Abstract
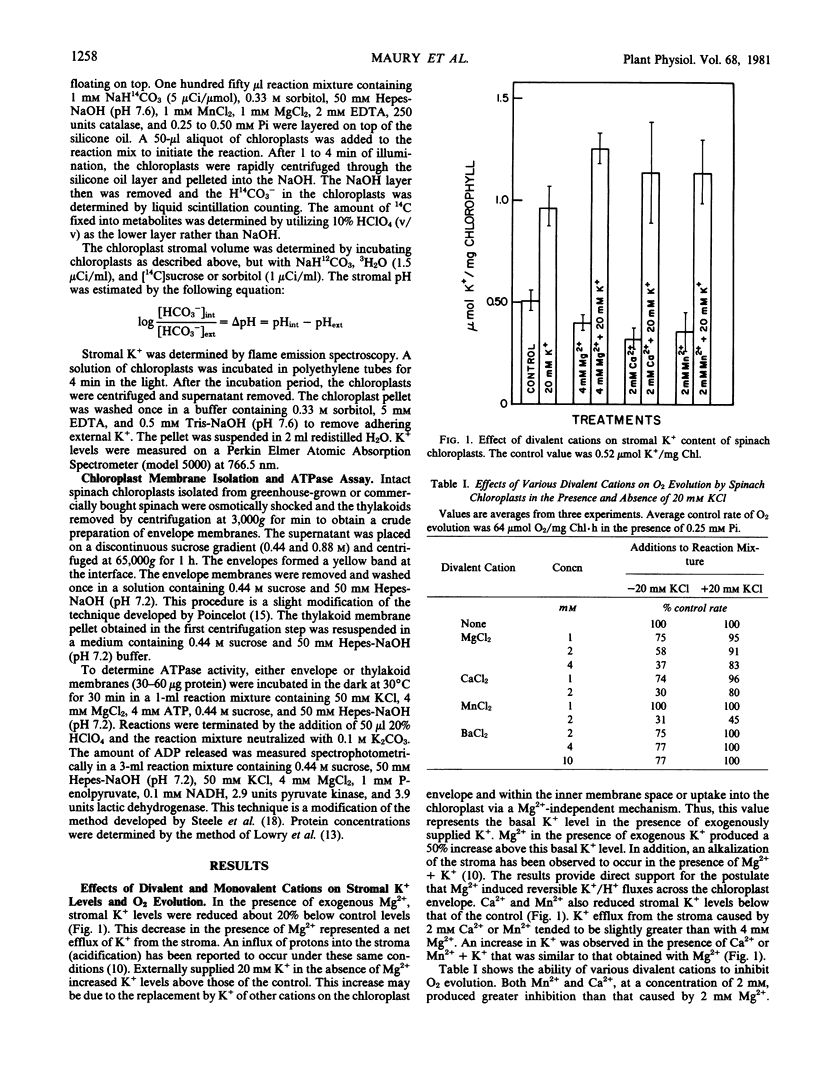
Addition of exogenous Mg2+ (2 millimolar) to illuminated intact spinach (Spinacia oleracea L.) chloroplasts caused acidification of the stroma and a 20% decrease in stromal K+. Addition of K+ (10-50 millimolar) reversed both stromal acidification and K+ efflux from the chloroplast caused by Mg2+. These data suggested that Mg2+ induced reversible H+/K+ fluxes across the chloroplast envelope. Ca2+ and Mn2+ (2 millimolar) were as effective as 4 millimolar Mg2+ in causing K+ efflux from chloroplasts and inhibition of O2 evolution. In contrast, 10 millimolar Ba2+ induced only a small amount of inhibition. The lack of strong inhibition by Ba2+ indicated that the effects of divalent cations such as Mg2+ cannot be attributed to generalized electrostatic interactions of the cation with the chloroplast envelope. With the chloroplasts used in this study, stromal acidification caused by 2 millimolar Mg2+ was small (0.07 to 0.15 pH units), but sufficient to account for the inhibition of O2 evolution (43%) induced by Mg2+.
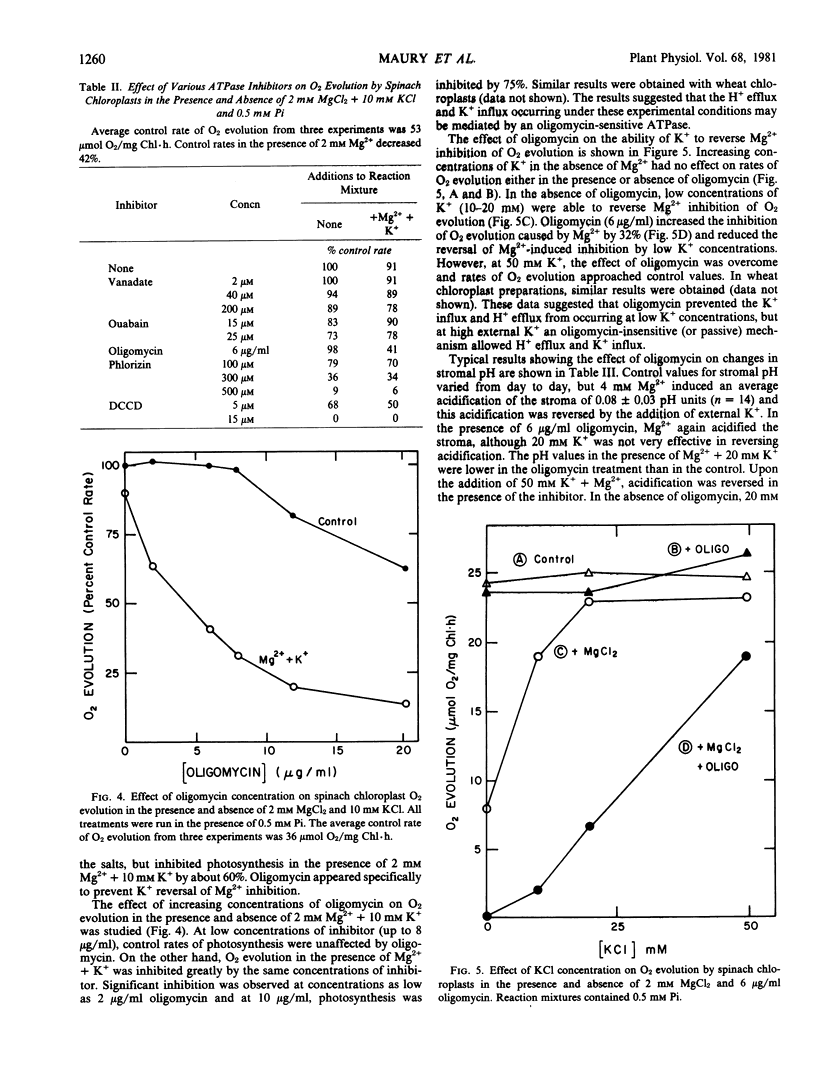
A variety of ATPase inhibitors were tested for effects on Mg2+-induced H+/K+ fluxes. Oligomycin was the only ATPase inhibitor which specifically inhibited photosynthesis in the presence of Mg2+ + K+, but had little or no effect in the absence of these cations. In the presence of oligomycin, much higher concentrations (50 millimolar) of exogenous K+ were required to reverse Mg2+-induced acidification and inhibition of O2 evolution than in its absence. Oligomycin (in the absence of divalent cations) increased the inhibition of photosynthesis caused by sodium acetate, which acts by causing stromal acidification. In addition, the chloroplast envelope ATPase was inhibited partially (45%) by oligomycin. These results suggested that H+ fluxes across the chloroplast envelope are regulated by two mechanisms: (a) an active, oligomycin-sensitive H+ efflux and (b) a reversible, Mg2+-dependent, oligomycin-insensitive H+/K+ exchange.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avron M., Shavit N. Inhibitors and uncouplers of photophosphorylation. Biochim Biophys Acta. 1965 Nov 29;109(2):317–331. doi: 10.1016/0926-6585(65)90160-3. [DOI] [PubMed] [Google Scholar]
- Carmeli C. Properties of ATPase in chloroplasts. Biochim Biophys Acta. 1969 Oct 21;189(2):256–266. doi: 10.1016/0005-2728(69)90052-8. [DOI] [PubMed] [Google Scholar]
- Douce R., Holtz R. B., Benson A. A. Isolation and properties of the envelope of spinach chloroplasts. J Biol Chem. 1973 Oct 25;248(20):7215–7222. [PubMed] [Google Scholar]
- Flügge U. I., Freisl M., Heldt H. W. Balance between Metabolite Accumulation and Transport in Relation to Photosynthesis by Isolated Spinach Chloroplasts. Plant Physiol. 1980 Apr;65(4):574–577. doi: 10.1104/pp.65.4.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldt H. W., Sauer F. The inner membrane of the chloroplast envelope as the site of specific metabolite transport. Biochim Biophys Acta. 1971 Apr 6;234(1):83–91. doi: 10.1016/0005-2728(71)90133-2. [DOI] [PubMed] [Google Scholar]
- Huber S. C. Effect of pH on chloroplast photosynthesis. Inhibition of O2 evolution by inorganic phosphate and magnesium. Biochim Biophys Acta. 1979 Jan 11;545(1):131–140. doi: 10.1016/0005-2728(79)90120-8. [DOI] [PubMed] [Google Scholar]
- Huber S. C., Maury W. Effects of Magnesium on Intact Chloroplasts: I. EVIDENCE FOR ACTIVATION OF (SODIUM) POTASSIUM/PROTON EXCHANGE ACROSS THE CHLOROPLAST ENVELOPE. Plant Physiol. 1980 Feb;65(2):350–354. doi: 10.1104/pp.65.2.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lilley R. M., Schwenn J. D., Walker D. A. Inorganic pyrophosphatase and photosynthesis by isolated chloroplasts. II. The controlling influence of orthophosphate. Biochim Biophys Acta. 1973 Dec 14;325(3):596–604. doi: 10.1016/0005-2728(73)90219-3. [DOI] [PubMed] [Google Scholar]
- Poincelot R. P. Isolation and lipid composition of spinach chloroplast envelope membranes. Arch Biochem Biophys. 1973 Nov;159(1):134–142. doi: 10.1016/0003-9861(73)90437-2. [DOI] [PubMed] [Google Scholar]
- Werdan K., Heldt H. W. Accumulation of bicarbonate in intact chloroplasts following a pH gradient. Biochim Biophys Acta. 1972 Dec 14;283(3):430–441. doi: 10.1016/0005-2728(72)90260-5. [DOI] [PubMed] [Google Scholar]
- Werdan K., Heldt H. W., Milovancev M. The role of pH in the regulation of carbon fixation in the chloroplast stroma. Studies on CO2 fixation in the light and dark. Biochim Biophys Acta. 1975 Aug 11;396(2):276–292. doi: 10.1016/0005-2728(75)90041-9. [DOI] [PubMed] [Google Scholar]


