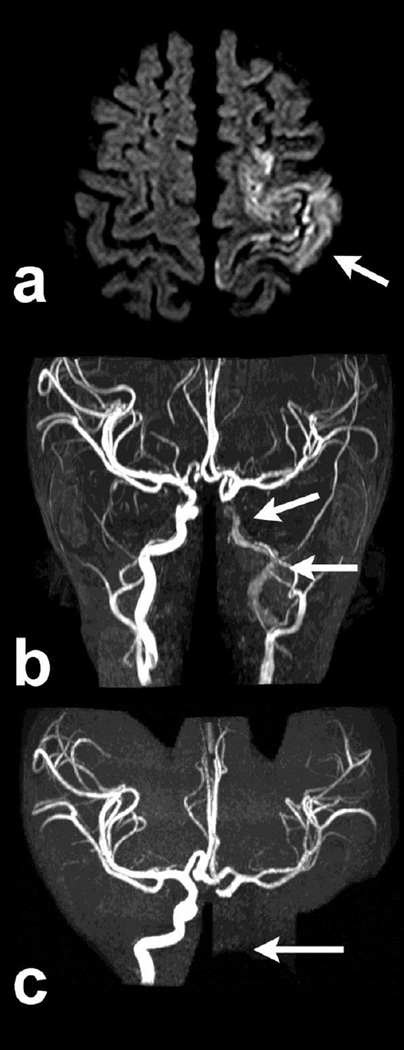
Figure 2.
Child with a left middle cerebral infarct (a, axial DWI, arrow), whose baseline MRA showed distal left internal carotid artery (ICA) stenosis (b, arrows), classified as a “definite arteriopathy” on baseline imaging review without clinical data. Clinical data revealed congenital heart disease with a intracardiac thrombus, suggesting the stenosis was due to embolus. Follow-up MRA 3 months later revealed complete occlusion of the ICA (c, arrows). The child’s final classification was “no arteriopathy,” cardioembolic etiology.
Another challenge is that childhood AIS is uncommon, so it is difficult for neuroradiologists and neurologists to gain experience in diagnosing childhood arteriopathies. Some arteriopathies are unique to childhood, and only rarely seen in adults with stroke. This includes TCA, a common arteriopathy among previously healthy children; the proximal middle cerebral artery banding seen in the very acute phase of this disease can be confused with fibromuscular dysplasia (FMD), an arteriopathy with a very different natural history (Figure 3).28 Adding further to the challenge, the arteriopathies that are more common in adults are rarely seen in children. We made no diagnoses of atherosclerosis in VIPS, even among adolescents, and also no diagnoses of primary CNS vasculitis. Lastly, the arteriopathies observed in children vary somewhat by geographic location: of 15 cases of secondary vasculitis in VIPS, 7 were enrolled in the Philippines, where tuberculosis meningitis remains a common cause of childhood AIS.
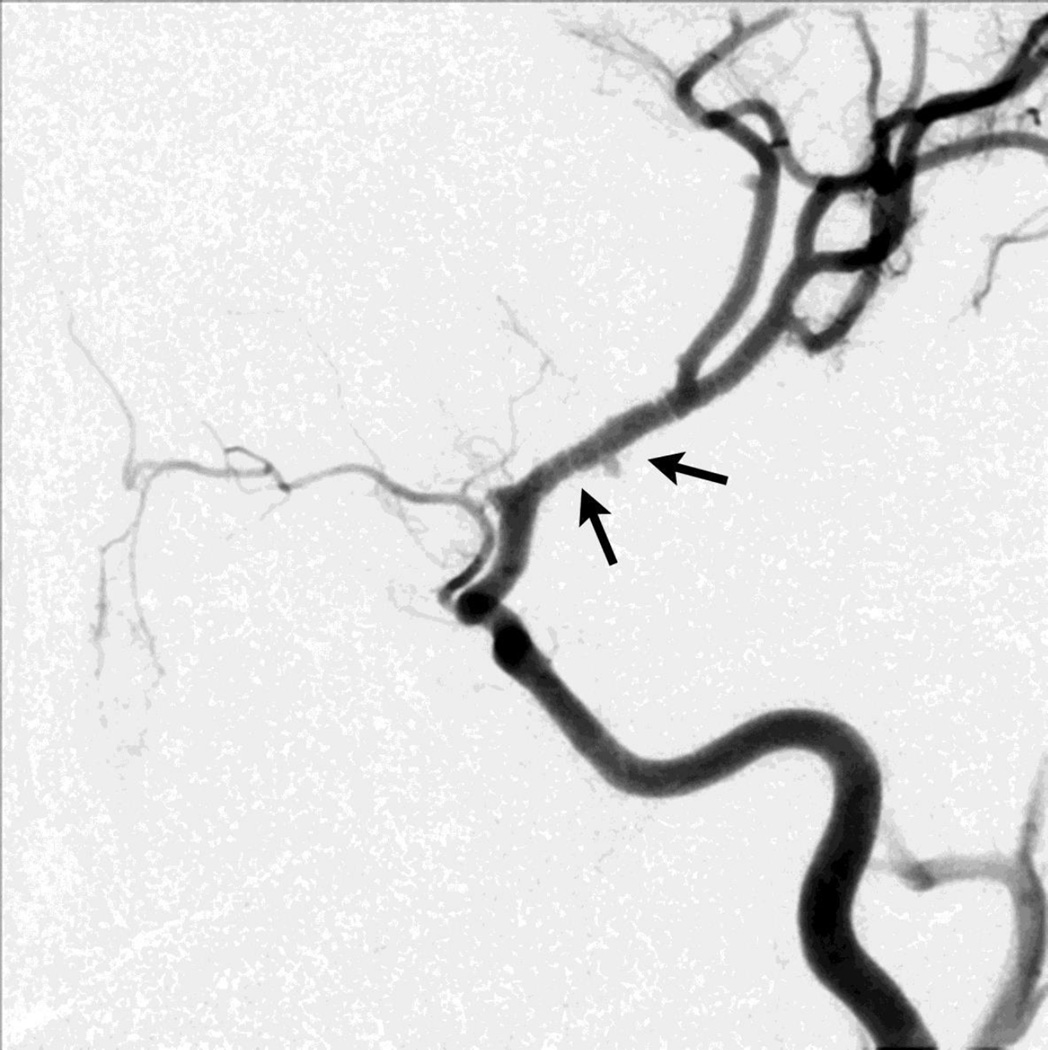
Figure 3.
Angiographic injection of the internal carotid artery showing banding (and small pseudoaneurysm) of the proximal middle cerebral artery (arrow) in a case of “transient cerebral arteriopathy” (TCA), a presumed inflammatory monophasic focal arteriopathy of the distal internal carotid artery and its proximal branches.
Lack of a reliable and user-friendly classification system for childhood arteriopathies remains another challenge. Definitions for childhood arteriopathies have been published,20 and were used in our study, but can be difficult to apply for all the reasons mentioned above. The best available system is the “Childhood AIS Standardized Classification and Diagnostic Evaluation” (CASCADE) system for classification of childhood AIS etiology, including arteriopathies.29 This system has taken a descriptive approach to arteriopathies (including categories such as “unilateral focal cerebral arteriopathy of childhood” and “bilateral cerebral arteriopathy of childhood”), and achieved good inter-rater reliability. But further work is needed to link these categories to both underlying mechanisms and prognosis, particularly recurrence risk.
The main limitation of our study was the variability in patient evaluation related to our complete reliance on clinically-obtained studies. Research imaging studies would have been prohibitively costly, and would have presented ethical issues related to the need for anesthesia in younger children. Although most baseline vascular imaging studies were MRAs of good quality, their timing with respect to the stroke ictus was variable. Only half of cases had cervical imaging, and only 14% had conventional angiography, which still is the gold standard for vascular imaging. The timing and frequency of follow-up vascular imaging was also variable, and many patients with normal vascular imaging at baseline did not receive follow-up, precluding an assessment of whether early MRA can be insensitive to acute arteriopathies like TCA. (In one VIPS case of TCA, the baseline MRA had been clinically interpreted as normal, then progressed to severe middle cerebral artery stenosis within days.) However, the VIPS study is the first-ever international study of childhood AIS to perform centralized imaging review, which allowed us to consistently apply methods for diagnosis of childhood arteriopathy.
Conclusions
Arteriopathy is common among children with AIS, particularly previously healthy children, but remains difficult to diagnose. Clinical data and follow-up imaging both aid in the diagnosis. More systematic use of follow-up vascular imaging, and advances in techniques such as arterial wall imaging, may prove helpful, and could assist both with prognostication in an individual child, and selection of children for secondary stroke prevention trials. We plan to perform further work with the VIPS cohort to determine predictors of arteriopathy subtypes, with the aim of developing simple algorithms that may assist a clinical neuroradiologist or neurologist in generating a reasonable differential diagnosis for arteriopathy in a child with AIS.
Supplementary Material
ACKNOWLEDGMENTS
The authors wish to thank the research coordinators at VIPS sites, and the patients and their families.
FUNDING: NIH R01 NS062820 (PIs Fullerton, DeVeber)
All authors receive NIH funding for this project. Dr. Wintermark received minimal grants from GE Healthcare and Philips Healthcare in the last three years which are not related to the topic of the manuscript.
APPENDIX
Dowling MM(University of Texas Southwestern Medical Center, Dallas), Benedict SL (Primary Children's Medical Center, Salt Lake City), Bernard TJ (Denver Children's Hospital), Fox CK (University of California San Francisco), DeVeber G (The Hospital for Sick Children, Toronto), Friedman NR (Cleveland Clinic Children's Hospital), Lo WD (The Ohio State University and Nationwide Children's Hospital, Columbus OH), Ichord RN (Children's Hospital of Philadelphia), Tan MA (University of the Philippines-Philippine General Hospital, Manila), Mackay MT (Royal Children's Hospital Melbourne), Kirton A (Alberta Children's Hospital), Hernandez Chavez MI (Pontificia Universidad Catolica de Chile), Humphreys P (Children's Hospital of Eastern Ontario), Jordan LC (Vanderbilt University Medical Center, Nashville), Sultan S (Columbia University Medical Center, New York), Rivkin MJ (Boston Children's Hospital), Rafay MF (Children's Hospital, Winnipeg, University of Manitoba), Titomanlio L (L Hôpital Robert Debré-Paris), Kovacevic GS (Mother and Child Health Care Institute, Serbia), Yager JY (Stollery Children's Hospital), Amlie-Lefond C (Seattle Children's Hospital), Dlamini N (Evelina London Children's Hospital), Condie J (Phoenix Children's Hospital), Yeh A (Women and Children's Hospital of Buffalo), Kneen R (Alder Hey Children's Hospital), Bjornson B (British Columbia Children's Hospital), Pergami P (West Virginia University), Zou LP (Chinese PLA General Hospital, Beijing), Elbers J (Stanford Children’s Health, Palo Alto), Abdalla A (Akron Children's Hospital), Chan AK (McMaster University Medical Centre, Hamilton), Farooq O (Women & Children's Hospital of Buffalo), Lim MJ (Evelina London Children's Hospital), Carpenter JL(Children's National Medical Center, Washington, D.C.), Pavlakis S (Maimonides Medical Center, Brooklyn), Wong VC (Queen Mary Hospital, Hong Kong), Forsyth R (Institute of Neuroscience, Newcastle University, UK)
Footnotes
DISCLOSURES: The authors have no commercial interests related to this project.
REFERENCES
- 1.Heron M. Deaths: Leading causes for 2010. Natl Vital Stat Rep. 2013;62:1–96. [PubMed] [Google Scholar]
- 2.Agrawal N, Johnston SC, Wu YW, Sidney S, Fullerton HJ. Imaging data reveal a higher pediatric stroke incidence than prior US estimates. Stroke. 2009;40:3415–3421. doi: 10.1161/STROKEAHA.109.564633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kleindorfer D, Khoury J, Kissela B, Alwell K, Woo D, Miller R, et al. Temporal trends in the incidence and case fatality of stroke in children and adolescents. J Child Neurol. 2006;21:415–418. doi: 10.1177/08830738060210050301. [DOI] [PubMed] [Google Scholar]
- 4.Giroud M, Lemesle M, Gouyon JB, Nivelon JL, Milan C, Dumas R. Cerebrovascular disease in children under 16 years of age in the city of Dijon, France: A study of incidence and clinical features from 1985 to 1993. J Clin Epidemiol. 1995;48:1343–1348. doi: 10.1016/0895-4356(95)00039-9. [DOI] [PubMed] [Google Scholar]
- 5.Mackay MT, Wiznitzer M, Benedict SL, Lee KJ, Deveber GA, Ganesan V. Arterial ischemic stroke risk factors: The International Pediatric Stroke Study. Ann Neurol. 2011;69:130–140. doi: 10.1002/ana.22224. [DOI] [PubMed] [Google Scholar]
- 6.Hills NK, Johnston SC, Sidney S, Zielinski BA, Fullerton HJ. Recent trauma and acute infection as risk factors for childhood arterial ischemic stroke. Ann Neurol. 2012;72:850–858. doi: 10.1002/ana.23688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kenet G, Lutkhoff LK, Albisetti M, Bernard T, Bonduel M, Brandao L, et al. Impact of thrombophilia on risk of arterial ischemic stroke or cerebral sinovenous thrombosis in neonates and children: A systematic review and meta-analysis of observational studies. Circulation. 2010;121:1838–1847. doi: 10.1161/CIRCULATIONAHA.109.913673. [DOI] [PubMed] [Google Scholar]
- 8.Fullerton HJ, Wu YW, Sidney S, Johnston SC. Risk of recurrent childhood arterial ischemic stroke in a population-based cohort: The importance of cerebrovascular imaging. Pediatrics. 2007;119:495–501. doi: 10.1542/peds.2006-2791. [DOI] [PubMed] [Google Scholar]
- 9.Amlie-Lefond C, Bernard TJ, Sebire G, Friedman NR, Heyer GL, Lerner NB, et al. Predictors of cerebral arteriopathy in children with arterial ischemic stroke: Results of the International Pediatric Stroke Study. Circulation. 2009;119:1417–1423. doi: 10.1161/CIRCULATIONAHA.108.806307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldenberg NA, Bernard TJ, Fullerton HJ, Gordon A, deVeber G. Antithrombotic treatments, outcomes, and prognostic factors in acute childhood-onset arterial ischaemic stroke: A multicentre, observational, cohort study. Lancet Neurol. 2009;8:1120–1127. doi: 10.1016/S1474-4422(09)70241-8. [DOI] [PubMed] [Google Scholar]
- 11.Danchaivijitr N, Cox TC, Saunders DE, Ganesan V. Evolution of cerebral arteriopathies in childhood arterial ischemic stroke. Ann Neurol. 2006;59:620–626. doi: 10.1002/ana.20800. [DOI] [PubMed] [Google Scholar]
- 12.Ganesan V, Prengler M, McShane MA, Wade AM, Kirkham FJ. Investigation of risk factors in children with arterial ischemic stroke. Ann Neurol. 2003;53:167–173. doi: 10.1002/ana.10423. [DOI] [PubMed] [Google Scholar]
- 13.Strater R, Becker S, von Eckardstein A, Heinecke A, Gutsche S, Junker R, et al. Prospective assessment of risk factors for recurrent stroke during childhood--a 5-year follow-up study. Lancet. 2002;360:1540–1545. doi: 10.1016/S0140-6736(02)11520-0. [DOI] [PubMed] [Google Scholar]
- 14.Chabrier S, Husson B, Lasjaunias P, Landrieu P, Tardieu M. Stroke in childhood: Outcome and recurrence risk by mechanism in 59 patients. J Child Neurol. 2000;15:290–294. doi: 10.1177/088307380001500504. [DOI] [PubMed] [Google Scholar]
- 15.Zimmer JA, Garg BP, Williams LS, Golomb MR. Age-related variation in presenting signs of childhood arterial ischemic stroke. Pediatr Neurol. 2007;37:171–175. doi: 10.1016/j.pediatrneurol.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 16.Fullerton HJ, Elkind MS, Barkovich AJ, Glaser C, Glidden D, Hills NK, et al. The Vascular effects of Infection in Pediatric Stroke (VIPS) study. J Child Neurol. 2011;26:1101–1110. doi: 10.1177/0883073811408089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sims JR, Gharai LR, Schaefer PW, Vangel M, Rosenthal ES, Lev MH, et al. ABC/2 for rapid clinical estimate of infarct, perfusion, and mismatch volumes. Neurology. 2009;72:2104–2110. doi: 10.1212/WNL.0b013e3181aa5329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hess CP, Fullerton HJ, Metry DW, Drolet BA, Siegel DH, Auguste KI, et al. Cervical and intracranial arterial anomalies in 70 patients with PHACE syndrome. AJNR Am J Neuroradiol. 2010;31:1980–1986. doi: 10.3174/ajnr.A2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frieden IJ, Reese V, Cohen D. Phace syndrome. The association of Posterior fossa brain malformations, Hemangiomas, Arterial anomalies, Coarctation of the aorta and cardiac defects, and Eye abnormalities. Arch Dermatol. 1996;132:307–311. doi: 10.1001/archderm.132.3.307. [DOI] [PubMed] [Google Scholar]
- 20.Sebire G, Fullerton H, Riou E, deVeber G. Toward the definition of cerebral arteriopathies of childhood. Curr Opin Pediatr. 2004;16:617–622. doi: 10.1097/01.mop.0000144441.29899.20. [DOI] [PubMed] [Google Scholar]
- 21.Chabrier S, Rodesch G, Lasjaunias P, Tardieu M, Landrieu P, Sebire G. Transient cerebral arteriopathy: A disorder recognized by serial angiograms in children with stroke. J Child Neurol. 1998;13:27–32. doi: 10.1177/088307389801300105. [DOI] [PubMed] [Google Scholar]
- 22.Jea A, Smith ER, Robertson R, Scott RM. Moyamoya syndrome associated with Down syndrome: Outcome after surgical revascularization. Pediatrics. 2005;116:e694–e701. doi: 10.1542/peds.2005-0568. [DOI] [PubMed] [Google Scholar]
- 23.Pearson E, Lenn NJ, Cail WS. Moyamoya and other causes of stroke in patients with Down syndrome. Pediatr Neurol. 1985;1:174–179. doi: 10.1016/0887-8994(85)90060-8. [DOI] [PubMed] [Google Scholar]
- 24.Zanin A, Blanc R, Alison M, Romanello S, Piotin M, Titomanlio L. Acute ischaemic stroke due to carotid dissection in a boy with Down syndrome. Acta Paediatr. 2013;102:e50–e51. doi: 10.1111/apa.12084. [DOI] [PubMed] [Google Scholar]
- 25.Ganesan V, Prengler M, Wade A, Kirkham FJ. Clinical and radiological recurrence after childhood arterial ischemic stroke. Circulation. 2006;114:2170–2177. doi: 10.1161/CIRCULATIONAHA.105.583690. [DOI] [PubMed] [Google Scholar]
- 26.Qiao Y, Steinman DA, Qin Q, Etesami M, Schar M, Astor BC, et al. Intracranial arterial wall imaging using three-dimensional high isotropic resolution black blood MRI at 3.0 tesla. J Magn Reson Imaging. 2011;34:22–30. doi: 10.1002/jmri.22592. [DOI] [PubMed] [Google Scholar]
- 27.Swartz RH, Bhuta SS, Farb RI, Agid R, Willinsky RA, Terbrugge KG, et al. Intracranial arterial wall imaging using high-resolution 3-tesla contrast-enhanced MRI. Neurology. 2009;72:627–634. doi: 10.1212/01.wnl.0000342470.69739.b3. [DOI] [PubMed] [Google Scholar]
- 28.Kirton A, Crone M, Benseler S, Mineyko A, Armstrong D, Wade A, et al. Fibromuscular dysplasia and childhood stroke. Brain. 2013;136:1846–1856. doi: 10.1093/brain/awt111. [DOI] [PubMed] [Google Scholar]
- 29.Bernard TJ, Manco-Johnson MJ, Lo W, MacKay MT, Ganesan V, DeVeber G, et al. Towards a consensus-based classification of childhood arterial ischemic stroke. Stroke. 2012;43:371–377. doi: 10.1161/STROKEAHA.111.624585. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.