Abstract
The (+) and (−) enantiomers for a cryptophane-7-bond-linker-benzenesulfonamide biosensor (C7B) were synthesized and their chirality confirmed by electronic circular dichroism (ECD) spectroscopy. Biosensor binding to carbonic anhydrase II (CAII) was characterized for both enantiomers by hyperpolarized (hp) 129Xe NMR spectroscopy. Our previous study of the racemic (+/−) C7B biosensor-CAII complex [Chambers, et al., J. Am. Chem. Soc. 2009, 131, 563–569], identified two “bound” 129Xe@C7B peaks by hp 129Xe NMR (at 71 and 67 ppm, relative to “free” biosensor at 64 ppm), which led to the initial hypothesis that (+) and (−) enantiomers produce diastereomeric peaks when coordinated to Zn2+ at the chiral CAII active site. Unexpectedly, the single enantiomers complexed with CAII also identified two “bound” 129Xe@C7B peaks: (+) 72, 68 ppm and (−) 68, 67 ppm. These results are consistent with X-ray crystallographic evidence for benzenesulfonamide inhibitors occupying a second site near the CAII surface. As illustrated by our studies of this model protein-ligand interaction, hp 129Xe NMR spectroscopy can be useful for identifying supramolecular assemblies in solution.
Keywords: 129Xe NMR spectroscopy, xenon biosensing, hyperpolarization
Introduction
The need for molecular-based medical diagnostic and treatment options (i.e., “molecular medicine”) motivates the development of spectroscopic and imaging techniques that can identify trace bio-analytes in complex media. In clinical practice, magnetic resonance imaging (MRI) is commonly used to scan deep tissues in human patients noninvasively and with high spatial resolution (< 100 μm3 per voxel). The challenge of identifying abnormal tissue based on proton signals from surrounding water and fat molecules often requires the use of contrast agents, e.g., gadolinium chelates and iron-oxide-based agents (1, 2). This combination typically provides valuable structural-anatomical information that helps to differentiate diseased from healthy tissues, but offers few additional molecular details. In particular, there have been challenges in developing contrast agents for in vivo imaging of medically relevant biomarkers, such as proteins. The importance of in situ molecular data for early and accurate diagnosis of disease continues to drive the development of complementary, molecule-specific, high-sensitivity contrast agents and detection strategies for MRI (3-7).
One option for increasing the magnetic spin reservoir is to use hyperpolarized (hp) nuclei (spin-1/2 options include 13C, 3He, 129Xe), which achieves magnetic resonance signal enhancements of several orders of magnitude compared to thermally equilibrated spin populations. 129Xe is an inert gas, and is abundant and readily harvested from the Earth's atmosphere. 129Xe can be hyperpolarized to near unity by spin-exchange optical pumping (8). Based on its significant polarizability, 129Xe binds void spaces in appropriately sized organic host molecules, e.g., cryptophane-A (9-14). Functionalizing the cryptophane produces significant chemical shift changes (15) (> 300 ppm window, important for simultaneous detection of multiple species in aqueous solution) and also imparts control over supramolecular interactions, which enables the sensitive hp 129Xe NMR detection of proteins, DNA, membranes, or metal ions in solution (16-18).
Our laboratory (7, 13, 15, 19, 20) and others (6, 17, 18, 21-29) have explored biosensing and bioimaging applications with hyperpolarized 129Xe magnetic resonance. A particular focus has been the development of hyperpolarized 129Xe NMR biosensors for biomedically relevant protein targets, which has led to the attachment of protein-targeting moieties to high-xenon-affinity host molecules. Cryptophane-A is chiral, but most studies are performed with racemic cryptophane as synthetic methods for generating useful quantities of enantiopure cryptophane material have been lacking until recently (30, 31). One protein target is carbonic anhydrase (CA), which plays important physiologic roles in carbon dioxide transport and pH homeostasis (32). Moreover, several CA isozymes are upregulated in cancer and other diseases, making CA a useful biomarker. Small-molecule drugs targeting CA typically contain a benzenesulfonamide moiety, which can coordinate as the deprotonated sulfonamidate to a buried Zn2+. This enzyme active site resides ~15 Å from the protein exterior surface. For these reasons, racemic cryptophane biosensors were generated in our lab with linkers of 6, 7, or 8 bonds (C6B, C7B, C8B) separating the cryptophane from the benzenesulfonamide terminus (33). This range of linker lengths was chosen for delicately tuning the interaction between cryptophane and the CA active-site channel. The three racemic biosensors were confirmed by isothermal titration calorimetry to bind with nanomolar dissociation constants to CA I and II. A 1.7-Å resolution structure determination of the 8-bond-linker biosensor, C8B, crystallized with CAII confirmed sulfonamidate-Zn2+ coordination, as well as occupancy of the biosensor within the active-site channel (34).
The racemic cryptophane biosensors yielded 129Xe NMR chemical shifts that varied considerably depending on the length of the linker as well as the CA isozyme (CAI vs. CAII) (33). When complexed with CAII, racemic C7B showed a bound resonance at 67.0 ppm, and a second bound resonance at 71.2 ppm (Δδ = 7.5 ppm, relative to the unbound resonance at 63.7 ppm). This protein-mediated shift was the largest ever reported for a xenon biosensor. We hypothesized that the two bound resonances were the result of diastereomerism, with the (+) and (−) C7B enantiomers interacting differently with the chiral CAII active site. Here, to test that hypothesis, we synthesized the enantiopure C7B biosensors and performed hp 129Xe NMR spectroscopy for each enantiomer in the presence of CAII.
Results and discussion
Synthesis
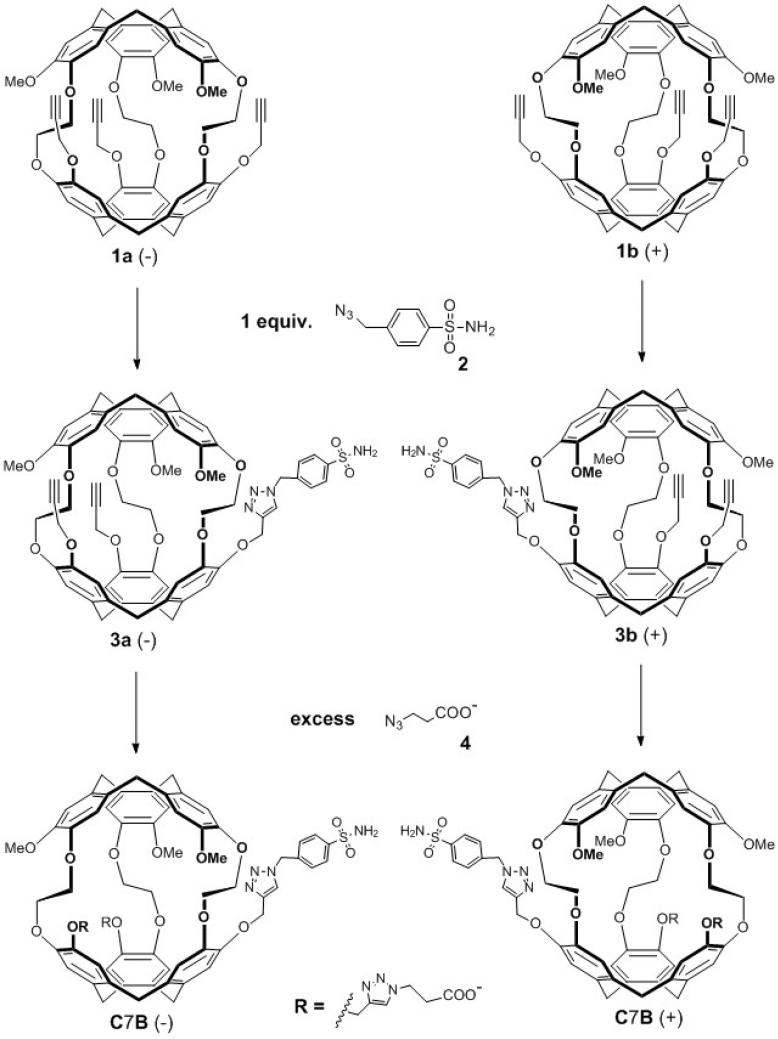
The final steps in enantiopure C7B biosensor synthesis are outlined in Scheme 1. The enantiopure precursors, tripropargyl cryptophanes 1a and 1b, were prepared in 9 overall steps in good yield. Briefly, this followed our previously published synthetic routes for racemic trihydroxy cryptophane (6 steps), subsequent installation of three Mosher's acids, purification of cryptophane diastereomers by column chromatography, removal of Mosher's acids to reveal enantiopure cryptophane (deprotection step), and, finally, installation of three propargyl moieties to give 1a (−) and 1b (+) (31). The azido-functionalized, para-substituted benzenesulfonamide linker 2 was readily synthesized in 1 step from the amine analogue to provide a 7-bond spacer between the cryptophane and benzenesulfonamide recognition unit (33). Linker 2 was then reacted with 1a and 1b via a copper (I)-catalyzed [3 + 2] cycloaddition process, to give the functionalized enantiopure cryptophanes 3a and 3b, as previously described for racemic tripropargyl cryptophane (33). Finally, to increase water solubility, the enantiopure cryptophanes 3a and 3b were reacted with excess 3-azidopropionic acid 4 by the same copper (I)-catalyzed [3+ 2] cycloaddition process to give the pair of water-soluble enantiomers (−)-C7B and (+)-C7B in ~50% yield.
Scheme 1.
Synthesis of enantiopure cryptophane biosensors (−) and (+) C7B from enantiopure tripropargyl cryptophanes (1a, 1b).
Circular dichroism spectroscopy
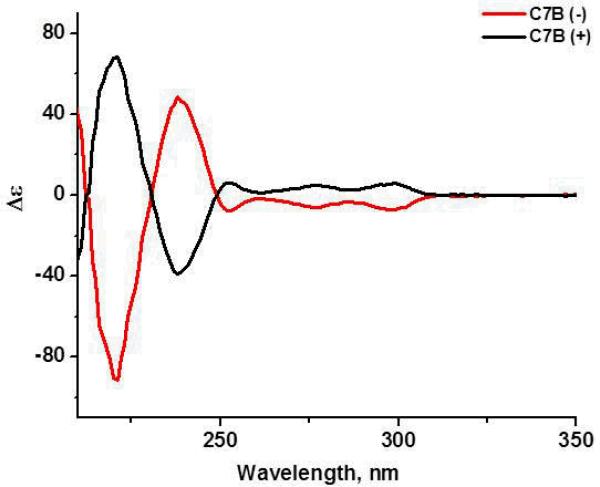
The enantiopurity of the isolated enantiomers (−)-3a and (+)-3b as well as enantiomers (−)-C7B and (+)-C7B was confirmed by electronic circular dichroism (ECD) spectroscopy showing the same peaks with opposite sign (Fig. 1). The ECD spectra, recorded in 1,4-dioxane (a solvent that is too large to enter the cryptophane cavity), were mirror images within experimental error, as expected for pairs of enantiomers. The molar ellipticity values, both in sign and magnitude, also agreed well with those measured for other chiral cryptophanes of C1 symmetry (35). In the absence of a X-ray crystal structure for the isolated enantiomers, the structural assignment for the two enantiomers was made by reacting (+)-trihydroxy cryptophane with methyl iodide to yield (+)-cryptophane-A. Its recorded ECD spectrum was found to be opposite of the previously reported spectrum for (−)-cryptophane-A (36).
Figure 1.
ECD spectra of enantiopure biosensors (−)-C7B and (+)-C7B in 1,4-dioxane at 298 K. Molar ellipticity (Δε) has units of M−1Lcm−1.
129Xe NMR spectroscopy
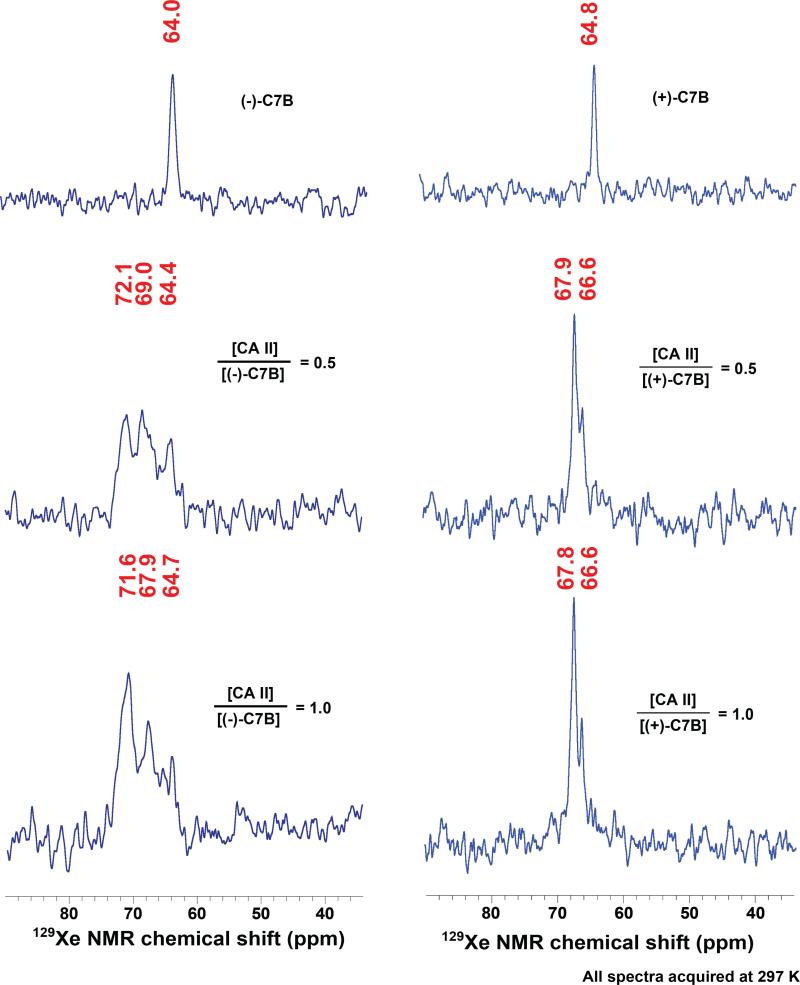
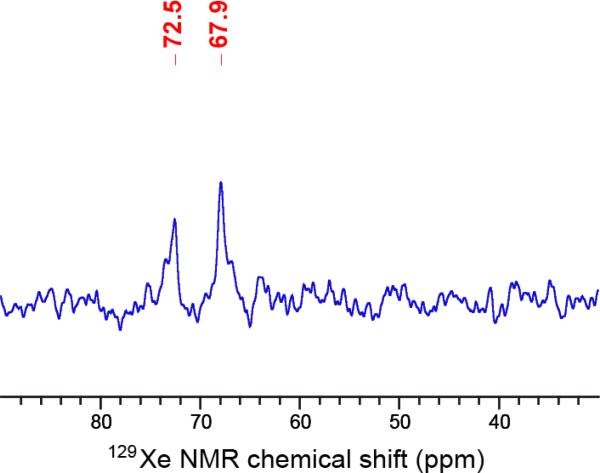
HP 129Xe NMR spectra were collected for both C7B enantiomers, either alone in solution or complexed with 0.5 or 1.0 equiv. of CAII (Fig. 2). As expected, one 129Xe NMR peak was observed for xenon bound to each enantiomer tumbling freely in solution: (−) 64.0 ppm and (+) 64.8 ppm. These chemical shifts are identical within experimental error (± 0.5 ppm) based on the temperature variability (297 K ± 1 K) within the NMR spectrometer. These chemical shifts were also in agreement, within error, of the “free” peak at 63.9 ppm observed previously for racemic C7B (33). However, titration of 0.5 or 1.0 equivalents of CAII into the enantiopure biosensor solutions led to the unexpected finding of two “bound” peaks for both C7B enantiomers. This ran counter to expectations of the (−) enantiomer producing one “bound” peak and the (+) enantiomer producing the other “bound” peak previously seen for 129Xe@racemic-C7B (33). Instead, stoichiometric 129Xe@(−)-C7B:CAII sample gave two similarly intense and broad peaks at 71.6 and 67.9 ppm and 129Xe@(+)-C7B:CAII gave a narrow-linewidth peak at 67.8 ppm and smaller peak at 66.6 ppm. The hp 129Xe NMR spectrum of the stoichiometric 129Xe@racemic-C7B complex was measured in the same buffer conditions as the enantiopure biosensors, and gave peaks at 72.5 and 67.9 ppm (Fig. 3), in agreement with our previous report (33). Thus, the observation of two “bound” peaks at ~72 ppm and ~68 ppm is consistent across the enantiopure 129Xe@(−)-C7B:CAII and racemic samples. Substantial amounts of “free” biosensor were observed (at 64.4–64.7 ppm, Fig. 2) in the 1:0.5 and 1:1 (−)-C7B:CAII samples. In contrast, no free biosensor was observed in either the 1:0.5 or 1:1 (+)-C7B:CAII samples (Fig. 2).
Figure 2.
HP 129Xe NMR spectra of (−)-C7B (left column) or (+)-C7B (right column) before and after binding to wild-type carbonic anhydrase II. The first row shows spectra of biosensors (138 μM, (−)-C7B; 147 μM (+)-C7B) dissolved in 90% Tris-SO4 buffer (50 mM, pH 8.0) and 10% glycerol. The second and third rows show spectra for biosensor-CA II binding. Solutions of 1.3 mM CA II in 50 mM Tris-SO4 buffer were titrated with 0.5 and 1.0 equivalents of biosensor, respectively.
Figure 3.
HP 129Xe NMR spectra of racemic C7B binding to wild-type carbonic anhydrase II. The C7B biosensor (176 μM) was dissolved in Tris-SO4 buffer (50 mM, pH 8.0) and 10% glycerol. Solution of 1.3 mM CAII in matched Tris-SO4 buffer was titrated to give 1:1 stoichiometry with racemic C7B. The chemical shifts for the two “bound” peaks (72.5, 67.9 ppm) are somewhat shifted from the spectrum in reference [33] due to differences in buffer conditions.
These hp 129Xe NMR data indicate that diastereomerism alone cannot explain the observation of two “bound” peaks in the CAII-C7B model system. A more compelling explanation arises from the fact that X-ray crystallographic studies with CAII showed binding of functionalized benzenesulfonamide inhibitors at both the canonical Zn2+ buried active site and a less buried ‘b’ site near the protein surface (37). The lack of free biosensor in the 1:0.5 (+)-C7B:CAII sample (Fig. 2), is consistent with (+)-C7B binding concurrently at both sites.
The peak observed at 72 ppm for CAII:(−)-C7B remains particularly striking as this represents a significant chemical shift change relative to the “free” biosensor peak at 64 ppm. In this example, the chirality of the biosensor is very important, as it clearly affects CAII binding interactions with the cryptophane biosensor. Interestingly, this effect is seen most profoundly for the intermediate linker length (C7), as neither the C6 nor the C8 biosensor produced a CAII:129Xe NMR resonance >68 ppm (33).
We hypothesize that the larger chemical shift change observed for (−)-C7B results from interaction with the CAII active-site pocket, leading to a significant perturbation of the xenon electron cloud within the cryptophane. This interaction could be enforced through Zn2+ coordination. Indeed, the greater linewidth of the 129Xe NMR peaks for the CAII:(−)-C7B sample suggests that the protein may be restricting the motions of the cryptophane, preventing free rotation. Notably, the narrow 129Xe NMR linewidths observed for the CAII:(+)-C7B sample make it more likely that the cryptophane, although “bound”, is freely rotating at some distance from CAII in solution, which our modeling suggests is more consistent with ‘b-site’ binding.. Further studies employing protein mutagenesis, protein crystallography, 129Xe NMR biosensors and spectroscopy are underway, with the goal of elucidating these different binding modes. It remains a compelling question how specific, supramolecular interactions contribute to differences in 129Xe NMR chemical shift.
Experimental
All commercially available chemicals were purchased from Sigma-Aldrich, Fisher or Acros Organics and used without further purification. All air- and moisture-sensitive reactions were performed under inert atmosphere in glassware flamed under vacuum, and using anhydrous solvents dried by standard methods. Standard workup procedures involved multiple (~3) extractions with the indicated organic solvent, followed by washing of the combined organic extracts with water or brine, drying over Na2SO4 and removal of solvents in vacuo. Column chromatography was performed using silica gel (60 Å pore size, 40-75 μm particle size) from Sorbent Technologies. Thin layer chromatography (TLC) was performed using silica gel plates (60 Å pore size, Whatman) with UV light at 254 nm as the detection method. High resolution mass spectrometry (HRMS) data were obtained using electrospray ionization (ESI) mass spectrometry on a Micromass Autospec at the Mass Spectrometry Center in the University of Pennsylvania Chemistry Department. 1H NMR (360 and 500 MHz) and 13C NMR (90 and 125 MHz) spectra were obtained on Bruker DMX 360 and AMX 500 spectrometers in the same department. Electronic circular dichroism (ECD) spectra were recorded at 298 K on a Chirascan™ ECD spectrometer using cells with a 0.1-cm pathlength. An in-house 129Xe hyperpolarizer based on the IGI.Xe.2000 system (Nycomed-Amersham, GE). Hyperpolarizer gas supply, purchased from Concorde Gases, NJ, was a mixture of 89% N2, 10% He, and 1% natural isotopic abundance Xe (26.4% 129Xe). 129Xe atoms were hyperpolarized to 10-15% after cryogenic separation, accumulation, thawing, and collection in degassed airtight NMR tubes (New Era) (33). These steps yielded 2-3 atm of hyperpolarized Xe in the tube. After hp 129Xe was collected, NMR tubes were shaken to mix hp 129Xe gas with biosensor solutions. All 129Xe NMR spectra were acquired on a 500 MHz Bruker BioDRX NMR spectrometer at the University of Pennsylvania NMR Facility. Radio-frequency pulse frequency for 129Xe was 138.12 MHz. Samples were observed using a Bruker 10 mm PABBO NMR probe. 129Xe spectra were processed using standard protocols from Bruker, and calibration was done by the same protocol as previous 129Xe NMR spectra of carbonic anhydrase biosensor solutions (33). NMR peak intensities were adjusted for best visibility relative to background.
Synthesis of enantiopure cryptophane biosensors
Precursors 1a, 1b and linker 4-(azidomethyl)-benzenesulfonamide 2, were prepared following published protocols from our laboratory (31, 33). 3-Azidopropionic acid 4 was prepared by a literature procedure and it matched the reported 1H NMR spectrum (38).
Cryptophane (−)-3a: Compound 1a (0.031 g, 0.032 mmol, 1 equiv.) was dissolved in dry DMSO (0.8 mL) at rt with stirring. Sulfonamide linker 2 (0.006 g, 0.03 mmol, 0.9 equiv.) dissolved in DMSO (0.2 mL) was added. CuSO4 (0.003 g, 0.02 mmol, 0.5 equiv., in 12 µL water) was added, followed by 2,6-lutidine (1 equiv., d = 0.925 g/mL), and (+)-sodium-L-ascorbate (0.062 g, 0.31 mmol, 10 equiv., in 45 μL water). The reaction was allowed to stir overnight and then poured into H2O (5 mL). This solution was extracted 3 times with ethyl acetate followed by standard workup. The yellow oil recovered was purified by silica gel column chromatography (CH2Cl2→THF:CH2Cl2 30:70, v/v) to give the pure product 3a (0.017 g, 0.014 mmol, 45% yield) as a white solid. 1H NMR and 13C NMR spectra for 3a were identical to the spectra of the corresponding racemic (±) compound (33). Confirmation of compound identity came from HRMS (m/z): [M+Na]+ calcd. for C67H62N4O14SNa 1201.3881; found, 1201.3862.
Cryptophane (+)-3b: Following the procedure for the synthesis of (−)-3a, compound 1b (0.031 g, 0.032 mmol, 1 equiv.) reacted with the sulfonamide linker 2 in dry DMSO (1 mL) in the presence of CuSO4 (0.003 g, 0.02 mmol, 0.5 equiv., in 12 μL water), 2,6-lutidine (1 equiv., d = 0.925 g/mL), and (+)-sodium-L-ascorbate (0.062 g, 0.31 mmol, 10 equiv., in 45 μL water) to give 0.018 g (0.015 mmol, 47% yield) of (+)-3b as a white solid. 1H NMR and 13C NMR spectra for 3b were identical to the spectra of 3a and the corresponding racemic (±) compound (33). Confirmation of compound identity came from HRMS (m/z): [M+H]+ calcd. for C67H63N4O14S 1179.4062; found, 1179.4133.
(−)-C7B: Following the same cycloaddition procedure, compound 3a (0.015 g, 0.013 mmol, 1 equiv.) reacted with the linker 4 in dry DMSO (0.8 mL) in the presence of CuSO4 (0.001 g, 0.009 mmol, 0.5 equiv., in 10 μL water), 2,6-lutidine (1 equiv., d = 0.925 g/mL), and (+)-sodium-L-ascorbate (0.025 g, 0.13 mmol, 10 equiv., in 35 μL water) to give 0.009 g (0.006 mmol, 49% yield) of (−)-C7B as a white solid. Pure product was extracted with ethyl acetate followed by standard workup without the need for column chromatography purification. 1H NMR and 13C NMR spectra for (−)-C7B are identical to the spectra of the corresponding racemic (±) compound. HRMS (m/z) confirmed compound identity: [M-H]+ calcd. for C73H71N10O18S 1407.4669; found, 1407.4666.
(+)-C7B: Following the same cycloaddition procedure, compound 3b (0.015 g, 0.013 mmol, 1 equiv.) reacted with the linker 7 in dry DMSO (0.8 mL) in the presence of CuSO4 (0.001 g, 0.009 mmol, 0.5 equiv., in 10 μL of water), 2,6-lutidine (1 equiv., d = 0.925 g/mL), and (+)-sodium-L-ascorbate (0.025 g, 0.13 mmol, 10 equiv., in 35 μL water) to give 0.01 g (0.007 mmol, 51% yield) of (+)-C7B as a white solid. Pure product was extracted with ethyl acetate followed by standard workup without the need for the column chromatography purification. 1H NMR and 13C NMR spectra for (+)-C7B were identical to the spectra of (−)-C7B and the corresponding racemic (±) compound (33). HRMS (m/z) confirmed compound identity: [M-H]+ calcd. for C73H71N10O18S 1407.4669; found, 1407.4698.
Conclusions
We described the 12-step synthesis, characterization and application of enantiopure C7B biosensors. The use of enantiopure biosensors helped to clarify the assignment of multiple “CAII-bound” 129Xe NMR peaks. These studies show that diastereomerism alone cannot explain the multiple peaks observed for the racemic C7B:CAII sample, as 129Xe NMR peaks positioned at 68 and 72 ppm were similarly observed for (−)-C7B:CAII sample. We hypothesize that these different peaks arise instead from interactions at two different benzenesulfonamide binding sites in CAII that were previously identified by X-ray crystallography (37). This study highlights a role for hp 129Xe NMR spectroscopy to investigate supramolecular interactions involving protein-ligand binding.
Guided by numerous crystal structures of CAII-inhibitor complexes, CAII has served as a useful model system for rational drug design (32, 39, 40). These inhibitor studies have largely focused on binding benzenesulfonamide moieties to the buried Zn2+ active site. The current study raises interesting questions about the relative occupancy of benzenesulfonamide inhibitors at multiple CAII binding sites.
Acknowledgments
This work was supported by the National Institutes of Health under Grant [GM097478] and the UPenn Chemistry Department. We thank George Furst (Chemistry Department, University of Pennsylvania) for NMR support.
References
- 1.Degani H, Gusis V, Weinstein D, Fields S, Strano S. Nature Med. 1997;3:780–782. doi: 10.1038/nm0797-780. [DOI] [PubMed] [Google Scholar]
- 2.Foster-Gareau P, Heyn C, Alejski A, Rutt BK. Magn. Reson. Med. 2003;49:968–971. doi: 10.1002/mrm.10417. [DOI] [PubMed] [Google Scholar]
- 3.Hancu I, Dixon WT, Woods M, Vinogradov E, Sherry AD, Lenkinski RE. Acta Radiol. 2010;51:910–923. doi: 10.3109/02841851.2010.502126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woods M, Woessner DE, Sherry AD. Chem. Soc. Rev. 2006;35:500–511. doi: 10.1039/b509907m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aime S, Castelli DD, Terreno E. Angew. Chem.-Int. Edit. 2005;44:5513–5515. doi: 10.1002/anie.200501473. [DOI] [PubMed] [Google Scholar]
- 6.Schroder L, Lowery TJ, Hilty C, Wemmer DE, Pines A. Science. 2006;314:446–449. doi: 10.1126/science.1131847. [DOI] [PubMed] [Google Scholar]
- 7.Bai Y, Hill AP, Dmochowski IJ. Anal. Chem. 2012;84:9935–9941. doi: 10.1021/ac302347y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nikolaou P, Coffey AM, Walkup LL, Gust BM, Whiting N, Newton H, Barcus S, Muradyan I, Dabaghyan M, Moroz GD, Rosen MS, Patz S, Barlow MJ, Chekmenev EY, Goodson BM. Proc. Natl. Acad. Sci. U. S. A. 2013;110:14150–14155. doi: 10.1073/pnas.1306586110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cherubini A, Bifone A. Prog. Nucl. Magn. Reson. Spec. 2003;42:1–30. [Google Scholar]
- 10.Collet A. Comp. Supramol. Chem. 1996;2:325–365. [Google Scholar]
- 11.Brotin T, Dutasta JP. Eur. J. Org. Chem. 2003;6:973–984. [Google Scholar]
- 12.Bartik K, Luhmer M, Dutasta JP, Collet A, Reisse J. J. Am. Chem. Soc. 1998;120:784–791. [Google Scholar]
- 13.Jacobson DR, Khan NS, Collé R, Fitzgerald R, Laureano-Pérez L, Bai Y, Dmochowski IJ. Proc. Natl. Acad. Sci. U.S.A. 2011;108:10969–10973. doi: 10.1073/pnas.1105227108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taratula O, Hill PA, Khan N, Carroll PJ, Dmochowski IJ. Nature Commun. 2010;1 doi: 10.1038/ncomms1151. doi:10.1038/ncomms1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hill PA, Wei Q, Troxler T, Dmochowski IJ. J. Am. Chem. Soc. 2009;131:3069–3077. doi: 10.1021/ja8100566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taratula O, Dmochowski IJ. Curr. Opin. Chem. Biol. 2010;14:97–104. doi: 10.1016/j.cbpa.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brotin T, Dutasta J-P. Chem. Rev. 2009;109:88–130. doi: 10.1021/cr0680437. [DOI] [PubMed] [Google Scholar]
- 18.Schröder L. Phys. Medica. 2013;29:3–16. doi: 10.1016/j.ejmp.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 19.Wei Q, Seward GK, Hill PA, Patton B, Dimitrov IE, Kuzma NN, Dmochowski IJ. J. Am. Chem. Soc. 2006;128:13274–13283. doi: 10.1021/ja0640501. [DOI] [PubMed] [Google Scholar]
- 20.Hill PA, Wei Q, Eckenhoff RG, Dmochowski IJ. J. Am. Chem. Soc. 2007;129:9262–9263. doi: 10.1021/ja072965p. [DOI] [PubMed] [Google Scholar]
- 21.Spence MM, Rubin SM, Dimitrov IE, Ruiz EJ, Wemmer DE, Pines A, Yao SQ, Tian F, Schultz PG. Proc. Natl. Acad. Sci. U. S. A. 2001;98:10654–10657. doi: 10.1073/pnas.191368398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spence MM, Ruiz EJ, Rubin SM, Lowery TJ, Winssinger N, Schultz PG, Wemmer DE, Pines A. J. Am. Chem. Soc. 2004;126:15287–15294. doi: 10.1021/ja0483037. [DOI] [PubMed] [Google Scholar]
- 23.Baumer D, Brunner E, Blümler P, Zänker PP, Spiess HW. Angew. Chem.-Int. Edit. 2006;45:7282–7284. doi: 10.1002/anie.200601008. [DOI] [PubMed] [Google Scholar]
- 24.Driehuys B, Moller HE, Cleveland ZI, Pollaro J, Hedlund LW. Radiology. 2009;252:386–393. doi: 10.1148/radiol.2522081550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boutin C, Desvaux H, Carrière M, Leteurtre F, Jamin N, Boulard Y, Berthault P. NMR Biomed. 2011;24:1264–1269. doi: 10.1002/nbm.1686. [DOI] [PubMed] [Google Scholar]
- 26.Boutin C, Stopin A, Lenda F, Brotin T, Dutasta J-P, Jamin N, Sanson A, Boulard Y, Leteurtre F, Huber G, Bogaert-Buchmann A, Tassali N, Desvaux H, Carrière M, Berthault P. Bioorg. Med. Chem. 2011;19:4135–4143. doi: 10.1016/j.bmc.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 27.Zhou X, Graziani D, Pines A. Proc. Natl. Acad. Sci. U. S. A. 2009;106:16903–16906. doi: 10.1073/pnas.0909147106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meldrum T, Seim KL, Bajaj VS, Palaniappan KK, Wu W, Francis MB, Wemmer DE, Pines A. J. Am. Chem. Soc. 2010;132:5936–5937. doi: 10.1021/ja100319f. [DOI] [PubMed] [Google Scholar]
- 29.Mugler JP, Altes TA, Ruset IC, Dregely IM, Mata JF, Miller GW, Ketel S, Ketel J, Hersman FW, Ruppert K. Proc. Natl. Acad. Sci. U. S. A. 2010;107:21707–21712. doi: 10.1073/pnas.1011912107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taratula O, Hill PA, Bai Y, Khan NS, Dmochowski IJ. Org. Lett. 2011;13:1414–1417. doi: 10.1021/ol200088f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taratula O, Kim MP, Bai Y, Philbin JP, Dmochowski IJ. Org. Lett. 2012;14:3580–3583. doi: 10.1021/ol300943w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Supuran CT. Nature Rev. Drug. Disc. 2008;7:168–181. doi: 10.1038/nrd2467. [DOI] [PubMed] [Google Scholar]
- 33.Chambers JM, Hill PA, Aaron JA, Han ZH, Christianson DW, Kuzma NN, Dmochowski IJ. J. Am. Chem. Soc. 2009;131:563–569. doi: 10.1021/ja806092w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aaron JA, Chambers JM, Jude KM, Di Costanzo L, Dmochowski IJ, Christianson DW. J. Am. Chem. Soc. 2008;130:6942–6943. doi: 10.1021/ja802214x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cavagnat D, Buffeteau T, Brotin T. J. Org. Chem. 2008;73:66–75. doi: 10.1021/jo701662w. [DOI] [PubMed] [Google Scholar]
- 36.Canceill J, Collet A, Gottarelli G, Palmieri P. J. Am. Chem. Soc. 1987;109:6454–6464. [Google Scholar]
- 37.Jude KM, Banerjee AL, Haldar MK, Manokaran S, Roy B, Mallik S, Srivastava DK, Christianson DW. J. Am. Chem. Soc. 2006;128:3011–3018. doi: 10.1021/ja057257n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang L, Chen X, Peng X, Herman HYS, Williams ID, Sharpless KB, Fokin VV, Gouchen J. J. Am. Chem. Soc. 2005;127:15998–15999. doi: 10.1021/ja054114s. [DOI] [PubMed] [Google Scholar]
- 39.Greer J, Erickson JW, Baldwin JJ, Varney MD. J. Med. Chem. 1994;37:1035 – 1054. doi: 10.1021/jm00034a001. [DOI] [PubMed] [Google Scholar]
- 40.Krishnamurthy VM, Kaufman GK, Urbach AR, Gitlin I, Gudiksen KL, Weibel DB, Whitesides GM. Chem. Rev. 2008;108:946–1051. doi: 10.1021/cr050262p. [DOI] [PMC free article] [PubMed] [Google Scholar]