Abstract
A protective effect of selenium on lipid levels has been reported in populations with relatively low selenium status. However, recent studies found that high selenium exposure may lead to adverse cardiometabolic effects, particularly in selenium-replete populations. We examined the associations of selenium status with changes in lipid levels in a 7-year follow up of an elderly Chinese cohort including participants from selenium-deplete areas. Study population consisted of 140 elderly Chinese aged 65 or older with nail selenium levels measured at baseline (2003-2005). Lipid concentrations were measured in fasting blood samples collected at baseline and the 7-year follow-up (2010-2012). Analysis of covariance (ANCOVA) models was used to determine the association between baseline selenium status and changes in lipid levels from baseline to follow-up adjusting for other covariates. Mean (±standard deviation) baseline selenium concentration was 0.41±0.2mg/kg. In prospective analysis, we found that individuals in the highest selenium quartile group showed 1.11 SD decrease on total-cholesterol (p<0.001), 0.41 SD increase on HDL-cholesterol (p<0.001) and 0.52 SD decrease on triglyceride after 7 years than those in the lowest selenium quartile group. The similar trends were seen with significant lipids changes in the 2th and 3th quartile groups. Selenium has modestly beneficial effects on blood lipid levels in a population with relatively low selenium status. Our result suggests adequate dietary selenium intake as a potential prevention strategy for lowering lipid levels in selenium deplete populations.
Keywords: selenium, lipids, elderly Chinese
Introduction
Selenium is an essential micronutrient with a narrow safety margin. Adequate selenium intake is needed to maximize antioxidant activities of glutathione peroxidases and other selenoproteins [1, 2]. In animal experiments, selenium supplementation decreased plasma total and low-density lipoprotein cholesterol levels and increased HDL cholesterol levels, whereas selenium deficiency had the opposite effect [3-5]. However, the relationship between selenium status and cardiovascular disease risk in human studies remains inconsistent [6-9].
Several cross-sectional studies have examined the association between selenium levels and lipids profile [10-15], or between selenium levels and the risk of cardiovascular diseases (CVD) [6-9, 16-21] in different populations. An association between higher selenium level and lower lipids has been reported particularly in populations with relatively low selenium status [6, 17-19]. However, in contrast to the putative benefits of selenium, recent observational studies have raised concern that high selenium exposure may lead to adverse cardiometabolic effects, particularly in selenium replete populations such as that of the US [10-11, 22-23]. Furthermore, a few randomized trials found no effect of selenium supplementation on serum cholesterol level [24] and cardiovascular disease prevention [7]. Nonetheless, most of these studies are cross-sectional, which cannot establish a causality relationship, and there is little longitudinal data on the relationship between selenium status and lipids profiles of the elderly.
Data from our populations where selenium supplementation is rare may be able to offer insight on the relationship between selenium and lipid levels. In this article, we report results from a longitudinal study of rural elderly Chinese cohort and examine the association between baseline selenium status and changes in lipid levels.
METHODS
Study Population
Participants for the current study were from the Selenium and Cognitive Decline study, a longitudinal epidemiologic project funded by the National Institute of Health examining the long-term impact of selenium on cognitive decline in rural elderly Chinese. Two thousand Chinese aged 65 and older from four counties in China were enrolled at baseline between December 2003 and May 2005. Details of the study and its methodological procedures have been previously described [25-26]. At baseline, venous blood samples were collected after the subjects had fasted for at least 12 hours from 10% of participants from each county (n=199); of these, 140 subjects were seen again in 2010-2012 and a second fasting blood sample was collected. Approximately 23.6% were lost among the two thousand participants between baseline and the seven years follow-up, but the characteristics of those lost were not significantly different from subjects with followed-up (data not shown). The Indiana University institutional review board and the Institute for Environmental Health and Related Product Safety, Chinese Center for Disease Control and Prevention approved the study.
Selenium Measures
At baseline, nail samples from all study subjects were collected at the time of interview and stored in clean plastic bags labeled with subject identification numbers. Fluorometric determination of trace amount of selenium with 2,3-diaminonaphthalene, described in details elsewhere[27], was used to determine trace amounts of selenium in nail samples. Quality control measures in the laboratory were described previously [25].
Lipids measurements
At both baseline and follow-up examination, serum total cholesterol, high-density lipoprotein (HDL) and triglyceride were tested by Roche Diagnostic kits (enzymic colorimetric method), using Hitachi 7180 automatic biochemistry analyzer. In addition to quality control samples, duplicate samples were used in the lab analysis. If the relative deviation of parallel samples was greater than 10%, we repeated measurement. The average of the two measurements was used in all analyses. The intra- and inter-assay coefficients of variation were less than 1.2% for total cholesterol, 0.9% for HDL cholesterol and 1.4% for triglyceride levels.
Other Information
Other information collected during the baseline interview included age, gender, years of education, body mass index (BMI), whether a participant is a life-long resident of the village since birth, history of alcohol and smoking, and self-reported history of stroke, hypertension, and heart attack. BMI (defined as body weight in kilograms divided by height in meters squared) was derived from height and weight measurements.
Statistical analysis
Descriptive data are expressed as means and standard deviation, or as percentages. Paired t-tests were used to detect significant change in cholesterol, HDL and triglyceride levels from baseline to 7-year follow-up. To detect potential non-linear relationship between selenium and lipid levels, participants were divided into quartile groups according to baseline selenium concentrations. Analysis of variance (ANOVA) was used to compare differences among group means, and chi-squared tests were used for differences in proportions between categorical variables.
For each participant with follow-up evaluation, changes in lipid levels were derived as the difference between follow-up and baseline measures. Analysis of covariance (ANCOVA) models was used to determine the association between baseline selenium quartile groups and changes in lipid level while adjusting for covariates. Separate ANCOVA models were used for cholesterol, HDL and triglyceride, respectively.
Results
Table 1 shows the baseline characteristics of the study population by quartiles of baseline nail selenium concentrations. In the 140 participants, mean (SD) nail selenium concentrations were 0.41(0.2) mg/kg, and mean (SD) cholesterol, HDL and triglyceride concentrations were 4.00(0.8), 1.15(0.3), and 1.11(0.3) (mmol/L), respectively. Higher selenium concentrations were associated with higher BMI, and higher systolic blood pressure measures. Age, sex, life-long residency, history of smoking and drinking alcohol, and other self-reported diseases history did not differ significantly across the selenium quartile groups. Among the baseline participants, selenium concentrations were inversely correlated with HDL (r=-0.21, P=0.02).
Table 1.
Participants’ Baseline Characteristics According to Baseline Selenium Levels Measured in Nail Samples.
| Means (SD) by Selenium Quartile Groups* | ||||||
|---|---|---|---|---|---|---|
| Baseline Characteristics | Total n=140 | Q1(low) n=35 | Q2 n=35 | Q3 n=35 | Q4(high) n=35 | P-value |
| Selenium level, mg/kg | 0.41(0.2) | 0.19(0.03) | 0.34(0.04) | 0.45(0.03) | 0.67(0.1) | <0.001 |
| Age | 69.35(4.1) | 68.70(3.3) | 69.68(4.9) | 69.08(3.6) | 69.96(4.1) | 0.409 |
| Male, % | 49.2 | 54.0 | 56.0 | 50.0 | 36.7 | 0.21 |
| Body Mass Index, kg/m2 | 22.94(3.6) | 22.04(2.7) | 21.92(3.2) | 23.38(3.5) | 24.44(4.5) | 0.001 |
| Systolic blood pressure, mmHg | 143.21(24.2) | 133.21(19.7) | 141.54(20.6) | 148.3(27.3) | 149.9(24.1) | 0.002 |
| Diastolic blood pressure, mmHg | 83.03(12.34) | 79.98(10.84) | 83.48(12.49) | 84.56(13.79) | 84.12(11.89) | 0.236 |
| life-long residency, % | 55.3 | 60.0 | 50.0 | 52.0 | 59.2 | 0.674 |
| Alcohol, % | 46.2 | 60.0 | 42.0 | 46.0 | 36.7 | 0.179 |
| Smoking, % | 50.8 | 23.8 | 25.7 | 28.7 | 21.8 | 0.592 |
| Self-reported history of, % | ||||||
| Diabetes | 4.0 | 4.0 | 4.0 | 6.0 | 2.0 | 0.800 |
| Hypertension | 15.6 | 10.0 | 8.0 | 20.0 | 24.5 | 0.07 |
| Stroke | 1.0 | 0 | 0 | 0 | 4.1 | 0.103 |
| Heart Attack | 3.5 | 6.0 | 2.0 | 4.0 | 2.0 | 0.66 |
| Cholesterol, mmol/L | 4.00(0.8) | 3.99(.7) | 3.68(.6) | 3.87(.7) | 4.24(.7) | 0.004 |
| HDL, mmol/L | 1.15(0.3) | 1.30(.3) | 1.13(.2) | 1.08(.2) | 1.13(.3) | 0.02 |
| Triglyceride, mmol/L | 1.11(0.3) | 1.2(.7) | .96(.3) | 1.11(8) | 1.09(.6) | 0.356 |
Range of baseline selenium levels is lower than 0.245 mg/kg in the lowest quartile (Q1), from 0.246 to 0.407 mg/kg in Q2, from 0.408 to 0.523 in Q3, and higher than 0.523 mg/kg in the highest quartile (Q4).
For the 140 participants who had 7-years follow-up evaluations, triglyceride level had decreased significantly at follow-up (p=0.003). The Pearson correlation coefficients between baseline and follow-up levels were 0.267, 0.395, and 0.456 for cholesterol, HDL, and triglyceride respectively (p<0.001 for all). (See in Table 2)
Table 2.
Changes in serum lipids levels between Baseline and 7-year Follow-up evaluations
| Cholesterol, mmol/L | HDL, mmol/L | Triglyceride, mmol/L | |
|---|---|---|---|
| Means(SD) at baseline | 4.00 (0.8) | 1.15 (0.3) | 1.11 (0.3) |
| Means(SD) at 7-year | 4.19 (0.8) | 1.26 (0.3) | 1.01 (0.3) |
| Change from baseline to 7-year | 0.19 | 0.11 | −0.1* |
| Pearson correlation coefficients between baseline and follow-up | 0.267** | 0.395** | 0.456** |
P<0.05
P<0.001
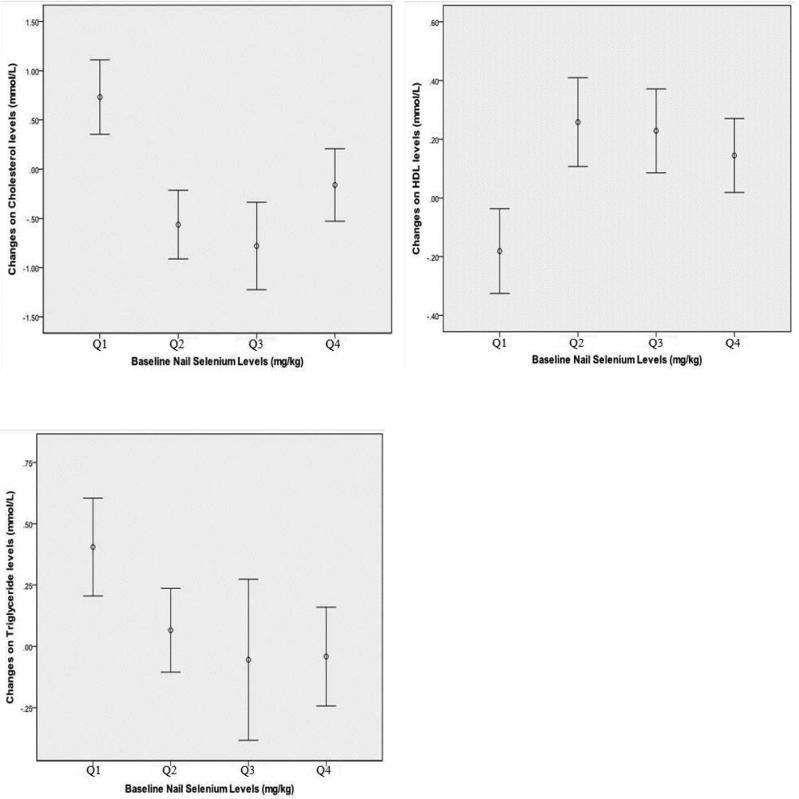
Results from separate ANCOVA models are presented in Table 3 showed that baseline selenium level was a strong predictor of the changes of serum total cholesterol (p<0.001), HDL-cholesterol (p<0.001) and triglyceride (p=0.015) between follow-up and baseline measures, after adjustment for BMI, systolic blood pressure and other covariates. Participants in the bottom selenium quartile group were used as the reference group in all models. Individuals in the highest selenium quartile group showed 1.11 SD decrease on total-cholesterol (p<0.001), 0.41 SD increase on HDL-cholesterol (p<0.001) and 0.52 SD decrease on triglyceride after 7 years than those in the lowest selenium quartile group. The same trends were seen with significant changes in lipids in the 2th and 3th quartile groups compared to the lowest quartile group. Predicted mean lipids changes by baseline selenium quartile groups are presented in Figure 1 and non-linear trends can be seen in all three plots.
Table 3.
Separate analysis of covariance (ANCOVA) models on changes in Cholesterol, HDL and triglyceride levels between baseline and 7-year follow-up examinations.
| Outcome Variable | Selenium Quartile groups, mg/kg | Parameter Estimate | Standard Error Estimate | p-value |
|---|---|---|---|---|
| Cholesterol | Q1 | Reference | -- | -- |
| Q2 | -1.222 | 0.27 | <0.001 | |
| Q3 | -1.637 | 0.28 | <0.001 | |
| Q4 | -1.109 | 0.29 | <0.001 | |
| HDL | Q1 | Reference | -- | -- |
| Q2 | 0.409 | 0.09 | <0.001 | |
| Q3 | 0.459 | 0.10 | <0.001 | |
| Q4 | 0.411 | 0.11 | <0.001 | |
| Triglyceride | Q1 | Reference | -- | -- |
| Q2 | -0.282 | 0.17 | 0.091 | |
| Q3 | -0.478 | 0.17 | 0.005 | |
| Q4 | -0.524 | 0.18 | 0.004 |
All models adjusted for age, gender, body mass index, smoking history, alcohol history, and self-reported history of stroke, hypertension, and angina.
Figure 1.
Relationship between baseline selenium and changes on Cholesterol, HDL and triglyceride levels after 7-year follow-up adjusted for age, gender, body mass index, smoking history, alcohol history, and self-reported history of stroke, hypertension, and angina. The horizontal axis represents baseline nail selenium quartile groups. The vertical axis represents the means and 95% confidence intervals of changes in the two lipids measurements, derived as the difference between follow-up and baseline lipids measures. Higher values on the vertical axis indicate greater lipid increase.
All models in table 3 were repeated with the additional adjustment for baseline lipid levels. Conclusions from all models were unchanged.
Discussion
In this study, we examined the associations between baseline selenium levels and changes in lipid levels in a 7-year follow up of elderly Chinese in the Selenium and Cognitive Decline study. We found that participants with the higher selenium levels had greater decrease in total cholesterol and greater increase in HDL from baseline to follow-up examination compared to those with lowest selenium levels. To our knowledge, this is the first longitudinal study examining the association of selenium status with changes in lipids in a Chinese population.
Cross-sectional studies examining the relationship between selenium status and lipids profiles have not been consistent [10-15]. Some studies found positive association between selenium and lipid levels, i.e. higher selenium was associated with higher lipid levels [10-11, 14-15], while others found no significant relationship [12-13]. Baseline selenium concentrations in our sample (mean 0.41±0.2mg/kg) indicate that our cohort population had relatively low dietary selenium intake. The selenium level in our samples was similar to the Finland urban men aged 55-69 y (mean toenail selenium concentration, 0.47 mg/kg) [28] and the Netherland subcohort populations which randomly drawn from the Netherland total Cohort study on diet and cancer (mean toenail selenium concentration, 0.553 mg/kg) [29]. It is noteworthy that the mean population selenium concentration in our sample was lower than toenail selenium levels in the American men (0.84mg/kg), American women (0.77mg/kg) [30], and young adults (0.86±0.15 mg/kg)[31], and also lower than the Northern Italy healthy controls (median level is 0.65 mg/kg) [32].
There had two previous longitudinal studies examining the relationship between selenium level and changes in lipids and both reported no association [33, 34]. In the adult male cohort of the Olivetti Heart Study, baseline serum selenium was a strong predictor of serum cholesterol (p=0.002) and LDL (p=0.001) at follow-up examination after adjusting for covariates. These associations, however, were no longer significant after additional adjustment for baseline lipids. Selenium at baseline did not predict changes in total cholesterol levels between the baseline and follow-up examinations [β-coefficient (±SE) = 0.09±0.12 (p=0.46)] [33]. However, non-linear trend was not explored in this study. Another cohort assessed cholesterol, HDL and LDL levels 5 times from 1980 to 2007 in Finland where selenium levels were the lowest in the world in the 1980s before a nationwide selenium fertilization program. This study found that selenium measured in 1986 was not associated with lipids assessed in 2001 and 2007 (p>0.05) [34]. Interestingly, cross-sectional analysis of both studies found that high selenium associated with adverse lipids profile, but failed to find baseline selenium associated with changes in lipid levels in prospective analyses.
There are several potential explanations for the difference in results between our study and the two previous ones. Firstly, our population had relatively low mean selenium levels compared with the Olivetti and Finland cohorts [33-34]. Like the findings from Rayman and her colleagues [35], we hypothesize that the association between selenium levels with lipids changes or antioxidant outcomes will probably be influenced by the baseline selenium status of the population studied. Besides, Cross-sectional study from the Third National Health and Nutrition Examination Survey showed an inverse association between serum selenium levels and cardiovascular mortality at low selenium levels (< 130 ng/ml) but a modest increase in mortality at high selenium levels (> 150 ng/ml) [20]. This supports our hypothesis from a biological mechanism view that selenium has narrow safety margin with large inter-individual variability in metabolic sensitivity [36-37]. It is plausible that a U-shaped relationship may exist between selenium level and lipid profiles in that both low and high selenium levels would lead to adverse effects on blood lipids [38, 39-40]. Neither of the two previous studies included results investigating a potential non-linear relationship.
A second explanation for the difference between our study and the two previous studies may be in the stability of the baseline selenium measures. In the Finland study, large scale selenium fertilization program was implemented after baseline selenium measures, thus it is possible that the baseline selenium measures may no longer represent participants’ selenium levels during follow-up. In contrast, the majority of our study participants were lifelong residents of the same villages where they were interviewed, and the participants were known not to take vitamin supplements; hence, the ascertained selenium levels can be inferred as lifelong exposure without the influence of supplements.
Our findings corroborated the antioxidant benefits of selenium on health outcomes [6, 17-19]. Our result was consistent with a randomized trial where 501 elderly volunteers in the UK received 100, 200 or 300μg selenium per day as high-selenium yeast or a yeast-based placebo for 6 months [35]. Compared with the placebo group, participants in the 100μg or 200μg selenium/day groups lowered serum total cholesterol by 0.22mmol/L(p=0.02) or 0.25mmol/L(p=0.008) after 6 months, respectively; Although HDL increased significantly at the 300μg/day group, there had no significant effect on total or non-HDL cholesterol at this dose. This proved that selenium has modestly beneficial effects on blood lipids in persons with relatively low selenium status. But it still should not be used to justify the use of selenium supplementation as additional or alternative therapy for dyslipidemia, particularly in higher selenium regions.
Our study has a number of strength. First, participants in the cohort were mostly life-long residents of the rural villages, thus their baseline selenium levels reflect dietary intake without the influence of selenium supplementation. Second, the availability of repeated blood samples provides the opportunity to examine changes in lipid levels.
This study also had important limitations. First, the sample size was relatively small, mostly due to the small number of participants with blood samples from baseline. Thus our results would need to be confirmed in other studies with larger sample sizes. Second, we cannot rule out the potential for confounding by unmeasured factors, such as the use of lipid lowering medications. Recent findings from the EVA study suggested that long-term use of fibrates (but not statins) increased plasma selenium concentrations in dyslipidemic aged patients [41]. However, such medication use cannot explain the significantly higher increase in selenium levels found in the lowest selenium quartile group. Third, GSHPx activity was not measured in this study and we are unable to determine the extent of GSHPx activity has in the relationship between selenium and lipids levels.
In conclusion, in this rural elderly Chinese cohort we found that participants with the higher selenium levels had greater decrease in total cholesterol and greater increase in HDL from baseline to follow-up examination compared to those with the lowest selenium levels. Our result, if confirmed in other studies, suggests adequate dietary selenium intake as a potential prevention strategy for lowering lipid levels in selenium deplete populations.
Highlights.
We examined the associations of selenium status with changes in lipid levels in a 7-year follow up of an elderly Chinese cohort including participants from selenium-deplete areas. In prospective analysis, we found that participants with the higher selenium levels had greater decrease in total cholesterol and greater increase in HDL from baseline to follow-up examination compared to those with lowest selenium levels, which suggested that adequate dietary selenium intake as a potential prevention strategy for lowering lipid levels in selenium deplete populations.
Acknowledgements
This research was supported by grant from the National Institutes of Health: R01 AG019181.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: There are no conflicts of interest to report.
Author Contributions: All authors participated in the study design and editing of the manuscript. Dr. Gao also contributed to the analysis and interpretation of the data.
References
- 1.Rayman MP. The importance of selenium to human health. Lancet. 2000;356:233–41. doi: 10.1016/S0140-6736(00)02490-9. [DOI] [PubMed] [Google Scholar]
- 2.Institute of Medicine, Panel on Dietary Antioxidants and Related Compounds . Dietary reference intakes for vitamin C, vitamin E, selenium and carotenoids. National Academy Press; Washington, DC: 2000. Subcommittees on Upper Reference Levels of Nutrients and Interpretation and Uses of Dietary Reference Intakes, Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. [Google Scholar]
- 3.Wolf NM, Mueller K, Hirche F, Most E, Pallauf J, Mueller AS. Study of molecular targets influencing homocysteine and cholesterol metabolism in growing rats by manipulation of dietary selenium and methionine concentrations. Br J Nutr. 2010;104(4):520–32. doi: 10.1017/S0007114510000899. [DOI] [PubMed] [Google Scholar]
- 4.Mazur A, Nassir F, Gueux E, Moundras C, Bellanger J, et al. Diets deficient in selenium and vitamin E affect plasma lipoprotein and apolipoprotein concentrations in the rat. Br J Nutr. 1996;76(6):899–907. doi: 10.1079/bjn19960096. [DOI] [PubMed] [Google Scholar]
- 5.Wójcicki J, Rózewicka L, Barcew-Wiszniewska B, Samochowiec L, Juźwiak S, et al. Effect of selenium and vitamin E on the development of experimental atherosclerosis in rabbits. Atherosclerosis. 1991;87(1):9–16. doi: 10.1016/0021-9150(91)90227-t. [DOI] [PubMed] [Google Scholar]
- 6.Flores-Mateo G, Navas-Acien A, Pastor-Barriuso R, et al. Selenium and coronary heart disease: a meta-analysis. Am J Clin Nutr. 2006;84:762–73. doi: 10.1093/ajcn/84.4.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stranges S, Marshall JR, Trevisan M, et al. Effects of selenium supplementation on cardiovascular disease incidence and mortality: secondary analyses in a randomized clinical trial. Am J Epidemiol. 2006;163(8):694–9. doi: 10.1093/aje/kwj097. [DOI] [PubMed] [Google Scholar]
- 8.Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. JAMA. 2007;297(8):842–57. doi: 10.1001/jama.297.8.842. [DOI] [PubMed] [Google Scholar]
- 9.Stranges S, Navas-Acien A, Rayman MP, Guallar E. Selenium status and cardiometabolic health: state of the evidence. Nutr Metab Cardiovasc Dis. 2010;20(10):754–60. doi: 10.1016/j.numecd.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 10.Stranges S, Laclaustra M, Ji C, et al. Higher selenium status is associated with adverse blood lipid profile in British adults. J Nutr. 2010;140:81–7. doi: 10.3945/jn.109.111252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laclaustra M, Stranges S, Navas-Acien A, Ordovas JM, Guallar E. Serum selenium and plasma lipids in US adults: National Health and Nutrition Examination Survey (NHANES) 2003–2004. Atherosclerosis. 2010;210:643–8. doi: 10.1016/j.atherosclerosis.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jossa F, Trevisan M, Krogh V, et al. Serum selenium and coronary heart disease risk factors in southern Italian men. Atherosclerosis. 1991;87:129–34. doi: 10.1016/0021-9150(91)90015-u. [DOI] [PubMed] [Google Scholar]
- 13.Ringstad J, Jacobsen BK, Thomassen Y. The Tromso Heart Study: relationships between the concentration of selenium in serum and risk factors for coronary heart disease. J Trace Elem Electrolytes Health Dis. 1987;1:27–31. [PubMed] [Google Scholar]
- 14.Berr C, Coudray C, Bonithon-Kopp C, et al. Demographic and cardiovascular risk factors in relation to antioxidant status: the EVA Study. Int J Vitam Nutr Res. 1998;68:26–35. [PubMed] [Google Scholar]
- 15.Yang KC, Lee LT, Lee YS, et al. Serum selenium concentration is associated with metabolic factors in the elderly: a cross-sectional study. Nutr Metab. 2010;7:38. doi: 10.1186/1743-7075-7-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suadicani P, Hein HO, Gyntelberg F. Serum selenium concentration and risk of ischaemic heart disease in a prospective cohort study of 3000 males. Atherosclerosis. 1992;96:33–42. doi: 10.1016/0021-9150(92)90035-f. [DOI] [PubMed] [Google Scholar]
- 17.Salonen JT, Alfthan G, Huttunen JK, Pikkarainen J, Puska P. Association between cardiovascular death and myocardial infarction and serum selenium in a matched-pair longitudinal study. Lancet. 1982;2:175–9. doi: 10.1016/s0140-6736(82)91028-5. [DOI] [PubMed] [Google Scholar]
- 18.Virtamo J, Valkeila E, Alfthan G, et al. Serum selenium and the risk of coronary heart disease and stroke. Am J Epidemiol. 1985;122:276–82. doi: 10.1093/oxfordjournals.aje.a114099. [DOI] [PubMed] [Google Scholar]
- 19.Wei WQ, Abnet CC, Qiao YL, et al. Prospective study of serum selenium concentrations and esophageal and gastric cardia cancer, heart disease, stroke, and total death. Am J Clin Nutr. 2004;79:80–5. doi: 10.1093/ajcn/79.1.80. [DOI] [PubMed] [Google Scholar]
- 20.Bleys J, Navas-Acien A, Guallar E. Serum selenium levels and all-cause, cancer, and cardiovascular mortality among US adults. Arch Intern Med. 2008;168:404–10. doi: 10.1001/archinternmed.2007.74. [DOI] [PubMed] [Google Scholar]
- 21.Bleys J, Navas-Acien A, Laclaustra M, et al. Serum selenium and peripheral arterial disease: results from the National Health and Nutrition Examination Survey (NHANES) 2003–2004. Am J Epidemiol. 2009;169:996–1003. doi: 10.1093/aje/kwn414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stranges S, Marshall JR, Natarajan R, et al. Effects of long-term selenium supplementation on the incidence of type 2 diabetes: a randomized trial. Ann Intern Med. 2007;147:217–23. doi: 10.7326/0003-4819-147-4-200708210-00175. [DOI] [PubMed] [Google Scholar]
- 23.Laclaustra M, Navas-Acien A, Stranges S, Ordovas JM, Guallar E. Serum selenium levels and hypertension in the US population. Circ Cardiovasc Qual Outcomes. 2009;2:369–76. doi: 10.1161/CIRCOUTCOMES.108.831552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu SY, Mao BL, Xiao P, et al. Intervention trial with selenium for the prevention of lung cancer among tin miners in Yunnan, China. A pilot study. Biol Trace Elem Res. 1990;24:105–8. doi: 10.1007/BF02917199. [DOI] [PubMed] [Google Scholar]
- 25.Gao S, Jin Y, Hall KS, Liang C, Unverzagt FW, Ji R, Murrell JR, Cao J, Shen J, Ma F, Matesan J, Ying B, Cheng Y, Bian J, Li P, Hendrie HC. Selenium level and cognitive function in rural elderly Chinese. Am J Epidemiol. 2007;165(8):955–65. doi: 10.1093/aje/kwk073. Epub 2007 Jan 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gao S, Jin Y, Unverzagt FW, et al. Trace element levels and cognitive function in rural elderly Chinese. J Gerontol A Biol Sci Med Sci. 2008 Jun;63(6):635–641. doi: 10.1093/gerona/63.6.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li M, Cao J, Sun S. Micro-fluoremetric determination of trace amount of selenium in blood, hair and milk powder. Chinese Journal of Public Health. 1991;10:306–308. [Google Scholar]
- 28.Ovaskainen ML, Virtamo J, Alfthan G, et al. Toenail selenium as an indicator of selenium intake among middle-aged men in an area with low soil selenium. Am J Clin Nutr. 1993;57(5):662–5. doi: 10.1093/ajcn/57.5.662. [DOI] [PubMed] [Google Scholar]
- 29.Steevens J, Schouten LJ, Driessen AL, Huysentruyt CJ, Keulemans YC, et al. Toenail selenium status and the risk of Barrett's esophagus: the Netherlands Cohort Study. Cancer Causes Control. 2010;21(12):2259–68. doi: 10.1007/s10552-010-9651-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park K, Rimm EB, Siscovick DS, Spiegelman D, Manson JE, Morris JS, Hu FB, Mozaffarian D. Toenail selenium and incidence of type 2 diabetes in U.S. men and women. Diabetes Care. 2012;35(7):1544–51. doi: 10.2337/dc11-2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xun P, Hou N, Daviglus M, Liu K, Morris JS, Shikany JM, Sidney S, Jacobs DR, He K. Fish oil, selenium and mercury in relation to incidence of hypertension: a 20-year follow-up study. J Intern Med. 2011;270(2):175–86. doi: 10.1111/j.1365-2796.2010.02338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vinceti M, Crespi CM, Malagoli C, Bottecchi I, Ferrari A, Sieri S, Krogh V, Alber D, Bergomi M, Seidenari S, Pellacani G. A case-control study of the risk of cutaneous melanoma associated with three selenium exposure indicators. Tumori. 2012;98(3):287–95. doi: 10.1700/1125.12394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stranges S, Galletti F, Farinaro E. Associations of selenium status with cardiometabolic risk factors: an 8-year follow-up analysis of the Olivetti Heart study. Atherosclerosis. 2011;217(1):274–8. doi: 10.1016/j.atherosclerosis.2011.03.027. [DOI] [PubMed] [Google Scholar]
- 34.Stranges S, Tabák AG, Guallar E, Rayman MP, Akbaraly TN. Selenium Status and Blood Lipids: The Cardiovascular Risk in Young Finns Study. J Intern Med. 2011;270(5):469–77. doi: 10.1111/j.1365-2796.2011.02398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rayman MP, Stranges S, Griffin BA, Pastor-Barriuso R, Guallar E. Effect of supplementation with high-selenium yeast on plasma lipids: a randomized trial. Ann Intern Med. 2011;154(10):656–65. doi: 10.7326/0003-4819-154-10-201105170-00005. [DOI] [PubMed] [Google Scholar]
- 36.Rayman MP. Food-chain selenium and human health: emphasis on intake. Br J Nutr. 2008;100:254–68. doi: 10.1017/S0007114508939830. [DOI] [PubMed] [Google Scholar]
- 37.Whanger P, Vendeland S, Park YC, Xia Y. Metabolism of subtoxic levels of selenium in animals and humans. Ann Clin Lab Sci. 1996;26:99–113. [PubMed] [Google Scholar]
- 38.Duffield AJ, Thomson CD, Hill KE, Williams S. An estimation of selenium requirements for New Zealanders. Am J Clin Nutr. 1999;70:896–903. doi: 10.1093/ajcn/70.5.896. [DOI] [PubMed] [Google Scholar]
- 39.Navas-Acien A, Bleys J, Guallar E. Selenium intake and cardiovascular risk : what is new? Curr Opin Lipidol. 2008;19:43–9. doi: 10.1097/MOL.0b013e3282f2b261. [DOI] [PubMed] [Google Scholar]
- 40.Mueller K, Wolf NM, Pallauf J. Selenium and diabetes: an enigma? Free Radic Res. 2009;43349(17):1029–59. 1605–13. doi: 10.1080/10715760903196925. [DOI] [PubMed] [Google Scholar]
- 41.Arnaud J, Akbaraly TN, Hininger-Favier I, Berr C, Roussel AM. Fibrates but not statins increase plasma selenium in dyslipidemic aged patients-The EVA study. J Trace Elem Med Biol. 2009;23:21–8. doi: 10.1016/j.jtemb.2008.08.001. [DOI] [PubMed] [Google Scholar]