Abstract
It is well established that healthy aging, amnestic Mild Cognitive Impairment (aMCI), and Alzheimer’s Disease (AD) are associated with substantial declines in episodic memory. However, there is still debate as to how two forms of episodic memory – recollection and familiarity – are affected by healthy and pathological aging. To address this issue we conducted a meta-analytic review of the effect sizes reported in studies using remember/know (RK), receiver operating characteristic (ROC) and process dissociation (PD) methods to examine recollection and familiarity in healthy aging (25 published reports), aMCI (9 published reports), and AD (5 published reports). The results from the meta-analysis revealed that healthy aging is associated with moderate-to-large recollection impairments. Familiarity was not impaired in studies using ROC or PD methods but was impaired in studies that used the RK procedure. aMCI was associated with large decreases in recollection whereas familiarity only tended to show a decrease in studies with a patient sample comprised of both single-domain and multiple-domain aMCI patients. Lastly, AD was associated with large decreases in both recollection and familiarity. The results are consistent with neuroimaging evidence suggesting that the hippocampus is critical for recollection whereas familiarity is dependent on the integrity of the surrounding perirhinal cortex. Moreover, the results highlight the relevance of method selection when examining aging, and suggest that familiarity deficits might be a useful behavioral marker for identifying individuals that will develop dementia.
Keywords: Aging, Recollection, Familiarity, Alzheimer’s disease, amnestic Mild Cognitive Impairment
Healthy aging is associated with impairments in episodic memory (Drag and Bieliauskas 2010; Hoyer and Verhaeghen 2006; Light 1991; Verhaeghen et al. 1993), although not all forms of episodic memory are equally impaired. For instance, healthy older adults show larger deficits on free recall tests compared to yes/no recognition tests (Craik and McDowd 1987; Schonfield and Robertson 1966; Whiting and Smith 1997; for review, see La Voie and Light 1994), and on tests of associative recognition compared to tests of single item recognition (for review, see Old and Naveh-Benjamin 2008; Spencer and Raz 1995). Such findings have been taken as evidence that healthy aging selectively impairs some episodic memory processes while leaving others unaffected.
A dual-process account of these findings suggests that aging leads to a relatively selective deficit in recollection, which is the ability to retrieve qualitative information about a prior study event, that leaves familiarity-based recognition judgments unaffected (Anderson et al. 2008; Luo et al. 2007; Parkin and Walter 1992; Yonelinas 2002). A growing number of studies have been conducted to assess this possibility using various methods to estimate the contributions of recollection and familiarity to overall performance in young and older adults. However, there is still disagreement about the nature of recollection and familiarity impairments in healthy aging. Some have reported that healthy aging is associated with selective declines in recollection-based episodic memory (Cohn et al. 2008; Jennings and Jacoby 1993; 1997; McCabe et al. 2009; Parkin and Walter 1992; Wolk et al. 2013; Yonelinas 2002), whereas others have found that healthy aging is associated with declines in both recollection- and familiarity-based episodic memory (Belleville et al. 2011; Duarte et al. 2006; Düzel et al. 2011; Friedman et al. 2010; Parks 2007; Peters and Daum 2008; Prull et al. 2006; Wang et al. 2012).
Understanding how recollection and familiarity-based episodic memory change during the course of healthy aging is critical not only in developing behavioral interventions aimed at slowing age-related cognitive decline, but also in determining the potential utility of using recollection and familiarity to identify individuals that will develop dementia. A hallmark of Alzheimer’s disease (AD) – the most common form of dementia associated with aging – is severe memory impairment, and a substantial body of work has indicated that symptoms predictive of developing AD occur well before a diagnosis can be made (for reviews, see Didic et al. 2011; Petersen 2004; Salmon 2012). Amnestic Mild Cognitive Impairment (aMCI) is believed to represent the transitional period between healthy aging and AD where early AD symptoms can be detected (Petersen 2004; Petersen et al. 2009; Salmon 2012). Although not all individuals with aMCI will progress to AD (Petersen et al. 2009), abnormally low episodic memory performance is associated with conversion to AD (e.g., Landau et al. 2010).
There is a growing interest in determining how aMCI and AD affect recollection-based and familiarity-based episodic memory. Results from studies comparing performance on tasks believed to rely primarily on recollection (e.g., free recall and associative recognition) to tasks thought to depend more on familiarity (e.g., old/new and forced-choice recognition tests) have led some to conclude that aMCI and AD are associated with declines in both recollection and familiarity (Algarabel et al. 2009, 2012; Bennett et al. 2006; Dudas et al. 2005;). For example, aMCI and AD patients show similarly large impairments on tests of free recall and item recognition (Bennett et al. 2006), which, from a dual-process perspective, indicates that recollection and familiarity are similarly impaired in aMCI and AD. However, Westerberg and colleagues (2006, 2013) reported that aMCI patients only show impairments on tasks thought to be rely relatively more on recollection (i.e., old/new recognition) whereas AD patients are impaired on tasks that rely on recollection and tasks that rely primarily on familiarity (i.e., forced choice-recognition; see Bastin and Van der Linden 2003). Such findings suggest that aMCI is associated with specific recollection impairments whereas AD is associated with declines in both recollection and familiarity. Moreover, results from studies that have used methods to estimate the contribution of recollection and familiarity have also led to mixed conclusions. For example, some studies showing that recollection is selectively affected in both aMCI (e.g., Anderson et al. 2008; Troyer et al. 2012) and AD patients (e.g., Genon et al. 2013), whereas other studies find that estimates of both recollection and familiarity are impaired in aMCI (Wolk et al. 2008, 2011, 2013) and AD (e.g., Ally et al. 2009; Wolk et al. 2011). Thus, similar to the studies examining healthy aging, the extant literature is mixed as to the fate of recollection and familiarity in aMCI and AD.
Our aim in this report is to address the above debates by conducting a meta-analytic review of the literature examining how healthy aging, aMCI, and AD affect recollection-based and familiarity-based episodic memory. The review is divided into four main sections. First, we provide an overview of the methods that have been used to measure recollection and familiarity. Second, we briefly discuss some variables to might lead to systematic differences (i.e., moderate) in pattern of recollection and familiarity decreases observed in the extant literature. Third, we present the quantitative meta-analysis of the literature examining recollection and familiarity differences in the three populations of interest. In particular, our goal is to determine if recollection, familiarity, or both are impaired to in these three populations, and to see these decreases are moderated by specific experimental variables. Fourth, we discuss the implications of the findings from the meta-analysis and relate the results to the emerging neuroimaging literature on age-related memory declines.
Measuring Recollection and Familiarity
Many different approaches have been developed to assess the contributions of recollection and familiarity to memory performance (for review, see Light 2011; Yonelinas 2002). The three most widely used process estimation methods are the Remember/Know (RK) procedure (Gardiner 1988; Tulving 1985), Process Dissociation (PD) procedure (Jacoby 1991; for review, see Yonelinas and Jacoby 2012), and the analysis of recognition memory receiver operating characteristics with the dual-process signal-detection model (i.e., the ROC procedure; Yonelinas 1994; 1999; for review, see Yonelinas and Parks 2007; see Supplemental Material)1. In the RK procedure, participants are asked to make introspective reports about their memory judgments (Gardiner 1988; Tulving 1985). For items judged to be from the study list, participants are instructed to make a ‘Remember’ response when they can recollect specific details of the prior encounter with the item and to make a ‘Know’ response if no details are recollected but there is a general sense the item was studied. Estimates of recollection and familiarity are derived from RK judgments using the independent RK formulas (Yonelinas and Jacoby 1995) that account for the fact that ‘Remember’ and ‘Know’ judgments are mutually exclusive.
The PD procedure relies on the logic that recollection and familiarity will lead to different memory judgments when placed in opposition (Jacoby 1991). In a typical PD experiment, a participant might study two lists of words presented in different modalities (e.g., auditory versus visual), followed by two recognition test phases. The inclusion test is a standard recognition test in which participants judge all studied items as ‘old’ and items that were not studied as ‘new’. On this test, recollection and familiarity act in concert to support accurate recognition decisions. In contrast, the exclusion test is a modified recognition test in which participants identify test items from one source dimension (e.g., aurally presented words during study) as ‘old’ and words from the other source dimension (e.g., visually presented words during study) as well as words that were not studied as ‘new’. On the exclusion tests, recollection and familiarity act in opposition when making recognition decisions about the to-be-excluded studied items (e.g., visually presented words). Specifically, familiarity in the absence of recollecting the critical source detail will lead to incorrect ‘old’ responses whereas recollecting the source detail will lead to the correct rejection of the to-be-excluded item. Recollection and familiarity estimates are derived from the proportion of ‘old’ responses given to items on the inclusion test and the proportion of ‘old’ responses given to to-be-excluded items on the exclusion test (Jacoby 1991; Yonelinas and Jacoby 1996a).
The ROC procedure involves a recognition memory test in which participants make their old/new memory decisions using a confidence scale (e.g., 6-‘sure old’, 5-‘maybe old’, 4-‘guess old’, 3-‘guess new’, 2-‘maybe new’, 1-‘sure new’). The confidence judgments are used to construct an ROC – a plot of the cumulative hit rate and false alarm rate across confidence bins (i.e., levels of response bias; Yonelinas and Parks 2007). For example, the first (i.e., left-most) point on the ROC is the proportion of old and new words that receive a 6-‘sure old’ judgments, and the second point of the ROC is the proportion of old and new words that receive a 6-‘sure old’ or a 5-‘sure new’ response. Each participant’s ROC is fit with the dual-process signal detection model to derive estimates of recollection and familiarity (Yonelinas 1994, 1999).
Although there are many similarities between the three methods regarding the assumptions underlying recollection and familiarity (e.g., the independence assumption), there are important differences between the methods in how the two processes are estimated. The RK method measures recollection and familiarity directly from introspective judgments of when recollection does and does not occur. In contrast, the PD procedure estimates recollection as the ability to remember a specific aspect (i.e., source detail) of the study event and estimates familiarity as the absence of recollecting the to-be-remembered detail. The ROC method is different from both of the above methods in that recollection and familiarity are not measured by asking participants to report a recollective experience per se. Instead, recollection and familiarity are inferred from the parameters obtained by fitting the dual-process signal detection model to the observed ROC constructed from confidence responses. As discussed below, the differences between these methods might account for some of the mixed findings across studies.
Variables Moderating Group Differences in Recollection and Familiarity
As mentioned above, the findings surrounding the effect of healthy aging, aMCI, and AD on recollection and familiarity are mixed. Although some of the variability is likely attributable to random factors across studies, it is possible that there are systematic influences underlying the mixed findings in the literature. One factor that could lead to systematic differences is the degree to which recollection contributes to overall recognition performance. Yonelinas (2002) proposed that high levels of recollection (e.g., recollection estimates greater than 0.60) can inflate estimates of familiarity derived from the RK, PD, and ROC procedures. To the extent that high levels of recollection are isolated to the control group, familiarity differences between older adults relative to young adults and aMCI/AD patients relative to healthy, age-matched controls might be due to artifacts in estimating recollection and familiarity. Yonelinas’s (2002) review of the literature supported this hypothesis in healthy aging studies by showing that familiarity estimates were age-invariant when studies had “normal” levels of recollection (i.e., estimates≤0.60) but the age-related decreases in familiarity were observed for studies with high levels of recollection (i.e., estimates>0.60). Thus, it could be the case that at least some of the variability across studies is accounted for by measurement artifacts caused by high levels of recollection.
A second factor is the method that is used to estimate recollection and familiarity (Light et al. 2000; Prull et al. 2006). As discussed in “Measuring Recollection and Familiarity”, this could come about because what constitutes recollection-based and familiarity-based recognition differs across the three estimation methods. Evidence in support of estimation method differences comes from a study by Prull and colleagues (2006) who examined age-related differences in recollection and familiarity derived from each estimation method in the same set of participants. Although the results from this study showed significant age-related differences in recollection in all three tasks, significant age-related differences in familiarity were only observed for the RK and ROC tasks. This difference could potentially arise because of differences in how the instructions are interpreted by different groups of participants, the type of memory judgment required (i.e., subjective or objective), or the assumptions underlying the different methods.
A third factor that might moderate the magnitude of group differences in recollection and familiarity is the type of materials (i.e., verbal or nonverbal materials). There is some evidence that age-related memory impairments might be greater for verbal than nonverbal materials. For example, age-related memory impairments are substantially reduced when pictorial stimuli are used compared to verbal stimuli (Ally et al. 2008; Park et al. 1983; Winograd et al. 1982). Such a finding would suggest that pictorial stimuli might lead to reductions in age-related declines in recollection, familiarity, or both processes. Consistent with this, a study by Luo and colleagues (2007) found that age-related differences in recollection were not present when words were studied with a corresponding picture. Moreover, using event-related potentials, Ally and colleagues (2008) showed that age-related differences in the left parietal old/new effect, which is the ERP correlate of recollection (Rugg and Curran 2007), were present for words but not pictures. Similarly, a study by Embree and colleagues (2012) reported that familiarity impairments in aMCI patients were robust for word stimuli, but absent for picture stimuli. Thus, reductions in recollection and familiarity in healthy older adults, aMCI, and AD might be dependent on the use of verbal or nonverbal materials.
One additional factor that may turn out to be important in aMCI studies is the inclusion of different subtypes of aMCI patients. As described by Petersen (2004), aMCI can be diagnosed as single-domain or multiple-domain. Single-domain aMCI is associated with selective memory impairments and no detectable impairments in other cognitive domains. By contrast, multiple-domain aMCI patients are impaired in at least one other cognitive domain (e.g., executive functioning) in addition to memory impairments. Although we are unaware of any study that has directly contrasted recollection and familiarity in single-domain and multiple-domain aMCI patients, some of the studies reporting spared familiarity-based recognition in aMCI only tested single-domain patients (Anderson et al. 2008; Serra et al. 2010; Troyer et al. 2012), whereas other studies that reported familiarity impairments in aMCI tested both single-domain and multiple-domain patients (Ally et al. 2009; Embree et al. 2012; Wolk et al. 2008, 2013).
The discussion above highlights that high levels of recollection, estimation method, type of materials, and single-domain or multiple-domain aMCI diagnosis might fully or partially account for the mixed findings in the literature. Thus, we included these variables in the meta-analysis reported below when the extant literature allowed us to do so. Specifically, the moderator variables examined for healthy aging studies included the level of recollection (i.e., high vs. “normal”), the process estimation method (i.e., RK, PD, or ROC), and the type of materials (verbal vs. nonverbal). Because of the limited number of relevant studies, the only moderator variable examined for aMCI studies was the type of patients examined (i.e., single-domain only vs. single-domain and multiple-domain patients). No moderator variables were examined for AD studies because only 5 articles reported enough data to be included in the meta-analysis.
Meta-Analysis of Published Studies
The primary focus of the meta-analysis was to determine if recollection, familiarity or both are impaired in healthy older adults, and aMCI and AD patients. A meta-analysis of the extant literature is useful in addressing the debates described above because it allows the magnitude of recollection and familiarity declines to be quantified across studies. We focused on studies that estimated recollection and familiarity using the RK, PD, or ROC procedures discussed previously. The studies examining healthy aging, aMCI, and AD were examined separately because the control group differed depending on the type of study; healthy aging studies used young adults as the control group, whereas studies with aMCI and AD patients used age-matched healthy older adults as the control group. In addition, we examined if the recollection and familiarity differences in these three groups varied systematically across the potential moderators discussed above.
Approximately half of the healthy aging studies that examined recognition memory with the RK, PD, or ROC procedures were excluded from the meta-analysis because estimates of recollection and familiarity were not reported (see Methods). Thus, we were concerned that the high proportion of excluded samples from the effect size analysis biased the results of the meta-analysis. To address this concern, we examined all studies with a complementary approach. Specifically, we adopted the approach used by Yonelinas (2002) whereby estimates of recollection and familiarity are derived for each sample from the average data reported in the paper. Although this approach allows us to obtain a recollection and familiarity estimate for all of the studies, it does not allow us to estimate an effect size measure (specifically Cohen’s d) that takes into account subject-level variability. The results from this approach are described in detail in the Supplemental Material.
Methods
Literature Search
PsycInfo and PubMed database searches were conducted to find articles that examined recollection- and familiarity-based recognition memory in samples of healthy older adults and in patients diagnosed with aMCI or AD. The following search terms were used: aging, amnestic mild cognitive impairment, Alzheimer’s disease, recognition memory, recollection, familiarity, process dissociation, remember, know, receiver-operating characteristic, dual-process theory. The search period for the database searches were between 1975 and 2013. We identified studies that reported using the RK, PD, or ROC procedures to examine episodic memory in healthy older adults, aMCI patients, or AD patients. Additional studies meeting our inclusion criteria, which are outlined below, were identified by searching the reference section of each article. The literature search was completed at the end of July 2013.
Inclusion/Exclusion Criteria
Only articles published in a peer-reviewed journal in English were included. Furthermore, to be included the meta-analysis, the study must have used the RK, PD, or ROC procedure to estimate recollection and familiarity using previously published formulas (e.g., Jacoby 1991; Yonelinas 1994, 1999; Yonelinas and Jacoby 1995, 1996a) in a recognition memory task. Studies using the RK procedure were excluded if they did not include false alarm rates for ‘Remember’ and ‘Know’ responses (e.g., Fell 1992; Friedman and Trott 2000; Larsson et al. 2006; Lovden et al. 2002; Mark and Rugg 1998).
The inclusion criteria specific to healthy aging studies were similar to Old and Naveh-Benjamin (2008). Specifically, experiments must have included a young adult group with a mean age below 30 years and a healthy (i.e., no memory pathology, such as AD), community-dwelling older adult group with a mean age of greater than 60 years. Across 49 published papers, we identified 68 independent comparisons of young and older adults (see Table 1). We treated each experiment in a multiple-experiment report as an independent observation when a new sample of participants were recruited (e.g., Healy et al. 2005; Jennings and Jacoby 1993; Luo et al. 2007; McCabe and Geraci 2009; Parks 2007; Skinner and Fernandes 2009a). Likewise, experiments that employed a between-subject manipulation crossed with age were treated as providing two independent observations (e.g., McCabe and Geraci 2009; Toth and Parks 2006). For example, the easy and hard source memory groups in Toth and Parks (2006) were treated as two independent samples examining recollection and familiarity in older adults. The one exception to this was Experiment 2 in Parks (2007), where the Easy and Broad groups were treated as a single group because this was the only way to estimate the effect size in this experiment. Studies reporting multiple older adults samples with a single young adult control group were treated as a single older adult group (Davidson and Glisky 2002; Duarte et al. 2006). Note that one experiment that met all of our inclusion criteria for this meta-analysis (Duarte et al. 2010) was excluded because the older adult sample was a subset of a larger sample from a previous study in the same lab (Duarte et al. 2008) that was selected to have low memory performance.
Table 1.
Studies examining recollection and familiarity differences between healthy young and older adults
| Young Adults | Older Adults | Effect Size d | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | R>.6 | Estimation Method |
Material Type |
n | R (prob.) |
F (prob.) |
n | R (prob.) |
F (prob.) |
R | F |
| Anderson et al. (2008) | Yes | PD | Verbal | 25 | 0.86 | 0.44 | 44 | 0.76 | 0.48 | −0.56 | 0.30 |
| Angel et al. (2013) | No | RK | Nonverbal | 20 | 0.40 | 0.55 | 20 | 0.31 | 0.61 | −0.72 | 0.43 |
| Bastin and Van der Linden (2003) | No | RK | Nonverbal | 64 | 0.35 | 0.42 | 64 | 0.24 | 0.42 | – | – |
| Bastin et al. (2004) | No | RK | Nonverbal | 48 | 0.44 | 0.82 | 48 | 0.26 | 0.74 | – | – |
| Belleville et al. (2011)a | No | RK | Verbal/Nonverbal | 29 | – | – | 29 | – | – | −0.66 | −1.04 |
| Benjamin and Craik (2001) – Exp. 2 | No | PD | Verbal | 18 | 0.58 | 0.42 | 34 | 0.38 | 0.53 | – | – |
| Boywitt et al. (2012) – Exp. 1 | No | RK | Verbal | 40 | 0.50 | 0.46 | 41 | 0.38 | 0.41 | – | – |
| Boywitt et al. (2012) – Exp. 2 | No | RK | Verbal | 44 | 0.31 | 0.27 | 44 | 0.23 | 0.20 | – | – |
| Bugaiska et al. (2007) | No | RK | Verbal | 24 | 0.28 | 0.35 | 24 | 0.15 | 0.28 | – | – |
| Bunce (2003) | No | RK | Verbal | 44 | 0.47 | 0.69 | 52 | 0.35 | 0.63 | – | – |
| Bunce and Macready (2005) | No | RK | Verbal | 52 | 0.54 | 0.54 | 52 | 0.47 | 0.48 | – | – |
| Clarys et al. (2002) | No | RK | Verbal | 27 | 0.22 | 0.28 | 55 | 0.14 | 0.30 | – | – |
| Clarys et al. (2009) | No | RK | Verbal | 44 | 0.37 | 0.43 | 44 | 0.27 | 0.36 | – | – |
| Cohn et al. (2008) – Exp. 1 | No | PD | Verbal | 24 | 0.48 | 0.21 | 24 | 0.25 | 0.30 | −1.07 | 0.18 |
| Comblain et al. (2004) | No | RK | Nonverbal | 20 | 0.54 | 0.47 | 20 | 0.29 | 0.29 | – | – |
| Daselaar et al. (2006) | No | ROC | Verbal | 12 | 0.40 | 0.36 | 12 | 0.20 | 0.46 | −0.72 | 0.34 |
| Davidson and Glisky (2002) | No | PD | Verbal | 32 | 0.40 | 0.84 | 48 | 0.21 | 0.69 | −0.69 | −0.69 |
| Duarte et al. (2006) | No | RK | Nonverbal | 18 | 0.55 | 0.36 | 24 | 0.44 | 0.24 | −0.95 | −0.61 |
| Duarte et al. (2008) | No | RK | Nonverbal | 17 | 0.51 | 0.58 | 27 | 0.49 | 0.46 | – | – |
| Düzel et al. (2011) | No | ROC | Nonverbal | 24 | 0.27 | 0.27 | 56 | 0.17 | 0.17 | −0.72 | −1.24 |
| Friedman et al. (2010) | No | RK | Nonverbal | 18 | 0.31 | 0.41 | 17 | 0.19 | 0.24 | −0.88 | −1.06 |
| Glanzer et al. (2004) – Exp. 5 | No | ROC | Verbal | 42 | 0.46 | 0.34 | 24 | 0.29 | 0.29 | – | – |
| Harkins et al. (1979) | No | ROC | Verbal | 8 | 0.44 | 0.27 | 16 | 0.27 | 0.18 | – | – |
| Healy et al. (2005) – Exp. 1d | No | ROC | Verbal | 59 | 0.41 | 0.44 | 60 | 0.28 | 0.51 | −0.67 | 0.31 |
| Healy et al. (2005) – Exp. 2d | No | ROC | Verbal | 31 | 0.47 | 0.50 | 33 | 0.34 | 0.48 | −0.65 | −0.08 |
| Healy et al. (2005) – Exp. 3d | No | ROC | Verbal | 25 | 0.37 | 0.58 | 36 | 0.32 | 0.48 | −0.29 | −0.29 |
| Howard et al. (2006) | No | ROC | Nonverbal | 43 | 0.30 | 0.22 | 33 | 0.25 | 0.24 | −0.57 | 0.36 |
| Jacoby (1999) –Exp.4 | No | PD | Verbal | 24 | 0.56 | 0.39 | 48 | 0.29 | 0.33 | −0.97a | 0b,c,d |
| Jennings and Jacoby (1993) – Exp. 1 | Yes | PD | Verbal | 24 | 0.60 | 0.14 | 24 | 0.25 | 0.10 | −1.52a | 0b,d |
| Jennings and Jacoby (1993) –Exp.2 | No | PD | Verbal | 16 | 0.42 | 0.63 | 16 | 0.25 | 0.60 | −1.02a | 0b,c,d |
| Jennings and Jacoby (1997) – Exp. 2 | Yes | PD | Verbal | 24 | 0.87 | 0.60 | 24 | 0.59 | 0.54 | – | – |
| Kapucu et al. (2008) | No | ROC | Verbal | 22 | 0.29 | 0.23 | 23 | 0.30 | 0.22 | – | – |
| Kilb & Naveh-Benjamin (2011) | No | RK | Nonverbal | 25 | 0.54 | 0.70 | 26 | 0.29 | 0.70 | – | – |
| Luo and Craik (2009) –Exp.1 | No | PD | Verbal | 32 | 0.27 | 0.09 | 32 | 0.03 | −0.05 | – | – |
| Luo and Craik (2009) – Exp. 2 | No | PD | Verbal | 24 | 0.40 | −0.02 | 24 | 0.27 | −0.02 | – | – |
| Luo et al. (2007) – Exp. 1 | No | PD | Verbal | 32 | 0.53 | 0.38 | 32 | 0.21 | 0.37 | −0.86 | 0.16d,f |
| Luo et al. (2007) – Exp. 2A | No | PD | Verbal | 27 | 0.44 | 0.36 | 27 | 0.31 | 0.41 | −0.47 | 0.24d |
| Luo et al. (2007) – Exp. 2B | No | PD | Verbal | 18 | 0.54 | 0.32 | 18 | 0.42 | 0.36 | −0.59 | 0.18d |
| Luo et al. (2007) – Exp. 2C | No | PD | Verbal | 32 | 0.44 | 0.39 | 32 | 0.31 | 0.54 | −0.57 | 0.74d |
| McCabe and Geraci (2009) – Exp. 1 (RK Group) | No | RK | Verbal | 36 | 0.29 | 0.27 | 36 | 0.24 | 0.26 | −0.53 | −0.10d |
| McCabe and Geraci (2009) – Exp. 1 (AB Group) | No | RK | Verbal | 37 | 0.38 | 0.33 | 36 | 0.25 | 0.31 | −0.90 | −0.35d |
| McCabe and Geraci (2009) –Exp.2 | No | RK | Verbal | 38 | 0.30 | 0.34 | 34 | 0.22 | 0.34 | −0.46 | 0.03d |
| McCabe et al. (2009) | No | RK | Verbal | 67 | 0.32 | 0.35 | 68 | 0.23 | 0.32 | −0.92 | −0.27d |
| Morcom et al. (2010) | No | RK | Verbal | 15 | 0.36 | 0.32 | 12 | 0.29 | 0.30 | −0.66 | 0b |
| Norman and Schacter (1997) – Exp. 1 (Exp. Group) | No | RK | Verbal | 12 | 0.52 | 0.48 | 12 | 0.46 | 0.34 | – | – |
| Norman and Schacter (1997) – Exp. 1 (No Exp. Group) | No | RK | Verbal | 12 | 0.53 | 0.48 | 12 | 0.45 | 0.40 | – | – |
| Parkin and Walter (1992) –Exp.1 | No | RK | Verbal | 20 | 0.51 | 0.49 | 20 | 0.18 | 0.49 | – | – |
| Parkin and Walter (1992) –Exp.2 | No | RK | Verbal | 30 | 0.36 | 0.65 | 60 | 0.14 | 0.54 | – | – |
| Parks (2007) – Exp. 1 | No | RK | Verbal | 51 | 0.41 | 0.51 | 41 | 0.27 | 0.37 | −0.74 | −0.86 |
| Parks (2007) – Exp. 2 | Yes | ROC | Verbal | 72 | 0.61 | 0.51 | 72 | 0.46 | 0.42 | −0.95a | −0.52b |
| Perfect and Dasgupta (1997) | Yes | RK | Verbal | 20 | 0.72 | 0.59 | 40 | 0.39 | 0.34 | – | – |
| Perfect et al. (1995) –Exp.1 | No | RK | Verbal | 22 | 0.48 | 0.44 | 22 | 0.11 | 0.61 | – | – |
| Perfect et al. (1995) – Exp. 2a (Deep Encoding) | Yes | RK | Verbal | 12 | 0.67 | 0.90 | 12 | 0.67 | 0.25 | – | – |
| Perfect et al. (1995) – Exp. 2a (Shallow Encoding) | No | RK | Verbal | 12 | 0.40 | 0.55 | 12 | 0.31 | 0.25 | – | – |
| Perfect et al. (1995) –Exp.2b | Yes | RK | Verbal | 12 | 0.87 | 0.81 | 12 | 0.22 | 0.44 | – | – |
| Peters and Daum (2008) | Yes | RK | Verbal | 22 | 0.61 | 0.55 | 23 | 0.36 | 0.32 | −1.10 | −0.83 |
| Prull et al. (2006) | No | RK/PD/ROC | Verbal | 36 | 0.45 | 0.41 | 36 | 0.19 | 0.37 | −1.28 | −0.49 |
| Schacter et al. (1997) – Exp. 1 | Yes | RK | Verbal | 32 | 0.78 | 0.28 | 32 | 0.65 | 0.16 | – | – |
| Schacter et al. (1997) – Exp. 2 | No | RK | Verbal | 16 | 0.58 | 0.43 | 16 | 0.24 | 0.10 | – | – |
| Skinner and Fernandes (2008) | No | RK | Verbal | 30 | 0.32 | 0.43 | 30 | 0.26 | 0.33 | −0.24 | −0.79 |
| Skinner and Fernandes (2009a) –Exp.1 | No | RK | Verbal | 15 | 0.45 | 0.29 | 15 | 0.34 | 0.14 | −0.55 | −0.71 |
| Skinner and Fernandes (2009a) –Exp.2 | No | RK | Verbal | 16 | 0.49 | 0.20 | 16 | 0.46 | 0.12 | −0.16 | −0.28 |
| Skinner and Fernandes (2009b) | No | RK | Verbal | 24 | 0.48 | 0.45 | 24 | 0.38 | 0.65 | – | – |
| Toth and Parks (2006) – Easy Group | No | PD | Verbal | 36 | 0.33 | 0.40 | 36 | 0.15 | 0.32 | −0.88 | −0.76 |
| Toth and Parks (2006) – Hard Group | No | PD | Verbal | 36 | 0.05 | 0.52 | 36 | 0.01 | 0.36 | −0.40 | −1.19 |
| Tse et al. (2010) | Yes | PD | Verbal | 30 | 0.78 | 0.66 | 105 | 0.63 | 0.77 | – | – |
| Wang et al. (2012) | No | RK | Verbal | 23 | 0.35 | 0.67 | 21 | 0.22 | 0.49 | −1.26b | −1.34b |
| Wolk et al. (2013) | Yes | PD | Verbal | 17 | 0.60 | 0.75 | 50 | 0.36 | 0.82 | −1.49 | 0.37 |
The recollection (R) and familiarity (F) estimates under the Young Adults and Older Adults headings refer to the probability (prob.) estimates derived from the Yonelinas (2002) method whereby R and F are calculated based on the average data reported in the study (see Supplemental Material for details). The n under these headings refers to the number of young and older adults in the study. The reported effects sizes for R and F are the uncorrected values calculated for each sample. The small-sample bias correction was applied to these effect sizes in the analysis reported in the main text. RK Remember-Know; PD Process Dissociation; ROC Receiver-Operating Characteristic
The probability estimates for recollection and familiarity could not be reliably calculated from the data reported
Effect size estimate based on t- or F-statistic
Group difference reported as non-significant, and effect size estimate set to 0
Subtracted the average false alarm rate from familiarity post hoc before calculating the effect size (see main text for details)
Two ROC estimates of recollection (i.e., Ro and Rn) were derived from an associative recognition task. The Ro measure was selected to compute the recollection effect size estimate because this is analogous to old/new discrimination in item recognition tests
The discrepancy between the age-related differences in the probability estimates of recollection and familiarity and the effect size measures is likely due to the former being calculated based on average data and the latter based on individual means and variance measures reported in the paper
A total of 36 (53 %) of the 68 independent comparisons identified in the literature search used the RK, PD, and ROC methods to provide estimates of recollection and familiarity and reported enough data to estimate effect sizes for both processes. These samples were distributed across 25 published articles. The proportion of samples excluded from the effect size analysis differed as a function of the process estimation method. Specifically, 62 %, 28 %, and 30 % of the excluded samples used the RK, PD, and ROC procedures, respectively2.
The inclusion criteria for the studies examining aMCI and AD were a healthy, age-matched control group determined to have no memory pathologies and a patient group diagnosed following published guidelines for aMCI (e.g., Petersen 2004; Petersen et al. 2009) and AD (e.g., McKhann et al. 1984). A list of the aMCI and AD studies meeting our inclusion criteria is provided in Tables 2 and 3, respectively. Nine published reports that examined patients diagnosed with aMCI met the inclusion criteria. Each study provided a single, independent sample of aMCI patients, and reported enough data to be included in the effect size analysis. Note that we excluded the aMCI sample reported by Wolk and colleagues (2011) because 12 out of the 14 aMCI patients were also included in a prior study by the same lab (Wolk et al. 2008). We also identified 7 published articles meeting the inclusion criteria that examined patients diagnosed with AD. Each article reported a single comparison of patients diagnosed with probable AD and healthy controls. Five of the seven AD samples provided enough data for the effect size analysis.
Table 2.
Studies that examined recollection and familiarity differences between healthy older adults and individuals diagnosed with amnestic Mild Cognitive Impairment (aMCI)
| Healthy Controls | aMCI Patients | Effect Size d | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Study | aMCI Subtypes | Estimation Method | n | R (prob.) | F (prob.) | n | R (prob.) | F (prob.) | R | F |
| Ally et al. (2009)a | SD & MD | ROC | 12 | 0.18 | 0.31 | 11 | 0.09 | 0.18 | −0.32 | −1.51 |
| Anderson et al. (2008) | SD | PD | 44 | 0.76 | 0.48 | 15 | 0.63 | 0.51 | −0.72 | 0.04 |
| Belleville et al. (2011)b | SD & MD | RK | 29 | – | – | 28 | – | – | −0.63 | 0.23 |
| Embree et al. (2012) | SD & MD | ROC | 16 | 0.43 | 0.54 | 16 | 0.15 | 0.45 | −1.83 | −0.45 |
| Hudon et al. (2009)a | SD & MD | RK | 23 | 0.64 | 0.56 | 20 | 0.43 | 0.59 | −1.26 | 0.39c |
| Serra et al. (2010) | SD | PD/RK | 23 | 0.29 | 0.18 | 19 | 0.18 | 0.19 | −0.27 | −0.12d |
| Troyer et al. (2012) | SD | PD | 21 | 0.58 | 0.31 | 24 | 0.26 | 0.39 | −1.29 | 0.43 |
| Wolk et al. (2008) | SD & MD | PD | 21 | 0.33 | 0.47 | 16 | 0.20 | 0.32 | −0.65 | −1.40 |
| Wolk et al. (2013) | SD & MD | PD | 50 | 0.36 | 0.82 | 32 | 0.21 | 0.50 | −0.99 | −1.75 |
The recollection (R) and familiarity (F) estimates under the Healthy Controls and aMCI patients headings refer to the probability (prob.) estimates derived from the Yonelinas (2002) method whereby R and F are calculated based on the average data reported in the study (see Supplemental Material for details). The n under these headings refers to the number of healthy controls and aMCI patients tested in the study. The reported effects sizes for R and F are the uncorrected values calculated for each sample. The small-sample bias correction was applied to these effect sizes in the analysis reported in the main text. The estimation method is provided for reference, and was not considered as a moderator variable in the analyses reported in the main text. RK Remember-Know; PD Process Dissociation; ROC Receiver-Operating Characteristic; SD Single-Domain aMCI; MD Multiple-Domain aMCI
This study examined both aMCI and AD with a single set of healthy controls
The probability estimates for recollection and familiarity could not be reliably calculated from the data reported
Subtracted the average false alarm rate from familiarity post hoc before calculating the effect size
The difference between the pattern of probability estimates and effect size measures is likely due to differences in the method used to calculate familiarity. Serra et al. (2010) use a signal-detection PDP calculator, whereas we used the formulas provided in Yonelinas (2002) (see Supplemental Materials for a more detailed description of the method)
Table 3.
Studies examining recollection and familiarity differences between healthy older adults and individuals diagnosed with Alzheimer’s disease (AD)
| Healthy Controls | AD Patients | Effect Size d | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Study | Estimation Method | n | R (prob.) | F (prob.) | n | R (prob.) | F (prob.) | R | F |
| Ally et al. (2009)a | ROC | 12 | 0.18 | 0.31 | 10 | −0.02 | −0.16 | −2.09 | −1.16 |
| Barba (1997) | RK | 12 | 0.34 | 0.21 | 12 | 0.15 | 0.18 | – | – |
| Genon et al. (2013)a | PD | 16 | 0.46 | 0.58 | 16 | 0.12 | 0.38 | −0.64 | −1.49b |
| Hudon et al. (2009) | RK | 23 | 0.64 | 0.56 | 10 | 0.25 | 0.22 | −1.97 | −0.89b |
| Smith and Knight (2002) | PD | 14 | 0.14 | 0.31 | 7 | 0.07 | 0.17 | −1.30 | −1.92 |
| Tse et al. (2010) | PD | 105 | 0.63 | 0.77 | 48 | 0.38 | 0.55 | – | – |
| Wolk et al. (2011)a | PD | 21 | 0.36 | 0.72 | 9 | 0.10 | 0.29 | −1.30 | −2.39 |
The recollection (R) and familiarity (F) estimates under the Healthy Controls and AD patients headings refer to the probability (prob.) estimates derived from the Yonelinas (2002) method whereby R and F are calculated based on the average data reported in the study (see Supplemental Material for details). The n under these headings refers to the number of healthy controls and
AD patients tested in the study. The reported effects sizes for R and F are the uncorrected values calculated for each sample. The small-sample bias correction was applied to these effect sizes in the analysis reported in the main text. The estimation method is provided for reference, and was not considered as a moderator variable in the analyses reported in the main text. RK Remember-Know; PD Process Dissociation; ROC Receiver-Operating Characteristic
This study examined both aMCI and AD with a single set of healthy controls
Subtracted the average false alarm rate from familiarity post hoc before calculating the effect size
Note that three studies examined recollection and familiarity in both healthy aging and aMCI (Anderson et al. 2008; Belleville et al. 2011; Wolk et al. 2013), and two studies compared an aMCI and AD sample to a single healthy older adult control group (Ally et al. 2009; Hudon et al. 2009). Table 4 shows the number of participants and demographic data for the samples of the healthy aging, aMCI, and AD studies included in the effect size meta-analysis.
Table 4.
Demographics of the healthy aging, aMCI, and AD studies included in the recollection and familiarity effect size analysis
| Age | Education | MMSE | |||||
|---|---|---|---|---|---|---|---|
| n | Mean | Range | Mean | Range | Mean | Range | |
| Healthy Aging Studies | |||||||
| Young Adults | 1080 | 21.47 | 18.90–30.00 | 14.55 | 12.80–18.30 | – | – |
| Older Adults | 1195 | 71.14 | 60.61–77.00 | 15.29 | 11.20–18.10 | – | – |
| aMCI Studies | |||||||
| Healthy Controls | 239 | 71.92 | 66.90–77.3 | 15.18 | 12.90–16.20 | 29.02 | 27.10–29.60 |
| aMCI Patients | 181 | 73.14 | 66.10–77.3 | 15.23 | 12.90–16.90 | 27.61 | 25.50–28.50 |
| AD Studies | |||||||
| Healthy Controls | 87 | 71.85 | 68.60–77.30 | 14.01 | 9.83–16.70 | 29.35 | 28.80–29.70 |
| AD Patients | 52 | 75.93 | 74.57–77.80 | 13.21 | 8.86–16.60 | 22.23 | 17.00–24.90 |
The means and ranges for age and education are based on studies that provided this data. For the healthy aging studies, one study did not report years of education for both young and older adults, one study did not report age or education for young adults, and three studies did not report education for older adults. The MMSE values were not reported for one AD study
Effect Size Meta-Analysis
Moderator Variable Coding
Each study that examined healthy aging was coded along three dimensions to test for potential moderators of recollection and familiarity declines. First, studies were coded as having high recollection estimates if the mean recollection estimate in the young adult group was≥.60. Four of the 36 samples reported high estimates of recollection (see Table 1).
Second, studies were coded based on the estimation method they employed. Of the 36 samples contributing to the effect size analysis, 15, 13, and 7 samples employed the RK, ROC, and PD methods, respectively. One study (Prull et al. 2006) used all three methods within the same sample of younger and older adults. Therefore, this study was excluded from the analysis examining the effect of the process estimation method.
Lastly, the studies were classified as verbal or nonverbal based on the materials used to test memory3. We made this distinction based on the types of materials used during the test phase. For example, a study that included words paired with a picture (Luo et al. 2007) or a face (Skinner and Fernandes 2009a), but only tested memory of the words was coded as a study using verbal materials. The type of nonverbal materials used in the studies included in the effect size analysis included travel scenes (Düzel et al. 2011; Howard et al. 2006), objects (Angel et al. 2013; Duarte et al. 2006), and symbols (Friedman et al. 2010). The only studies in the effect size meta-analysis that used nonverbal materials according to our definition were Angel et al. (2013), Duarte et al. (2006), Düzel et al. (2011), Friedman et al. (2010), and Howard et al. (2006). One study examined memory for words and melodies in the same sample, and was excluded from any comparisons involving this variable (Belleville et al. 2011).
The aMCI studies were coded based on the composition of aMCI subtypes in the sample (Petersen 2004). Three reported a homogenous single-domain aMCI sample and the remaining 6 reported examining an aMCI mixed sample of both single-domain and multiple-domain aMCI patients (see Table 2).
Effect Size Calculation
The effect size measure used to quantify differences between young and older adults and healthy controls and patient groups (aMCI or AD) was the standardized mean difference (Cohen’s d; Cohen 1988). Two effect size measures were calculated for each sample – one for recollection and one for familiarity. Note that some studies did not subtract out baseline familiarity from the reported familiarity estimates (Anderson et al. 2008; Genon et al. 2013; Hudon et al. 2009; Jacoby 1999). Given that our primary focus is on examining how familiarity is useful in discriminating between studied and new events, we post hoc corrected the estimate of familiarity reported for each group by subtracting out the average baseline familiarity estimate. For PD studies, the value subtracted from the familiarity estimate was the average false alarm rate to new items on the inclusion and exclusion conditions. For RK studies, the value subtracted from the reported familiarity estimate was the familiarity estimate for new items.
The effect size for each study was calculated using the online calculator that is a complement to the Lipsey and Wilson (2001) text (http://www.campbellcollaboration.org/resources/effect_size_input.php). In most cases, means and standard deviations (or standard errors) were used to calculate the effect size4. If unavailable, the relevant F or t statistic was used to estimate the effect size. Some studies reported the effect of healthy aging on estimates of familiarity to be non-significant, but did not provide means and variance measures or a precise test statistic value (e.g., F<1). In such cases, the effect size was conservatively estimated to be 0. Multiple effect size estimates of recollection and familiarity could be derived for some studies (e.g., deep versus shallow encoding; Ally et al. 2009). In these situations, we averaged across the multiple effect size estimates. Next, the small sample bias correction (Hedges 1981) was applied to the effect sizes measures. Negative effect size values indicate impairments in healthy older adults relative to young adults or impairments in aMCI or AD patients relative to controls.
Data Analysis
All of the analyses reported below were carried out using the metafor package (Viechtbauer 2010) in R, version 3.0.1 (R Core Team 2013). The mean weighted effect sizes (dw) and the corresponding 95 % confidence intervals were calculated by fitting the effect sizes with a random effects model using restricted maximum likelihood estimation (Viechtbauer 2005). A random effects model was used because our goal was to make inferences about the magnitude of recollection and familiarity impairments in the population (Hedges and Vevea 1998; Viechtbauer 2010). A mean weighted effect size for both recollection and familiarity estimates were calculated separately for healthy aging studies, aMCI studies, and AD studies. Each study’s effect size was weighted by the inverse variance weight (Lipsey and Wilson 2001). The Knapp and Hartung (2003) correction was used to account for uncertainty in the estimated amount of heterogeneity. The test for individual coefficients in the random effects models is based on a t-distribution with k − p degrees of freedom, where k is the number of effect sizes, and p is the total number of coefficients (including the intercept). Homogeneity tests were conducted using the Q-statistic (Lipsey and Wilson 2001). This statistic has a χ2 distribution with k − 1 degrees of freedom. A significant Q indicates that the distribution of effect sizes is heterogeneous (i.e., the variability in effect sizes is larger than expected from sampling error alone).
A series of mixed effects models were fit to the recollection and familiarity effect sizes to investigate if any of the moderator variables influenced the magnitude of recollection and familiarity differences. Specifically, these models examined if high estimates of recollection, the estimation method, and materials moderated differences in recollection and familiarity estimates between healthy older adults and young adults. A mixed effect model was fit to the aMCI data to examine how the composition of the aMCI sample (i.e., single-domain only vs. single-domain and multiple-domain patients) affected recollection and familiarity declines. With the Knapp and Hartung (2003) adjustment, the omnibus test to determine if the moderators accounted for a significant portion of the variance (i.e., determine if there was a significant difference between the levels of the moderator variable) is represented by an F-distribution with m and k – p degrees of freedom, where m is the number of moderators in the model excluding the intercept. This F-statistic tests the null hypothesis that the coefficients of the model (excluding the intercept) are equal to zero (e.g., H0: β1=β2=0). In the models reported below, one of the levels of a factor (e.g., RK studies when examining the effects of the estimation method) was defined as the reference group (i.e., intercept) of the model. Thus, a significant F-statistic indicates that the different levels of a factor have significantly different effect sizes. The significance test for individual coefficients is the same as described above for the random effects models. The specific details of each model will be described in more detail when presenting the results.
Publication Bias
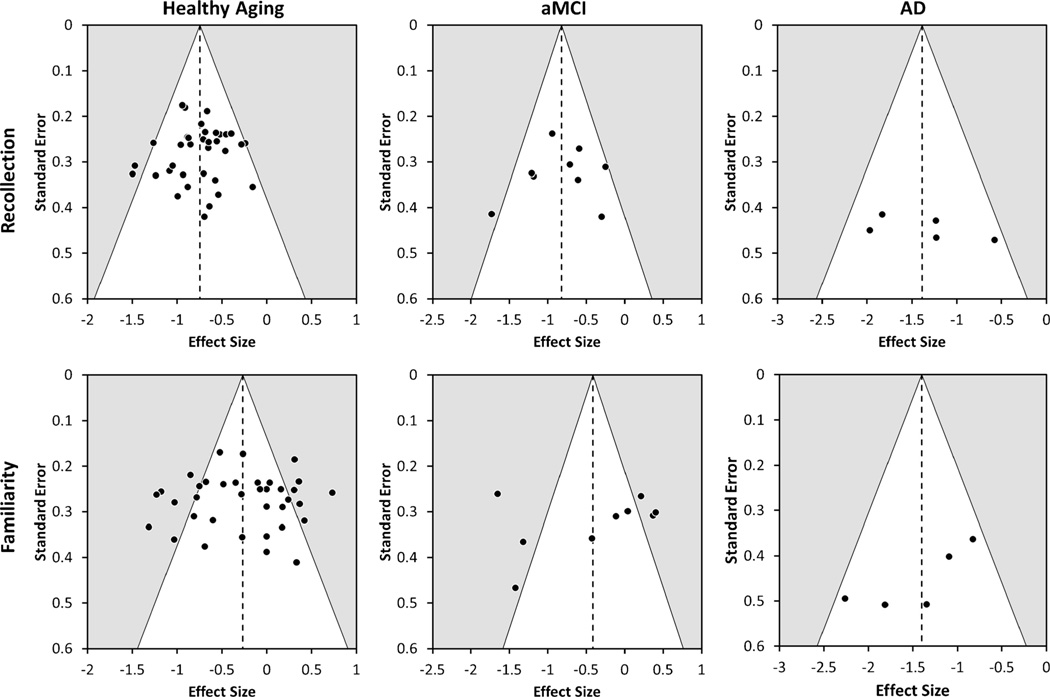
One limitation of meta-analysis is that the results might be influenced by certain publication biases, such as reporting overestimates of the true effect size in experiments with small sample sizes. We addressed the above form of publication bias by examining asymmetry in funnel plots. Specifically, we performed a random-effects version of the Egger’s test in which the effect sizes are regressed on the standard error of the effect sizes (Egger et al. 1997; Sterne and Egger 2005). The funnel plots for healthy aging, aMCI, and AD studies are shown in Fig. 1.
Fig. 1.
Funnel plots with the 95 % pseudo-confidence interval (white area) for recollection (top row) and familiarly (bottom row) differences in healthy aging, amnestic Mild Cognitive Impairment (aMCI), and Alzheimer’s disease (AD) studies. Note that the range of the effect sizes on the x-axis is differs across the plots.
Estimating Recollection and Familiarity for Each Study Using the Yonelinas (2002)
In addition to the effect size analysis, we also examined recollection and familiarity deficits in healthy aging, aMCI, and AD following the method outlined by Yonelinas (2002). This approach calculates an estimate of recollection and familiarity for each group in a study (e.g., young and older adults) using the average proportion of responses and the formulas for the corresponding method. The recollection and familiarity estimates derived from this method are provided in Tables 1 for young and healthy older adults, in Table 2 for healthy controls and aMCI patients, and in Table 3 for healthy controls and AD patients. The dependent variable of interest in this analysis was the absolute difference score between young and older adults or between healthy controls and aMCI/AD patients. This difference score was calculated for both recollection and familiarity, with negative values indicating lower estimates of recollection and familiarity in older adults, patients, and AD. Note that our primary purpose for conducting this analysis is to determine if excluding approximately half of the samples biased the results from the effect size meta-analysis reported in the main text. This method and results from this approach are presented in detail in the Supplemental Material. With one exception (discussed below), these results did not alter our conclusions.
Results
Healthy Aging
Overall
The mean effect sizes for recollection and familiarity differences between young and healthy older adults are shown Fig. 2. Healthy aging led to a significant decrease in recollection, [dw = −0.75; LB95% CI = −0.85; UB95% CI=−0.65; t(35)=15.28; p<0.001]. The Egger’s test did not find any evidence for publication bias; the coefficient for the standard error (bSE), which indexes funnel plot asymmetry, was not significant, [bSE=−.49, t(34)=0.55, p=.59]. The homogeneity test was not significant, [Q(34)=41.84; p=0.19], indicating that the variability present in the recollection effect sizes was within that expected from sampling error alone.
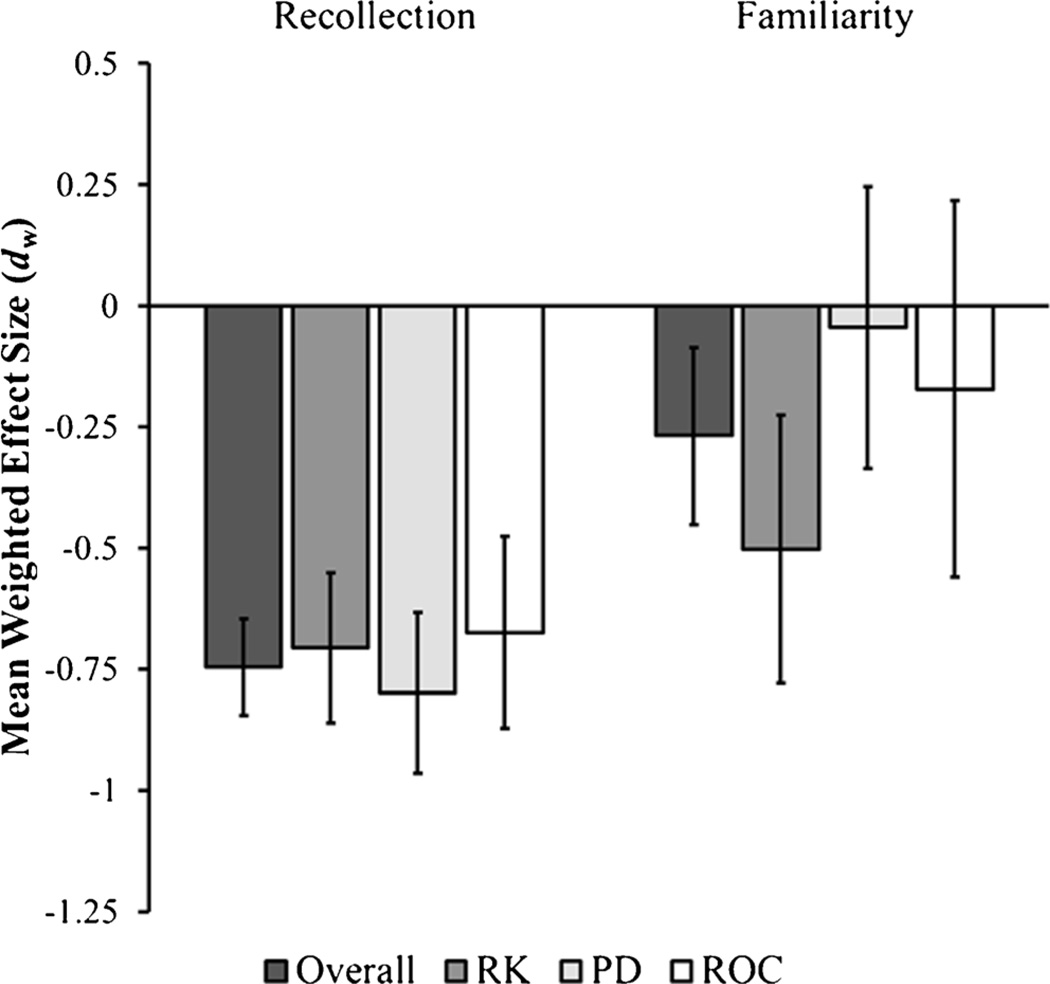
Fig. 2.
The overall mean weighted recollection and familiarity effect size estimates for studies examining healthy aging, and the mean weighted effect sizes for recollection and familiarity divided by the method used to estimate recollection and familiarity. Negative values represent decreases in older adults relative to young adults. Error bars represent the 95 % confidence interval of the effect size estimate. RK = Remember/Know Task; PD = Process Dissociation Procedure; ROC = Receiver-Operating Characteristic Procedure
Similar to recollection, the overall familiarity effect size was significant [dw=−0.27; LB95% CI=−0.45; UB95% CI= −0.09; t(35)=2.99; p<0.01], suggesting that healthy aging is associated with reductions in familiarity. However, the mean effect size for familiarity was approximately a third of the magnitude of the recollection effect sizes. Moreover, the 95% confidence intervals did not overlap, which suggests that aging leads to larger declines in recollection compared to familiarity. The Egger’s test did not provide any evidence to suggest that publication bias was present for the familiarity effect sizes, [bSE=−.22, t(34)=0.14, p=.89]. A significant amount of heterogeneity was present in the effect sizes for familiarity, [Q(34)=139.54; p<0.001]. Below, a series of mixed effects models were fit to the data to determine high levels of recollection, the process estimation method, or the type of materials accounts for a portion of the heterogeneity of the familiarity effect sizes. For consistency, the same mixed effects models were conducted on the recollection effect sizes.
High Levels of Recollection
The first model examined if high levels of recollection moderated recollection and familiarity effect sizes. The recollection effect size for studies with high levels of recollection (i.e., Recollection estimates≥0.60), [dw=−0.96; LB95% CI=−1.23; UB95% CI=−0.69; t(34)= −7.19; p<0.001], was numerically higher than the effect size for studies with “normal” levels of recollection (i.e., Recollection estimates<0.60), [dw=−0.72; LB95% CI=−0.82; UB95% CI=−0.61; t(34)=−14.13; p<0.001]. However, a mixed-effect regression model with “normal” recollection studies as the reference group indicated that this difference was not reliable, [F(1, 34)=2.86, p=0.10]. The mean familiarity effect sizes nearly identical for studies with “normal” levels of recollection, [dw=−0.27; LB95% CI=−0.47; UB95% CI=−0.07; t(34)=2.79; p<0.01] and studies high levels of recollection, [dw=−0.26; LB95% CI=−0.80; UB95% CI=0.29; t(34)=0.95; p=0.35]. A mixed-effect regression model identical to that used for the recollection estimates indicated that these effect sizes did not statistically differ, [F(1, 34)=0.003, p=0.96]. This suggests that high levels of recollection do not result in larger age-related recollection or familiarity impairments. However, the lack of an effect on high levels of recollection on age differences in familiarity might have arisen because a large number of studies with high levels of recollection, some of which were included in Yonelinas (2002), were excluded in the effect size analysis reported here. Using the Yonelinas (2002) method, the studies excluded from the effect size meta-analysis showed evidence that age differences in both recollection and familiarity are inflated with high levels of recollection, whereas the studies included showed no such effect (see Table S1 in the Supplemental Material).
Process Estimation Method
A second mixed effects model examined the influence of the process estimation method on the magnitude of effect size estimates for age differences in recollection and familiarity. Note that this analysis was performed only on 35 of the samples in the meta-analysis; the Prull et al. (2006) sample was excluded because it used multiple methods with the same group of participants. Figure 2 depicts the recollection and familiarity effect size estimates for the RK, PD, and ROC methods. The recollection effect sizes for RK studies, [dw=−0.71; LB95% CI=−0.86; UB95% CI=−0.55; t(32)=9.24; p<0.001], PD studies, [dw= −0.80; LB95% CI=−0.97; UB95% CI=−0.63; t(32)=9.77; p<0.001], and ROC studies, [dw=−0.67; LB95% CI=−0.887; UB95% CI=−0.48; t(32)=6.96; p<0.001], were all similar in magnitude and significantly different from zero. The familiarity effect size for RK studies was significantly different from zero, [dw=−0.50; LB95% CI=−0.78; UB95% CI=−0.23; t(32)= 3.70 p<0.001], whereas the effect size for PD studies, [dw= −0.04; LB95% CI=−0.34; UB95% CI=0.25; t(32)=0.31; p= 0.76], and ROC studies, [dw=−0.17; LB95% CI=−0.56; UB95% CI=0.22; t(32)=0.90; p=0.37], did not significantly differ from zero. This suggests that the use of the RK procedure might account for some of the variance in reported familiarity differences, but not recollection differences..
To test if the effect sizes were significantly different between the three estimation methods, we created a mixed effects model with two dummy coded variables. One variable coded the studies that used the PD method, and the other coded that studies that used the ROC method, thus making studies using the RK task the reference group. This model revealed no significant effect of estimation method on recollection effect sizes, [F(2, 32)=0.57, p=0.57]. However, there was a marginally significant effect of the estimation method on familiarity, [F(2, 32)=2.83, p=0.07]. An examination of the model coefficients, which measures the difference between the RK effect sizes with the PD and ROC effect sizes, demonstrated that the mean effect size for PD studies was significantly different from the RK studies, [t(32)=2.32, p<0.05], whereas the mean familiarity effect size for the ROC studies did not significantly differ from the RK studies, [t(32)=1.41, p=0.17]. The ROC and PD tasks were compared by modifying the above model such that the intercept of the mixed effects model reflected the mean effect size for the PD studies. The coefficient for the ROC studies in this model was not significant, [t(32)=0.53, p=0.60], indicated that the PD and ROC effect sizes did not significantly differ from each other. These results show that age differences in familiarity, not recollection, are affected by the estimation method. Specifically, familiarity differences between young adults and healthy older adults were only significant when the RK task was used to assess recollection and familiarity, but not when the PD and ROC tasks were employed. The estimation method accounted for approximately 7.8 % of the estimated heterogeneity in the familiarity effect sizes, and a significant portion of residual heterogeneity remained, [Q(32)=122.60; p<0.001].
Verbal Versus Non-Verbal Materials
Lastly, a mixed effects model was specified to examine whether the type of materials moderated the magnitude of the recollection and familiarity effect sizes for healthy aging studies. The recollection effect sizes were nearly identical for studies using verbal materials, [dw=−0.75; LB95% CI=−0.86; UB95% CI=−0.64; t(33)=13.75; p<0.001], and studies using nonverbal materials, [dw=−0.73; LB95% CI=−1.02; UB95% CI=−0.41; t(33)=5.04; p=.11]. A mixed-effects regression model with studies using verbal materials as the reference group indicated that these two effect sizes did not reliably differ from each other, [F(1, 33)=0.02, p=0.88]. There was a numerical difference between the familiarity effect sizes for studies using verbal materials, [dw=−0.22; LB95% CI=−0.42; UB95% CI=−0.02; t(33)=2.29; p<0.05], and nonverbal materials, [dw=−0.40; LB95% CI=−0.89; UB95% CI=0.10; t(33)=1.63; p=.11]. However, the difference was not reliable, [F(1, 33)=0.45, p=0.51]. Thus, there was little evidence that the age effects on recognition were different for verbal and nonverbal materials.
The results from the healthy aging studies provide evidence that healthy older adults show large decreases in recollection relative the healthy young adults. Familiarity decreases were also significant, but much smaller than the decrease in recollection. Moreover, age differences in familiarity were moderated by the estimation method such that familiarity differences were only significant in studies using the RK method and not in studies using the PD and ROC methods. However, the estimation method only accounted for a small portion of the variance across studies, suggesting that other variables not examined here need to be accounted for in future studies. We will return to this issue in the General Discussion.
Amnestic Mild Cognitive Impairment
Figure 3 plots the mean effect sizes for recollection and familiarity differences between aMCI and healthy controls. The recollection effect size was significantly negative, [dw=−0.82; LB95% CI=−1.16; UB95% CI=−0.49; t(8)=5.64; p<0.001], indicating decreased levels of recollection in aMCI patients compared to healthy controls. The test for heterogeneity test approached significance, [Q(8)=13.81; p=0.09], and there was no indication of publication bias as the Egger’s test was not significant, [bSE=−1.31, t(7)=.46, p=.66]. The mean familiarity effect size across studies was negative, but not significantly different from zero, [dw=−0.41; LB95% CI=−1.04; UB95% CI= 0.21; t(8)=1.52; p=0.17]. There was a significant amount of heterogeneity in the familiarity effect sizes, [Q(8)=55.87; p<0.001]. Similar to the recollection effect sizes, the Egger’s test was not significant, indicating that there was no evidence of publication bias for familiarity estimates in the aMCI studies, [bSE=−5.46, t(7)=1.18, p=.28].
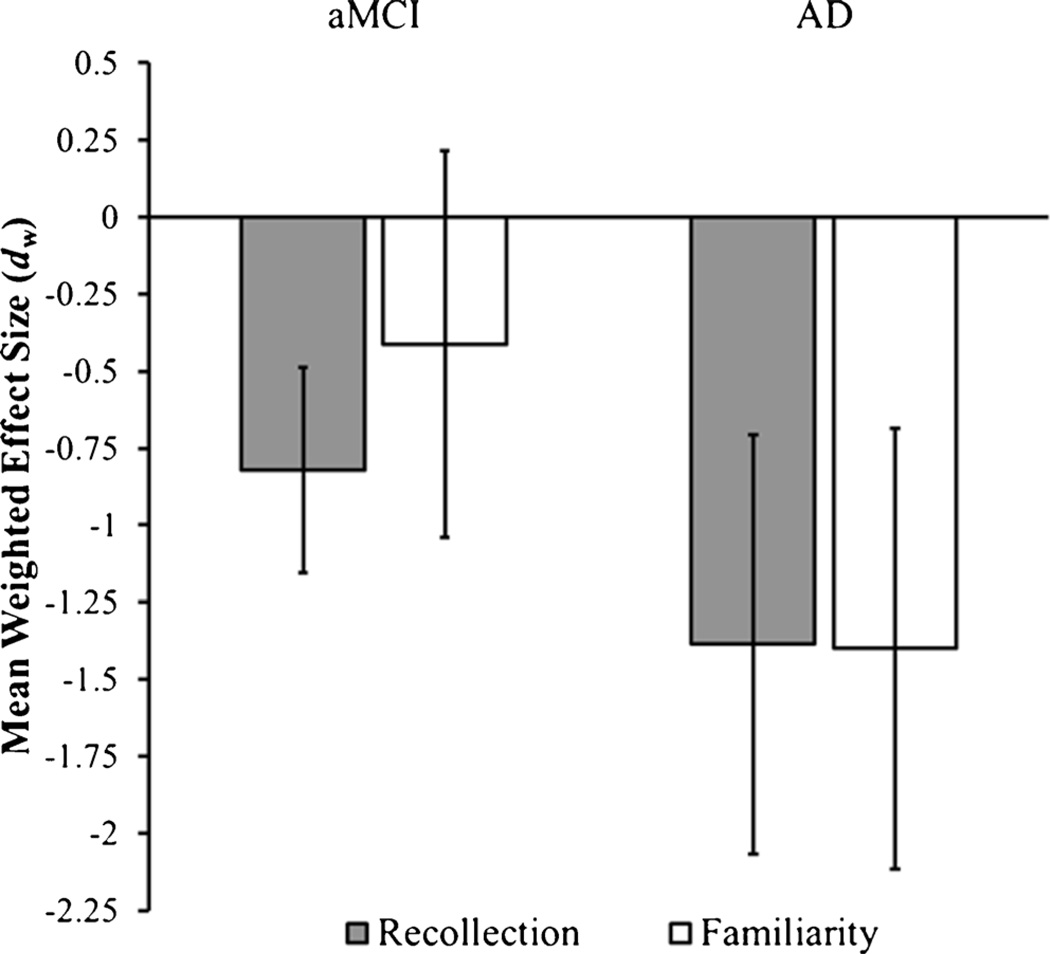
Fig. 3.
Mean weighted effect size estimates for recollection and familiarity differences in studies comparing patients diagnosed with amnestic Mild Cognitive Impairment (aMCI) and Alzheimer’s disease (AD) compared to healthy, age-matched controls. Negative values represent decreases in aMCI and AD patients relative to controls. Error bars represent the 95 % confidence interval of the effect size estimate
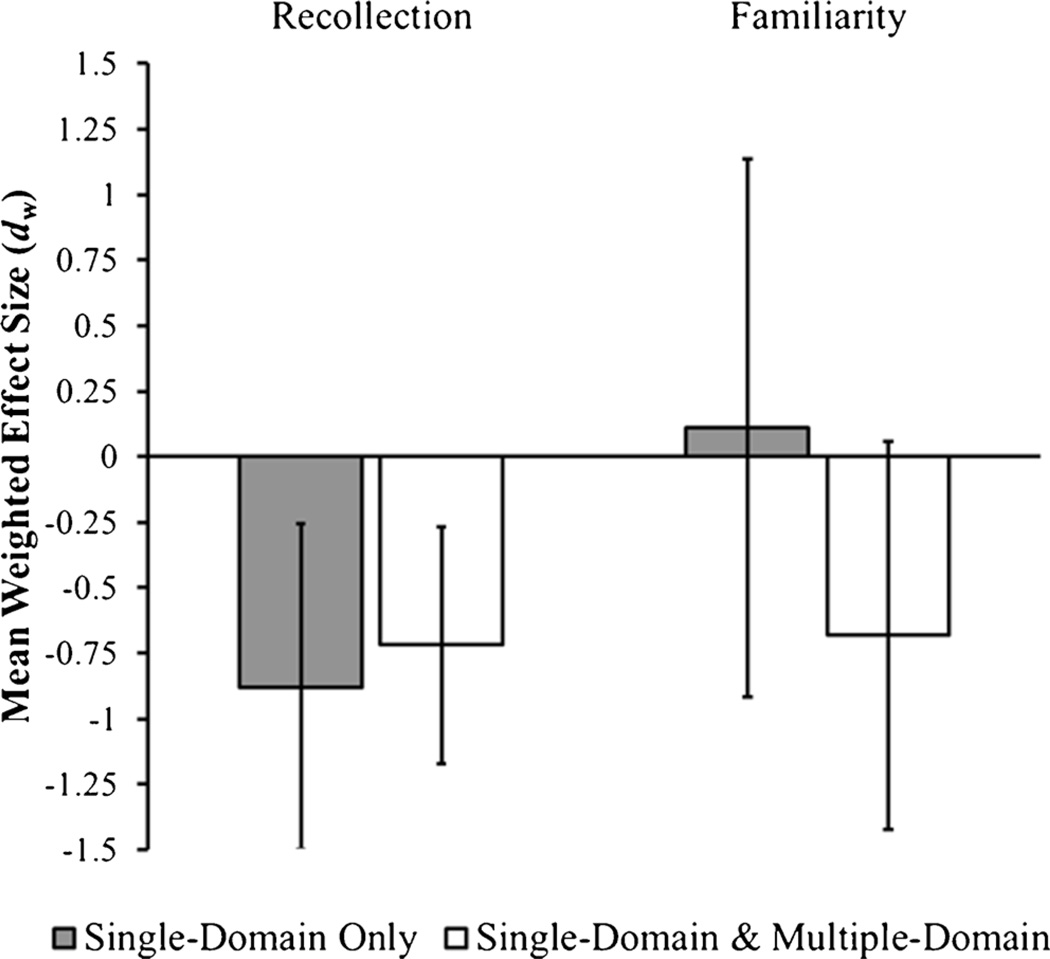
As discussed previously, some studies only tested single-domain aMCI patients whereas others tested a patient sample comprised of both single-domain and multiple-domain aMCI patients. To examine if this moderated the magnitude of the recollection and familiarity effect sizes, this variable was dummy coded such that the reference group was studies that only examined single-domain aMCI patients. The recollection effect size for the studies examining only single-domain patients, [dw=−0.72; LB95% CI=−1.34; UB95% CI=−0.10; t(7)=2.72; p<0.05], and the studies that examined both single-domain and multiple-domain patients, [dw=−0.88; LB95% CI=−1.33; UB95% CI=−0.43; t(7)=4.60; p<0.01], were both significantly different from zero, but did not significantly differ from each other, [F(1, 7)=0.24, p=0.64] (see Fig. 4). The mean familiarity effect size for the studies exclusively testing single-domain aMCI patients was not significant, [dw=0.11; LB95% CI=−0.91; UB95% CI=1.14; t(7)=0.26; p=0.80], whereas the effect size for studies testing a mixture of single-domain and multiple-domain aMCI patients was marginally significant, [dw=−0.68; LB95% CI=−1.42; UB95% CI=0.06; t(7)=2.18; p=.07]. Although there was a fairly large numerical difference between the two effect sizes, the difference was not reliable, [F(1, 7)=2.41, p=0.16], which might reflect the small number of studies included in this analysis. Overall, these results show that the aMCI groups exhibited reduced recollection estimates compared to aged controls. Familiarity estimates were numerically reduced as well but only approached significance for studies that examined a mixture of single-domain and multiple-domain aMCI patients.
Fig. 4.
Mean weighted effect size estimates for recollection and familiarity differences in studies that compared a sample of single-domain amnestic Mild Cognitive Impairment (aMCI) to healthy, age-matched controls and studies that compared a mixture of single-domain and multiple-domain aMCI patients to healthy, age-matched controls. Negative values represent decreases in aMCI patients relative to controls. Error bars represent the 95 % confidence interval of the effect size estimate
Alzheimer’s Disease
The effect sizes for both recollection, [dw=−1.39; LB95% CI= −2.07; UB95% CI=−0.71; t(4)=5.66; p<0.01], and familiarity, [dw=−1.40; LB95% CI=−2.12; UB95% CI=−0.68; t(4)=5.43; p<0.01], were significant in the AD studies (see Fig. 3), suggesting that AD is associated with large deficits in both recollection and familiarity relative to age-matched controls. The test for heterogeneity was not significant for recollection, [Q(4)=6.00; p=0.20], nor familiarity, [Q(4)=6.74; p=0.15]. The Egger’s test was not significant for the recollection effect sizes, [bSE=13.68, t(3)=1.33, p=.28]. However, the Egger’s test approached significance for familiarity effect sizes, [bSE= −6.81, t(3)=2.63, p=.08], suggesting that publication bias might be present for familiarity. However, it is important to point out that the Egger’s test can be unreliable when the sample size is small (Moreno et al. 2009). These above findings indicate that AD is associated with large decreases in both recollection and familiarity-based recognition.
General Discussion
The results from the meta-analysis reported above revealed that healthy aging is associated with significant reductions in recollection, with an effect size in the moderate-to-large range (Cohen 1988).A significant age-related decrease in familiarity was also observed, albeit a small effect size. However, these familiarity differences depended on the test method. Specifically, the familiarity effect size was moderate in magnitude and significant in studies using the RK method, whereas familiarity was not impaired in studies using the PD and ROC methods. There was little evidence that high levels of recollection or the type of materials moderated recollection or familiarity effect sizes.
Large decreases in recollection were observed in aMCI patients. Familiarity deficits in aMCI patients were not significant overall. However, the available data suggests that familiarity decreases in aMCI might depend on the type of patients included in the sample. Specifically, a moderate-to-large decrease in familiarity, which approached significance, was observed in studies that included a mixture of single-domain and multiple-domain aMCI patients whereas no familiarity decrease was evident in studies that included only single-domain aMCI patients.
Finally, the meta-analysis of the AD studies revealed that AD is associated with large and significant decreases in both recollection and familiarity. There were however, too few studies to determine whether those effects were moderated by other experimental or individual group differences. We discuss the implications of each of the above findings in turn.
Healthy Aging
The results from all three test procedures converged in showing that recollection is disrupted in healthy aging, a finding that is in good agreement with the widely held belief that recollection is impaired in healthy aging. In addition, the results showing significant decreases in familiarity in the studies using the RK test procedure but not the PD or the ROC test procedures partially explains why there has been disagreement in the literature about the fate of familiarity (e.g., Davidson and Glisky 2002; Duarte et al. 2006; Jennings and Jacoby 1993; Prull et al. 2006; Wang et al. 2012; Yonelinas 2002). While it is often assumed that the different estimation methods are interchangeable (Yonelinas 2001a; 2002), the aging literature reviewed here makes it quite clear that this is not always the case (see also, Prull et al. 2006). Why is it that familiarity is impaired in healthy older adults when it is measured using the RK procedure, but not when it is measured using the ROC or PDP procedures? The current review cannot provide a definitive answer to this question, and future studies that directly contrast the three methods are necessary. However, there are several potential explanations for the difference between the estimation methods that are worth considering.
First, the comparison between the estimation methods presented here relied on comparisons across studies, and so it is possible that older adults sampled in the RK studies had a fundamentally different demographic or neuropsychological profile compared to older adults in the PD and ROC studies. However, the studies included in this meta-analysis randomly sampled older adults from the population, which presumably would result in similar sample characteristics on average. Breaking up the data reported in Table 4 by the estimation method is consistent with this notion; the average age (RK=70.84 years; PD=71.37 years; ROC=70.84 years) and education (RK=15.34 years; PD=14.65 years; ROC=16.22 years) were fairly similar across studies. Although we cannot fully rule out this possibility, we believe this account to be unlikely.
Second, it could be the case that the RK studies are telling us the true story (i.e., healthy aging leads to similar decreases in both recollection and familiarity) and that the PD and ROC methods are biased in such a way to make it appear that familiarity is spared in healthy aging. However, several findings argue against this possibility. First, results from numerous task dissociation studies indicate that recollection is more disrupted that familiarity (e.g., recall and associative recognition are significantly more impaired than item recognition; La Voie and Light 1994; Old and Naveh-Benjamin 2008), which is consistent with the conclusions the PD and ROC methods, and suggests that it is the RK finding that might be anomalous. In addition, the factors that are known to produce potential biasing effects on the PD and ROC procedures do not appear to be playing a role in the current aging studies. For example, in the PD procedure, familiarity estimates can be inflated by noncriterial recollection (i.e., recollection of specific details that do not support the required source memory discrimination; Yonelinas and Jacoby 1996b). However, noncriterial recollection should occur more often in young adults than in older adults because young adults have higher levels of recollection, which would inflate the apparent familiarity deficits in aging rather than eliminate them (Parks 2007; Toth and Parks 2006). In the ROC procedure, familiarity decreases in healthy aging could be masked because recollection and familiarity are not as reliably estimated in older adults compared to young adults. However, existing data provides no evidence indicating that the dual-process signal detection model fits data from older adults more poorly than the data from younger adults (e.g., Healy et al. 2005; Parks 2007). Finally, as discussed below, there is growing empirical evidence that the RK procedure may be providing biased estimates in studies of aging.
A third possibility is that the PD and ROC methods are telling the true story (i.e., recollection, not familiarity, is impaired in healthy aging) and that the RK method is biased in some way. One aspect of that could lead to the findings observed for the RK studies is that older adults might interpret the RK instructions differently than younger adults. The RK procedure is unique in that it measures recollection and familiarity on the basis of the participants’ introspective reports. The extant literature demonstrates that the specific instructions of the RK procedure significantly impact the results (Geraci and McCabe 2006; Geraci et al. 2009; McCabe and Geraci 2009; Rotello et al. 2005; Yonelinas 2001b). For example, using the standard instructions (e.g., Gardiner 1988; Rajaram 1993) some individuals might not fully understand the distinction between ‘Remember’ and ‘Know’ judgments, particularly those individuals with memory impairments (Aggleton et al. 2005; Baddeley et al. 2001; Yonelinas et al. 2002). This has led some researchers to adopt a set of strict instructions whereby participants are instructed to make a ‘Remember’ response only if they can report specific details about the study event to the experimenter to ensure that ‘Remember’ responses reflect instances in which qualitative information is retrieved (e.g., Yonelinas et al. 2002). Importantly, it is only under these strict instructions that the results of the RK procedure converge with those obtained using the ROC procedure (e.g., Koen and Yonelinas 2010; Yonelinas 2001b; for caveats, see Rotello et al. 2005).
The vast majority of the RK studies included in the current meta-analysis used the standard instructions. Thus, it is possible that, compared to young adults, older adults in these RK studies spontaneously adopted a more lenient definition of what constitutes a’Remember’ response or were more likely to confuse the two response options. In either case, if older adults make ‘Remember’ responses to items that are highly familiar rather than responding ‘Know’ to these items, this would lead to an apparent reduction in familiarity-based responses. It is not possible to verify that older and younger adults use the RK responses in the same way based on the data presented in the meta-analysis, but there is some evidence in the extant literature favoring this explanation. First, a recent review indicated that older adults typically give more false ‘Remember’ responses compared to young adults (McCabe et al. 2009). This increased propensity to give a ‘Remember’ response to new items could potentially decrease familiarity estimates (Yonelinas and Jacoby 1995). Second, McCabe and Geraci (2009) found that source-specific instructions, which placed a prominent emphasis on recollecting specific details of the study episode, increased familiarity estimates in older adults but not in younger adults. These results indicate that older adults are particularly sensitive to the content of the RK instructions. Finally, a recent study (Koen & Yonelinas, under review) that directly compared the RK, PD, and ROC methods in the same sample of participants used strict RK instructions, and found no evidence for age-related familiarity impairments in any of the three estimation procedures (but see Prull et al. 2006). Although the above findings suggest that the RK instructions might play a pivotal role, future work that systematically examines how aged and young subjects differ in their interpretation of the RK instructions would be beneficial.
In addition to assessing the moderating effects of the estimation methods, we examined if high levels of recollection and material type moderated age-related differences in recollection and familiarity. In neither case did we find compelling evidence that the results depended critically on either of these factors. The results from the meta-analysis reported above suggested that high levels of recollection – which can inflate familiarity estimates – did not significantly impact the age-related effects on recollection or familiarity. This finding stands in contrast to Yonelinas (2002) who reported that familiarity differences due to healthy aging were more evident when the recollection estimate of young adults was high (i.e., ≥0.60). One potential reason for this difference is that the studies included in the current effect size meta-analysis tended to have lower overall levels of recollection than those considered by Yonelinas (2002), and thus minimized the biasing effects of high performance on the observed familiarity effect sizes (see Supplemental Material).
The results of the current meta-analysis also failed to provide support for the idea that the type of materials moderated recollection or familiarity differences between young and healthy older adults. If anything, the familiarity reduction in healthy older adults was larger for studies using nonverbal materials. However, the meta-analysis might not have been sufficiently sensitive to material effects because the type of nonverbal stimuli varied across the studies and included faces, scenes, visual objects, and symbols. Future studies that use a variety of different types of materials will also be important to determine if and how the type of materials moderates age-related declines in recollection and familiarity. It could be the case that age-related differences in recollection and familiarity might vary with the type of nonverbal materials used. Evidence from individuals with medial temporal lobe damage is consistent with this notion (Aly et al. 2010; Bird and Burgess 2008; Carlesimo et al. 2001). For example, Aly and colleagues (2010) showed that, compared to healthy controls, patients with medial temporal lobe damage have significantly reduced estimates of familiarity for words but normal familiarity estimates for faces. To our knowledge, no study has directly examined age-related differences in recollection and familiarity for verbal stimuli compared to different types of nonverbal stimuli (e.g., scenes, faces, objects).
It is important to point out that although the estimation method accounted for part of the across study variance, a large portion of the variance was unexplained by the moderator variables included in this meta-analysis. While it is possible that the across study variance regarding age differences in familiarity might be due to increased noise in familiarity estimates in both young and older subjects, relative to recollection estimates, this explanation likely cannot account for all of the across study variance. We believe that there are other systematic influences that need to be investigated further. While there are many candidate variables, one factor that we see as critical is how healthy and pathological aging influences the structural and functional integrity of neural regions that support familiarity age-related and disease-related differences in the neural correlates of familiarity. Indeed, as we discuss in more detail below, healthy older adults and aMCI patients show differences in regions thought to support recollection and familiarity. However, it is important to point out that other factors, such as the neuropsychological profile of the sample and age differences in encoding and retrieval strategies might also play an important role.
Amnestic Mild Cognitive Impairment and Alzheimer’s Disease
The results indicated that both recollection and familiarity were significantly reduced in AD. In addition, although recollection was clearly impaired in aMCI, there was only a numerical reduction in familiarity estimates that was not significant. However, the results provided evidence that the aMCI subtype might play a role in the familiarity decline. Specifically, the mean familiarity effect size for studies that only tested single-domain aMCI patients was near zero and slightly positive, whereas the corresponding effect size for studies that tested a mixture of single-domain and multiple-domain aMCI patients was negative and approached significance. This finding is consistent with recent explanations for why familiarity may be spared in some aMCI patients (Serra et al. 2010; Troyer et al. 2012). The findings from the literature comparing tasks believed to differentially rely on recollection and familiarity (e.g., free recall compared to old/new recognition) are also consistent with the pattern of data reported here. In particular, studies exclusively testing multiple-domain aMCI patients found impairments on tasks believed to rely heavily on familiarity (Algarabel et al. 2009; 2012; but see Westerberg et al. 2006). However, although limited by a small sample of single-domain patients, a recent study by Westerberg and colleagues (2013) did not find differences between single and multiple-domain aMCI patients on a four-alternative forced choice test, which they argued relies heavily on familiarity. Future research with larger samples that directly contrast recollection and familiarity estimates between single-domain and multiple-domain aMCI patients is needed to fully address this question.
Why might familiarity be spared in single-domain patients but impaired in multiple-domain patients? Although the extant literature has no addressed this question directly, one possibility is that multiple-domain aMCI patients have more dense amnesia compared to single-domain patients. However, some evidence indicates that single-domain and multiple-domain aMCI patients only differ in impairments on non-memory cognitive domains (Seo et al. 2007). Alternatively, it could be the case that impairments in non-memory domains are what underlie familiarity decreases in multiple-domain aMCI patients. However, some non-memory domains that are affected in multiple-domain aMCI, such as executive function, are typically associated with recollection, not familiarity (e.g., Bugaiska et al. 2007; Davidson and Glisky 2002; but see Bunce and Macready 2005). Thus, it could be the case that familiarity impairments in multiple-domain aMCI patients are not ubiquitous, but depend on the specific neuropsychological profile of the patient. A third possibility discussed by Wolk and colleagues (2013) is that multiple-domain aMCI patients may have more AD-related neuropathology and contain a higher proportion of prodromal AD individuals. Compared to single-domain aMCI patients, multiple-domain aMCI patients have an increased presence of AD biomarkers (Wolk et al. 2009) and also convert to AD at a higher rate (Han et al. 2012). Determining which of the above factors, if any, contribute to familiarity differences in aMCI and AD is an important endeavor for future research.
The results of the meta-analysis are consistent with a recent proposal that “context free” memory, which includes familiarity-based recognition, is impaired early in the course of AD (Didic et al. 2011). These data suggest that familiarity impairments might be predictive of which individuals will convert to AD and who will remain stable or even revert back to normal. As discussed previously, the finding that healthy aging can be associated with selective recollection deficits, whereas AD is associated with pronounced deficits in recollection and familiarity suggest that familiarity impairments might be indicative of pathological aging. In addition, the finding that familiarity decreases were evident only in aMCI studies that tested both single-domain and multiple-domain aMCI patients in conjunction with findings that multiple-domain aMCI patients convert to AD at a higher rate (Han et al. 2012) suggest that familiarity may be useful in predicting conversion to AD. Future research, and in particular longitudinal data, is needed to determine if baseline levels of the rate of decline in recollection and familiarity predict conversion to AD.
It is also important to consider whether or not other factors play a role in recollection and familiarity decreases observed in aMCI and AD patients. Currently, there is little evidence examining the role the estimation method, high levels of recollection, or the type of stimuli role in recollection and familiarity differences in aMCI and AD. Although few studies have critically examined these issues, the available evidence suggests that only the type of stimuli might moderate familiarity differences in aMCI patients. For instance, Serra and colleagues (2010) reported similar patterns of recollection and familiarity deficits in aMCI patients tested with the RK and PD methods, and Wolk and colleagues (2013) reported that the pattern of recollection and familiarity deficits were unchanged when individuals with high levels of recollection were excluded from the analysis. Regarding the type of stimuli, a study by Embree and colleagues (2012) recently demonstrated that familiarity deficits in aMCI patients were eliminated when the to-be-learned stimuli were pictures instead of words. Additional work replicating and extending the above findings are needed before any firm conclusions can be drawn.
Linking Memory Changes to Medial Temporal Lobe Changes
What are the neural changes that underlie the declines in recollection and familiarity that are observed in aging? It is well established that the medial temporal lobes (MTL) play a critical role in episodic memory, with damage to these regions causing severe impairments in the ability to form new episodic memories (e.g., Scoville and Milner 1957; Squire and Zola-Morgan 1991). In addition, a growing body of research from studies of lesion patients, neuroimaging and animal studies has indicated that within the MTL, the hippocampus is critical for recollection, whereas the surrounding perirhinal cortex is critical for familiarity (for reviews see Diana et al. 2007; Eichenbaum et al. 2007; Yonelinas et al. 2010; but see Squire et al. 2007). A number of structural and functional neuroimaging studies have begun to explore how age related changes in these regions are related to declines in recollection and familiarity. Aging is associated with a large variety of brain changes and a full neural account of age-related memory declines is undoubtedly quite complex. For instance, aging is associated with robust volumetric reductions in the frontal cortex (e.g., Raz 2005), and a full understanding of age differences in memory must account for these changes. Here, we focus on the MTL because, as discussed above, the extant literature provides strong evidence for a dissociation between the involvement of the hippocampus and perirhinal cortex in recollection and familiarity, respectively. The existing results suggest that changes in these two MTL regions are directly related to the types of memory impairment that are observed in different age and patient groups.
Structural neuroimaging studies have shown decreases in hippocampal volume in normal aging, aMCI and AD (for reviews, see Raz 2005; Raz and Rodrigue 2006; Ries et al. 2008). AD and aMCI patients show pronounced volumetric decreases in the parahippocampal gyrus, including the perirhinal and entorhinal cortices (e.g., Ries et al. 2008; Troyer et al. 2012; Westerberg et al. 2013; Wolk et al. 2011), whereas perirhinal and entorhinal volumes are less affected in healthy aging (e.g., Raz et al. 2004; Yonelinas et al. 2007). Thus, it might be the case that recollection impairments in healthy aging, aMCI and AD are due to decreases in hippocampal integrity, whereas the familiarity deficits seen in AD and some aMCI groups could be explained by damage to regions outside the hippocampus, such as the entorhinal and perirhinal cortices.
Support for this idea comes from studies examining the relationship between age-related brain changes and memory performance. A number of studies have revealed that age reductions in MTL volumes correlate with decreases in behavioral measures of episodic memory (e.g., Rajah et al. 2010; Troyer et al. 2012; Westerberg et al. 2013; Wolk et al. 2011; Yonelinas et al. 2007; but see Van Petten 2004). Only a few studies to date have used process estimation methods to examine the relationship between estimates of recollection and familiarity with MTL volumes. Importantly, these studies appear to converge in showing that hippocampal volume correlates with recollection whereas entorhinal/perirhinal volume correlates with familiarity. For example, Yonelinas and colleagues (2007) examined the relationship between hippocampal and entorhinal/perirhinal volumes and recollection and familiarity estimates derived from structural equation modeling (cf., Quamme et al. 2004) in 157 older adults between 65 and 80 years-of-age. The results showed that hippocampal volume correlated with recollection where entorhinal/perirhinal volumes correlated with familiarity. Moreover, hippocampal volume was found to mediate the relationship between age and recollection. Wolk and colleagues (2011) found a similar pattern of results in a sample of healthy older adults, aMCI patients, and AD patients. Specifically, after controlling for age and diagnostic status, the volume of the hippocampus, but not parahippocampal gyrus (i.e., the perirhinal, entorhinal, and parahippocampal cortices) predicted recollection estimates derived from a PD procedure. In contrast, parahippocampal gyrus volume predicted familiarity, but not recollection estimates. Likewise, Troyer and colleagues (2012) reported that hippocampal volume correlated with estimates recollection but not familiarity derived from the PD procedure.
Recently, Wolk and colleagues (2013) examined how cortical thickness in a network of regions which is hypothesized to be a “cortical signature of AD” (see Dickerson et al. 2009) correlated with recollection and familiarity estimates in healthy older adults and aMCI patients. The network includes nine bilateral regions distributed across the frontal cortex (superior frontal gyrus and inferior frontal sulcus), parietal cortex (angular gyrus, superior parietal lobule, supramarginal gyrus, and precuneus) and temporal cortex (anterior medial temporal lobe, temporal pole, inferior temporal gyrus). Critically, the anterior medial temporal lobe encompassed the entorhinal and perirhinal regions that have been shown to correlate with familiarity estimates (Wolk et al. 2011; Yonelinas et al. 2007). The mean cortical thickness of the above 9 bilateral regions correlated with familiarity, but not recollection, in both healthy older adults and aMCI patients, such that cortical thinning was associated with lower levels of familiarity. The above finding suggests that familiarity impairments in apparently healthy older adults, when they are observed, may be a result of underlying AD neuropathology that does not result in clinical impairments.
The evidence from functional neuroimaging studies has suggested that young and older subjects recruit similar regions in recollection and familiarity related recognition, but that there might be subtle differences in the roles of these regions and how they interact with other cortical regions to support episodic memory. Some studies have reported that older and younger adults show similar patterns of recollection and familiarity related activity in the hippocampus and perirhinal cortex, respectively (Angel et al., 2013; de Chastelaine et al. 2011; Duarte et al. 2008; 2010). For example, using an RK task, Duarte and colleagues (2008, 2010) found increased hippocampal activity associated with recollection and decreased activity in the perirhinal cortex activity associated with familiarity during retrieval in both young and healthy older adults. However, Daselaar and colleauges (2006) reported that increased hippocampal activity associated with recollection in young adults was reduced in healthy older adults. However, healthy older adults showed larger decreases in perirhinal activity associated with familiarity than did young controls (see also Morcom et al. 2010). Moreover, these changes were associated with differences in the connectivity profiles of the hippocampus and perirhinal cortex, such hippocampal activity correlated more strongly with retrosplenial cortex in young adults and perirhinal activity correlated more strongly with prefrontal cortical regions in older adults (for similar results, see Dennis et al. 2008). In contrast, Duverne et al. (2008) reported that aging was associated with increased recollection-related activity in the hippocampus. Finally, Genon and colleagues (2013) reported decreased recollection related connectivity between the posterior cingulate and hippocampus in AD patients, which suggests that decreased hippocampal function might underlie recollection impairments in AD.
The structural and functional neuroimaging results are broadly consistent with the results showing that the hippocampus and perirhinal cortex are critical for recollection and familiarity in healthy older adults, aMCI patients, and AD patients. Moreover, the above discussion suggests that age-related or disease-related changes in MTL regions might underlie decreases in recollection and familiarity. The extant data also suggest that AD-related neuropathology in aMCI patients and healthy older adults might underlie age-related declines in familiarity.
Future Directions and Conclusions
One general limitation of the studies examined in the current meta-analysis is that they examined cross-sectional samples and compared healthy older adults to college-aged young adults. Future work should focus on examining recollection and familiarity declines with longitudinal experimental designs as well as with cross-sectional designs that treat age as a continuous variable. These studies are needed to address important theoretical questions, such as determining if the recollection and familiarity declines onset simultaneously, or if one happens later than the other. Another important issue is to determine if the two processes show linear or nonlinear age-related decreases. A few studies have examined age-related differences in recollection and familiarity using cross-sectional samples, with the results showing that age is negatively correlated with recollection but not familiarity (Koen & Yonelinas, under review; McCabe et al. 2009; Yonelinas et al. 2007). However, these studies did not examine if nonlinear age trends were present in recollection and familiarity.
Additionally, only a small number of studies have examined recollection and familiarity in AD and aMCI, and further studies will be essential in determining the fate of recollection and familiarity in these two patient populations. In AD, the impairments in both recollection and familiarity were large and consistent across studies suggesting that the deficits observed in this group are quite robust. This is important because it suggests that the deficits seen in AD are distinct from those seen in normal aging where only recollection is consistently impaired. In aMCI, recollection impairments were also quite consistent but the fate of familiarity was less clear. The results did suggest that multiple-domain, but not single-domain, aMCI patients might exhibit familiarity deficits, but no study that we are aware of has directly compared recollection and familiarity estimates between these two aMCI subtypes. Additional research directly assessing this issue is needed.
Moreover, the results from this meta-analysis suggested that familiarity does not generally decline in healthy aging when measured with the PD and ROC procedures, but that age-related declines in familiarity are often observed in studies using the RK task. This might be related to differences in how elderly individuals interpret the RK instructions; however, further studies directly contrasting the different measurement methods in aged groups will be critical in assessing this possibility. Moreover, findings from Wolk and colleagues (2013) suggest that another cause of familiarity deficits in healthy older adults might be the presence and load of underlying AD neuropathology.
Taken together, the results presented here highlight the importance of assessing recollection and familiarity when examining the effects of age on episodic memory. In addition, they suggest that the distinction between recollection and familiarity has the potential to play an important role in differentiating between memory changes in healthy aging and neurodegenerative diseases.
Supplementary Material
Acknowledgments
This work was supported by National Science Foundation Graduate Research Fellowship 1148897 awarded to Joshua D. Koen and by National Institute of Mental Health Grants 5R01-MH059352-13 and 5R01-MH083734-05 awarded to Andrew P. Yonelinas.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s11065-014-9266-5) contains supplementary material, which is available to authorized users.
Here, we adopt the dual-process interpretation of the RK, PD, and ROC procedures. However, we acknowledge that there is still debate surrounding how data from these three procedures should be interpreted (e.g., Donaldson 1996; Dunn 2004; 2008; Wixted 2007; Yonelinas 2001a; 2002). Although this is an important theoretical issue that demands further investigation, we do not intend to add to this debate here because it is beyond the scope of the present article.
This was not too surprising because the RK procedure was not initially developed to estimate recollection and familiarity; this development occurred almost a decade after the procedure was introduced (e.g., Yonelinas and Jacoby 1995). In contrast, the motivation underlying both the PD (Jacoby 1991) and dual-process signal detection model that has been applied to recognition ROCs (Yonelinas 1994) was to quantify the contribution of recollection and familiarity.
The studies that used nonverbal materials only did so in conjunction with the RK and ROC estimation methods. The data reported in the Results were similar when considering the materials effect using only the RK and ROC studies (data not shown).
The metric reported for estimates of recollection and familiarity varies across the three estimation methods, and also across studies using the same estimation method. For example, recollection in the ROC procedure is calculated as a probability, whereas recollection and familiarity in the RK task can be calculated as a probability or a discrimination index (d’; e.g., McCabe and Geraci 2009). Likewise, in the PD and RK tasks, familiarity can be calculated as a probability (e.g., Jennings and Jacoby 1993) or a discrimination index (e.g., Davidson and Glisky 2002). Effect size estimates were based on means and variances of the metric used to calculate recollection and familiarity in a given study.
Contributor Information
Joshua D. Koen, Email: joshua.koen@utdallas.edu, Department of Psychology, University of California, Davis, 1 Shields Avenue, Davis, CA 95616, USA; Center for Vital Longevity, University of Texas at Dallas, 1600 Viceroy Drive, Suite 800, Dallas, TX 75235, USA.
Andrew P. Yonelinas, Department of Psychology, University of California, Davis, 1 Shields Avenue, Davis, CA 95616, USA Center for Mind and Brain, University of California, Davis, 1 Shields Avenue, Davis, CA 95616, USA.
References
- Aggleton JP, Vann SD, Denby C, Dix S, Mayes AR, Roberts N, Yonelinas AP. Sparing of the familiarity component of recognition memory in a patient with hippocampal pathology. Neuropsychologia. 2005;43(12):1810–1823. doi: 10.1016/j.neuropsychologia.2005.01.019. [DOI] [PubMed] [Google Scholar]
- Algarabel S, Escudero J, Mazón J-F, Pitarque A, Fuentes M, Peset V, Lacruz L. Familiarity-based recognition in the young, healthy elderly, mild cognitive impaired and Alzheimer’s patients. Neuropsychologia. 2009;47(10):2056–2064. doi: 10.1016/j.neuropsychologia.2009.03.016. [DOI] [PubMed] [Google Scholar]
- Algarabel S, Fuentes M, Escudero J, Pitarque A, Peset V, Mazón J-F, Meléndez J-C. Recognition memory deficits in mild cognitive impairment. Aging, Neuropsychology, and Cognition: A Journal on Normal and Dysfunctional Development. 2012;19(5):608–619. doi: 10.1080/13825585.2011.640657. [DOI] [PubMed] [Google Scholar]
- Ally BA, Waring JD, Beth EH, McKeever JD, Milberg WP, Budson AE. Aging memory for pictures: using high-density event-related potentials to understand the effect of aging on the picture superiority effect. Neuropsychologia. 2008;46(2):679–689. doi: 10.1016/j.neuropsychologia.2007.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ally BA, Gold CA, Budson AE. An evaluation of recollection and familiarity in Alzheimer’s disease and mild cognitive impairment using receiver operating characteristics. Brain and Cognition. 2009;69(3):504–513. doi: 10.1016/j.bandc.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aly M, Knight RT, Yonelinas AP. Faces are special but not too special: spared face recognition in amnesia is based on familiarity. Neuropsychologia. 2010;48(13):3941–3948. doi: 10.1016/j.neuropsychologia.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson ND, Ebert PL, Jennings JM, Grady CL, Cabeza R, Graham SJ. Recollection- and familiarity-based memory in healthy aging and amnestic mild cognitive impairment. Neuropsychology. 2008;22(2):177–187. doi: 10.1037/0894-4105.22.2.177. [DOI] [PubMed] [Google Scholar]
- Angel L, Bastin C, Genon S, Balteau E, Phillips C, Luxen A, Maquet P, Salmon E, Collette F. Differential effects of aging on the neural correlates of recollection and familiarity. Cortex. 2013;49:1585–1597. doi: 10.1016/j.cortex.2012.10.002. [DOI] [PubMed] [Google Scholar]
- Baddeley A, Vargha-Khadem F, Mishkin M. Preserved recognition in a case of developmental amnesia: implications for the acaquisition of semantic memory? Journal of Cognitive Neuroscience. 2001;13(3):357–369. doi: 10.1162/08989290151137403. [DOI] [PubMed] [Google Scholar]
- Barba GD. Recognition memory and recollective experience in Alzheimer’s disease. Memory. 1997;5(6):657–672. doi: 10.1080/741941546. [DOI] [PubMed] [Google Scholar]
- Bastin C, Van der Linden M. The contribution of recollection and familiarity to recognition memory: a study of the effects of test format and aging. Neuropsychology. 2003;17(1):14–24. [PubMed] [Google Scholar]
- Bastin C, Van der Linden M, Michel A-P, Friedman WJ. The effects of aging on location-based and distance-based processes in memory for time. Acta Psychologica. 2004;116(2):145–171. doi: 10.1016/j.actpsy.2003.12.014. [DOI] [PubMed] [Google Scholar]
- Belleville S, Ménard M-C, Lepage E. Impact of novelty and type of material on recognition in healthy older adults and persons with mild cognitive impairment. Neuropsychologia. 2011;49(10):2856–2865. doi: 10.1016/j.neuropsychologia.2011.06.011. [DOI] [PubMed] [Google Scholar]
- Benjamin AS, Craik FIM. Parallel effects of aging and time pressure on memory for source: evidence from the spacing effect. Memory & Cognition. 2001;29(5):691–697. doi: 10.3758/bf03200471. [DOI] [PubMed] [Google Scholar]
- Bennett IJ, Golob EJ, Parker ES, Starr A. Memory evaluation in mild cognitive impairment using recall and recognition tests. Journal of Clinical and Experimental Neuropsychology. 2006;28(8):1408–1422. doi: 10.1080/13803390500409583. [DOI] [PubMed] [Google Scholar]
- Bird CM, Burgess N. The hippocampus supports recognition memory for familiar words but Not unfamiliar faces. Current Biology. 2008;18(24):1932–1936. doi: 10.1016/j.cub.2008.10.046. [DOI] [PubMed] [Google Scholar]
- Boywitt CD, Kuhlmann BG, Meiser T. The role of source memory in older adults’ recollective experience. Psychology and Aging. 2012;27(2):484–497. doi: 10.1037/a0024729. [DOI] [PubMed] [Google Scholar]
- Bugaiska A, Clarys D, Jarry C, Taconnat L, Tapia G, Vanneste S, Isingrini M. The effect of aging in recollective experience: the processing speed and executive functioning hypothesis. Consciousness and Cognition. 2007;16(4):797–808. doi: 10.1016/j.concog.2006.11.007. [DOI] [PubMed] [Google Scholar]
- Bunce D. Cognitive support at encoding attenuates age differences in recollective experience among adults of lower frontal lobe function. Neuropsychology. 2003;17(3):353–361. doi: 10.1037/0894-4105.17.3.353. [DOI] [PubMed] [Google Scholar]
- Bunce D, Macready A. Processing speed, executive function, and age differences in remembering and knowing. The Quarterly Journal of Experimental Psychology Section A: Human Experimental Psychology. 2005;58(1):155–168. doi: 10.1080/02724980443000197. [DOI] [PubMed] [Google Scholar]
- Carlesimo GA, Fadda L, Turriziani P, Tomaiuolo F, Caltagirone C. Selective sparing of face learning in a global amnesic patient. Journal of Neurology, Neurosurgery & Psychiatry. 2001;71(3):340–346. doi: 10.1136/jnnp.71.3.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarys D, Isingrini M, Gana K. Mediators of age-related differences in recollective experience in recognition memory. Acta Psychologica. 2002;109(3):315–329. doi: 10.1016/s0001-6918(01)00064-6. [DOI] [PubMed] [Google Scholar]
- Clarys D, Bugaiska A, Tapia G, Alexia Baudouin A. Ageing, remembering, and executive function. Memory. 2009;17(2):158–168. doi: 10.1080/09658210802188301. [DOI] [PubMed] [Google Scholar]
- Cohen JD. Statistical power analysis for the social sciences. 2nd ed. Hillsdale: Lawrence Erlbaum; 1988. [Google Scholar]
- Cohn M, Emrich SM, Moscovitch M. Age-related deficits in associative memory: the influence of impaired strategic retrieval. Psychology and Aging. 2008;23(1):93–103. doi: 10.1037/0882-7974.23.1.93. [DOI] [PubMed] [Google Scholar]
- Comblain C, D’Argembeau A, Van der Linden M, Aldenhoff L. The effect of ageing on the recollection of emotional and neutral pictures. Memory. 2004;12(6):673–684. doi: 10.1080/09658210344000477. [DOI] [PubMed] [Google Scholar]
- Craik FIM, McDowd JM. Age differences in recall and recognition. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1987;13(3):474–479. [Google Scholar]
- Daselaar SM, Fleck MS, Dobbins IG, Madden DJ, Cabeza R. Effects of healthy aging on hippocampal and rhinal memory functions: an event-related fMRI study. Cerebral Cortex. 2006;16(12):1771–1782. doi: 10.1093/cercor/bhj112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson PSR, Glisky EL. Neuropsychological correlates of recollection and familiarity in normal aging. Cognitive, Affective, & Behavioral Neuroscience. 2002;2(2):174–186. doi: 10.3758/cabn.2.2.174. [DOI] [PubMed] [Google Scholar]
- de Chastelaine M, Wang TH, Minton B, Muftuler LT, Rugg MD. The effects of age, memory performance, and callosal integrity on the neural correlates of successful associative encoding. Cerebral Cortex. 2011;21:2166–2176. doi: 10.1093/cercor/bhq294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis NA, Hayes SM, Prince SE, Madden DJ, Huettel SA, Cabeza R. Effects of aging on the neural correlates of successful item and source memory encoding. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2008;34(4):791–808. doi: 10.1037/0278-7393.34.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diana RA, Yonelinas AP, Ranganath C. Imaging recollection and familiarity in the medial temporal lobe: a three-component model. Trends in Cognitive Sciences. 2007;11(9):379–386. doi: 10.1016/j.tics.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Dickerson BC, Bakkour A, Salat DH, Feczko E, Pacheco J, Greve DN, et al. The cortical signature of Alzheimer’s disease: regionally specific cortical thinning relates to symptom severity in very mild to mild AD dementia and is detectable in asymptomatic amyloid-positive individuals. Cerebral Cortex. 2009;19(3):497–510. doi: 10.1093/cercor/bhn113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Didic M, Barbeau EJ, Felician O, Tramoni E. Which memory system is impaired first in Alzheimer’s disease? Journal of Alzheimer's Disease. 2011;27(1):11–22. doi: 10.3233/JAD-2011-110557. [DOI] [PubMed] [Google Scholar]
- Donaldson W. The role of decision processes in remembering and knowing. Memory & Cognition. 1996;24(4):523–533. doi: 10.3758/bf03200940. [DOI] [PubMed] [Google Scholar]
- Drag LL, Bieliauskas LA. Contemporary review 2009: cognitive aging. Journal of Geriatric Psychiatry and Neurology. 2010;23(2):75–93. doi: 10.1177/0891988709358590. [DOI] [PubMed] [Google Scholar]
- Duarte A, Ranganath C, Trujillo C, Knight RT. Intact recollection memory in high-performing older adults: ERP and behavioral evidence. Journal of Cognitive Neuroscience. 2006;18(1):33–47. doi: 10.1162/089892906775249988. [DOI] [PubMed] [Google Scholar]
- Duarte A, Henson RN, Graham KS. The effects of aging on the neural correlates of subjective and objective recollection. Cerebral Cortex. 2008;18(9):2169–2180. doi: 10.1093/cercor/bhm243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte A, Graham KS, Henson RN. Age-related changes in neural activity associated with familiarity, recollection and false recognition. Neurobiology of Aging. 2010;31(10):1814–1830. doi: 10.1016/j.neurobiolaging.2008.09.014. [DOI] [PubMed] [Google Scholar]
- Dudas RB, Clague F, Thompson SA, Graham KS, Hodges JR. Episodic and semantic memory in mild cognitive impairment. Neuropsychologia. 2005;43(9):1266–1276. doi: 10.1016/j.neuropsychologia.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Dunn JC. Remember-know: a matter of confidence. Psychological Review. 2004;111(2):524–542. doi: 10.1037/0033-295X.111.2.524. [DOI] [PubMed] [Google Scholar]
- Dunn JC. The dimensionality of the remember-know task: a state-trace analysis. Psychological Review. 2008;115(2):426–446. doi: 10.1037/0033-295X.115.2.426. [DOI] [PubMed] [Google Scholar]
- Duverne S, Habibi A, Rugg MD. Regional specificity of age effects on the neural correlates of episodic retrieval. Neurobiology of Aging. 2008;29:1902–1916. doi: 10.1016/j.neurobiolaging.2007.04.022. [DOI] [PubMed] [Google Scholar]
- Düzel E, Schütze H, Yonelinas AP, Heinze H-J. Functional phenotyping of successful aging in long-term memory: preserved performance in the absence of neural compensation. Hippocampus. 2011;21(8):803–814. doi: 10.1002/hipo.20834. [DOI] [PubMed] [Google Scholar]
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. British Medical Journal. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Annual Review of Neuroscience. 2007;30(1):123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Embree LM, Budson AE, Ally BA. Memorial familiarity remains intact for pictures but not for words in patients with amnestic mild cognitive impairment. Neuropsychologia. 2012;50(9):2333–2340. doi: 10.1016/j.neuropsychologia.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fell M. Encoding, retrieval and age effects on recollective experience. The Irish Journal of Psychology. 1992 [Google Scholar]
- Friedman D, Trott C. An event-related potential study of encoding in young and older adults. Neuropsychologia. 2000;38(5):542–557. doi: 10.1016/s0028-3932(99)00122-0. [DOI] [PubMed] [Google Scholar]
- Friedman D, de Chastelaine M, Nessler D, Malcolm B. Changes in familiarity and recollection across the lifespan: an ERP perspective. Brain Research. 2010;1310:124–141. doi: 10.1016/j.brainres.2009.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner J. Functional aspects of recollective experience. Memory & Cognition. 1988;16(4):309–313. doi: 10.3758/bf03197041. [DOI] [PubMed] [Google Scholar]
- Genon S, Collette F, Feyers D, Phillips C, Salmon E, Bastin C. Item familiarity and controlled associative retrieval in Alzheimer’s disease: an fMRI study. Cortex. 2013;49(6):1566–1584. doi: 10.1016/j.cortex.2012.11.017. [DOI] [PubMed] [Google Scholar]
- Geraci L, McCabe DP. Examining the basis for illusory recollection: the role of remember/know instructions. Psychonomic Bulletin & Review. 2006;13(3):466–473. doi: 10.3758/bf03193871. [DOI] [PubMed] [Google Scholar]
- Geraci L, McCabe DP, Guillory JJ. On interpreting the relationship between remember–know judgments and confidence: The role of instructions. Consciousness and Cognition. 2009;18(3):701–709. doi: 10.1016/j.concog.2009.04.010. [DOI] [PubMed] [Google Scholar]
- Glanzer M, Hilford A, Kim K. Six regularities of source recognition. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2004;30(6):1176–1195. doi: 10.1037/0278-7393.30.6.1176. [DOI] [PubMed] [Google Scholar]
- Han JW, Kim TH, Lee SB, Park JH, Lee JJ, Huh Y, et al. Predictive validity and diagnostic stability of mild cognitive impairment subtypes. Alzheimer's & Dementia. 2012;8(6):553–559. doi: 10.1016/j.jalz.2011.08.007. [DOI] [PubMed] [Google Scholar]
- Harkins SW, Chapman CR, Eisdorfer C. Memory loss and response bias in senescence. Journal of Gerontology. 1979;34(1):66–72. doi: 10.1093/geronj/34.1.66. [DOI] [PubMed] [Google Scholar]
- Healy MR, Light LL, Chung C. Dual-process models of associative recognition in young and older adults: evidence from receiver operating characteristics. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2005;31(4):768–788. doi: 10.1037/0278-7393.31.4.768. [DOI] [PubMed] [Google Scholar]
- Hedges LV. Distribution theory for Glass’s estimator of effect size and related estimators. Journal of Educational and Behavioral Statistics. 1981;6(2):107–128. [Google Scholar]
- Hedges LV, Vevea JL. Fixed- and random-effects models in meta-analysis. Psychological Methods. 1998;3(4):486–504. [Google Scholar]
- Howard MW, Bessette-Symons B, Zhang Y, Hoyer WJ. Aging selectively impairs recollection in recognition memory for pictures: evidence from modeling and receiver operating characteristic curves. Psychology and Aging. 2006;21(1):96–106. doi: 10.1037/0882-7974.21.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyer WJ, Verhaeghen P. Memory aging. In: Birren JE, Schaie KW, editors. Handbook of the psychology of aging. 6th ed. Boston: Elsevier; 2006. pp. 209–232. [Google Scholar]
- Hudon C, Belleville S, Gauthier S. The assessment of recognition memory using the remember/know procedure in amnestic mild cognitive impairment and probable Alzheimer’s disease. Brain and Cognition. 2009;70(1):171–179. doi: 10.1016/j.bandc.2009.01.009. [DOI] [PubMed] [Google Scholar]
- Jacoby LL. A process dissociation framework: separating automatic from intentional uses of memory. Journal of Memory and Language. 1991;30(5):513–541. [Google Scholar]
- Jacoby LL. Ironic effects of repetition: measuring age-related differences in memory. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1999;25(1):3–22. doi: 10.1037//0278-7393.25.1.3. [DOI] [PubMed] [Google Scholar]
- Jennings JM, Jacoby LL. Automatic versus intentional uses of memory: aging, attention, and control. Psychology and Aging. 1993;8(2):283–293. doi: 10.1037//0882-7974.8.2.283. [DOI] [PubMed] [Google Scholar]
- Jennings JM, Jacoby LL. An opposition procedure for detecting age-related deficits in recollection: telling effects of repetition. Psychology and Aging. 1997;12(2):352–361. doi: 10.1037//0882-7974.12.2.352. [DOI] [PubMed] [Google Scholar]
- Kapucu A, Rotello CM, Ready RE, Seidl KN. Response bias in “remembering” emotional stimuli: a new perspective on age differences. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2008;34(3):703–711. doi: 10.1037/0278-7393.34.3.703. [DOI] [PubMed] [Google Scholar]
- Kilb A, Naveh-Benjamin M. The effects of pure pair repetition on younger and older adults’ associative memory. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2011;37(3):706–719. doi: 10.1037/a0022525. [DOI] [PubMed] [Google Scholar]
- Knapp G, Hartung J. Improved tests for a random effects meta-regression with a single covariate. Statistics in Medicine. 2003;22(17):2693–2710. doi: 10.1002/sim.1482. [DOI] [PubMed] [Google Scholar]
- Koen JD, Yonelinas AP. Memory variability is due to the contribution of recollection and familiarity, not to encoding variability. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2010;36(6):1536–1542. doi: 10.1037/a0020448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Voie D, Light LL. Adult age differences in repetition priming: a meta-analysis. Psychology and Aging. 1994;9(4):539–553. doi: 10.1037//0882-7974.9.4.539. [DOI] [PubMed] [Google Scholar]
- Landau SM, Harvey D, Madison CM, Reiman EM, Foster NL, Aisen PS, et al. Comparing predictors of conversion and decline in mild cognitive impairment. Neurology. 2010;75(3):230–238. doi: 10.1212/WNL.0b013e3181e8e8b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson M, Oberg C, Bäckman L. Recollective experience in odor recognition: influences of adult age and familiarity. Psychological Research. 2006;70(1):68–75. doi: 10.1007/s00426-004-0190-9. [DOI] [PubMed] [Google Scholar]
- Light LL. Memory and aging: four hypotheses in search of data. Annual Review of Psychology. 1991;42(1):333–376. doi: 10.1146/annurev.ps.42.020191.002001. [DOI] [PubMed] [Google Scholar]
- Light LL. Dual-process theories of memory in old age: An update. In: Naveh-Benjamin M, Ohta N, editors. Memory and Aging: Current Issues and Future Directions. Psychology Press; 2011. [Google Scholar]
- Light LL, Prull MW, La Voie D, Healy MR. Dual-process theories of memory in old age. In: Perfect TJ, Maylor EA, editors. Models of cognitive aging. Oxford: Oxford University Press; 2000. [Google Scholar]
- Lipsey MW, Wilson DB. Practical meta-analysis. Thousand Oaks: SAGE Publications; 2001. [Google Scholar]
- Lovden M, Ronnlund M, Nilsson L-G. Remembering and knowing in adulthood: effects of enacted encoding and relations to processing speed. Aging, Neuropsychology, and Cognition: A Journal on Normal and Dysfunctional Development. 2002;9(3):184–200. [Google Scholar]
- Luo L, Craik FIM. Age differences in recollection: specificity effects at retrieval. Journal of Memory and Language. 2009;60(4):421–436. [Google Scholar]
- Luo L, Hendriks T, Craik FIM. Age differences in recollection: three patterns of enhanced encoding. Psychology and Aging. 2007;22(2):269–280. doi: 10.1037/0882-7974.22.2.269. [DOI] [PubMed] [Google Scholar]
- Mark RE, Rugg MD. Age effects on brain activity associated with episodic memory retrieval. An electrophysiological study. Brain. 1998;121(5):861–873. doi: 10.1093/brain/121.5.861. [DOI] [PubMed] [Google Scholar]
- McCabe DP, Geraci LD. The influence of instructions and terminology on the accuracy of remember–know judgments. Consciousness and Cognition. 2009;18(2):401–413. doi: 10.1016/j.concog.2009.02.010. [DOI] [PubMed] [Google Scholar]
- McCabe DP, Roediger HL, III, McDaniel MA, Balota DA. Aging reduces veridical remembering but increases false remembering: neuropsychological test correlates of remember–know judgments. Neuropsychologia. 2009;47(11):2164–2173. doi: 10.1016/j.neuropsychologia.2008.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of alzheimer“s disease: report of the NINCDS-ADRDA work group* under the auspices of department of health and human services task force on alzheimer”s disease. Neurology. 1984;34(7):939. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Morcom AM, Bullmore ET, Huppert FA, Lennox B, Praseedom A, Linnington H, Fletcher PC. Memory encoding and dopamine in the aging brain: a psychopharmacological neuroimaging study. Cerebral Cortex. 2010;20(3):743–757. doi: 10.1093/cercor/bhp139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno SG, Sutton AJ, Ades AE, Stanley TD, Abrams KR, Peters JL, Cooper NJ. Assessment of regression-based methods to adjust for publication bias through a comprehensive simulation study. BMC Medical Research Methodology. 2009;9(3) doi: 10.1186/1471-2288-9-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman KA, Schacter DL. False recognition in younger and older adults: exploring the characteristics of illusory memories. Memory & Cognition. 1997;25(6):838–848. doi: 10.3758/bf03211328. [DOI] [PubMed] [Google Scholar]
- Old SR, Naveh-Benjamin M. Differential effects of age on item and associative measures of memory: a meta-analysis. Psychology and Aging. 2008;23(1):104–118. doi: 10.1037/0882-7974.23.1.104. [DOI] [PubMed] [Google Scholar]
- Park DC, Puglisi JT, Sovacool M. Memory for pictures, words, and spatial location in older adults: evidence for pictorial superiority. Journal of Gerontology. 1983;38(5):582–588. doi: 10.1093/geronj/38.5.582. [DOI] [PubMed] [Google Scholar]
- Parkin AJ, Walter BM. Recollective experience, normal aging, and frontal dysfunction. Psychology and Aging. 1992;7(2):290–298. doi: 10.1037//0882-7974.7.2.290. [DOI] [PubMed] [Google Scholar]
- Parks CM. The role of noncriterial recollection in estimating recollection and familiarity. Journal of Memory and Language. 2007;57(1):81–100. doi: 10.1016/j.jml.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perfect TJ, Dasgupta ZRR. What underlies the deficit in reported recollective experience in old age? Memory & Cognition. 1997;25(6):849–858. doi: 10.3758/bf03211329. [DOI] [PubMed] [Google Scholar]
- Perfect TJ, Williams RB, Anderton-Brown C. Age differences in reported recollective experience are Due to encoding effects, not response bias. Memory. 1995;3(2):169–186. doi: 10.1080/09658219508258964. [DOI] [PubMed] [Google Scholar]
- Peters J, Daum I. Differential effects of normal aging on recollection of concrete and abstract words. Neuropsychology. 2008;22(2):255–261. doi: 10.1037/0894-4105.22.2.255. [DOI] [PubMed] [Google Scholar]
- Petersen RC. Mild cognitive impairment as a diagnostic entity. Journal of Internal Medicine. 2004;256(3):183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Roberts RO, Knopman DS, Boeve BF, Geda YE, Ivnik RJ, et al. Mild cognitive impairment: Ten years later. Archives of Neurology. 2009;66(12):1447–1455. doi: 10.1001/archneurol.2009.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prull MW, Dawes LLC, Martin AM, Rosenberg HF, Light LL. Recollection and familiarity in recognition memory: adult age differences and neuropsychological test correlates. Psychology and Aging. 2006;21(1):107–118. doi: 10.1037/0882-7974.21.1.107. [DOI] [PubMed] [Google Scholar]
- Quamme JR, Yonelinas AP, Widaman KF, Kroll NEA, Sauve MJ. Recall and recognition in mild hypoxia: using covariance structural equation modeling to test competing theories of explicit memory. Neuropsychologia. 2004;42(5):672–691. doi: 10.1016/j.neuropsychologia.2003.09.008. [DOI] [PubMed] [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: 2013. Retrieved from http://www.R-project.org/ [Google Scholar]
- Rajah MN, Kromas M, Han JE, Pruessner JC. Group differences in anterior hippocampal volume and in the retrieval of sptial and temporal context memory in healthy young versus older adults. Neuropsychologia. 2010;48:4020–4030. doi: 10.1016/j.neuropsychologia.2010.10.010. [DOI] [PubMed] [Google Scholar]
- Rajaram S. Remembering and knowing: Two means of access to the personal past. Memory & Cognition. 1993;21(1):89–102. doi: 10.3758/bf03211168. [DOI] [PubMed] [Google Scholar]
- Raz N. The aging brain observed in vivo: Differential changes and their modifiers. In: Cabeza R, Nyberg L, Park D, editors. Cognitive neuroscience of aging. New York: Oxford University Press; 2005. pp. 19–57. [Google Scholar]
- Raz N, Rodrigue KM. Differential aging of the brain: patterns, cognitive correlates, and modifiers. Neuroscience and Biobehavioral Reviews. 2006;30:730–748. doi: 10.1016/j.neubiorev.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM, Head D, Kennedy KM, Acker JD. Differential aging of the medial temporal lobe. Neurology. 2004;62:433–438. doi: 10.1212/01.wnl.0000106466.09835.46. [DOI] [PubMed] [Google Scholar]
- Ries ML, Carlsson CM, Rowley HA, Sager MA, Gleason CE, Asthana S, Johnson SC. Magnetic resonance imaging characterization of brain structure and functino in mild cognitive impairment: a review. Journal of the American Geriatrics Society. 2008;56:920–934. doi: 10.1111/j.1532-5415.2008.01684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotello C, Macmillan N, Reeder J, Wong M. The remember response: subject to bias, graded, and not a process-pure indicator of recollection. Psychonomic Bulletin & Review. 2005;12(5):865–873. doi: 10.3758/bf03196778. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Curran T. Event-related potentials and recognition memory. Trends in Cognitive Sciences. 2007;11(6):251–257. doi: 10.1016/j.tics.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Salmon D. Neuropsychological features of mild cognitive impairment and preclinical Alzheimer’s disease. In: Pardon M-C, Bondi MW, editors. Behavioral neurobiology of aging. Vol. 10. Berlin: Springer; 2012. pp. 187–212. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Koutstaal W, Johnson MK, Gross MS, Angell KE. False recollection induced by photographs: a comparison of older and younger adults. Psychology and Aging. 1997;12(2):203–215. doi: 10.1037//0882-7974.12.2.203. [DOI] [PubMed] [Google Scholar]
- Schonfield D, Robertson BA. Memory storage and aging. Canadian Journal of Psychology. 1966;20(2):228–236. doi: 10.1037/h0082941. [DOI] [PubMed] [Google Scholar]
- Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. Journal of Neurology, Neurosurgery, and Psychiatry. 1957;20(1):11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo SW, Im K, Lee J-M, Kim Y-H, Kim ST, Kim SY, et al. Cortical thickness in single- versus multiple-domain amnestic mild cognitive impairment. NeuroImage. 2007;36(2):289–297. doi: 10.1016/j.neuroimage.2007.02.042. [DOI] [PubMed] [Google Scholar]
- Serra L, Bozzali M, Cercignani M, Perri R, Fadda L, Caltagirone C, Carlesimo GA. Recollection and familiarity in amnesic mild cognitive impairment. Neuropsychology. 2010;24(3):316–326. doi: 10.1037/a0017654. [DOI] [PubMed] [Google Scholar]
- Skinner EI, Fernandes MA. Interfering with remembering and knowing: effects of divided attention at retrieval. Acta Psychologica. 2008;127(2):211–221. doi: 10.1016/j.actpsy.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Skinner EI, Fernandes MA. Age-related changes in the use of study context to increase recollection. Aging, Neuropsychology, and Cognition: A Journal on Normal and Dysfunctional Development. 2009a;16(4):377–400. doi: 10.1080/13825580802573052. [DOI] [PubMed] [Google Scholar]
- Skinner EI, Fernandes MA. Illusory recollection in older adults and younger adults under divided attention. Psychology and Aging. 2009b;24(1):211–216. doi: 10.1037/a0014177. [DOI] [PubMed] [Google Scholar]
- Smith JA, Knight RG. Memory processing in Alzheimer’s disease. Neuropsychologia. 2002;40(6):666–682. doi: 10.1016/s0028-3932(01)00137-3. [DOI] [PubMed] [Google Scholar]
- Spencer WD, Raz N. Differential effects of aging on memory for content and context: a meta-analysis. Psychology and Aging. 1995;10(4):527–539. doi: 10.1037//0882-7974.10.4.527. [DOI] [PubMed] [Google Scholar]
- Squire LS, Zola-Morgan S. The medial temporal lobe memory system. Science. 1991;253(5026):1380–1386. doi: 10.1126/science.1896849. [DOI] [PubMed] [Google Scholar]
- Squire LS, Wixted JT, Clark RE. Recognition memory and the medial temporal lobes: a new perspective. Nature Reviews Neuroscience. 2007;8(11):872–883. doi: 10.1038/nrn2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterne JAC, Egger M. Regression methods to detect publication and other bias in meta-analysis. In: Rothstein HR, Sutton AJ, Borenstein M, editors. Publication bias in meta-analysis: Prevention, assessment, and adjustments. Chichester: Wiley; 2005. pp. 99–110. [Google Scholar]
- Toth JP, Parks CM. Effects of age on estimated familiarity in the process dissociation procedure: the role of noncriterial recollection. Memory & Cognition. 2006;34(3):527–537. doi: 10.3758/bf03193576. [DOI] [PubMed] [Google Scholar]
- Troyer AK, Murphy KJ, Anderson ND, Craik FIM, Moscovitch M, Maione A, Gao F. Associative recognition in mild cognitive impairment: relationship to hippocampal volume and apolipoprotein E. Neuropsychologia. 2012;50(14):3721–3728. doi: 10.1016/j.neuropsychologia.2012.10.018. [DOI] [PubMed] [Google Scholar]
- Tse C-S, Balota DA, Moynan SC, Duchek JM, Jacoby LL. The utility of placing recollection in opposition to familiarity in early discrimination of healthy aging and very mild dementia of the Alzheimer’s type. Neuropsychology. 2010;24(1):49–67. doi: 10.1037/a0014887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulving E. Memory and consciousness. Canadian Psychology. 1985;26(1):1–12. [Google Scholar]
- Van Petten C. Relationship between hippocampal volume and memory ability in healthy individuals across the lifespan: review and meta-analysis. Neuropsychologia. 2004;42:1394–1413. doi: 10.1016/j.neuropsychologia.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Verhaeghen P, Marcoen A, Goossens L. Facts and fiction about memory aging: a quantitative integration of research findings. Journal of Gerontology. 1993;48(4):157–171. doi: 10.1093/geronj/48.4.p157. [DOI] [PubMed] [Google Scholar]
- Viechtbauer W. Bias and efficiency of meta-analytic variance estimators in the random-effects model. Journal of Educational and Behavioral Statistics. 2005;30(3):261–293. [Google Scholar]
- Viechtbauer W. Conducting meta-analyses in R with the metafor package. Journal of Statistical Software. 2010;36(3):1–48. [Google Scholar]
- Wang TH, de Chastelaine M, Minton B, Rugg MD. Effects of Age on the neural correlates of familiarity as indexed by ERPs. Journal of Cognitive Neuroscience. 2012;24(5):1055–1068. doi: 10.1162/jocn_a_00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerberg CE, Paller KA, Weintraub S, Mesulam MM, Holdstock JS, Mayes AR, Reber PJ. When memory does not fail: familiarity-based recognition in mild cognitive impairment and Alzheimer’s disease. Neuropsychology. 2006;20(2):193–205. doi: 10.1037/0894-4105.20.2.193. [DOI] [PubMed] [Google Scholar]
- Westerberg CE, Mayes AR, Florczak SM, Chen Y, Creery J, Parrish T, et al. Distinct medial temporal contributions ot diffeerent forms of recognition in amnestic mild cognitive impairment and Alzheimer’s disease. Neuropsychologia. 2013;51:2450–2461. doi: 10.1016/j.neuropsychologia.2013.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiting WL, Smith AD. Differential age-related processing limitations in recall and recognition tasks. Psychology and Aging. 1997;12(2):216–224. doi: 10.1037//0882-7974.12.2.216. [DOI] [PubMed] [Google Scholar]
- Winograd E, Smith AD, Simon EW. Aging and the picture superiority effect in recall. Journal of Gerontology. 1982;37(1):70–75. doi: 10.1093/geronj/37.1.70. [DOI] [PubMed] [Google Scholar]
- Wixted JT. Dual-process theory and signal-detection theory of recognition memory. Psychological Review. 2007;114(1):152–176. doi: 10.1037/0033-295X.114.1.152. [DOI] [PubMed] [Google Scholar]
- Wolk DA, Signoff ED, Dekosky ST. Recollection and familiarity in amnestic mild cognitive impairment: a global decline in recognition memory. Neuropsychologia. 2008;46(7):1965–1978. doi: 10.1016/j.neuropsychologia.2008.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolk DA, Price JC, Saxton JA, Snitz BE, James JA, Lopez OL, et al. Amyloid imaging in mild cognitive impairment subtypes. Annals of Neurology. 2009;65(5):557–568. doi: 10.1002/ana.21598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolk DA, Dunfee KL, Dickerson BC, Aizenstein HJ, Dekosky ST. A medial temporal lobe division of labor: insights from memory in aging and early Alzheimer disease. Hippocampus. 2011;21(5):461–466. doi: 10.1002/hipo.20779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolk DA, Mancuso L, Kliot D, Arnold SE, Dickerson BC. Familiarity-based memory as an early cognitive marker of preclinical and prodromal AD. Neuropsychologia. 2013;51(6):1094–1102. doi: 10.1016/j.neuropsychologia.2013.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonelinas AP. Receiver-operating characteristics in recognition memory: evidence for a dual-process model. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1994;20(6):1341–1354. doi: 10.1037//0278-7393.20.6.1341. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP. The contribution of recollection and familiarity to recognition and source-memory judgments: a formal dual-process model and an analysis of receiver operating characteristics. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1999;25(6):1415–1434. doi: 10.1037//0278-7393.25.6.1415. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP. Components of episodic memory: the contribution of recollection and familiarity. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 2001a;356(1413):1363–1374. doi: 10.1098/rstb.2001.0939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonelinas AP. Consciousness, control, and confidence: the 3 Cs of recognition memory. Journal of Experimental Psychology: General. 2001b;130(3):361–379. doi: 10.1037//0096-3445.130.3.361. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP. The nature of recollection and familiarity: a review of 30 years of research. Journal of Memory and Language. 2002;46(3):441–517. [Google Scholar]
- Yonelinas AP, Jacoby LL. The relation between remembering and knowing as bases for recognition: effects of size congruency. Journal of Memory and Language. 1995;34(5):622–643. [Google Scholar]
- Yonelinas AP, Jacoby LL. Response bias and the process-dissociation procedure. Journal of Experimental Psychology: General. 1996a;125(4):422–434. [Google Scholar]
- Yonelinas AP, Jacoby LL. Noncriterial recollection: familiarity as automatic, irrelevant recollection. Consciousness and Cognition. 1996b;5(1–2):131–141. doi: 10.1006/ccog.1996.0008. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP, Jacoby LL. The process-dissociation approach two decades later: convergence, boundary conditions, and new directions. Memory & Cognition. 2012;40(5):663–680. doi: 10.3758/s13421-012-0205-5. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP, Parks CM. Receiver operating characteristics (ROCs) in recognition memory: a review. Psychological Bulletin. 2007;133(5):800–832. doi: 10.1037/0033-2909.133.5.800. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP, Kroll NEA, Quamme JR, Lazzara MM, Sauvé M-J, Widaman KF, Knight RT. Effects of extensive temporal lobe damage or mild hypoxia on recollection and familiarity. Nature Neuroscience. 2002;5(11):1236–1241. doi: 10.1038/nn961. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP, Widaman K, Mungas D, Reed B, Weiner MW, Chui HC. Memory in the aging brain: doubly dissociating the contribution of the hippocampus and entorhinal cortex. Hippocampus. 2007;17(11):1134–1140. doi: 10.1002/hipo.20341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonelinas AP, Aly M, Wang W-C, Koen JD. Recollection and familiarity: examining controversial assumptions and new directions. Hippocampus. 2010;20(11):1178–1194. doi: 10.1002/hipo.20864. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.