Abstract
Evidence suggests the involvement of the cannabinoid system in the pathogenesis of multiple sclerosis (MS). We studied cannabinoid receptor (CB)1 and CB2 receptor gene expression in B, natural killer (NK) and T cells from MS patients before and after 1 year of interferon beta therapy, and compared these levels to those of healthy controls. We also measured the production of the endocannabinoids anandamide (AEA) and 2-arachidonoylglycerol (2-AG) and the gene expression of the endocannabinoid-degrading enzyme fatty acid amide hydrolase (FAAH) in these cells. Prior to interferon therapy, MS patients showed significantly elevated CB2 expression in B cells, but not in T or NK cells. These levels decreased gradually within 6 months to 1 year of interferon treatment. CB1 expression was elevated in all cell subsets, but only reached statistical significance in T cells; all levels decreased progressively over time. Before treatment, AEA but not 2-AG levels were significantly elevated in the three cell populations; after 1 year of treatment, all values decreased to control levels. The expression of FAAH was unchanged. The different expression of cannabinoid receptor genes and the increased level of AEA in lymphocytes point to a possible role of the cannabinoid system in MS immune response and its modulation by interferon.
Keywords: cannabinoid receptors, endocannabinoids, interferon beta, multiple sclerosis
Introduction
Multiple sclerosis (MS) is a demyelinating disease in which acute and chronic inflammation are associated with glial scarring and neuroaxonal damage. In the early phases of the disease, inflammation caused by mononuclear cells infiltrating the central nervous system (CNS) is a prominent pathological feature. With time, CNS inflammation decreases, and the degeneration of neurones and nerve fibres predominate along with atrophy. Therefore, MS is commonly considered a two-phase (inflammatory and degenerative) disease [1]. Interferon (IFN)-β has been used for almost 20 years as a first-line therapy for relapsing–remitting MS because it significantly reduces the frequency of clinical relapses and diminishes the inflammatory response. These effects are related to complex mechanisms of action that modulate the immune response [2].
The cannabinoid system is composed of the endogenous cannabinoid compounds (endocannabinoids), their targeted cannabinoid receptors (CBrs) and the enzymes involved in endocannabinoid synthesis and breakdown. Two main CBrs have been identified: the CB1 receptor, which is expressed mainly in the CNS but also, to a lesser extent, in peripheral blood lymphocytes; and the CB2 receptor, which is expressed primarily in immune system cells and microglial cells [3,4]. CBrs are G protein-coupled receptors linked to Gi/o-proteins, and negatively regulate adenylate cyclase and the accumulation of intracellular cyclic AMP (cAMP) [5].
Given their presence in immune-related cells, CBr-mediated signalling pathways are considered relevant for immune modulation; in fact, CB2 activation has been shown to affect cytokine secretion, immune cell trafficking, cell survival and antigen presentation [6]. An important feature of the CB2 receptor is the lack of psychoactive side effects after stimulation. Furthermore, studies performed in animals with experimental autoimmune encephalomyelitis (EAE), an MS model, showed that cannabinoids ameliorate symptoms (spasticity, tremor, ataxia and pain), reduce inflammation and favour remyelination via CB1- and CB2-mediated mechanisms [7,8]. Finally, most immunomodulatory/anti-inflammatory activities of cannabinoids in peripheral blood mononuclear cells (PBMCs) are mediated through CB2 receptors [9–11].
Several reports have shown the modulation of the endocannabinoid system in MS; CBrs are expressed specifically in CNS plaques [12], and endocannabinoid levels are altered significantly either in the cerebrospinal fluid (CSF) [13,14] or in the plasma [15]. In this paper, we have studied the expression of CBr genes in separated subsets of B, natural killer (NK) and T cells from MS patients before and after 1 year of IFN-β therapy, and we have compared these values with representative controls. The production of two endocannabinoids, anandamide (AEA) and 2-arachidonoylglycerol (2-AG), and the gene expression of the endocannabinoid-degrading enzyme fatty acid amide hydrolase (FAAH) were also analysed longitudinally.
This work was initiated to investigate how MS and MS therapy modulate the endocannabinoid system in patients. Considering the strong association of CB2 with immune regulation, we first analysed the expression of this receptor in immune cells of the peripheral blood from MS patients and the influence of the standard MS therapy, IFN-β, in a longitudinal study. Taking into account the involvement of T and B cells in the pathogenesis of MS and the reported differential expression of CB2 in human leucocyte subpopulations according to a rank order of B > NK > T cells [16,17], our study was also designed to analyse the expression of CB2 in these three cell populations before and after therapy. Next, we analysed the levels of CB1, the production of the endocannabinoids AEA and 2-AG and the expression of the catabolic enzyme FAAH in these cell populations at the same time-points.
Materials and methods
Ethics statement
This study was approved by the institutional scientific ethics commission, and all individuals participating in this study provided written informed consent.
Subjects
Twenty-six patients with relapsing–remitting MS were selected for the study; all had been diagnosed according to McDonald criteria [18] and had an active disease defined by at least one attack in the previous year. Blood samples were collected before the initiation of therapy and after 6 and 12 months with IFN-β-1a (Avonex® and Rebif®) or IFN-β-1b (Betaferon®). Patients were compared with 21 matched, drug-free healthy volunteers with no previous inflammatory or neurological conditions. Demographic and clinical characteristics are collected in Table 1.
Table 1.
Demographic and clinical characteristics of relapsing–remitting multiple sclerosis (RR-MS) patients
| Number of patients (Female/male) | Age (years) | Relapse rate previous 3 years | Relapse rate end of study | Basal EDSS |
|---|---|---|---|---|
| 19F/7M | 34·5 ± 10·6 (18–58) | 0·79 ± 0·29 (0·3–1·3) | 0·37 ± 0·68* (0–1) | 1·66 ± 1·53 (0–5) |
P-values < 0·05 (*) were considered significant between groups. The relapse rate changed significantly from 0·79 ± 0·29 (relapse rate previous 3 years) to 0·37 ± 0·68 (relapse rate at end of study). Expanded Disability Status Scale (EDSS) before therapy (basal EDSS) and EDSS after therapy with interferon (IFN)-β (EDSS end of the study). Data are expressed as the means ± standard deviation (range).
Isolation of cell subsets
Approximately 30 ml of blood from healthy donors and patients were processed for separation of B, NK and T cell populations (10 ml each) using 50 μl of RosetteSep human B, NK and T cell enrichment cocktails (Stem Cell Technologies, Inc., Vancouver, BC, Canada) per ml of blood. We added these cocktails, mixed them, and incubated the mixtures at room temperature for 20 min. Next, blood was diluted 1 : 1 with sterile phosphate-buffered saline (PBS) with 2% fetal bovine serum (FBS; Gibco® Life Technologies, Grand Island, NY, USA) layered over Ficoll-Hypaque (GE Life Sciences, Uppsala, Sweden) and centrifuged at 1100 g for 20 min at room temperature. The interphase layer of B, NK and T cells was collected, washed with ×1 PBS, and counted using trypan blue.
We evaluated the recovery and purity of separated cell populations by flow cytometry using CD19-peridinin chlorophyll (PerCp), CD56-allophycocyanin (APC) and CD3-fluorescein isothiocyanate (FITC) antibodies (BD Biosciences, San Jose, CA, USA) for B, NK and T cells, respectively. The purity of the subpopulations ranged from 92 to 98% with a recovery rate of 86%.
Measurement of endocannabinoid levels
After subset purification, cells were centrifuged at 1300 g for 5 min, and pellets were snap-frozen in liquid nitrogen at −80°C. Concentrations of AEA and 2-AG were measured using a method based on isotope dilution gas chromatography–mass spectrometry (GC-MS), as described previously [19]. Endocannabinoid levels were expressed as pmol/number of cells, and values were presented on a logarithmic scale. Due to the limited availability of biological samples, only 11 patients out of the whole 26 could be included for these measurements.
Analysis of CB1, CB2 and fatty acid amide hydrolase (FAAH) gene expression by real-time quantitative PCR
Total RNA was extracted using the RNeasy Mini Kit (Qiagen, Valencia, CA, USA), according to the manufacturer's recommendations. cDNA was synthesized from 500 ng of RNA by GeneAmp gold RNA polymerase chain reaction (PCR) Core Kit for reverse transcription–polymerase chain reaction (RT–PCR) (Applied Biosystems, Carlsbad, CA, USA) in a final volume of 20 μl.
Real-time quantitative PCR was performed in a LightCycler 480 apparatus (Roche Diagnostics, Basel, Switzerland) using the LightCycler 480 SYBR Green I Master kit (Roche Diagnostics) or the LightCycler 480 Probes Master (Roche Applied Science, Barcelona, Spain), depending on the primers or probes used for the amplification. The following CB2 and succinate dehydrogenase complex, subunit A (SDHA) primers were used: CB2 forward, 5′-AGCCACCCACAACACAACC-3′, reverse, 5′-GAGCCATTGGCTATCTCTGTC-3′; SDHA forward, 5′-TGGGAACAAGAGGGCATCTG-3′, reverse, 5′-CCACCACTGCATCAAATTCATG-3′.
To analyse the gene expression of CB1 and FAAH, the following highly specific fluorogenic primer/probe sets were used [Universal Probe Library (UPL) of Roche Applied Science]: CB1 (ID: 104051), FAAH (ID: 115042) and SDHA (ID: 102136).
To analyse the expression of CB2 and SDHA, cDNA obtained from B, NK and T cells was amplified in triplicate with 4 μl of cDNA template (corresponding to cDNA from 500 ng of RNA), 5 μl SYBR Green containing PCR master mix (LightCycler 480 SYBR Green I Master kit; Roche Diagnostics) and 0·5 μl of 10 μΜ concentrations for each primer; the total volume of the real-time PCR mixture was 10 μl. For CB1 and FAAH expression, the cDNA was amplified in triplicate with 5 μl of template cDNA, 10 μl Probes Master containing PCR master mix and 1 μl real-time ready assay containing primers (8 pmol of each primer) and probe mixture; the total volume of the real-time PCR mixture was 20 μl. A negative control (water) was included for each primer and probe.
The PCR reaction was first carried out at 95°C for 10 min followed by 45 cycles of thermal cycling at 95°C for 10 s, 59°C (CB2) or 60°C (CB1 and FAAH) for 30 s, and extension at 72°C for 1 s.
Real-time quantitative PCR was performed on the samples. The detected Ct values of the target genes (CB1, CB2 and FAAH) versus the housekeeping gene (SDHA) were transformed into ratios by the 2-ΔΔCt method (LightCycler 480 version 1·2 software; Roche Diagnostics), and values were normalized with the log of the ratios.
Statistical analysis
Statistical analysis was performed using one-way analysis of variance (anova) with Bonferroni's multiple comparison test for the study of cannabinoid receptor and FAAH expression and endocannabinoid production. A paired Wilcoxon test and paired Student's t-test were used to identify the effect of IFN-β on the relapse rate and expanded disability status scale (EDSS) evolution, respectively. Because the distribution of the gene expression and endocannabinoid values were not normally distributed (Kolmogorov–Smirnov test), we log-normalized the data distribution to conduct the statistical analysis. P-values < 0·05 (*) were considered significant between groups.
Results
IFN-β therapy affects CB2 and CB1 mRNA expression in MS patients
MS patients showed the same rank order of CB2 expression described in human lymphocytes, with the highest values in B cells and the lowest in T cells. Specifically, by quantitative real-time PCR, we observed a significant increase of CB2 expression in B cells from untreated patients that was progressively down-regulated by IFN-β therapy after 12 months compared to the basal and 6-month levels (Fig. 1a). In NK cells, the basal levels of CB2 did not differ from controls, but its expression was also diminished significantly after 1 year of IFN-β (Fig. 1b). In T cells, we observed a similar pattern as observed for NK cells, with the lowest expression at 1 year (Fig. 1c).
Fig. 1.

Comparison of cannabinoid receptor (CB)2 mRNA expression in healthy controls (HC) and relapsing–remitting multiple sclerosis (RR-MS) patients. Effects of interferon (IFN)-β therapy. CB2 mRNA expression (log scale) in B, natural killer (NK) and T cells (a,b,c, respectively) from HC (n = 25), MS (n = 26), MS + IFN-β 6 months (n = 26) and MS + IFN-β 12 months (n = 26) patients. P-values < 0·05 (*) were considered statistically significant between groups. Data are expressed as the means ± standard deviation.
CB1 receptor gene expression has been reported in mononuclear cells of human peripheral blood with a CB1/CB2 receptor ratio of ∼1 : 3 [20]. In addition, the CB1 gene is up-regulated in experimental models of inflammation [21]. Börner et al. have described the possible amplification of anti-inflammatory cannabinoid effects by the up-regulation of CB1 receptors [22]. Therefore, it was important to determine whether the expression of these receptors was also modified by IFN-β therapy in the same cell subpopulations. Contrary to CB2, when compared to healthy controls (HCs), the basal expression of CB1 in MS patients was elevated significantly in T cells but not in B cells; in the latter cell subset, as well as in NK cells, the values were close to significance (P = 0·065). In the three subsets, IFN-β induced a progressive decrease of CB1 gene expression levels at 6 months. At 12 months, all three cell populations had a marked reduction compared to basal, 6-month and HC levels (Fig. 2a–c).
Fig. 2.

Comparison of cannabinoid receptor (CB)1 mRNA expression in healthy controls (HC) and relapsing–remitting multiple sclerosis (RR-MS) patients. Effects of interferon (IFN)-β therapy. CB1 mRNA expression (log scale) in B, natural killer (NK) and T cells (a,b,c, respectively) from HC (n = 11), MS (n = 11), MS + IFN-β 6 months (n = 11) and MS + IFN-β 12 months (n = 11) patients. P-values < 0·05 (*) were considered statistically significant between groups. Data are expressed as the means ± standard deviation.
Endocannabinoid and FAAH measurements
Because 2-AG and AEA exert most of their biological effects via activation of CB1 and CB2 receptors, we measured the levels of these endocannabinoids in B, NK and T cells before and after 1 year of therapy. As in the case of CB1/CB2 mRNA levels, all values were compared to HCs. Before IFN-β therapy, the AEA levels in MS patients were elevated significantly in the three cell populations compared to controls. After 1 year of medication, these values were decreased to control levels (Fig. 3a–c).
Fig. 3.

Anandamide (AEA) levels in healthy controls (HC) and relapsing–remitting multiple sclerosis (RR-MS) patients. Effects of interferon (IFN)-β therapy. AEA expression (log scale) in B, natural killer (NK) and T cells (a,b,c, respectively) in HC (n = 11), MS (n = 11) and MS + IFN-β therapy (n = 11) patients. P-values < 0·05 (*) were considered statistically significant between groups. AEA is expressed as pmol/number of cells, and values are presented on a logarithmic scale.
2-AG levels produced by B and T cells from patients did not differ from those of HCs before and after therapy. Interestingly, the levels in NK cells were higher than controls and remained elevated for the duration of the study (Fig. 4a–c).
Fig. 4.

2-arachidonoylglycerol (2-AG) levels in healthy controls (HC) and relapsing–remitting multiple sclerosis (RR-MS) patients. Effects of interferon (IFN)-β therapy. 2-AG expression (log scale) in B, natural killer (NK) and T cells (a,b,c, respectively) in HC (n = 11), MS (n = 11) and MS + IFN-β therapy (n = 11) patients. P-values < 0·05 (*) were considered statistically significant between groups. 2-AG is expressed as pmol/number of cells, and values are presented on a logarithmic scale.
FAAH is a broad specificity enzyme involved in the hydrolysis of AEA and 2-AG [23]. Whereas other hydrolases have a more specific role for 2-AG, FAAH is the critical regulator of the endogenous levels of AEA [24]. To assess whether the high AEA production observed in lymphoid cells was due to abnormal expression of its catabolic enzyme, we measured FAAH immunoreactivity in these cells. FAAH levels were similar to those observed in HCs at all the time-points investigated (Fig. 5a–c), suggesting that AEA elevation was not due primarily to FAAH down-regulation.
Fig. 5.
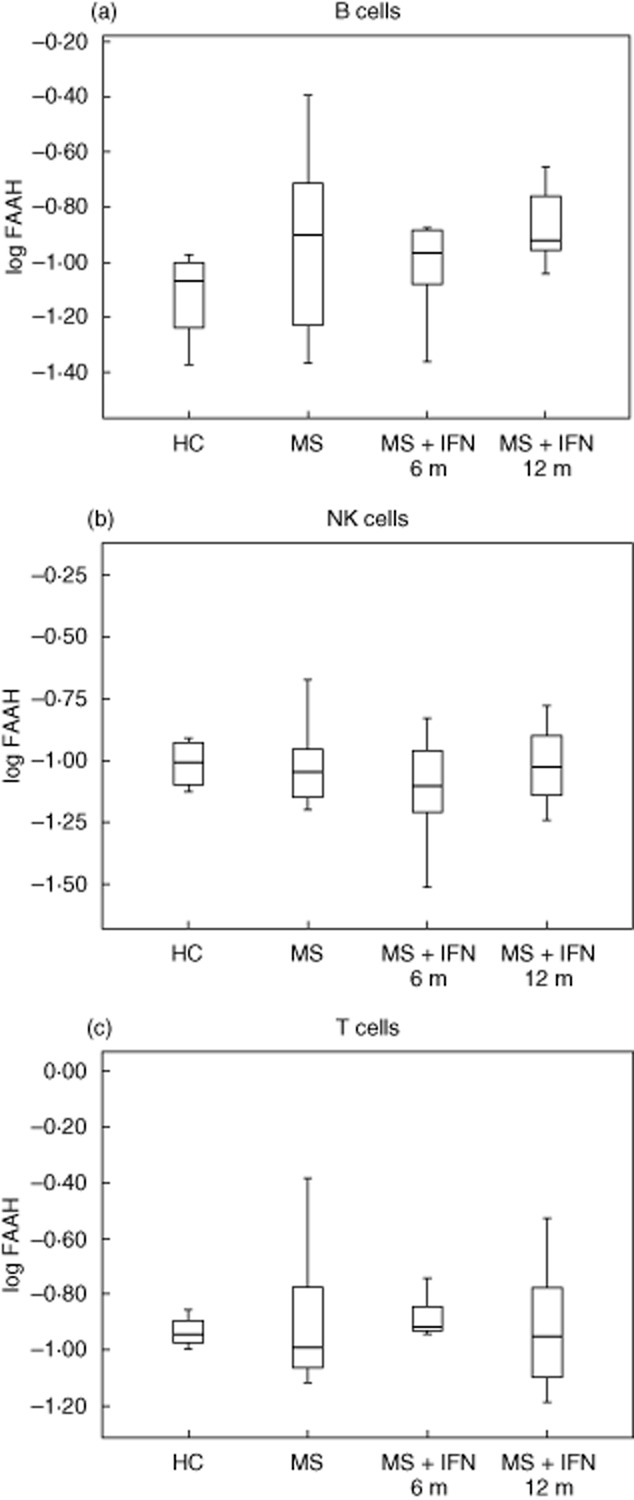
Comparison of fatty acid amide hydrolase (FAAH) mRNA expression in healthy controls (HC) and relapsing–remitting multiple sclerosis (RR-MS) patients. Effects of interferon (IFN)-β therapy. FAAH mRNA expression (log scale) in B, natural killer (NK) and T cells (a,b,c, respectively) from HC (n = 11), MS (n = 11), MS + IFN-β 6 months (n = 11) and MS + IFN-β 12 months (n = 11) patients. P-values < 0·05 (*) were considered statistically significant between groups. Data are expressed as the means ± standard deviation (range).
Clinical data
At the end of the study, the relapse rate changed significantly (P < 0·05) from 0·79 ± 0·29 to 0·37 ± 0·68. However, EDSS scores remained unchanged over the entire period (Table 1). No correlations could be established between EDSS and gender, clinical features or type of interferon, cannabinoid receptor expression, endocannabinoid production or FAAH expression.
Discussion
In our study, we confirmed that the endocannabinoid system is altered in untreated MS patients and that it is modulated by IFN-β therapy over a period of 1 year. AEA was elevated in untreated patients in the three cell subsets, and these levels decreased significantly after 1 year of IFN-β therapy to a level similar to that of HCs. 2-AG was unmodified in B and T cells, but NK cells synthesized more 2-AG than controls and maintained these levels until the end of the study. FAAH mRNA expression was unmodified in patients with or without medication.
CBr expression in immune cells is modulated by cell activation; numerous stimuli, including lipopolysaccharide (LPS), phorbol myristate acetate, phytohaemagglutinin, anti-CD40, anti-CD3 and IFN-γ, have been associated with an increase of CB2, and possibly CB1, in different cell populations [25]. In cell membranes from primary human T lymphocytes, LPS or anti-CD3 stimulation elevates CB2 receptors by sixfold at the mRNA level and 2·5-fold at the protein level; CB1 receptor levels were changed less significantly [9]. In actively relapsing MS, proinflammatory cytokines are a pathogenic component of the disease [26], and it seems plausible to hypothesize that the increased expression of CBr could reflect a response to the proinflammatory milieu. Proinflammatory cytokines might increase CBr expression by activating transcription factors, such as NF-kB, NF-AT or AP-1, all of which have binding sites at the promoters of both CB genes [27]. Our data show that the proinflammatory status of actively relapsing MS affects both CB1 and CB2 genes and differs depending on the cell population. CB2 gene expression was clearly high in B cells before therapy, whereas CB1 was higher in T cells. In all cases, the levels were decreased significantly by IFN-β therapy over the course of 1 year, although treatment for longer than 6 months was necessary to see a significant reduction. Interestingly, CB1 expression was lower than that of controls after 1 year of therapy, but this tendency was significant only for NK cells; this finding might indicate that this gene, at least in this cell subset, is more susceptible to the immunomodulatory effects of IFN-β than CB2. However, additional work should address this possibility.
The mechanisms of IFN-β activity in MS are complex, pleiotropic and incompletely understood. The induction of a myriad immunological effects has been described, including a deviation of the cytokine profile towards an anti-inflammatory phenotype in the systemic circulation [2,28]. The modulation of markers associated with IFN-β efficacy in MS occurs over time [29,30]. In patients, the response to IFN-β is clinically measurable only after a relatively prolonged treatment, a fact that may reflect a progressive and sustained down-regulation of the proinflammatory milieu [29]. In line with this idea, the gradual and progressive reduction of CBr expression levels observed in this study may be consistent with the idea that an initial response to global immune activation before therapy is moderated by the IFN-β anti-inflammatory effect.
Altered endocannabinoid metabolism has been reported previously both in EAE and MS. In EAE brains, we found reduced AEA concentrations [31], whereas others observed normal [32] or higher levels of this endocannabinoid [13]. These discrepancies may be related to the EAE model (rats versus mice), analytical methods or sampling time. In MS brains, the AEA concentration was higher in active than in chronic plaques [33]. In MS cerebrospinal fluid, Centonze et al. found high levels of AEA but normal levels of 2-AG [13], whereas Di Filippo et al. observed a significant reduction of both endocannabinoids compared to controls [14]; the latter authors found a tendency towards higher but still below control levels during relapses. High levels of AEA have been reported in plasma from MS patients [15] as well as an increased production of the same lipid by peripheral lymphocytes [13].
In MS, the source of endocannabinoids in peripheral blood has been a matter of debate. It has been shown that AEA can be released from blood cells [34]; furthermore, significant sources of endocannabinoids include leucocytes, platelets, endothelial cells and peripheral lymphocytes [13]. In the MS brain, AEA is increased in active plaques, but this increase may be due to the infiltrating lymphocytes [35] because the number of CBrs is scarce in the white matter; it could also be produced by the degeneration of the adjacent nervous tissue. In addition, a possible contribution from neurones and glial cells across a defective blood–brain barrier has been suggested [15].
The reduced number of samples analysed for endocannabinoid production by the three lymphocyte subsets limits the interpretation of our results. However, the data are consistent with the reported elevation of AEA in MS for the cell types examined and point towards the increased synthesis of AEA rather than reduced catabolism, as suggested elsewhere [13,15], as the mRNA levels of the degrading enzyme FAAH did not differ from controls in our study. However, this finding does not exclude that FAAH protein could be reduced due to translation alterations or the possibility of changes in FAAH activity independent of protein expression.
In activated human lymphocytes in culture, AEA is a strong modulator of proinflammatory mediators, including interleukin (IL)-17 [9]. Thus, in pathological conditions such as MS, which is characterized by the presence of proinflammatory cytokines, AEA increase may represent an adaptive response to control a dysregulated immune balance. In addition to the activities in the peripheral blood, AEA secreted by infiltrating lymphocytes may help to protect the nervous tissue not only as an anti-inflammatory but also as a neuroprotective agent through CB1- and CB2-mediated mechanisms [13,33].
2-AG levels produced by T and B cells from patients before and after IFN-β therapy were similar to those of HCs. However, in NK cells, 2-AG levels remained high during the study. Available information on NK cells and 2-AG is very limited. It has been reported that this endocannabinoid induces the migration of human NK cells, an effect abolished by the CB2 antagonist SR144528 [36]. In our study, the meaning of the observed 2-AG elevation in NK cells is unclear; additional experimental work will be required to confirm and clarify these findings.
Conclusions
In conclusion, our investigation confirms the increased expression of CBr and AEA elevation in peripheral blood lymphocytes from MS patients. We have also described for the first time the specific contribution of B, NK and T lymphocytes to this elevation and the gradual but marked reduction of CBr levels after IFN-β therapy, an observation consistent with the immunomodulatory effects of this drug. These findings supplement the current body of evidence showing the involvement of the endocannabinoid system in the immune dysregulation of MS.
Acknowledgments
The authors would like to thank V. García Barberan for valuable discussions, L. Campos Ruíz for the expert technical assistance and I. Millán (Biostatistics Unit) for the statistical support. Special thanks are also due to the donors and the Biobanco Hospital Universitario Puerta de Hierro Majadahonda – Instituto de Investigación Sanitaria Puerta de Hierro, for the human specimens used in this study. This work was supported by the Fondo de Investigaciones Sanitarias (FIS), Instituto de Salud Carlos III, Spain (PS09/02004 and PI12/02672), and by Fundación ‘La Caixa’ (Project: 23379).
Disclosure
The authors declare that they have no conflicts of interest.
Author contributions
A. J. S. L., L. R. V. and A. G. M. conceived and designed the experiments. A. J. S. L., L. R. V., E. R. T. and A. G. performed the experiments. A. J. S. L., A. G. and A. G. M. analysed the data. A. J. S. L., L. R. V., E. R. T. and A. G. contributed reagents/materials/analysis tools. A. J. S. L., L. R. V. and A. G. M. wrote the paper.
References
- 1.Frischer JM, Bramow S, Dal-Bianco A, et al. The relation between inflammation and neurodegeneration in multiple sclerosis brains. Brain. 2009;132:1175–1189. doi: 10.1093/brain/awp070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yong VW, Chabot S, Stuve O, Williams G. Interferon beta in the treatment of multiple sclerosis: mechanisms of action. Neurology. 1998;51:682–689. doi: 10.1212/wnl.51.3.682. [DOI] [PubMed] [Google Scholar]
- 3.Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- 4.Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- 5.Howlett AC, Barth F, Bonner TI, et al. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev. 2002;54:161–202. doi: 10.1124/pr.54.2.161. [DOI] [PubMed] [Google Scholar]
- 6.Sanchez AJ, Garcia-Merino A. Neuroprotective agents: cannabinoids. Clin Immunol. 2012;142:57–67. doi: 10.1016/j.clim.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 7.Pryce G, Ahmed Z, Hankey DJ, et al. Cannabinoids inhibit neurodegeneration in models of multiple sclerosis. Brain. 2003;126:2191–2202. doi: 10.1093/brain/awg224. [DOI] [PubMed] [Google Scholar]
- 8.Fernandez-Ruiz J, Pazos MR, Garcia-Arencibia M, Sagredo O, Ramos JA. Role of CB2 receptors in neuroprotective effects of cannabinoids. Mol Cell Endocrinol. 2008;286:S91–S96. doi: 10.1016/j.mce.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 9.Cencioni MT, Chiurchiu V, Catanzaro G, et al. Anandamide suppresses proliferation and cytokine release from primary human T-lymphocytes mainly via CB2 receptors. PLOS ONE. 2010;5:e8688. doi: 10.1371/journal.pone.0008688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ihenetu K, Molleman A, Parsons M, Whelan C. Pharmacological characterisation of cannabinoid receptors inhibiting interleukin 2 release from human peripheral blood mononuclear cells. Eur J Pharmacol. 2003;464:207–215. doi: 10.1016/s0014-2999(03)01379-7. [DOI] [PubMed] [Google Scholar]
- 11.Malfitano AM, Laezza C, D'Alessandro A, et al. Effects on immune cells of a new 1,8-naphthyridin-2-one derivative and its analogues as selective CB2 agonists: implications in multiple sclerosis. PLOS ONE. 2013;8:e62511. doi: 10.1371/journal.pone.0062511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benito C, Romero JP, Tolon RM, et al. Cannabinoid CB1 and CB2 receptors and fatty acid amide hydrolase are specific markers of plaque cell subtypes in human multiple sclerosis. J Neurosci. 2007;27:2396–2402. doi: 10.1523/JNEUROSCI.4814-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Centonze D, Bari M, Rossi S, et al. The endocannabinoid system is dysregulated in multiple sclerosis and in experimental autoimmune encephalomyelitis. Brain. 2007;130:2543–2553. doi: 10.1093/brain/awm160. [DOI] [PubMed] [Google Scholar]
- 14.Di FM, Pini LA, Pelliccioli GP, Calabresi P, Sarchielli P. Abnormalities in the cerebrospinal fluid levels of endocannabinoids in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2008;79:1224–1229. doi: 10.1136/jnnp.2007.139071. [DOI] [PubMed] [Google Scholar]
- 15.Jean-Gilles L, Feng S, Tench CR, et al. Plasma endocannabinoid levels in multiple sclerosis. J Neurol Sci. 2009;287:212–215. doi: 10.1016/j.jns.2009.07.021. [DOI] [PubMed] [Google Scholar]
- 16.Galiegue S, Mary S, Marchand J, et al. Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. Eur J Biochem. 1995;232:54–61. doi: 10.1111/j.1432-1033.1995.tb20780.x. [DOI] [PubMed] [Google Scholar]
- 17.Graham ES, Angel CE, Schwarcz LE, Dunbar PR, Glass M. Detailed characterisation of CB2 receptor protein expression in peripheral blood immune cells from healthy human volunteers using flow cytometry. Int J Immunopathol Pharmacol. 2010;23:25–34. doi: 10.1177/039463201002300103. [DOI] [PubMed] [Google Scholar]
- 18.Polman CH, Reingold SC, Edan G, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the ‘McDonald criteria’. Ann Neurol. 2005;58:840–846. doi: 10.1002/ana.20703. [DOI] [PubMed] [Google Scholar]
- 19.Hardison S, Weintraub ST, Giuffrida A. Quantification of endocannabinoids in rat biological samples by GC/MS: technical and theoretical considerations. Prostaglandins Other Lipid Mediat. 2006;81:106–112. doi: 10.1016/j.prostaglandins.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 20.Nong L, Newton C, Cheng Q, Friedman H, Roth MD, Klein TW. Altered cannabinoid receptor mRNA expression in peripheral blood mononuclear cells from marijuana smokers. J Neuroimmunol. 2002;127:169–176. doi: 10.1016/s0165-5728(02)00113-3. [DOI] [PubMed] [Google Scholar]
- 21.Izzo AA, Fezza F, Capasso R, et al. Cannabinoid CB1-receptor mediated regulation of gastrointestinal motility in mice in a model of intestinal inflammation. Br J Pharmacol. 2001;134:563–570. doi: 10.1038/sj.bjp.0704293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Borner C, Hollt V, Sebald W, Kraus J. Transcriptional regulation of the cannabinoid receptor type 1 gene in T cells by cannabinoids. J Leukoc Biol. 2007;81:336–343. doi: 10.1189/jlb.0306224. [DOI] [PubMed] [Google Scholar]
- 23.Ueda N. Endocannabinoid hydrolases. Prostaglandins Other Lipid Mediat. 2002;68–69:521–534. doi: 10.1016/s0090-6980(02)00053-9. [DOI] [PubMed] [Google Scholar]
- 24.Fezza F, De SC, Amadio D, Maccarrone M. Fatty acid amide hydrolase: a gate-keeper of the endocannabinoid system. Subcell Biochem. 2008;49:101–132. doi: 10.1007/978-1-4020-8831-5_4. [DOI] [PubMed] [Google Scholar]
- 25.Klein TW, Newton C, Larsen K, et al. The cannabinoid system and immune modulation. J Leukoc Biol. 2003;74:486–496. doi: 10.1189/jlb.0303101. [DOI] [PubMed] [Google Scholar]
- 26.Sospedra M, Martin R. Immunology of multiple sclerosis. Annu Rev Immunol. 2005;23:683–747. doi: 10.1146/annurev.immunol.23.021704.115707. [DOI] [PubMed] [Google Scholar]
- 27.Jean-Gilles L, Gran B, Constantinescu CS. Interaction between cytokines, cannabinoids and the nervous system. Immunobiology. 2010;215:606–610. doi: 10.1016/j.imbio.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 28.Kieseier BC. The mechanism of action of interferon-beta in relapsing multiple sclerosis. CNS Drugs. 2011;25:491–502. doi: 10.2165/11591110-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 29.Killestein J, Polman CH. Determinants of interferon beta efficacy in patients with multiple sclerosis. Nat Rev Neurol. 2011;7:221–228. doi: 10.1038/nrneurol.2011.22. [DOI] [PubMed] [Google Scholar]
- 30.Malucchi S, Gilli F, Caldano M, et al. Predictive markers for response to interferon therapy in patients with multiple sclerosis. Neurology. 2008;70:1119–1127. doi: 10.1212/01.wnl.0000304040.29080.7b. [DOI] [PubMed] [Google Scholar]
- 31.Cabranes A, Venderova K, de Lago E, et al. Decreased endocannabinoid levels in the brain and beneficial effects of agents activating cannabinoid and/or vanilloid receptors in a rat model of multiple sclerosis. Neurobiol Dis. 2005;20:207–217. doi: 10.1016/j.nbd.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 32.Witting A, Chen L, Cudaback E, et al. Experimental autoimmune encephalomyelitis disrupts endocannabinoid-mediated neuroprotection. Proc Natl Acad Sci USA. 2006;103:6362–6367. doi: 10.1073/pnas.0510418103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eljaschewitsch E, Witting A, Mawrin C, et al. The endocannabinoid anandamide protects neurons during CNS inflammation by induction of MKP-1 in microglial cells. Neuron. 2006;49:67–79. doi: 10.1016/j.neuron.2005.11.027. [DOI] [PubMed] [Google Scholar]
- 34.Vogeser M, Hauer D, Christina AS, Huber E, Storr M, Schelling G. Release of anandamide from blood cells. Clin Chem Lab Med. 2006;44:488–491. doi: 10.1515/CCLM.2006.065. [DOI] [PubMed] [Google Scholar]
- 35.Baker D, Pryce G. The endocannabinoid system and multiple sclerosis. Curr Pharm Des. 2008;14:2326–2336. doi: 10.2174/138161208785740036. [DOI] [PubMed] [Google Scholar]
- 36.Kishimoto S, Muramatsu M, Gokoh M, Oka S, Waku K, Sugiura T. Endogenous cannabinoid receptor ligand induces the migration of human natural killer cells. J Biochem. 2005;137:217–223. doi: 10.1093/jb/mvi021. [DOI] [PubMed] [Google Scholar]


