Abstract
Swift and regulated clearance of apoptotic cells prevents the accumulation of cell remnants in injured tissues and contributes to the shift of macrophages towards alternatively activated reparatory cells that sustain wound healing. Environmental signals, most of which are unknown, in turn control the efficiency of the clearance of apoptotic cells and as such determine whether tissues eventually heal. In this study we show that vessel-associated stem cells (mesoangioblasts) specifically modulate the expression of genes involved in the clearance of apoptotic cells and in macrophage alternative activation, including those of scavenger receptors and of molecules that bridge dying cells and phagocytes. Mesoangioblasts, but not immortalized myoblasts or neural precursor cells, enhance CD163 membrane expression in vitro as assessed by flow cytometry, indicating that the effect is specific. Mesoangioblasts transplanted in acutely or chronically injured skeletal muscles determine the expansion of the population of CD163+ infiltrating macrophages and increase the extent of CD163 expression. Conversely, macrophages challenged with mesoangioblasts engulf significantly better apoptotic cells in vitro. Collectively, the data reveal a feed-forward loop between macrophages and vessel-associated stem cells, which has implications for the skeletal muscle homeostatic response to sterile injury and for diseases in which homeostasis is jeopardized, including muscle dystrophies and inflammatory myopathies.
Keywords: apoptosis, inflammation, macrophage, phagocytosis
OTHER ARTICLES PUBLISHED IN THIS SERIES
Dying autologous cells as instructors of the immune system. Clinical and Experimental Immunology 2015, 179: 1–4.
Anti-dsDNA antibodies as a classification criterion and a diagnostic marker for systemic lupus erythematosus: critical remarks. Clinical and Experimental Immunology 2015, 179: 5–10.
The effect of cell death in the initiation of lupus nephritis. Clinical and Experimental Immunology 2015, 179: 11–16.
Desialylation of dying cells with catalytically active antibodies possessing sialidase activity facilitate their clearance by human macrophages. Clinical and Experimental Immunology 2015, 179: 17–23.
Instructive influences of phagocytic clearance of dying cells on neutrophil extracellular trap generation. Clinical and Experimental Immunology 2015, 179: 24–29.
Developmental regulation of p53-dependent radiation-induced thymocyte apoptosis in mice Clinical and Experimental Immunology 2015, 179: 30–38.
Loading of nuclear autoantigens prototypically recognized by systemic lupus erythematosus sera into late apoptotic vesicles requires intact microtubules and myosin light chain kinase activity. Clinical and Experimental Immunology 2015, 179: 39–49.
Low and moderate doses of ionizing radiation up to 2 Gy modulate transmigration and chemotaxis of activated macrophages, provoke an anti-inflammatory cytokine milieu, but do not impact upon viability and phagocytic function. Clinical and Experimental Immunology 2015, 179: 50–61.
Acetylated histones contribute to the immunostimulatory potential of neutrophil extracellular traps in systemic lupus erythematosus. Clinical and Experimental Immunology 2015, 179: 68–74.
Unconventional apoptosis of polymorphonuclear neutrophils (PMN): staurosporine delays exposure of phosphatidylserine and prevents phagocytosis by MΦ-2 macrophages of PMN. Clinical and Experimental Immunology 2015, 179: 75–84.
Introduction
Tissue regeneration after acute muscle damage is controlled by a fine-tuned interaction between muscle stem cells and macrophages (MFs), which dispose of myofibre debris and apoptotic cells [1–3]. The regenerating skeletal muscle represents an optimal scenario to study the functional plasticity of tissue-recruited MFs. Macrophages that infiltrate the tissue early after injury produce inflammatory cytokines, such as tumour necrosis factor (TNF)-α and interleukin (IL)-1β, and effectively clear myofibre remnants and apoptotic cells while sustaining the activation and the proliferation of muscle stem cells [1]. As such, their features reflect those of the classically activated inflammatory macrophages, also referred to as ‘M1 cells’. At later times, in contrast, ‘alternatively activated’ or ‘M2’ macrophages predominate, i.e. cells that play a key role in the tissue homeostasis by regulating development, healing and termination of the inflammatory responses [4]. They produce IL-10 and transforming growth factor (TGF)-β and sustain the fusion of myoblasts and the formation and assembly of novel myofibres [1,5] and are required for eventual effective myofibre regeneration [6]. The ability to clear cell remnants and macrophage alternative activation both abate when adenosine monophosphate-activated protein kinase (AMPK)-α1, a trimeric AMP kinase, is inhibited by genetic or pharmacological tools, indicating that the two events are causally linked [7], further supporting the contention that the recognition and clearance of apoptotic cells represent critical events in the M1/M2 shift.
Of importance, the pattern of MF activation controls the expression of several iron-related genes and MF ability to manage iron [8–10], and alternative activation is typically associated with an increased expression of scavenger receptors, in particular the haemoglobin/haptoglobin receptor CD163 [11,12], a pattern recognition receptor that, on one hand, physiologically scavenges haptoglobin by prompting the endocytosis of haptoglobin/haemoglobin complexes, with processing and eventual rescue of haem–iron components, and on the other hand contributes indirectly to the anti-inflammatory response associated with alternative activation [13,14].
Macrophages in injured skeletal muscles interact productively with vessel-associated stem cells (mesoangioblasts) [5,15], myogenic precursors defined by the expression of pericyte markers and by their ability to cross vessel walls [16]. In this study we show that mesoangioblasts instruct MFs, sustaining their conversion into cells that acquire CD163 receptor expression, clear apoptotic cells and promote tissue remodelling.
Materials and methods
Macrophage–mesoangioblast culture
Bone marrow-derived macrophages and mesoangioblasts were isolated and differentiated as described previously [5]. Briefly, bone marrow precursors from C57BL/6 female mice were isolated and propagated for 7 days in α-modified Eagle's medium (MEM) (Gibco/Invitrogen, Carlsbad, CA, USA) containing 10% fetal bovine serum (FBS) in the presence of recombinant mouse macrophage colony-stimulating factor (rm-M-CSF) (100 ng/ml). Adult mesoangioblasts were isolated from tibialis anterior muscles of C57BL/6 female mice and cloned by limiting dilution at 0·3 cell/well. Mesoangioblasts were added to macrophages at a 1:10 ratio either in physical contact (co-culture) or in the upper chamber of a 0·4 μm membrane-separated Transwell system for 48 h (Transwell; Nunc, Waltham, MA, USA). Macrophages were also challenged at the indicated ratio with immortalized C2C12 mouse myoblasts, mouse embryonic fibroblasts (10T1/2), and mouse neuronal precursor stem cells (NPC), propagated as described [17].
Gene expression profile and data analysis
Total cellular RNA was extracted from M0 and M0 Transwell mesoangioblasts (MAB) using the RNeasy midi kit, following the manufacturer's recommendations. Disposable RNA chips (Agilent RNA 6000 Nano LabChip kit, Santa Clara, CA, USA) were used to determine the concentration and purity/integrity of RNA samples using the Agilent 2100 bioanalyser. cDNA synthesis, biotin-labelled target synthesis, HG-U133 plus 2·0 GeneChip (Affymetrix, Santa Clara, CA, USA) array hybridization, staining and scanning were performed according to the standard protocol supplied by Affymetrix. The GeneChip mouse expression, set 430 2·0, which provides comprehensive coverage of the mouse transcriptome, was used. Raw data were acquired using the Affymetrix® GeneChip® Command Console® (AGCC) software. Data processing and appropriate statistical analysis and all data quality controls were performed using the r (Bioconductor) and Partek® Genomic Suite. The functional analyses were generated through the use of Ingenuity Pathways Analysis (Ingenuity® Systems, http://www.ingenuity.com).
Intramuscular mesoangioblast transplantation
Tibialis anterior and quadriceps muscles of 6-week-old C57Bl/6 mice were injured by injection of cardiotoxin, as described [5]. Mesoangioblasts (5 × 105 /mouse) were transplanted 1 day after treatment with cardiotoxin or 15 days before killing in αSG−/− dystrophic mice (8–10 weeks old). Immunohistochemistry on muscle sections, isolation of leucocytes from the skeletal muscle and flow cytometry were performed as described previously [5]. All procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of the San Raffaele Scientific Institute.
Phagocytosis
RMA cells were plated (5 × 105 cells/ml) in RPMI + 10% FCS. Cells were then irradiated using an ultraviolet (UV) source at 0·1 J/cm3. After 16 h, apoptosis was verified as described [18] and cells stained with CMTMR (Invitrogen, Carlsbad, CA, USA), according to the manufacturer's instructions. Bone-marrow derived macrophages were seeded in the presence or absence of MAB in a Transwell system as described above. After 48 h macrophages were challenged with apoptotic cells for 2 h at a 1:1 ratio at 37°C or 4°C. Cells were washed and phagocytosis verified by flow cytometry after staining with allophycocyanin(APC)-conjugated anti-CD11b (clone M1/70; BD Biosciences, San Jose, CA, USA). The phagocytosis was calculated as %CD11b+CMTMR+ cells at 37°C – %CD11b+CMTMR+ cells at 4°C. Data were acquired on a fluorescence activated cell sorter (FACS)Calibur (BD Biosciences).
Statistics
All data are presented as mean ± standard error of the mean (s.e.m.). The two-tailed Student's t-test was used for comparisons between experimental groups. Significant differences were defined at P < 0·05.
Results
Mesoangioblasts promote the expression of genes associated with macrophage alternative activation
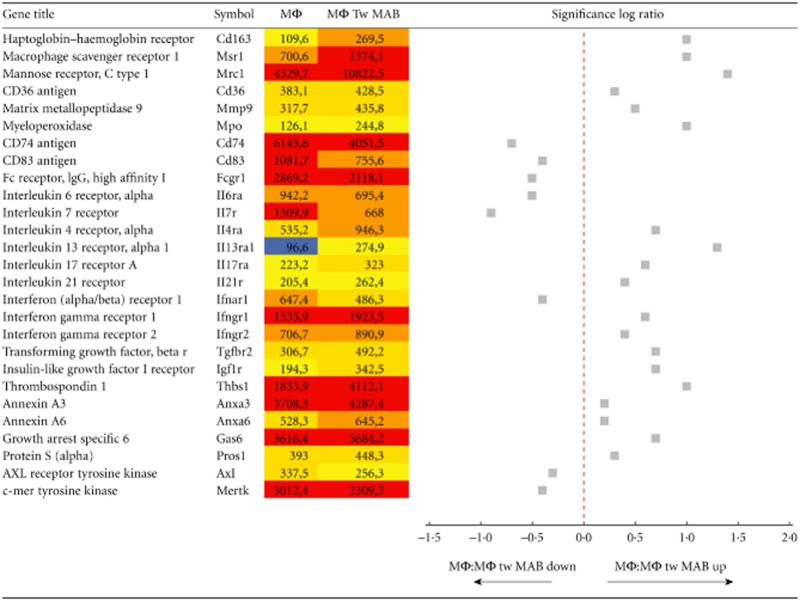
We assessed the gene expression of macrophages propagated from the mouse bone marrow that had been challenged or not with mesoangioblasts in a Transwell system, which prevents direct cell-to-cell contact but allows the diffusion of soluble moieties. The expression of scavenger receptors involved in: (i) the alternative activation of macrophages (CD163, CD206) [11,19,20] and (ii) the phagocytic clearance of soluble and particulate substrates, including cell remnants (MSR1, CD36) [21,22] is significantly up-regulated (Table 1). Moreover, macrophages exposed to mesoangioblasts express significantly higher amounts of genes coding for thrombospondin 1 (TSP-1) and Gas6, which bridge macrophages to the apoptotic substrate (Table 1).
Table 1.
Values refer to the Affymetrix® GeneChip® Command Console® (AGCC). Macrophages alone (Mφ) or cultured in Transwell (Tw) for 48 h with mesoangioblast stem cells (Mφ Tw MAB) were screened for gene array analysis. Signals for transcripts with a present call are shown in gradient-coloured cells from blue (low abundance) to dark red (very highly abundant transcript). Expression of macrophages genes that were modulated in a statistically significant manner after culture with mesoangioblasts are reported. Data are from three independent experiments
 |
Mesoangioblasts modulate macrophages phenotype and function in vitro and in vivo
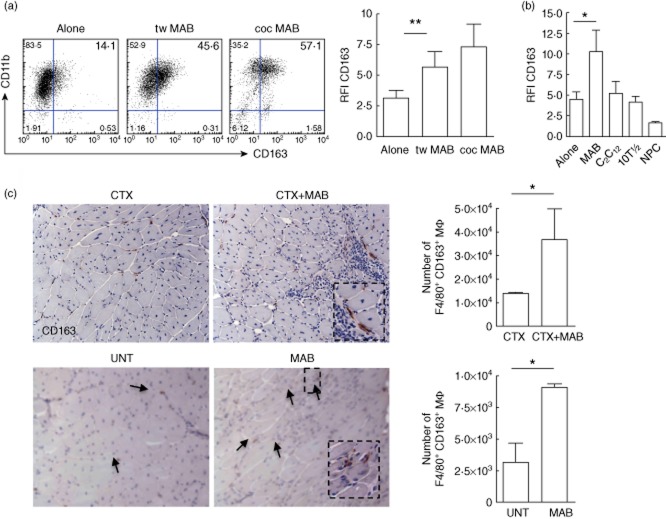
The fraction of macrophages expressing the haptoglobin–haemoglobin receptor CD163 and the overall CD163-associated fluorescence are both significantly higher in macrophages challenged with mesoangioblasts than in those cultured alone (Fig. 1a). CD163 up-regulation is similar when macrophages are in direct contact with mesoangioblasts or physically separated via a Transwell system (Fig. 1a). In contrast, immortalized myoblasts (C2C12), embryonic primary fibroblasts (10T1/2) and NPC fail to modulate macrophage CD163 expression (Fig. 1b). Mesoangioblasts transplanted in muscles damaged by the injection of cardiotoxin or dystrophic because of the genetic deficiency of alpha-sarcoglycan not only favour tissue regeneration (not shown and [16]), but also expand the fraction of inflammatory phagocytes expressing the CD163, as detected by immunohistochemistry and by flow cytometry (Fig. 1c). Macrophages that had been challenged previously with mesoangioblasts phagocytose apoptotic CMTMR-labelled RMA cells significantly more effectively than macrophages challenged with satellite cells or cultured alone (Fig. 2). The mechanism by which mesoangioblasts modulate macrophage function remains to be elucidated. However, they express an array of inflammatory molecules, which are important for their ability to migrate to sites of injury and to interact with inflammatory and tissue cells. Inflammatory molecules expressed by mesoangioblasts comprise signals that are known to modulate the alternative activation of macrophages, in particular TGF-β and M-CSF (Supporting information, Table S1).
Fig. 1.
Mesoangioblasts regulate macrophage CD163 expression. Macrophages cultured for 48 h alone (alone), or challenged with mesoangioblasts (MAB) either in culture conditions allowing physical cell-to-cell contact (co-culture, coc) or separated by a semi-permeable membrane (Transwell, Tw) were analysed by flow cytometry for CD163 expression. (a) Representative dot-plots indicating frequency of CD11b+CD163+ cells and CD163 relative fluorescence intensity (RFI). Error bars indicate the mean ± standard error of the mean (s.e.m.) of 11 independent samples. **P < 0·01. (b) CD163-associated fluorescence (RFI) was assessed in macrophages cultured alone or challenged with MAB, C2C12 immortalized myoblasts, 10T1/2 embryonic fibroblasts or neural precursor cells (NPC). Error bars indicate the mean ± s.e.m. of three independent experiments. *P < 0·05. (c) CD163 expression was assessed by immunohistochemistry: in the skeletal muscle of C57Bl6 mice in which mesoangioblasts had been transplanted (CTX+MAB) or not (CTX) 24 h after injury (top panels); in the skeletal muscle of C57Bl6 αSG−/− dystrophic mice (bottom panel) transplanted (MAB) or not (untreated, UNT) with mesoangioblasts. (d) Mononuclear cells were retrieved after enzymatic digestion of the skeletal muscle. F4/80+CD163+ macrophages were identified within CD45+CD11b+ leucocytes by flow cytometry. Error bars indicate the mean ± s.e.m. of three independent experiments with three mice per group. *P < 0·05.
Fig. 2.
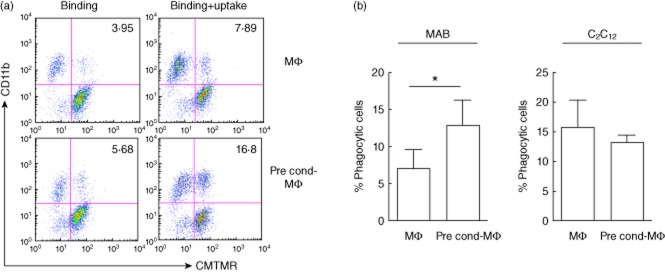
Mesoangioblasts increase macrophage capacity to phagocyte apoptotic cells. Macrophages were cultured alone (Mφ) or challenged with mesoangioblasts (pre-cond-Mφ) for 48 h. Macrophages were then incubated with CellTracker CMTMR-labelled apoptotic RMA cells for 2 h. (a) Physical interaction and phagocytosis were verified by flow cytometry at 4°C (binding) and 37°C (binding+uptake). Percentage of CD11b+CMTMR+ phagocytic cells is normalized with the control (binding at 4°C). (b) Macrophages cultured alone or in the Transwell system for 48 h with immortalized myoblasts (C2C12) were subjected to phagocytosis assay, as reported above.
Discussion
Macrophages reprogramme their function in response to signals derived from microbes [23,24], damaged tissues [25] and resting or activated lymphocytes [26–28], an event which is critical for tissue plasticity [29]. The interaction between mesoangioblasts and macrophages has been investigated in previous studies [5,15], and macrophages have been shown to orchestrate the survival and the differentiation of mesoangioblasts transplanted into injured skeletal muscles. The latter event is strictly linked to the alternative activation of macrophages within the tissue [5,7]. Here we reveal the existence of a feed-forward loop, by which mesoangioblasts sustain the activation program of macrophages which, in turn, enable them to provide stem cells with survival and differentiation signals. Of interest, the action of mesoangioblasts on macrophages appears somewhat selective, as other sources of stem cells, including NPCs, which have well-characterized immunoregulatory properties [17], fail to modulate macrophage characteristics.
Mesoangioblasts are derived from a subset of pericytes found in the skeletal muscle, and pericytes have been shown to regulate the traffic of leucocytes through the subendothelial matrix in inflamed tissues [30–32]. It is tempting to speculate that mesoangioblasts' ability to modulate the activation of macrophages reflect a more complex action of pericytes associated physiologically with muscular vessels. Further studies are necessary to verify whether this is the case and whether the up-regulation of bridging proteins that have been implicated in the phagocytic synapse elicited by mesoangioblasts controls macrophage behaviour in vivo.
Acknowledgments
We thank T. Touvier and L. Zanotti (San Raffaele, Milan) for kindly providing satellite cells and neural precursor cells and G. Cossu (Manchester, UK) for providing alpha-sarcoglycan-deficient mice. This work was supported by the Italian Ministry of Health (Fondo per gli Investimenti della Ricerca di Base-IDEAS to P. R.-Q. and Ricerca Finalizzata to P. R.-Q. and A. A. M.) by the Associazione Italiana Ricerca sul Cancro (AIRC IG1176 to A. A. M.) and by the Italian Ministry of University and Research (PRIN 2010 to A. A. M.).
Disclosure
The authors have no financial conflicts of interest.
Supporting information
Additional Supporting information may be found in the online version of this article at the publisher's web-site:
Table S1. Values refer to the Affymetrix® GeneChip® Command Console® (AGCC). Mesoangioblasts were screened for gene array analysis. Signals for transcripts with a present call are shown in gradient-coloured cells from blue (low abundance) to dark red (very highly abundant transcript). Data are from two independent experiments.
References
- 1.Arnold L, Henry A, Poron F, et al. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J Exp Med. 2007;204:1057–1069. doi: 10.1084/jem.20070075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tidball JG, Villalta SA. Regulatory interactions between muscle and the immune system during muscle regeneration. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1173–1187. doi: 10.1152/ajpregu.00735.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kharraz Y, Guerra J, Mann CJ, Serrano AL, Munoz-Canoves P. Macrophage plasticity and the role of inflammation in skeletal muscle repair. Mediators Inflamm. 2013;2013:491–497. doi: 10.1155/2013/491497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davies LC, Jenkins SJ, Allen JE, Taylor PR. Tissue-resident macrophages. Nat Immunol. 2013;14:986–995. doi: 10.1038/ni.2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bosurgi L, Corna G, Vezzoli M, et al. Transplanted mesoangioblasts require macrophage IL-10 for survival in a mouse model of muscle injury. J Immunol. 2012;188:6267–6277. doi: 10.4049/jimmunol.1102680. [DOI] [PubMed] [Google Scholar]
- 6.Ruffell D, Mourkioti F, Gambardella A, et al. A CREB-C/EBPbeta cascade induces M2 macrophage-specific gene expression and promotes muscle injury repair. Proc Natl Acad Sci USA. 2009;106:17475–17480. doi: 10.1073/pnas.0908641106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mounier R, Theret M, Arnold L, et al. AMPKalpha1 regulates macrophage skewing at the time of resolution of inflammation during skeletal muscle regeneration. Cell Metab. 2013;18:251–264. doi: 10.1016/j.cmet.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 8.Corna G, Campana L, Pignatti E, et al. Polarization dictates iron handling by inflammatory and alternatively activated macrophages. Haematologica. 2010;95:1814–1822. doi: 10.3324/haematol.2010.023879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gaetano C, Massimo L, Alberto M. Control of iron homeostasis as a key component of macrophage polarization. Haematologica. 2010;95:1801–1803. doi: 10.3324/haematol.2010.030239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bories G, Colin S, Vanhoutte J, et al. Liver X receptor activation stimulates iron export in human alternative macrophages. Circ Res. 2013;113:1196–1205. doi: 10.1161/CIRCRESAHA.113.301656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bacci M, Capobianco A, Monno A, et al. Macrophages are alternatively activated in patients with endometriosis and required for growth and vascularization of lesions in a mouse model of disease. Am J Pathol. 2009;175:547–556. doi: 10.2353/ajpath.2009.081011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Capobianco A, Monno A, Cottone L, et al. Proangiogenic Tie2(+) macrophages infiltrate human and murine endometriotic lesions and dictate their growth in a mouse model of the disease. Am J Pathol. 2011;179:2651–2659. doi: 10.1016/j.ajpath.2011.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Etzerodt A, Kjolby M, Nielsen MJ, Maniecki M, Svendsen P, Moestrup SK. Plasma clearance of hemoglobin and haptoglobin in mice and effect of CD163 gene targeting disruption. Antioxid Redox Signal. 2013;18:2254–2263. doi: 10.1089/ars.2012.4605. [DOI] [PubMed] [Google Scholar]
- 14.Etzerodt A, Moestrup SK. CD163 and inflammation: biological, diagnostic, and therapeutic aspects. Antioxid Redox Signal. 2013;18:2352–2363. doi: 10.1089/ars.2012.4834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lolmede K, Campana L, Vezzoli M, et al. Inflammatory and alternatively activated human macrophages attract vessel-associated stem cells, relying on separate HMGB1- and MMP-9-dependent pathways. J Leukoc Biol. 2009;85:779–787. doi: 10.1189/jlb.0908579. [DOI] [PubMed] [Google Scholar]
- 16.Sampaolesi M, Torrente Y, Innocenzi A, et al. Cell therapy of alpha-sarcoglycan null dystrophic mice through intra-arterial delivery of mesoangioblasts. Science. 2003;301:487–492. doi: 10.1126/science.1082254. [DOI] [PubMed] [Google Scholar]
- 17.Pluchino S, Zanotti L, Rossi B, et al. Neurosphere-derived multipotent precursors promote neuroprotection by an immunomodulatory mechanism. Nature. 2005;436:266–271. doi: 10.1038/nature03889. [DOI] [PubMed] [Google Scholar]
- 18.Rovere P, Vallinoto C, Bondanza A, et al. Bystander apoptosis triggers dendritic cell maturation and antigen-presenting function. J Immunol. 1998;161:4467–4471. [PubMed] [Google Scholar]
- 19.Stein M, Keshav S, Harris N, Gordon S. Interleukin 4 potently enhances murine macrophage mannose receptor activity: a marker of alternative immunologic macrophage activation. J Exp Med. 1992;176:287–292. doi: 10.1084/jem.176.1.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moestrup SK, Moller HJ. CD163: a regulated hemoglobin scavenger receptor with a role in the anti-inflammatory response. Ann Med. 2004;36:347–354. doi: 10.1080/07853890410033171. [DOI] [PubMed] [Google Scholar]
- 21.Savill J. Recognition and phagocytosis of cells undergoing apoptosis. Br Med Bull. 1997;53:491–508. doi: 10.1093/oxfordjournals.bmb.a011626. [DOI] [PubMed] [Google Scholar]
- 22.Platt N, da Silva RP, Gordon S. Class A scavenger receptors and the phagocytosis of apoptotic cells. Immunol Lett. 1999;65:15–19. doi: 10.1016/s0165-2478(98)00118-7. [DOI] [PubMed] [Google Scholar]
- 23.Martinon F, Chen X, Lee AH, Glimcher LH. TLR activation of the transcription factor XBP1 regulates innate immune responses in macrophages. Nat Immunol. 2010;11:411–418. doi: 10.1038/ni.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nau GJ, Richmond JF, Schlesinger A, Jennings EG, Lander ES, Young RA. Human macrophage activation programs induced by bacterial pathogens. Proc Natl Acad Sci USA. 2002;99:1503–1508. doi: 10.1073/pnas.022649799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.London A, Itskovich E, Benhar I, Kalchenko V, Mack M, Jung S, Schwartz M. Neuroprotection and progenitor cell renewal in the injured adult murine retina requires healing monocyte-derived macrophages. J Exp Med. 2011;208:23–39. doi: 10.1084/jem.20101202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tiemessen MM, Jagger AL, Evans HG, van Herwijnen MJ, John S, Taams LS. CD4+CD25+Foxp3+ regulatory T cells induce alternative activation of human monocytes/macrophages. Proc Natl Acad Sci USA. 2007;104:19446–19451. doi: 10.1073/pnas.0706832104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wong SC, Puaux AL, Chittezhath M. Macrophage polarization to a unique phenotype driven by B cells. Eur J Immunol. 2010;40:2296–2307. doi: 10.1002/eji.200940288. [DOI] [PubMed] [Google Scholar]
- 28.Liu G, Ma H, Qiu L, Li L, Cao Y, Ma J, Zhao Y. Phenotypic and functional switch of macrophages induced by regulatory CD4+CD25+ T cells in mice. Immunol Cell Biol. 2011;89:130–142. doi: 10.1038/icb.2010.70. [DOI] [PubMed] [Google Scholar]
- 29.Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 2010;11:889–896. doi: 10.1038/ni.1937. [DOI] [PubMed] [Google Scholar]
- 30.Proebstl D, Voisin MB, Woodfin A, et al. Pericytes support neutrophil subendothelial cell crawling and breaching of venular walls in vivo. J Exp Med. 2012;209:1219–1234. doi: 10.1084/jem.20111622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stark K, Eckart A, Haidari S, et al. Capillary and arteriolar pericytes attract innate leukocytes exiting through venules and ‘instruct’ them with pattern-recognition and motility programs. Nat Immunol. 2013;14:41–51. doi: 10.1038/ni.2477. [DOI] [PubMed] [Google Scholar]
- 32.Alon R, Nourshargh S. Learning in motion: pericytes instruct migrating innate leukocytes. Nat Immunol. 2013;14:14–15. doi: 10.1038/ni.2489. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Values refer to the Affymetrix® GeneChip® Command Console® (AGCC). Mesoangioblasts were screened for gene array analysis. Signals for transcripts with a present call are shown in gradient-coloured cells from blue (low abundance) to dark red (very highly abundant transcript). Data are from two independent experiments.