Abstract
In addition to disturbed apoptosis and insufficient clearance of apoptotic cells, there is recent evidence for a role of neutrophils in the aetiopathogenesis of systemic lupus erythematosus (SLE). In response to various stimuli, neutrophils can rapidly release DNA fibres decorated with citrullinated histones and anti-microbial peptides. These structures are referred to as neutrophil extracellular traps (NETs). In addition to apoptotic cell-derived microparticles, these NETs may comprise a further source of autoantigens, able to drive the autoimmune response in SLE. Our group recently identified specific histone modifications occurring during apoptosis that play an important role in the autoimmune response in SLE. In the current study, we evaluated the presence and immunostimulatory potential of these previously identified histone modifications in NETs. Compared to NETs from healthy donors, the histones present in NETs formed by SLE-derived neutrophils contain increased amounts of acetylated and methylated residues, which we previously observed to be associated with apoptosis and SLE. Treatment of neutrophils with histone deacetylase (HDAC) inhibitor Trichostatin A (TSA), prior to induction of NETosis, induced NETs containing hyperacetylated histones, endowed with an increased capacity to activate macrophages. This implies that specific histone modifications, in particular acetylation, might enhance the immunostimulatory potential of NETs in SLE.
Keywords: histone acetylation, NETosis, NETs, neutrophil, SLE
OTHER ARTICLES PUBLISHED IN THIS SERIES
Dying autologous cells as instructors of the immune system. Clinical and Experimental Immunology 2015, 179: 1–4.
Anti-dsDNA antibodies as a classification criterion and a diagnostic marker for systemic lupus erythematosus: critical remarks. Clinical and Experimental Immunology 2015, 179: 5–10.
The effect of cell death in the initiation of lupus nephritis. Clinical and Experimental Immunology 2015, 179: 11–16.
Desialylation of dying cells with catalytically active antibodies possessing sialidase activity facilitate their clearance by human macrophages. Clinical and Experimental Immunology 2015, 179: 17–23.
Instructive influences of phagocytic clearance of dying cells on neutrophil extracellular trap generation. Clinical and Experimental Immunology 2015, 179: 24–29.
Developmental regulation of p53-dependent radiation-induced thymocyte apoptosis in mice Clinical and Experimental Immunology 2015, 179: 30–38.
Loading of nuclear autoantigens prototypically recognized by systemic lupus erythematosus sera into late apoptotic vesicles requires intact microtubules and myosin light chain kinase activity. Clinical and Experimental Immunology 2015, 179: 39–49.
Low and moderate doses of ionizing radiation up to 2 Gy modulate transmigration and chemotaxis of activated macrophages, provoke an anti-inflammatory cytokine milieu, but do not impact upon viability and phagocytic function. Clinical and Experimental Immunology 2015, 179: 50–61.
Vessel-associated myogenic precursors control macrophage activation and clearance of apoptotic cells. Clinical and Experimental Immunology 2015, 179: 62–67.
Unconventional apoptosis of polymorphonuclear neutrophils (PMN): staurosporine delays exposure of phosphatidylserine and prevents phagocytosis by MΦ-2 macrophages of PMN. Clinical and Experimental Immunology 2015, 179: 75–84.
Introduction
Systemic lupus erythematosus (SLE) is an autoimmune disease that mainly affects women of childbearing age. SLE is a prototype type III hypersensitivity reaction in which immune complex depositions of chromatin and anti-chromatin autoantibodies cause a wide spectrum of clinical symptoms such as fever, rashes, photosensitivity, joint and muscle pain, pericarditis and nephritis. It is widely thought that abnormalities in apoptosis play an important role in SLE autoimmunization (reviewed in [1]). Apoptosis is a highly organized and immunologically silent cell death pathway that plays an important role in tissue homeostasis. Increased apoptosis as well as defects in the clearance of apoptotic material have both been implicated in the aetiopathogenesis of SLE [2–9]. During apoptosis, cellular constituents – in particular of a nuclear nature – are susceptible to modifications and translocate into apoptotic blebs. When apoptotic cells or blebs are improperly cleared, they may progress into a state known as late apoptosis or secondary necrosis, thereby leaking their modified constituents into the cells' vicinity. Uncleared apoptotic material may then be presented to antigen-presenting cells which subsequently instruct the adaptive immune system to produce the hallmark autoantibodies that characterize SLE. Autoantibodies found in SLE patients are directed mainly against nuclear components, i.e. nucleosomes, DNA, histones and ribonucleoproteins [10]. In a substantial subset of SLE patients, autoantibodies also target proteins from the cytoplasm of neutrophils [11]. The field of SLE research entered a new phase when a novel way of neutrophil cell death was described in 2004, involving the release of neutrophil extracellular traps (NETs) in the extracellular milieu, suggesting that NETs might also instigate the autoimmune response in SLE.
NETs are extracellular DNA fibres decorated with citrullinated histones and neutrophil granule peptides such as elastase, cathepsin G and lactoferrin [12]. NETs are formed by activated neutrophils in a process called NETosis that can be initiated by bacteria, viruses, fungi and parasites, but also by non-microbial mediators such as monosodium urate (MSU) crystals, interleukin (IL)-8, tumour necrosis factor (TNF)-α or phorbol 12-myristate 13-acetate (PMA) (reviewed in [13]). The formation of NETs has evolved as a strategy for killing extracellular pathogens. The sticky chromatin fibres of NETs immobilize and eliminate pathogens via anti-microbial peptides decorating the chromatin within NETs in high concentrations. While the benefits of NETosis during periods of infection appear evident, NETs also operate prominently in various states of disease. In addition to a suspected involvement in tumour spreading [14] and thrombus formation [15], NETs are thought to play a pivotal role in the aetiopathogenesis of autoimmune diseases such as SLE, as they contain vast amounts of antigens that are targeted by SLE autoantibodies (reviewed in [16]). The latter are often associated with proinflammatory mediators.
We have described previously that specific histone modifications are associated with both apoptosis and the autoimmune response in patients with SLE [17–20]. In this report we investigated whether the same SLE-associated histone modifications are to be detected in NETs, as 70% of their proteins are histones.
Materials and methods
Patients and controls
In this study, seven consecutive patients who fulfilled the American College of Rheumatology (ACR) classification criteria for SLE were included. Informed consent for whole blood donation was obtained from all patients. The SLE patients (six female, one male) with a median age of 53 years (range 32–72 years) had a mean duration of disease of 14·3 years (range 9–26 years) and were in clinical remission (mean duration 10 years; range 7–16 years). All patients were treated with hydroxychloroquine (mean 400 mg/day), except one who was treated with mycophenolate mofetil (750 mg/day). In addition to hydroxychloroquine, one patient received methotrexate (20 mg/day), one patient prednisone (5 mg/day) and two patients azathioprine (50 and 150 mg/day). Three staff members (two male, one female) with a median age of 26 years (range 21–29 years) served as controls.
Isolation of neutrophils
Neutrophils were isolated from heparinized (20 U/ml) blood by Ficoll density gradient centrifugation using Lymphoprep™ (Stemcell Technologies, Oslo, Norway). Briefly, whole blood was diluted 1:1 in phosphate-buffered saline (PBS) to a volume of 30 ml and slowly layered on top of 15 ml Lymphoprep™. The blood was then centrifuged at 800 g for 20 min and neutrophils were collected from the lower cellular fraction. Residual erythrocytes were eliminated by repetitive hypotonic lysis until no erythrocytes were remaining. Viable neutrophils were counted by trypan blue exclusion in a Neubauer chamber.
Visualization and quantification of histone modifications in NETs
Neutrophils at a density of 2 × 106 per ml in 20 mM HEPES buffer (pH 7·5) supplemented with 5 mM CaCl2 were stimulated with 20 nM phorbol myristate acetate (PMA) (Sigma-Aldrich, St Louis, MO, USA) for 2 h at 37°C to form NETs. After stimulation, neutrophils and NETs were suspended in paraformaldehyde (final concentration 1%) to a cell density of 1 × 106 per ml and then centrifuged at 800 g for 10 min with a cytospin cuvette (Hettich Cyto System, Tuttlingen, Germany) on glass slides. DNA was stained for 30 min with 1 μg/ml 4′,6-diamidino-2-phenylindole (DAPI) (Invitrogen, Eugene, OR, USA) and histones were stained employing our panel of lupus-derived monoclonal antibodies consisting of #34 (anti-H3; [17]), KM-2 (anti-H4-K8,12,16ac [18]), LG11-2 (anti-H2B-K12ac [19]) and BT164 (anti-H3-K27me3 [20]). The specificity of these four monoclonal antibodies was determined originally by epitope mapping using random peptide phage display. Furthermore, the fine specificity was determined originally in peptide competition enzyme-linked immunosorbent assays (ELISAs) using specifically modified histone peptides and their unmodified counterparts. Purification of these monoclonal antibodies was performed as described previously [17–20]. The specificity for each batch of purified monoclonal antibody is routinely validated by comparing the reactivity in ELISA with the respective histone peptides. The antibodies #34, KM-2, LG11-2 and BT164 in this study were used at concentrations of 5, 6, 0·1 and 4 μg/ml, respectively. Alexa Fluor 488-labelled goat anti-mouse immunoglobulin (Ig)G(H + L) (Invitrogen) was used for detection at a concentration of 10 μg/ml. Slides were inspected by fluorescence microscopy using standard filter sets. Histone modifications in unstimulated viable neutrophils or in NETs were quantified with Adobe Photoshop CS5 by quantifying the mean Alexa488 signal intensity.
Immunostimulatory potential of acetylated NET histones
For the respective production of hyperacetylated and hypoacetylated NETs, we preincubated neutrophils (5 × 106 per ml) from three SLE patients for 3 h in phosphate-buffered saline (PBS) supplemented with 100 ng/ml of the histone deacetylase (HDAC) inhibitor Trichostatin A (TSA) (Sigma-Aldrich) or 10 μM of the histone acetyltransferase (HAT) inhibitor anacardic acid (Sigma-Aldrich), prior to stimulation of NETosis. For the isolation of NETs, we centrifuged neutrophils and NETs at 1000 g for 5 min and washed the pellet in warm PBS. This washing step was repeated four times. To subsequently separate NETs from neutrophils, the pellet of neutrophils and NETs was resuspended in 20 mM HEPES buffer (pH 7·5) supplemented with 5 mM CaCl2 and 500 mU/ml Micrococcal Nuclease (MNase) (Worthington Biochemical Corporation, Lakewood, NJ, USA). After 20 min of incubation at 37°C, neutrophils were pelleted at 1000 g for 5 min and the supernatants containing the NETs was mixed 1:1 with RPMI-1640 medium supplemented with 20% autologous SLE plasma. Macrophages were differentiated from HL-60 cells with 20 nM PMA for 48 h as described previously [21] and were kept in this medium for 24 h, after which the expression of the activation marker CD71 was determined by flow cytometry after staining with a fluorescein isothiocyanate (FITC)-labelled anti-CD71 antibody (eBioscience, San Diego, CA, USA; cat. no. 11-0719). Addition of lipopolysaccharide (LPS) to a final concentration of 100 ng/ml served as positive control of cell activation.
Notably, the differentiation of HL-60 cells into macrophages or granulocytes by exposure to, respectively, PMA or dimethylsulphoxide (DMSO) has been described extensively in the literature [21]. We confirmed both neutrophil or macrophage-like phenotypes with light microscopy, i.e. neutrophils clearly showed lobed nuclei, whereas macrophages increased in size and displayed more granularity.
Statistical analysis
We performed statistical analyses with spss PASW statistics version 18. The results are represented as mean ± standard error of the mean (s.e.m.) of at least three independent experiments. The Mann–Whitney U-test was used for the repeated-measurements analysis of variance. The P-value for significance was set at 0·05.
Results
NETs contain apoptosis-associated histone modifications
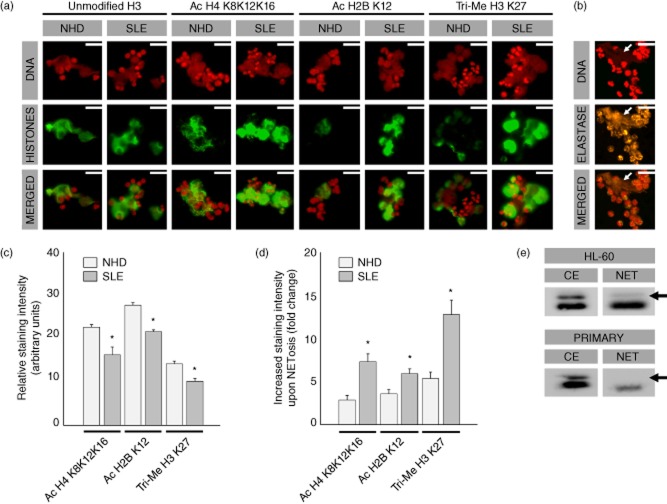
Immunofluorescence staining of NETs using our panel of monoclonal anti-histone antibodies revealed that histone modifications, that had been observed previously in chromatin of apoptotic cells, can also be observed in NETs (Fig. 1a). Extracellular DNA co-localized with modified histones and also with neutrophil elastase, thereby confirming that the shown structures represent NETs (Fig. 1b). Modified histones of NETs formed by neutrophils of patients with SLE and of healthy donors were compared. Our data show that unmodified H3 is equally present in both kinds of NETs, whereas acetylated H4-K8,12,16, acetylated H2B-K12 and tri-methylated H3-K27 is more abundant in NETs of SLE patients when compared to those of healthy donors (Fig. 1a).
Fig. 1.
Neutrophil extracellular traps (NETs) contain high amounts of apoptosis-associated histone modifications. (a) Modified histones co-localize with extracellular DNA from NETs. NETs from patients with systemic lupus erythematosus (SLE) contain high amounts of tri-acetylated H4-K8,12,16, acetylated H2B-K12 and tri-methylated H3-K27, whereas unmodified H3 is equally present in NETs of patients with SLE and of healthy donors (NHD). (b) Extracellular DNA also co-localizes with neutrophil elastase (white arrows), confirming that the structures shown represent NETS. (c) Histones from unstimulated viable SLE neutrophils are relatively hypoacetylated and hypomethylated (P < 0·05) when compared to neutrophils from healthy donors. (d) NETs from SLE patients, however, contain higher amounts of H4-K8,12,16, acetylated H2B-K12 and tri-methylated H3-K27 (P < 0·05). (e) In contrast to apoptosis, histone H2A becomes hypoacetylated during NETosis in granulocytes differentiated from the HL-60 cell line as well as in primary neutrophils, while H4 is hyperacetylated. Histones extracted from unstimulated viable cells (CE = cell extract) served as reference. Scale bar = 50 μM.
Histones from SLE patients are more susceptible to modifications upon NETosis
Next, we quantified histone modifications more precisely by analysing a large cohort of single NETs and unstimulated viable neutrophils. In unstimulated viable SLE neutrophils we observed hypoacetylation of H2B-K12 and H4-K8,12,16 as well as hypomethylation of H3-K27 when compared to unstimulated viable healthy donor neutrophils (P < 0·05) (Fig. 1c). In contrast, histones from NETs from SLE patients are two- to threefold more acetylated on H2B-K12 and H4-K8,12,16 and methylated on H3-K27 when compared to healthy donor NETs (P < 0·05) (Fig. 1d).
In addition to high reactivity with acetylated H4-K8,12,16, our monoclonal antibody KM-2 also reacts with a homologous (acetylated) epitope on histone H2A. From previous work, we know by using KM-2 that histone H2A becomes hyperacetylated during apoptosis [18]. Interestingly, during NETosis histone H2A appears to become hypoacetylated. This hypoacetylation of histone H2A was to be seen in primary neutrophils as well as in granulocytes differentiated in vitro from the HL-60 cell line with DMSO (Fig. 1e).
Acetylated histones contribute substantially to the immunostimulatory potential of NETs
With the knowledge that NETs from SLE patients contain more acetylated H4-K8,12,16 and H2B-K12, we wanted to evaluate specifically whether or not these hyperacetylated histones contribute to the immunostimulatory potential of NETs. Therefore, we induced hyperacetylation, prior to NETosis, by incubating neutrophils from three SLE patients for 3 h with TSA or, alternatively, hypoacetylation with anacardic acid (AA). With TSA we were able to generate hyperacetylated H4-K8,12,16 in NETs, whereas the amount of unmodified H3 did not change (Fig. 2a). Anacardic acid did not influence the amount of acetylated H4-K8,12,16 in NETs (data not shown). Finally, we cultured macrophages, derived from the HL-60 cell line, for 24 h in the presence of normal and hyperacetylated NETs. Differentiation of HL-60 cells led to a typical increase in both side- and forward-scatter in flow cytometry and in corresponding morphological changes in light microscopy, thereby confirming a macrophage-like phenotype (data not shown). Interestingly, the amount of macrophages that up-regulated activation marker CD71 was significantly higher when cultured in the presence of hyperacetylated NETs compared to normal NETs (P < 0·05) (Fig. 2b).
Fig. 2.
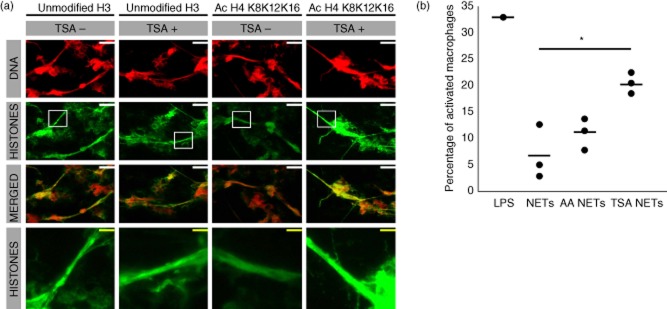
Hyperacetylated neutrophil extracellular traps (NETs) show an increased capacity to activate macrophages. (a) Preincubation with histone deacetylase inhibitor Trichostatin A (TSA) prior to the induction of NETosis, renders NETs hyperacetylated at H4-K8,12,16, whereas the amount of unmodified H3 remains constant. (b) Hyperacetylated NETs (TSA NETs) activate more macrophages based on up-regulation of the activation marker CD71 (P < 0·05). Anacardic acid (AA NETs) did not affect the amount of acetylated histones in NETs and did not differ significantly in their macrophage activating potential when compared with NETs obtained from untreated neutrophils (P < 0·05). Lipopolysaccharide (LPS) (100 ng/ml) served as positive control. Scale bar (white) = 40 μM, (yellow) = 8 μM.
Discussion
During the past few years, several mechanisms have been proposed for a potential role of NETs in the aetiopathogenesis of SLE. It is assumed that SLE patients extensively externalize NETs, as inflammatory cytokines and autoantibodies can initiate NETosis [13,22]. Moreover, Denny et al. described a distinct subset of SLE neutrophils, so-called low-density granulocytes, with increased NET releasing capabilities [23]. It has also been reported that SLE patients display a reduced NET clearance capacity due to a decreased DNase I activity and/or NET-bound C1q/autoantibodies preventing the accessibility to the NETs of DNase I [24,25]. Both increased NETosis and reduced degradation/removal of NETs may lead to an enduring exposure of NET antigens to the immune system, thereby evoking or amplifying an anti-nuclear autoimmune response. In addition to the aforementioned, we describe in the current report that NETs from SLE patients contain many methylated and acetylated residues in their histones that may increase the immunostimulatory potential of NETs.
We show that when neutrophils undergo NETosis, histones are highly susceptible to acetylation and methylation of certain residues that were reportedly associated with apoptosis [17–20]. Whereas histones from unstimulated viable SLE neutrophils are relatively hypoacetylated at H4-K8,12,16 and H2B-K12 and hypomethylated at H3-K27 (in concordance with earlier reported hypoacetylation and hypomethylation in splenocytes [26] and CD4+ T cells [27], respectively), they become hyperacetylated and hypermethylated upon NETosis. To test the hypothesis of whether NETs and the histone modifications they harbour might be capable of inducing anti-histone autoantibodies, Liu et al. analysed whether IgG autoantibodies from histone-reactive SLE patients react with targets present in NETs [28]. They showed that SLE IgG autoantibodies preferentially recognize acetylated H2B-K5, H2B-K12 and H2B-K20. However, they did not manage to identify these histone modifications within NETs formed by primary neutrophils due to significant proteolysis of the core histones during NETosis. Acetylated H2B-K5, H2B-K12 and H2B-K20 could also not be identified in NETs formed by the human HL-60 or murine early promyelocytes (EPRO) cell line. It is therefore not surprising that EPRO-derived NETs were weakly immunostimulatory in vivo, as there appears to be a discordance between the anti-histone autoantibodies found in SLE sera and the presence of their targets within NETs formed by murine EPRO cells. In contrast to Liu et al., we were able to identify acetylated H2B-K12, among other modifications, in NETs formed by primary neutrophils. NETs from primary neutrophils may therefore display a different immunostimulatory potential when compared to HL-60 or EPRO-derived NETs, as they harbour different histone modifications. Furthermore, Liu et al. did not confirm reactivity of anti-histone autoantibodies with acetylated H4-K8,12,16 and tri-methylated H3-K27, as we have described [18,20].
Garcia-Romo et al. reported that NETs from SLE patients, in contrast to those of healthy donors, are highly capable of activating plasmacytoid dendritic cells (pDCs), a process accompanied by an increased secretion of the proinflammatory cytokines interferon (IFN)-α, interferon gamma-induced protein 10 (IP-10), TNF-α and IL-6 [22]. Furthermore, they showed that the anti-microbial peptides LL-37 and HMGB1 co-localize with the globular structures along NETs from SLE patients, whereas neutrophils from healthy donors tend to retain both proteins within cytoplasmic remnants upon NETosis. It was demonstrated by Lande et al. that the anti-microbial peptides in NETs are required for the ability of NETs to activate pDCs [29]. It is speculated that histone modifications within NETs enhance the binding of anti-microbial peptides such as LL-37, HMGB1 and HNPs. The increased content of acetylated and methylated residues on histones from NETs of SLE patients may, therefore, indirectly increase the immunostimulatory potential of NETs via an enhanced binding of anti-microbial peptides within the NET. However, it appears that modified histones themselves are also highly immunostimulatory. In the past, our group demonstrated that hyperacetylated nucleosomes from apoptotic cells have a higher capacity to activate bone marrow-derived DCs in vitro compared to normal nucleosomes [18]. Moreover, administration of hyperacetylated histone H4 peptides to lupus-prone mice enhanced mortality and aggravated disease symptoms, while non-acetylated H4 peptides had no pathogenic effect. We suggest that, in addition to apoptotic material, hyperacetylated chromatin of NETs may activate myeloid and plasmacytoid DCs and subsequently challenge self-tolerance. This ultimately causes activation of autoreactive T and B cells to produce the plethora of autoantibodies that characterizes SLE.
Acknowledgments
This study was supported by grants from the Dutch Arthritis Association (grant 09-1-308; J. vd V.), Dutch Kidney Foundation (K. S. B. S. 12·073; E. P. and K. J. P. B. 11·021; J. H.), RadboudUMC Honours Acadamy (E. P.) and German Research Council (D. F. G.) (SFB643-TP-B5; M. H.).
Disclosure
The authors declare no financial or other conflicts of interest.
Author contributions
E. P. performed the experiments; E. P., J. D. and J. vd V. designed the study; J. H., J. B. provided patients and clinical data; E. P., M. H., J. B., J. D., J. H. and J. vd V. wrote the paper.
References
- 1.Munoz LE, van Bavel C, Franz S, Berden J, Herrmann M, van der Vlag J. Apoptosis in the pathogenesis of systemic lupus erythematosus. Lupus. 2008;17:371–375. doi: 10.1177/0961203308089990. [DOI] [PubMed] [Google Scholar]
- 2.Jin O, Sun LY, Zhou KX, et al. Lymphocyte apoptosis and macrophage function: correlation with disease activity in systemic lupus erythematosus. Clin Rheumatol. 2005;24:107–110. doi: 10.1007/s10067-004-0972-x. [DOI] [PubMed] [Google Scholar]
- 3.Ren Y, Tang J, Mok MY, Chan AW, Wu A, Lau CS. Increased apoptotic neutrophils and macrophages and impaired macrophage phagocytic clearance of apoptotic neutrophils in systemic lupus erythematosus. Arthritis Rheum. 2003;48:2888–2897. doi: 10.1002/art.11237. [DOI] [PubMed] [Google Scholar]
- 4.Kaplan MJ, Lewis EE, Shelden EA, et al. The apoptotic ligands TRAIL, TWEAK, and Fas ligand mediate monocyte death induced by autologous lupus T cells. J Immunol. 2002;169:6020–6029. doi: 10.4049/jimmunol.169.10.6020. [DOI] [PubMed] [Google Scholar]
- 5.Shoshan Y, Shapira I, Toubi E, Frolkis I, Yaron M, Mevorach D. Accelerated Fas-mediated apoptosis of monocytes and maturing macrophages from patients with systemic lupus erythematosus: relevance to in vitro impairment of interaction with iC3b-opsonized apoptotic cells. J Immunol. 2001;167:5963–5969. doi: 10.4049/jimmunol.167.10.5963. [DOI] [PubMed] [Google Scholar]
- 6.Gaipl US, Kuhn A, Sheriff A, et al. Clearance of apoptotic cells in human SLE. Curr Dir Autoimmun. 2006;9:173–187. doi: 10.1159/000090781. [DOI] [PubMed] [Google Scholar]
- 7.Herrmann M, Voll RE, Zoller OM, Hagenhofer M, Ponner BB, Kalden JR. Impaired phagocytosis of apoptotic cell material by monocyte-derived macrophages from patients with systemic lupus erythematosus. Arthritis Rheum. 1998;41:1241–1250. doi: 10.1002/1529-0131(199807)41:7<1241::AID-ART15>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 8.Licht R, Dieker JW, Jacobs CW, Tax WJ, Berden JH. Decreased phagocytosis of apoptotic cells in diseased SLE mice. J Autoimmun. 2004;22:139–145. doi: 10.1016/j.jaut.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 9.Muñoz LE, Lauber K, Schiller M, Manfredi AA, Herrmann M. The role of defective clearance of apoptotic cells in systemic autoimmunity. Nat Rev Rheumatol. 2010;6:280–289. doi: 10.1038/nrrheum.2010.46. [DOI] [PubMed] [Google Scholar]
- 10.van der Vlag J, Berden JH. Lupus nephritis: role of antinucleosome autoantibodies. Semin Nephrol. 2011;31:376–389. doi: 10.1016/j.semnephrol.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 11.Pradhan VD, Badakere SS, Bichile LS, Almeida AF. Anti-neutrophil cytoplasmic antibodies (ANCA) in systemic lupus erythematosus: prevalence, clinical associations and correlation with other autoantibodies. J Assoc Physicians India. 2004;52:533–537. [PubMed] [Google Scholar]
- 12.Brinkmann V, Reichard U, Goosmann C, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 13.Cheng OZ, Palaniyar N. NET balancing: a problem in inflammatory lung diseases. Front Immunol. 2013;4:1. doi: 10.3389/fimmu.2013.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cools-Lartigue J, Spicer J, McDonald B, et al. Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. J Clin Invest. 2013;123:3446–3458. doi: 10.1172/JCI67484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fuchs TA, Brill A, Duerschmied D, et al. Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci USA. 2010;107:15880–15885. doi: 10.1073/pnas.1005743107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu Y, Su K. Neutrophil extracellular traps and systemic lupus erythematosus. J Clin Cell Immunol. 2013;4:pii: 139. doi: 10.4172/2155-9899.1000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van der Heijden GW, Dieker JW, Derijck AA, et al. Asymmetry in histone H3 variants and lysine methylation between paternal and maternal chromatin of the early mouse zygote. Mech Dev. 2005;122:1008–1022. doi: 10.1016/j.mod.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 18.Dieker JW, Fransen JH, van Bavel CC, et al. Apoptosis-induced acetylation of histones is pathogenic in systemic lupus erythematosus. Arthritis Rheum. 2007;56:1921–1933. doi: 10.1002/art.22646. [DOI] [PubMed] [Google Scholar]
- 19.van Bavel CC, Dieker J, Muller S, et al. Apoptosis-associated acetylation on histone H2B is an epitope for lupus autoantibodies. Mol Immunol. 2009;47:511–516. doi: 10.1016/j.molimm.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 20.van Bavel CC, Dieker JW, Kroeze Y, et al. Apoptosis-induced histone H3 methylation is targeted by autoantibodies in systemic lupus erythematosus. Ann Rheum Dis. 2011;70:201–207. doi: 10.1136/ard.2010.129320. [DOI] [PubMed] [Google Scholar]
- 21.Murao S, Gemmell MA, Callaham MF, Anderson NL, Huberman E. Control of macrophage cell differentiation in human promyelocytic HL-60 leukemia cells by 1,25-dihydroxyvitamin D3 and phorbol-12-myristate-13-acetate. Cancer Res. 1983;43:4989–4996. [PubMed] [Google Scholar]
- 22.Garcia-Romo GS, Caielli S, Vega B, et al. Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus. Sci Transl Med. 2011;3:73ra20. doi: 10.1126/scitranslmed.3001201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Denny MF, Yalavarthi S, Zhao W, et al. A distinct subset of proinflammatory neutrophils isolated from patients with systemic lupus erythematosus induces vascular damage and synthesizes type I IFNs. J Immunol. 2010;184:3284–3297. doi: 10.4049/jimmunol.0902199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Skiljevic D, Jeremic I, Nikolic M, et al. Serum DNase I activity in systemic lupus erythematosus: correlation with immuno serological markers, the disease activity and organ involvement. Clin Chem Lab Med. 2013;51:1083–1091. doi: 10.1515/cclm-2012-0521. [DOI] [PubMed] [Google Scholar]
- 25.Leffler J, Martin M, Gullstrand B, et al. Neutrophil extracellular traps that are not degraded in systemic lupus erythematosus activate complement exacerbating the disease. J Immunol. 2012;188:3522–3531. doi: 10.4049/jimmunol.1102404. [DOI] [PubMed] [Google Scholar]
- 26.Garcia BA, Busby SA, Shabanowitz J, Hunt DF, Mishra N. Resetting the epigenetic histone code in the MRL-lpr/lpr mouse model of lupus by histone deacetylase inhibition. J Proteome Res. 2005;4:2032–2042. doi: 10.1021/pr050188r. [DOI] [PubMed] [Google Scholar]
- 27.Hu N, Qiu X, Luo Y, et al. Abnormal histone modification patterns in lupus CD4+ T cells. J Rheumatol. 2008;35:804–810. [PubMed] [Google Scholar]
- 28.Liu CL, Tangsombatvisit S, Rosenberg JM, et al. Specific post-translational histone modifications of neutrophil extracellular traps as immunogens and potential targets of lupus autoantibodies. Arthritis Res Ther. 2012;14:R25. doi: 10.1186/ar3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lande R, Ganguly D, Facchinetti V, et al. Neutrophils activate plasmacytoid dendritic cells by releasing self-DNA-peptide complexes in systemic lupus erythematosus. Sci Transl Med. 2011;3:73ra19. doi: 10.1126/scitranslmed.3001180. [DOI] [PMC free article] [PubMed] [Google Scholar]