Abstract
The triple-helical structure of collagen has been accurately reproduced in numerous chemical and recombinant model systems. Triple-helical peptides and proteins have found application for dissecting collagen-stabilizing forces, isolating receptor- and protein-binding sites in collagen, mechanistic examination of collagenolytic proteases, and development of novel biomaterials. Introduction of native-like sequences into triple-helical constructs can reduce the thermal stability of the triple-helix to below that of the physiological environment. In turn, incorporation of nonnative amino acids and/or templates can enhance triple-helix stability. We presently describe approaches by which triple-helical structure can be modulated for use under physiological or near-physiological conditions.
Keywords: Collagen, Triple-helix, Template, Peptide-amphiphile, Proline analogs, Disulfide knot
1 Introduction
1.1 Collagen
Collagen is the most abundant protein in animals, and is the major structural protein found in the connective tissues such as basement membranes, tendons, ligaments, cartilage, bone, and skin. The collagen family consists of at least 28 members [1–5]. The most defining feature of collagen is the supersecondary structure, composed of three parallel extended left-handed polyproline type II alpha chains of primarily repeating Gly-Xxx-Yyy triplets. Three left-handed strands intertwine in a right-handed fashion around a common axis to form a triple-helix (Fig. 1). The formation of the triple-helix creates a shallow superhelical pocket inside it. The collagen triple-helix is essential for the integrity of multiple connective tissues.
Fig 1.
“Ball and stick” computer-generated models of (top) a continuous collagen triple-helix (peptide T3-785, 3[(Pro-Hyp-Gly)3-Ile-Thr-Gly-Ala-Arg-Gly-Leu-Ala-Gly-(Pro-Hyp-Gly)4]) and (bottom) an unwound (heat denatured) version of the same sequence
Depending on the primary structure of the alpha chains [4], collagens have been classified into two main categories, homotrimeric and heterotrimeric. Homotrimeric collagens have three identical alpha chain sequences (designated α1). Examples of such type of collagens are types II and III. Alternatively, heterotrimeric collagens have either three alpha chains of different sequence, designated as α1, α2, and α3 (i.e., type V) or two alpha chains of identical sequence (α1) and third alpha chain of different sequence (α2), such as types I and VI [6]. Furthermore, based on their quaternary structure, collagens are grouped into subfamilies such as fibrillar, collagen associated with banded fibrils (i.e., fibril-associated collagens with interrupted triple-helices—FACITs), network-forming (i.e., basement membrane and short chain), transmembranous, and membrane associated with interrupted triple helices (MACITs) [6].
1.2 Stability of Collagen-Model Triple-Helical Peptides
For several decades triple-helical peptides (THPs) or “mini-collagens” consisting of collagen-model sequences and their three-dimensional folds have been constructed and studied to fully investigate the structural and biological roles of collagenous proteins [5, 7–13]. A model for the collagen triple-helix was first proposed in the 1950s [14, 15] and subsequently refined over time [16–20]. To understand the stability of the collagen triple-helix, it is important to understand its compositional elements. In the collagen Gly-Xxx-Yyy triplet, the residue in the Xxx position is often L-proline (Pro) and the residue in the Yyy position is often 4(R)-hydroxy-L-proline (Hyp), accounting for 20 % of the total amino acid composition in collagen [21]. The other commonly found amino acids are Ala, Lys, Arg, Leu, Val, Ser, and Thr [21]. The packing of the triple-helical coiled-coil structure requires Gly in every third position. Because of its compact structure, assembly of the triple-helix puts this residue at the interior of the helix and the side chain of the Gly, an H atom, is small enough to fit into the center of the helix.
Several factors influence the stability of triple-helical peptides. The chains are held together by hydrogen bonds that form between the peptide amine of Gly residues and peptide carbonyl groups in an adjacent polypeptide chain (Fig. 2) [9, 18]. Interstrand hydrogen bonds formed between the GlyN-H and XxxC=O are critical for triple-helix stability, as replacement of a central amide bond of (Pro-Pro-Gly)10 with either an ester or an (E)-alkene, or replacement of the central Pro-Pro with a Pro-trans-Pro isostere, substantially destabilizes the THP [22–24].
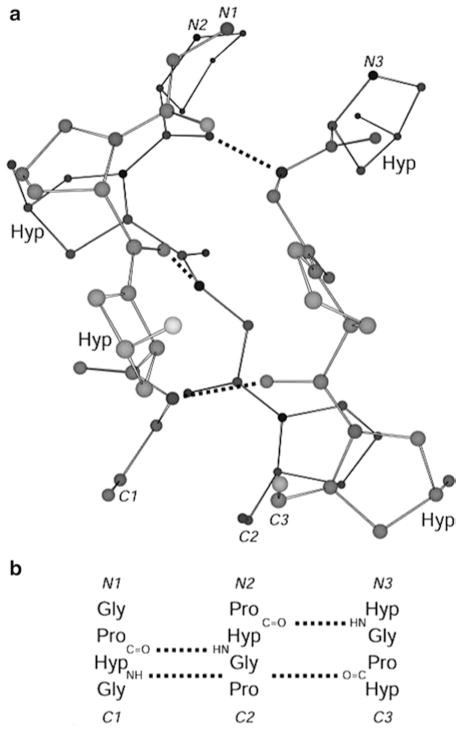
Fig 2.
Segment of a (Pro-Hyp-Gly)n triple-helix. (a) Ball-and-stick representation indicating 4-hydroxy-L-proline residues and XaaC=O–H–NGly hydrogen bonds. (b) Register of the residues in the three strands of panel (a). Atomic coordinates are from Science 1994, 266:75 (PDB entry 1CAG)
The role of hydrogen bonding versus inductive effects in triple-helix stabilization is an intensive area of THP research [5]. Incorporation of several nonnative amine acids in the proper position within the peptide sequence often remarkably enhance triple-helix stability. One such example of a stabilizing nonnative amino acid is 4R-fluoroproline (4R-Flp) (Fig. 3). Incorporation of 4R-fluoroproline has been shown to induce hyperstability in the triple-helix of (Pro-4RFlp-Gly)10 compared to (Pro-4RHyp-Gly)10 [25, 26]. Alternatively, 4S-Flp destabilizes the triple-helix of (Pro-4SFlp-Gly)7 compared to (Pro-4RHyp-Gly)7 [27]. More specifically, substitution of all of the 4R-Hyp residues in (Pro-4RHyp-Gly)10 or (Pro-4RHyp-Gly)7 by 4R-Flp increased Tm by 22 and 9 °C, respectively [25–27]. Conversely, an analogous substitution in (Pro-4RHyp-Gly)7 by 4S-Flp dramatically decreased Tm by greater than 26 °C [27]. The relative effects of 4R-Hyp, 4R-Flp, and 4S-Flp coincide with their propensity for forming trans-peptide bonds compared to cis-peptide bonds [27, 28]. Studies of collagen mimics have indicated that this increased stability arises from the inductive effects of an electronegative substituent in the 4R position, as in 4(R)-fluoroproline [9, 25, 26].
Fig 3.
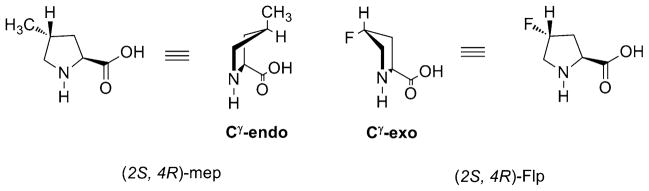
Ring conformations of 4-substituted L -prolines
It has long been noted that the thermal stability of the collagen triple-helix is enhanced by Hyp residues. It was hypothesized that this stability is due to Hyp residues involved in the hydrogen-bonded network mediated by water molecules, which connect the hydroxyl group of Hyp in one strand to the main chain amide carbonyl of another chain [29]. Substituting Pro for Hyp in the chain significantly decreased the Tm in collagen-model peptides [29]. However, collagen peptides containing the nonnative amino acid 4R-Flp exhibited higher stability than peptide with Hyp residues [25, 26] and thus the stabilizing effect arising from Hyp might not be due to hydrogen bonding but rather from the electronegative oxygen preorganizing the main chain in the proper conformation for triple-helix formation [30]. X-ray crystallographic analysis indicated that the 4-OH group in Hyp has no effect on the hydration pattern and the resulting molecular structures [31]. The O-methylation of hydroxyproline in Yyy stabilizes the triple-helix more than Hyp itself, most likely because the pyrrolidine ring of 4-methoxyproline adopts a Cγ-exo ring pucker. The conformational stability of the triple-helix arising from O-methylation provides strong evidence that the hydroxyl group of Hyp acts primarily through stereoelectronic effects [30].
Both the position of the residue containing the electronegative substituent and the stereochemistry of that electronegative substituent play a role in triple-helix stability. The positional effects of γ-substitution are illustrated by the stabilities, relative to (Pro-Pro-Gly)10, of (Pro-4RHyp-Gly)10 (increased triple-helix stability) and (4RHyp-Pro-Gly)10 (decreased stability) [32]. For many years, it was believed that peptides containing 4R-Hyp in the Xxx position of Gly-Xxx-Yyy repeats do not form collagen-like triple-helices. However, incorporation of 4R-Hyp in the Xxx position and Thr or Val in the Yaa position triggers triple-helix formation [33, 34]. For example, acetyl-(Gly-4RHyp-Thr)10-NH2 forms a stable triple-helix in water [33]. Both the hydroxyl and methyl groups of Thr with their stereochemical configuration appear to induce the triple-helix stability. Molecular modeling showed that the Thr methyl group shields the interchain hydrogen bond between the carbonyl group of Hyp of the adjacent chain and the amino group of the next Gly residue in the same chain.
Other significant factors that influence the stability of collagen triple helix are the trans/cis ratio of the Xaai-1-Proi peptide bond and the ring pucker of Proi [35, 36]. The trans/cis ratio is crucial as all of the peptide bonds in the triple-helix prefer the trans conformation. Pro in solution can adopt either exo or endo ring puckers (Fig. 3). The first Pro in the Pro-Pro-Gly triplet prefers an endo ring pucker conformation while the second Pro prefers the exo ring pucker conformation [35]. This observation suggested that Pro derivatives preferring the Cγ-endo ring pucker could pre-organize triple-helix formation when in the Xaa position, whereas those that prefer the Cγ-exo ring pucker could preorganize triple-helix formation when in the Yaa position [37].
As discussed earlier, incorporation of 4R-Flp (Fig. 3) in the Yyy position of Xxx-Yyy-Gly triplets induces hyperstability in the triple-helix.Thisisbecausethe4R-fluorogroupin4R-fluoroproline stabilizes the Cγ-exo pucker conformation. These stereoelectronic effects markedly enhance the conformational stability of a collagen triple-helix [38].
Similar to 4R-Flp, incorporation of 4R-methylproline (4R-mep) (Fig. 3) within the peptide sequence significantly increases triple-helix stability. Raines and colleagues studied the effect of 4-methylproline in conformational stability of (Xxx-Yyy-Gly)7. 4S-Methylproline (4S-Mep) in the Yyy position enhanced triple-helix stability more so than 4R-mep in the Xxx position [38]. A (4R-mep-4S-Mep-Gly)7 triple-helix is more stable than (4R-mep-Pro-Gly)7 or (Pro-4S-Mep-Gly)7, simply because the steric effects are additive. In contrast, (4S-flp-4R-Flp-Gly)7 is less stable than (4S-flp-Pro-Gly)7 or (Pro-4R-Flp-Gly)7 where the stereoelectronic effects induced by the hetero atom are not additive [38].
The combination of stereoelectronic and steric effects can also induce the preorganization of a polypeptide chain. Integration of both effect results in the most stable triple-helix known so far. Among four triple-helices formed from the four different polypeptide chains, (Pro-Pro-Gly)7, (Pro-4R-Hyp-Gly)7, (4S-flp-4S-Mep-Gly)7, and (4R-mep-4R-Flp-Gly)7, only the latter two showed hyperstability with Tm values of 51 and 58 °C, respectively [37].
The stability of THPs may also be regulated by pH. To create a triple-helix that was pH dependent, Hyp was modified by O-alkylation to a carboxylate group [39]. The incorporation of 1 or 3 Hyp(CO2) residues within a Pro-Hyp-Gly template did not result in a pH-sensitive triple-helix. However, acetyl-[Pro-Hyp(CO2)-Gly]7-OH formed a triple-helix with a Tm = 17 °C at pH 2.7 but had no triple-helical structure at pH 7.2 [39]. pH-dependent triple-helical stability was also observed for (Pro-4R-Amp-Gly)6 sequences, where 4R-Amp is (2S,4R)-4-aminoproline [40, 41].
Self-association of triple-helical structures has been used to “sandwich” a collagen-model sequence between repeats of Gly-Pro-Hyp to obtain THPs of reasonable stability (Fig. 4). Self-associated triple-helical peptides were used to study the structural aspects of collagen via the “host–guest” approach, using sequences such as (Pro-Hyp-Gly)n-Xxx-Yyy-Gly-(Pro-Hyp-Gly)n [42–44]. Compared with Gly-Pro-Hyp, none of the 20 natural amino acids provides enhanced thermal stability. The most stable residues in the Yyy position were Hyp > Arg> Met, while for the Xxx position Pro > charged residues> Ala> Gln [43–45]. Within a host–guest THP 4R-Flp in the Yyy position is slightly destabilizing compared with Hyp, in contrast to (Pro-4R-Flp-Gly)n and (Pro-Hyp-Gly)n THPs [46]. It has been suggested that different mechanisms are in place when Pro-4R-Flp-Gly is inserted within (Pro-Hyp-Gly)n compared with (Pro-4R-Flp-Gly)n [46].
Fig 4.

Modular structures of (top) sandwiched associated triple-helical peptide and (bottom) sandwiched associated triple-helical peptide-amphiphile. The associated THP features repeats of Gly-Pro-Hyp [(GPO)n] on both the N- and C-termini to induce or stabilize triple-helical structure, and a diverse collagen-like sequence [(GXY)n] in the middle for structural and/or biological studies. The peptide-amphiphile additionally possesses a pseudo-lipid attached to the N-terminus to further enhance triple-helical stability via hydrophobic interactions
Electrostatic effects can also contribute favorably to the stability of THPs, as found in host–guest studies [45, 47, 48]. Favorable electrostatic interactions were observed for Gly-Lys-Asp and Gly-Arg-Asp within the acetyl-(Gly-Pro-Hyp)3-Gly-Xxx-Yyy-(Gly-Pro-Hyp)4-Gly-Gly-NH2 host [49]. Conversely, guests Gly-Arg-Lys, Gly-Lys-Arg, and Gly-Glu-Asp exhibit charge repulsion [49]. The sequence Gly-Pro-Lys-Gly-[Asp/Glu]-Hyp is found to be as stabilizing as (Gly-Pro-Hyp)2 due to interchain axial ion pair interactions [50, 51]. Arg in the Yyy position can offer high stability, possibly based on its ability to form a hydrogen bond with C=O in a neighboring strand [45, 52]. Heterotrimeric host–guest peptide approaches show a decreased thermal stability for an acetyl-(Gly-Pro-Hyp)3-Gly-Pro-Yyy-(Gly-Pro-Hyp)4-Gly-Gly-NH2 sequence with an increased number of Arg residues [53]. For example, Hyp residues in all Yyy positions results in a THP with a Tm = 47.2 °C compared to 44.5, 40.8, and 37.5 °C, respectively, when all Hyp is replaced by Arg residues in one chain, two chains, or three chains [53].
1.3 Synthesis of Fmoc-Pro Derivatives
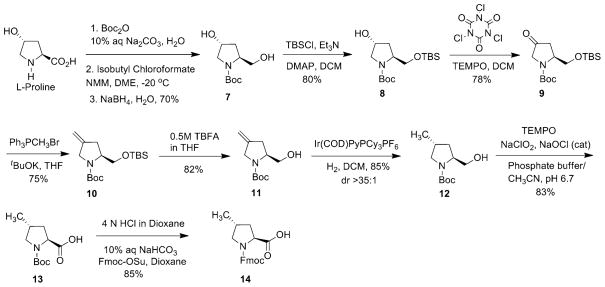
To efficiently incorporate (2S, 4R)-4-fluoroproline (Flp) and (2S, 4R)-4-methylproline (mep) into peptide sequences, convenient synthetic routes for the preparation of derivatized Flp and Mep residues such as Fmoc-trans-Flp 6 and Fmoc-trans-Mep 14 are required (Figs. 5 and 6). Several methods have been reported in the literature for the synthesis of N-protected-4(R)-Flp from N-protected-4(R)-Hyp derivatives [54–63]. Fields et al. previously reported the synthesis of Fmoc-trans-Flp starting from expensive cis-4(S)-hydroxy-L-proline [64]. The removal of benzyl ester was problematic under standard Pd/C hydrogenation, as the Fmoc group started decomposing upon prolonged exposure to Pd/C-hydrogen [65, 66]. Recently, we developed a more convenient synthetic route for the preparation of Fmoc-trans-Flp from readily available (and inexpensive) trans-4(R)-hydroxy-L-proline (Fig. 5). A one-pot, three-step procedure is deployed involving a bis-deprotection of the N- and C-termini under catalytic hydrogenation conditions followed by selective capping of the N-terminus with an Fmoc group to yield Fmoc-trans-Flp.
Fig 5.
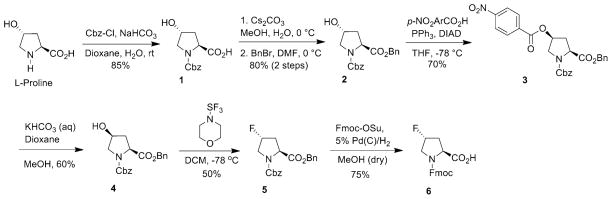
Synthesis of Fmoc-(2S,4R )-4-fluoroproline (Flp)
Fig 6.
Synthesis of Fmoc-(2S,4R )-4-methylproline (mep)
The strategy for the synthesis of the Fmoc-trans-Flp entails initial Cbz-protection of the amine nitrogen followed by benzylation of the carboxylic acid group. Cbz protection of trans-4(R)-hydroxy-L-proline is performed by using standard protocols to obtain 1 in 85 % of yield (Fig. 5) [67]. The conversion of N-Cbz-4(R)-Hyp 1 to N-Cbz-4(R)-Hyp-OBzl 2 is achieved in 80 % yield by reaction with CsCO3 and BnBr [68]. Mitsunobu reaction of N-Cbz-4(R)-Hyp-OBzl 2 followed by hydrolysis results in the N-Cbz-4-Hyp derivative 4 in 42 % (two-step overall yield) yield with inversion of configuration at C-4 position [69]. The next step is the synthesis of diastereomeric fluoroproline by a stereospecific displacement of the hydroxyl group of Hyp by fluorine. Fluorination of N-Cbz-4(S)-Hyp-OBzl 4 is performed using diethylaminosulfur trifluoride (DAST) as fluorinating agent to yield N-Cbz-4(R)-Flp-OBzl 5 in 50 % yield [69]. Finally, catalytic hydrogenation of 5 with 5 % Pd/C under hydrogen (atm. pressure) in the presence of Fmoc-OSu affords desired N-Fmoc-4-Flp 6 in good yields (Fig. 5) [70]. This one-pot reaction involved the simultaneous removal of the Cbz- and benzyl-protecting groups and re-protection of amine nitrogen with Fmoc group. Optimal yield (~75 %) is observed with a reaction time of 2 h, whereas prolonged exposure of the reaction mixture to hydrogenation resulted in partial Fmoc deprotection.
To synthesize Fmoc-trans-mep 14 (Fig. 6), initially N-Boc-trans-mep 13 is synthesized by following a straightforward method reported by Goodman et al. [71]. The reaction starts with initial Boc protection of commercially available trans-4(R)-hydroxy-L-proline followed by carboxylic acid group reduction to provide Boc-protected alcohol 7. Selective protection of the primary alcohol with TBDMSCl gave compound 8, which is then oxidized with trichlorocyanuric acid and catalytic TEMPO to afford pyrrolidinone 9 in 78 % yields. Reaction of pyrrolidinone 9 with methyltriphenylphosphonium bromide gave olefin 10 in 75 % yields. Silyl-ether deprotection of 10 was first carried out to unmask the hydroxyl directing groups. Treatment of the resulting olefin 11 with 3 mol% of the Crabtree catalyst, under H2 atm, gave excellent selectivity of trans-4-methyl pyrrolidine 12 in 85 % yields. Oxidation of the indolylprolinol derivative 12 in the presence of TEMPO, bleach, and sodium chlorite provided N-Boc-trans-mep 13. Finally, deprotection of Boc group with 4 N HCl followed by Fmoc installation using Fmoc-OSu and aqueous NaHCO3 in dioxane provided Fmoc-trans-mep 14 in 85 % yield [38].
1.4 Covalent Modification of Triple-Helical Peptides
Several covalent modification strategies have been employed to induce triple-helix structure formation and/or enhance their stability. Substantial stabilization can be achieved by use of a self-assembly approach where alignment of amphiphilic compounds at the lipid–solvent interface facilitates peptide alignment and structure initiation and propagation. Addition of lipophilic molecules at the N-terminus of the peptide, known as the “peptide-amphiphile” (PA) approach, often stabilizes self-associated peptides. The term peptide-amphiphile was first used in 1984, when an alanine residue was interposed between a charged head group and a double-chain pseudo-lipid tail [72]. In this approach pseudo-lipids such as mono- and di-alkyl chains are covalently attached to peptides to create peptide-amphiphiles that then associate via hydrophobic interactions (Fig. 4) [73–77]. Peptide-amphiphiles are advantageous in that they present a multivalent ligand [78] that is chemically well defined, avoiding loss of activity that can occur during nonspecific coupling of peptides to lipids [79]. The amphiphilic character of peptide-amphiphiles allows for the control of assembled structures by manipulating their molecular composition [80]. The thermal stability of triple-helical peptide-amphiphile head groups can be modulated by the length of the lipophilic moiety [73, 74, 76, 81, 82]. The thermal stability of the triple-helical structure in the peptide-amphiphile was found to increase as the monoalkyl tail chain length was increased over a range of C6–C16 [74]. Conversely, alterations in the pseudo-lipid tail composition affect peptide-amphiphile aggregate structures [76, 80]. Desirable peptide head group melting temperature values can be achieved for in vivo use, as triple-helical PAs have been constructed with Tm values ranging from 30 to 70 °C [64, 73, 74, 76, 81–85].
The stability of associated THPs may also be regulated by photolysis. Modification of the sequence (Gly-Pro-Hyp)3-Gly-Cys-Hyp-Gly-Pro-Hyp-Gly-Pro-Cys-(Gly-Pro-Hyp)5-Gly-Gly-NH2 with an azobenzene bridge between the Cys residues resulted in a THP whose stability decreased up irradiation at λ =330 nm at 27 °C [86]. Unfortunately, the poor solubility of this peptide prohibited quantitative analysis of its stability [86].
The use of template-assisted approaches is another important strategy to create stable triple-helical conformation. In recent years much progress has been made in template-assisted synthetic methodologies. These include orthogonal protection schemes, chemoselective ligation, and the design of novel and increasingly flexible templates. Many templates have been used to create stable THPs. The main categories of template strategies involve a scaffold to which the peptides are covalently attached or the use of metal ions for non-covalent association. The covalent templates include (1) multi-Lys branching [87–91], (2) a double-disulfide “knot” (cystine knots) [53, 92–96], (3) cis,cis-1,3,5-trimethylcyclohexane-1,3,5-tricarboxylic acid (Kemp triacid; KTA) [97–101], (4) tris(2-aminoethyl)amine (TREN) [102], (5) cyclotriveratrylene (CVT) [103], (6) macrocyclic scaffold [104], or (7) carbohydrates (Fig. 7) [105, 106]. Alternatively, the metal ions Ca2+, Ru2+, Ni2+, Fe2+, Cd2+, and Hg2+ have been used as templates in conjugation with amino acids or N-terminal pyridyl or bipyridyl functionalities to create multistrand linkages [107–109].
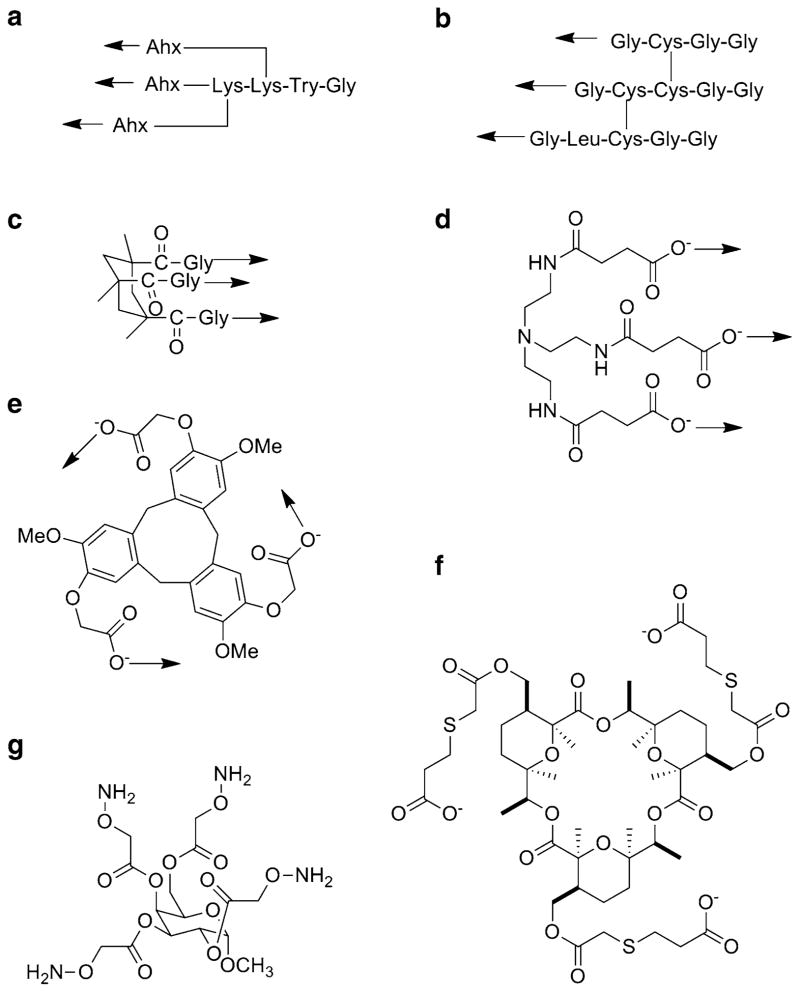
Fig 7.
Templates used for the chemical synthesis of triple-helical peptides: (a) di-Lys branch after coupling to 6-aminohexanoic acid (Ahx); (b) disulfide bridge (cystine knot); (c) cis,cis-1,3,5-trimethylcyclohexane-1,3,5-tricarboxylic acid (KTA) after coupling to Gly; (d) tris(2-aminoethyl)amine (TREN) after coupling to succinic acid; (e) cyclotriveratrylene (CTV) after coupling to a bromoethanoic acid; (f) macrocyclic; and (g) methyl 2,3,4,6-tetra-O-Aoa-α-D-Galp. The arrows indicate the direction of collagen-like sequence incorporation
A significant strategy to stabilize THPs is the covalent attachment of three strands via a C-terminal branch. The C-terminal branch is expected to align and entropically stabilize the C-terminus of the THP and thus enhance triple-helical thermal stability [90] and to provide a model of the disulfide-linked C-terminus of type III collagen [110, 111]. Branching can be achieved by selective deprotection of Lys Nα- and Nε-amino groups.
Solid-phase assembly of triple-helical collagen-model peptides using a Lys-Lys C-terminal branch requires three different protecting group strategies (Fig. 8): Nα-amino protection (A); Lys Nε-amino side-chain protection (B), which must be stable to the Nα-amino group removal conditions; and Cα-carboxyl protection (linker), which must be stable to both the Nα- and Nε-amino-protecting group removal conditions. Based on the three different protecting group strategies, four different branching combinations have been developed [90, 112]. Branching is achieved by synthesizing A-[Lys(B)]2-Tyr(C)-Gly-linker resin and deprotecting the Nα- and Nε-amino groups. Tyr was incorporated prior to branching to provide a convenient chromophore for eventual concentration determination. Incorporation of Ahx (6-aminohexanoic acid) onto the N-termini of all three strands provided a flexible spacer. All solid-phase methods to prepare THPs were based on Fmoc Nα-amino group protection. For example, the type (IV) collagen-derived α(IV)1263-1277 branch was assembled by a three-dimensional orthogonal strategy, where B was tert-butyloxycarbonyl (Boc) and linker was 4-trityloxy-Z-but-2-enyloxyacetic acid [cleaved by (Ph3P)4Pd-catalyzed nucleophilic transfer] [91]. A (Gly-Pro-Hyp)8 branch has also been assembled by using the same orthogonal strategy, where B was allyloxycarbonyl (Aloc) [cleaved by (Ph3P)4Pd-catalyzed nucleophilic transfer] and linker was hydroxymethylphenoxy (HMP; cleaved by TFA). The third three-dimensional orthogonal strategy was developed using the 1-(4,4-dimethyl-2,6-dioxocyclohex-1-ylidene)ethyl (Dde) group as B (cleaved by hydrazine/DMF) and the linker was HMP [90]. Due to the mild conditions the Fmoc/Dde combination (with ivDde replacing Dde) is now the most preferred. For example, Farndale, Barnes, and colleagues reported the synthesis of THPs, based on the bovine α1(III)CB4 fragment, via this C-terminal covalent attachment strategy [113–115].
Fig 8.
General scheme for the synthesis of branched, triple-helical peptides. Ahx is 6-aminohexanoic acid. Reprinted with permission from Biopolymers, copyright 1993, Wiley and Sons
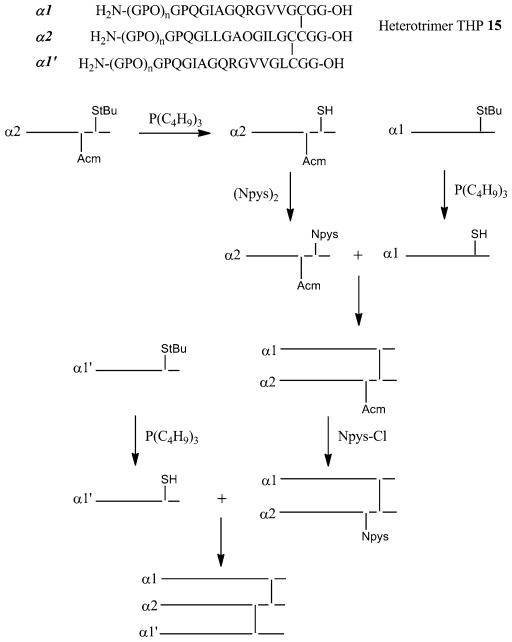
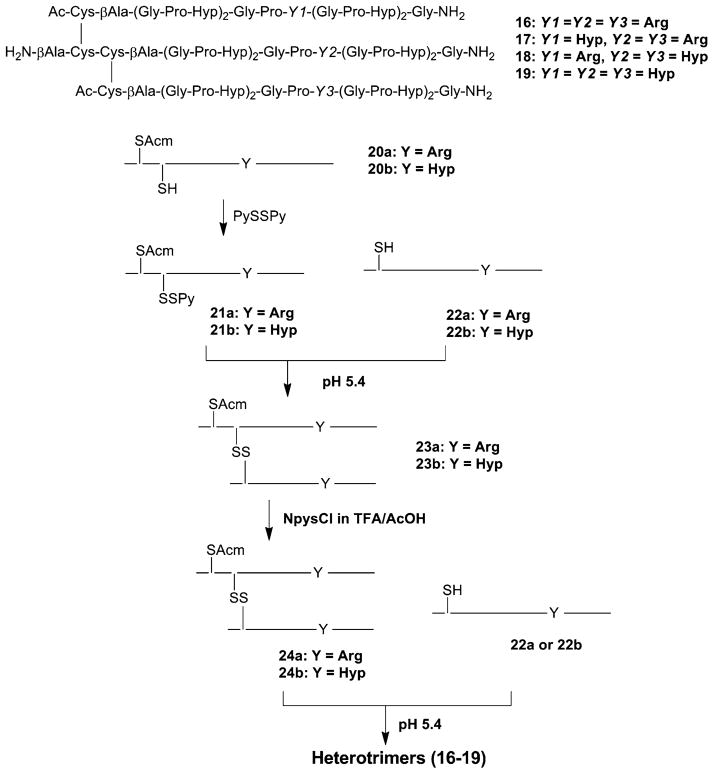
A double-disulfide “knot” (cystine knots) either at the C-terminal region or at the N-terminal region of three peptide strands is another frequently used strategy to stabilize THPs [3, 92, 94–96]. One representative example of a C-terminal cysteine knot is construction of branched heterotrimeric THP 15 (Fig. 9). For this peptide all three individual chains are constructed by solid-phase methodology using Fmoc chemistry. The α1 chain contained either 3 or 5 Gly-Pro-Hyp repeats on the N-terminus of Gly-Pro-Gln-Gly-Ile-Ala-Gly-Gln-Arg-Gly-Val-Val-Gly-Cys(Acm)-Gly-Gly-OH, where Acm was acetamidomethyl, the α2 chain contained 3 or 5 Gly-Pro-Hyp repeats on the N-terminus of Gly-Pro-Gln-Gly-Leu-Leu-Gly-Ala-Hyp-Gly-Ile-Leu-Gly-Cys(Acm)-Cys(StBu)-Gly-Gly-OH, and the α1’ chain contained either 3 or 5 Gly-Pro-Hyp repeats on the N-terminus of Gly-Pro-Gln-Gly-Ile-Ala-Gly-Gln-Arg-Gly-Val-Val-Gly-Leu-Cys(StBu)-Gly-Gly-OH. The three chains are assembled into the heterotrimer by stepwise regioselective cross-linking. Peptide α1 is treated with 3-nitro-2-pyridine-sulfenyl chloride (Npys-Cl) to convert Cys(Acm) to Cys(Npys). Peptide α2 is treated with P(C4H9)3 to remove the StBu group. Peptides α1 and α2 are reacted at pH 4.5 to form a dimer. The dimer is treated with Npys-Cl to convert the α2 chain Cys(Acm) to Cys(Npys). The α1’ chain is treated with P(C4H9)3 to remove the StBu group. Peptide α1’ and the dimer are reacted at pH 4.5 to form a trimer.
Fig 9.
Heterotrimer THP 15 and general scheme for regioselective assembly of the α1, α2, and α1′ cysteine peptides into heterotrimers with the α1α2α1′ register
Another example of an N-terminal cysteine knot is construction of branched THPs 16–19 (Fig. 10) using the same protocol described for the C-terminally branched cysteine knot heterotri-meric THP [53]. Heterologous trimerization is achieved by stepwise disulfide bond-forming reactions (Fig. 9). First, the free thiol groups of the biscysteinyl peptides (20a, 20b) are converted to 2-pyridylthio (PyS) groups by treatment with PySSPy. The purified PyS-activated peptides (21a, 21b) are then mixed with monocys-teinyl peptides (22a, 22b) to yield corresponding heterodimeric peptides (23a, 23b). Formation of small amounts of homodimers is also observed in this reaction. The S-Acm groups in the heterodimers are converted to S-3-nitro-2-pyridinesulfenyl (Npys) groups by treating them with NpysCl. Even under optimized conditions, this reaction generated some by-products, including homodimers. Finally, the Npys-activated dimers (24a, 24b) were mixed with monocysteinyl peptides (22a, 22b) to yield desired heterotrimers.
Fig 10.
Synthesis of heterotimeric THPs utilizing an N-terminal cystine knot
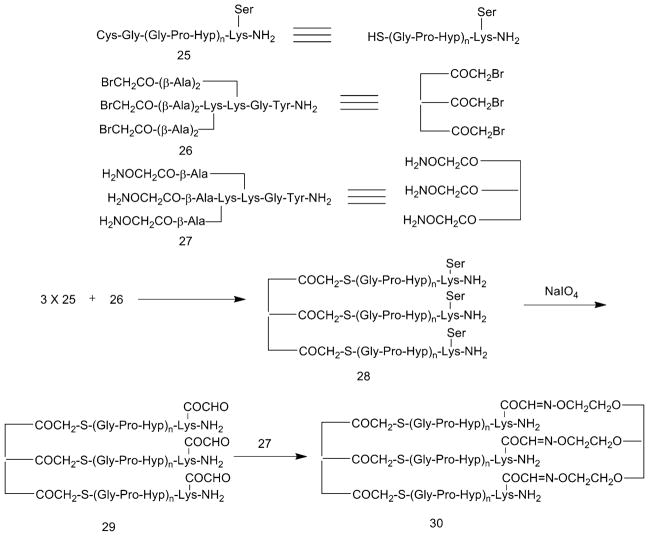
Stabilization of THPs can also be achieved by cross-linking both the C- and N-terminal ends of three peptide chains. This strategy required synthesis of three different fragments (Fig. 11) [88]. The first synthesis was of linear peptides of the sequence Cys-Gly-(Gly-Pro-Hyp)n-Lys(Ser)-NH2 (n =3–10) 25 using Fmoc chemistry, starting from Fmoc-Lys[Boc-Ser(tBu)]-OH, which in turn was prepared from reaction of Boc-Ser(tBu)-OSu with Fmoc-Lys-OH. The second synthesis was of two different trivalent anchor molecules: tris-bromoacetylated Lys-Lys dimer 26 and tris-aminooxyacetylated branch peptide 27 (synthesized from Bocaminooxyacetic acid). Chemoselective ligation of 25 and 26 followed by oxidation of the Lys(Ser) residue created an aldehyde group for the next ligation site. Final ligation of the N-terminus-coupled peptide with the tris-aminooxyacetyl group of anchor molecule 27 in aq. buffer produced di-cross-linked peptide 30.
Fig 11.
Structures of the collagen-model peptide 25 and the linker peptides 26 and 27, and the synthetic scheme for the assembly of cross-linked THP 30
Use of the conformationally constrained organic template cis,cis-1,3,5-trimethylcyclohexane 1,3,5-tricarboxylic acid (Kemp triacid, KTA, Fig. 7) is another strategy to induce or stabilize triple-helical structures [97–101]. This template possesses three carboxyl groups which can be coupled to the N-termini of three peptide chains. Attachment of a Gly residue as a spacer between each peptide chain and each carboxyl group on KTA is vital to compensate for the difference in diameters between the KTA and the collagen triple-helix and to facilitate the synthesis [97]. Two synthetic routes have been used to prepare KTA template-assembled collagen-based structures. The first method exclusively utilized solid-phase methodology [101], whereas in the second method formation of the peptide bond between KTA and peptide–peptoid chains was in solution [99]. In the solid-phase method Boc-(Gly-Pro-Hyp)n MBHA resin is prepared and then the Boc group is removed. KTA-(Gly-OH)3 is then coupled to the N-termini of peptide chains using DIPCDI and HOBt. In the second method peptide–peptoid chains are synthesized by solid-phase methods and cleaved from the resin. The free amine peptide–peptoid chains are then coupled with KTA-(Gly-OH)3 in solution using EDC and HOBt.
TREN (Fig. 7) is another beneficial scaffold for the synthesis of template THPs [102]. The TREN molecule is a flexible tripodal structure with three aminoethyl groups. TREN-(suc-OH)3 template is prepared by coupling TREN to monobenzylated succinate, and removing the benzyl ester groups by hydrogenation. The succinic acid groups extend the flexibility of the scaffold and provide terminal carboxylates for attachment of peptide chains. The succinic acid spacers release any steric hindrance by extending the reactive sites. TREN-(suc-OH)3 is acylated to three peptide strands simultaneously on the solid phase using DIC and HOBt.
Another synthetic scaffold molecule, which can be attached to both model and native collagen sequences, is cone-shaped CVT (Fig. 7) [103]. Attachment to the N-terminus of the collagen peptides with CVT is done in solution using BOP as a coupling reagent.
Stable collagen triple-helices are also synthesized by using a macrocyclic scaffold [104]. This scaffold belongs to a class of 18-membered cyclic hydropyran oligolides with alternating ester and ether linkages. In this scaffold, three ligand attachment sites form an equilateral triangle on one face. Macrocyclic template is coupled to the N-terminus of collagen-model peptides in solution using PyBOP and DIEA.
The use of carbohydrates as potential templates is another strategy for the de novo design of triple-helical peptides (Fig. 7) [105, 106]. Carbohydrates are multifunctional molecules having comparative rigidity of ring forms, simplicity in regioselective manipulation of functional groups, and access to mono- and di-saccharide stereoisomers. The primary and secondary hydroxyl of mono- and di-saccharides provide a more flexible control of the directionality and distances among attachment points of the peptide chains. This carbopeptide strategy starts with aminooxy-acetyl (Aoa) functionalization of the methyl α-D-galactopyranose (d-Galp) derivative to obtain template molecules followed by C-terminal peptide aldehyde coupling to the template via oxime formation.
The simplified N-terminal di-Glu template Gly-Phe-Gly-Glu-Glu-Gly was assembled by solid-phase Fmoc methodology, isolated as Fmoc-Gly-Phe-Gly-Glu-Glu-Gly, and acylated to three peptide strands simultaneously on the solid phase using 2-(1H-benzotriazole-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate (HBTU)/1-hydroxybenzotriazole (HOBt) [89].
As discussed earlier, heterotrimeric triple-helical peptides have usually been prepared using (1) disulfide-mediated cross-linking or (2) electrostatic-assisted self-assembly. More recently, “2 + 1 strand click synthesis” has been utilized to synthesize discrete homo- and heterotrimeric triple-helical peptides whereby the C-termini of the peptides are chemically stapled using the Huisgen Cu(I)-catalyzed azide-alkyne cycloaddition reaction [116]. The “2 + 1 strand click synthesis” requires assembly of a two-stranded branched peptide possessing a C-terminal 6-azidelysine residue, which has the capacity to “click” to a single-stranded peptide bearing a (2S)-propargylglycinamide at the C-terminus.
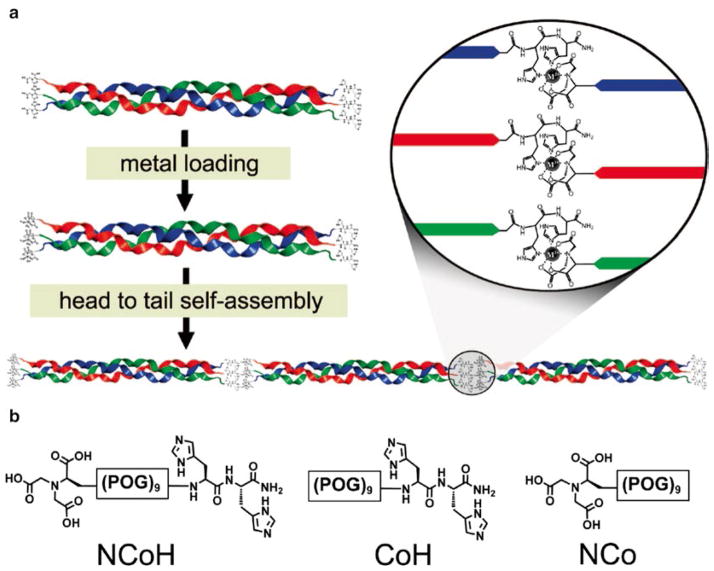
Metal chelation also plays an important role in the construction of templated triple-helices [107–109]. As an example the addition of a dopamine residue on the N-terminus of (Gly-Nleu-Pro)6 via a flexible succinic acid linker followed by incubation with Fe3+ converted a non-triple-helical peptide to a triple-helical peptide with a Tm value of 28 °C [107]. In similar fashion, addition of hydroxamic acid on the C-terminus of Boc-β-Ala-TRIS-[(Gly-Pro-Nleu)6]3, followed by incubation with Fe3+, resulted in a stabilized triple-helical structure (7 °C increase in Tm) [108]. Based on metal ion chelation, Chiemelewski et al. reported the self-assembly of THPs to form microflorettes [109]. To obtain collagen-based macromolecular assemblies with temporal control, the peptide design consisted of a central collagen-based core composed of nine repeating units of the tripeptide Pro-Hyp-Gly and distinct metal-binding ligands at each termini (NCoH, Fig. 12). The metal-binding ligands were a His2 moiety on the C-terminus and a nitrilotriacetic acid unit on the N-terminus. The core triple-helical unit has been used in the formation of collagen-like peptide fibers in solution upon the introduction of Zn2+, Cu2+, Ni2+, or Co2+. This assembly process was found to be fully reversible using EDTA as a metal ion chelator. The assembly process proceeds under mild conditions using neutrally buffered aqueous solution at room temperature.
Fig 12.
(a) Schematic representation of the design of the NCoH THP and assembly into higher order structures. Following triple-helix formation, the addition of metal ions triggers an initial assembly directed by the NTA and His2 moieties. (b) Structures of peptides NCoH, CoH, and NCo. Reprinted with permission from J Am Chem Soc, copyright 2009, American Chemical Society
The effect of templates is often to enhance the thermal stability of collagen-like sequences. For example, the THP acetyl-Gly-Gly-(Pro-Hyp-Gly)5-NH2 has a Tm value of 9.2 °C, while template versions of the same sequence, using either (+)CTV or KTA, have Tm values of 58 and 62 °C, respectively [103]. Similarly, (Gly-Pro-Hyp)6 has a Tm =25.4 °C, (Pro-Hyp-Gly)6 with a C-terminal branch has a Tm =39.4 °C, (Gly-Pro-Hyp)6 with an N-terminal branch has a Tm = 56.2 °C, and (Gly-Pro-Hyp)6 with both an N- and C-terminal branch has a Tm =69.7 °C [88, 117]. An N-terminal macrocyclic template induced triple-helical structure of the sequence Gly-(Pro-Pro-Gly)7-NH2 (Tm = 39.9 °C), whereas the non-templated sequence was not triple-helical [104]. Triple-helices containing peptoid residues, such as N-isobutylglycine (Nleu), have been stabilized by templates. Acetyl-(Gly-Nleu-Pro)6-NH2 had a Tm =26 °C, while KTA-[Gly-(Gly-Nleu-Pro)6-NH2]3, TREN-[suc-(Gly-Nleu-Pro)6-NH2]3, and Boc-β-Ala-TRIS-[(Gly-Nleu-Pro)6-OCH3]3 had Tm values of 36, 46, and 33 °C, respectively [100, 102, 118]. The thermal stability of the α2β1 integrin-binding sequence (Gly-Pro-Hyp)3-Gly-Phe-Hyp-Gly-Glu-Arg-(Gly-Pro-Hyp)3 was enhanced by an N-terminal Gly-Phe-Gly-Glu-Glu-Gly template, resulting in an increase in Tm from 25 to 44 °C [89]. Acetyl-(Pro-Hyp-Gly)5-Pro-Cys(StBu)-Cys(StBu)-Gly-Gly-Gly-NH2 had a Tm = 20.3 °C, while the double-disulfide-linked [acetyl-(Pro-Hyp-Gly)5-Pro-Cys-Cys-Gly-Gly-Gly-NH2]3 had a Tm =68.1 °C [119]. Formation of the cysteine knot in (Gly-Pro-Pro)3-Gly-Pro-Arg-Gly-Glu-Lys-Gly-Glu-Arg-Gly-Pro-Arg-(Gly-Pro-Pro)3-Gly-Pro-Cys-Cys-Gly increased Tm from 35 to 43 °C [120].
2 Materials
Dimethylformamide (DMF), N-methylpyrrolidone (NMP), N,N-diisopropylethylamine (DIEA), trifluoroacetic acid (TFA, peptide synthesis grade), methyl-tert-butyl ether (MTBE), 1,8-diaza-bicyclo[5.40.]undec-7-ene (DBU), dimethyl sulfoxide (DMSO), acetonitrile (peptide synthesis or HPLC grade), sodium acetate, ammonium acetate, NH4HCO3, isopropanol (Optima/HPLC grade), β-mercaptoethanol (molecular biology grade), thioanisole, ethanedithiol, phenol, Tris–HCl, NaCl, CaCl2, and brij-35 are purchased from Fisher Scientific (Atlanta, GA).
Piperidine is purchased from AnaSpec (San Jose, CA).
Fmoc-amino acids, N-[(1H-benzotriazol-1-yl)(dimethyl-amino)methylene]-N-methylmethanaminium hexafluoro-phosphate N-oxide (HBTU), 1-H-benzotriazolium-1-[bis (dimethylamino)methylene]-5-chloro-hexafluorophos-phate-(1-), 3-oxide (HCTU), 1-hydroxybenzotriazole (HOBt), Fmoc-Gly-Sasrin resin, Fmoc-Rink Amide 4-methyl-benzhydrylamine (MBHA) resin, and NovaSyn TGR PEG resin (see Note 1) are purchased from EMD Biosciences (San Diego, CA).
5,5’-Dithiobis(2-nitrobenzoic acid), α-cyano-4-hydroxycinnamic acid (MS grade), and 2-hydroxyethyl disulfide are purchased from Sigma-Aldrich (St. Louis, MO).
Alamar Blue is obtained from BioSource International (Camarillo, CA).
The monoalkyl chains hexanoic acid [CH3–(CH2)4–CO2H, designated C6], octanoic acid [CH3–(CH2)6–CO2H, designated C8], decanoic acid [CH3–(CH2)8–CO2H, designated C10], dodecanoic acid [CH3–(CH2)10–CO2H, designated C12], tetradecanoic acid [CH3–(CH2)12–CO2H, designated C14], palmitic acid [CH3–(CH2)14–CO2H, designated C16], and stearic acid [CH3–(CH2)16–CO2H, designated C18] are purchased from Sigma-Aldrich.
3 Methods
3.1 Synthesis and Characterization of Peptide-Amphiphiles
Incorporation of individual amino acids is by Fmoc solid-phase methodology using cycles described previously [90, 91].
For Fmoc removal, a solution of DBU–piperidine–NMP (1:5:44) is used for 5–10 and 10–15 min.
All couplings are performed with HCTU/HOBt.
Acylation of the peptide–resin involves condensing a fourfold molar excess of alkyl acid to the Nα-deprotected resin, with a 3.8-fold molar excess each of HBTU and HOBt in DMF for 2 h. The reaction is initiated by the addition of an eightfold molar excess of DIEA and proceeds for 1 h.
Cleavage and side-chain deprotection of the lipidated peptide–resins is accomplished by treating the resin for 1–2 h with either H2O–TFA (1:19) or ethanedithiol–thioanisole–phenol–H2O–TFA (2.5:5:5:5:82.5) [121, 122].
Resins are filtered and rinsed with TFA, and the combined filtrate and wash reduced under vacuum at room temperature to ~0.5 mL and precipitated with MTBE.
The precipitate is centrifuged and washed three times with MTBE, dissolved in acetonitrile–H2O (1:9–1:4), and purified by preparative reversed-phase HPLC.
Preparative reversed-phase HPLC is performed on a Rainin AutoPrep System with a Vydac 218TP152022 C18 or 214TP152022 C4 column (15–20 μm particle size, 300 Å pore size, 250 mm × 22 mm) at a flow rate of 10 mL/min with 0.1 % TFA in H2O (A) and 0.1 % TFA in acetonitrile (B), and detection at λ = 220 nm. For very hydrophobic samples, it is advantageous to use 0.1 % TFA in isopropanol instead of acetonitrile [73, 74]. Analytical HPLC is performed on a Hewlett-Packard 1100 Liquid Chromatograph equipped with an ODS Hypersil C-18 column (5 μm particle size, 100 mm × 2.1 mm).
PA composition can be determined by MALDI-TOF mass spectrometry (MS) [64, 73, 84, 85, 123–125]. PA homogeneity can also be evaluated by diphenyl and nonporous C18 reversed-phase HPLC [124] and/or hydrophobic interaction HPLC.
Triple-helicity is monitored by circular dichroism (CD) spectroscopy in the far UV wavelengths [64, 73, 74, 83–85, 90, 91, 125]. Peptides are dissolved in the appropriate buffer at concentrations of 2–500 μM. Conditions should reflect final relevant assay conditions as much as possible based on limitations imposed by sample availability and/or instrumentation. For example, if the peptides are to be used in a cell-binding assay at 10 μM in PBS, then the CD spectra should be acquired at that peptide concentration in a similar buffer. Since chloride ions interfere with the acquisition of data, a phosphate buffer can be substituted for PBS. A typical spectrum for a triple-helix shows a positive molar ellipticity at λ ~ 225 nm and a negative molar ellipticity at λ ~ 205 nm. In addition to the far UV wavelength scan of λ ~ 195–250 nm, one can determine the melting temperature by monitoring the change in molar ellipticity at λ = 225 nm with a constant change in temperature from 5 to 85 °C. For samples exhibiting sigmoidal melting curves, the inflection point in the transition region (first derivative) is defined as Tm. Alternatively, Tm is evaluated from the midpoint of the transition.
NMR spectroscopy can also be used to study the relative thermal stability, alignment, and flexibility (backbone mobilities) of triple-helical PAs [73, 74, 126].
3.2 Template-Assembled Synthetic THP Methods
3.2.1 Preparation of [N-tris(Fmoc-Ahx)-Lys-Lys]-Tyr-Gly Branched Template
Deprotect 1 g of Fmoc-Gly-Sasrin resin with 20 % piperidine in DMF for 30 min followed by a second 5-min treatment.
Wash the resin three times with DMF. If the substitution level is higher than 0.5 mmol/g, add 0.2 mmol benzoic anhydride dissolved in 25 ml DMF and 0.4 mmol DIEA, mix for 1 h, and wash the resin three times with DMF.
Dissolve 3 eq of Fmoc-Tyr(tBu) in approximately 10 mL DMF. Add 2.7 eq of HBTU and 3 eq of HOBt, and then add the mixture to resin. Add 6 eq of DIEA, mix for 30–60 min, and wash the resin with DMF three times.
Remove Fmoc as indicated above and wash the resin three times with DMF.
Dissolve 3 eq of Fmoc-Lys(Dde) (see Note 1) in approximately 10 mL DMF. Add 2.7 eq of HBTU and 3 eq of HOBt and then add the mixture to the resin. Add 6 eq of DIEA, shake for 30–60 min, and wash with DMF three times.
Repeat the Fmoc removal and Fmoc-Lys(Dde) coupling steps such that the resin contains two adjacent Lys(Dde) residues (see Note 2).
If the desired construct is homotrimeric (see Note 3), the Dde and Fmoc groups can be simultaneously removed by treatment with 2 % hydrazine in DMF for 30 min followed by three rinses with DMF. An optional spacer of Fmoc-Ahx acid can be added to reduce the steric hindrance of the branch.
Keeping in mind that the chain is now trimeric, dissolve 3 eq of Fmoc-Ahx in approximately 10 mL DMF, add 2.7 eq of HBTU and 3 eq of HOBt, and add the mixture to the resin. Add 6 eq of DIEA, mix for 60 min, and wash three times with DMF.
A small volume of template is cleaved from the resin using H2O–TFA (5:95) for 1 h and analyzed by MALDI-TOF mass spectrometry to confirm proper branch assembly. This scale of template is sufficient for three 0.25 mmol syntheses.
3.2.2 Peptide Synthesis, Side-Chain Deprotection, and Peptide–Resin Cleavage
Incorporation of individual amino acids is by Fmoc solid-phase methodology [90, 91].
For Fmoc removal, a solution of DBU–piperidine–NMP (1:5:44) is used for 5–10 and 10–15 min.
All couplings are performed with HCTU/HOBt.
Peptide–resins are cleaved and side-chain deprotected by treatment with H2O–TFA (5:95), H2O–thioanisole–TFA (5:5:90), orethanedithiol–H2O–thioanisole–phenol–TFA(2.5:5:5:5:82.5) for ≥2 h depending upon the peptide sequence [121, 122].
Resins are filtered and rinsed with TFA, and the combined filtrate and wash reduced under vacuum at room temperature to ~0.5 mL and precipitated with MTBE.
The precipitate is centrifuged, washed three times with MTBE, dissolved in acetonitrile–H2O (1:9–1:4), and purified by preparative reversed-phase HPLC.
3.2.3 Purification and Characterization of Peptide-Template
Preparative reversed-phase HPLC is performed with a Vydac 218TP152022 C18 column (15–20 μm particle size, 300 Å pore size, 250 mm × 22 mm) at a flow rate of 10 mL/min with 0.1 % TFA in H2O (A) and 0.1 % TFA in acetonitrile (B), and detection at λ=220 nm.
The eluent is lyophilized to a powder and repurified using a semipreparative Vydac 219TP54 diphenyl column (5 μm particle size, 300 Å pore size, 250 mm × 4.6 mm) at a flow rate of 2 mL/min with detection at λ=220 nm.
Analytical HPLC is performed with an ODS Hypersil C-18 column (5 μm particle size, 100 mm × 2.1 mm).
Chain assembly is confirmed by Edman degradation and MALDI-TOF mass spectrometry using a specialized matrix mixture containing 2,5-dihydroxybenzoic acid/2-hydroxy-5-methoxybenzoic acid (9:1, v/v) [84, 117].
Footnotes
By utilizing the array of readily available orthogonally protected Lys derivatives, including, but not limited to, Fmoc-Lys(Dde), Dde-Lys(Fmoc), Fmoc-Lys(Fmoc), Fmoc-Lys(Mmt), Fmoc-Lys(Mtt), or Fmoc-Lys(ivDde), an array of topological designs can be created. The dilute acid utilized for side-chain deprotection of the Mmt and Mtt groups will cleave the template from the Gly-SASRIN resin. Thus, a more acid-stable resin such as Rink amide MBHA should be utilized in combination with Fmoc-Lys(Mmt) and Fmoc-Lys(Mtt).
One can create a variety of templates based on the simple Lys-branching method. The template can accommodate 2–8 branches with ease and can also create a number of homo- or hetero-multimeric sequences. However, as the number of branching points increases care must be taken during N-α-amino or N-ε-amino deprotection and subsequent couplings. The resin becomes increasingly “sticky” as the number of free amino groups increases, which can complicate subsequent deprotection and coupling reactions. Ensure that a proper solution volume or resin mixing action is taken to avoid deletions during peptide chain assembly.
One can also utilize the branching strategy to construct heterotrimeric triple-helical peptides [112]. The synthesis of triple-helical peptides with one chain of different sequence from the other two requires a four-dimensional orthogonal scheme, where HMP is the linker and Dde or 1-(4,4-dimethyl-2,6-dioxocyclohex-1-ylidene)-3-methylbutyl (ivDde) [127, 128] side-chain protection of Fmoc-Lys is used. Synthesis by Fmoc strategy of one chain (i.e., the α2 collagen chain sequence) proceeds through the α-amino of Lys; thus, the Dde/ivDde and Fmoc groups are not removed simultaneously. The α2 chain sequence is assembled by Fmoc chemistry as described above. After incorporation of the α2 chain sequence, the peptide chain is “capped” by using allyloxycarbonyl (Aloc) chloride or Aloc-Gly, creating an N-terminal Aloc group. The Lys α-amino Dde or ivDde groups are then removed with hydrazine (see above), which does not remove Aloc, and synthesis by Fmoc strategy proceeds for the other two chains (i.e., α1 collagen chain sequence). Once all three chains are equivalent in terms of number of residues incorporated, the Aloc group is removed by treatment with (Ph3P)4Pd in CHCl3–acetic acid–N-methyl morpholine (20:1:0.5) [129] and the Fmoc groups removed as described above. Finally, Fmoc-Gly, -Pro, and -Hyp(tBu) are incorporated into all three chains. Cleavage and side-chain deprotection are by TFA as described above.
References
- 1.Myllyharju J, Kivirikko KI. Collagens and collagen-related diseases. Ann Med. 2001;33:7–21. doi: 10.3109/07853890109002055. [DOI] [PubMed] [Google Scholar]
- 2.Hashimoto T, Wakabayashi T, Watanabe A, Kowa H, Hosoda R, Nakamura A, Kanazawa I, Arai T, Takio K, Mann DM, Iwatsubo T. CLAC: a novel Alzheimer amyloid plaque component derived from a transmembrane precursor, CLAC-P/collagen type XXV. EMBO J. 2002;21:1524–1534. doi: 10.1093/emboj/21.7.1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boulegue C, Musiol HJ, Gotz MG, Renner C, Moroder L. Natural and artificial cystine knots for assembly of homo- and heterotrimeric collagen models. Antioxid Redox Signal. 2008;10:113–125. doi: 10.1089/ars.2007.1868. [DOI] [PubMed] [Google Scholar]
- 4.Gordon MK, Hahn RA. Collagens. Cell Tissue Res. 2010;339:247–257. doi: 10.1007/s00441-009-0844-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shoulders MD, Raines RT. Collagen structure and stability. Annu Rev Biochem. 2009;78:929–958. doi: 10.1146/annurev.biochem.77.032207.120833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cole WG. Collagen genes: mutations affecting collagen structure and expression. Prog Nucleic Acid Res Mol Biol. 1994;47:29–80. doi: 10.1016/s0079-6603(08)60249-4. [DOI] [PubMed] [Google Scholar]
- 7.Brodsky B, Shah NK. Protein motifs. 8. The triple-helix motif in proteins. FASEB J. 1995;9:1537–1546. doi: 10.1096/fasebj.9.15.8529832. [DOI] [PubMed] [Google Scholar]
- 8.Fields GB, Prockop DJ. Perspectives on the synthesis and application of triple-helical, collagen-model peptides. Biopolymers. 1996;40:345–357. doi: 10.1002/(SICI)1097-0282(1996)40:4%3C345::AID-BIP1%3E3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 9.Jenkins CL, Raines RT. Insights on the conformational stability of collagen. Nat Prod Rep. 2002;19:49–59. doi: 10.1039/a903001h. [DOI] [PubMed] [Google Scholar]
- 10.Koide T. Triple helical collagen-like peptides: engineering and applications in matrix biology. Connect Tissue Res. 2005;46:131–141. doi: 10.1080/03008200591008518. [DOI] [PubMed] [Google Scholar]
- 11.Koide T. Designed triple-helical peptides as tools for collagen biochemistry and matrix engineering. Philos Trans R Soc Lond B Biol Sci. 2007;362:1281–1291. doi: 10.1098/rstb.2007.2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brodsky B, Thiagarajan G, Madhan B, Kar K. Triple-helical peptides: an approach to collagen conformation, stability, and self-association. Biopolymers. 2008;89:345–353. doi: 10.1002/bip.20958. [DOI] [PubMed] [Google Scholar]
- 13.Fields GB. Synthesis and biological applications of collagen-model triple-helical peptides. Org Biomol Chem. 2010;8:1237–1258. doi: 10.1039/b920670a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramachandran GN, Kartha G. Structure of collagen. Nature. 1954;174:269–270. doi: 10.1038/174269c0. [DOI] [PubMed] [Google Scholar]
- 15.Ramachandran GN, Kartha G. Structure of collagen. Nature. 1955;176:593–595. doi: 10.1038/176593a0. [DOI] [PubMed] [Google Scholar]
- 16.Rich A, Crick FH. The structure of collagen. Nature. 1955;176:915–916. doi: 10.1038/176915a0. [DOI] [PubMed] [Google Scholar]
- 17.Rich A, Crick FH. The molecular structure of collagen. J Mol Biol. 1961;3:483–506. doi: 10.1016/s0022-2836(61)80016-8. [DOI] [PubMed] [Google Scholar]
- 18.Bella J, Eaton M, Brodsky B, Berman HM. Crystal and molecular structure of a collagen-like peptide at 1.9 A resolution. Science. 1994;266:75–81. doi: 10.1126/science.7695699. [DOI] [PubMed] [Google Scholar]
- 19.Riddihough G. Structure of collagen. Nat Struct Biol. 1998;5 [Google Scholar]
- 20.Okuyama K, Miyama K, Mizuno K, Bachinger HP. Crystal structure of (Gly-Pro-Hyp)(9): implications for the collagen molecular model. Biopolymers. 2012;97:607–616. doi: 10.1002/bip.22048. [DOI] [PubMed] [Google Scholar]
- 21.Woodhead-Galloway J. Collagen: the anatomy of a protein. Edward Arnold Limited; London: 1980. [Google Scholar]
- 22.Jenkins CL, Vasbinder MM, Miller SJ, Raines RT. Peptide bond isosteres: ester or (E)-alkene in the backbone of the collagen triple helix. Org Lett. 2005;7:2619–2622. doi: 10.1021/ol050780m. [DOI] [PubMed] [Google Scholar]
- 23.Dai N, Wang XJ, Etzkorn FA. The effect of a trans-locked Gly-Pro alkene isostere on collagen triple helix stability. J Am Chem Soc. 2008;130:5396–5397. doi: 10.1021/ja711021m. [DOI] [PubMed] [Google Scholar]
- 24.Dai N, Etzkorn FA. Cis-trans proline isomerization effects on collagen triple-helix stability are limited. J Am Chem Soc. 2009;131:13728–13732. doi: 10.1021/ja904177k. [DOI] [PubMed] [Google Scholar]
- 25.Holmgren SK, Taylor KM, Bretscher LE, Raines RT. Code for collagen’s stability deciphered. Nature. 1998;392:666–667. doi: 10.1038/33573. [DOI] [PubMed] [Google Scholar]
- 26.Holmgren SK, Bretscher LE, Taylor KM, Raines RT. A hyperstable collagen mimic. Chem Biol. 1999;6:63–70. doi: 10.1016/S1074-5521(99)80003-9. [DOI] [PubMed] [Google Scholar]
- 27.Bretscher LE, Jenkins CL, Taylor KM, Derider ML, Raines RT. Conformational stability of collagen relies on a stereoelectronic effect. J Am Chem Soc. 2001;123:777–778. doi: 10.1021/ja005542v. [DOI] [PubMed] [Google Scholar]
- 28.Eberhardt ES, Panisik N, Jr, Raines RT. Inductive effects on the energetics of prolyl peptide bond isomerization: implications for collagen folding and stability. J Am Chem Soc. 1996;118:12261–12266. doi: 10.1021/ja9623119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sakakibara S, Inouye K, Shudo K, Kishida Y, Kobayashi Y, Prockop DJ. Synthesis of (Pro-Hyp-Gly) n of defined molecular weights. Evidence for the stabilization of collagen triple helix by hydroxypyroline. Biochim Biophys Acta. 1973;303:198–202. doi: 10.1016/0005-2795(73)90164-5. [DOI] [PubMed] [Google Scholar]
- 30.Kotch FW, Guzei IA, Raines RT. Stabilization of the collagen triple helix by O-methylation of hydroxyproline residues. J Am Chem Soc. 2008;130:2952–2953. doi: 10.1021/ja800225k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nagarajan V, Kamitori S, Okuyama K. Structure analysis of a collagen-model peptide with a (Pro-Hyp-Gly) sequence repeat. J Biochem. 1999;125:310–318. doi: 10.1093/oxfordjournals.jbchem.a022288. [DOI] [PubMed] [Google Scholar]
- 32.Inouye K, Sakakibara S, Prockop DJ. Effects of the stereo-configuration of the hydroxyl group in 4-hydroxyproline on the triple-helical structures formed by homogenous peptides resembling collagen. Biochim Biophys Acta. 1976;420:133–141. doi: 10.1016/0005-2795(76)90352-4. [DOI] [PubMed] [Google Scholar]
- 33.Bann JG, Bachinger HP. Glycosylation/hydroxylation-induced stabilization of the collagen triple helix. 4-trans-hydroxyproline in the Xaa position can stabilize the triple helix. J Biol Chem. 2000;275:24466–24469. doi: 10.1074/jbc.M003336200. [DOI] [PubMed] [Google Scholar]
- 34.Mizuno K, Hayashi T, Bachinger HP. Hydroxylation-induced stabilization of the collagen triple helix. Further characterization of peptides with 4(R)-hydroxyproline in the Xaa position. J Biol Chem. 2003;278:32373–32379. doi: 10.1074/jbc.M304741200. [DOI] [PubMed] [Google Scholar]
- 35.Derider ML, Wilkens SJ, Waddell MJ, Bretscher LE, Weinhold F, Raines RT, Markley JL. Collagen stability: insights from NMR spectroscopic and hybrid density functional computational investigations of the effect of electronegative substituents on prolyl ring conformations. J Am Chem Soc. 2002;124:2497–2505. doi: 10.1021/ja0166904. [DOI] [PubMed] [Google Scholar]
- 36.Vitagliano L, Berisio R, Mastrangelo A, Mazzarella L, Zagari A. Preferred pro-line puckerings in cis and trans peptide groups: implications for collagen stability. Protein Sci. 2001;10:2627–2632. doi: 10.1110/ps.ps.26601a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shoulders MD, Satyshur KA, Forest KT, Raines RT. Stereoelectronic and steric effects in side chains preorganize a protein main chain. Proc Natl Acad Sci U S A. 2010;107:559–564. doi: 10.1073/pnas.0909592107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shoulders MD, Hodges JA, Raines RT. Reciprocity of steric and stereoelectronic effects in the collagen triple helix. J Am Chem Soc. 2006;128:8112–8113. doi: 10.1021/ja061793d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee SG, Lee JY, Chmielewski J. Investigation of pH-dependent collagen triple-helix formation. Angew Chem Int Ed Engl. 2008;47:8429–8432. doi: 10.1002/anie.200802224. [DOI] [PubMed] [Google Scholar]
- 40.Babu IR, Ganesh KN. Enhanced triple helix stability of collagen peptides with 4R-aminoprolyl (Amp) residues: relative roles of electrostatic and hydrogen bonding effects. J Am Chem Soc. 2001;123:2079–2080. doi: 10.1021/ja002165d. [DOI] [PubMed] [Google Scholar]
- 41.Umashankara M, Babu IR, Ganesh KN. Two prolines with a difference: contrasting stereoelectronic effects of 4R/S--aminoproline on triplex stability in collagen peptides [pro(X)-pro(Y)-Gly]n. Chem Commun (Camb) 2003;20:2606–2607. doi: 10.1039/b308581c. [DOI] [PubMed] [Google Scholar]
- 42.Shah NK, Ramshaw JA, Kirkpatrick A, Shah C, Brodsky B. A host-guest set of triple-helical peptides: stability of Gly-X-Y triplets containing common nonpolar residues. Biochemistry. 1996;35:10262–10268. doi: 10.1021/bi960046y. [DOI] [PubMed] [Google Scholar]
- 43.Persikov AV, Ramshaw JA, Brodsky B. Collagen model peptides: sequence dependence of triple-helix stability. Biopolymers. 2000;55:436–450. doi: 10.1002/1097-0282(2000)55:6<436::AID-BIP1019>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 44.Persikov AV, Ramshaw JA, Kirkpatrick A, Brodsky B. Amino acid propensities for the collagen triple-helix. Biochemistry. 2000;39:14960–14967. doi: 10.1021/bi001560d. [DOI] [PubMed] [Google Scholar]
- 45.Berisio R, DSA, Ruggiero A, Improta R, Vitagliano L. Role of side chains in collagen triple helix stabilization and partner recognition. J Pept Sci. 2008;15:131–140. doi: 10.1002/psc.1082. [DOI] [PubMed] [Google Scholar]
- 46.Persikov AV, Ramshaw JA, Kirkpatrick A, Brodsky B. Triple-helix propensity of hydroxyproline and fluoroproline: comparison of host-guest and repeating tripeptide collagen models. J Am Chem Soc. 2003;125:11500–11501. doi: 10.1021/ja036673+. [DOI] [PubMed] [Google Scholar]
- 47.Persikov AV, Ramshaw JA, Brodsky B. Prediction of collagen stability from amino acid sequence. J Biol Chem. 2005;280:19343–19349. doi: 10.1074/jbc.M501657200. [DOI] [PubMed] [Google Scholar]
- 48.Venugopal MG, Ramshaw JA, Braswell E, Zhu D, Brodsky B. Electrostatic interactions in collagen-like triple-helical peptides. Biochemistry. 1994;33:7948–7956. doi: 10.1021/bi00191a023. [DOI] [PubMed] [Google Scholar]
- 49.Persikov AV, Ramshaw JA, Kirkpatrick A, Brodsky B. Peptide investigations of pairwise interactions in the collagen triple-helix. J Mol Biol. 2002;316:385–394. doi: 10.1006/jmbi.2001.5342. [DOI] [PubMed] [Google Scholar]
- 50.Fallas JA, Dong J, Tao YJ, Hartgerink JD. Structural insights into charge pair interactions in triple helical collagen-like proteins. J Biol Chem. 2012;287:8039–8047. doi: 10.1074/jbc.M111.296574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Persikov AV, Ramshaw JA, Kirkpatrick A, Brodsky B. Electrostatic interactions involving lysine make major contributions to collagen triple-helix stability. Biochemistry. 2005;44:1414–1422. doi: 10.1021/bi048216r. [DOI] [PubMed] [Google Scholar]
- 52.Yang W, Chan VC, Kirkpatrick A, Ramshaw JA, Brodsky B. Gly-Pro-Arg confers stability similar to Gly-Pro-Hyp in the collagen triple-helix of host-guest peptides. J Biol Chem. 1997;272:28837–28840. doi: 10.1074/jbc.272.46.28837. [DOI] [PubMed] [Google Scholar]
- 53.Koide T, Nishikawa Y, Takahara Y. Synthesis of heterotrimeric collagen models containing Arg residues in Y-positions and analysis of their conformational stability. Bioorg Med Chem Lett. 2004;14:125–128. doi: 10.1016/j.bmcl.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 54.Hudlicky MMaJM. New stereospecific syntheses and x-ray diffraction structures of (-)-D-erythro- and (+)-L-threo-4-fluoroglutamic. Tetrahedron Lett. 1990;31:7403–7406. [Google Scholar]
- 55.Hudlicky M. Stereospecific syntheses of all four stereoisomers of 4-fluoroglutamic acid. J Fluorine Chem. 1993;60:193–210. [Google Scholar]
- 56.Panasik N, Jr, Eberhardt ES, Edison AS, Powell DR, Raines RT. Inductive effects on the structure of proline residues. Int J Pept Protein Res. 1994;44:262–269. doi: 10.1111/j.1399-3011.1994.tb00169.x. [DOI] [PubMed] [Google Scholar]
- 57.Kronenthal DR, Mueller RH, Kuester TP, Kissick TP, Johnson EJ. Stereospecific friedel-crafts alkylation of benzene with 4-mesyloxy-L-prolines. A new synthesis of 4-phenylprolines. Tetrahedron Lett. 1990;31:1241–1244. [Google Scholar]
- 58.Gottlieb AA, Yoshimasa F, Undenfriend S, Witkop B. Incorporation of cis- and trans-4-fluoro-L-prolines into proteins and hydroxylation of the trans isomer during collagen biosynthesis. Biochemistry. 1965;4:2507–2513. [Google Scholar]
- 59.Shirota FN, Nagasawa HT, Elberling JA. Potential inhibitors of collagen biosynthesis. 5,5-Difluoro-DL-lysine and 5, 5-dimethyl-DL-lysine and their activation by lysyl-tRNA ligase. J Med Chem. 1977;20:1623–1627. doi: 10.1021/jm00222a017. [DOI] [PubMed] [Google Scholar]
- 60.Hart BP, Coward JK. The synthesis of DL-3,3-Difluoroglutamic acid from a 3-oxoprolinol derivative. Tetrahedron Lett. 1993;34:4917–4920. [Google Scholar]
- 61.Avent AG, Bowler AN, Doyle PM, Marchand CM, Young DW. Stereospecific synthesis of 4-fluoroglutamic acids. Tetrahedron Lett. 1992;33:1509–1512. [Google Scholar]
- 62.Demange L, Menez A, Dugave C. Practical synthesis of Boc and Fmoc protected 4-fluoro and 4-difluoroprolines from trans-4-hydroxyproline. Tetrahedron Lett. 1998;39:1169–1172. [Google Scholar]
- 63.Tran TT, Patino N, Condom R, Frogier T, Guedj R. Fluorinated peptides incorporating a 4-fluoroproline residue as potential inhibitors of HIV protease. J Fluorine Chem. 1997;82:125–130. [Google Scholar]
- 64.Malkar NB, Lauer-Fields JL, Borgia JA, Fields GB. Modulation of triple-helical stability and subsequent melanoma cellular responses by single-site substitution of fluoroproline derivatives. Biochemistry. 2002;41:6054–6064. doi: 10.1021/bi012071w. [DOI] [PubMed] [Google Scholar]
- 65.Martinez J, Tolle JC, Bodanszky M. Side reactions in peptide synthesis. 12. Hydrogenolysis of the 9-fluorenylmethyloxycarbonyl group. J Org Chem. 1979;44:3596–3598. [Google Scholar]
- 66.Atherton E, Bury C, Sheppard RC, Williams BJ. Stability of fluorenylemthoxycar-bonylamino groups in peptide synthesis. Cleavage by hydrogenolysis and by dipolar aprotic solvents. Tetrahedron Lett. 1979;20:3041–3042. [Google Scholar]
- 67.Vnek V, Budesinsky M, Rinnová M, Rosenberg I. Prolinol-based nucleoside phos-phonic acids: new isosteric conformationally flexible nucleotide analogues. Tetrahedron. 2009;65:862–876. [Google Scholar]
- 68.Doi M, Nishi Y, Kiritoshi N, Iwata T, Nago M, Nakano H, Uchiyama S, Nakazawa T, Wakamiya T, Kobayashi Y. Simple and efficient syntheses of Boc- and Fmoc- protected 4(R)- and 4(S)-fluoroproline solely from 4(R)-hydroxyproline. Tetrahedron. 2002;58:8453–8459. [Google Scholar]
- 69.Hodges JA, Raines RT. Stereoelectronic effects on collagen stability: the dichotomy of 4-fluoroproline diastereomers. J Am Chem Soc. 2003;125:9262–9263. doi: 10.1021/ja035881z. [DOI] [PubMed] [Google Scholar]
- 70.Bhowmick M, Sappidi RR, Fields GB, Lepore SD. Efficient synthesis of Fmoc-protected phosphinic pseudodipeptides: building blocks for the synthesis of matrix metalloproteinase inhibitors. Biopolymers. 2011;96:1–3. doi: 10.1002/bip.21425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Goodman M, Del Valle JR. Asymmetric hydrogenations for the synthesis of Boc-protected 4-alkylprolinols and prolines. J Org Chem. 2003;68:3923–3931. doi: 10.1021/jo034214l. [DOI] [PubMed] [Google Scholar]
- 72.Murakami Y, Nakano A, Yoshimatsu A, Uchitomi K, Mastsuda Y. Characterization of molecular aggregates of peptide amphiphiles and kinetics of dynamic processes performed by single-walled vesicles. J Am Chem Soc. 1984;106:3613–3623. [Google Scholar]
- 73.Yu Y-C, Brendt P, Tirrell M, Fields GB. Self-assembling amphiphiles for construction of protein molecular architecture. J Am Chem Soc. 1996;118:12515–12520. [Google Scholar]
- 74.Yu YC, Berndt P, Tirrell M, Fields GB. Minimal lipidation stabilizes protein-like molecular architecture. J Am Chem Soc. 1998;120:9979–9987. [Google Scholar]
- 75.Pakalns T, Haverstick KL, Fields GB, Mccarthy JB, Mooradian DL, Tirrell M. Cellular recognition of synthetic peptide amphiphiles in self-assembled monolayer films. Biomaterials. 1999;20:2265–2279. doi: 10.1016/s0142-9612(99)00157-x. [DOI] [PubMed] [Google Scholar]
- 76.Forns P, Lauer-Fields JL, Gao S, Fields GB. Induction of protein-like molecular architecture by monoalkyl hydrocarbon chains. Biopolymers. 2000;54:531–546. doi: 10.1002/1097-0282(200012)54:7<531::AID-BIP60>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 77.Borgia JA, Fields GB. Chemical synthesis of proteins. Trends Biotechnol. 2000;18:243–251. doi: 10.1016/s0167-7799(00)01445-1. [DOI] [PubMed] [Google Scholar]
- 78.Mammen M, Choi SK, Whitesides GM. Polyvalent interactions in biological systems: implications for design and use of multivalent ligands and inhibitors. Angew Chem Int Ed. 1998;37:2754–2794. doi: 10.1002/(SICI)1521-3773(19981102)37:20<2754::AID-ANIE2754>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 79.García M, Alsina M, Reig F, Haro I. Liposomes as vehicles for the presentation of a synthetic peptide containing an epitope of hepatitis A virus. Vaccine. 2000;18:276–283. doi: 10.1016/s0264-410x(99)00198-x. [DOI] [PubMed] [Google Scholar]
- 80.Gore T, Dori Y, Talmon Y, Tirrell M, Bianco-Peled H. Self-assembly of model collagen peptide amphiphiles. Langmuir. 2001;17:5352–5360. [Google Scholar]
- 81.Malkar NB, Lauer-Fields JL, Juska D, Fields GB. Characterization of peptide-amphiphiles possessing cellular activation sequences. Biomacromolecules. 2003;4:518–528. doi: 10.1021/bm0256597. [DOI] [PubMed] [Google Scholar]
- 82.Fields GB, Lauer JL, Dori Y, Forns P, Yu YC, Tirrell M. Protein-like molecular architecture: biomaterial applications for inducing cellular receptor binding and signal transduction. Biopolymers. 1998;47:143–151. doi: 10.1002/(SICI)1097-0282(1998)47:2<143::AID-BIP3>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 83.Lauer-Fields JL, Tuzinski KA, Shimokawa K, Nagase H, Fields GB. Hydrolysis of triple-helical collagen peptide models by matrix metalloproteinases. J Biol Chem. 2000;275:13282–13290. doi: 10.1074/jbc.275.18.13282. [DOI] [PubMed] [Google Scholar]
- 84.Lauer-Fields JL, Nagase H, Fields GB. Use of Edman degradation sequence analysis and matrix-assisted laser desorption/ionization mass spectrometry in designing substrates for matrix metalloproteinases. J Chromatogr A. 2000;890:117–125. doi: 10.1016/s0021-9673(00)00396-4. [DOI] [PubMed] [Google Scholar]
- 85.Lauer-Fields JL, Broder T, Sritharan T, Chung L, Nagase H, Fields GB. Kinetic analysis of matrix metalloproteinase activity using fluorogenic triple-helical substrates. Biochemistry. 2001;40:5795–5803. doi: 10.1021/bi0101190. [DOI] [PubMed] [Google Scholar]
- 86.Kusebauch U, Cadamuro SA, Musiol HJ, Moroder L, Renner C. Photocontrol of the collagen triple helix: synthesis and conformational characterization of bis-cysteinyl collagenous peptides with an azobenzene clamp. Chemistry. 2007;13:2966–2973. doi: 10.1002/chem.200601162. [DOI] [PubMed] [Google Scholar]
- 87.Hojo H, Akamatsu Y, Yamauchi K, Kinoshita M, Miki S, Nakamura Y. Synthesis and structural characterization of triple-helical peptides which mimic the ligand binding site of the human macrophage scavenger receptor. Tetrahedron. 1997;53:14263–14274. [Google Scholar]
- 88.Tanaka Y, Suzuki K, Tanaka T. Synthesis and stabilization of amino and car-boxy terminal constrained collagenous peptides. J Pept Res. 1998;51:413–419. doi: 10.1111/j.1399-3011.1998.tb00639.x. [DOI] [PubMed] [Google Scholar]
- 89.Khew ST, Tong YW. Template-assembled triple-helical peptide molecules: mimicry of collagen by molecular architecture and integrin-specific cell adhesion. Biochemistry. 2008;47:585–596. doi: 10.1021/bi702018v. [DOI] [PubMed] [Google Scholar]
- 90.Fields CG, Lovdahl CM, Miles AJ, Hagen VL, Fields GB. Solid-phase synthesis and stability of triple-helical peptides incorporating native collagen sequences. Biopolymers. 1993;33:1695–1707. doi: 10.1002/bip.360331107. [DOI] [PubMed] [Google Scholar]
- 91.Fields CG, Mickelson DJ, Drake SL, Mccarthy JB, Fields GB. Melanoma cell adhesion and spreading activities of a synthetic 124-residue triple-helical “mini-collagen”. J Biol Chem. 1993;268:14153–14160. [PubMed] [Google Scholar]
- 92.Ottl J, Moroder L. Disulfide-bridged heterotrimeric collagen peptides containing the collagenase cleavage site of collagen type I. Synthesis and conformational properties. J Am Chem Soc. 1999;121:653–661. [Google Scholar]
- 93.Ottl J, Gabriel D, Murphy G, Knauper V, Tominaga Y, Nagase H, Kroger M, Tschesche H, Bode W, Moroder L. Recognition and catabolism of synthetic heterotrimeric collagen peptides by matrix metalloprotein-ases. Chem Biol. 2000;7:119–132. doi: 10.1016/s1074-5521(00)00077-6. [DOI] [PubMed] [Google Scholar]
- 94.Ottl J, Battistuta R, Pieper M, Tschesche H, Bode W, Kuhn K, Moroder L. Design and synthesis of heterotrimeric collagen peptides with a built-in cystine-knot. Models for collagen catabolism by matrix-metalloproteases. FEBS Lett. 1996;398:31–36. doi: 10.1016/s0014-5793(96)01212-4. [DOI] [PubMed] [Google Scholar]
- 95.Muller JC, Ottl J, Moroder L. Heterotrimeric collagen peptides as fluorogenic collagenase substrates: synthesis, conformational properties, and enzymatic digestion. Biochemistry. 2000;39:5111–5116. doi: 10.1021/bi992724x. [DOI] [PubMed] [Google Scholar]
- 96.Sacca B, Fiori S, Moroder L. Studies of the local conformational properties of the cell-adhesion domain of collagen type IV in synthetic heterotrimeric peptides. Biochemistry. 2003;42:3429–3436. doi: 10.1021/bi0206762. [DOI] [PubMed] [Google Scholar]
- 97.Goodman M, Feng Y, Melacini G, Taulane JP. A template-induced incipient collagen-like triple-helical structure. J Am Chem Soc. 1996;118:5156–5157. [Google Scholar]
- 98.Goodman M, Melacini G, And Feng Y. Collagen-like triple helices incorporating peptoid residues. J Am Chem Soc. 1996;118:10928–10929. [Google Scholar]
- 99.Feng Y, Melacini G, Taulane JP, Goodman M. Collagen-based structures containing the peptoid residue N-isobutylglycine (Nleu): synthesis and biophysical studies of Gly-Pro-Nleu sequences by circular dichroism, ultraviolet absorbance, and optical rotation. Biopolymers. 1996;39:859–872. doi: 10.1002/(SICI)1097-0282(199612)39:6%3C859::AID-BIP10%3E3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 100.Feng Y, Melacini G, Goodman M. Collagen-based structures containing the pep-toid residue N-isobutylglycine (Nleu): synthesis and biophysical studies of Gly-Nleu-Pro sequences by circular dichroism and optical rotation. Biochemistry. 1997;36:8716–8724. doi: 10.1021/bi962980z. [DOI] [PubMed] [Google Scholar]
- 101.Feng Y, Melacini G, Taulane JP, Goodman M. Acetyl-terminated and template-assembled collagen-based polypeptides composed of Gly-Pro-Hyp sequences. 2. Synthesis and conformational analysis by circular dichroism, ultraviolet absorbance, and optical rotation. J Am Chem Soc. 1996;118:10351–10358. [Google Scholar]
- 102.Kwak J, De Capua A, Locardi E, Goodman M. TREN (Tris(2-aminoethyl)amine): an effective scaffold for the assembly of triple helical collagen mimetic structures. J Am Chem Soc. 2002;124:14085–14091. doi: 10.1021/ja0209621. [DOI] [PubMed] [Google Scholar]
- 103.Rump ET, Rijkers DT, Hilbers HW, De Groot PG, Liskamp RM. Cyclotriveratrylene (CTV) as a new chiral triacid scaffold capable of inducing triple helix formation of collagen peptides containing either a native sequence or Pro-Hyp-Gly repeats. Chemistry. 2002;8:4613–4621. doi: 10.1002/1521-3765(20021018)8:20<4613::AID-CHEM4613>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 104.Horng JC, Hawk AJ, Zhao Q, Benedict ES, Burke SD, Raines RT. Macrocyclic scaffold for the collagen triple helix. Org Lett. 2006;8:4735–4738. doi: 10.1021/ol061771w. [DOI] [PubMed] [Google Scholar]
- 105.Brask J, Jensen KJ. Carboproteins: a 4-alpha-helix bundle protein model assembled on a D-galactopyranoside template. Bioorg Med Chem Lett. 2001;11:697–700. doi: 10.1016/s0960-894x(01)00035-x. [DOI] [PubMed] [Google Scholar]
- 106.Thulstrup PW, Brask J, Jensen KJ, Larsen E. Synchrotron radiation circular dichroism spectroscopy applied to metmyoglobin and a 4-alpha-helix bundle carboprotein. Biopolymers. 2005;78:46–52. doi: 10.1002/bip.20253. [DOI] [PubMed] [Google Scholar]
- 107.Cai W, Kwok SW, Taulane JP, Goodman M. Metal-assisted assembly and stabilization of collagen-like triple helices. J Am Chem Soc. 2004;126:15030–15031. doi: 10.1021/ja0442062. [DOI] [PubMed] [Google Scholar]
- 108.Kinberger GA, Taulane JP, Goodman M. Fe(III)-binding collagen mimetics. Inorg Chem. 2006;45:961–963. doi: 10.1021/ic0520059. [DOI] [PubMed] [Google Scholar]
- 109.Pires MM, Chmielewski J. Self-assembly of collagen peptides into microflorettes via metal coordination. J Am Chem Soc. 2009;131:2706–2712. doi: 10.1021/ja8088845. [DOI] [PubMed] [Google Scholar]
- 110.Thakur S, Vadolas D, Germann HP, Heidemann E. Influence of different tripeptides on the stability of the collagen triple helix II. An experimental approach with appropriate variations of a trimer model oligotripeptide. Biopolymers. 1986;25:1081–1086. doi: 10.1002/bip.360250608. [DOI] [PubMed] [Google Scholar]
- 111.Germann HP, Heidemann E. A synthetic model of collagen: an experimental investigation of the triple-helix stability. Biopolymers. 1988;27:157–163. doi: 10.1002/bip.360270112. [DOI] [PubMed] [Google Scholar]
- 112.Fields CG, Lauer GB, Miles JL, Yu AJ, Fields Y-CGB. Lett Peptide Sci. 1996;3:3–16. [Google Scholar]
- 113.Barnes MJ, Knight CG, Farndale RW. The use of collagen-based model peptides to investigate platelet-reactive sequences in collagen. Biopolymers. 1996;40:383–397. doi: 10.1002/(sici)1097-0282(1996)40:4<383::aid-bip4>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 114.Morton LF, Peachey AR, Knight CG, Farndale RW, Barnes MJ. The platelet reactivity of synthetic peptides based on the collagen III fragment alpha1(III)CB4. Evidence for an integrin alpha2beta1 recognition site involving residues 522–528 of the alpha1(III) collagen chain. J Biol Chem. 1997;272:11044–11048. doi: 10.1074/jbc.272.17.11044. [DOI] [PubMed] [Google Scholar]
- 115.Knight CG, Morton LF, Onley DJ, Peachey AR, Ichinohe T, Okuma M, Farndale RW, Barnes MJ. Collagen-platelet interaction: Gly-Pro-Hyp is uniquely specific for platelet Gp VI and mediates platelet activation by collagen. Cardiovasc Res. 1999;41:450–457. doi: 10.1016/s0008-6363(98)00306-x. [DOI] [PubMed] [Google Scholar]
- 116.Byrne C, Mcewan PA, Emsley J, Fischer PM, Chan WC. End-stapled homo and hetero collagen triple helices: a click chemistry approach. Chem Commun (Camb) 2011;47:2589–2591. doi: 10.1039/c0cc04795c. [DOI] [PubMed] [Google Scholar]
- 117.Henkel W, Vogl T, Echner H, Voelter W, Urbanke C, Schleuder D, Rauterberg J. Synthesis and folding of native collagen III model peptides. Biochemistry. 1999;38:13610–13622. doi: 10.1021/bi9905157. [DOI] [PubMed] [Google Scholar]
- 118.Cai W, Wong D, Kinberger GA, Kwok SW, Taulane JP, Goodman M. Facile and efficient assembly of collagen-like triple helices on a TRIS scaffold. Bioorg Chem. 2007;35:327–337. doi: 10.1016/j.bioorg.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 119.Barth D, Kyrieleis O, Frank S, Renner C, Moroder L. The role of cystine knots in collagen folding and stability, part II. Conformational properties of (Pro-Hyp-Gly) n model trimers with N- and C-terminal collagen type III cystine knots. Chemistry. 2003;9:3703–3714. doi: 10.1002/chem.200304918. [DOI] [PubMed] [Google Scholar]
- 120.Krishna OD, Kiick KL. Supramolecular assembly of electrostatically stabilized, hydroxyproline-lacking collagen-mimetic peptides. Biomacromolecules. 2009;10:2626–2631. doi: 10.1021/bm900551c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.King DS, Fields CG, Fields GB. A cleavage method which minimizes side reactions following Fmoc solid phase peptide synthesis. Int J Pept Protein Res. 1990;36:255–266. doi: 10.1111/j.1399-3011.1990.tb00976.x. [DOI] [PubMed] [Google Scholar]
- 122.Fields CG, Fields GB. Minimization of tryptophan alkylation following 9-fluorenylmethoxycarbonyl solid-phase peptide synthesis. Tetrahedron Lett. 1993;34:6661–6664. [Google Scholar]
- 123.Berndt P, Fields GB, Tirrell M. Synthetic lipidation of peptides and amino acids: monolayer structure and properties. J Am Chem Soc. 1995;117:9515–9522. [Google Scholar]
- 124.Fields CG, Grab B, Lauer JL, Fields GB. Purification and analysis of synthetic, triple-helical “minicollagens” by reversed-phase high-performance liquid chromatography. Anal Biochem. 1995;231:57–64. doi: 10.1006/abio.1995.1503. [DOI] [PubMed] [Google Scholar]
- 125.Grab B, Miles AJ, Furcht LT, Fields GB. Promotion of fibroblast adhesion by triple-helical peptide models of type I collagen-derived sequences. J Biol Chem. 1996;271:12234–12240. doi: 10.1074/jbc.271.21.12234. [DOI] [PubMed] [Google Scholar]
- 126.Yu YC, Roontga V, Daragan VA, Mayo KH, Tirrell M, Fields GB. Structure and dynamics of peptide-amphiphiles incorporating triple-helical proteinlike molecular architecture. Biochemistry. 1999;38:1659–1668. doi: 10.1021/bi982315l. [DOI] [PubMed] [Google Scholar]
- 127.Chhabra SR, Hothi B, Evans DJ, White PD, Bycroft BW, Chan WC. An appraisal of new variants of Dde amine protecting group for solid phase peptide synthesis. Tetrahedron Lett. 1998;39:1603–1606. [Google Scholar]
- 128.Rohwedder B, Mutti Y, Dumy P, Mutter M. Hydrazinolysis of Dde: complete orthogonality with Aloc protecting groups. Tetrahedron Lett. 1998;39:1175–1178. [Google Scholar]
- 129.Kates SA, Daniels SB, Albericio F. Automated allyl cleavage for continuous-flow synthesis of cyclic and branched peptides. Anal Biochem. 1993;212:303–310. doi: 10.1006/abio.1993.1334. [DOI] [PubMed] [Google Scholar]