Abstract
Background
Since 2000, there has been a marked rise in acute hepatitis C virus (HCV) in HIV-positive men who have sex with men (MSM). We conducted an international phylogenetic study to investigate the existence of an HCV transmission network among MSM.
Methods
HIV-positive MSM diagnosed with recent HCV (n=226) in England (107), Netherlands (58), France (12), Germany (25) and Australia (24) between 2000 and 2006 were enrolled into a molecular phylogenetic study. Using real-time PCR, the NS5B region of the HCV genome (436 bp) was amplified, sequenced and compared with unrelated NS5B sequences.
Results
NS5B sequences were obtained from 200 (89%) cases. Circulating HCV genotypes were 1a (59%); 4d (23%), 3a (11%), 1b (5%), and 2b/c (3%). Phylogenetic analysis revealed 156 (78%) sequences that formed 11 clusters (bootstrap value >70%) containing between 4 and 37 individual sequences. Country mixing was associated with larger cluster size (17 vs 4.5 sequences; p=0.03). ‘Molecular clock’ analysis indicated that the majority (85%) of transmissions occurred since 1996.
Discussion
Phylogenetic analysis revealed a large international network of HCV transmission among HIV-positive MSM. The rapid spread of HCV among neighboring countries is supported by the large proportion (74%) of European MSM infected with an HCV strain co-circulating in multiple European countries; the low evolutionary distances among HCV isolates from different countries; and the trend toward increased country mixing with increasing cluster size. Temporally, this epidemic coincides with the introduction of highly active anti-retroviral therapy and associated increases in sexual risk behaviors. International collaborative public health efforts are needed to mitigate HCV transmission among this population.
Introduction
Over the last 5 years an increasing number of hepatitis C virus (HCV) outbreaks have been reported among HIV-positive men who have sex with men (MSM) in large urban centres in Europe [1-3], USA [4,5] and Australia [6]. Longitudinal cohort studies have confirmed a recent rise in the incidence of HCV among HIV-positive MSM in England [7] and the Netherlands [8] and phylogenetic analysis has revealed the presence of MSM-specific HCV transmission networks in the UK [2], the Netherlands [8] and France [3]. In contrast to the usual pattern of HCV transmission in such regions, with 80% of incident cases attributable to injecting drug use (IDU) [9], the vast majority of MSM recently diagnosed with HCV deny IDU. This strongly suggests that the epidemiology of HCV in this population is changing, with permucosal (sexual) factors becoming a potentially important route of transmission.
Following the introduction of highly active antiretroviral therapy (HAART) for HIV and the consequent reduction in HIV-related morbidity and mortality, the number of people living with HIV is steadily increasing. Outbreaks of syphilis, lymphogranuloma venereum (LGV), Shigella sonnei and HCV, suggest a considerable and increasing burden of various STI among HIV-positive MSM [10-12]. A recent molecular epidemiological study revealed that ten hepatitis A virus (HAV) outbreaks among MSM in seven European countries between 1997 and 2005 actually represented one large outbreak among interconnected MSM communities, within which HAV transmission has become endemic [13]. These emergent high-risk sexual networks are susceptible to the introduction and spread of various infectious agents, such as HCV, for which sexual contact is not usually considered to be a primary mode of transmission [14].
The emergence of HCV as an STI among HIV-positive MSM has potentially serious clinical implications, as HCV/HIV co-infection profoundly influences the natural history of HCV, and possibly also HIV. HCV/HIV co-infection been associated with lower rates of spontaneous HCV clearance, accelerated HCV-related liver disease, less favourable HCV treatment outcomes, delayed CD4 cell recovery after initiation of HAART and increased HAART-associated hepatotoxicity [15-18]. Recent findings among HIV-positive MSM with newly diagnosed HCV in New York suggested a fibrosis progression rate 5 times greater than that of HIV-negative individuals with recent HCV-infection [19].
The aim of this study was to determine whether reported cases of acute HCV among HIV-positive MSM represent small isolated HCV outbreaks, or whether these HCV outbreaks form part of a larger interconnected international transmission network. Using a phylogenetic approach we analysed HCV strains obtained from HIV-positive MSM that were recently diagnosed with acute HCV in Europe and Australia. If indeed a high-risk sexual network exists that facilitates the rapid and ongoing transmission of HCV among HIV-positive MSM, it would have important implications for clinicians and public health agencies responsible for control, education, therapy and surveillance of HCV in those regions.
Materials and methods
Study population and case definition
We studied 226 HIV-positive MSM diagnosed with acute HCV infection in England, the Netherlands, Germany, France and Australia. The majority of cases, except German, had been previously reported. Acute HCV infection was defined as a documented anti-HCV seroconversion within 12 months, and/or clinical and biochemical criteria (acute hepatitis in individuals without pre-existing liver disease, excluding other infective, metabolic, toxic and drug causes for hepatitis, and a serum alanine aminotransferase (ALT) level ≥ 10 times the upper limit of normal) [20]. All HCV infections were diagnosed after 2000. Although recruitment of MSM varied for each country, all participants were HIV-positive MSM attending urban HIV treatment centres and/or participating in longitudinal cohort studies. English MSM were recruited from two large urban HIV clinics in London (n=95) and one in Brighton (n=12) [2]. Dutch participants included notified cases from HIV and/or STI clinics in Amsterdam (n=44) and Rotterdam (n=9), as well as participants of the Amsterdam Cohort Studies among MSM (n=5) [1,8]. German MSM (n=25) were recruited from HIV clinics/practises in Berlin [21], and French MSM (n=12) from two large centres for infectious diseases and one liver unit in Paris [3]. All Australian subjects (n=24) were participating in the Australian Trial in Acute Hepatitis C (ATAHC); a prospective study of the natural history and treatment outcomes in acute HCV ongoing in 21 sites in Australia [6].
Reverse transcriptase polymerase Chain Reaction (RT-PCR) method
Using the first available HCV RNA positive sample for each participant, RNA isolation was performed on 100 μl of serum using the TriPure method (Roche Diagnostics). Pellets containing the HCV RNA were resuspended in a volume of 50 μl containing 10 mM Tris buffer pH = 8.0 and 10 U RNAsin. Of each isolate, 3 μl was used as input for a nested multiplex RT-PCR which targets the NS5B region of the HCV genome. This NS5B PCR was devised to amplify HCV RNA (436 bp) of genotypes 1-4; the exact conditions and primers have been described elsewhere [22]. All sera and RNA isolates had been stored at -80 °C. RNA isolation and amplification of the 202 European samples was performed at the Health Service Laboratory in Amsterdam, the Netherlands. To prevent cross-country contamination, samples originating from different European countries were always handled separately. Using the same protocol, RNA isolation and amplification of the 24 Australian samples was performed at the University of New South Wales in Sydney, Australia.
Sequencing & Phylogenetic analysis
Sequence reactions were performed as described by van de Laar et al. (2005) [22]. NS5B PCR products were ethanol precipitated. Sense and antisense strands were separately cycle-sequenced using the BigDye Terminator system (version 1.1; Applied Biosystems), and subsequently purified using DyeEx spin kits (Qiagen). Viral epidemic history was assessed by constructing phylogenetic trees of the case sequences plus available unrelated reference sequences from GenBank (www.ncbi.nlm.nih.gov) and the Los Alamos HCV Sequence Database (www.hcv.lanl.gov). Phylogenies were estimated by the maximum likelihood approach implemented in PAUP*4, the Hasegawa-Kishino-Yano substitution model plus a gamma distribution model of among-site rate heterogeneity (HKY-Γ) [23]. Statistical robustness of phylogenies was assessed by bootstrapping with 1000 replicates (bootstrap values > 70 represent robust clusters). Phylogenetic trees were visualised using Figtree v1.0 software. The date of the common ancestor for each HCV cluster was estimated using a molecular clock approach, which places the phylogenetic history of HCV onto a real timescale of months and years. Based on previous estimates [24,25], the inferred rate of nucleotide evolution for our NS5B sequences was 5.0 × 10-4 substitutions per site per year. Dates of origin for each cluster were subsequently calculated using a Bayesian MCMC approach implemented in BEAST v1.3 [26]. In common with previous studies [2,27] a ‘coalescent based’ reconstruction of epidemic history [24,25] cannot be applied to this data, because the proportion of sampled lineages is too high. However, lineage splits in the phylogenetic tree do represent past transmission events and reflect the actual spread of the virus within the population. Therefore we used the molecular clock based phylogenies to calculate the number of phylogenetic lineage splits in each cluster that took place after 1996, 1998 and 2000. These three years mark different events in the MSM community; the introduction of HAART which successfully decreased HIV-related morbidity and mortality (1996); rise in sexual risk-taking and resurgence of STI among MSM following success of HAART (1998), and the first reports of acute HCV in HIV-positive MSM (2000).
Nucleotide sequence accession numbers
Sequences reported in this study have been submitted to GenBank and can be retrieved under accession numbers FJ386690 to FJ386850. The majority (39/49) HCV cases from Amsterdam had previously been submitted; GenBank accession numbers DQ897975 to DQ898026 and EF123061 to EF123064 [8].
Results
Study population
The demographics of the study population are shown in Table 1. The vast majority (96%) were diagnosed based on anti-HCV or HCV RNA seroconversion rather than a clinical/biochemical hepatitis. The median age at HCV diagnosis was 38 years (interquartile range [IQR], 33 - 42 years). The median CD4-count was 518 cells/μl [IQR: 359 - 695 cells/μl] and 62% of participants received HAART (Table 1). As Dutch participants were anonymously collected through centralised health services in Amsterdam and Rotterdam, clinical information regarding CD4 count and use of HAART were mostly unavailable. The number of MSM reporting IDU in the 12 months prior to HCV seroconversion was low on the European mainland (2.9%), intermediate in the UK (17%), and high in Australia (50%). Despite the relatively high percentage of English MSM reporting IDU, only one subject from the UK (1.6%) reported needle sharing.
Table 1.
Patient demographics of HIV positive men who have sex with men (MSM) on diagnosis of acute hepatitis C virus (HCV) infection.
| Number | Median Age (yrs) | Median CD4 cells (μ/ml) | HAART (%) | IDU (%) | |
|---|---|---|---|---|---|
| UK | 107 | 35 [IQR: 32-39] | 510 [IQR: 341-703] | 61/102 (60) | 10/60 (17) |
| Netherlands | 58 | 40 [IQR: 35-46] | na | 4/9 (44)* | 2/58 (3) |
| Germany | 25 | 38 [IQR: 30-41] | 429 [IQR: 343-628] | 15/25 (60) | 1/25 (4) |
| France | 12 | 40 [IQR: 34-44] | 604 [IQR: 569-697] | 9/12 (75) | 0/12 (0) |
| Australia | 24 | 40 [IQR: 34-46] | 596 [IQR: 417-807] | 18/24 (75) | 12/24 (50) |
| Overall | 226 | 38 [IQR: 33-42] | 518 [IQR: 359-695] | 107/172 (62) | 25/179 (14) |
Data on HAART are known for the Rotterdam cases only
RT-PCR and sequencing
The HCV NS5B fragment was amplified from 200/226 (88%) HIV-positive MSM enrolled. Subsequent sequencing and genotyping was successful in all 200 patients. The HCV genotype distribution according to country of origin is given in Table 2. The majority of isolates were genotype 1a (59%) and genotype 4d (23%) with some genotype 1b, 2b/c and 3a infections. The difficult-to-treat HCV genotypes 1 and 4 accounted for 158/176 (90%) of amplified sequences from Europe, and 16/24 (67%) of amplified Australian sequences. HCV genotype 3a was relatively more prevalent (33%) among Australian MSM. As HCV genotype 3a in western countries has particularly been associated with IDU [28], the relatively high proportion of HCV genotype 3a among Australian MSM could be explained by the larger proportion of Australian MSM reporting IDU. However, HCV genotype 3a was equally prevalent among MSM who denied IDU.
Tabel 2.
HCV genotype distribution of acute HCV infections among HIV-positive men who have sex with men (MSM) in England (UK), the Netherlands (NL), Germany (GE), France (FR) and Australia (AUS) based on sequencing part of the NS5B region (436 bp).
| UK Nr (%) |
NL Nr (%) |
GE Nr (%) |
FR Nr (%) |
AUS Nr (%) |
Total Nr (%) |
|
|---|---|---|---|---|---|---|
| Isolates | 107 | 58 | 25 | 12 | 24 | 226 |
| Untyped | 20 (19) | 1 (2) | 3 (12) | 2 (17) | 0 (0) | 26* (12) |
| Genotyped | 87 (81) | 57 (98) | 22 (88) | 10 (83) | 24 (100) | 200 (88) |
| Genotype | ||||||
| 1a | 53 (61) | 31 (54) | 17 (77) | 1 (10) | 16 (67) | 118 (59) |
| 1b | 6 (7) | 3 (5) | 1 (5) | - | - | 10 (5) |
| 2b | 2 (2) | 1 (2) | - | - | - | 3 (2) |
| 2c | 2 (2) | - | - | - | - | 2 (1) |
| 3a | 9 (10) | 3 (5) | - | 1 (10) | 8 (33) | 21 (11) |
| 4d | 15 (17) | 19 (33) | 4 (18) | 8 (80) | - | 46 (23) |
19/26 (83%) could be genotyped using VERSANT HCV genotype assay (LIPA), 17/19 (89%) were infected with difficult-to-treat HCV genotypes 1 and 4.
Phylogenetic analysis
Phylogenetic trees were constructed for each HCV genotype separately. These phylogenetic trees included our 200 HCV NS5B case sequences plus 850 homologous reference sequences of concordant HCV genotypes. Phylogenetic analysis revealed 11 strongly-supported monophyletic clusters (bootstrap > 70) of MSM-specific strains, 6 homologous pairs of genetically similar MSM sequences and 32 ‘singleton’ MSM sequences that were more closely related to reference strains than to another MSM strain from our study. Overall, 168/200 (84%) MSM harboured an HCV strain that was most similar to HCV present in another MSM participant of this study.
MSM clusters ranged in size from 4-37 sequences, and were predominantly of genotype 1a and 4d (Table 3). The genotype 1a phylogeny is depicted in figure 1 and shows 7 monophyletic clusters, 3 homologous pairs, and 12 singleton MSM sequences. Five of the seven clusters contained sequences from more than one European country; the two remaining clusters (clusters 9 and 10) contained only Australian isolates. All MSM sequences of HCV genotype 4d were part of two monophyletic clusters containing 34 (cluster 2) and 12 (cluster 6) sequences respectively (Figure 1). Phylogenies of less prevalent HCV genotypes are not shown (available on request). The genotype 3a phylogeny revealed one MSM-specific cluster (Cluster 8) which contained 5 English and one French sequence, plus one homologous pair of Australian sequences (Pair F), and 13 unrelated MSM sequences. The genotype 1b tree contained two unrelated MSM sequences, two homologous pairs of MSM sequences (an English pair D and a Dutch pair E) and 4 nearly identical English MSM strains (Cluster 11) that were part of a larger paraphyletic cluster together with 12 European and American reference strains. All genotype 2 HCV sequences appeared unrelated to any other MSM study strain. As half of the Australian MSM reported IDU, we compared sequences of Australian MSM who reported IDU with those who denied IDU. Australian clusters 9 and 10 both contained MSM reporting and denying IDU. The two homologous pairs of Australian MSM (pair C and F) both consisted of MSM reporting IDU, and of the 10 unrelated MSM sequences 4 did and 6 did not report IDU.
Table 3.
Identified Hepatitis C virus (HCV) clusters among HIV-positive MSM with acute HCV infection after 2000 in England (UK), the Netherlands (NL), France (FR), Germany (GE) and Australia (AUS)
| Nr of sequences per country | Country mixing |
||||||
|---|---|---|---|---|---|---|---|
| Cluster (n) | UK | NL | GE | FR | AUS | ||
| 1 | genotype 1a (37) | 32 | 5 | - | - | - | yes |
| 2 | genotype 4d (34) | 3 | 19 | 4 | 8 | - | yes |
| 3 | genotype 1a (19) | 1 | 17 | 1 | - | - | yes |
| 4 | genotype 1a (17) | 11 | - | 6 | - | - | yes |
| 5 | genotype 1a (12) | 1 | 2 | 8 | - | 1 | yes |
| 6 | genotype 4d (12) | 12 | - | - | - | - | no |
| 7 | genotype 1a (6) | - | 5 | 1 | - | - | yes |
| 8 | genotype 3a (6) | 5 | - | - | 1 | - | yes |
| 9 | genotype 1a (5) | - | - | - | - | 5 | no |
| 10 | genotype 1a (4) | - | - | - | - | 4 | no |
| 11 | genotype 1b (4) | 4 | - | - | - | - | no |
Figure 1. NS5B phylogentic trees of HCV genotypes 1a (left) and 4d (right).
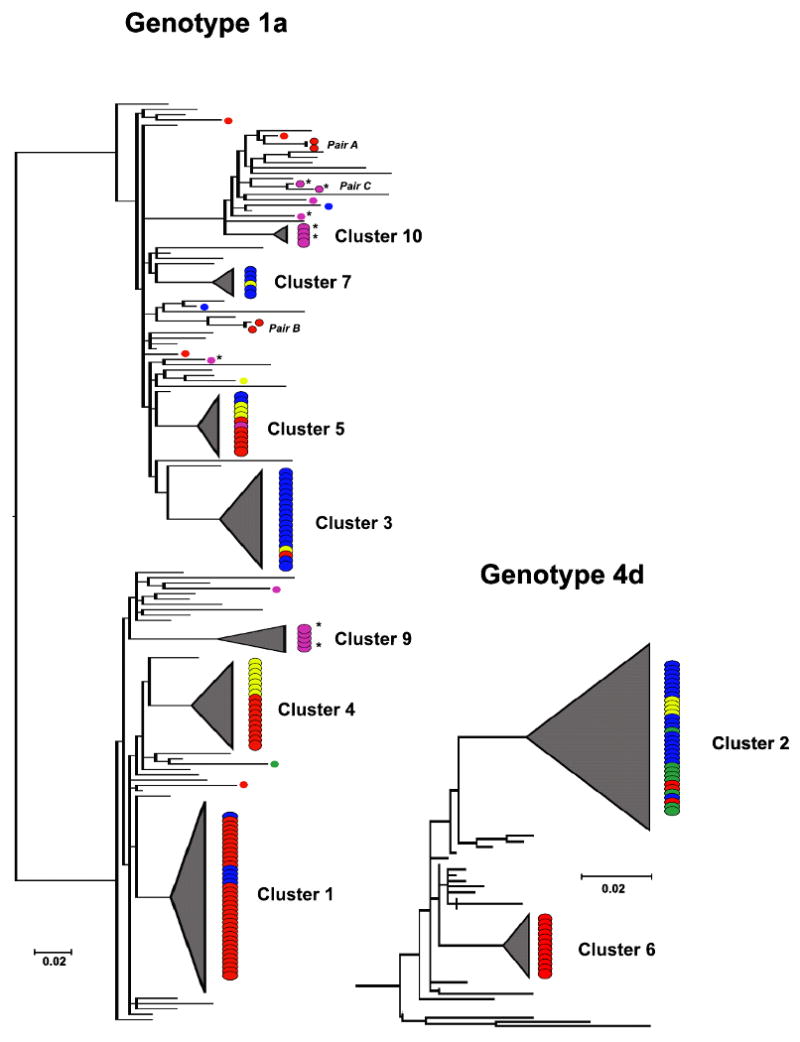
Monophyletic clusters are shaded, country of origin coded: ( ) England, (
) England, ( ) Netherlands, (
) Netherlands, ( ) Germany, (
) Germany, ( ) France, (
) France, ( ) Australia. Australian MSM with reported IDU are marked IDU*
) Australia. Australian MSM with reported IDU are marked IDU*
The phylogenies of the predominant HCV genotypes 1a and 4d demonstrate that the majority of MSM clusters contain sequences from different countries. Country mixing occurred in seven of the eleven clusters and these clusters were significantly larger when compared with country-specific clusters (no country mixing) (17 versus 4.5 sequences; p=0.03) (Table 3). There was a non-statistically significant trend to increased country mixing with increased median cluster size when comparison was made between country-specific clusters, clusters containing isolates from two countries (intermediate degree of country mixing) and clusters containing isolates from three or more countries (high degree of country mixing) (4.5, 11.5 and 19 sequences; p=0.08 by Kruskall-Wallis). This trend was statistically significant when the 6 homologous pairs of MSM-sequences, which represent possible newly evolving clusters, were included in our analysis (2, 11.5 and 19 sequences; p=0.005 by Kruskall-Wallis). Overall, 131/200 (66%) of MSM were infected with an HCV lineage that circulated in more than one country. This percentage increased to 74% (130/176) when Australian MSM were excluded. Although Australian MSM do cluster together, only one Australian MSM was infected with a strain that also circulates in Europe (cluster 5).
For each MSM-specific HCV cluster, we used a Bayesian MCMC approach to calculate the year of the most common recent ancestor in the MSM-community. The calculated year of origin varied from 1975 (cluster 2) to 2001 (cluster 10). Due to the high rate of chronicity and long asymptomatic course of HCV infection, the year of the MRCA does not directly reflect the rate of spread of the virus within a population. Viral spread is reflected by the temporal distribution of lineage splits in each cluster. Except for clusters 8 and 9, the vast majority of lineage splits (85-100%) occurred since 1996, regardless of the year of cluster origin. Overall, the percentage of lineage splits across all clusters decreased from 85% (n=120) since 1996 to 78% (n=111) since 1998 and 63% (n=90) since 2000 (Figure 4).
Discussion
It has been estimated that parenteral risk factors, particularly IDU, account for 80% of HCV transmission in developed countries [9]. However, the increasing number of HIV-positive MSM diagnosed with acute HCV-infection in Europe, Australia and USA suggests that the epidemiology of HCV transmission in this population is changing. Using a phylogenetic approach, our study reveals the existence of a large European MSM-specific transmission network, linking the independently reported national HCV outbreaks in England, the Netherlands, Germany and France. Based on the individual cohorts evaluation of risk factors, which did not form part of this study, the majority of these infections relate to permucosal risk factors in the context of (traumatic) sexual practices [1-3,8]. A second MSM specific transmission network, possibly related to both IDU and sexual behaviour, was identified in Australia. Solely based on phylogenetic evidence, this Australian network at present shows very limited overlap with the network that exists in Europe.
Increased permucosal transmission of HCV implies that HCV is emerging as an STI, which is rapidly affecting an increasing proportion of HIV-positive MSM communities in Europe, Australia and probably the USA. Our molecular analysis revealed 11 robust HCV transmission clusters among HIV-positive MSM in Europe and Australia, which included 86% of the European and 42% of the Australian MSM infections we studied. Such clustering is typical of multiple independent, parallel chains of transmission, each seeded by a single source of infection. Three strong lines of evidence suggest that, once introduced, each strain rapidly spreads to neighbouring countries via a joint transmission network: (i) the large proportion (74%) of European MSM that is infected with an HCV strain that circulates in multiple European countries, (ii) the observed low evolutionary distances among HCV isolates from different countries, and (iii) the fact that the degree of country mixing tends to increase with increasing cluster size. As MSM communities across Europe are large and interconnected, HCV transmission might become endemic among HIV-positive MSM, similar to what has been observed for hepatitis A virus (HAV) and hepatitis B virus (HBV) [13,29]. Recently, an increasing incidence of non-IDU acquired HCV was reported in HIV-negative MSM in Brighton, United Kingdom, raising the possibility of the infection bridging between HIV-positive and HIV-negative MSM populations [30].
Using an evolutionary analysis of HCV lineages circulating among European and Australian MSM, we calculated that the majority (85%) of transmission has occurred since 1996, with 63% of sampled lineage splits occurring after 2000. This observed discontinuity in the course of HCV infection among HIV-positive MSM over time, would imply occasional introduction and transmission of HCV in the MSM population between 1975 and 1996, followed by a more rapid expansion of HCV transmission among HIV-positive MSM since 1996. Interestingly, recent longitudinal cohort studies from London and Amsterdam support our finding of a marked increase in HCV transmission since 2000. Before 2000, HCV-incidence among HIV-positive MSM in both cities did not exceed 1 per 1000 person years (PY) follow-up and, despite occasional introductions in the MSM community, sexual transmission of HCV remained rare before 2000 [8,31]. After 2000, the estimated annual HCV-incidence in HIV-positive MSM attending HIV/genitourinary medicine clinics in London and Brighton has risen by 20% each year, from 7/1000 PY follow-up in 2002 to 12/1000 PY follow-up in the first six months of 2006 [7]. In Amsterdam, HCV incidence rose tenfold to 8.7/1000 PY in the period 2000-2003 compared to 0.8/1000 PY in the period 1984-1999 [8]. Significantly, in 2007 15.5% of HIV-positive MSM attending the Amsterdam STI clinic during an anonymous survey, tested positive for HCV infection [32]. Our evolutionary analysis suggests that there has been a significant change in the pattern of HCV transmission since the late 1990s that has given rise to the spread of HCV in HIV-positive MSM all over Europe.
The reason for a change in HCV transmission pattern since the late 1990s remains unclear, but it probably relates to biological and behavioural factors. It is striking that permucosal transmission of HCV is almost exclusively described in HIV-positive MSM. HIV co-infection might facilitate HCV transmission by increasing both viral infectiousness due to higher HCV viral loads in serum and semen, and viral susceptibility through impaired immunological control of HCV [33,34]. However, HIV-positive MSM diagnosed with acute HCV infection in our study had relatively preserved CD4 counts, with median CD4 counts exceeding 500 cells/μl. Moreover, high rates of HCV acquisition soon after diagnosis with primary HIV infection among MSM in the UK argue for behavioural risk factors rather than a role for HIV-infection itself [35]. Biologically, our data also argue against a change in the virulence of HCV, because the identified co-circulating strains in this population belong to several different genotypes and subtypes. The timescale of the current HCV epidemic coincides with the introduction of HAART, which has been associated with a rise in sexual risk behaviour and increased STI rates among MSM. Less concern about HIV given the availability of HAART and the belief that HAART reduces HIV transmission have been associated with increased sexual risk-taking in MSM [36]. Furthermore, unprotected anal intercourse (UAI) within the context of serosorting (i.e. engaging in sexual risk only with HIV seroconcordant partners) is now considered a ‘harm reduction’ strategy by reducing HIV transmission in those having UAI [37]. However, this practice places these men at increased risk of HIV superinfection and STIs. Ulcerative STIs might potentiate sexual transmission of HCV through mucosal lesions, in particular lymphogranuloma venereum and syphilis have been associated with acute HCV in HIV-positive MSM [1,3]. In a recent case-control study, HCV/HIV co-infected MSM were more like to report UAI, traumatic sexual techniques (e.g. fisting and group sex), a history of STI, and the recreational use of party drugs, compared to HIV monoinfected controls [2]. The popularity of Internet, cheaper international travel, growing MSM tourism and organised sex-parties have probably extended sexual networks and increased the connectivity of high-risk MSM communities in different parts of the world. In a large survey among British residents, 62% of MSM outside a committed relationship reported a sexual partner overseas in the last two holidays. Among MSM, travelling has been associated with increased risk for UAI, especially among those with positive HIV-status [38,39].
Australian sequences lie in two distinct country-specific HCV clusters, as might be expected given its relative geographic separation. In Australia, but also in Europe, it is plausible that HCV initially entered the MSM community through incidental transfer from the injection drug user population. In contrast to the European cases, IDU explains a significant proportion of HCV infections within the Australian MSM population. Importantly, however, MSM reporting IDU clustered with MSM who denied ever injecting, emphasising that parenteral as well as permucosal risk factors contribute to the transmission of HCV among Australian MSM. Interestingly, one Australian MSM was infected with a HCV strain that also circulates in Europe, implying at least some HCV transmission between the two continents [40]. These results highlight the potential of HCV to rapidly infect other MSM populations. Importantly, while small HCV outbreaks among HIV-positive MSM have been observed [4,5], US health authorities have not yet reported spread of HCV on a scale similar to that in Europe or Australia. Phylogenetic analysis of HCV strains circulating in the US would provide valuable information on the global spread of HCV in this population, especially as different European MSM have reported sexual contact in the States during the acute phase of HCV infection [1].
In conclusion, Europe and Australia are currently facing a new epidemic of sexually transmitted HCV, which is rapidly infecting an increasing proportion of HIV-positive MSM through large international transmission networks. The first cases of HCV superinfection and reinfection have already been described [41,42]. HCV/HIV co-infection negatively influences the natural history of HCV [16-18], in particular as HCV is acquired after HIV [19] and at an older age (>40 years) [43]. Therefore, the current epidemic among HIV-positive MSM has serious clinical implications, exacerbated by the high frequency of difficult-to-treat HCV genotypes 1 and 4 in MSM in Europe and Australia. Regional collaborative measures are necessary which should include targeted prevention, education of patients and clinicians, screening and therapy of HCV. Annual HCV antibody screening should become part of the routine care of all HIV-positive MSM, and should be considered for MSM with negative or unknown HIV-status who report high-risk behaviours [44]. In particular, those participating in mucosal traumatic sex practices, group sex, unprotected anal intercourse with casual partners, injecting drug use, and those diagnosed with ulcerative STIs should be more frequently screened. Early detection of HCV will allow early effective treatment and identification of risk factors, which may mitigate ongoing transmission [20]. In immunocompromised individuals with CD4 counts below 200 cells/mm3, HCV RNA testing may be appropriate [44]. If no action is taken, there is a risk that the current HCV epidemic might bridge into the HIV-negative MSM population.
Table 4.
Estimated year of origin for each identified hepatitis C virus (HCV) cluster, and the calculated number of lineage divergent events over time.
| Cluster | Genotype | Number of sequences | Year of origin (95% CI) |
Number (%) of lineage splits since |
||
|---|---|---|---|---|---|---|
| 1996 | 1998 | 2000 | ||||
| 1 | 1a | 37 | 1985 (1975-1993) | 30(83) | 27(75) | 22(61) |
| 2 | 4d | 34 | 1975 (1961-1989) | 28(85) | 26(78) | 21(64) |
| 3 | 1a | 19 | 1993 (1985-1999) | 17(94) | 17(94) | 13(72) |
| 4 | 1a | 17 | 1989 (1978-1998) | 15(94) | 14(88) | 11(69) |
| 5 | 1a | 12 | 1997 (1990-2001) | 11(100) | 10(91) | 9(82) |
| 6 | 4d | 12 | 1996 (1990-2000) | 11(100) | 10(91) | 7(64) |
| 7 | 1a | 6 | 1997 (1989-2003) | 5(100) | 4(80) | 4(80) |
| 8 | 3a | 6 | 1984 (1974-1993) | 0(0) | 0(0) | 0(0) |
| 9 | 1a | 5 | 1984 (1972-1994) | 0(0) | 0(0) | 0(0) |
| 10 | 1a | 4 | 2000 (1995-2004) | 3(100) | 3(100) | 3(100) |
Year of origin and number of lineage splits could not be calculated for HCV cluster 11, as these 4 English MSM were part of a larger paraphyletic cluster that also contained HCV reference strains from Europe and the Unites States.
Acknowledgments
This research was supported by: Special Trustees of Royal Free & University College Medical School Fellowship (MD), Peter Samuel Fellowship (MD), UNSW Faculty Research Grant 2008 (MD), and Health Service Amsterdam; Netherlands Organization for Health Research and Development Grant 912-03-005 (TvdL), National Institutes of Health Grant R01 DA 15999 (GD, GM)
Footnotes
Support and Financial Disclosures: None of the authors have any financial disclosures or conflicts of interest related to the work contained within this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Gotz HM, van Doornum G, Niesters HG, den Hollander JG, Thio HB, de Zwart O. A cluster of acute hepatitis C virus infection among men who have sex with men--results from contact tracing and public health implications. AIDS. 2005;19:969–74. doi: 10.1097/01.aids.0000171412.61360.f8. [DOI] [PubMed] [Google Scholar]
- 2.Danta M, Brown D, Bhagani S, Pybus OG, Sabin CA, Nelson M, et al. Recent epidemic of acute hepatitis C virus in HIV-positive men who have sex with men linked to high-risk sexual behaviours. AIDS. 2007;21:983–91. doi: 10.1097/QAD.0b013e3281053a0c. [DOI] [PubMed] [Google Scholar]
- 3.Serpaggi J, Chaix ML, Batisse D, Dupont C, Vallet-Pichard A, Fontaine H, et al. Sexually transmitted acute infection with a clustered genotype 4 hepatitis C virus in HIV-1-infected men and inefficacy of early antiviral therapy. AIDS. 2006;20:233–40. doi: 10.1097/01.aids.0000200541.40633.56. [DOI] [PubMed] [Google Scholar]
- 4.Luetkemeyer A, Hare CB, Stansell J, Tien PC, Charlesbois E, Lum P, et al. Clinical presentation and course of acute hepatitis C infection in HIV-infected patients. J Acquir Immune Defic Syndr. 2006;41(1):31–6. doi: 10.1097/01.qai.0000191281.77954.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bateman KM, Factor S, Gutierrez JA, Fierer DS, Uriel AJ, Carriero DC, et al. Evidence for sexual transmission ofhepatitis C virus infection in men who have sex with men (MSM) in New York City. 14th International Symposium on Hepatitis C Virus and related Viruses; 9-13th September; Glasgow. 2007. abstract P48. [Google Scholar]
- 6.Matthews GV, Hellard M, Kaldor J, Lloyd A, Dore G. Further evidence of sexual transmission among HIV-positive men who have sex with men; response to Danta et al. AIDS. 2007;21:2112–2113. doi: 10.1097/QAD.0b013e3282ef3873. [DOI] [PubMed] [Google Scholar]
- 7.Giraudon I, Ruf M, Maguire H, Charlett A, Ncube F, Turner J, et al. Increase in newly acquired hepatitis C in HIV positive men who have sex with men across London and Brighton, 2002-2006. Is this an outbreak? Sex Transm Infect. 2007;84:111–115. doi: 10.1136/sti.2007.027334. [DOI] [PubMed] [Google Scholar]
- 8.van de Laar TJ, Van der Bij AK, Prins M, Bruisten SM, Brinkman K, Ruys TA, et al. Increase in HCV incidence among men who have sex with men in Amsterdam most likely caused by sexual transmission. J Infect Dis. 2007;196:230–8. doi: 10.1086/518796. [DOI] [PubMed] [Google Scholar]
- 9.Alter MJ. Prevention of spread of hepatitis C. Hepatology. 2002;36:S93–S98. doi: 10.1053/jhep.2002.36389. [DOI] [PubMed] [Google Scholar]
- 10.Marcus U, Zucs P, Bremer V, Hamouda O, Prager R, Tschaepe H, et al. Shigellosis - a re-emerging sexually transmitted infection: outbreak in men having sex with men in Berlin. Int J STD AIDS. 2004;15:533–7. doi: 10.1258/0956462041558221. [DOI] [PubMed] [Google Scholar]
- 11.Van der Bij AK, Spaargaren J, Morre SA, Fennema HS, Mindel A, Coutinho RA, et al. Diagnostic and clinical implications of anorectal lymphogranuloma venereum in men who have sex with men: a retrospective case-control study. Clin Infect Dis. 2006;42:186–94. doi: 10.1086/498904. [DOI] [PubMed] [Google Scholar]
- 12.Macdonald N, Dougan S, McGarrigle CA, Baster K, Rice BD, Evans BG, et al. Recent trends in diagnoses of HIV and other sexually transmitted infections in England and Wales among men who have sex with men. Sex Transm Infect. 2004;80:492–7. doi: 10.1136/sti.2004.011197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stene-Johansen K, Tjon G, Schreier E, Bremer V, Bruisten S, Ngui SL, et al. Molecular epidemiological studies show that hepatitis A virus is endemic among active homosexual men in Europe. J Med Virol. 2007;79:356–65. doi: 10.1002/jmv.20781. [DOI] [PubMed] [Google Scholar]
- 14.Marcus U, Voss L, Kollan C, Hamouda O. HIV incidence increasing in MSM in Germany: factors influencing infection dynamics. Euro Surveill. 2006;11:157–60. [PubMed] [Google Scholar]
- 15.Danta M, Semmo N, Fabris P, Brown D, Pybus OG, Sabin CA, et al. Impact of HIV on Host-Virus Interactions during Early Hepatitis C Virus Infection. J Infect Dis. 2008;197:1558–1566. doi: 10.1086/587843. [DOI] [PubMed] [Google Scholar]
- 16.Graham CS, Baden LR, Yu E, Mrus JM, Carnie J, Heeren T, et al. Influence of human immunodeficiency virus infection on the course of hepatitis C virus infection: a meta-analysis. Clin Infect Dis. 2001;33:562–9. doi: 10.1086/321909. [DOI] [PubMed] [Google Scholar]
- 17.Greub G, Ledergerber B, Battegay M, Grob P, Perrin L, Furrer H, et al. Clinical progression, survival, and immune recovery during antiretroviral therapy in patients with HIV-1 and hepatitis C virus coinfection: the Swiss HIV Cohort Study. Lancet. 2000;356:1800–5. doi: 10.1016/s0140-6736(00)03232-3. [DOI] [PubMed] [Google Scholar]
- 18.Soriano V, Puoti M, Garcia-Gasco P, Rockstroh JK, Benhamou Y, Barreiro P, et al. Antiretroviral drugs and liver injury. AIDS. 2008;22:1–13. doi: 10.1097/QAD.0b013e3282f0e2fd. [DOI] [PubMed] [Google Scholar]
- 19.Fierer DS, Uriel AJ, Carriero DC, Klepper A, Dieterich DT, Mullen MP, et al. Liver fibrosis during an outbreak of acute hepatitis C virus infection in HIV-infected men: a prospective cohort study. J Infect Dis. 2008;198:683–6. doi: 10.1086/590430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gerlach JT, Diepolder HM, Zachoval R, Gruener NH, Jung MC, Ulsenheimer A, et al. Acute hepatitis C: high rate of both spontaneous and treatment-induced viral clearance. Gastroenterology. 2003;125:80–8. doi: 10.1016/s0016-5085(03)00668-1. [DOI] [PubMed] [Google Scholar]
- 21.Vogel M, Bieniek B, Jessen H, Schewe CK, Hoffmann C, Baumgarten A, et al. Treatment of acute hepatitis C infection in HIV-infected patients: a retrospective analysis of eleven cases. J Viral Hepat. 2005;12:207–11. doi: 10.1111/j.1365-2893.2005.00580.x. [DOI] [PubMed] [Google Scholar]
- 22.van de Laar TJ, Langendam MW, Bruisten SM, Welp EA, Verhaest I, Van Ameijden EJ, et al. Changes in risk behavior and dynamics of hepatitis C virus infections among young drug users in Amsterdam, the Netherlands. J Med Virol. 2005;77:509–18. doi: 10.1002/jmv.20486. [DOI] [PubMed] [Google Scholar]
- 23.Swofford DL, Waddell PJ, Huelsenbeck JP, Foster PG, Lewis PO, Rogers JS. Bias in phylogenetic estimation and its relevance to the choice between parsimony and likelihood methods. Syst Biol. 2001;50:525–39. [PubMed] [Google Scholar]
- 24.Tanaka Y, Hanada K, Mizokami M, Yeo AE, Shih JW, Gojobori T, et al. Inaugural Article: A comparison of the molecular clock of hepatitis C virus in the United States and Japan predicts that hepatocellular carcinoma incidence in the United States will increase over the next two decades. Proc Natl Acad Sci U S A. 2002;99:15584–9. doi: 10.1073/pnas.242608099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pybus OG, Charleston MA, Gupta S, Rambaut A, Holmes EC, Harvey PH. The epidemic behavior of the hepatitis C virus. Science. 2001;292:2323–5. doi: 10.1126/science.1058321. [DOI] [PubMed] [Google Scholar]
- 26.Drummond AJ, Ho SY, Phillips MJ, Rambaut A. Relaxed phylogenetics and dating with confidence. PLoS Biol. 2006;4:e88. doi: 10.1371/journal.pbio.0040088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Oliveira T, Pybus OG, Rambaut A, Salemi M, Cassol S, Ciccozzi M, et al. Molecular epidemiology: HIV-1 and HCV sequences from Libyan outbreak. Nature. 2006;444:836–7. doi: 10.1038/444836a. [DOI] [PubMed] [Google Scholar]
- 28.Morice Y, Cantaloube JF, Beaucourt S, Barbotte L, de Gendt S, Lopes Goncales F, et al. Molecular epidemiology of hepatitis C virus subtype 3a in injecting drug users. J Med Virol. 2006;78:1296–1303. doi: 10.1002/jmv.20692. [DOI] [PubMed] [Google Scholar]
- 29.Van Houdt R, Sonder GJ, Dukers NH, Bovee LP, Van Den HA, Coutinho RA, et al. Impact of a targeted hepatitis B vaccination program in Amsterdam, The Netherlands. Vaccine. 2007;25:2698–705. doi: 10.1016/j.vaccine.2006.06.058. [DOI] [PubMed] [Google Scholar]
- 30.Richardson D, Fisher M, Sabin CA. Sexual Transmission of Hepatitis C in MSM May Not Be Confined to Those with HIV Infection. J Infect Dis. 2008;197:1213–4. doi: 10.1086/533454. [DOI] [PubMed] [Google Scholar]
- 31.Browne R, Asboe D, Gilleece Y, Atkins M, Mandalia S, Gazzard B, et al. Increased numbers of acute hepatitis C infections in HIV positive homosexual men; is sexual transmission feeding the increase? Sex Transm Infect. 2004;80:326–7. doi: 10.1136/sti.2003.008532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Urbanus AT, van de Laar TJW, Schinkel J, Thiesbrummel H, Coutinho RA, Prins M. HCV is emerging as an STI among HIV-infected MSM: a threat to the MSM community?. Internatonal AIDS Conference; 3-8th August; Mexico-City. Poster Discussion A-072-0128-04472. [Google Scholar]
- 33.Mattapallil JJ, Douek DC, Hill B, Nishimura Y, Martin M, Roederer M. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature. 2005;434:1093–7. doi: 10.1038/nature03501. [DOI] [PubMed] [Google Scholar]
- 34.Matthews-Greer JM, Caldito GC, Adley SD, Willis R, Mire AC, Jamison RM, et al. Comparison of hepatitis C viral loads in patients with or without human immunodeficiency virus. Clin Diagn Lab Immunol. 2001;8:690–4. doi: 10.1128/CDLI.8.4.690-694.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fox J, Nastouli E, Thomson E, Muir D, McClure M, Weber J, et al. Increasing incidence of acute hepatitis C in individuals diagnosed with primary HIV in the United Kingdom. AIDS. 2008;22:666–8. doi: 10.1097/QAD.0b013e3282f5f4cf. [DOI] [PubMed] [Google Scholar]
- 36.Crepaz N, Passin WF, Herbst JH, Rama SM, Malow RM, Purcell DW, et al. Meta-analysis of cognitive-behavioral interventions on HIV-positive persons' mental health and immune functioning. Health Psychol. 2008;27:4–14. doi: 10.1037/0278-6133.27.1.4. [DOI] [PubMed] [Google Scholar]
- 37.Parsons JT, Schrimshaw EW, Wolitski RJ, Halkitis PN, Purcell DW, Hoff CC, et al. AIDS. 2005;19:S13–S15. doi: 10.1097/01.aids.0000167348.15750.9a. [DOI] [PubMed] [Google Scholar]
- 38.Mercer CH, Fenton KA, Wellings K, Copas AJ, Erens B, Johnson AM. Sex partner acquisition while overseas: results from a British national probability survey. Sex Transm Infect. 2007;83:517–22. doi: 10.1136/sti.2007.026377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Clift SM, Forrest SP. Factors associated with gay men's sexual behaviours and risk on holiday. AIDS Care. 1999;11:281–95. doi: 10.1080/09540129947929. [DOI] [PubMed] [Google Scholar]
- 40.Cochrane A, Searle B, Hardie A, Robertson R, Delahooke T, Cameron S, et al. A genetic analysis of hepatitis C virus transmission between injection drug users. J Infect Dis. 2002;186:1212–21. doi: 10.1086/344314. [DOI] [PubMed] [Google Scholar]
- 41.Ghosn J, Thibault V, Delaugerre C, Fontaine H, Lortholary O, Rouzioux C, et al. Sexually transmitted hepatitis C virus superinfection in HIV/hepatitis C virus co-infected men who have sex with men. AIDS. 2008;22:658–61. doi: 10.1097/QAD.0b013e3282f4e86f. [DOI] [PubMed] [Google Scholar]
- 42.Jones R, Brown D, Nelson M, Bhagani S, Atkins M, Danta M, et al. Hepatitis C viremia following sustained virological response to pegylated interferon and ribavirin in HIV+ men who have sex with men - Reinfection or late relapse?. 15th Conference on Retroviruses and Opportunistic Infections (CROI); February 3-6; Boston. 2008. Abstract 61LB. [Google Scholar]
- 43.Thomas DL, Astemborski J, Rai RM, Anania FA, Schaffer M, Galai N, et al. The natural history of hepatitis C virus infection: host, viral and environmental factors. JAMA. 2000;284:450–6. doi: 10.1001/jama.284.4.450. [DOI] [PubMed] [Google Scholar]
- 44.Rockstroh JK, Bhagani S, Benhamou Y, Bruno R, Mauss S, Peters L, et al. European AIDS Clinical Society (EACS) guidelines for the clinical management and treatment of chronic hepatitis B and C coinfection in HIV-infected adults. HIV Med. 2008;9:82–8. doi: 10.1111/j.1468-1293.2007.00535.x. [DOI] [PubMed] [Google Scholar]


