Abstract
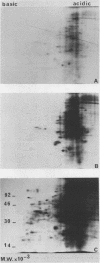
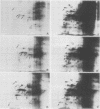
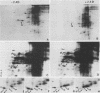
Two-dimensional separation of proteins newly synthesized by tobacco mesophyll protoplasts cultivated in vitro allows us to detect, reproducibly, 257 spots. The pattern is extremely stable throughout the three days of culture, the intensity of only 24 spots varying during this time. The absence of cytokinin (N6-benzyladenine) in the culture medium prohibits entry into S phase but does not modify the pattern, indicating that none of the observed proteins is specifically synthesized in S, G2, or M phases. The presence of 2,4-dichlorophenoxyacetic acid is necessary for the mitotic development of protoplasts. It induces the appearance of one protein, increases the level of another, and reduces that of eight others. All proteins sensitive to auxin belong to the group of proteins the levels of which vary during culture.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Bravo R., Celis J. E. A search for differential polypeptide synthesis throughout the cell cycle of HeLa cells. J Cell Biol. 1980 Mar;84(3):795–802. doi: 10.1083/jcb.84.3.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fosket D. E., Volk M. J., Goldsmith M. R. Polyribosome Formation in Relation to Cytokinin-induced Cell Division in Suspension Cultures of Glycine max [L.] Merr. Plant Physiol. 1977 Oct;60(4):554–562. doi: 10.1104/pp.60.4.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivarie R. D., O'Farrell P. H. The glucocorticoid domain: steroid-mediated changes in the rate of synthesis of rat hepatoma proteins. Cell. 1978 Jan;13(1):41–55. doi: 10.1016/0092-8674(78)90136-8. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Legocki R. P., Verma D. P. Identification of "nodule-specific" host proteins (nodoulins) involved in the development of rhizobium-legume symbiosis. Cell. 1980 May;20(1):153–163. doi: 10.1016/0092-8674(80)90243-3. [DOI] [PubMed] [Google Scholar]
- Mocquot B., Prat C., Mouches C., Pradet A. Effect of anoxia on energy charge and protein synthesis in rice embryo. Plant Physiol. 1981 Sep;68(3):636–640. doi: 10.1104/pp.68.3.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M., O'Farrell P. H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977 Dec;12(4):1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- Zurfluh L. L., Guilfoyle T. J. Auxin-induced changes in the patterns of protein synthesis in soybean hypocotyl. Proc Natl Acad Sci U S A. 1980 Jan;77(1):357–361. doi: 10.1073/pnas.77.1.357. [DOI] [PMC free article] [PubMed] [Google Scholar]