Abstract
Tuberculosis (TB) is the number one bacterial killer worldwide and the current increase in type 2 diabetes mellitus patients (DM), particularly in countries where TB is also endemic, has led to the re-emerging importance of DM2 as a risk factor for TB. There is an urgent need to implement strategies for TB prevention among the millions of DM patients exposed to Mycobacterium tuberculosis (Mtb) worldwide, but knowledge is limited on how and when DM2 alters the natural history of this infection. In this review we summarize the current epidemiological, clinical and immunologic studies on TB and DM and discuss the clinical and public health implications of these findings. Specifically, we evaluate the mechanisms by which DM patients have a higher risk of Mtb infection and TB development, present with signs and symptoms indicative of a more infectious TB infection, and are more likely to have adverse TB treatment outcomes, including death. Emphasis is placed on type 2 DM given its higher prevalence in contemporary times, but the underlying role of hyperglycemia and of type 1 DM is also discussed.
Keywords: tuberculosis, diabetes, immunity, review, hyperglycemia, epidemiology, clinical
1. Introduction
Tuberculosis (TB) has plagued mankind for thousands of years, and even though the first anti-TB drugs were discovered 60 years ago, TB still kills an estimated 1.3 million people yearly [1]. Immunological impairment has played a major role in TB control throughout history. Today, the most common causes of compromised immunity that favor TB development are HIV/AIDS, malnutrition, aging, and most recently, smoking and diabetes mellitus (DM) [2;3]. At the individual level HIV/AIDS has the greatest effect by increasing TB risk by more than 20-fold [4]. In contrast, the other factors cause a relatively mild immunological impairment but affect a significant proportion of the population; hence, these factors contribute substantially to the global TB incidence. In this review we focus on the contribution of DM, particularly type 2 DM in adults. The association between DM and TB has been known for centuries [5] and in the 1950s the DM patient not dying from a diabetic comma was likely to do so from TB [6–8]. This association was reduced with the advent of insulin for DM and antibiotics to treat TB, but in the 1980s the co-occurrence began to re-emerge as a consequence of the DM ‘pandemic’ which is predicted to reach 439 million patients by 2030 and is primarily attributed to type 2 DM [9;10]. Consequently, the World Health Organization has identified DM as a neglected, important and re-emerging risk factor for TB [11–13]. In this review ‘DM’ will refer mostly to type 2 DM since it is the most prevalent form. The current literature suggests that DM has an impact at every stage of the natural history of TB and the goal of this review is evaluate how these findings provide insights for improved TB control. For this we summarize current epidemiological, clinical and immunological knowledge on TB and DM and then discuss the possible mechanisms by which DM affects the increased risk of Mycobacterium tuberculosis (Mtb) infection and TB development, and modifies its clinical presentation and treatment outcomes. Emphasis is placed on the contemporary literature, but relevant data prior to the 1950s are also mentioned.
2. The natural history of TB and contribution of innate and adaptive immunity to its outcomes
TB is an airborne infection transmitted between humans, but only 30% of the close contacts of a pulmonary TB patient become successfully infected with Mtb based on a positive tuberculin skin test (TST) or an Interferon-gamma release assay (IGRA). An “enhanced” innate immune response is believed to abrogate Mtb infection (negative TST or IGRAs) in the remaining 70% of the contacts, but the mechanisms comprising this protective immunity remain a major knowledge gap in the TB field (Figure). Among those infected, the most frequent outcome is the progression to a latent TB infection (LTBI) where most mycobacteria are killed and the few that remain viable are in a latent state characterized by altered metabolism and persistence. To reach this host-bacterium balance (i.e., sterile immunity is not achieved), innate immunity and particularly mononuclear phagocytes in the alveolar space allow for initial intracellular growth of the inhaled Mtb. As the Mtb burden increases, this innate immune response orchestrates development of adaptive immunity with activation of a localized T-helper 1 (Th1) inflammatory response (e.g. TNF-α, IL-1β, IL-12) and recruitment of monocytes, lymphocytes, natural killer T cells and B lymphocytes for the formation of granulomas that kill most Mtb and restrict the growth of those that remain viable. Dendritic cells contribute to this response by transporting Mtb from the lungs to regional thoracic nodes for antigen presentation to T cells, which then differentiate into effector and memory T cells specific for Mtb [14]. Granulomas form throughout the lungs, but they can also be detected in other organs given that lympho-hematogenous spread accompanies primary infection. Among these individuals with LTBI, most (90%) will effectively contain Mtb growth and will never progress to TB during their lifetime, while the remaining 10% will eventually progress to TB (reactivation TB), 80% of the time occurring in the lungs. Alternatively, progression to TB that occurs shortly after exposure is referred to as primary TB and occurs most commonly in individuals with severe immunocompromise such as HIV/AIDS [15]. The lessons learned from HIV/AIDS, genetic defects in the IL12-IFN-γ axis and the clinical use of TNF-α blockers have highlighted the critical role of T-cells and their derived lymphokines like IFN-γ and TNF-α for preventing reactivation of Mtb (progression from LTBI to TB) [14;16]. CD4+ T lymphocytes orchestrate the activation of CD8+ T cells that are cytotoxic for Mtb-infected cells, and of macrophages which are important effectors for enhanced MHC-II antigen presentation to T lymphocytes, granuloma formation and activation of bactericidal mechanisms (e.g. cathelicidin) that ultimately kill Mtb [17]. However, this adaptive immunity only results in the arrest (but not elimination) of Mtb replication. The re-emerging contribution of DM to TB provides an additional opportunity to identify new molecules and mechanisms that are critical for Mtb control or progression.
Figure.
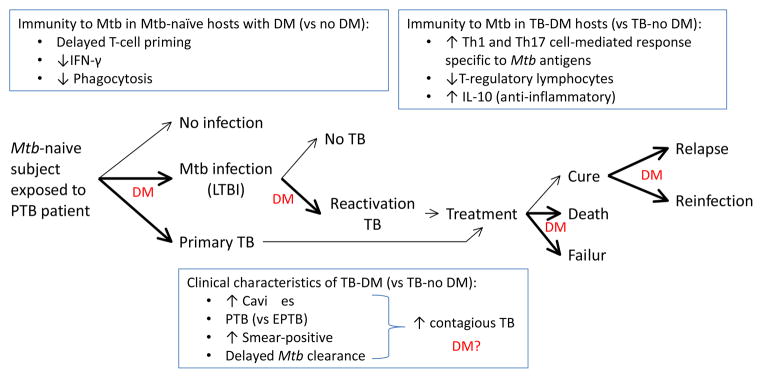
Impact of DM on the natural history of TB: association with dysfunctional immunity and clinical characteristics. Exposure of Mtb-naïve individuals without DM to a pulmonary TB patient results in no infection (70%) or in Mtb infection (30%) or primary TB. Among those infected, the lifetime risk of reactivation TB is 10%. Once TB develops, possible treatment outcomes include cure, treatment failure or death. Among presumably cured individuals TB relapses can ocurr. A previous history of TB does not confer immunity against all strains and re-exposure to another Mtb strain can lead to re-infection. Bold arrows and “DM” indicate stages of TB where DM appears to have an impact. However, further research is needed at all of these stages to confirm these findings, have a more precise estimate of the effects, and elucidate the underlying molecular mechanisms involved. Current evidence is described in the text. As the natural history of TB evolves in the DM host, so does the immune response with characteristics that contrast with the non-DM host (upper text boxes). The TB-DM host is more likely to present with clinical characteristics associated with enhanced TB transmission, but the impact of disease spread in the community is unclear (bottom text box; “DM?”). The dysfunctional immune response of the DM host to Mtb antigens is likely to influence the development, clinical presentation and outcomes of TB but the mechanisms involved are poorly understood. PTB, pulmonary TB; EPTB, extrapulmonary TB; LTBI, latent TB infection
3. Dysfunctional innate and adaptive immunity to Mtb in DM patients
Detailed reviews on the dysfunctional innate and adaptive immune response to Mtb in DM patients have been published recently and in this section we summarize the key findings in order to provide the reader with the foundation for assessing the contribution of these alterations to the clinical outcomes of TB in these patients [18;19]. While we will not discuss all of the possible mechanisms by which DM affects the immune response to Mtb, it is worth mentioning that the most consistent (although not universal) underlying factor across studies is chronic hyperglycemia in both human and animal studies.
3.1 Innate immunity to Mtb in the DM host
We have standardized an in-vitro assay to determine if the initial encounter between Mtb and a TB-naïve host is affected by DM. For this we infect human blood monocytes from TB-naïve hosts with diabetes (versus no diabetes) with Mtb, and then evaluate differences in the host-pathogen outcomes. Current findings indicate that diabetic monocytes have significantly reduced association (binding and phagocytosis) of Mtb and this defect appears to be attributed to alterations in the diabetic monocyte as well as in serum opsonins for Mtb, particularly the C3 component of complement which mediates Mtb phagocytosis [20;21]. These in-vitro findings in humans are consistent with in-vivo observations in mice with chronic DM where there is also a reduced uptake of Mtb by alveolar macrophages within 2 weeks of infection [19]. Furthermore, this model of chronic DM is associated with delayed innate immunity to Mtb due to late delivery of Mtb-bearing antigen-presenting cells to the lung draining lymph nodes [22]. Efficient phagocytosis and priming of the adaptive immune response are necessary to activate the cell-mediated immune response that restricts initial Mtb growth [22] and these delays likely contribute to the higher risk of DM patients for Mtb infection and persistence (Figure).
3.2 Innate and adaptive immunity to Mtb in the DM host with LTBI and TB
There are no data on the type of immunity to Mtb that facilitates the progression from LTBI to TB in individuals with underlying DM (versus no DM), nor animal studies in whom reproduction of the latent stage of TB is a challenge. In contrast, there are an increasing number of immunological studies in DM patients who have developed TB that indicate a paradoxical hyper-inflammatory response. Despite some variation, most studies indicate that ex-vivo and in-vitro stimulation of peripheral white blood cells with mycobacterial antigens results in higher Th1 and Th17 responses, including higher IFN-γ and IL-17 secretion [23–25]. Other cytokines promoting immunity have been also reported to be higher in TB-DM vs TB-no DM, such as IL-2 and GM-CSF [23;24]. TB-DM cases also have a higher frequency of single- and double-cytokine producing CD4+ Th1 cells in response to Mtb antigens (for IFN-γ, TNF-α or IL-2) [24]. These hyper-active responses in peripheral blood contrast with the results from few studies conducted at the site of infection (in bronchoalveolar lavage) where TB-DM patients appear to have reduced activation of immunity; one reported a lower proportion of activated alveolar macrophages [26], and another higher IL-10 (which is anti-inflammatory) and lower IFN-γ[27]. The impact of the host compartment (peripheral blood versus lung) requires further study. In mice with chronic (but not acute) DM who have already progressed to TB (2 months post-infection), there is higher pulmonary Mtb burden and more extensive inflammation with higher expression of pro-inflammatory cytokines like IFN-γ [22;28;29]. Thus, these findings in the lungs of mice resemble the hyper-response to Mtb antigens in the peripheral blood of TB-DM (versus TB-no DM) patients. The contribution of this response to the natural history of DM is discussed in the following sections.
4. Impact of DM on the natural history of TB: Risk of Mtb infection, persistence and LTBI
The detection of a delayed and defective innate immune response to Mtb in DM provides indirect support for the higher risk of LTBI among contacts with DM. However, the relative risk of LTBI among contacts of TB patients with DM (versus no DM) has not been systematically measured in contemporary epidemiological studies. In the Philadelphia survey from 1952, Boucot conducted chest x-rays for the diagnosis of active or LTBI and found that alterations suggestive of TB were positive in 8.4% of DM versus 4.3% of non-DM, with 2.6% of the DM already having active TB [30]. Our observations in a recent pilot study among 79 adult contacts from 55 TB patients indicated a 2-fold higher prevalence of DM or chronic hyperglycemia among contacts with LTBI versus no LTBI (odds ratio 2.39; 95% CI 0.69, 8.54; Restrepo, unpublished). Even though these findings are preliminary, they also suggest that DM patients (versus no DM) may be more likely to become infected with Mtb (Figure). Given that successful Mtb infection is the first requirement for development of TB, there is a need for epidemiologic studies to confirm these early events, along with complementary basic science studies to identify critical molecules and mechanisms that influence the risk of Mtb infection in DM patients. This is a major knowledge gap in the TB field and the resurgence of DM provides a novel opportunity to evaluate it.
5. Impact of DM on progression to TB
The higher risk of DM patients to TB is the most solid and clinically important finding, and hence, the best-characterized aspect of the association between TB and DM throughout history. While we focus our analysis on the contemporary literature on TB and DM which is based on studies in adults who are more likely to have type 2 DM, we should keep in mind that type 1 DM is likely to have played a more significant role prior to the 1950s. This is inferred from the low frequency of obesity worldwide during these earlier times and the characteristics of the patients, including a significant proportion of young adults and the frequent descriptions of ‘severe diabetes’ and diabetic coma [7;8;30]. The recognition of DM as a risk factor for TB received its highest acceptance in the contemporary literature with a publication of a meta-analysis of 13 observational studies in 2008, where Jeon et al concluded that at an individual level, DM patients have a 3-fold higher risk of developing TB (relative risk 3.11; 95% CI 2.27–4.26; Figure) [31]. Most subsequent studies have confirmed that there is a significant TB risk in DM patients, but with variable effect sizes and even with some not showing a significant risk [32–34]. Such variability is also a hallmark of the immunological studies of DM patients as described in the previous section. This variation is likely the result of interaction or additive effects between the relatively “mild” contribution of DM to immune dysfunction and a variety of additional host factors that also depress immunity, vary across study populations, and are not always taken into account. In epidemiological studies, the most notable are poor glucose control and older age. For example, i) Leung et al showed that the higher risk of TB in individuals 65 years or older is poorly-controlled DM (and not DM in itself) [33]; ii) Baker et al showed that the risk of TB increased among DM patients with more than two co-morbidities, a surrogate for chronic and poor glucose control [35]; finally, iii) we found that the relative risk of DM patients for TB was 5.1 (95%CI 2.6–10.2) among individuals 35–64 years old, but a risk was not detected among younger or older adults.[36] The relationship between DM and other co-factors that enhance TB risk are emerging (e.g. DM plus smoking) and are likely to become a common theme as additional studies are conducted [37].
At the population level, the contribution of DM to TB can also vary substantially, even within a country. In the UK the general population attributable risk is 10%, but rises to 20% in Asian males.[38] In countries where TB and DM are endemic such as India or Mexico, the population attributable risk reaches 20%.[39;40] In the Texas-Mexico border our findings are even more striking with data suggesting that DM is the underlying attributable risk for nearly one-third (28%) of the TB cases in this region [36]. Here again, age and a chronic history of poor glucose control are likely to provide the “perfect storm” for TB development. In this community the DM patients had a chronic history of DM prior to TB development (median 6 years) and high HbA1c (66% of DM patients). More than half of the TB patients were between 35–64 years old, the age group where DM is most prevalent.
In summary, most epidemiological studies indicate a higher risk of TB in DM patients, with cohort studies suggesting directionality from DM to TB. We calculated that only 3% of the ‘DM’ in TB-DM patients in our community is due to stress hyperglycemia and not DM per se [36]. Variation in the magnitude of the association between studies is likely the result of variability in the characteristics of study populations, and emphasizes the importance of a thorough characterization of DM and other host factors with multivariable analysis in order to reach reliable conclusions. These basic principles are not always followed given that the results from most of the epidemiological studies are derived from secondary analyses of datasets collected for surveillance purposes. Such lessons in design and analysis need to be extended to clinical and immunological studies in these patients where consensus is often reached based on the most frequent findings, but in the midst of some contradictory results.
6. Impact of DM on TB treatment outcomes
In addition to the well-established contribution of DM to enhanced TB risk, there is growing evidence from observational studies that this co-morbidity is associated with delays in Mtb clearance during treatment, treatment failures, death, relapse and re-infection (Figure) [35;41;42]. Therefore, we summarize the adverse outcomes in order to evaluate their possible underlying mechanisms. We refer the reader to recent systematic reviews and meta-analyses on this topic for a more detailed description of the individual studies [35;41;43]. DM is most often associated with delayed mycobacterial clearance from sputum during the course of treatment (reviewed by [41;43]). That is, TB-DM versus TB-no DM patients have a higher proportion of smear-positives after completion of the intensive phase of treatment, or longer median days-to-mycobacterial clearance from sputum. These outcomes are early predictors of treatment failure (sputum smear or culture positivity at five months or later during treatment), which is also more likely in TB-DM versus TB-no DM [42;44;45]. Another adverse outcome of TB-DM is death as reported by Root in 1934: “During the latter half of the nineteenth century the diabetic patient appeared to be doomed to die of pulmonary TB if he succeeded in escaping coma” [8]. In a systematic review and meta-analysis of contemporary literature, Baker et al concluded that the risk of death in 23 unadjusted studies was nearly 2-fold (RR 1.89; 95% CI 1.52–2.36), and this increased to 4.95 (95% CI 2.69-9-10) in 4 studies that adjusted for age and potential confounders [45]. This difference in results exemplifies the importance of controlling for confounders in TB-DM studies. Finally, TB-DM patients also appear to have a higher risk of relapse. The review by Baker et al reported a nearly 4-fold risk of relapse in TB-DM versus TB-no DM (RR 3.89; 95% CI 2.43–6.23) [45]. A more recent prospective study in southern Mexico with 1262 TB patients further distinguished between relapses and re-infections by complementing the data with Mtb genotyping, and found higher adjusted odds of both in DM vs no DM (OR= 1.8 for both recurrence and relapse) [42].
Given that efficient Mtb killing by anti-mycobacterial antibiotics requires cooperation with a properly functioning immune system, the higher frequency of adverse outcomes in DM patients suggests that the hyper-reactive immune response to mycobacterial antigens in TB-DM patients is not effective for Mtb killing. There are several possible explanations for the contribution of dysfunctional immunity to these adverse treatment outcomes. First, the higher Th1 and Th17 response is only present in the peripheral blood of TB-DM patients, while an anti-inflammatory response that facilitates Mtb growth is located at the site of infection, the lungs. Alternatively, there is a higher cell-mediated immunity in the lungs of humans (as observed in mice), but it is not effective for Mtb killing. Furthermore, this hyper-reaction to Mtb antigens may be deleterious and contribute to lung tissue damage and hence, to the higher frequency of death in these patients.
Together, these results indicate the need for prospective cohort studies aimed at confirming the clinical findings and identifying the underlying factors leading to treatment failure in DM. Two underlying factors are prime suspects. The first is poor glucose control. Chronic hyperglycemia is associated with the dysfunctional immunity to Mtb in DM patients, and hence, likely to reduce the efficiency of anti-mycobacterial treatment. Hyperglycemia may also compromise Mtb killing by affecting the microvasculature and reducing lung tissue perfusion for optimal immune surveillance [46]. However, the need for intervention studies to assess the effect of glucose control on TB treatment outcomes is questionable [43], and the WHO considers that the available data is sufficient to recommend optimized glucose control as part of the management of TB-DM patients for improved TB outcomes [47]. The second suspect is possible suboptimal plasma levels of anti-mycobacterial antibiotics in the DM versus non-DM patients [48–50]. While these studies have conflicting results, further assessment of suboptimal drug levels and its relationship to treatment failure among TB-DM patients is required, particularly in the continuation phase of treatment [51]. This may not only lead to treatment failure, but can favor the development of MDR-TB, which has been reported in TB-DM patients, although in a meta-analysis it was not found to be significantly higher [45;52].
7. Impact of DM on other aspects of the association between TB and DM
Primary versus reactivation TB
The literature often refers to DM as a risk factor for reactivation TB, but the relative risk of primary vs reactivation TB has never been systematically studied in DM. Is the delayed and underperforming innate immune response following the initial encounter with Mtb sufficient to increase the risk of primary TB? Is the ensuing hyper-reactive adaptive response effective to compensate for this initial delay or causes additional problems? Indirect evidence from a population-based study in TB patients from Southern Mexico suggested no difference in the proportion of primary and reactivation TB between TB-DM versus TB-no DM patients. These obervations were based on Mtb strain genotypes where detection of clusters (suggesting recent infection) and non-clustered Mtb (suggesting re-activation TB) among TB-DM versus TB-no DM were no different (Mtb genotype clusters in 24% and non-clustered in 76% in both study groups) [40]. However, other findings suggest that DM may increase the risk of primary TB. First, a cohort of close contacts of TB patients in China reported that DM was a risk factor for TB development at both early (3-months; adjusted OR 9.1, 95%CI 2.6–31.7) and later times during the study (up to 5-years; adjusted OR 3.4, 95%CI 1.0–11.3) time periods after the diagnosis of the index TB case [53]. Second, analysis of chest x-rays indicate that TB-DM patients are more likely to have lower lobe lung infiltrates [54;55]. Given that inhaled Mtb is initially localized in the lower lung lobes where it implants (versus reactivation TB which primarly affects the upper lung after lympho-hematogenous dissemination has occurred), these data suggest that DM patients may be more likely to have primary TB, although alternative explanations for the higher frequency of lower lung lobe infiltrates in DM are possible (older age also favors lower lung lobe infiltrates). Additional prospective studies are required to confirm these findings. The possibility that DM patients may have a higer risk of primary TB alerts us to the need for systematic assessment of DM among adults during contact investigations for preventive treatment in low-prevalence settings or a closer follow-up for early TB detection in TB-endemic regions.
Does immune dysfunction in DM compromise the performance of the TST or IGRAs for detection of LTBI?
The in-vivo TST and ex-vivo IGRAs (QuantiFERON-TB Gold assays and T.Spot-TB; Qiagen and Oxford Immunotec, respectively) are used in the clinical setting to diagnose LTBI in individuals with high risk of TB progression, or support the diagnosis of TB. They are based on the detection of immunological memory to Mtb (or mycobacteria spp. for TST) and therefore their performance depends on intact immune function. Given the importance of these tools for TB prevention, there is a need to determine whether the immune dysfunction in DM compromises their performance. Most of the studies done to date have been in TB patients +/− DM and results are inconsistent. In two studies DM was associated with reduced sensitivity by TST or a higher proportion of false-negatives by QFT-IT [56;57]. However, these findings require further analysis given that in the study by Rawat et al., the DM patients were older and multivariable analysis was not conducted and in the study by Faurholt-Jepsen et al., only the proportion of negative QFT-IT results (and not the positive results) was analyzed, without taking into account the significantly higher proportion of indeterminate results in the non-DM group. Two more studies found no differences in the performance of TST and/or IGRAs by DM status [58;59]. Finally, we found no difference in the performance of the T.Spot-TB, and higher sensitivity of the QFT-Gold in individuals with DM [25]. Thus, most of the studies in TB patients suggest that DM status does not compromise the detection of TB using TSTs or IGRAs, but further studies are required to address the limitations in previous designs and assess the underlying factors for variable performances in different populations.
There is little information on the performance of the TST or IGRAs for the diagnosis of LTBI in DM patients. Faurholt-Jepsen et al. evaluated individuals without TB using a community-based approach and concluded that the proportion of QFT-IT negatives was higher among individuals with pre-DM or DM (vs no DM), but this difference was not significant after controlling for confounders [57]. Thus, additional studies are also needed among DM patients at risk for TB since the diagnosis of LTBI will guide decisions for preventive TB treatment during contact investigations or among individuals at high risk for TB progression.
Pulmonary versus extrapulmonary TB
Pulmonary TB accounts for 70–80% of the cases, and it is generally accepted that compromised immunity facilitates early and late hematogenous dissemination of Mtb, predisposing to extrapulmonary TB. Factors determining the outcome of extrapulmonary TB are not fully understood, but may be influenced by the strain of Mtb and the host’s immune status. Such is the case in HIV patients with advanced AIDS [60] or following treatment with TNF blockers [61]. This contrasts with current findings in DM patients which consistently show lower extrapulmonary TB.[33;62–65] This may be due to a hyper-reactive cell-mediated immune response to Mtb in DM patients that may be suboptimal for containing Mtb growth within the lung, but effective for preventing its dissemination and reactivation elsewhere [23;25;66].
Cavitary and smear-positive TB
The strong cell-mediated immune response elicited by Mtb antigens leads to the formation of a granuloma (tubercle) that becomes a double-edged sword for the host [67]. While it initially contains and limits Mtb growth, in some hosts (most likely with immunocompromise) this structure enlarges and undergoes central caseation with rupture and spilling of thousands of viable bacilli into the airways. This is known as cavitary TB and is associated with smear-positivity [68]. TB-DM patients are more likely than TB-no DM to present with cavitary TB, particularly with poor glycemic control, which is in accordance with their robust cell-mediated immunity to Mtb antigens [54;69;70]. Cavitary TB is accompanied by higher bacillary burden in the sputa of TB-DM patients, as shown in several studies [63;65]. Thus, the CXR findings in TB-DM contrast with those in HIV, where the patients with the lowest CD4+ T cells are less likely to have cavitary TB or positive smears [71;72].
Are TB-DM patients more infectious than TB-no DM?
The higher frequency of PTB vs extrapulmonary TB, cavitary TB, and smear-positive TB would predict that TB-DM patients are more infectious than TB-no DM [73]. This is be further supported by data indicating delays in their conversion to smear- or for prolonged culture-positivity during the course of treatment (discussed above). Thus, studies designed to evaluate whether TB-DM patients contribute to enhanced TB transmission would have a tremendous public-health impact and are likely to reveal another distinction between the HIV and DM co-morbidities.
What can we learn about the association between TB and DM from the studies of other DM complications?
A unifying factor from epidemiological, clinical and immunological studies in the past and current literature on TB and DM is the importance of glucose control [43]. This is consistent with the clinical management and outcome of other DM complications, where the beneficial effects of glucose control for the prevention of microvascular and macrovascular complications are generally accepted. In fact, the risk of microvascular complications among DM patients provided the basis for identifying the normal cut-off for HbA1c levels used today in the clinical setting [74]. However, a more in-depth analysis of this literature indicated a more complex landscape. These guidelines for HbA1c are based on results from the Diabetes Control and Complications Trial published in 1993. But in this trial only 11% of the variation in retinopathy risk was attributed to HbA1c levels or to the duration of DM, suggesting that in the remaining 89% there are other factors that also contribute. Thereafter, it has been suggested that the extent of glucose variability rather than the average glucose levels (which is measured by HbA1c) is a better predictor of DM complications [75;76]. Host genetics further determines the capacity of each individual to deal with this glucose variability. These conclusions are based on combined observations from clinical and basic science studies. Namely, high glucose variability is correlated with epigenetic changes and the generation of reactive oxidative species, the latter being a common effector mechanism for microvasculature damage [77–80]. Some of these findings are beginning to be adopted in the clinical management of DM patients by monitoring not just HbA1c, but also glucose variability, particularly in type 1 DM where this is inevitable. Thus, our studies in TB and DM may need to look beyond HbA1c. This will require a multi-disciplinary approach with the integration of epidemiological, clinical and basic science that leads to the identification of simple and measurable risk factors for TB development at the individual level. Only then will we be able to stratify TB risk with more precision for targeted TB prevention among the millions of DM patients at risk for TB.
The current rise in diabetes mellitus (DM) worldwide has led to the re-emerging importance of DM to tuberculosis control
We review the impact of type 2 DM on every stage of the natural history of TB
We postulate mechanisms by which DM increases the risk of TB development or its more adverse treatment outcomes
Dysfunctional immunity in the poorly-controlled DM patient is the best-characterized underlying factor for promoting TB
Acknowledgments
We thank the physicians, researchers, health authorities, field and laboratory workers from the Texas-Mexico border who have contributed throughout the years to the development of basic and epidemiological studies of TB and diabetes.
Funding: NIH Centers for Translational Science Award (NIH CTSA) grant UL1 TR000371. The content is solely the responsibility of the authors and these sponsors had no role in the study design, collection, analysis or interpretation of data. NIH had no role in the decision to publish.
Footnotes
Conflict of interest
The authors have no conflicts of interest in relation to this work
Author contributions: BIR and LSS wrote and approved the final version of the review
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.World Health Organization. Global tuberculosis report 2013. 2013 http://appswhoint/iris/bitstream/10665/91355/1/9789241564656_engpdf.
- 2.Fox GJ, Menzies D. Epidemiology of tuberculosis immunology. Adv Exp Med Biol. 2013;783:1–32. doi: 10.1007/978-1-4614-6111-1_1. [DOI] [PubMed] [Google Scholar]
- 3.Rieder HL. The dynamics of tuberculosis epidemiology. Indian J Tuberc. 2014;61(1):19–29. [PubMed] [Google Scholar]
- 4.Getahun H, Gunneberg C, Granich R, Nunn P. HIV infection-associated tuberculosis: the epidemiology and the response. Clin Infect Dis. 2010;50 (Suppl 3):S201–S207. doi: 10.1086/651492. [DOI] [PubMed] [Google Scholar]
- 5.Morton R. Phthisiologia, Or a Treatise of Consumptions. London, England: 1694. [Google Scholar]
- 6.Banyai AL. Diabetes and pulmonary tuberculosis. Am Rev Tuberc. 1931;24:650–67. [Google Scholar]
- 7.Oscarsson PN, Silwer H. Incidence of pulmonary tuberculosis among diabetics. Acta Med Scand. 1958;335:23–48. [PubMed] [Google Scholar]
- 8.Root HF. The association of diabetes and tuberculosis. N Engl J Med. 1934;210:1–13. [Google Scholar]
- 9.Chen L, Magliano DJ, Zimmet PZ. The worldwide epidemiology of type 2 diabetes mellitus--present and future perspectives. Nat Rev Endocrinol. 2012;8(4):228–36. doi: 10.1038/nrendo.2011.183. [DOI] [PubMed] [Google Scholar]
- 10.International Diabetes Federation. Diabetes Atlas. 2014 http://wwwdiabetesatlasorg/ [cited 2014 Nov 12];(6th)Available from: URL: http://www.idf.org/sites/default/files/EN_6E_Atlas_Full_0.pdf.
- 11.Dye C. Global epidemiology of tuberculosis. Lancet. 2006;367(9514):938–40. doi: 10.1016/S0140-6736(06)68384-0. [DOI] [PubMed] [Google Scholar]
- 12.Jeon CY, Harries AD, Baker MA, Hart JE, Kapur A, Lonnroth K, et al. Bi-directional screening for tuberculosis and diabetes: a systematic review. Tropical Medicine & International Health. 2010;15(11):1300–14. doi: 10.1111/j.1365-3156.2010.02632.x. [DOI] [PubMed] [Google Scholar]
- 13.Ottmani SE, Murray MB, Jeon CY, Baker MA, Kapur A, Lonnroth K, et al. Consultation meeting on tuberculosis and diabetes mellitus: meeting summary and recommendations. Int J Tuberc Lung Dis. 2010;14(12):1513–7. [PubMed] [Google Scholar]
- 14.Flynn JL, Chan J. Immunology of tuberculosis. Annu Rev Immunol. 2001;19:93–129. doi: 10.1146/annurev.immunol.19.1.93. [DOI] [PubMed] [Google Scholar]
- 15.Guirado E, Schlesinger LS, Kaplan G. Macrophages in tuberculosis: friend or foe. Semin Immunopathol. 2013;35(5):563–83. doi: 10.1007/s00281-013-0388-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nicod LP. Immunology of tuberculosis. Swiss Med Wkly. 2007;137(25–26):357–62. doi: 10.4414/smw.2007.11499. [DOI] [PubMed] [Google Scholar]
- 17.Fabri M, Stenger S, Shin DM, Yuk JM, Liu PT, Realegeno S, et al. Vitamin D is required for IFN-gamma-mediated antimicrobial activity of human macrophages. Sci Transl Med. 2011;3(104):104ra102. doi: 10.1126/scitranslmed.3003045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Restrepo BI, Schlesinger LS. Host-pathogen interactions in tuberculosis patients with type 2 diabetes mellitus. Tuberculosis (Edinb ) 2013;93(S1):S10–S14. doi: 10.1016/S1472-9792(13)70004-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martinez N, Kornfeld H. Diabetes and immunity to tuberculosis. Eur J Immunol. 2014;44(3):617–26. doi: 10.1002/eji.201344301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gomez DI, Twahirwa M, Schlesinger LS, Restrepo BI. Reduced Mycobacterium tuberculosis association with monocytes from diabetes patients that have poor glucose control. Tuberculosis. 2013;93(2):192–7. doi: 10.1016/j.tube.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Restrepo BI, Twahirwa M, Rahbar MH, Schlesinger LS. Phagocytosis via Complement or Fc-Gamma Receptors Is Compromised in Monocytes from Type 2 Diabetes Patients with Chronic Hyperglycemia. PLoS ONE. 2014;9(3):e92977. doi: 10.1371/journal.pone.0092977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vallerskog T, Martens GW, Kornfeld H. Diabetic mice display a delayed adaptive immune response to Mycobacterium tuberculosis. J Immunol. 2010;184(11):6275–82. doi: 10.4049/jimmunol.1000304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Restrepo BI, Fisher-Hoch S, Pino P, Salinas A, Rahbar MH, Mora F, et al. Tuberculosis in poorly controlled type 2 diabetes: altered cytokine expression in peripheral white blood cells. Clin Infect Dis. 2008;47:634–41. doi: 10.1086/590565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumar NP, Sridhar R, Banurekha VV, Jawahar MS, Nutman TB, Babu S. Expansion of pathogen-specific T-helper 1 and T-helper 17 cells in pulmonary tuberculosis with coincident type 2 diabetes mellitus. J Infect Dis. 2013;208(5):739–48. doi: 10.1093/infdis/jit241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walsh M, Camerlin A, Miles R, Pino P, Martinez P, Mora-Guzman F, et al. Sensitivity of Interferon-gamma release assays is not compromised in tuberculosis patients with diabetes. Int J Tuberc Lung Dis. 2010;15(2):179–84. [PMC free article] [PubMed] [Google Scholar]
- 26.Wang CH, Yu CT, Lin HC, Liu CY, Kuo HP. Hypodense alveolar macrophages in patients with diabetes mellitus and active pulmonary tuberculosis. Tuber Lung Dis. 1999;79(4):235–42. doi: 10.1054/tuld.1998.0167. [DOI] [PubMed] [Google Scholar]
- 27.Sun Q, Zhang Q, Xiao H, Cui H, Su B. Significance of the frequency of CD4+CD25+C. Respirology. 2012;17(5):876–82. doi: 10.1111/j.1440-1843.2012.02184.x. [DOI] [PubMed] [Google Scholar]
- 28.Martens GW, Arikan MC, Lee J, Ren F, Greiner D, Kornfeld H. Tuberculosis Susceptibility of Diabetic Mice. Am J Respir Cell Mol Biol. 2007;37:518–24. doi: 10.1165/rcmb.2006-0478OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamashiro S, Kawakami K, Uezu K, Kinjo T, Miyagi K, Nakamura K, et al. Lower expression of Th1-related cytokines and inducible nitric oxide synthase in mice with streptozotocin-induced diabetes mellitus infected with Mycobacterium tuberculosis. Clin Exp Immunol. 2005;139(1):57–64. doi: 10.1111/j.1365-2249.2005.02677.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boucot KR, Dillon ES, Cooper DA, Meier P, Richardson R. Tuberculosis among diabetics: the Philadelphia survey. Am Rev Tuberc. 1952;65(1:2):1–50. [PubMed] [Google Scholar]
- 31.Jeon CY, Murray MB. Diabetes mellitus increases the risk of active tuberculosis: a systematic review of 13 observational studies. PLoS Med. 2008;5(7):1091–101. doi: 10.1371/journal.pmed.0050152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leegaard A, Riis A, Kornum JB, Prahl JB, Thomsen VO, Sorensen HT, et al. Diabetes, glycemic control, and risk of tuberculosis: a population-based case-control study. Diabetes Care. 2011;34(12):2530–5. doi: 10.2337/dc11-0902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leung CC, Lam TH, Chan WM, Yew WW, Ho KS, Leung GM, et al. Diabetic control and risk of tuberculosis: a cohort study. Am J Epidemiol. 2008;167(12):1486–94. doi: 10.1093/aje/kwn075. [DOI] [PubMed] [Google Scholar]
- 34.Dobler CC, Flack JR, Marks GB. Risk of tuberculosis among people with diabetes mellitus: an Australian nationwide cohort study. BMJ Open. 2012;2(1):e000666. doi: 10.1136/bmjopen-2011-000666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baker MA, Lin HH, Chang HY, Murray MB. The risk of tuberculosis disease among persons with diabetes mellitus: a prospective cohort study. Clin Infect Dis. 2012;54(6):818–25. doi: 10.1093/cid/cir939. [DOI] [PubMed] [Google Scholar]
- 36.Restrepo BI, Camerlin A, Rahbar M, Restrepo M, Zarate I, Wing R, et al. Cross-sectional assessment reveals high diabetes prevalence among newly-diagnosed tuberculosis patients. Bull World Health Organ. 2011;89:352–9. doi: 10.2471/BLT.10.085738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reed GW, Choi H, Lee SY, Lee M, Kim Y, Park H, et al. Impact of diabetes and smoking on mortality in tuberculosis. PLoS ONE. 2013;8(2):e58044. doi: 10.1371/journal.pone.0058044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Walker C, Unwin N. Estimates of the impact of diabetes on the incidence of pulmonary tuberculosis in different ethnic groups in England. Thorax. 2010 doi: 10.1136/thx.2009.128223. [DOI] [PubMed] [Google Scholar]
- 39.Stevenson CR, Forouhi NG, Roglic G, Williams BG, Lauer JA, Dye C, et al. Diabetes and tuberculosis: the impact of the diabetes epidemic on tuberculosis incidence. BMC Public Health. 2007;7(147):234. doi: 10.1186/1471-2458-7-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ponce-De-Leon A, Garcia-Garcia MdL, Garcia-Sancho MC, Gomez-Perez FJ, Valdespino-Gomez JL, Olaiz-Fernandez G, et al. Tuberculosis and diabetes in southern Mexico. Diabetes Care. 2004;27(7):1584–90. doi: 10.2337/diacare.27.7.1584. [DOI] [PubMed] [Google Scholar]
- 41.Jeon CY, Murray MB, Baker MA. Managing tuberculosis in patients with diabetes mellitus: why we care and what we know. Expert Rev Anti Infect Ther. 2012;10(8):863–8. doi: 10.1586/eri.12.75. [DOI] [PubMed] [Google Scholar]
- 42.Jimenez-Corona ME, Cruz-Hervert LP, Garcia-Garcia L, Ferreyra-Reyes L, Delgado-Sanchez G, Bobadilla-del-Valle M, et al. Association of diabetes and tuberculosis: impact on treatment and post-treatment outcomes. Thorax. 2013;68(3):214–20. doi: 10.1136/thoraxjnl-2012-201756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jorgensen ME, Faurholt-Jepsen D. Is there an effect of glucose lowering treatment on incidence and prognosis of tuberculosis? A systematic review Curr Diab Rep. 2014;14(7):505. doi: 10.1007/s11892-014-0505-1. [DOI] [PubMed] [Google Scholar]
- 44.Viswanathan V, Vigneswari A, Selvan K, Satyavani K, Rajeswari R, Kapur A. Effect of diabetes on treatment outcome of smear-positive pulmonary tuberculosis--a report from South India. J Diabetes Complications. 2014;28(2):162–5. doi: 10.1016/j.jdiacomp.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 45.Baker MA, Harries AD, Jeon CY, Hart JE, Kapur A, Lonnroth K, et al. The impact of diabetes on tuberculosis treatment outcomes: a systematic review. BMC Med. 2011;9:81. doi: 10.1186/1741-7015-9-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koziel H, Koziel MJ. Pulmonary complications of diabetes mellitus. Pneumonia Infect Dis Clin North Am. 1995;9(1):65–96. [PubMed] [Google Scholar]
- 47.WHO, International Union Against TB Lung Disease. Collaborative framework for care and control of tuberculosis and diabetes. 2011 [PubMed] [Google Scholar]
- 48.Requena-Mendez A, Davies G, Ardrey A, Jave O, Lopez-Romero SL, Ward SA, et al. Pharmacokinetics of rifampin in Peruvian tuberculosis patients with and without comorbid diabetes or HIV. Antimicrob Agents Chemother. 2012;56(5):2357–63. doi: 10.1128/AAC.06059-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nijland HM, Ruslami R, Stalenhoef JE, Nelwan EJ, Alisjahbana B, Nelwan RH, et al. Exposure to rifampicin is strongly reduced in patients with tuberculosis and type 2 diabetes. Clin Infect Dis. 2006;43(7):848–54. doi: 10.1086/507543. [DOI] [PubMed] [Google Scholar]
- 50.Babalik A, Ulus IH, Bakirci N, Kuyucu T, Arpag H, Dagyildizi L, et al. Plasma concentrations of isoniazid and rifampin are decreased in adult pulmonary tuberculosis patients with diabetes mellitus. Antimicrob Agents Chemother. 2013;57(11):5740–2. doi: 10.1128/AAC.01345-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ruslami R, Nijland HM, Adhiarta IG, Kariadi SH, Alisjahbana B, Aarnoutse RE, et al. Pharmacokinetics of antituberculosis drugs in pulmonary tuberculosis patients with type 2 diabetes. Antimicrob Agents Chemother. 2010;54(3):1068–74. doi: 10.1128/AAC.00447-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fisher-Hoch SP, Whitney E, McCormick JB, Crespo G, Smith B, Rahbar MH, et al. Type 2 diabetes and multidrug-resistant tuberculosis. Scand J Infect Dis. 2008;40(11–12):888–93. doi: 10.1080/00365540802342372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee MS, Leung CC, Kam KM, Wong MY, Leung MC, Tam CM, et al. Early and late tuberculosis risks among close contacts in Hong Kong. Int J Tuberc Lung Dis. 2008;12(3):281–7. [PubMed] [Google Scholar]
- 54.Perez-Guzman C, Torres-Cruz A, Villarreal-Velarde H, Vargas MH. Progressive age-related changes in pulmonary tuberculosis images and the effect of diabetes. Am J Respir Crit Care Med. 2000;162(5):1738–40. doi: 10.1164/ajrccm.162.5.2001040. [DOI] [PubMed] [Google Scholar]
- 55.Bacakoglu F, Basoglu OO, Cok G, Sayiner A, Ates M. Pulmonary tuberculosis in patients with diabetes mellitus. Respiration. 2001;68(6):595–600. doi: 10.1159/000050578. [DOI] [PubMed] [Google Scholar]
- 56.Rawat J, Sindhwani G, Biswas D. Effect of age on presentation with diabetes: Comparison of nondiabetic patients with new smear-positive pulmonary tuberculosis patients. Lung India. 2011;28(3):187–90. doi: 10.4103/0970-2113.83975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Faurholt-Jepsen D, Aabye MG, Jensen AV, Range N, PrayGod G, Jeremiah K, et al. Diabetes is associated with lower tuberculosis antigen-specific interferon gamma release in Tanzanian tuberculosis patients and non-tuberculosis controls. Scand J Infect Dis. 2014;46(5):384–91. doi: 10.3109/00365548.2014.885657. [DOI] [PubMed] [Google Scholar]
- 58.Syed Ahamed KB, Raman B, Thomas A, Perumal V, Raja A. Role of QuantiFERON-TB gold, interferon gamma inducible protein-10 and tuberculin skin test in active tuberculosis diagnosis. PLoS ONE. 2010;5(2):e9051. doi: 10.1371/journal.pone.0009051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jung JY, Lim JE, Lee HJ, Kim YM, Cho SN, Kim SK, et al. Questionable role of interferon-gamma assays for smear-negative pulmonary TB in immunocompromised patients. J Infect. 2012;64(2):188–96. doi: 10.1016/j.jinf.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 60.Leeds IL, Magee MJ, Kurbatova EV, del RC, Blumberg HM, Leonard MK, et al. Site of extrapulmonary tuberculosis is associated with HIV infection. Clin Infect Dis. 2012;55(1):75–81. doi: 10.1093/cid/cis303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Keane J. TNF-blocking agents and tuberculosis: new drugs illuminate an old topic. Rheumatology (Oxford) 2005;44(6):714–20. doi: 10.1093/rheumatology/keh567. [DOI] [PubMed] [Google Scholar]
- 62.Reis-Santos B, Locatelli R, Horta BL, Faerstein E, Sanchez MN, Riley LW, et al. Socio-Demographic and Clinical Differences in Subjects with Tuberculosis with and without Diabetes Mellitus in Brazil - A Multivariate Analysis. PLoS ONE. 2013;8(4):e62604. doi: 10.1371/journal.pone.0062604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Restrepo BI, Fisher-Hoch SP, Crespo JG, Whitney E, Perez A, Smith B, et al. Type 2 diabetes and tuberculosis in a dynamic bi-national border population. Epidemiol Infect. 2007;135(3):483–91. doi: 10.1017/S0950268806006935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lin JN, Lai CH, Chen YH, Lee SS, Tsai SS, Huang CK, et al. Risk factors for extra-pulmonary tuberculosis compared to pulmonary tuberculosis. Int J Tuberc Lung Dis. 2009;13(5):620–5. [PubMed] [Google Scholar]
- 65.Viswanathan V, Kumpatla S, Aravindalochanan V, Rajan R, Chinnasamy C, Srinivasan R, et al. Prevalence of diabetes and pre-diabetes and associated risk factors among tuberculosis patients in India. PLoS ONE. 2012;7(7):e41367. doi: 10.1371/journal.pone.0041367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kumar N, Nutman T, Babu S. Expansion of pathogen-specific Th1 and Th17 cells in pulmonary tuberculosis with coincident type 2 diabetes mellitus. 2013:100. doi: 10.1093/infdis/jit241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Guirado E, Schlesinger LS. Modeling the Mycobacterium tuberculosis Granuloma - the Critical Battlefield in Host Immunity and Disease. Front Immunol. 2013;4:98. doi: 10.3389/fimmu.2013.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Russell DG. Who puts the tubercle in tuberculosis? Nat Rev Microbiol. 2007;5(1):39–47. doi: 10.1038/nrmicro1538. [DOI] [PubMed] [Google Scholar]
- 69.Moran A, Harbour DV, Teeter LD, Musser JM, Graviss EA. Is alcohol use associated with cavitary disease in tuberculosis? Alcohol Clin Exp Res. 2007;31(1):33–8. doi: 10.1111/j.1530-0277.2006.00262.x. [DOI] [PubMed] [Google Scholar]
- 70.Chiang CY, Lee JJ, Chien ST, Enarson DA, Chang YC, Chen YT, et al. Glycemic control and radiographic manifestations of tuberculosis in diabetic patients. PLoS ONE. 2014;9(4):e93397. doi: 10.1371/journal.pone.0093397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Perlman DC, El-Sadr WM, Nelson ET, Matts JP, Telzak EE, Salomon N, et al. Variation of chest radiographic patterns in pulmonary tuberculosis by degree of human immunodeficiency virus-related immunosuppression. The Terry Beirn Community Programs for Clinical Research on AIDS (CPCRA). The AIDS Clinical Trials Group (ACTG) Clin Infect Dis. 1997;25(2):242–6. doi: 10.1086/514546. [DOI] [PubMed] [Google Scholar]
- 72.Peter JG, Theron G, Singh N, Singh A, Dheda K. Sputum induction to aid diagnosis of smear-negative or sputum-scarce tuberculosis in adults in HIV-endemic settings. Eur Respir J. 2014;43(1):185–94. doi: 10.1183/09031936.00198012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Behr MA, Warren SA, Salamon H, Hopewell PC, Ponce de LA, Daley CL, et al. Transmission of Mycobacterium tuberculosis from patients smear-negative for acid-fast bacilli. Lancet. 1999;353(9151):444–9. doi: 10.1016/s0140-6736(98)03406-0. [DOI] [PubMed] [Google Scholar]
- 74.The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329(14):977–86. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 75.Hirsch IB, Brownlee M. The effect of glucose variability on the risk of microvascular complications in type 1 diabetes. Diabetes Care. 2007;30(1):186–7. doi: 10.2337/dc06-1610. [DOI] [PubMed] [Google Scholar]
- 76.Hirsch IB, Brownlee M. Beyond hemoglobin A1c--need for additional markers of risk for diabetic microvascular complications. Jama. 2010;303(22):2291–2. doi: 10.1001/jama.2010.785. [DOI] [PubMed] [Google Scholar]
- 77.Monnier L, Mas E, Ginet C, Michel F, Villon L, Cristol JP, et al. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. Jama. 2006;295(14):1681–7. doi: 10.1001/jama.295.14.1681. [DOI] [PubMed] [Google Scholar]
- 78.Monnier L, Colette C, Leiter L, Ceriello A, Hanefeld M, Owens D, et al. The effect of glucose variability on the risk of microvascular complications in type 1 diabetes. Diabetes Care. 2007;30(1):185–6. doi: 10.2337/dc06-1594. [DOI] [PubMed] [Google Scholar]
- 79.El-Osta A, Brasacchio D, Yao D, Pocai A, Jones PL, Roeder RG, et al. Transient high glucose causes persistent epigenetic changes and altered gene expression during subsequent normoglycemia. J Exp Med. 2008;205(10):2409–17. doi: 10.1084/jem.20081188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107(9):1058–70. doi: 10.1161/CIRCRESAHA.110.223545. [DOI] [PMC free article] [PubMed] [Google Scholar]


