Abstract
Background
Anaphylaxis-related deaths in the United States (US) have not been well characterized in recent years.
Objectives
To define epidemiological features and time trends of fatal anaphylaxis in the US from 1999 to 2010.
Methods
Anaphylaxis-related deaths were identified by the 10th clinical modification of the International Classification of Diseases (ICD-10) system diagnostic codes on death certificates from the US National Mortality Database. Rates were calculated using census population estimates.
Results
There were a total of 2,458 anaphylaxis deaths in the US from 1999 to 2010. Medications were the most common cause (58.8%), followed by unspecified anaphylaxis (19.3%), venom (15.2%), and food (6.7%). There was a significant increase in fatal drug anaphylaxis over twelve years: from 0.27 (95%CI: 0.23-0.30) in 1999-2001 to 0.51 (95%CI: 0.47-0.56) per million in 2008-2010, P<0.001. Fatal anaphylaxis due to medications, food, and unspecified allergens was significantly associated with African-American race and older age, P<0.001. Fatal anaphylaxis to venom was significantly associated with White race, older age, and male gender, P<0.001. The rates of fatal anaphylaxis to foods in African-American males increased from 0.06 (95%CI 0.01-0.17) in 1999-2001 to 0.21 (95%CI 0.11-0.37) per million in 2008-2010, P<0.001. The rates of unspecified fatal anaphylaxis decreased overtime from 0.30 (95%CI: 0.26-0.34) in 1999-2001 to 0.09 (95%CI: 0.07-0.11) per million in 2008-2010, P<0.001.
Conclusion
There are strong and disparate associations between race and specific classes of anaphylaxis mortality in the United States. The increase in medication-related anaphylaxis deaths likely relates to increased medication and radiocontrast use, enhanced diagnosis, and coding changes.
Keywords: fatal anaphylaxis, drug, food, venom, death certificate, epidemiology
Introduction
Anaphylaxis, has been dubbed “the latest allergy epidemic”. 1 The US and Australia have some of the highest rates of severe anaphylaxis among developed countries.2 Analyzing fatal anaphylaxis trends is challenging in the US, as unlike the United Kingdom (UK),3 it does not have a national registry for anaphylaxis deaths. There is a voluntary registry of fatal food-induced anaphylaxis, which documented 32 cases of fatal anaphylaxis due to food between 1994 and 1998 and additional an 31 cases between 2001 and 2006.4,5 Anaphylaxis admissions have increased overtime in the US, Australia and in the United Kingdom for both food and non-food related causes.6-11 In Australia, the incidence of fatal anaphylaxis doubled between 2002 and 2004 as compared to 1997-2001.9 It has been suggested that overall rates of fatal anaphylaxis in the US is stable.6 However, little is known about changes in anaphylaxis-related mortality rates by cause and how they differ among racial/ethnic groups and by age and gender. Furthermore, while racial disparities have been reported for other allergic conditions, such as food allergies and asthma,12-14 it is not clear whether the same tendencies exist in fatal anaphylaxis. The ICD-10 system introduced drug anaphylaxis as a new category, and also added specific codes, which allow for more exact identification of the cause of anaphylaxis. This enabled the identification of medications involved in anaphylactic shock in the UK and probable causes of fatal anaphylaxis in Australia.11,15
To understand the features and time trends in fatal anaphylaxis we analyzed the National Mortality Database in the United States between 1999 and 2010, using ICD-10 coding of US death certificates. With this study we characterized recent trends in fatal anaphylaxis and its associations with demographic characteristics such as age, gender, race, and geographical distribution in the US.
Methods
Case identification and selection
Mortality data were obtained from the Vital Statistics of the National Center for Health Statistics' (NCHS) Multiple Cause of Death Data,16 which containes the codes provided on all annually reported death certificates from the US. Data were analyzed for the time period starting 1999 (the first year ICD-10 codes were used),17 through 2010 (last year for which data were available in the Vital Statistics database at the time of the analysis). All mortality data for this period were entered from death certificates into the database by the Automated Mortality Medical Data System.18
Categories were assigned using specific anaphylaxis codes (food, drug, serum, unspecified), and certain causality codes (e.g., venom, medications) (see Table E1 in this article's Online Repository at www.jacionline.org).
In the Multiple Cause of Death Database, anaphylaxis is disallowed as the underlying cause-of-death,19 therefore, anaphylactic deaths were defined as all deaths that had at least one anaphylaxis code among the remaining causes of death fields. Additional deaths that fulfilled algorithmic definitions of anaphylaxis were identified (see Table E2 in this article's Online Repository).20,21
The following groupings were identified as probable causes of fatal anaphylaxis:15
-
-
Drug anaphylaxis: T88.6 (anaphylactic shock due to adverse effect of correct drug or medicament properly administered) with or without medication-specific codes Y40.0 through Y59.0 and/or T88.7 (specific or unspecified adverse drug reactions, including allergic), or T78.2 (anaphylactic shock, unspecified) if recorded in combination with Y40.0-Y59.0 or T88.7.
-
-
Anaphylactic shock due to serum: T80.5.
-
-
Food anaphylaxis: T78.0 (anaphylactic shock due to adverse food reaction) and/or T78.1 (other adverse reaction (including allergic) to ingested food) if it was recorded in combination with T78.2.
-
-
Venom anaphylaxis: T63.4 (toxic effect of contact with venom of other arthropods (insect bite or sting), X23 (contact (accidental) with bees, wasps, hornets, including yellow jacket), X25 (contact (accidental) or sting by venomous arthropod, insect, or ants), if it was recorded in combination with T78.2.
-
-
Unspecified anaphylaxis: T78.2 in the absence of other anaphylactic codes or X/Y codes.
Anaphylactic deaths were further analyzed for the place of death: (i) either in a medical facility (including nursing home or hospice) as inpatient (inpatient); (ii) in an outpatient medical facility or emergency room (ER) (outpatient); (iii) or decedent's home, or other, or dead on arrival (community).
Anaphylactic deaths were analyzed for the presence of asthma,22 angioedema or hypotension on death certificates (see Table E2 in this article's Online Repository).
Data and statistical analysis
Each of the above categories (drug, venom, food, or unspecified) was evaluated by age, sex, and race. Race was specified based on categories used by the US Census Bureau and race information was obtained from the death certificates. Race and ethnicity groupings were created as follows: White, African-American, Hispanics, and others.23 Age was grouped by 20 years intervals: 0-19 years, 20-39 years, 40-59 years, 60-79 years, 80 years and above). Three-year time intervals were used as follows: 1999-2001, 2002-2004, 2005-2007, and 2008-2010.
Per persons rates were calculated using US census population data. For years 2000 and 2010 the actual population counts were employed. Population estimates were used for the remaining years.24 Mortality rates by age, sex, race, and time period per million persons were calculated. Rates were not calculated for other race due to the small number of cases in each category and a heterogeneous mix.
95% confidence intervals (95%CI) were calculated for each time point of the analysis by applying gamma method for estimates based on fewer than 100 deaths.25-27 Negative binomial regression models (including all of the predictors) were used to assess the predictor effects of age, sex, race, time, and region on anaphylactic deaths. A dichotomous race variable was used in regression models as either White vs. non-White, African-American vs. non-African American, or Hispanics vs. non-Hispanics for each of the four anaphylactic death groups. Information on US census geographical regions (Northeast, Midwest, South, and West)28 was available only for years 1999 through 2004.16 Modeling which included geographic region was performed as a subset analysis. STATA 11.2 (College Station, TX) and SAS 9.3 (Cary, NC) were used for statistical analysis. The study was classified as exempt by the Albert Einstein College of Medicine Institutional Review Board.
Results
General characteristics of anaphylactic deaths
From 1999 through 2010, there were 2,458 fatal anaphylaxis cases due to all causes, with a prevalence of fatal anaphylaxis of 0.69 people per million. Among anaphylaxis causes, medications were the most common cause of fatal anaphylaxis in the US, followed by unspecified anaphylaxis, venom, and food. The demographic characteristics for each anaphylaxis cause are shown in Table 1.
Table 1. Characteristics of fatal anaphylaxis, by specific cause.
| Characteristic | Anaphylaxis N=2,458 |
|||
|---|---|---|---|---|
| Drug and serum N=1,446 (58.8%) |
Venom N=374 (15.2%) |
Food N=164 (6.7%) |
Unspecified N=474 (19.3%) |
|
| Males, n (%) | 701 (48.1) | 301 (80.3) | 97 (59.2) | 238 (50.2) |
| Age, median (IQR§) | ||||
| Females | 61 (47-73) | 51 (42-64) | 44 (20-60) | 57 (43-71) |
| Males | 59 (47-70) | 52 (44-62) | 37 (22-50) | 54 (40-68) |
| Race, n (%) | ||||
| Non-Hispanic White | 1,071 (73.7) | 328 (87.7) | 98 (59.8) | 342 (72.2) |
| African-American | 240 (16.5) | 24 (6.4) | 41 (25.0) | 93 (19.6) |
| Hispanic | 93(6.4) | 17 (4.6) | 9 (6.0) | 29 (6.1) |
| Place of death | ||||
| Inpatient | 852 (58.6%) | 152 (40.6%) | 56 (34.2%) | 270 (57.0%) |
| Outpatient (including ER*) | 429 (29.5%) | 154 (41.2%) | 86 (52.4%) | 134 (28.3%) |
| Community (including dead onarrival) | 156 (10.75%) | 67 (17.9%) | 21 (12.8%) | 64 (13.5%) |
| Associations with asthma, angioedema, and hypotension | ||||
| Asthma | 54 (3.7%) | 3 (0.8) | 27 (16.5) | 37 (7.8) |
| Angioedema | 49 (3.4%) | 2 (0.5) | 5 (3.1) | 26 (5.5) |
| Hypotension | 46 (3.2%) | 12 (3.2) | 0 | 18 (3.8) |
IQR–interquartile range
ER – emergency room
Drug-induced anaphylaxis (including serum)
There were 1,446 fatal anaphylaxis deaths attributable to medications comprising 58.8% of fatal anaphylaxis deaths in the US during the twelve years of the study. Forty-nine (3.4%) of the fatalities were in persons ≤19 years of age. Most deaths (58.6%) occurred in inpatient facilities, Table 1.
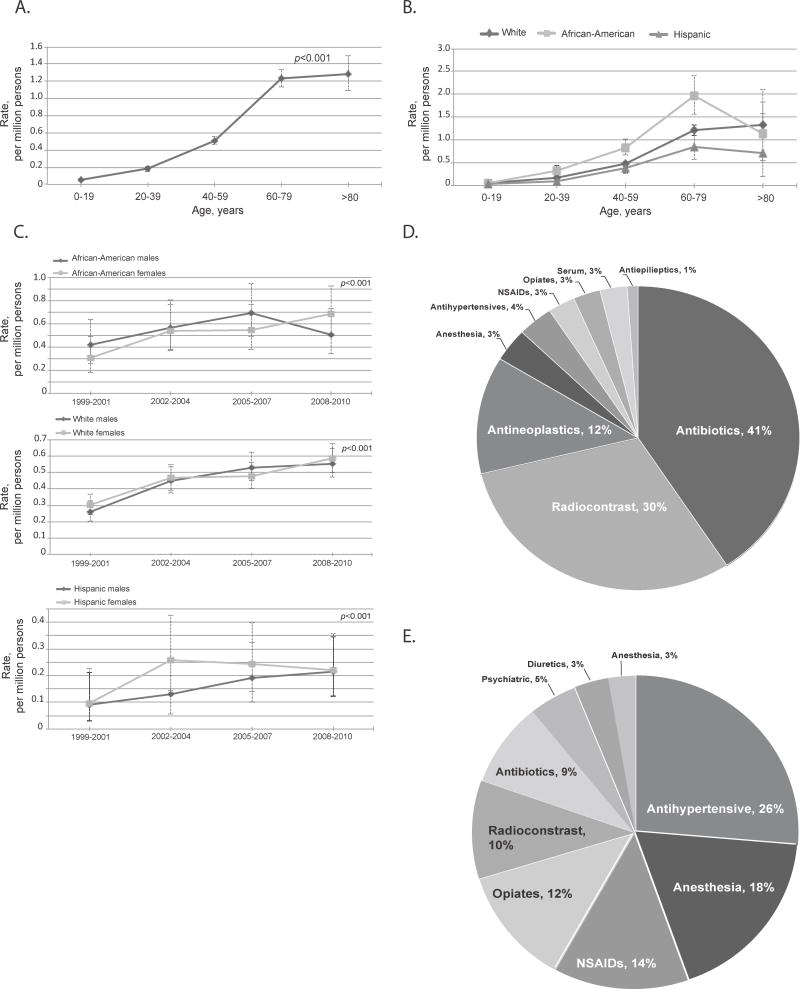
Higher rates of drug anaphylaxis were observed with increasing age and in African-Americans: 0.05 (95%CI 0.04-0.07) per million among ≤19 years old vs. 1.28 (95%CI 1.09-1.50) per million among people ≥80 years old, and 0.54 (95%CI 0.47-0.61) in African-Americans, while 0.19 (95%CI 0.15-0.23) per million in Hispanics, and 0.45 (95%CI 0.43-0.48) per million in Whites,P < 0.001 for all (Figures 1A and 1B and see Tables E3 and E4 in this article's Online Repository).
Figure 1.
Fatal drug anaphylaxis features. A. Rates by age groups, all races and genders combined. Drug anaphylaxis was significantly associated with age, P<0.001. B. Rates by race and age groups, both genders. Drug anaphylaxis was significantly associated with African-American race, P<0.001. C. Rates over time by race and gender. Fatal anaphylaxis to drug significantly increased over 12 years among all races and both genders, P<0.001. No significant difference was observed between females and males. D: Culprit medications in fatal drug anaphylaxis with specified medications, N=368. E: Culprit medications in fatal algorithm-identified anaphylaxis with specified medications, N=148. The error bars show 95%CI.
The rates of drug-induced fatal anaphylaxis increased overtime for all races from 0.27 (95%CI 0.23-0.30) per million in 1999-2001 to 0.51 (95%CI 0.47-0.56) per million in 2008-2010, P<0.001 (Figure 1C and see Table E5 in this article's Online Repository). There was no significant difference in rates of fatal drug anaphylaxis between genders, (Figure 1C and see Table E5 in this article's Online Repository).
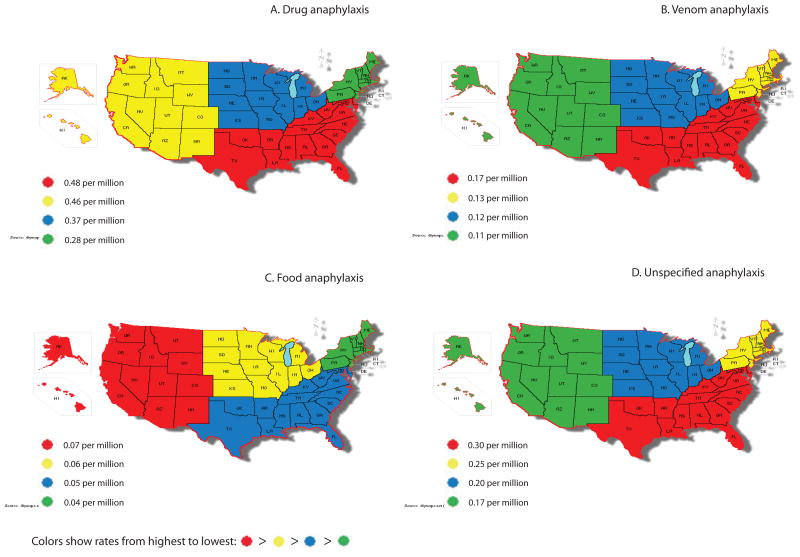
Between 1999 and 2004, the Northeast region had significantly lower rates of drug anaphylaxis, P= 0.01 (Figure 2A). African-American race, year and age remained significant after adjusting for region, P<0.001 for all.
Figure 2.
Fatal anaphylaxis by geographic region performed as a subset analysis, 1999-2004. A. Drug anaphylaxis rates were lower in Northeast region, P<0.001. B. Venom anaphylaxis rates were higher in the South region, P<0.001. C. There was no significant association with geographic region for food-induced anaphylaxis. D. West region had lower unspecified anaphylaxis rates, P=0.03.
Maps were created using an interactive map tool at http://diymaps.net
In 1,078 (74.5%) of all drug anaphylaxis fatalities the culprit drug was not specified. Among 368 (25.4%) fatalities in which a culprit drug class was identified, 40.5% were due to antibiotics (149 deaths). Of these, only 77 had a specific antibiotic class assigned. Penicillins were identified most often (35), followed by cephalosporins (33 deaths), sulfa-containing medications and macrolides. The remainder was due to unspecified antibiotics.
The next most common group of specified drug anaphylaxis causes consisted of reactions to radiocontrast agents (100 deaths). When combined with the adverse reactions due to other diagnostic agents code, this group comprised 112 (30.4%) deaths.
The next most frequent group was anaphylactic deaths due to anti-neoplastic drugs (46). The remaining specified drug anaphylaxis deaths were due to serum (10), opiates, anti-hypertension agents, non-steroidal anti-inflammatory drugs (NSAIDs), and anesthetic agents, Figure 1D.
Venom-induced anaphylaxis
Three hundred seventy-four fatalities were attributed to venom anaphylaxis, which comprised 15.2% of all anaphylaxis cases (Table 1). Six fatalities were in persons ≤19 years of age. Venom anaphylaxis had the largest proportion of deaths occurring in the community (17.9%), Table 1.
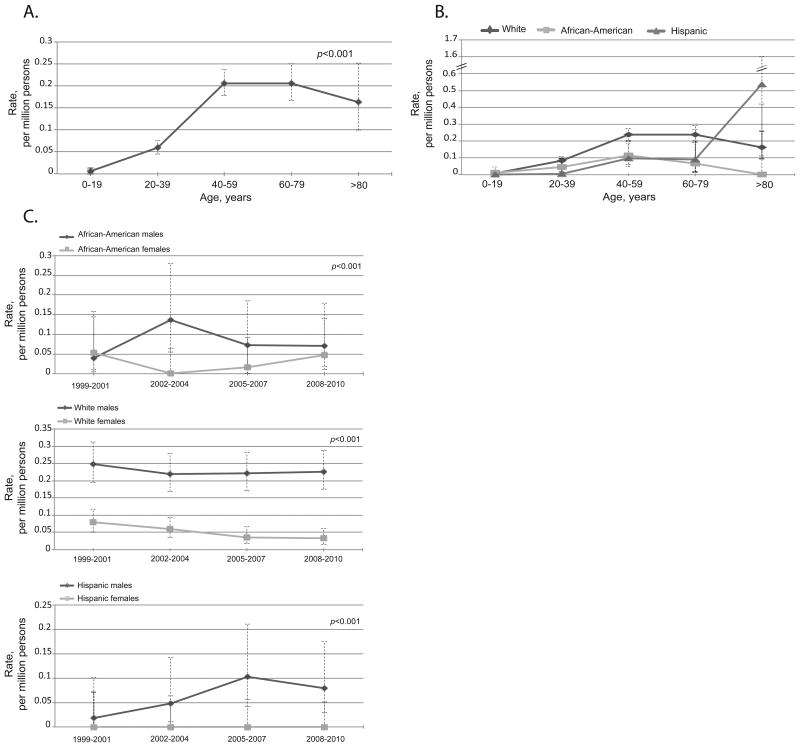
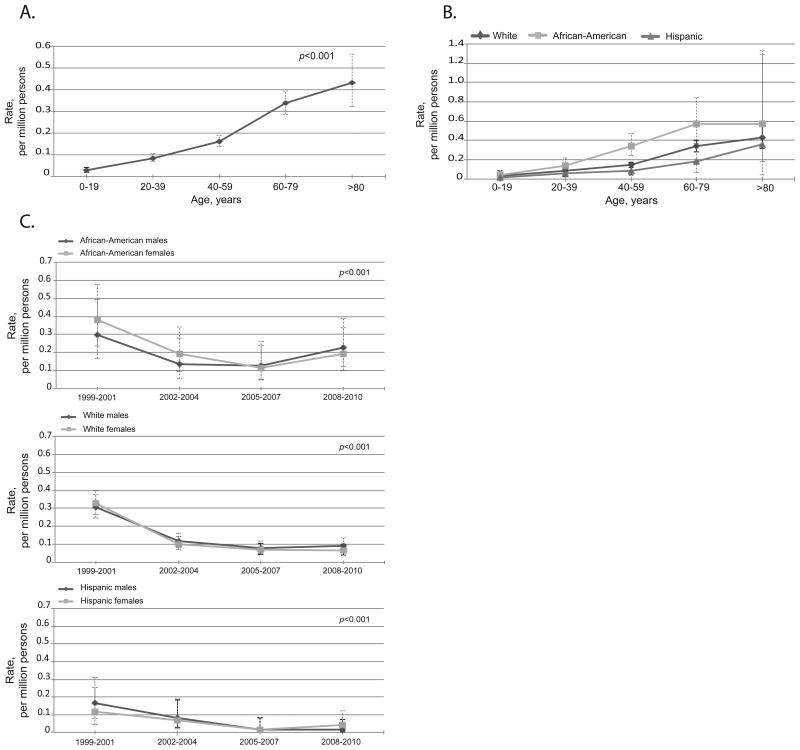
Fatal anaphylaxis from venom was significantly associated with older age: 0.01 (95%CI 0.00-0.01) per million in ≤19 years old vs. 0.20 (95%CI 0.17-0.25) per million among 60-79 years old), White race: 0.14 (95%CI 0.12-0.15) in Whites vs. 0.05 (95%CI 0.03-0.08) per million in African-Americans, and 0.03 (95%CI 0.02-0.05) per million in Hispanics, and male gender: 0.18 (95%CI 0.16-0.21) per million males vs. 0.04 (95%CI 0.03-0.05) per million females, P<0.001 for all (Figure 3A, 3B and 3C and see Tables E3, E4, and E5 in this article's Online Repository).
Figure 3.
Fatal venom anaphylaxis features. A. Rates by age groups, all races and genders combined. Venom anaphylaxis was significantly associated with age, P<0.001. B. Rates by race and age groups, both genders. Venom anaphylaxis was significantly associated with White race, P<0.001. C. Rates over time by race and gender. Venom anaphylaxis was significantly associated with male gender, P<0.001.There was no significant increase overtime in fatal anaphylaxis to venom. The error bars show 95%CI.
There was no significant increase in venom anaphylaxis rates overtime (Figure 3C and see Table E5 in this article's Online Repository). Between 1999 and 2004, the South had significantly higher rates of fatal venom anaphylaxis, P=0.02 (Figure 2B). Age, male gender, and White race remained significant after adjusting for region, P<0.001 for all.
Food-induced anaphylaxis
Anaphylaxis to food was identified in 164 fatalities and was the least common (6.7%) cause of fatal anaphylaxis (Table 1). Thirty-seven (22.6%) deaths were in persons ≤19 years of age. This group had the highest (52.4%) proportion of deaths occurring in outpatient facilities, Table 1.
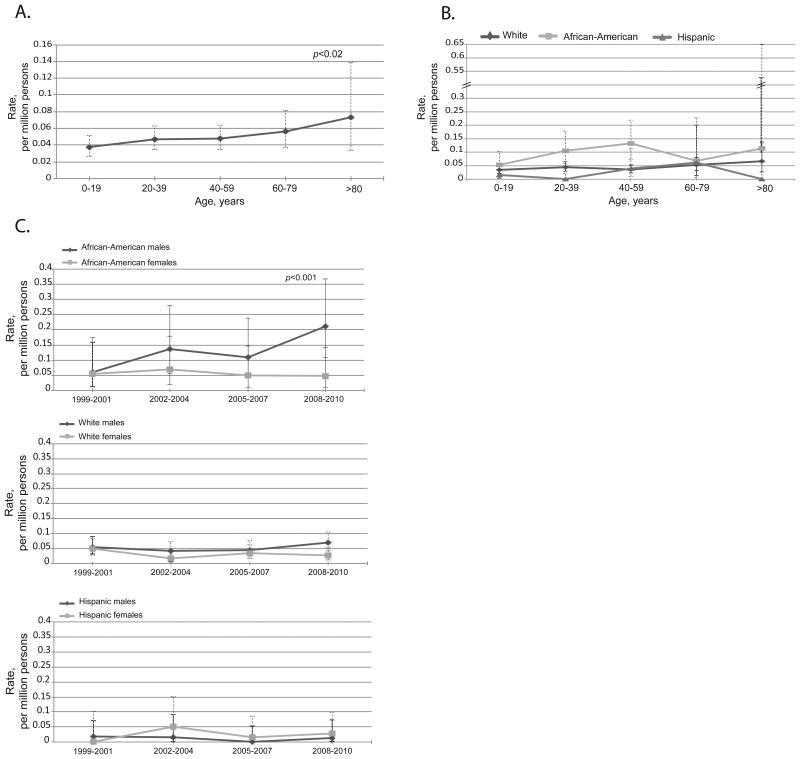
Age, African-American race, and male gender were significantly associated with food anaphylaxis: 0.04 (95%CI 0.03-0.05) per million among ≤19 years old vs. 0.06 (95%CI 0.04-0.08) among 60-79 years old, P-value 0.02); 0.09 (95%CI 0.07-0.12) in African-Americans vs. 0.04 (95%CI 0.03-0.05) per million in Whites, and 0.02 (95%CI 0.01-0.03) per million in Hispanics, P-value<0.001; 0.06 (95%CI 0.05-0.07) per million males vs. 0.03 (95%CI 0.02-0.04) per million females, P-value<0.01 (Figures 4A, 4B, and 4C and see Tables E3, E4, and E5 in this article's Online Repository).
Figure 4.
Fatal food anaphylaxis features. A. Rates by age groups, all races and genders combined. Fatal food anaphylaxis was significantly associated with age, P=0.02. B. Rates by race and age groups, both genders. Food anaphylaxis was significantly associated with African-American race, P<0.001. C. Rates over time by race and gender. There was significant increase overtime in fatal anaphylaxis to food among African-American males, P<0.001.
The error bars show 95%CI.
There was no significant overall increase in food anaphylaxis over time. Analysis of time trends by race and gender subgroups suggested a time effect in African-American males: the rates increased from 0.06 (95%CI 0.01-0.17) per million in 1999-2001 to 0.21 (95%CI 0.11-0.37) per million in 2008-2010, P<0.001 (Figure 4C and see Table E5 in this article's Online Repository).
There was no significant geographic effect in the food-related anaphylaxis (Figure 2C).
Unspecified anaphylaxis
There were 474 (19.3%) fatal anaphylaxis deaths that had no specified cause (Table 1). Twenty-seven deaths (5.7%) were in persons ≤19 years of age. Most deaths (57.0%) occurred in inpatient facilities, Table 1.
Unspecified fatal anaphylaxis was significantly associated with older age and African-American race: 0.03 (95%CI 0.02-0.04) per million in ≤19 years old vs. 0.43 (9%%CI 0.32-0.56) per million in ≥80 years old; 0.21 (95%CI 0.17-0.25) in African-Americans vs. 0.15 (95%CI 0.13-0.16) per million in Whites, and 0.06 (95%CI 0.04-0.08) per million in Hispanics, P<0.001 for all (Figures 5A and 5B and see Tables E3 and E4 in this article's Online Repository). The rates of unspecified fatal anaphylaxis decreased overtime from 0.30 (95%CI 0.26-0.34) per million in 1999-2001 to 0.09 (95%CI 0.07-0.11) per million in 2008-2010, P <0.001 (Figure 5C and see Table E5 in this article's Online Repository).
Figure 5.
Unspecified fatal anaphylaxis features. A. Rates by age groups, all races and genders combined. Unspecified fatal anaphylaxis was significantly associated with age, P<0.001. B. Rates by race and age groups, both genders. Unspecified anaphylaxis was significantly associated with African-American race, P<0.001. C. Rates over time by race and gender. There was significant decrease overtime in unspecified fatal anaphylaxis, among all races and both genders, P<0.001.
The error bars show 95%CI.
Between 1999 and 2004, there were significant geographical differences in unspecified fatal anaphylaxis. The West region had significantly lower rates of unspecified anaphylaxis, P=0.03 (Figure 2D). The associations between unspecified fatal anaphylaxis and age, African-American race, and time remained significant after adjusting for region, P <0.001, 0.01, and <0.001, respectively.
Algorithm-identified deaths and additional analyses
The algorithm identified 593 deaths. Combining anaphylaxis and algorithm-defined deaths results in the prevalence for fatal anaphylaxis of 0.86 people per million. The demographic characteristics for each type of algorithm-identified deaths are shown in Table 2. Three hundred six deaths were due to drugs, 48 to venom, 24 to food, and 215 to non-specified allergic reactions. Among drug-induced reactions, 148 (48.4%) deaths had an identified possible culprit medication, Figure 1E. Combining algorithm-identified cases into the four anaphylactic groups did not result in abrogation of the age, race, gender, and time effects noted for non-algorithmically-defined anaphylaxis (data not shown). More deaths occurred in the community, especially the non-drug-related algorithm-defined anaphylaxis, Table 2.
Table 2. Characteristics of additional algorithmically defined anaphylaxis cases, by specific cause.
| Characteristic | Fatal allergic reactions and anaphylaxis deaths identified by algorithmN=593 | |||
|---|---|---|---|---|
| Drug and serum N=306 (51.6%) | Venom N=48 (8.1%) | Food N=24 (4.0%) | Unspecified N=215 (36.3%) | |
| Males, n (%) | 139 (45.4) | 38 (79.2) | 13 (54.2) | 83 (38.6) |
| Age, median (IQR§) | ||||
| Females | 65 (49.0-79.0) | 51 (39-64) | 64 (25-75) | 64.5 (51-79) |
| Males | 61.0 (44-75) | 54.5 (45-65) | 41 (34-63) | 63 (50-76) |
| Race, n (%) | ||||
| Non-Hispanic White | 190 (62.1) | 38 (79.2) | 14 (58.3) | 171 (79.5) |
| African-American | 93 (30.4) | 7 (14.6) | 3 (12.5) | 31 (14.4) |
| Hispanic | 16 (5.2) | 2 (4.2) | 1 (4.2) | 10 (4.7) |
| Place of death | ||||
| Inpatient | 210 (68.6) | 11(22.9) | 8 (33.3) | 95 (44.2) |
| Outpatient (including ER*) | 59 (19.3) | 22 (45.8) | 10 (41.7) | 59 (27.4) |
| Community (including deadon arrival) | 37 (12.1) | 15 (31.3) | 6 (25.0) | 60 (27.9) |
| Associations with asthma, angioedema, and hypotension | ||||
| Asthma | 119 (38.9) | 8 (16.7) | 10 (41.7) | 142 (66.1) |
| Angioedema | 99 (32.4) | 10 (20.8) | 12 (50.0) | 45 (20.9) |
| Hypotension | 47 (15.4) | 19 (39.6) | 1 (4.2) | 25 (11.6) |
IQR – interquartile range
ER – emergency room
As drug and unspecified anaphylaxis had opposing time effects, we examined the hypothetical scenario that all unspecified cases were miscoded drug cases by merging the two groups for regression modeling for the specified effects. No time effect was noted on the combined anaphylaxis cases: 0.55 (95%CI 0.50-0.60) per million in 1999-2001 and 0.58 (95%CI 0.53-0.63) per million in 2008-2010 (see Figure E1 in this article's Online Repository).
Discussion
The analysis of the US Death Database from 1999 to 2010 suggested that fatal anaphylaxis during this time period was mostly due to the use of medications, followed by unspecified anaphylaxis, venom, and food. Based on these data, the overall prevalence of fatal anaphylaxis for recent years in the US was 0.69 people per million – similar to the prevalence found in a recent US population-based study.6 This prevalence is higher than that reported from UK (0.33 per million).22 While the rate reported from Australia is 0.64 per million,15 the Australian anaphylaxis data used a more expansive case by including angioedema (T78.3) and allergy, unspecified (T78.4). If algorithm-identified anaphylaxis deaths are included, the US mortality rate is higher than that in Australia – 0.86 per million.
Drugs were the most common cause of fatal anaphylaxis in the US, similar to the reports from UK, Australia, and New Zealand.3,15,29,30 Fatal drug anaphylaxis in the UK has been reported to be mostly due to general anesthetics,29 while in Australia,15 and in France,2 antibiotics predominate. Most (∼74%) of the drug anaphylaxis deaths in the current study had no identified culprit drug. Among those with the identified culprit drug (368), nearly a half (149) were antibiotics. This underscores the continued importance of identifying antibiotic allergy in the US population.
In fatal drug anaphylaxis, 15% in UK, 7% in Australia, and 20% in New Zealand (Auckland county) of cases with specified drug were due to radiocontrast agents.15,22,30 The high number of anaphylactic deaths in this study were due to radiocontrast and diagnostic agents in the US could be in part explained by the greater use of diagnostic imaging studies compared to other countries.31 In the US 228 CT and 91 MRI exams were performed per 100,000 persons in 2008.31
Chemotherapy was another frequently identified cause of fatal drug anaphylaxis. With increasing cancer prevalence predictions, especially in developed countries,32 awareness chemotherapy anaphylaxis is particularly relevant.
Drug anaphylaxis fatalities in the US significantly increased over the twelve years of the study period without a significant influence of gender. A similar increase in fatal drug anaphylaxis was noted in Australia.9,15 Over the course of the study, there was also a significant decrease in unspecified anaphylaxis. The lack of time effect in combined drug and unspecified anaphylaxis supports the possibility that at least some of the unspecified cases may have been drug-related. However, unexplained death has been linked to fatal anaphylaxis due to insect stings33,34 raising the possibility that some unspecified anaphylaxis cases were due to venom.
The rates of venom anaphylactic deaths were relatively constant over the 12-year period. Our results are consistent with the previously published data: earlier US reports suggested similar rates of ∼40 deaths per year due to venom anaphylaxis.35,36
Due to the lack of the specific code for venom anaphylaxis in ICD-10, the number of anaphylaxis cases due to venom is possibly underestimated.20 Other studies reported that fatal venom anaphylaxis comprises 18% to 23% of all fatal anaphylaxis cases.3,15,37 Not having a specific code for venom anaphylaxis can result in diagnostic inaccuracies and currently available codes reflecting venom toxic effect or venomous insects contact are not always related to allergic reactions.
Higher rates for venom anaphylaxis were observed among white males. This finding is consistent with the previously reported male predilection of fatal anaphylaxis to venom: 95% of fatal anaphylaxis to venom was observed in males in Australia and significantly more males than females died in Florida from hymenoptera anaphylaxis.15,37 Indolent systemic mastocytosis (ISM) with insect sting anaphylaxis is also significantly associated with the male gender, which itself is one clinical criterion for predicting bone marrow mast cell clonality and systemic mastocytosis in the absence of skin lesions.38,39 White race was associated with higher rates of venom anaphylaxis possibly reflecting differential occupational and/or outdoor exposure by race.
Fatal food anaphylaxis rates remained relatively unchanged over the 12-year time period. The proportion of anaphylactic deaths from food in the US is similar to that reported in Australia. In this study, 6.7% of all fatal anaphylaxis was food-related, while an Australian study reported food being responsible for 6% of all fatal allergic reactions.15 Fatal food-induced allergic reactions are commonly caused by asthma and angioedema and less commonly associated with shock22 and in this study the diagnosis of asthma was more common in food-related anaphylaxis. African-American race has been reported to be a risk factor for food allergies40,41 and in this study higher food-related fatal anaphylaxis rates were observed in African-Americans. Male gender predominated among fatal food-induced anaphylaxis. This contrasts with an Australian study, which observed that five out of seven food-induced fatalities were in females15 and with a UK study reported that 75% of UK nut-allergic fatalities were in females.22 Overall, the prevalence of food-related anaphylaxis was lower than for anaphylaxis due to other causes, consistent with findings from other fatal anaphylaxis studies.42
African-American race was a significant risk factor for all anaphylaxis causes with the exception of venom anaphylaxis. Previous US reports suggested racial/ethnic disparity in atopic diseases with African-American persons more likely to be sensitized to foods, to have self-reported allergy or clinic-based diagnosis of food allergy.12 Also, African-Americans have higher asthma mortality than white persons.43,44 Higher prevalence of fatal drug anaphylaxis in African-Americans may reflect more comorbidities, a greater medication use, and less access to care.
Older age was associated with increased fatal anaphylaxis rate regardless of the anaphylaxis cause. Even in food-induced anaphylaxis (which had the highest proportion of childhood fatalities), the rates nearly doubled from 0.04 per million in persons ≤19 years of age to 0.07 per million in individuals aged ≥80 years, emphasizing that coping with severe anaphylaxis is more challenging for elderly.45,46
Geography has been reported to play an important role in allergies and in anaphylaxis, specifically. The latitude and solar radiation were linked to more admissions for anaphylaxis and greater sales of epinephrine autoinjectors in the United States and in Australia.47-49 In this study, there was no overall predominance of a geographic area associated with fatal anaphylaxis. While drug anaphylaxis was less common in the Northeast, unspecified anaphylaxis was less common in the West. Fatal venom anaphylaxis was observed more in the South, which could relate to greater/longer exposure to venomous insects in that area. Interestingly, fatal food anaphylaxis had no significant association with a geographic region. Although latitude was reported to be an important risk factor for hospital admissions for food-induced anaphylaxis,48 it is possible that the lack of such association in this study is due to a relatively low number of food anaphylaxis cases.
As previously observed, the drug-induced anaphylaxis happened more frequently among inpatients50 and the deaths from food anaphylaxis were more frequent in the outpatient settings.51 Interestingly, the highest proportion of cases occurring in the community was due to venom. It is possible that venom-induced anaphylaxis occurs more frequently in areas remote from medical assistance making timely treatment difficult. In addition, people allergic to venom frequently neglect using epinephrine-autoinjectors.52,53 This underscores the use of venom immunotherapy as a highly effective treatment option for venom-allergic patients.51 Algorithm-identified fatal allergic reactions to food also had a high proportion of deaths occurring in the community. Educating all allergic patients at risk on self-management of anaphylaxis emergencies is an important task of practicing physicians.51
This study has several limitations. Overall, there is a possibility of under- and mis-reporting of anaphylaxis fatalities on death certificates. Thus the presented findings should be interpreted with caution. The large number of unspecified anaphylaxis codes used in the registry necessitated the use of adverse/toxic effect or contact codes to classify etiology. While drug anaphylaxis code was added to ICD-10, a venom anaphylaxis code was not. It is important to consider the introduction of such a code. Culprit foods in food-related anaphylaxis could not be assessed as they are coded with Z-codes in ICD-10 system, which are considered therapeutic codes and are not reported on death certificates. It would be important to consider creating ICD-10 codes specific to anaphylaxis due to certain foods like it exists for serum anaphylaxis, so that the actual foods could be identified and preventive strategies implemented. Finally, there was no information on the time course of the anaphylactic events and attempted treatment. Many of the above limitations could be overcome if US would have a National Registry on Fatal Anaphylaxis. While it exists for food allergies,4,5 other anaphylaxis causes should also be considered.
Nevertheless, the present study also has several strengths, including analysis of the National Mortality Database, which includes all anaphylactic deaths from the study period. To our knowledge, this is the largest study of anaphylaxis-related mortality that is based on the entire US population during the study period and examines demographic features of fatal anaphylaxis by cause. Understanding the patterns of fatal anaphylaxis may help in identifying specific risk factors and formulating preventative approaches.
Supplementary Material
Clinical implications.
Drugs are the most frequent culprits of fatal 54 anaphylaxis in the US. Anaphylactic deaths from medications and diagnostic agents increased overtime. Practitioners should be aware of therapeutic and prevention strategies.
Acknowledgments
The authors would like to thank David M. Homa, PhD, MPH, and Kenneth D. Kochanek, MA, from the Centers for Disease Control and Prevention for their statistical consultation; Betzaida Tejada-Vera, MS, from the Centers for Disease Control and Prevention for her support with Multiple Cause of Death Database; David Rosenstreich, M.D. and Gabriele de Vos, M.D., M.Sc. for their critical comments and review of the manuscript.
Declaration of funding sources: There was no financial or material support for this study
Abbreviations
- ICD-10
International Classification of Diseases, 10th revision
- 95%CI
95% confidence interval
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Warner JO. Anaphylaxis; the latest allergy epidemic. Pediatr Allergy Immunol. 2007;18:1–2. doi: 10.1111/j.1399-3038.2007.00532.x. [DOI] [PubMed] [Google Scholar]
- 2.Moneret-Vautrin DA, Morisset M, Flabbee J, Beaudouin E, Kanny G. Epidemiology of life-threatening and lethal anaphylaxis: a review. Allergy. 2005;60:443–51. doi: 10.1111/j.1398-9995.2005.00785.x. [DOI] [PubMed] [Google Scholar]
- 3.Pumphrey RS. Fatal anaphylaxis in the UK, 1992-2001. Novartis Found Symp. 2004;257:116–28. discussion 28-32, 57-60, 276-85. [PubMed] [Google Scholar]
- 4.Bock SA, Munoz-Furlong A, Sampson HA. Fatalities due to anaphylactic reactions to foods. J Allergy Clin Immunol. 2001;107:191–3. doi: 10.1067/mai.2001.112031. [DOI] [PubMed] [Google Scholar]
- 5.Bock SA, Munoz-Furlong A, Sampson HA. Further fatalities caused by anaphylactic reactions to food, 2001-2006. J Allergy Clin Immunol. 2007;119:1016–8. doi: 10.1016/j.jaci.2006.12.622. [DOI] [PubMed] [Google Scholar]
- 6.Ma L, Danoff TM, Borish L. Case fatality and population mortality associated with anaphylaxis in the United States. J Allergy Clin Immunol. 2013 doi: 10.1016/j.jaci.2013.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin RY, Anderson AS, Shah SN, Nurruzzaman F. Increasing anaphylaxis hospitalizations in the first 2 decades of life: New York State, 1990 -2006. Ann Allergy Asthma Immunol. 2008;101:387–93. doi: 10.1016/S1081-1206(10)60315-8. [DOI] [PubMed] [Google Scholar]
- 8.Brown AF, McKinnon D, Chu K. Emergency department anaphylaxis: A review of 142 patients in a single year. J Allergy Clin Immunol. 2001;108:861–6. doi: 10.1067/mai.2001.119028. [DOI] [PubMed] [Google Scholar]
- 9.Poulos LM, Waters AM, Correll PK, Loblay RH, Marks GB. Trends in hospitalizations for anaphylaxis, angioedema, and urticaria in Australia, 1993-1994 to 2004-2005. J Allergy Clin Immunol. 2007;120:878–84. doi: 10.1016/j.jaci.2007.07.040. [DOI] [PubMed] [Google Scholar]
- 10.Sheikh A, Alves B. Hospital admissions for acute anaphylaxis: time trend study. BMJ. 2000;320:1441. doi: 10.1136/bmj.320.7247.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilson R. Upward trend in acute anaphylaxis continued in 1998-9. BMJ. 2000;321:1021–2. [PMC free article] [PubMed] [Google Scholar]
- 12.Greenhawt M, Weiss C, Conte ML, Doucet M, Engler A, Camargo CA., Jr Racial and ethnic disparity in food allergy in the United States: a systematic review. J Allergy Clin Immunol Pract. 2013;1:378–86. doi: 10.1016/j.jaip.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 13.Meyer PA, Yoon PW, Kaufmann RB Centers for Disease C, Prevention. Introduction: CDC Health Disparities and Inequalities Report - United States, 2013. MMWR Surveill Summ. 2013;62(Suppl 3):3–5. [PubMed] [Google Scholar]
- 14.Moorman JE, Akinbami LJ, Bailey CM, et al. National surveillance of asthma: United States, 2001-2010. Vital Health Stat 3. 2012:1–67. [PubMed] [Google Scholar]
- 15.Liew WK, Williamson E, Tang ML. Anaphylaxis fatalities and admissions in Australia. J Allergy Clin Immunol. 2009;123:434–42. doi: 10.1016/j.jaci.2008.10.049. [DOI] [PubMed] [Google Scholar]
- 16.Multiple Cause of Death. National Bureau of Economic Research,1999-2010. [Accessed 04/03/2014]; at http://www.nber.org/data/multicause.html.
- 17.Anderson RN, Minino AM, Hoyert DL, Rosenberg HM. Comparability of cause of death between ICD-9 and ICD-10: preliminary estimates. Natl Vital Stat Rep. 2001;49:1–32. [PubMed] [Google Scholar]
- 18.About Mortality Medical Data System. [Accessed 10/01/2013];2010 at http://www.cdc.gov/nchs/nvss/mmds/about_mmds.htm.
- 19.Instructions for Classifying the Underlying Cause-of-Death, ICD-10. [Accessed 02/16/2014];2014 at http://www.cdc.gov/nchs/nvss/instruction_manuals.htm.
- 20.Harduar-Morano L, Simon MR, Watkins S, Blackmore C. Algorithm for the diagnosis of anaphylaxis and its validation using population-based data on emergency department visits for anaphylaxis in Florida. J Allergy Clin Immunol. 2010;126:98–104. e4. doi: 10.1016/j.jaci.2010.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sampson HA, Munoz-Furlong A, Campbell RL, et al. Second symposium on the definition and management of anaphylaxis: summary report--Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. J Allergy Clin Immunol. 2006;117:391–7. doi: 10.1016/j.jaci.2005.12.1303. [DOI] [PubMed] [Google Scholar]
- 22.Pumphrey R. Anaphylaxis: can we tell who is at risk of a fatal reaction? Curr Opin Allergy Clin Immunol. 2004;4:285–90. doi: 10.1097/01.all.0000136762.89313.0b. [DOI] [PubMed] [Google Scholar]
- 23.What is Race? [Accessed 02/16/2014];2012 at http://www.census.gov/population/race/
- 24.Population Estimates. [Accessed 03/01/2014];2012 at http://www.census.gov/popest/data/intercensal/
- 25.Kochanek KD, Xu J, Murphy SL, Minino AM, Kung HC. Deaths: final data for 2009. Natl Vital Stat Rep. 2011;60:1–116. [PubMed] [Google Scholar]
- 26.Fay MP, Feuer EJ. Confidence intervals for directly standardized rates: a method based on the gamma distribution. Stat Med. 1997;16:791–801. doi: 10.1002/(sici)1097-0258(19970415)16:7<791::aid-sim500>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 27.CDC Centers for Disease Control and Prevention, National Center for Health Statistics. Vital statistics of the United States: mortality. 1999 technical appendix. 1999 [Google Scholar]
- 28.Geographic Terms and Concepts - Census Divisions and Census Regions. [Accessed 02/16/2014];2013 at http://www.census.gov/geo/reference/gtc/gtc_census_divreg.html.
- 29.Pumphrey RS. Lessons for management of anaphylaxis from a study of fatal reactions. Clin Exp Allergy. 2000;30:1144–50. doi: 10.1046/j.1365-2222.2000.00864.x. [DOI] [PubMed] [Google Scholar]
- 30.Low I, Stables S. Anaphylactic deaths in Auckland, New Zealand: a review of coronial autopsies from 1985 to 2005. Pathology. 2006;38:328–32. doi: 10.1080/00313020600820831. [DOI] [PubMed] [Google Scholar]
- 31.Squires DA. The U.S. health system in perspective: a comparison of twelve industrialized nations. Issue Brief (Commonw Fund) 2011;16:1–14. [PubMed] [Google Scholar]
- 32.Bray F, Jemal A, Grey N, Ferlay J, Forman D. Global cancer transitions according to the Human Development Index (2008-2030): a population-based study. Lancet Oncol. 2012;13:790–801. doi: 10.1016/S1470-2045(12)70211-5. [DOI] [PubMed] [Google Scholar]
- 33.Schwartz HJ, Sutheimer C, Gauerke MB, Yunginger JW. Hymenoptera venom-specific IgE antibodies in post-mortem sera from victims of sudden, unexpected death. Clin Allergy. 1988;18:461–8. doi: 10.1111/j.1365-2222.1988.tb02896.x. [DOI] [PubMed] [Google Scholar]
- 34.Schwartz HJ, Yunginger JW, Schwartz LB. Is unrecognized anaphylaxis a cause of sudden unexpected death? Clin Exp Allergy. 1995;25:866–70. doi: 10.1111/j.1365-2222.1995.tb00029.x. [DOI] [PubMed] [Google Scholar]
- 35.Barnard JH. Studies of 400 Hymenoptera sting deaths in the United States. J Allergy Clin Immunol. 1973;52:259–64. doi: 10.1016/0091-6749(73)90044-4. [DOI] [PubMed] [Google Scholar]
- 36.Graft DF. Insect sting allergy. Med Clin North Am. 2006;90:211–32. doi: 10.1016/j.mcna.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 37.Simon MR, Mulla ZD. A population-based epidemiologic analysis of deaths from anaphylaxis in Florida. Allergy. 2008;63:1077–83. doi: 10.1111/j.1398-9995.2008.01737.x. [DOI] [PubMed] [Google Scholar]
- 38.Alvarez-Twose I, Gonzalez de Olano D, Sanchez-Munoz L, et al. Clinical, biological, and molecular characteristics of clonal mast cell disorders presenting with systemic mast cell activation symptoms. J Allergy Clin Immunol. 2010;125:1269–78. e2. doi: 10.1016/j.jaci.2010.02.019. [DOI] [PubMed] [Google Scholar]
- 39.Alvarez-Twose I, Zanotti R, Gonzalez-de-Olano D, et al. Nonaggressive systemic mastocytosis (SM) without skin lesions associated with insect-induced anaphylaxis shows unique features versus other indolent SM. J Allergy Clin Immunol. 2014;133:520–8. doi: 10.1016/j.jaci.2013.06.020. [DOI] [PubMed] [Google Scholar]
- 40.Liu AH, Jaramillo R, Sicherer SH, et al. National prevalence and risk factors for food allergy and relationship to asthma: results from the National Health and Nutrition Examination Survey 2005-2006. J Allergy Clin Immunol. 2010;126:798–806. e13. doi: 10.1016/j.jaci.2010.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sicherer SH, Munoz-Furlong A, Sampson HA. Prevalence of seafood allergy in the United States determined by a random telephone survey. J Allergy Clin Immunol. 2004;114:159–65. doi: 10.1016/j.jaci.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 42.Umasunthar T, Leonardi-Bee J, Hodes M, et al. Incidence of fatal food anaphylaxis in people with food allergy: a systematic review and meta-analysis. Clin Exp Allergy. 2013;43:1333–41. doi: 10.1111/cea.12211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Akinbami LJ, Moorman JE, Bailey C, et al. Trends in asthma prevalence, health care use, and mortality in the United States, 2001-2010. NCHS Data Brief. 2012:1–8. [PubMed] [Google Scholar]
- 44.Gold DR, Wright R. Population disparities in asthma. Annu Rev Public Health. 2005;26:89–113. doi: 10.1146/annurev.publhealth.26.021304.144528. [DOI] [PubMed] [Google Scholar]
- 45.Simons FE, Frew AJ, Ansotegui IJ, et al. Risk assessment in anaphylaxis: current and future approaches. J Allergy Clin Immunol. 2007;120:S2–24. doi: 10.1016/j.jaci.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 46.Simons FE, Ardusso LR, Bilo MB, et al. World allergy organization guidelines for the assessment and management of anaphylaxis. World Allergy Organ J. 2011;4:13–37. doi: 10.1097/WOX.0b013e318211496c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Camargo CA, Jr, Clark S, Kaplan MS, Lieberman P, Wood RA. Regional differences in EpiPen prescriptions in the United States: the potential role of vitamin D. J Allergy Clin Immunol. 2007;120:131–6. doi: 10.1016/j.jaci.2007.03.049. [DOI] [PubMed] [Google Scholar]
- 48.Hoyos Bachiloglu R, P SM, Cerda J, et al. Higher latitude and lower solar radiation influence on anaphylaxis in Chilean children. Pediatr Allergy Immunol. 2014 doi: 10.1111/pai.12211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mullins RJ, Clark S, Camargo CA., Jr Regional variation in epinephrine autoinjector prescriptions in Australia: more evidence for the vitamin D-anaphylaxis hypothesis. Ann Allergy Asthma Immunol. 2009;103:488–95. doi: 10.1016/S1081-1206(10)60265-7. [DOI] [PubMed] [Google Scholar]
- 50.Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients: a meta-analysis of prospective studies. JAMA. 1998;279:1200–5. doi: 10.1001/jama.279.15.1200. [DOI] [PubMed] [Google Scholar]
- 51.Lieberman P, Nicklas RA, Oppenheimer J, et al. The diagnosis and management of anaphylaxis practice parameter: 2010 update. J Allergy Clin Immunol. 2010;126:477–80. e1–42. doi: 10.1016/j.jaci.2010.06.022. [DOI] [PubMed] [Google Scholar]
- 52.Demain JG, Minaei AA, Tracy JM. Anaphylaxis and insect allergy. Curr Opin Allergy Clin Immunol. 2010;10:318–22. doi: 10.1097/ACI.0b013e32833a6c72. [DOI] [PubMed] [Google Scholar]
- 53.Simons FE, Clark S, Camargo CA., Jr Anaphylaxis in the community: learning from the survivors. J Allergy Clin Immunol. 2009;124:301–6. doi: 10.1016/j.jaci.2009.03.050. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.