Abstract
The presence of Crimean-Congo hemorrhagic fever virus (CCHFV) in Iran was first identified in studies of livestock sera and ticks in the 1970s, but the first human infection was not diagnosed until 1999. Since that time, the number of cases of CCHF in Iran has markedly increased. Through January 2012, articles in the published literature have reported a total of 870 confirmed cases, with 126 deaths, for a case fatality rate (CFR) of 17.6%. The disease has been seen in 26 of the country’s 31 provinces, with the greatest number of cases in Sistan and Baluchestan, Isfahan, Fars, Tehran, Khorasan, and Khuzestan provinces. The increase in CCHF in Iran has paralleled that in neighboring Turkey, though the number of cases in Turkey has been much larger, with an overall CFR of around 5%. In this article, we review the features of CCHF in Iran, including its history, epidemiology, animal and tick reservoirs, current surveillance and control programs, diagnostic methods, clinical features and experience with ribavirin therapy, and consider possible explanations for the difference in the CFR of CCHF between Iran and Turkey. The emergence of CCHF in Iran calls for countermeasures at many levels to protect the population, but also provides opportunities for studying the epidemiology, diagnosis and management of the disease.
I. Introduction
Crimean-Congo hemorrhagic fever (CCHF) is a tick-borne disease caused by a virus in the genus Nairovirus, family Bunyaviridae, which is endemic in many countries in Africa, southeastern Europe and Asia. The disease was first described in the Crimea in 1944 and given the name Crimean hemorrhagic fever. In 1969, it was recognized that the same pathogen was responsible for an illness identified in 1956 in the Congo; linkage of the two place names resulted in the current designation of Crimean-Congo hemorrhagic fever virus (CCHFV). CCHF patients may develop either a mild, nonspecific febrile syndrome or a more severe illness with vascular leak, hemorrhage and shock. Most cases occur sporadically, as a result of tick bite or direct contact with the blood or tissues of an infected animal.
CCHFV circulation in Iran was first demonstrated in the early 1970s, through the detection of virus-specific antibodies in serum samples from livestock and humans (Chumakov, 1972; Saidi, 1974), but the first case of CCHF in a patient was not diagnosed until 1999 (Table 1). Since then, there has been a marked increase in human infections, with 870 confirmed cases reported by the end of 2011.
Table 1.
Published studies reporting cases of CCHF in Iran since 1999.
| Years | Location | Number of cases | Summary | Possible mode of transmission | Reference |
|---|---|---|---|---|---|
| 1999–2004 | Sistan and Baluchestan | 255 patients | Epidemiologic, laboratory and clinical description | Animal contact, nosocomial, tick bite, slaughtering | (Alavi-Naini, et al., 2006) |
| 2001–2004 | Fars, southern Iran | 16 patients, 3 deaths | Epidemiologic, clinical and laboratory data are described. | Slaughtering, animal contact, tick bite | (Fakoorziba, et al., 2006) |
| 1999–2007 | Sistan and Baluchestan | 123 patients, 19 fatalities | N/A | (Sharifi-Mood, et al., 2009) | |
| 1999–2001 | Shahrekord (central Iran) | 3 healthcare workers, 1 death | Nosocomial | (Mardani, et al., 2009) | |
| 2000–2009 | All involved provinces | 635 patients, 89 fatalities | Risk factors and geographic distribution | Slaughtering, animal contact, nosocomial | (Chinikar, et al., 2010) |
| 2009 | Mashhad (north-eastern Iran) | 6 healthcare workers, 2 deaths | Nosocomial | (Naderi et al., 2011) | |
| 2011 | Khorasan (north-eastern) Province | 1 healthcare worker, fatal | A medical student who died within one week of exposure. | Nosocomial | (Naderi, et al., 2012) |
In this article, we review the features of CCHF in Iran. We first examine the older literature for evidence that the disease had been preset in the country long before the first case was diagnosed. We then review the history of confirmed CCHF cases since 1999; the clinical features, diagnosis and management of the disease, including ribavirin; detection of virus in animal and tick reservoirs; and current surveillance and control programs. We then consider possible explanations for the difference in the CFR of CCHF between Iran and Turkey, and conclude by identifying important questions for future research.
II. Early history of diseases consistent with CCHF
Although the first case was only diagnosed in 1999, it seems likely that CCHF has occurred in Iran for hundreds, perhaps thousands of years. The oldest known reference to a hemorrhagic febrile illness in Persian medical textbooks dates to the Zakhīra-yi Khrāzmshāhī, written by Jurjānī in the late 12th century (Table 2) (Jurjani, 1203). Under the heading of different animal bites, he states “The vulture louse… is a creature similar to a louse and to a tick, and it is extremely small. Galen states that it is so small that one can not escape it, and it is difficult to see. However, the harm caused by its bite is quite large. There is bleeding from the nose, bladder, rectum, and teeth. There is hematemesis from the stomach and hemoptysis from the lungs, and once it is out of control no treatment will cure it.” Although it has been claimed that this is the oldest description of CCHF (Arda and Aciduman, 2007; Hoogstraal, 1979), Jurjānī’s description is actually identical to that given earlier by Galen, as cited in (Albertus Magnus, 1260). The description might also be consistent with other hemorrhagic fevers in the region. We were not able to find any other references to hemorrhagic fevers in the available works of Tabari, Razi, Majusi, Ibn Sina or other early Persian physicians.
Table 2.
History of Crimean-congo hemorrhagic fever in Persia/ Iran.
| Date | Description | Comment | Reference |
|---|---|---|---|
| 1203 CE | Detailed description of hemorrhagic fever and its putative causative agent (vulture louse) | Description identical to Galen’s, thus may not be specific to CCHF | (Jurjānī, 1203 CE) |
| 1887–1888 | Description of a fatal hemorrhagic disease among the nomadic Yomut Turkomen in northern Iran | Likely CCHF, but key details, such as fever and season, are missing | (Brown, 1893) |
| 19th century | Reports of a sometimes fatal disease though to be caused by Argas persicus in the Mianeh region in NW Iran | Unlikely to be CCHF, though some clinical features suggestive | (Nuttal, 1908) |
| 1940’s–1960’s | Seasonal and sometimes fatal hemorrhagic fever known locally as Gara Mikh typhoid fever in East Azerbaijan, Iran. | Clinical and epidemiologic features consistent with CCHF. | (Aminolashrafi and Nooranian, 1966) |
| 1966–69 | Report of 41 cases of hemorrhagic fever from East Azerbaijan, Iran. | Possible CCHF outbreak | (Aminolashrafi, 1970) |
| 1970–71 | Sheep serum sent tested positive for CCHFV antibodies. | First documentation of CCHFV in livestock | (Chumakov, 1972) |
| 1971–3 | Report of 60 cases of hemorrhagic fever from East Azerbaijan, Iran. | First suspected cases of CCHF in humans. | (Asefi, 1973) |
| 1970–1971 | Sera of humans in northern Iran tested positive for anti-CCHFV antibodies | First documentation of CCHFV infection | (Saidi, 1974) |
| 1974–1975 | Hemorrhagic fever epidemic in northern Iran | Suspected CCHF, but not proven | (Ardoin and Karimi, 1982) |
| 1999 | Nosocomial transmission of CCHF | First confirmed cases of CCHF in Iran | (Mardani, 2001) |
In the more recent literature, a disease consistent with CCHF was described in Edward Brown’s account of his stay in Persia from 1887–8, when he was present at a meeting describing an outbreak of fatal hemorrhagic disease among the Yomut Turkomen in the north of the country (Brown, 1893). No further details are available; given the limited clinical information, the differential diagnosis might also include leptospirosis. In the 19th and 20th centuries, there were also reports of an occasionally fatal disease transmitted by Argas persicus ticks (Nuttal, 1908). Many different syndromes were ascribed to the bite of this tick, from intense itching to a rickettsia-like eschar and fever, sometimes ending in death, but we did not find any accounts of hemorrhagic disease. In 1966, 9 cases of a febrile, hemorrhagic disease occurred in villages near Sarab in East Azerbaijan, in the northwest of Iran (Aminolashrafi and Nooranian, 1966). The physicians suspected a viral hemorrhagic fever, and noted that they had seen similar cases since 1963. The illness was characterized by 3–4 days of fever, followed by petechial hemorrhages, purpura and bleeding from the gums or nose that sometimes led to internal bleeding and death. Laboratory studies revealed thrombocytopenia and leukocytosis. An epidemiologic study revealed an additional 41 cases, with 2 deaths (Aminolashrafi, 1969). Local residents said the disease had occurred since the late 1940s, typically between April and November; it was known as Ghara Mikh typhoid fever, named after the characteristic black petechiae. The authors suspected that the disease was transmitted from mice, rabbits or cattle by ticks (O. Laboransis or O. Tholozani). Another 60 cases of a similar disease, suspected at the time to be CCHF, were described in 1971–3 in northwestern Iran (Asefi, 1973). The case fatality rate (CFR) was 5%.
In 1974–75, a hemorrhagic fever epidemic was again noted in a rural province in northwest Iran, with a particularly high incidence in July (Ardoin and Karimi, 1982). A tick-borne virus or a toxic etiology was suspected, but neither was proved. Despite awareness of tick reservoirs and serologic evidence of human infection, there was little research and no reports of new cases during the next several decades, possibly due to the turmoil of the 1979 Iranian Revolution and the 1981–88 war with Iraq. Although it is possible that new cases did not occur, it is more likely that CCHF patients were misdiagnosed.
III. Cases of CCHF in Iran since 1999
The first confirmed case of CCHF in Iran was diagnosed in August, 1999, when a patient died of severe gastrointestinal bleeding at a hospital in the central part of the country. During the course of his medical care, he coughed and splashed blood on the face of a physician trying to insert a nasogastric tube. Two weeks later, the doctor became mildly ill, and he subsequently tested positive for CCHF by IgG. At the same time, his wife developed intermittent fever, vomiting, diarrhea and vaginal bleeding. Her physical examination and laboratory values on hospital admission were strongly suggestive of a viral hemorrhagic fever. She died on day 6 of her illness, and following her death a blood sample tested positive for CCHFV by PCR. A subsequent investigation identified more individuals who had been exposed to the patients, but did not become ill (Mardani, 2001).
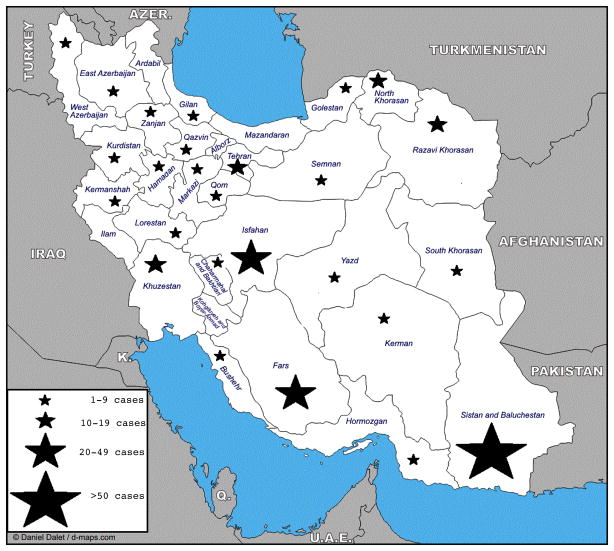
Since those first recognized cases, numerous papers have appeared in the medical literature describing human infections with CCHFV in Iran (Table 1). These studies describe different modes of transmission including animal contact, tick bite, nosocomial and occupational exposure among the general population and healthcare workers in different parts of Iran. Tick bites occurring during animal contact are the most common mode of virus transmission. Since 1999, CCHF has been reported in 26 of Iran’s 31 provinces, with the most cases in Sistan and Baluchestan, Isfahan, Fars, Tehran, Khorasan, and Khuzestan. Only 5 provinces (Mazandaran, Ardabil, Ilam, Kohgiluyeh and Boyer-Ahmad, and Alborz) have not reported human infections, but at least two of them are known to harbor CCHFV in cattle and ticks (Figure 1) (Chinikar et al., 2012). As discussed below, Hyalomma ticks have been found in all habitable regions (Nabian et al., 2009); acquisition of CCHF is therefore theoretically possible throughout the country.
Figure 1.
Total number of cases of CCHF reported by each province in Iran, over the period 2000–12. Data were obtained from the Pasteur Institute of Iran and from (Chinikar et al., 2012).
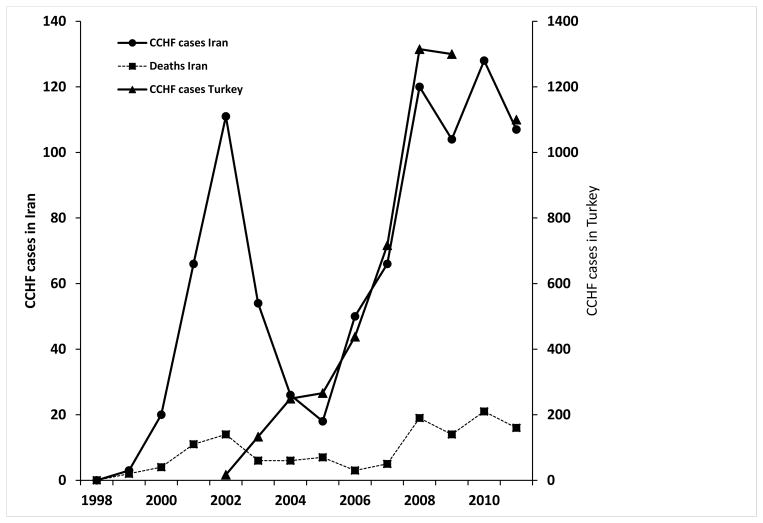
The most recent cumulative data show a total of 870 confirmed cases of CCHF in Iran, with 126 deaths from June, 2000 through January, 2012 (Chinikar et al., 2012). Forty-eight cases and 3 deaths have occurred in children (Chinikar et al., 2011). The graph of annual reported cases shows an initial rise from 1999–2002, followed by a drop from 2003–5, then an increase and possible plateau in the number of cases (Figure 2). It was at one time thought that the initial wave was caused by sudden awareness of the problem of CCHF by the public health system, and that the subsequent decline had been brought about through preventive and control measures (Chinikar et al., 2008). However, the increase in cases and fatalities since 2005 has challenged those conclusions.
Figure 2.
Total number of confirmed CCHF cases in Iran and Turkey reported each year for the period 2000–12, with the total number of fatal cases in Iran. Data were obtained from the Pasteur Institute of Iran and from (Chinikar, et al., 2012; Yilmaz et al., 2008; Ergonul, 2009; Maltezou et al., 2010; Burki, 2012).
IV. Clinical features, diagnosis and management
A. Clinical syndrome
CCHF occurs in four phases: incubation, prehemorrhagic, hemorrhagic and convalescent (Hoogstraal, 1979). After an incubation period of 1–9 days, the patient presents with a nonspecific febrile syndrome that typically lasts less than one week. In some cases, the hemorrhagic phase develops rapidly, beginning between days 3–5 of illness. Circulatory shock and disseminated intravascular coagulation (DIC) occur in severe cases (Mardani et al., 2003).
B. Diagnosis
A diagnosis of CCHF is suspected on the basis of clinical, epidemiologic and laboratory criteria, including: signs and symptoms including fever, muscle pain and bleeding; history of tick bite, travel to or residence in provinces with the most CCHF cases, contact with persons suspected to have the disease, or exposure to animals known to harbor the virus; and suggestive laboratory data, including a platelet count <150,000/mm3 and a total white blood cell count <3000 or >9000/mm3 (Mardani et al. 2003). Confirmed cases are those who are positive for CCHFV by RT-PCR, or have anti-CCHFV IgM or a four-fold increase in specific IgG (Chinikar et al., 2008; Keshtkar-Jahromia et al., 2011). However, an antibody response may not be detected in severely ill patients.
Since 2000, the Pasteur Institute’s Laboratory of Arboviruses and Viral Hemorrhagic Fevers has tested specimens from suspected CCHF patients and from animals. Human samples are submitted in three phases. When a patient is first identified as a suspected case, serum is collected and tested for the S-segment of the virus genome by RT-PCR and for anti-CCHFV IgM and IgG by ELISA. If the first sample was negative and the patient is still alive 5–10 days after disease onset, a second sample is tested by ELISA for anti-CCHF IgM and IgG. If that sample is negative, a third sample is collected on day 10–15 and tested again by ELISA for anti-CCHF IgM and IgG (Chinikar et al., 2008). Acute infection is diagnosed on the basis of either a positive RT-PCR, positive anti-CCHFV IgM or 4 fold-increase in anti-CCHFV IgG. All samples are processed free of charge, and the results are followed by the CDC for the appropriate response and patient follow-up.
C. Treatment
Treatment of CCHF in Iran follows World Health Organization (WHO) guidelines (Mardani, 2003). In suspected cases, the patient is treated with an initial loading dose of 30 mg/kg of oral ribavirin, followed by 15 mg/kg every 6 hours for 4 days, then 7.5 mg/kg every 8 hours for 6 days, for a total of 10 days. Patients with nausea and vomiting are given ribavirin by nasogastric tube. Because the drug was not available, the cases diagnosed in 1999 did not receive ribavirin. Since 2000, Iran’s CDC has supervised the timely diagnosis and treatment of suspected cases until they have the final diagnosis.
D. Patient isolation
Patients with suspected CCHF are isolated in a private room with negative-pressure respiratory isolation, if available, but because the disease most commonly occurs in rural areas with limited facilities, airborne isolation is not possible in the majority of cases. Transfer of patients to other facilities is discouraged, because of the risk of disease transmission, unless a higher level of patient care is needed. Contact isolation with gloves and gown; droplet protection by face shield; and respiratory isolation with an N95 mask are recommended for everyone entering the patient area.
E. Postexposure prophylaxis
Oral ribavirin, 200 mg twice daily for 5 days, is recommended for those who have had mucous membrane exposure, including kissing or sexual contact, with a CCHF patient or a percutaneous injury involving body fluids. Prophylaxis is also recommended for those involved in patient care before isolation measures are initiated. Exposed health care providers are placed under medical surveillance and instructed to record their temperature twice daily for two weeks. Treatment with oral ribavirin is recommended if they develop a fever of 38.3° C or higher; however, no data have been reported on its efficacy.
V. Experience in Iran in treating CCHF with oral ribavirin
When the first case of CCHF was identified in Iran in 1999, fear of an expanding outbreak led to the use of potentially useful medical treatments and supportive measures. At that time, the published literature on ribavirin therapy of CCHF was limited to an observational study in South Africa (van de Wal et al., 1985) and another in Pakistan (Fisher-Hoch et al., 1995). Based on these reports and WHO recommendations for the treatment of viral hemorrhagic fever, the NECVHF decided to include ribavirin as part of treatment for patients suspected of having CCHF. Patients were given a full 10-day course unless the diagnosis was ruled out.
The results of ribavirin treatment from 1999–2002 were published in 2003 (Mardani et al., 2003). Fatality rates were compared among 139 suspected and 69 confirmed CCHF patients, based on oral ribavirin treatment. For those with confirmed infection, the survival rate was 69.8% for treated and 41.7% for untreated patients, while for those with suspected disease, the rates were 88.4% and 54.2%, respectively. The overall efficacy of ribavirin treatment was 80% among confirmed and 34% among suspected cases. The drug’s lower efficacy among suspected patients presumably reflected the fact that many of them did not have CCHF.
Since that time, ribavirin has been a standard part of treatment of CCHF in Iran, and multiple studies have been conducted to evaluate its efficacy (Table 3). At least 679 confirmed cases have been described in reports published since 1999, with an overall mortality rate of 17.7%. Because the vast majority of these patients appear to have received ribavirin, there are not enough untreated cases to determine a baseline fatality rate.
Table 3.
Experience with the treatment of CCHF with ribavirin in Iran from 1999–2011. In Column 2, some studies compared the outcome of cases treated with ribavirin, beginning at different time points in the disease course. Column 3 lists the number of patients in each study who were treated with ribavirin and the total number of confirmed cases of CCHF. Column 4 lists the number of deaths among patients who were treated with ribavirin.
| Years | Study type | Treated/confirmed | Deaths among treated cases | Province | Reference |
|---|---|---|---|---|---|
| 1999–2001 | Historical comparison | 61/69 | 8/61 (13.1%) | All involved provinces | (Mardani et al., 2003) |
| 1999–2004 | Historical comparison | 236/255 | 37/236 (15.7%) | Sistan and Baluchestan | (Alavi-Naini et al., 2006) |
| 2000–2005 | Comparison to evaluate | 184/184 | 38/184 (20.65 %) | Sistan and Baluchestan | (Metanat et al., 2006) |
| 2000–2006 | Comparison to evaluate timing | 63/63 | 16/63 (25.4%) | Sistan and Baluchestan | (Izadi et al., 2009) |
| 2004–2006 | Observational | 6/6 | 0/6 (00.0%) | Golestan | (Jabbari et al., 2006) |
| 2009 | Observational | 6/6 | 2/6 (33.3%) | Khorasan | (Naderi et al., 2011) |
| Total of all 7 studies | 679/706 | 120/679 (17.67%) |
The efficacy of ribavirin in the treatment of CCHF continues to be a matter of debate (Keshtkar-Jahromi, et al., 2011; Koksal, et al., 2010; Duygu, et al., 2012). We and others have observed that the outcome is better in those who receive the drug earlier in the disease course (Izadi, et al., 2009). The relatively low total numbers of CCHF cases, coupled with ethical concerns in designing a randomized, placebo-controlled trial (Arda, et al., 2012) likely means that this issue will not be resolved in the near future. Other approaches, such as the use of CCHF immune globulin, have not yet been studied in Iran. Reports from Turkey and Bulgaria have shown its possible effectiveness, though these studies did not include control groups (Kubar, et al., 2011; Vassilenko, et al., 1990).
The Iranian experience of CCHF is large enough to support a basic epidemiological study of risk factors for fatal infections, led by the NECVHF. Factors that could be studied include quality control and independent testing of the ribavirin used, strain-specific virologic or virulence factors, host genetic polymorphism and comparison of different supportive care measures. In terms of the latter, given the continued high CFR, active measures should be undertaken to safely transfer patients from rural areas to larger medical centers, rather than keeping them isolated and potentially inadequately treated.
VI. Detection of the circulation of CCHFV in Iran
A. Serosurveys
The presence of CCHFV in Iran was first demonstrated when antibodies to the virus were detected in serum samples from sheep that were sent from Tehran to Moscow for testing (Table 2) (Chumakov, 1972). Screening of 580 serum samples collected in several regions of the country detected antibodies in 49% of sheep and 19% of camels, but human specimens were negative. A subsequent study of serum samples from preschool children in the Caspian Sea area in 1970–71 found that 4% were positive (Saidi, 1974). Serological studies of humans and cattle in 1974 clearly showed that CCHFV was endemic in Iran, with 45 of 351 serum samples from men near the Caspian Sea and in East Azerbaijan province positive for CCHFV antibodies (Saidi et al., 1975).
The extent of animal infection with CCHFV in Iran has been further investigated since 1999. Of nearly 5000 serum samples from livestock in high-risk areas sent to the National Reference Laboratory for testing from June, 2000 through December, 2009, more than 2000 were positive for anti-CCHFV IgG (Chinikar et al., 2010). Localized studies have shown variable results. In a recent investigation, sera from 5.9% of 876 dairy cattle in 16 cities in Razavi Khorasan, Southern Khorasan, Chaharmahal Bakhtiari, Sistan and Baluchestan and Semnan provinces were positive by ELISA (Lotfollahzadeh et al., 2011). Antibody prevalence was 3.7% in desert areas, but increased to 9% in regions with a steppe climate. Interestingly, even though the province of Sistan and Baluchestan has the most cases of CCHF, there was a lower antibody prevalence in cattle. In Ardabil, in the northwest of the country, 39% of 56 serum samples from sheep, cattle and goats were positive for anti-CCHFV IgG (Telmadarraiy, et al., 2010), while a serosurvey of sheep in Mazandaran showed that only 3.7% were positive (Mostafavi et al., 2012). The role of small mammals in the transmission of CCHFV in Iran has not yet been adequately studied.
The human seroprevalence rate of anti-CCHFV IgG in the Sistan and Baluchestan province in 2000–04 was 2.4% (7/297 samples). All seropositive subjects, but only 56% of seronegative subjects, had a history of animal contact within the previous 12 months (Izadi et al., 2006). However, this study suffers from small sample size and lack of representation of the entire study area.
B. Recovery of CCHFV from ticks
Ecologic studies have shown that hard (ixodid) ticks of the genus Hyalomma are the primary vector for transmission of CCHFV, but the virus has also been isolated from members of the genera Rhipicephalus and Amblyomma (Tahmasebi et al., 2010, Zeller et al., 1997, Bursali et al., 2011). Although CCHFV has also been detected in soft ticks of the genera Argasidae and Ornithodoros, there is no evidence that these species are competent vectors; the detection of virus in a soft tick simply indicates that it has recently fed on a viremic vertebrate (Turell, 2007, Durden et al., 1993).
In Iran, CCHFV has been recovered from both hard and soft ticks (Table 4). The virus was first isolated in 1978 from ticks of the species Ornithodoros lahorensis (Sureau and Klein, 1980). In a 2010 report on ticks collected in Ardabil province, RT-PCR was positive for CCHFV in 26.2% of hard ticks, including the species Rhipicephalus bursa (17.5%) and members of the Hyalomma genus (8.7%), and was positive in 100% of soft ticks, which were all of the species Ornithodoros lahorensis (Telmadarraiy et al., 2010). In a study of ticks collected from livestock in Hamadan province in Iran, 19.2% were positive by RT-PCR (16.32% of Hyalomma detritum, 18.18% of H. anatolicum, 55% of Rhipicephalus sanguineus, and 100% of Argas reflexus) (Tahmasebi et al., 2010). No Ornithodoros ticks were positive. In another study of ixodid ticks recovered from sheep, goats and cattle in the Sanandaj and Kamiaran districts of Kurdistan, 70% were members of the Hyalomma genus (Fakoorziba et al., 2012). Of 90 adult ticks tested by RT-PCR, 5 Hyalomma ticks were positive for CCHFV.
Table 4.
Species of ticks from which CCHFV has been recovered in Iran. As discussed in the text, only Hyalomma spp. and certain other species of ixodid (hard) ticks are considered to be competent vectors for the virus.
| Date | Tick | Location | Virus detection | Reference |
|---|---|---|---|---|
| 1978 | Ornithodoros lahorensis | Khorasan | Virus isolated for the first time in ticks in Iran | (Sureau and Klein, 1980) |
| 2004–2005 | Rhipicephalus bursa Hyalomma genus Ornithodoros lahorensis | Ardabil | Ticks were tested by RT-PCR | (Telmadarraiy et al., 2010) |
| 2007 | Hyalomma genus Rhipicephalus sanguineus Argas reflexus | Hamadan | Tested by RT-PCR | (Tahmasebi et al., 2010) |
| 2007 | Hyalomma genus | Kurdistan | Tested by RT-PCR | (Fakoorziba et al., 2012) |
VII. Disease surveillance
In response to the recognition of CCHF in 1999, the Iranian Center for Disease Control (CDC), the Pasteur Institute and the National Veterinary Organization formed the National Expert Committee of Viral Hemorrhagic Fevers (NECVHFs). Comprised of clinicians, epidemiologists, entomologists, veterinarians and virologists, this committee is dedicated to identifying and investigating CCHF cases and is responsible for surveillance and prevention programs.
Activities are carried out at two levels. At the national level, the committee is primarily involved in developing guidelines for case identification, treatment, prevention, case investigation and data collection. Activities at Level II comprise the local response to CCHF, primarily through universities and health centers, involving diagnosis and treatment, isolation measures for suspected cases, prevention of secondary transmission and reporting of new cases to the CDC through a detailed questionnaire. The committee also holds workshops and other training and education programs for persons at high risk of infection, including health care workers, laboratory technicians, persons in contact with livestock and the general population of endemic areas. Guidelines for disease identification and treatment have been sent to all universities and public health systems in Iran, with a special focus on regions with the highest case numbers.
Suspected or confirmed CCHF is a reportable disease in Iran, and physicians are required to complete a surveillance form and submit it the CDC. The form includes demographic data, date of first symptoms, epidemiologic risk factors, clinical signs and symptoms, laboratory findings, description of ribavirin treatment, and disease outcome. The CDC maintains an active database for research and patient follow-up.
VIII. Control of CCHFV in ticks and livestock
Since 2000, in an effort to prevent human exposure to infected ticks, all livestock in industrial breeding centers have been treated with insecticide showers. It is currently recommended that animals should be treated two weeks before slaughter, but no strict guidelines exist. Workers in industrial slaughterhouses are educated about routes of virus transmission from animals to humans, and are required to wear gloves, goggles and plastic gowns when in contact with animals, their blood or a freshly dead carcass. However, local or traditional slaughtering activities employ few or no preventive measures (Chinikar, 2007). No studies have examined the efficacy of control methods such as insecticide use or compared industrial breeding centers to traditional centers.
Because CCHF has been reported in most of Iran’s neighboring countries, the control of imported livestock may play an important role in reducing disease incidence (Chinikar et al., 2010). However, providing animal quarantine along some borders, such as that with Afghanistan, is not practical, because most animal transport occurs illegally.
Guidelines for the prevention of CCHF have recently been described, based on a 5-level stratification of the risk of disease acquisition (Mertens, M., et al, 2013). These guidelines recommend control of tick populations and of the size of wildlife populations (highest level) for countries with endemic areas of CCHF.
IX. Genetic diversity of CCHFV in Iran
Phylogenetic analysis of all known strains of CCHFV has shown that they can be divided into 7 lineages, which have been given different names by different investigators (Supplementary Figure 1) (Deyde et al., 2006; Mild et al., 2010; Han and Rayner, 2011). Viruses recovered in Iran fall into 4 of the 7 groupings, the broadest genetic diversity of any country (Mild et al., 2010). These will be briefly reviewed by genotype (Table 5).
Table 5.
Genetic diversity of CCHFV isolated from ticks, livestock and humans in Iran. See also Supplementary Figure 1 for the locations of these isolates in a phylogenetic tree of the viral S segment (Mild et al., 2010).
| Genotype | Year | Comment | Reference |
|---|---|---|---|
| 1 (Africa 1) | 2002 | The dominant isolate in Iran in the 2000s. | (Chinikar et al., 2004; Mild et al., 2010) |
| 2007 | (Tahmasebi et al., 2010) | ||
| 2008 | Likely circulating in Iran. | (Chinikar et al., 2012) | |
| 2 (Asia 2) | 2002 | Only isolate of this strain from Iran. | (Chinikar et al., 2004; Mild et al., 2010) |
| 4 (Europe 1) | 2002 | (Chinikar et al., 2004; Mild et al., 2010) | |
| 2012 | Currently circulating in Iran. | (Mahzounieh et al., 2012) | |
| 7 (Africa 1) | 1978 | Only isolate of this strain from Iran. | (Sureau and Klein, 1980; Mild et al., 2010) |
Genotype 1 (Asia 1)
When intensive study of CCHFV began in Iran following the cluster of cases in 1999, the first isolates to be reported were found to resemble the Matin strain from Pakistan (Chinikar et al., 2004; Mild et al., 2010), a finding compatible with the large number of cases seen in the Sistan and Baluchestan province, which borders Pakistan. In 2007, a study of viruses isolated from ticks on sheep in Hamadan found that all but one strain was related to Genotype 1 (Asia 1) (Tahmasebi et al., 2010). In 2008, viruses recovered from ticks in Isfahan were also shown to be related to genotype 1, mostly to Pakistani strains, with one related to an Iraqi strain (Chinikar et al., 2012).
Genotype 2 (Asia 2)
One of the viruses reported in 2002 was related to CCHFV strains from Central Asia and China (Mile et al., 2010).
Genotype 4 (Europe 1)
One of the isolates reported in 2002 fell within the genotype 4 clade, resembling strains circulating in Turkey in 2004–7 (Mild et al., 2010). Recently, it was found that viruses recovered from sheep in the Chaharmahal Bakhtiari province were also closely related to genotype 4 strains seen in Turkey, specifically Turkish group II (Mahzounieh et al., 2012). The authors do not state the exact year they obtained the samples. Another recent publication described a genotype 4 strain from a patient in northern Iran (Chinikar et al., 2012).
Genotype 7
The first virus isolated in Iran in 1978 (ArTeh 193-3) is classified in genotype 7 (Africa 1), a clade which also contains viruses isolated in Senegal in 1969, in Mauritania in 1988 and in South Africa in 1989 and 1998 (Mild et al., 2010).
The broad genetic diversity of CCHFV in Iran reflects the variety of viruses found in neighboring countries. To the southeast, Iran shares genotype 1 (Asia 1) strains with Pakistan and Afghanistan, while genotype 2 (Asia 2) strains are found to the northeast, in Central Asia, and to the northwest Iran shares a border and genotype 4 (Europe 1) viruses with Turkey. Considering that the incidence of CCHF has increased in parallel in Turkey and Iran during the past decade, it is tempting to attribute the surge in cases in Iran to genotype 4 (Europe 1) strains, but data are still lacking to test this hypothesis.
X. Possible ecological basis of the increase in CCHF in Iran
The ecology and epidemiology of CCHF in Iran are only beginning to be understood. Turkey, Iran’s neighbor to the northwest, has seen a dramatic increase in the number of human infections since 2008, but the causes of this increase are not clear. While there is a cumulative temperature requirement for molting of Hyaloma ticks, climate change appears to be only one of many factors (Estrada-Peña et al., 2010, Purnak et al., 2007). Alterations in habitat and in small mammal populations, migratory birds, and other factors are also being investigated in studies focusing on the potential for CCHF to spread within Europe (Estrada-Peña et al., 2010, Gale et al., 2010, Estrada-Peña et al., 2012, Randolph and Rogers, 2007, Estrada-Peña et al., 2013, Gale et al., 2012, Maltezou and Papa, 2010).
It is noteworthy that the increase in CCHF cases in Turkey in 2008, followed by a leveling-out of case numbers, mirrored what occurred in Iran during the same period (Figure 2). It should also be noted that cases in Iran were first identified in 1999 and increased through 2002, when the first patient was recognized in Turkey (Ergonul et al., 2004, Gozalan et al., 2004). Although Iran has not been included in studies of the ecology and epidemiology of CCHF, the apparently simultaneous increase in cases across such distant and different geographic areas raises questions about CCHFV transmission and the conclusions of epidemiologic studies that have focused only on Turkey. Surprisingly, regions of northwestern Iran that most closely resemble Turkey do not have the highest rates of infection in the country.
Sistan and Baluchestan province, the region with the largest numbers of cases of CCHF in Iran, borders Pakistan in the southeast and is sparsely populated. Its desert terrain has a completely different ecosystem than northern Iran or Turkey, but Hyalomma ticks harboring CCHFV are present (Mehravaran et al., 2012). Unfortunately, until now there have been no comprehensive studies of the life cycle of CCHFV in that province. Further investigation of factors including changes in ecologic factors such as rainfall, humidity and temperature across a wide range of areas may be still be useful, despite the lack of findings from previous studies focusing on Africa and Europe (Estrada-Peña, 2008). Studies of the unique ecological niche of Sistan and Baluchestan could provide new information about the establishment, persistence, and transmission of CCHFV.
XI. Difference in case fatality rate between Iran and Turkey
The CFR of confirmed cases of CCHF in Iran appears to be several times higher than the rate of 5% seen for untreated cases in Turkey (Koksal et al., 2010, Duygu et al., 2012). This difference, and indeed the wide range in CFR reported in various endemic countries, is somewhat puzzling. It could simply reflect better surveillance in Turkey, and the detection of greater number of infected persons with few or no symptoms. However, other factors, such as the quality of ribavirin, level of supportive care, and virus and host differences should also be considered. It should also be noted that the case fatality of epidemics in Iran in the 1960’s and 70’s bordering Turkey were also around 5%. While these were not proven to be CCHF, the similarities in patient presentation and epidemiology are striking. Thus, possibilities such as differences in mortality based on viral strain differences and ribavirin usage should be considered.
Regarding the former, it should be noted that the CFR of one large study in Pakistan was around 15% (Durrani et al., 2007), thus it may be that the genotype 1 (Asia) viruses are more virulent than genotype 4 (Europe), but more studies will be needed to test this hypothesis. As noted above, the Iranian experience is large enough to support basic epidemiological research on risk factors for mortality. Greater national cooperation in CCHF research between Iran and Turkey would be useful in carrying out such studies.
XII. Questions for further research
Since the first human infection was identified in 1999, CCHF has been a major public health concern in Iran, with a large number of cases observed all over the country. Because the epidemiologic picture is only 13 years old, we do not have enough information to predict how the disease will behave in the future. Surveillance and control programs initially appeared to be successful in limiting the number of cases, but the increase since 2005 has challenged those assumptions. The recent surge highlights the potential role of environmental factors, and more studies are needed to understand those factors that affect sylvatic and human infection cycles. This is particularly true for Sistan and Baluchestan province, which has the most cases of CCHF, but in which its ecology and epidemiology are poorly understood.
Current preventive and treatment measures in Iran also provide an opportunity to study the role of insecticide treatment of cattle and early therapy for humans. The high CFR in Iran despite ribavirin treatment is alarming. Formal studies assessing risk factors for mortality and further measures to reduce this high rate should be addressed. Comparative epidemiological studies of CCHF in Turkey and Iran will also help us better understand and control the disease.
Supplementary Material
Supplementary Figure 1: Midpoint-rooted ML tree using the 450 nt dataset of the S segment of CCHFV. Viruses isolated in Iran are highlighted in yellow. Abbreviations: CAR; Central African Republic; DRC; Democratic Republic of the Congo. Bar, 0.04 nucleotide substitutions per site. From (Mild et al., 2010), with permission.
Highlights.
Iran has been challenged with Crimean-Congo hemorrhagic fever since 1999.
The emergence of CCHF provides opportunities for studying its epidemiology, diagnosis and management.
Iran has one of the largest experiences in the treatment of CCHF with ribavirin.
The CCHF case fatality rate in Iran appears to be several times greater than in its neighbor, Turkey.
Acknowledgments
The authors acknowledge the contribution of all present and past members of the National Expert Committee of Viral Hemorrhagic Fevers (NECVHFs) in Iran. We would also like to thank Professor Cyrus Abivardi, who provided guidance on historical entomology.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alavi-Naini R, Moghtaderi A, Koohpayeh HR, Sharifi-Mood B, Naderi M, Metanat M, Izadi M. Crimean-Congo haemorrhagic fever in Southeast of Iran. J Infect. 2006;52(5):378–82. doi: 10.1016/j.jinf.2005.07.015. [DOI] [PubMed] [Google Scholar]
- Magnus Albertus. 1260 (translated 1987) Man and the beasts (De animalibus, books 22–26) In: Scanlan JJ, editor. Medieval & Renaissance Texts & Studies. Binghampton, NY: p. 439. [Google Scholar]
- Aminolashrafi T, Nooranian A. Observance of a small epidemic of an hemmorhagic epidemic virus with several deaths in East Azarbaijan, Iran. Journal of the Medical and Pharmacologic School of Tabriz. 1345 [1966] 1966;6(1):1–36. (in Persian) [Google Scholar]
- Aminolashrafi T. Viral Hemmorhagic Fever of Sarab. Journal of the Medical and Pharmacologic School of Tabriz. 1349 [1970] 1969;10(4):377–458. (in Persian) [Google Scholar]
- Arda B, Aciduman A. A historical perspective of infectious diseases with reference to Crimean-Congo hemorrhagic fever. In: Ergonul O, Whitehouse CA, editors. Crimean-Congo Hemorrhagic Fever—A Global Perspective. Springer; Vienna, Austria: 2007. pp. 13–22. [Google Scholar]
- Arda B, Aciduman A, Johnston JC. A randomized controlled trial of ribavirin in Crimean Congo haemorrhagic fever: ethical considerations. J Med Ethics. 2012;38(2):117–20. doi: 10.1136/medethics-2011-100107. [DOI] [PubMed] [Google Scholar]
- Ardoin A, Karimi Y. A focus of thrombocytopenic purpura in East Azerbaidjan province, Iran (1974–1975) Med Trop. 1982;42(3):319–26. [PubMed] [Google Scholar]
- Asefi V. A clinical study of 60 patients with an infectious hemmoragic syndrome in East Azerbaijan Province (Iran) J Med Council. 1973;4(3):182–188. (in Persian) [Google Scholar]
- Brown EG. A year amongst the Persians. Adam and Charles Black; UK, London: 1893. pp. 97–8. [Google Scholar]
- Bursali A, Tekin S, Keskin A, Ekici M, Dundar E. Species diversity of ixodid ticks feeding on humans in Amasya, Turkey: seasonal abundance and presence of Crimean-Congo hemorrhagic fever virus. J Med Entomol. 2011;48(1):85–93. doi: 10.1603/me10034. [DOI] [PubMed] [Google Scholar]
- Burki TK. Ticks and Turkey. Lancet. 2012;380(9857):1897–8. doi: 10.1016/s0140-6736(12)62097-2. [DOI] [PubMed] [Google Scholar]
- Chinikar S. Crimean-Congo haemorrhagic Fever in Iran. In: Ergonul O, Whitehouse CA, editors. Crimean-Congo Hemorrhagic Fever—A Global Perspective. Springer; Vienna, Austria: 2007. pp. 89–98. [Google Scholar]
- Chinikar S, Goya MM, Shirzadi MR, Ghiasi SM, Mirahmadi R, Haeri A, Moradi M, Afzali N, Rahpeyma M, Zeinali M, Meshkat M. Surveillance and laboratory detection system of Crimean-Congo haemorrhagic fever in Iran. Transbound Emerg Dis. 2008;55(5–6):200–4. doi: 10.1111/j.1865-1682.2008.01028.x. [DOI] [PubMed] [Google Scholar]
- Chinikar S, Ghiasi SM, Hewson R, Moradi M, Haeri A. Crimean-Congo haemorrhagic fever in Iran and neighboring countries. J Clin Virol. 2010;47(2):110–4. doi: 10.1016/j.jcv.2009.10.014. [DOI] [PubMed] [Google Scholar]
- Chinikar S, Ghiasi SM, Moradi M, Goya MM, Shirzadi MR, Zeinali M, Meshkat M, Bouloy M. Geographical distribution and surveillance of Crimean-Congo haemorrhagic fever in Iran. Vector Borne Zoonotic Dis. 2010;10(7):705–8. doi: 10.1089/vbz.2009.0247. [DOI] [PubMed] [Google Scholar]
- Chinikar S, Mirahmadi R, Moradi M, Ghiasi M, Khakifirouz S, Sadat Varai F, Asl Solaimani M, Hasan Zehi A. A Molecular and Serological Survey on Crimean Congo Haemorrhagic Fever in Iranian Children. Pediatric Research. 2011;70:430–430. [Google Scholar]
- Chinikar S, Shah-Hoseini N, Khakifirouz S, Rasi Varaie FS, Rafigh M, Hasanzehi A. Serological and molecular evaluation of Crimean-Congo Haemorrhagic Fever in Iranian probable patients. 15th International Congress on Infectious Diseases, Session Emerging Infectious Diseases; Thailand, Bangkok. 2012. Jun 13–16, ( http://www.xcdsystem.com/icid2012/46.011.html. [Google Scholar]
- Chinikar S, Ghiasi SM, Moradi M, Goya MM, Shirzadi MR, Zeinali M, Mostafavi E, Pourahmad M, Haeri A. Phylogenetic analysis in a recent controlled outbreak of Crimean-Congo haemorrhagic fever in the south of Iran, December 2008. Euro Surveill. 2010;15(47) doi: 10.2807/ese.15.47.19720-en. [DOI] [PubMed] [Google Scholar]
- Chinikar S, Persson SM, Johansson M, Bladh L, Goya M, Houshmand B, Mirazimi A, Plyusnin A, Lundkvist A, Nilsson M. Genetic analysis of Crimean-congo hemorrhagic fever virus in Iran. J Med Virol. 2004;73(3):404–11. doi: 10.1002/jmv.20106. [DOI] [PubMed] [Google Scholar]
- Chinikar S, Shah-Hosseini N, Bouzari S, Jalali T, Shokrgozar MA, Mostafavi E. New circulating genomic variant of Crimean-Congo hemorrhagic fever virus in Iran. Arch Virol. 2012 doi: 10.1007/s00705-012-1588-0. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Chinikar S, Moghadam AH, Parizadeh SJ, Moradi M, Bayat N, Zeinali M, Mostafavi E. Seroepidemiology of crimean congo hemorrhagic Fever in slaughterhouse workers in north eastern iran. Iran J Public Health. 2012;41(11):72–7. [PMC free article] [PubMed] [Google Scholar]
- Chinikar S, Ghiasi SM, Naddaf S, Piazak N, Moradi M, Razavi MR, Afzali N, Haeri A, Mostafavizadeh K, Ataei B, Khalilifard-Brojeni M, Husseini SM, Bouloy M. Serological evaluation of Crimean-Congo hemorrhagic fever in humans with high-risk professions living in enzootic regions of Isfahan province of Iran and genetic analysis of circulating strains. Vector Borne Zoonotic Dis. 2012;12(9):733–8. doi: 10.1089/vbz.2011.0634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chumakov MP, Smirnova SE. Detection of antibodies to Crimean hemorrhagic fever virus in serum of wild and domestic animals from Iran and Africa. In: Chumakov MP, editor. Current problems in virology and the prevention of viral diseases. Academy of Medical Sciences of the USSR; Moscow [Russian]: 1972. pp. 367–8. [Google Scholar]
- Deyde VM, Khristova ML, Rollin PE, Ksiazek TG, Nichol ST. Crimean-Congo hemorrhagic fever virus genomics and global diversity. J Virol. 2006;80(17):8834–42. doi: 10.1128/JVI.00752-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durden LA, Logan TM, Wilson ML, Linthicum KJ. Experimental vector incompetence of a soft tick, Ornithodoros sonrai (Acari: Argasidae), for Crimean-Congo hemorrhagic fever virus. J Med Entomol. 1993;30(2):493–6. doi: 10.1093/jmedent/30.2.493. [DOI] [PubMed] [Google Scholar]
- Durrani AB, Shaikh M, Khan Z. Congo crimean hemorrhagic Fever in balochistan. J Coll Physicians Surg Pak. 2007;17(9):543–5. [PubMed] [Google Scholar]
- Ergönül O, Celikbaş A, Dokuzoguz B, Eren S, Baykam N, Esener H. Characteristics of patients with Crimean-Congo hemorrhagic fever in a recent outbreak in Turkey and impact of oral ribavirin therapy. Clin Infect Dis. 2004;39(2):284–7. doi: 10.1086/422000. [DOI] [PubMed] [Google Scholar]
- Ergonul O. Clinical and pathologic features of Crimean-Congo hemorrhagic fever. In: Ergonul O, Whitehouse CA, editors. Crimean-Congo Hemorrhagic Fever—A Global Perspective. Springer; Vienna, Austria: 2007. pp. 207–220. [Google Scholar]
- Ergonul O. International Meeting on Emerging Diseases and Surveillance; Feb 14th; Vienna, Austria. 2009. [Google Scholar]
- Estrada-Peña A. The Use of Climactic Spatial Maps. European Society of Clinical Microbiology and Infectious Disease Conference of Viral Hemorrhagic Fevers; June 27–28; Istanbul, Turkey. 2008. [Google Scholar]
- Estrada-Peña A, Vatansever Z, Gargili A, Ergönul O. The trend towards habitat fragmentation is the key factor driving the spread of Crimean-Congo haemorrhagic fever. Epidemiol Infect. 2010;138(8):1194–203. doi: 10.1017/S0950268809991026. [DOI] [PubMed] [Google Scholar]
- Estrada-Peña A, Jameson L, Medlock J, Vatansever Z, Tishkova F. Unraveling the ecological complexities of tick-associated Crimean-Congo hemorrhagic fever virus transmission: a gap analysis for the western Palearctic. Vector Borne Zoonotic Dis. 2012;12(9):743–52. doi: 10.1089/vbz.2011.0767. [DOI] [PubMed] [Google Scholar]
- Estrada-Peña A, Ruiz-Fons F, Acevedo P, Gortazar C, de la Fuente J. Factors driving the circulation and possible expansion of Crimean-Congo haemorrhagic fever virus in the western Palearctic. J Appl Microbiol. 2013;114(1):278–86. doi: 10.1111/jam.12039. [DOI] [PubMed] [Google Scholar]
- Fakoorziba MR, Neghab M, Alipour H, Moemenbellah-Fard MD. Tick Borne Crimean-Congo Haemorrhagic Fever in Fars Province, Southern Iran: Epidemiologic Characteristics and Vector Surveillance. Pakistan Journal of Biological Sciences. 2006;9(14):2681–4. [Google Scholar]
- Fakoorziba MR, Golmohammadi P, Moradzadeh R, Moemenbellah-Fard MD, Azizi K, Davari B, Alipour H, Ahmadnia S, Chinikar S. Reverse Transcription PCR-Based Detection of Crimean-Congo Hemorrhagic Fever Virus Isolated from Ticks of Domestic Ruminants in Kurdistan Province of Iran. Vector Borne Zoonotic Dis. 2012 May 31; doi: 10.1089/vbz.2011.0743. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Fisher-Hoch SP, Khan JA, Rehman S, Mirza S, Khurshid M, McCormick JB. Crimean Congo-haemorrhagic fever treated with oral ribavirin. Lancet. 1995;346:472–475. doi: 10.1016/s0140-6736(95)91323-8. [DOI] [PubMed] [Google Scholar]
- Gale P, Estrada-Peña A, Martinez M, Ulrich RG, Wilson A, Capelli G, Phipps P, de la Torre A, Muñoz MJ, Dottori M, Mioulet V, Fooks AR. The feasibility of developing a risk assessment for the impact of climate change on the emergence of Crimean-Congo haemorrhagic fever in livestock in Europe: a review. J Appl Microbiol. 2010;108(6):1859–70. doi: 10.1111/j.1365-2672.2009.04638.x. [DOI] [PubMed] [Google Scholar]
- Gale P, Stephenson B, Brouwer A, Martinez M, de la Torre A, Bosch J, Foley-Fisher M, Bonilauri P, Lindström A, Ulrich RG, de Vos CJ, Scremin M, Liu Z, Kelly L, Muñoz MJ. Impact of climate change on risk of incursion of Crimean-Congo haemorrhagic fever virus in livestock in Europe through migratory birds. J Appl Microbiol. 2012;112(2):246–57. doi: 10.1111/j.1365-2672.2011.05203.x. [DOI] [PubMed] [Google Scholar]
- Gözalan A, Akin L, Rolain JM, Tapar FS, Oncül O, Yoshikura H, Zeller H, Raoult D, Esen B. Epidemiological evaluation of a possible outbreak in and nearby Tokat province. Mikrobiyol Bul. 2004;38(1–2):33–44. [PubMed] [Google Scholar]
- Han N, Rayner S. Epidemiology and mutational analysis of global strains of crimean-congo haemorrhagic fever virus. Virologica Sinica. 2011;26(4):229–244. doi: 10.1007/s12250-011-3211-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogstraal H. The epidemiology of tick-borne Crimean-Congo hemorrhagic fever in Asia, Europe, and Africa. J Med Entomol. 1979;15(4):307–417. doi: 10.1093/jmedent/15.4.307. [DOI] [PubMed] [Google Scholar]
- Ibn Sīnā. In: [The canon of medicine]. Bulaq Edition. Sharafkandi A, translator. University of Tehran Press; Tehran, Iran: 1978. [Google Scholar]
- Izadi S, Holakouie-Naieni K, Majdzadeh SR, Chinikar S, Nadim A, Rakhshani F, Hooshmand B. Seroprevalence of Crimean-Congo hemorrhagic fever in Sistan-va-Baluchestan province of Iran. Jpn J Infect Dis. 2006;59(5):326–8. [PubMed] [Google Scholar]
- Izadi S, Salehi M. Evaluation of the efficacy of ribavirin therapy on survival of Crimean-Congo hemorrhagic fever patients: a case–control study. Jpn J Infect Dis. 2009;62:11–15. [PubMed] [Google Scholar]
- Jabbari A, Besharat S, Abbasi A, Moradi A, Kalavi K. Crimean-Congo hemorrhagic fever: case series from a medical center in Golestan province, Northeast of Iran (2004) Indian J Med Sci. 2004;60(8):327–9. [PubMed] [Google Scholar]
- Jurjānī . [The Treasure of Khwarazm Shah], Sirjani Edition. (1203 CE) (Persian) Bonyad-e Farhag-e Iran; Tehran: 1976. p. 643. [Google Scholar]
- Keshtkar-Jahromi M, Kuhn JH, Christova I, Bradfute SB, Jahrling PB, Bavari S. Crimean-Congo hemorrhagic fever: current and future prospects of vaccines and therapies. Antiviral Res. 2011;90(2):85–92. doi: 10.1016/j.antiviral.2011.02.010. [DOI] [PubMed] [Google Scholar]
- Koksal I, Yilmaz G, Aksoy F, Aydin H, Yavuz I, Iskender S, Akcay K, Erensoy S, Caylan R, Aydin K. The efficacy of ribavirin in the treatment of Crimean-Congo hemorrhagic fever in Eastern Black Sea region in Turkey. J Clin Virol. 2010;47:65–68. doi: 10.1016/j.jcv.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Kubar A, Haciomeroglu M, Ozkul A, Bagriacik U, Akinci E, Sener K, Bodur H. Prompt administration of Crimean-Congo hemorrhagic fever (CCHF) virus hyperimmunoglobulin in patients diagnosed with CCHF and viral load monitorization by reverse transcriptase-PCR. Jpn J Infect Dis. 2011;64(5):439–43. [PubMed] [Google Scholar]
- Lotfollahzadeh S, Nikbakht Boroujeni GR, Mokhber Dezfouli MR, Bokaei S. Zoonoses Public Health. A serosurvey of Crimean-Congo haemorrhagic fever virus in dairy cattle in Iran. 2011;58(1):54–9. doi: 10.1111/j.1863-2378.2009.01269.x. [DOI] [PubMed] [Google Scholar]
- Mahzounieh M, Dincer E, Faraji A, Akin H, Akkutay AZ, Ozkul A. Relationship between Crimean-Congo hemorrhagic fever virus strains circulating in Iran and Turkey: possibilities for transborder transmission. Vector Borne Zoonotic Dis. 2012;12(9):782–5. doi: 10.1089/vbz.2011.0928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltezou HC, Papa A. Crimean-Congo hemorrhagic fever: risk for emergence of new endemic foci in Europe? Travel Med Infect Dis. 2010;8(3):139–43. doi: 10.1016/j.tmaid.2010.04.008. [DOI] [PubMed] [Google Scholar]
- Maltezou HC, Andonova L, Andraghetti R, Bouloy M, Ergonul O, Jongejan F, Kalvatchev N, Nichol S, Niedrig M, Platonov A, Thomson G, Leitmeyer K, Zeller H. Crimean-Congo hemorrhagic fever in Europe: current situation calls for preparedness. Euro Surveill. 2010;15(10):1–4. [PubMed] [Google Scholar]
- Mardani M. Nosocomial Crimean-Congo hemorrhagic fever in Iran, 1999–2000. Clin Microbiol Infect. 2001;7(Suppl 1):213. [Google Scholar]
- Mardani M, Keshtkar-Jahromi M, Holakouie Naieni K, Zeinali M. The efficacy of oral ribavirin in the treatment of crimean-congo haemorrhagic fever in Iran. Clin Infect Dis. 2003;36(12):1613–8. doi: 10.1086/375058. [DOI] [PubMed] [Google Scholar]
- Mardani M, Keshtkar-Jahromi M, Ataie B, Adibi P. Crimean-Congo hemorrhagic fever virus as a nosocomial pathogen in Iran. Am J Trop Med Hyg. 2009;81(4):675–8. doi: 10.4269/ajtmh.2009.09-0051. [DOI] [PubMed] [Google Scholar]
- Metanat M, Sharifi-Mood B, Salehi M, Alavi-Naini R. Clinical outcomes in Crimean-Congo hemorrhagic fever: a five-years experience in the treatment of patients with oral ribavirin. Int J Virol. 2006;2:21–24. [Google Scholar]
- Mertens M, Schmidt K, Ozkul A, Groschup MH. The impact of Crimean-Congo hemorrhagic fever virus on public health. Antiviral Res. 2013;98(2):248–60. doi: 10.1016/j.antiviral.2013.02.007. [DOI] [PubMed] [Google Scholar]
- Mild M, Simon M, Albert J, Mirazimi a. Towards an understanding of the migration of Crimean–Congo hemorrhagic fever virus. Journal of General Virology. 2010;91:199–207. doi: 10.1099/vir.0.014878-0. [DOI] [PubMed] [Google Scholar]
- Mostafavi E, Chinikar S, Esmaeili S, Amiri FB, Tabrizi AM, Khakifirouz S. Seroepidemiological Survey of Crimean-Congo Hemorrhagic Fever Among Sheep in Mazandaran Province, Northern Iran. Vector Borne Zoonotic Dis. 2012;12(9):739–42. doi: 10.1089/vbz.2011.0958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabian S, Rahbari S, Changizi A, Shayan P. The distribution of Hyalomma spp. ticks from domestic ruminants in Iran. Med Vet Entomol. 2009;23(3):281–3. doi: 10.1111/j.1365-2915.2009.00804.x. [DOI] [PubMed] [Google Scholar]
- Naderi HR, Sarvghad MR, Bojdy A, Hadizadeh MR, Sadeghi R, Sheybani F. Nosocomial outbreak of Crimean-Congo haemorrhagic fever. Epidemiol Infect. 2011;139(6):862–6. doi: 10.1017/S0950268810002001. [DOI] [PubMed] [Google Scholar]
- Naderi H, Sheybani F, Bojdi A, Khosravi N, Mostafavi I. Fatal Nosocomial Spread of Crimean-Congo Hemorrhagic Fever with Very Short Incubation Period. Am J Trop Med Hyg. 2012 Dec 26; doi: 10.4269/ajtmh.2012.12-0337. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuttall GHF. Ticks: A Monograph of the Ixodoidea. Cambrige University Press; Cambridge, England: 1908. pp. 85–86. [Google Scholar]
- Purnak T, Selvi NA, Altundag K. Global warming may increase the incidence and geographic range of Crimean-Congo Hemorrhagic Fever. Med Hypotheses. 20067;68(4):924–5. doi: 10.1016/j.mehy.2006.09.020. [DOI] [PubMed] [Google Scholar]
- Randolph SE, Rogers DJ. Ecology of Tick-Borne Disease and the Role of Climate. In: Ergonul O, Whitehouse CA, editors. Crimean-Congo Hemorrhagic Fever—A Global Perspective. Springer; Vienna, Austria: 2007. pp. 167–186. [Google Scholar]
- Razi . In: Kholāse Kitāb Al-Hawi [Continens Liber, abridged] Tabatabaie SM, editor. Entesharate Daneshgahe Oloom Pezeshki Mashhad; Mashhad, Iran: 2008. [Google Scholar]
- Saidi S. Viral antibodies in preschool children from the Caspian area, Iran. Iranian Journal of Public Health. 1974;3:83–91. [Google Scholar]
- Saidi S, Casals J, Faghih MA. Crimean haemorrhagic fever-Congo (CHF-C) virus antibodies in man and in domestic and small mammals in Iran. Am J Trop Med Hyg. 1975;24(2):353–7. doi: 10.4269/ajtmh.1975.24.353. [DOI] [PubMed] [Google Scholar]
- Sharifi-Mood B, Metanat M, Ghorbani-Vaghei A, Fayyaz-Jahani F, Akrami E. The outcome of patients with Crimean-Congo hemorrhagic Fever in zahedan, southeast of iran: a comparative study. Arch Iran Med. 2009;12(2):151–3. [PubMed] [Google Scholar]
- Sureau P, Klein JM. Arboviruses in Iran. Med Trop. 1980;40(5):549–54. [PubMed] [Google Scholar]
- Tabarī IR. In: [The Paradise of Wisdom] (Persian) Madani SA, Boroujerdi A, translators. Mehr Amin Press; Tehran, Iran: 2008. [Google Scholar]
- Tahmasebi F, Ghiasi SM, Mostafavi E, Moradi M, Piazak N, Mozafari A, Haeri A, Fooks AR, Chinikar S. Molecular epidemiology of Crimean-Congo hemorrhagic fever virus genome isolated from ticks of Hamadan province of Iran. J Vector Borne Dis. 2010;47(4):211–6. [PubMed] [Google Scholar]
- Telmadarraiy Z, Ghiasi SM, Moradi M, Vatandoost H, Eshraghian MR, Faghihi F, Zarei Z, Haeri A, Chinikar S. A survey of Crimean-Congo haemorrhagic fever in livestock and ticks in Ardabil Province, Iran during 2004–2005. Scand J Infect Dis. 2010;42(2):137–41. doi: 10.3109/00365540903362501. [DOI] [PubMed] [Google Scholar]
- Turell M. Role of ticks in the transmission of Crimean-Congo hemorrhagic fever. In: Ergonul O, Whitehouse CA, editors. Crimean-Congo Hemorrhagic Fever—A Global Perspective. Springer; Vienna, Austria: 2007. pp. 143–154. [Google Scholar]
- van de Wal BW, Joubert JR, van Eeden PJ, King JB. A nosocomial outbreak of Crimean-Congo haemorrhagic fever at Tygerberg Hospital. Part IV. Preventive and prophylactic measures. S Afr Med J. 1985;68:729–732. [PubMed] [Google Scholar]
- Vassilenko SM, Vassilev TL, Bozadjiev LG, Bineva IL, Kazarov GZ. Specific intravenous immunoglobulin for Crimean-Congo haemorrhagic fever. Lancet. 1990;335:791–792. doi: 10.1016/0140-6736(90)90906-l. [DOI] [PubMed] [Google Scholar]
- Yilmaz GR, Buzgan T, Torunoglu MA, Safran A, Irmak H, Com S, Uyar Y, Carhan A, Ozkaya E, Ertek M. A preliminary report on Crimean-Congo haemorrhagic fever in Turkey March - June 2008. Euro Surveill. 2008;13(33):1–2. doi: 10.2807/ese.13.33.18953-en. [DOI] [PubMed] [Google Scholar]
- Zeller HG, Cornet JP, Diop A, Camicas JL. Crimean-Congo hemorrhagic fever in ticks (Acari: Ixodidae) and ruminants: field observations of an epizootic in Bandia, Senegal (1989–1992) J Med Entomol. 1997;34(5):511–6. doi: 10.1093/jmedent/34.5.511. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: Midpoint-rooted ML tree using the 450 nt dataset of the S segment of CCHFV. Viruses isolated in Iran are highlighted in yellow. Abbreviations: CAR; Central African Republic; DRC; Democratic Republic of the Congo. Bar, 0.04 nucleotide substitutions per site. From (Mild et al., 2010), with permission.