Abstract
Recently we reported the first known incidence of antibodies possessing catalytic sialidase activity (sialidase abzymes) in the serum of patients with multiple myeloma and systemic lupus erythematosus (SLE). These antibodies desialylate biomolecules, such as glycoproteins, gangliosides and red blood cells. Desialylation of dying cells was demonstrated to facilitate apoptotic cell clearance. In this study we assessed the possibility to facilitate dying cell clearance with the use of F(ab)2 fragments of sialidase abzymes. Two sources of sialidase abzymes were used: (i) those isolated from sera of patients with SLE after preliminary screening of a cohort of patients for sialidase activity; and (ii) by creating an induced sialidase abzyme through immunization of a rabbit with synthetic hapten consisting of a non-hydrolysable analogue of sialidase reaction conjugated with bovine serum albumin (BSA) or keyhole limpet haemocyanin (KLH). Antibodies were purified by ammonium sulphate precipitation, protein-G affinity chromatography and size exclusion-high performance liquid chromatography (HPLC-SEC). Effect of desialylation on efferocytosis was studied using human polymorphonuclear leucocytes (PMN), both viable and aged, as prey, and human monocyte-derived macrophages (MoMa). Treatment of apoptotic and viable prey with both disease-associated (purified from blood serum of SLE patients) and immunization-induced (obtained by immunization of rabbits) sialidase abzymes, its F(ab)2 fragment and bacterial neuraminidase (as positive control) have significantly enhanced the clearance of prey by macrophages. We conclude that sialidase abzyme can serve as a protective agent in autoimmune patients and that artificial abzymes may be of potential therapeutic value.
Keywords: abzyme, apoptosis, desialylation, efferocytosis, sialidase
OTHER ARTICLES PUBLISHED IN THIS SERIES
Dying autologous cells as instructors of the immune system. Clinical and Experimental Immunology 2015, 179: 1–4.
Anti-dsDNA antibodies as a classification criterion and a diagnostic marker for systemic lupus erythematosus: critical remarks. Clinical and Experimental Immunology 2015, 179: 5–10.
The effect of cell death in the initiation of lupus nephritis. Clinical and Experimental Immunology 2015, 179: 11–16.
Instructive influences of phagocytic clearance of dying cells on neutrophil extracellular trap generation. Clinical and Experimental Immunology 2015, 179: 24–29.
Developmental regulation of p53-dependent radiation-induced thymocyte apoptosis in mice. Clinical and Experimental Immunology 2015, 179: 30–38.
Loading of nuclear autoantigens prototypically recognized by systemic lupus erythematosus sera into late apoptotic vesicles requires intact microtubules and myosin light chain kinase activity Clinical and Experimental Immunology 2015, 179: 39–49.
Low and moderate doses of ionizing radiation up to 2 Gy modulate transmigration and chemotaxis of activated macrophages, provoke an anti-inflammatory cytokine milieu, but do not impact upon viability and phagocytic function. Clinical and Experimental Immunology 2015, 179: 50–61.
Vessel-associated myogenic precursors control macrophage activation and clearance of apoptotic cells. Clinical and Experimental Immunology 2015, 179: 62–67.
Acetylated histones contribute to the immunostimulatory potential of neutrophil extracellular traps in systemic lupus erythematosus. Clinical and Experimental Immunology 2015, 179: 68–74.
Unconventional apoptosis of polymorphonuclear neutrophils (PMN): staurosporine delays exposure of phosphatidylserine and prevents phagocytosis by MΦ-2 macrophages of PMN. Clinical and Experimental Immunology 2015, 179: 75–84.
Introduction
Catalytically active antibodies (abzymes) attract much attention due to a few unique features being clearly associated with some disorders (particularly autoimmune) where their role could be protective or pathogenic, depending on the type of disease and abzymes characteristics. Recently we have discovered a novel class of catalytically active antibodies possessing sialidase activity (sialidase abzymes: SA) [1]. SA were found in patients with multiple myeloma [1] or systemic lupus erythematosus (SLE) [2], but not in rheumatoid arthritis, paraprotein syndrome, multiple sclerosis patients or normal healthy blood donors (NHD) [3].
It is generally accepted that impaired clearance of apoptotic cells and the accumulation of necrotic material in various tissues may cause chronic inflammatory autoimmune diseases, such as SLE [4]. Artificial desialylation of dying lymphocytes with bacterial neuraminidase greatly enhanced clearance by human monocyte-derived macrophages (MoMa) of both apoptotic cells and apoptotic cell-derived microvesicles (ACMV) [5]. Cellular sialidase Neu1 is activated during the execution phase of apoptosis and exposed on the surfaces of apoptotic cells/ACMV, as well as other intracellular molecules [6]. At the cell surface, sialidase is responsible for the desialylated glycoprofile of dying cells [7] and their neighbours [8]. Recently it was demonstrated that sialylation of ‘eat-me’ signals regulates their functions [9].
In this study we analysed whether F(ab)2 fragments of SA facilitate the clearance of dying cells. Viable and aged human peripheral blood-derived granulocytes served as prey for MoMa. Our data demonstrated that F(ab)2 fragments of abzymes with sialidase activity significantly facilitate the clearance by human MoMa of apoptotic prey.
Materials and methods
Patients
Peripheral blood serum samples of 62 patients diagnosed with SLE, including 52 female and 10 males, with Systemic Lupus Erythematosus Disease Activity Index (SLEDAI) > 4 [10], mean value = 10·7; age range 17–70 years, mean American College of Rheumatology (ACR) 5·83; mean disease duration of 100 months (2–420 months) were analysed; clinical data are detailed in the Supporting Information, Table S1. An informed consent was obtained from all patients and was approved by the Review Board of the Lviv National Medical University, in accordance with the regulations of the Ministry of Health Protection of Ukraine.
Animals
Eight rabbits were used – two were immunized with 2,3-dehydro-2-deoxy-N-acetylneuraminic acid (DANA)-BSA, two with bovine serum albumin (BSA), two with DANA-keyhole limpet haemocyanin (KLH) and two with KLH. Immunization was performed according to Kit et al. [11] using a mixture of DANA-protein conjugates (20 μg/injection) synthesized as described [3], and Freund's adjuvant (50 μl/injection) in phosphate-buffered saline (PBS) (total volume 400 μl) (complete Freund's adjuvant was used during the first immunization and incomplete Freund's adjuvant at the second and third immunizations; the fourth immunization was with protein adjuvants alone. Blood was collected 10 days after the fourth immunization. Food and water were available ad libitum and all experiments with animals were approved by the Bioethics Committee of Institute of Cell Biology, NAS, Ukraine.
Culture and isolation of cells
Human leukaemia Jurkat T cells, primary human polymorphonuclear leucocytes (PMN) and MoMa from healthy volunteers were used. Monocytes were isolated from peripheral blood by LymphoPrep® gradient according to the manufacturer's recommendations for isolation of the peripheral blood mononuclear cell (PBMC) fraction. Plastic-attached cells of the PBMC fraction were then cultured for 7 days in the presence of granulocyte–macrophage colony-stimulating factor (GM-CSF) (100 U/ml) and autologous serum (added at days 1, 3 and 5) to generate MoMa. After 7 days of differentiation, the MoMa population was tested. They typically contain > 95% CD11b+ cells, > 90% CD14+ cells and > 85% CD89+ cells. Phagocytosis was assessed by incubation of PMN (freshly isolated or aged for 24 h) with SA, its F(ab)2 fragment or Clostridium perfringens neuraminidase (each at normalized activity of 30 mU) for 3 h at 37°C in Ringer buffer. Cells were washed thoroughly three times with Ringer solution and incubated with human MoMa. Uningested PMN were analysed by flow cytometry (after prestaining with carboxyfluorescein diacetate succinimidyl ester (CFSE) [12]) or in the haemocytometric chamber using a Zeiss AxioImager A1 microscope. The percentage of prey cells that had been bound to or taken up by MoMa was calculated (prey disappearance) and normalized according to prey disappearance values treated in the same way, but added to wells with full medium without MoMa.
Induction and inhibition of apoptosis
Cell viability was controlled by annexin V/PI staining. Apoptosis was induced by irradiation of Jurkat cells with ultraviolet light type B (UV-B) (180 mJ/cm2, 60 s), or by ageing of PMN.
Flow cytometry
Analyses employing fluorescence-labelled lectins [13] were performed using a fluorescence activated cell sorter (FACS)Scan flow cytometer (BD Biosciences, San Jose, CA, USA). Propidium iodide (PI) was used to counterstain necrotic cells further excluded from analysis. Lectins PNA (peanut agglutinin) and SNA (Sambucus nigra agglutinin II, α2,6-sialil specific) were from Lectinotest Laboratory (Lviv, Ukraine).
Antibody purification
Isolation of immunoglobulin (Ig)G fractions from blood serum was performed according to the reported procedure [2]; the purification methods used in this study are summarized in the Supporting Information, Fig. S1. Specifically, blood serum proteins were precipitated three times with ammonium sulphate (50% saturation), the pellet was dissolved in 150 mM NaCl, 20 mM Tris-HCl buffer, pH 7·5, and dialyzed against the same buffer. IgGs were purified by affinity chromatography employing a protein G-sepharose column. IgG was eluted from the column with 0·1 M glycine-HCl, pH 2·6, neutralized immediately by 1 M Tris-HCl buffer, pH 8·8, and dialyzed for 18 h against 100 mM NaCl, 20 mM Tris-HCl buffer, pH 7·5. Protein concentration was measured using the NanoDrop ND-1000 spectrophotometer using the extinction coefficient of IgG, preloaded in the device (NanoDrop Technologies, Wilmington, DE, USA). The IgG antibodies were tested for sialidase activity.
High-performance liquid chromatography (HPLC)
Size exclusion (HPLC-SEC) was performed in PBS, pH 6·8, on the Perkin Elmer HPLC series 200 HPLC system using a Bio-Sil SEC 250 7·8 × 300 mm column (Bio-Rad, Hercules, CA, USA) at a 1 ml/min flow rate. The fractions corresponding to the main peak were collected and used for further analysis. Analysis of immune complexes was performed using the same setup in 0·1 M glycin-HCl with 0·05% azide, pH 2·6, supplied at a 1 ml/min flow rate.
Strong cation exchange (HPLC-SCX) was performed using a Shiseido Capcell SCX UG80 1·5 × 150 mm column with 20 mM MES plus gradient of NaCl (60–200 mM) with a flow rate of 0·3 ml/min.
Preparation of F(ab)2
Antibodies were digested with pepsin and undigested antibodies were removed with protein G sepharose; F(ab)2 fragments were purified by gel filtration chromatography with the Toyopearl HW-55F column (0·5 × 20 cm) in 50 mM sodium phosphate, 100 mM NaCl, Tween 20 0·25 M buffer, pH 7·0, 0·1% NaN3 or by HPLC-SEC using the Bio-Sil SEC 250 column to remove potential contamination with immune complexes.
Fluorometric assay
Sialidase activity was measured as described previously [14,15]. Briefly, protein samples (50 μg of protein per sample) were diluted to equal protein concentrations (estimated by Bradford protein assay) with 0·2 M acetate buffer (with 5 mM CaCl2 and MgCl2), pH 4·2, in a final sample volume at 190 μl; 10 μl of 0·5 mM 4-methylumbelliferole (MU)-NA or 4-MU-Gal (both from BioSynth, Staad, Switzerland) were added and incubated for 3 h. In some cases 4-MU-Gal was used as substrate specificity control. If indicated, sialidase inhibitor DANA (final concentration 15 μM; Sigma, St Louis, MO, USA) was added to protein samples. Cleavage of the substrate by sialidase yields MU, a fluorescent product. Substrate used without antibody served as a blank to determine the non-specific degradation of the substrate. Reaction was stopped by the addition of 1 ml of glycine-carbonate buffer, pH 10·7 and fluorescence was measured using a Turner Quantech FM109510 (Barnstead Thermolyne Corporation, Dubuque, IA, USA) fluorometer (excitation: 365 nm and emission: 430 nm).
Sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE)
Antibodies and their (Fab)2 fragments (3–5 μg per well) under non-reducing or reducing conditions [in the presence of 2% β-mercaptoethanol (β-ME), correspondingly] was performed in 5–16% gradient gels containing 0·1% SDS using the Laemmli system.
Statistics
Statistical significance was assessed with Student's t-test. Three levels of significance were used: *P < 0·05; **P < 0·01; ***P < 0·001.
Results
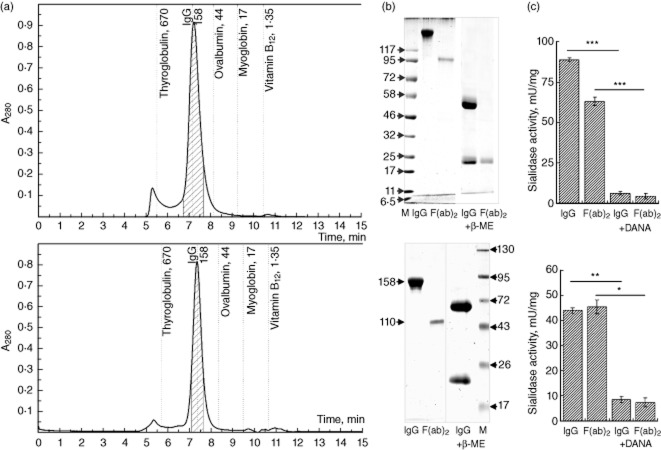
Two sources of abzymes with sialidase activity and their F(ab)2 were used in the current study: (i) those isolated from sera of SLE patients after preliminary screening of a cohort of patients for sialidase activity in the globulin fraction of their sera; and (ii) by creating immunization-induced abzymes after rabbit immunization with haptens consisting of DANA, a non-hydrolysable analogue of the sialidase reaction, conjugated to BSA or KLH via a zero-length cross-linking reaction [3]. The IgG fractions obtained after ammonium sulphate precipitation and protein-G affinity chromatography were further subjected to HPLC-SEC chromatography (the antibody purification scheme is summarized in the Supporting Information, Fig. S1); the middle of the peaks corresponding to IgG were collected and used for analysis by SDS-PAGE for testing neuraminidase activity and its inhibition with DANA (Fig. 1). This type of purification (ammonium sulphate precipitation, protein G-sepharose affinity chromatography, HPLC-SEC separation) was reported previously to provide essentially pure IgG preparations containing SA and lack immune complexes. The purity was confirmed by HPLC-SEC at pH 2·6, HPLC-SCX at NaCl gradient (60–200 mM range; Supporting Information Fig. S2) and by in-gel detection of abzyme activity (zymogram) [1]. Kinetic parameters of sialidase reaction were calculated at 0·1–100 mM concentrations of 4-MU-NA. Computer analysis with the EnzymeKinetics module for SigmaPlot software demonstrated that the observed reaction belongs to a single substrate enzymatic reaction, described by the classical Michaelis–Menten equation, which allowed us to determine basic kinetic constants: Km = 67·7 μM; Vmax = 0·028 μmol/min/mg and Kcat = 4·451 min−1. The amount of cleaved reaction product (MU) was linearly proportional to the SA amount in the reaction mixture (Supporting Information, Fig. S3). IgG fractions containing SA were treated with pepsin to obtain F(ab)2 fragments which would maintain the sialidase activity but, due to the lack of the Fc region, would not be able to opsonize the treated cells.
Fig. 1.
Catalytically active antibodies (abzymes) used for testing. After precipitation with ammonium sulphate and affinity chromatography on protein-G sepharose, immunoglobulin (Ig)G preparations from patients with systemic lupus erythematosus (SLE) (upper row) or from immunized rabbits (lower row) were further purified by size exclusion-high performance liquid chromatography (HPLC-SEC) and the shaded fraction was collected (a) and used to prepare F(ab)2 fragments, subjected to polyacrylamide gel electrophoresis (PAGE) in denaturating and non-denaturating conditions (b), as well as testing for the presence of sialidase activity and its inhibition with 2,3-dehydro-2-deoxy-N-acetylneuraminic acid (DANA) (c).
Treatment of viable and apoptotic (UV-B irradiated) Jurkat cells with SA, their F(ab)2 fragments or C. perfringens neuraminidase, all at 30 mU, 3 h incubation at 37°C in Ringer buffer, revealed the decrease of 2,6-sialic acid residues [quantified by SNA-fluorescein isothiocyanate (FITC) lectin binding]. The mean fluorescence intensity (MFI) of lectin binding (normalized to untreated cells) was 68 and 78% for viable cells and apoptotic cells treated with SA, respectively. The relative MFI values for the treatments with F(ab)2 fragment of the abzyme and for C. perfringens neuraminidase were 84/87% (V/A) and 47/60% (V/A), respectively. At the same time, desialylation of human THP-1 cells or HeLa cells with SA (60 mU/106 cells for 1 h) had no significant influence on their adhesive properties.
The SA and their F(ab)2 fragments were used to desialylate viable and apoptotic (aged 24 h) human PMN cells and to estimate the effect on phagocytosis by human allogenic and autologous MoMa. As depicted in Fig. 2, treatment of PMN with F(ab)2 fragments of abzymes with sialidase activity isolated from blood serum of patients with SLE significantly increased prey numbers disappeared from the system (bound and ingested by MoMa). Similarly, treatment of PMN with F(ab)2 fragments of abzymes with sialidase activity isolated from immunized rabbits also significantly increased the clearance of apoptotic prey.
Fig. 2.
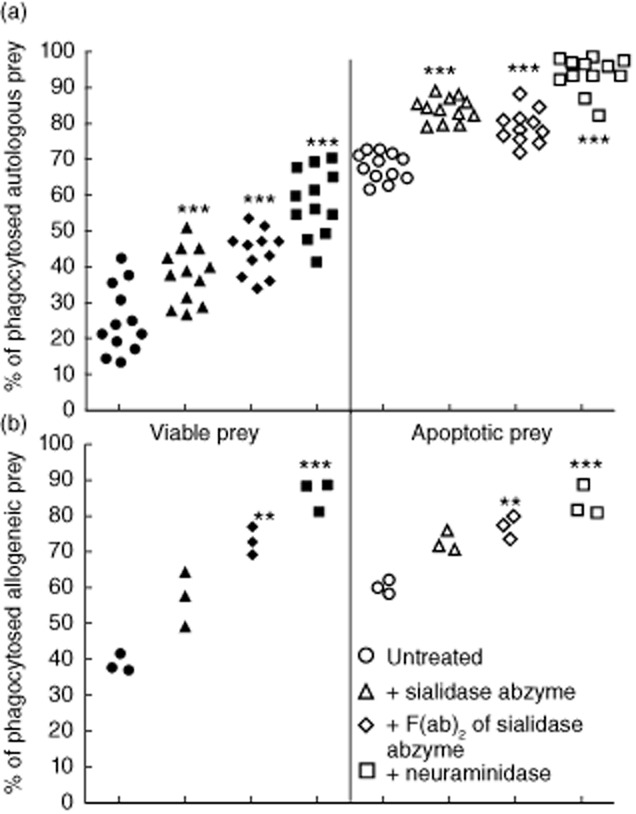
Sialidase abzymes significantly facilitate clearance of viable and apoptotic prey [polymorphonuclear (PMN) cells] by human monocyte-derived macrophages (MoMa). Prey cells were pretreated with indicated desialylating agents and co-incubated with macrophages; uneaten cells were counted afterwards. (a) Human autologous model (PMN+MoMa), treatment with sialidase abzymes from patients with systemic lupus erythematosus (SLE) blood. (b) Human allogeneic model (PMN+MoMa), treatment with sialidase-abzymes from immunized rabbits. A typical experiment (of two replicates, each including an abzyme isolated from distinct patient/rabbit) is shown.
Discussion
Treatment with F(ab)2 fragments of SA resulted in lower levels of Jurkat cells desialylation and reduced engulfment of apoptotic prey by MoMa in comparison to the bacterial neuraminidase with the same enzymatic activity. Nevertheless, the possibility of using F(ab)2 fragments of SA opens new perspectives for the development of novel treatment strategies for SLE and other clearance-related disorders. Moreover, in this study we demonstrated that abzymes with sialidase activity formed by rabbit immunization with specific conjugates of sialidase inhibitors results in functionally active abzymes, serving as a potential base for the development of future drugs. Interestingly, until now SA were found only in patients with SLE or multiple myeloma, but not in NHD or other rheumatic disorders [3]. While multiple myeloma is characterized by overgrowth of hyperactive antibody-producing cells, the reason for the appearance of these abzymes in the blood of patients with SLE remains a mystery. Interestingly, DNA-hydrolyzing abzymes have also been reported to be most prevalent in patients with SLE [16].
A possible explanation for the appearance of abzymes with sialidase activity in the sera of patients with SLE is that increased sialidase activity on the surface of apoptotic cells [1,17] and its prolonged exposure to the immune system during impaired cell clearance results in the formation of anti-idiotypic antibodies against the active centre of a sialidase which, in turn, allows stabilization of the transitional intermediate of the catalyzed sialidase reaction and reduction of the energy barrier needed to perform sialic acid cleavage [18,19]. This hypothesis is supported by the fact that all four known human sialidases share a significant structural similarity to the active centre [20]. Monospecific antibodies against each particular sialidase display cross-reactivity in blocking sialidase activity (probably by binding to the active centre); for example, as reported previously by us, an increase in Neu1 activity at apoptosis [7] was inhibited by anti-Neu1 antibodies to 11·6 ± 2·6% of initial activity; by anti-Neu2 antibody to 47·5 ± 4·4%; by anti-Neu3 antibody to 33·4 ± 4·9%; by anti-Neu4 to 22·6 ± 4·1; and by a combination of Neu2 and Neu3 to 20·2 ± 4·8% of initial activity. Abzymes with sialidase activity are prone to play a protective role in the organism by enhancing apoptotic cell desialylation and subsequent efferocytosis. Although abzymes can be both protective (e.g. secretory sIgA abzymes hydrolysing HIV gp120) [21] or deleterious (hydrolysing myelin basic protein) [22], or even combining two distinct activities [23], growing evidence suggests important functional roles for catalytic antibodies in homeostasis, autoimmune disease and protection against infection [21,22,24]. One should also keep in mind the multifarious roles of sialic acids in immunity [25], probably causing abzymes with sialidase activity to influence a plethora of functions. Despite the well-characterized functions of all four human sialidases, none of them was found in relevant amounts in blood or other body fluids (lymph) [26].
The hypothesis of abzyme formation as antibody against transitional intermediates of catalytic reactions was the base to employ conjugates of DANA with BSA and KLH, perform immunization and obtain abzymes with sialidase activity. DANA is a sialidase inhibitor, an uncleavable analogue of transitional state intermediate during sialic acid trimming [27]. Recent studies have shown that by utilizing haptens for immunization one can induce abzymes with exotic specificities, such as hydrolyzing cocaine [28,29] or organophosphorus nerve poisons [30].
In summary, the ability of catalytically active antibodies, endowed with sialidase activity to facilitate the clearance of apoptotic cells by human macrophages, opens possibilities for the development of new therapeutic strategies for clearance-associated disorder. Induced formation of monoclonal SA will eventually allow determination of their sequence and production of humanized therapeutic SA as a single-domain antibody or other small forms, still maintaining sialidase activity to be used for efferocytosis modulation in clearance-related disorders [3].
Acknowledgments
This work was supported partially by grants from NASU, State Agency of Ukraine for Science, Innovation and Information and the President of Ukraine (R. B., T. D., A. T.), CRDF (R. B., T. D., R. S.), WUBMRC (T. D., R. B.), the intramural Emerging Fields Initiative (EFI) of the FAU Erlangen-Nuremberg (M. H.) and by the integrated research training group GK SFB 643 from the DFG (M. H.). The authors are grateful to N. Korniy for technical assistance.
Disclosure
The authors report no conflicts of interest. R. B., E. B. and Y. K. are the inventors of a method for sialidase abzyme production, protected by UA patent application a201210528, PCT application PCT/IB2013/001908; the commercial rights belong to ICB NASU. The authors alone are responsible for the content and writing of the paper.
Supporting information
Additional Supporting information may be found in the online version of this article at the publisher's web-site:
Fig S1. Scheme of immunoglobulin (Ig)G and F(ab)2 purification, used in the current study.
Fig S2. Abzymes obtained after purification with ammonium sulphate precipitation, protein-G affinity chromatography and size exclusion-high performance liquid chromatography (HPLC-SEC) are essentially pure, as can be seen for rabbit abzymes at (a) further fractionated using HPLC- strong cation exchange (SCX) in the NaCl gradient and subsequent measurement of sialidase activity of each fraction; they are also not contaminated with immune complexes, as demonstrated for systemic lupus erythematosus (SLE) abzyme at (b) during HPLC-SEC at acidic conditions, pH 2·6.
Fig S3. Kinetic parameters of sialidase reaction catalyzed by sialidase-abzymes from immunized rabbits. (a) Michaelis–Menten plot; (b) Lineweaver–Burk plot: the incubation time for all samples was 180 min; (c) dose-dependent effect on reaction product [methylumbelliferole (MU)] accumulation at different amounts of abzymes in the reaction medium. Reaction was carried out at 100 μM of 4-Mu-NA for 240 min. Main kinetic constants are indicated on the Lineweaver–Burk plot.
Table S1. Serological features and cinical data of studied systemic lupus erythematosus (SLE) patients.
References
- 1.Bilyy R, Tomin A, Mahorivska I, et al. Antibody-mediated sialidase activity in blood serum of patients with multiple myeloma. J Mol Recognit. 2011;24:576–584. doi: 10.1002/jmr.1071. [DOI] [PubMed] [Google Scholar]
- 2.Bilyy R, Tomin A, Tolstyak Y. Cell surface glycans at SLE: changes during cells death, utilization for disease detection and molecular mechanism underlying their modification. In: Mavragani C, et al., editors. Autoimmune disorder/book 1: INTECH. Rijeka, Croatia: InTech; 2011. pp. 89–110. [Google Scholar]
- 3.Bilyy RO, Bila EE, Kit YY. inventors. Catalytically-active antibodies (abzymes) with sialidase activity and method for the preparation thereof. UA201210528, PCT/IB2013/0019082014.
- 4.Munoz LE, Lauber K, Schiller M, Manfredi AA, Herrmann M. The role of defective clearance of apoptotic cells in systemic autoimmunity. Nat Rev Rheumatol. 2010;6:280–289. doi: 10.1038/nrrheum.2010.46. [DOI] [PubMed] [Google Scholar]
- 5.Meesmann HM, Fehr E-M, Kierschke S, et al. Decrease of sialic acid residues as an eat-me signal on the surface of apoptotic lymphocytes. J Cell Sci. 2010;123:3347–3356. doi: 10.1242/jcs.066696. [DOI] [PubMed] [Google Scholar]
- 6.Schiller M, Heyder P, Ziegler S, et al. During apoptosis HMGB1 is translocated into apoptotic cell-derived membraneous vesicles. Autoimmunity. 2013;46:342–346. doi: 10.3109/08916934.2012.750302. [DOI] [PubMed] [Google Scholar]
- 7.Bilyy RO, Shkandina T, Tomin A, et al. Macrophages discriminate glycosylation patterns of apoptotic cell-derived microparticles. J Biol Chem. 2012;287:496–503. doi: 10.1074/jbc.M111.273144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shkandina T, Herrmann M, Bilyy R. Sweet kiss of dying cell: sialidase activity on apoptotic cell is able to act toward its neighbors. Autoimmunity. 2012;45:574–578. doi: 10.3109/08916934.2012.719951. [DOI] [PubMed] [Google Scholar]
- 9.Malagolini N, Catera M, Osorio H, Reis C, Chiricolo M, Dall'Olio F. Apoptotic cells selectively uptake minor glycoforms of vitronectin from serum. Apoptosis. 2013;18:373–384. doi: 10.1007/s10495-013-0812-z. [DOI] [PubMed] [Google Scholar]
- 10.Gladman DD, Ibañez D, Urowitz MB. Systemic lupus erythematosus disease activity index 2000. J Rheumatol. 2002;29:288–291. [PubMed] [Google Scholar]
- 11.Kit Y, Bilyy R, Stoika R, Mitina N, Zaichenko A. Immunogenicity and adjuvant properties of novel biocompatible nanoparticles. In: Kumar A, editor. Biocompatible nanomaterials: synthesis, characterization and applications. New York: Nova Publisher; 2010. pp. 209–223. [Google Scholar]
- 12.Rodel F, Franz S, Sheriff A, et al. The CFSE distribution assay is a powerful technique for the analysis of radiation-induced cell death and survival on a single-cell level. Strahlenther Onkol. 2005;181:456–462. doi: 10.1007/s00066-005-1361-3. [DOI] [PubMed] [Google Scholar]
- 13.Franz S, Frey B, Sheriff A, et al. Lectins detect changes of the glycosylation status of plasma membrane constituents during late apoptosis. Cytometry A. 2006;69:230–239. doi: 10.1002/cyto.a.20206. [DOI] [PubMed] [Google Scholar]
- 14.Warner TG, O'Brien JS. Synthesis of 2′-(4-methylumbelliferyl)-alpha-D-N-acetylneuraminic acid and detection of skin fibroblast neuraminidase in normal humans and in sialidosis. Biochemistry. 1979;18:2783–2787. doi: 10.1021/bi00580a014. [DOI] [PubMed] [Google Scholar]
- 15.Tomin A, Shkandina T, Bilyy R. Novel assay for direct fluorescent imaging of sialidase activity. Proc SPIE. 2011;80871Z:1–10. [Google Scholar]
- 16.Parkhomenko TA, Buneva VN, Doronin BM, et al. IgGs containing λ- and κ-type light chains and of all subclasses (IgG1–IgG4) from the sera of patients with autoimmune diseases and viral and bacterial infections hydrolyze DNA. J Mol Recognit. 2012;25:383–392. doi: 10.1002/jmr.2185. [DOI] [PubMed] [Google Scholar]
- 17.Bilyy R, Stoika R. Sweet taste of cell death: role of carbohydrate recognition systems. Ukrain'skyi Biokhimichnyi Zhurnal. 2013;85:183–196. [Google Scholar]
- 18.Friboulet A, Izadyar L, Avalle B, Roseto A, Thomas D. Abzyme generation using an anti-idiotypic antibody as the ‘internal image’ of an enzyme active site. Appl Biochem Biotechnol. 1994;47:229–237. doi: 10.1007/BF02787937. discussion 37–9. [DOI] [PubMed] [Google Scholar]
- 19.Nevinsky GA, Buneva VN. Natural catalytic antibodies – abzymes. In: Ehud K, editor. Catalytic antibodies. Weinheim: Wiley-VCH Verlag GmbH & Co. KGaA; 2005. pp. 505–569. [Google Scholar]
- 20.Magesh S, Suzuki T, Miyagi T, Ishida H, Kiso M. Homology modeling of human sialidase enzymes NEU1, NEU3 and NEU4 based on the crystal structure of NEU2: hints for the design of selective NEU3 inhibitors. J Mol Graph Model. 2006;25:196–207. doi: 10.1016/j.jmgm.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 21.Paul S, Planque SA, Nishiyama Y, Hanson CV, Massey RJ. Nature and nurture of catalytic antibodies. Adv Exp Med Biol. 2012;750:56–75. doi: 10.1007/978-1-4614-3461-0_5. [DOI] [PubMed] [Google Scholar]
- 22.Mahendra A, Sharma M, Rao DN, et al. Antibody-mediated catalysis: induction and therapeutic relevance. Autoimmun Rev. 2013;12:648–652. doi: 10.1016/j.autrev.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 23.Magorivska IB, Bilyy RO, Havrylyuk AM, Chop'yak VV, Stoika RS, Kit YY. Anti-histone H1 IgGs from blood serum of systemic lupus erythematosus patients are capable of hydrolyzing histone H1 and myelin basic protein. J Mol Recognit. 2010;23:495–502. doi: 10.1002/jmr.1033. [DOI] [PubMed] [Google Scholar]
- 24.Kozyr AV, Gabibov AG. DNA-hydrolyzing Ab: is catalytic activity a clue for physiological significance? Autoimmunity. 2009;42:359–361. doi: 10.1080/08916930902832009. [DOI] [PubMed] [Google Scholar]
- 25.Varki A, Gagneux P. Multifarious roles of sialic acids in immunity. Ann NY Acad Sci. 2012;1253:16–36. doi: 10.1111/j.1749-6632.2012.06517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miyagi T, Yamaguchi K. Mammalian sialidases: physiological and pathological roles in cellular functions. Glycobiology. 2012;22:880–896. doi: 10.1093/glycob/cws057. [DOI] [PubMed] [Google Scholar]
- 27.Chavas LM, Tringali C, Fusi P, et al. Crystal structure of the human cytosolic sialidase Neu2. Evidence for the dynamic nature of substrate recognition. J Biol Chem. 2005;280:469–475. doi: 10.1074/jbc.M411506200. [DOI] [PubMed] [Google Scholar]
- 28.Pan Y, Gao D, Zhan CG. Modeling the catalysis of anti-cocaine catalytic antibody: competing reaction pathways and free energy barriers. J Am Chem Soc. 2008;130:5140–5149. doi: 10.1021/ja077972s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Treweek JB, Janda KD. An antidote for acute cocaine toxicity. Mol Pharm. 2012;9:969–978. doi: 10.1021/mp200588v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smirnov I, Belogurov A, Jr, Friboulet A, Masson P, Gabibov A, Renard PY. Strategies for the selection of catalytic antibodies against organophosphorus nerve agents. Chem Biol Interact. 2013;203:196–201. doi: 10.1016/j.cbi.2012.10.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1. Scheme of immunoglobulin (Ig)G and F(ab)2 purification, used in the current study.
Fig S2. Abzymes obtained after purification with ammonium sulphate precipitation, protein-G affinity chromatography and size exclusion-high performance liquid chromatography (HPLC-SEC) are essentially pure, as can be seen for rabbit abzymes at (a) further fractionated using HPLC- strong cation exchange (SCX) in the NaCl gradient and subsequent measurement of sialidase activity of each fraction; they are also not contaminated with immune complexes, as demonstrated for systemic lupus erythematosus (SLE) abzyme at (b) during HPLC-SEC at acidic conditions, pH 2·6.
Fig S3. Kinetic parameters of sialidase reaction catalyzed by sialidase-abzymes from immunized rabbits. (a) Michaelis–Menten plot; (b) Lineweaver–Burk plot: the incubation time for all samples was 180 min; (c) dose-dependent effect on reaction product [methylumbelliferole (MU)] accumulation at different amounts of abzymes in the reaction medium. Reaction was carried out at 100 μM of 4-Mu-NA for 240 min. Main kinetic constants are indicated on the Lineweaver–Burk plot.
Table S1. Serological features and cinical data of studied systemic lupus erythematosus (SLE) patients.