Abstract
Background
Results of previous studies suggest that β-adrenoreceptor activation may augment pain, and that β-adrenoreceptor antagonists may be effective in reducing pain, particularly in individuals not homozygous for the catechol-O-methyltransferase (COMT) high activity haplotype.
Methods
Consenting patients admitted for thermal burn injury at participating burn centers were genotyped; those who were not high activity COMT homozygotes were randomized to propranolol 240mg/day or placebo. Primary outcomes were study feasibility (consent rate, protocol completion rate) and pain scores on study days 5-19. Secondary outcomes assessed pain and posttraumatic stress disorder (PTSD) symptoms 6 weeks post-injury.
Results
Seventy-seven (61/79) percent of eligible patients were consented and genotyped, and 77% (47/61) were genotype-eligible and randomized. Ninety-one percent (43/47) tolerated study drug and completed primary outcome assessments. In intention to treat and per protocol analyses, patients randomized to propranolol had worse pain scores on study days 5-19.
Conclusions
Genotype-specific pain medication interventions are feasible in hospitalized burn patients. Propranolol is unlikely to be a useful analgesic during the first few weeks after burn injury.
Keywords: propranolol, burn, randomized control trial, catechol-O-methyltransferase
Introduction
More than 700,000 individuals seek care for burn injury in the United States each year, and more than 50,000 require hospitalization.1 Even with currently available medications, moderate or severe pain after burn injury is common and remains a substantial clinical challenge.2,3 New medication treatments which target novel mechanisms are needed in order to reduce the suffering of patients with major burn injury.4
Results of both preclinical and clinical studies suggest that β-adrenoreceptor activation may contribute to hyperalgesia and allodynia. In animal models, catecholamines induce hyperalgesia and allodynia via β2- and β3- adrenoreceptor stimulation5,6, and this enhanced pain sensitivity can be prevented by the administration of the nonselective β-adrenergic antagonist propranolol.7,8 In human observational studies, genetic variants in the gene coding for the β2-adrenoreceptor (ADRB2) have been found to be associated with vulnerability to develop pain conditions.9,10,11 In a case-crossover study of propranolol treatment for patients with chronic musculoskeletal pain (n = 40), more patients reported a reduction in pain during propranolol treatment.12 Participants in that study were also genotyped at the gene coding for the enzyme catechol-O-methyltransferase (COMT), the primary enzyme that metabolizes catecholamines.13 When stratified by common COMT haplotypes, a beneficial effect of propranolol on pain was found among participants not carrying a high activity COMT haplotype.12 (Individuals without a high activity COMT haplotype have less COMT enzyme activity and relatively high synaptic catecholamine levels.14) A diminished benefit of propranolol on pain was observed in COMT high activity haplotype heterozygotes, and no benefit was noted among homozygotes.12
Based on these results, we hypothesized that propranolol administration would reduce pain severity in patients hospitalized with major thermal burn injury who were not homozygous for the high activity COMT haplotype. We performed a pilot randomized double-blind clinical trial to (1) assess the feasibility of performing a genotype-based multisite trial in patients with major thermal burn injury and (2) evaluate the potential efficacy of propranolol in reducing pain in burn patients without a high activity COMT haplotype.
Materials and Methods
This was a pilot genotype-based, multisite, double-blind randomized clinical trial. Local Institutional Review Board (IRB) approval was obtained from study site IRBs (University of North Carolina IRB, Chapel Hill, NC; MedStar Health Research Institute IRB, Washington, DC; Wake Forest University Health System IRB, Wake Forest, NC; Crozer-Keystone Health System IRB, Upland, PA). Written informed consent was obtained from all subjects. Participant safety data was monitored quarterly by the University of North Carolina Data Safety Monitoring Board (DSMB). Participant recruitment occurred between June 2009 and January 2011.
Eligibility Criteria
Individuals admitted to participating burn centers within 72 hours of sustaining a thermal burn injury involving ≤20% total body surface area (TBSA) were eligible for study participation. Patients with major burns >20% were deemed too critically ill for this study. Exclusion criteria also included an estimated hospital stay at the time of admission of <5 days or >40 days, intentional injury, substantial concomitant non-burn injury, and greater than first degree cardiac conduction blockade. Patients who were already taking a β-adrenergic antagonist medication or who were non-English speaking, clinically unstable, a prisoner, or who had a history of asthma, diabetes, coronary artery disease, psychotic disorder, or hepatic, renal, or congestive heart failure were also excluded. Also ineligible were patients whose highest pain score between admission and recruitment was < 4 (0-10 numeric rating scale (NRS)) or who were on opioid medications for chronic pain prior to their burn injury.
Study Procedures Prior to Randomization
All admitted burn patients were screened for potential eligibility via hospital electronic records; potentially eligible patients were approached by research staff for participation within 48 hours of admission. Blood samples for genotyping were collected from consenting patients via EDTA Vacutainer collection tubes (BD, Franklin Lakes, New Jersey, USA). DNA was subsequently purified from these samples using QIAamp DNA Mini Kit (Qiagen, Valencia, California, USA). Genotyping at rs4818 was performed on study day 1 or 2 using the TaqMan Assay on a Bio-Rad CFX96 Real-time PCR Detection System (Bio-Rad, Hercules, California, USA). SNP rs4818 was used to identify the high activity COMT haplotype, because a C genotype at rs4818 identifies the high activity COMT haplotype with approximately 95% accuracy.14
Randomization
A computer generated protocol created a numbered sequence of treatment assignments (allocation list) at each study site using permuted blocks (block sizes of 2 and 4) stratified by race (European American, African American, or other) and sex. On study day three, study participants who were not homozygous for the high activity COMT haplotype were randomized to propranolol hydrochloride or placebo using the allocation list at their study site.
Blinding Procedures
Other than the study biostatistician, all burn unit staff, research data collectors, and investigators were blinded to randomization schedule. Investigational drug pharmacy personnel at each study site maintained the unblinded study site allocation list and assigned participants to treatment arm. Capsules (sight, taste, smell) and medication bottles were identical across treatment arm. Bottles were marked by unique study ID numbers only. These methods were used to help ensure that if a single patient was unblinded (for whatever reason), the entire study would not be unblinded.
Dosing, Escalation, and Taper of Study Medication
Propranolol hydrochloride is used routinely in adults at doses of 240-320mg/day for indications such as migraine prophylaxis15,16 and essential tremor17-19 and has been used safely in burn patients to decrease the metabolic consequences of burn injury.20-22 Based on this safety profile and lack of established dose regimen for burn pain, a dose of 120mg BID of propranolol (240mg/day) was selected for patients receiving active study drug. Propranolol hydrochloride extended release capsules (Par Pharmaceuticals) were used, because this formulation has been rated by the FDA as bioequivalent to Inderal LA.23
Following randomization, study participants received an initial test dose of 40mg of short-acting propranolol or placebo on study day three. Heart rate and blood pressure were assessed 30 and 60 minutes after test dose administration, and participants were observed by study personnel for at least one hour for potential adverse events. After test dose administration, participants received study drug (propranolol 60mg ER or placebo capsules) according to the following schedule: one capsule BID × two doses, then two capsules BID until three weeks following hospital discharge, and then a 20 day taper. This 20 day study drug taper consisted of two capsules in the AM and one in the PM for 5 days, one capsule in the AM and one in the PM for 5 days, one capsule in the AM for 10 days, and then discontinuation.
Evaluation of Participant Adherence
During hospitalization study drug was administered by burn center nurses along with other medications. After discharge, adherence to the study medication was assessed via child-resistant Medication Event Monitoring System (MEMS) medication bottle caps.24 These caps contain an electronic microcircuit chip which records each date and time that a pill bottle is opened. After completion of the study taper, participants were asked to return their pill bottle with MEMS cap via a postage-paid container supplied by the study team. MEMS cap data were then downloaded to a desktop computer using MEMS software (Powerview, Aardex Ltd, Zug, Switzerland), and the percent of standard doses taken as prescribed (BID) and the percentage of days at least one dose was taken were calculated. Consistent with previous studies, for sensitivity analyses “adherent” patients were defined as those with ≥ 80% of prescribed doses removed from the MEMS device according to protocol.25
Participant Assessments
Participant demographic information (e.g. education, income), baseline pain measures, and distress symptoms (Peritraumatic Distress Inventory26) were assessed during initial interview evaluation. During hospitalization study participants received daily assessments evaluating adverse events and pain severity (waking, worst, least, and average pain severity during the past 24 hours). Each pain severity assessment was performed using a 0-10 NRS. NRS scales are a valid method of assessing pain27 and have advantages in burn patients who often are unable to record visual analogue scale responses due to upper extremity injuries.
Following discharge, study participants continued to receive the same adverse event and pain severity assessments via telephone on study days 5, 7, 10, 13, 17, and 19. Between study day 19 and the beginning of the patient's taper, subjects received adverse event assessments twice a week if in the hospital and once a week if outpatient. All patients also completed a structured six week outcome evaluation via telephone or in-person interview. This evaluation included an assessment of waking, worst, and least pain intensity (0-10 NRS) and an assessment of posttraumatic stress disorder (PTSD) symptoms (Posttraumatic Symptom Scale - Interview Version28 (PSS-I)) during the past week. Participants were paid $50 for completing this six week follow-up assessment.
Primary Outcomes
Primary study outcomes were study feasibility and acute pain differences between treatment arms. Consent and protocol completion rates were selected prior to study start as main study feasibility outcomes (consent rate, protocol completion rate). Average pain scores during the past 24 hours on study days 5, 7, 10, 13, 17, and 19 were selected prior to study start as primary acute pain outcomes. However, soon after starting the study it was observed that patients sometimes provided an “average” pain rating greater than their worst reported pain or less than their least reported pain. These observations, together with the high degree of educational disadvantage in this population, led us to appreciate that our interview question regarding “average pain” was poorly designed for the study population and did not reliably yield valid data. We therefore used linear mixed modeling to combine the pain measurements assessed from all participants on primary outcome days (waking, worst and least pain) into an “overall pain” score for each of these days. This alternative primary acute pain outcome was defined after the study start and prior to study analyses (secondary analyses also evaluated our original primary outcome measure, average pain severity). A reduction of two or more points (on a 0 - 10 NRS) was considered the threshold for a clinically relevant improvement in pain.29
Secondary Outcomes
Secondary analyses assessed for the potential evidence of propranolol efficacy in several other domains. Because β-adrenoreceptor blockade may influence risk of persistent pain development, pain severity six weeks after study enrollment between study groups was assessed using ANOVA tests. Also, because some studies30-33 (but not others34,35) suggest that propranolol may reduce PTSD symptoms, PTSD symptom severity and diagnosis (based on PSS-I criteria36) six weeks after enrollment were also assessed. Finally, as described above, secondary analyses examined primary study outcomes using our average pain measure.
Sample Size Estimation
At the time of study design, there was little information with which to generate sample size estimates. The most applicable data came from a report of pain scores after median thoracotomy among patients randomized to a beta-antagonist vs. placebo.37 Based on these data, and our use of repeated measures within each subject, we felt that randomization group sizes of n = 20 (total n = 40) would be sufficient to assess study feasibility and to generate useful estimates of potential propranolol efficacy.
Statistical Analyses
To evaluate study feasibility, descriptive statistics were used to evaluate consent and protocol completion rates. Outcomes of patients randomized to propranolol vs. placebo through study day 19 were compared using repeated measures (or correlated outcomes) analysis. The following model was used: Yij = β0 + β1×treatment + β2×day + (β1β2)×treatment×day + eij, where Yij is the overall pain score for subject i on day j; β0 is the intercept; β1 is the main effect for treatment (propranolol vs. placebo), β2 is the time effect (change in pain scores over time), (β1β2) is the treatment x time interaction coefficient, and eij is the random error associated with measurement of the ith subject on the jth day. Missing patient data were not included in the model. The correlation among measures within each subject was taken into account by the covariance structure of the random errors, using generalized estimating equations (GEE)38. After fitting the model, the significance of the interaction term (β1β2) was assessed. If significant, it would mean that the effect of treatment on pain trajectory slope varies by time. Six week pain and PTSD symptoms according to treatment group were compared using the following ANOVA model: Yi = β0 + β1×treatment + ei, where Yi is the pain score assessed in the ith subject at week 6; β0 is the intercept; β1 is the main effect for treatment (propranolol vs. placebo), and ei is the random error associated with the measurement on the ith subject.
Ninety five percent confidence intervals around observed treatment group differences in intention to treat, per protocol, and adherent patient analyses were obtained using the above model. For the purposes of graphic data representation, the model was modified to treat the time variable as categorical. Least square means of overall pain scores (combining waking, worst and least pain) were obtained for each day by treatment group, together with standard errors of the mean. All analyses were conducted using SAS (version 9.2, SAS Institute Inc., Cary, NC). P-values < .05 were considered statistically significant.
Results
Enrollment and feasibility assessments
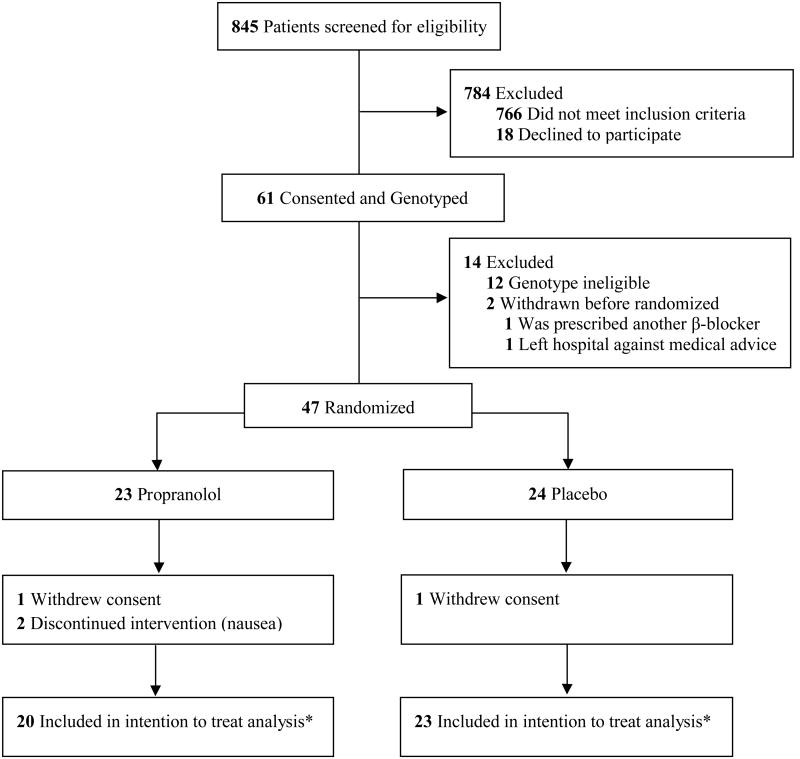
Most patients (766/845, 91%) did not meet the relatively strict eligibility criteria (designed to limit potential adverse events) in this proof of concept trial (Figure 1). The most common reasons for exclusion were non-thermal burn injury, age > 60 years, and hospital admission > 72 hours after burn. Of those meeting initial eligibility criteria, 61 (77%) consented. Among those providing initial consent, 12 (20%) were genotype ineligible and 2 (3%) were withdrawn before randomization. Twenty-three participants were randomized to the propranolol arm and 22 participants received the intervention (one refused further participation after randomization but before receiving the study medication). Twenty-four participants were randomized to placebo and 23 received the intervention (one refused further participation after randomization but before receiving the study medication). Two participants (9%) in the propranolol arm dropped out after randomization (during dose escalation) due to adverse events (nausea). The final numbers of patients who completed the primary outcome assessments for intention to treat analysis was 20 patients receiving propranolol and 23 patients receiving placebo. However, during the study one patient assigned to the placebo group erroneously received propranolol. Thus, for the per protocol analysis, the number of patients in the propranolol and placebo groups were 21 and 22, respectively.
Figure 1. Study Enrollment and Randomization.
* One patient randomized to placebo arm received propranolol. Hence for the per protocol analysis, the number of patients in the propranolol and placebo groups are 21 and 22, respectively.
Characteristics of the 43 participants are shown in Table 1. Most patients were young European American males, had some education past high-school, and had suffered a partial thickness burn that was ≤ 10% TBSA (average TBSA ∼6%). Median annual family income reported by study participants was $20,000-39,000. Baseline clinical characteristics of patients in the two treatment groups were similar. On Day 1, mean pain scores in both arms were close to 6. Dressing changes were performed daily on all in-hospital patients except during post-operative days 1-3, during which time patients' dressings were irrigated regularly.
Evaluation of Participant Adherence
Median length of hospital stay was 11 days (range 3 - 41 days). All study participants were adherent during hospitalization, when their study medication was administered by a hospital nurse. Adherence following discharge (determined via analysis of Medication Event Monitoring System (MEMS) data) is shown in Table 2. Participants in the placebo arm tended to have higher rates of adherence in terms of both the percentage of days the correct dose was taken (p=.189) and the percentage of days at least one dose was taken (p=.137).
Primary Outcomes
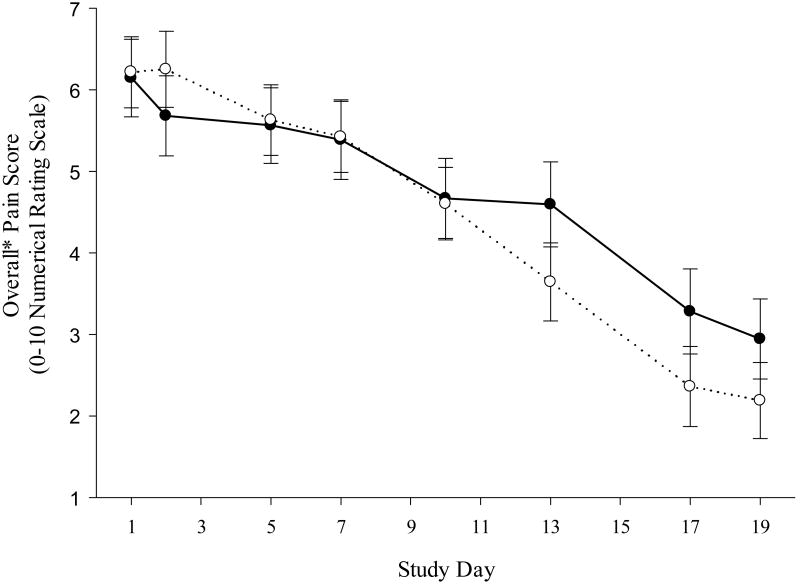
The effects of propranolol on overall pain score by treatment arm are shown in Figure 2. Patients randomized to propranolol had slightly worse overall pain outcomes across the primary assessment period. The 95% confidence intervals around observed treatment group differences in overall pain in intention to treat, per protocol, and adherent patient analyses did not include a clinically relevant treatment benefit for propranolol (Table 3). Outcomes for average pain over time were very similar to results for overall pain (Table 3). In per protocol and intention to treat analyses, there was a statistically significant (but clinically non-meaningful) worsening of average pain scores in the propranolol vs. the placebo groups.
Figure 2. Effect of Propranolol on Overall* Pain Score.
*Linear mixed modeling was used to combine pain measurements (waking, worst and least pain assessed each day for primary outcome days) for each individual into an overall pain score.
Secondary Outcomes
There was no significant difference in opioid use during hospitalization between randomization arms (average morphine equivalent dose per day 68.1±14.1 in patients randomized to propranolol vs. 77.4±12.6 in patients randomized to placebo, p=.63). Follow-up data to assess secondary outcomes assessing the effects of propranolol at six weeks were available for 38/43 (88%) of randomized patients (16/20 (80%) patients randomized to propranolol and 22/23 (96%) patients randomized to placebo). Comparison of six week overall and average pain scores according to treatment group are shown in Table 4. Point estimates for overall pain were lower for propranolol patients in per protocol, intention to treat, and adherent analyses. Confidence intervals around point estimates for overall pain did not include a clinically relevant treatment effect. Comparison for average pain differences at 6 weeks are also shown in Table 4. Confidence intervals for average pain included a clinically relevant treatment effect for adherent patients, but not for per protocol or intention to treat analyses. There was a non-significant reduction in PTSD symptoms (PSS-I score 8.1±11.4 vs. 10.7±13.1, p = .51) among patients randomized to propranolol vs. control, as well as a non-significant reduction in the percentage of patients who met criteria for PTSD diagnosis (3/17 (19%) vs. 6/22 (27%), p = .71).
Adverse Events
Table 5 presents adverse events according to treatment arm. No significant differences were observed in the number of events between treatment groups. The most common side effects in the control arm were gastrointestinal and dermatologic (6 cases each). The most common side effect in the treatment arm was metabolic/laboratory (11 cases).
Discussion
Our results support the feasibility of performing clinical trials of pain medications in a genotype-selected subgroup of patients with major thermal burn injury. Seventy seven percent of eligible patients consented to the protocol, and 91% of randomized patients tolerated study drug and completed primary outcome assessments. Overall medication adherence after discharge from the burn unit (median length of stay 11 days) was relatively poor, even compared to other electronic monitoring adherence studies, which have found that patients are only adherent to BID medications on 70-80% of study days.39-41 Patients randomized to propranolol tended to be less adherent than placebo patients (adherent with one or more doses on 70% vs. 86% of study days, BID adherence on 48% vs. 62% of study days). This trend towards decreased adherence in the propranolol group may be due to increased side effects and/or a lack of pain relief efficacy.42-45 The overall reduced adherence in this study vs. other studies may also be due to the relatively low socioeconomic status of the patient population.46,47
Individuals randomized to propranolol tended to have worse pain scores during the 19 day study period. The 95% confidence intervals around observed treatment group differences in overall pain (and average pain) in per protocol, intention to treat, and adherent patient analyses did not include a clinically relevant treatment effect for propranolol on acute pain. In secondary analyses, patients randomized to propranolol had slightly lower overall and average pain scores than patients randomized to placebo at six weeks post-burn. Only the 95% confidence interval for average pain scores among adherent patients included a clinically relevant reduction in pain. Patients randomized to propranolol also showed non-significant reductions in PTSD symptom scores and in the proportion of patients meeting criteria for the diagnosis of PTSD.
A challenge to the development of new medications is that large scale clinical trials are extremely expensive and require preliminary data, but at the same time the results of small preliminary trials may be inaccurate. For this reason, current guidelines recommend that small trials focus on confidence intervals rather than point estimates of effect size.48 For acute pain during the first 19 days after randomization, the primary outcome of this trial, confidence intervals of trial treatment effects did not include a clinically relevant pain reduction for propranolol in the selected study population. Similarly, in secondary analyses examining the influence of the propranolol intervention on pain symptoms six weeks after randomization, confidence intervals of trial treatment effects also did not include a clinically relevant reduction in pain, except in a single secondary analysis of average pain scores among adherent patients. Together, these findings suggest that propranolol is unlikely to be a useful intervention in this patient population.
Tolstoy's famous line, “Happy families are all alike; every unhappy family is unhappy in its own way,”49 is applicable to the results of clinical trials. There are a great many different reasons that a clinical trial may be negative, including wrong patient population, wrong dose, wrong timing of intervention or outcome assessment, wrong (imprecise) assessment tool, wrong (unpowerful) study design, or wrong (ineffective) drug. While the results of a clinical trial provide little information to distinguish between these possibilities, several comments may be useful when considering our results and the available literature.
First, while the etiology of burn pain is unknown, the presence of thermal injury to peripheral structures and the quality of patient symptoms suggest a neuropathic component.50 A previous case cross-over trial found no evidence of effectiveness with propranolol in patients with neuropathic pain51, whereas a trial in patients with a myogenous pain disorder suggested a benefit with propranolol in our study population.52 Thus it is conceivable that β-adrenoreceptor antagonists in general (or propranolol in particular) are more effective in nociceptive than neuropathic pain.
Second, it is possible that our genotype-based eligibility criteria selected a patient population less likely to respond to propranolol. In a recent publication using pre-randomization observational data from the present trial, we found that patients who are not homozygous for the high activity COMT haplotype (patients selected for inclusion in this trial) did indeed have higher overall pain scores (6.3 (0.4) vs. 5.4 (0.4), p = .037) during the first two days of hospital admission (prior to randomization).53 COMT haplotype was a stronger predictor of overall pain severity among study patients than other factors including burn size or depth.53 These data suggest that the effect of COMT haplotype on burn pain may occur via mechanisms other than via the activation of β-adrenoreceptors, or that this mechanism was not effectively modulated with propranolol among the population of burn patients selected for inclusion in our study. It is also possible that our genotype-based eligibility criteria were too liberal, in that we allowed individuals with one copy of the high activity COMT haplotype into the study. Such patients were allowed into the study based on evidence from another pain population that patients with one copy of the high activity COMT haplotype respond to propranolol.12 We performed secondary analyses evaluating treatment response according to high activity COMT haplotype copy number, and found no evidence for an effect (non-significant differences (p >.25) and effect size well below a clinically significant change in pain (< 1 unit change in 0-10 NRS pain score)). These findings suggest that including only patients with no copies of the high activity COMT haplotype would not have changed our study results.
Third, it is possible that other, non-genotype-related criteria might identify burn patients in the study population who would experience pain relief with propranolol treatment. One possibility that we explored in secondary analyses was whether initial cardiovascular response to study drug (heart rate or blood pressure response) would predict treatment response. Unfortunately, while a reduction in heart rate and blood pressure 60 minutes after test dose administration predicted drug response, this was equally true among patients receiving propranolol and patients receiving placebo. Thus this reduction is consistent with a placebo response rather than a response specifically linked to β-adrenoreceptor antagonism.
Finally, it is possible that propranolol and/or other beta-antagonists would have a therapeutic effect only after more prolonged treatment or more long term follow-up assessments. Even though patients completed the propranolol intervention prior to the six week follow-up assessment, point estimates suggest a more favorable (though marginal) effect of propranolol on persistent post-burn pain in comparison with acute burn pain. We do not have data past 6 weeks to determine if any more substantive analgesic effect of propranolol emerged over time. However, we believe that confidence intervals observed for propranolol at 6 weeks, together with the poor observed adherence (and suggestion of differentially poor adherence), indicate that propranolol is unlikely to be a useful analgesic in the selected burn population during the first months after injury.
In addition to the limitations described above, several other limitations should also be considered when interpreting our study results. First, our eligibility criteria limited study participation to patients with TBSA burns ≤ 20%, and most patients in our sample had burns that were less than 10% TBSA. Study inclusion criteria limited enrollment to patients with TBSA burns ≤ 20%, because patients with larger burn injuries are sometimes intubated or more heavily sedated, making burn pain assessment difficult, and because patients with TBSA burns ≤ 20% constitute the great majority (>86%) of admissions to major burn centers.54 It is possible that patients with larger burn injuries, which are associated with greater catabolism and hypermetabolism, may have a different analgesic response to propranolol intervention. In addition, to reduce the potential risk of study participation, patients with greater than first-degree atrioventricular block, patients taking a β-adrenergic antagonist medication, and patients with asthma, diabetes, coronary artery disease, and congestive heart failure were excluded from study participation. Therefore the generalizability of study findings to these burn patient groups cannot be assessed. Finally, as described in the methods section, we erred when we assumed that patients would be consistently familiar with the term “average”. Based on responses of initial participants to this question, we changed our primary outcome measure to “overall pain”, a combination of waking, least, and worst pain. However, as shown above, results for both overall and average pain were generally consistent and did not suggest a clinically relevant reduction in pain with propranolol intervention. To prevent this error in future burn studies assessing symptom outcomes, the term “average” should be avoided in favor of another term (e.g. pain “most of the time”).
In conclusion, our study results indicate that genotype-based randomized controlled trials of patients with major thermal burn injury are feasible, and that propranolol is unlikely to be a useful adjunct to reducing pain during the first months after injury among the population of burn patients selected for this study. Further studies are needed to better understand mechanisms of post-burn pain and to continue to test interventions to reduce the suffering of patients with major thermal burn injury.
Supplementary Material
Acknowledgments
Funding: This project was supported by Award Number UL1RR025747 from the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, the NC Jaycee Burn Center Fund, the Firefighters Research Fund, the DC Firefighters Burn Foundation, and UNC Institutional Resources.
Information for LWW regarding depositing manuscript into PubMed Central: This research was funded by National Institutes of Health grant number UL1RR025747.
This report describes human research. IRB contact information: Office of Human Research Ethics, The University of North Carolina at Chapel Hill, Ph: 919-966-3113, Fax: 919-966-7879This study was conducted with written informed consent from the study subjects. This report describes a prospective randomized clinical trial. The author states that the report includes every item in the CONSORT checklist for a prospective randomized clinical trial. This was not an observational clinical study.
Contributor Information
Danielle C Orrey, Email: danielle_orrey@med.unc.edu, The University of North Carolina at Chapel Hill.
Omar I Halawa, Email: ohalawa@gmail.com, The University of North Carolina at Chapel Hill.
Andrey V Bortsov, Email: abortsov@aims.unc.edu, The University of North Carolina at Chapel Hill.
Jeffrey W Shupp, Email: Jeffrey.W.Shupp@medstar.net, Washington Hospital Center.
Samuel W Jones, Email: samuel_jones@med.unc.edu, The University of North Carolina at Chapel Hill.
Linwood R Haith, Email: linwood.haith@verizon.net, Crozer Chester Medical Center.
Janelle M Hoskins, Email: jmhoskin@email.unc.edu, The University of North Carolina at Chapel Hill.
Marion H Jordan, Email: Marion.H.Jordan@medstar.net, Washington Hospital Center.
Shrikant I Bangdiwala, Email: kant@unc.edu, The University of North Carolina at Chapel Hill.
Brandon Roane, Email: brandon_roane@med.unc.edu, UNC Chapel Hill.
Timothy F Platts-Mills, Email: tim_platts-mills@med.unc.edu, UNC Chapel Hill.
James H Holmes, Email: jholmes@wakehealth.edu, Wake Forest Baptist Medical Center.
James Hwang, Email: james_hwang@med.unc.edu, UNC Chapel Hill.
Bruce A Cairns, Email: bruce_cairns@med.unc.edu, UNC Chapel Hill.
Samuel A McLean, Email: smclean@aims.unc.edu, UNC Chapel Hill.
References
- 1.LaBorde P. Burn epidemiology: the patient, the nation, the statistics, and the data resources. Crit Care Nurs Clin North Am. 2004;16:13–25. doi: 10.1016/j.ccell.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 2.Gallagher G, Rae CP, Kinsella J. Treatment of pain in severe burns. Am J Clin Dermatol. 2000;1:329–335. doi: 10.2165/00128071-200001060-00001. [DOI] [PubMed] [Google Scholar]
- 3.Perry S, Heidrich G, Ramos E. Assessment of pain by burn patients. Journal of Burn Care & Research. 1981;2:322–326. [Google Scholar]
- 4.Summer GJ, Puntillo KA, Miaskowski C, et al. Burn injury pain: the continuing challenge. J Pain. 2007;8:533–548. doi: 10.1016/j.jpain.2007.02.426. [DOI] [PubMed] [Google Scholar]
- 5.Khasar SG, Green PG, Miao FJ, et al. Vagal modulation of nociception is mediated by adrenomedullary epinephrine in the rat. Eur J Neurosci. 2003;17:909–915. doi: 10.1046/j.1460-9568.2003.02503.x. [DOI] [PubMed] [Google Scholar]
- 6.Khasar SG, McCarter G, Levine JD. Epinephrine produces a beta-adrenergic receptor-mediated mechanical hyperalgesia and in vitro sensitization of rat nociceptors. J Neurophysiol. 1999;81:1104–1112. doi: 10.1152/jn.1999.81.3.1104. [DOI] [PubMed] [Google Scholar]
- 7.Nackley AG, Tan KS, Fecho K, et al. Catechol-O-methyltransferase inhibition increases pain sensitivity through activation of both β2- and β3-adrenergic receptors. Pain. 2007;128:199–208. doi: 10.1016/j.pain.2006.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaplan R, Robinson CA, Scavulli JF, et al. Propranolol and the treatment of rheumatoid arthritis. Arthritis Rheum. 1980;23:253–255. doi: 10.1002/art.1780230220. [DOI] [PubMed] [Google Scholar]
- 9.Hocking LJ, Smith BH, Jones GT, et al. Genetic variation in the beta2-adrenergic receptor but not catecholamine-O-methyltransferase predisposes to chronic pain: results from the 1958 British Birth Cohort Study. Pain. 2010;149:143–151. doi: 10.1016/j.pain.2010.01.023. [DOI] [PubMed] [Google Scholar]
- 10.Diatchenko L, Anderson AD, Slade GD, et al. Three major haplotypes of the β2 adrenergic receptor define psychological profile, blood pressure, and the risk for development of a common musculoskeletal pain disorder. Am J Med Genet. 2006;141B:449–462. doi: 10.1002/ajmg.b.30324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vargas-Alarcon G, Fragoso JM, Cruz-Robles D, et al. Association of adrenergic receptor gene polymorphisms with different fibromyalgia syndrome domains. Arthritis Rheum. 2009;60:2169–2173. doi: 10.1002/art.24655. [DOI] [PubMed] [Google Scholar]
- 12.Tchivileva IE, Lim PF, Smith SB, et al. Effect of catechol-O-methyltransferase polymorphism on response to propranolol therapy in chronic musculoskeletal pain: a randomized, double-blind, placebo-controlled, crossover pilot study. Pharmacogenet Genom. 2010;20:239–248. doi: 10.1097/FPC.0b013e328337f9ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mannisto PT, Kaakkola S. Catechol-O-methyltransferase (COMT): biochemistry, molecular biology, pharmacology, and clinical efficacy of the new selective COMT inhibitors. Pharmacol Rev. 1999;51:593–628. [PubMed] [Google Scholar]
- 14.Diatchenko L, Slade GD, Nackley AG, et al. Genetic basis for individual variations in pain perception and the development of a chronic pain condition. Hum Mol Genet. 2005;14:135–143. doi: 10.1093/hmg/ddi013. [DOI] [PubMed] [Google Scholar]
- 15.Rosen JA. Observations on the efficacy of propranolol for the prophylaxis of migraine. Ann Neurol. 1983;13:92–93. doi: 10.1002/ana.410130119. [DOI] [PubMed] [Google Scholar]
- 16.Diamond S, Kudrow L, Stevens J, et al. Long-term study of propranolol in the treatment of migraine. Headache. 1982;22:268–271. doi: 10.1111/j.1526-4610.1982.hed2206268.x. [DOI] [PubMed] [Google Scholar]
- 17.Murray TJ. Long-term therapy of essential tremor with propranolol. Can Med Assoc J. 1976;115(9):892–894. [PMC free article] [PubMed] [Google Scholar]
- 18.Sweet RD, Blumberg J, Lee JE, et al. Propranolol treatment of essential tremor. Neurology. 1974;24:64–67. doi: 10.1212/wnl.24.1.64. [DOI] [PubMed] [Google Scholar]
- 19.Winkler GF, Young RR. Efficacy of chronic propranolol therapy in action tremors of the familial, senile or essential varieties. N Engl J Med. 1974;290:984–988. doi: 10.1056/NEJM197405022901802. [DOI] [PubMed] [Google Scholar]
- 20.Pereira CT, Jeschke MG, Herndon DN. Beta-blockade in burns. Novartis Found Symp. 2007;280:238–248. doi: 10.1002/9780470059593.ch16. discussion 248-251. [DOI] [PubMed] [Google Scholar]
- 21.Pereira CT, Herndon DN. The pharmacologic modulation of the hypermetabolic response to burns. Adv Surg. 2005;39:245–261. doi: 10.1016/j.yasu.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 22.Herndon DN, Tompkins RG. Support of the metabolic response to burn injury. Lancet. 2004;363:1895–1902. doi: 10.1016/S0140-6736(04)16360-5. [DOI] [PubMed] [Google Scholar]
- 23.Eldon MA, Kinkel AW, Daniel JE, et al. Bioavailability of propranolol hydrochloride tablet formulations: Application of multiple dose crossover studies. Biopharm Drug Dispos. 1989;10:69–76. doi: 10.1002/bdd.2510100108. [DOI] [PubMed] [Google Scholar]
- 24.Aardex Group. [Accessed 3/5/2012];2012 http://www.aardexgroup.com/aardex_index.php?group=aardex.
- 25.Nuesch R, Schroeder K, Dieterle T, et al. Relation between insufficient response to antihypertensive treatment and poor compliance with treatment: a prospective case-control study. BMJ. 2001;323:142–146. doi: 10.1136/bmj.323.7305.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brunet A, Weiss D, Metzler T, et al. The peritraumatic distress inventory: a proposed measure of PTSD criterion A2. Am J Psychiatry. 2001;158:1480–1485. doi: 10.1176/appi.ajp.158.9.1480. [DOI] [PubMed] [Google Scholar]
- 27.Bijur PE, Latimer CT, Gallagher EJ. Validation of a verbally administered numerical rating scale of acute pain for use in the emergency department. Acad Emerg Med. 2003;10:390–392. doi: 10.1111/j.1553-2712.2003.tb01355.x. [DOI] [PubMed] [Google Scholar]
- 28.Foa EB, Tolin DF. Comparison of the PTSD symptom scale-interview version and the clinician-administered PTSD scale. J Trauma Stress. 2000;13:181–191. doi: 10.1023/A:1007781909213. [DOI] [PubMed] [Google Scholar]
- 29.Farrar JT, Young JP, Jr, LaMoreaux L, et al. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94:149–158. doi: 10.1016/S0304-3959(01)00349-9. [DOI] [PubMed] [Google Scholar]
- 30.Pitman RK, Sanders KM, Zusman RM, et al. Pilot study of secondary prevention of posttraumatic stress disorder with propranolol. Biol Psychiatry. 2002;51:189–192. doi: 10.1016/s0006-3223(01)01279-3. [DOI] [PubMed] [Google Scholar]
- 31.Vaiva G, Ducrocq F, Jezequel K, et al. Immediate treatment with propranolol decreases posttraumatic stress disorder two months after trauma. Biol Psychiatry. 2003;54:947–949. doi: 10.1016/s0006-3223(03)00412-8. [DOI] [PubMed] [Google Scholar]
- 32.Taylor F, Cahill L. Propranolol for reemergent posttraumatic stress disorder following an event of retraumatization: A Case Study. J Trauma Stress. 2002;15:433–437. doi: 10.1023/A:1020145610914. [DOI] [PubMed] [Google Scholar]
- 33.Famularo R, Kinscherff R, Fenton T. Propranolol treatment for childhood posttraumatic stress disorder, Acute TypeA Pilot Study. Arch Pediatr Adolesc Med. 1988;142:1244–1247. doi: 10.1001/archpedi.1988.02150110122036. [DOI] [PubMed] [Google Scholar]
- 34.McGhee LL, Maani CV, Garza TH, et al. The effect of propranolol on posttraumatic stress disorder in burned service members. J Burn Care Res. 2009;30:92–97. doi: 10.1097/BCR.0b013e3181921f51. [DOI] [PubMed] [Google Scholar]
- 35.Stein MB, Kerridge C, Dimsdale JE, et al. Pharmacotherapy to prevent PTSD: Results from a randomized controlled proof-of-concept trial in physically injured patients. J Trauma Stress. 2007;20:923–932. doi: 10.1002/jts.20270. [DOI] [PubMed] [Google Scholar]
- 36.Foa EB, Riggs DS, Dancu CV, et al. Reliability and validity of a brief instrument for assessing posttraumatic stress disorder. J Trauma Stress. 1993;6:459–473. [Google Scholar]
- 37.Shyong EQ, Lucchinetti E, Tagliente TM, et al. Interleukin balance and early recovery from anesthesia in elderly surgical patients exposed to beta-adrenergic antagonism. J Clin Anesth. 2003;15:170–178. doi: 10.1016/s0952-8180(03)00033-3. [DOI] [PubMed] [Google Scholar]
- 38.Zeger S, Liang K. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–130. [PubMed] [Google Scholar]
- 39.Eisen SA, Kang HK, Murphy FM, et al. Gulf War veterans' health: medical evaluation of a U.S. cohort. Ann Intern Med. 2005;142:881–890. doi: 10.7326/0003-4819-142-11-200506070-00005. [DOI] [PubMed] [Google Scholar]
- 40.Parker C, Chen Z, Price M, et al. Adherence to warfarin assessed by electronic pill caps, clinician assessment, and patient reports: results from the IN-RANGE study. J Gen Intern Med. 2007;22:1254–1259. doi: 10.1007/s11606-007-0233-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cramer J, Vachon L, Desforges C, et al. Dose frequency and dose interval compliance with multiple antiepileptic medications during a controlled clinical trial. Epilepsia. 1995;36:1111–1117. doi: 10.1111/j.1528-1157.1995.tb00469.x. [DOI] [PubMed] [Google Scholar]
- 42.Liang SY, Yates P, Edwards H, et al. Factors influencing opioid-taking self-efficacy and analgesic adherence in Taiwanese outpatients with cancer. Psycho-Oncology. 2008;17:1100–1107. doi: 10.1002/pon.1326. [DOI] [PubMed] [Google Scholar]
- 43.Catz SL, Kelly JA, Bogart LM, et al. Patterns, correlates, and barriers to medication adherence among persons prescribed new treatments for HIV disease. Health psychol. 2000;19:124–133. [PubMed] [Google Scholar]
- 44.Bartlett JA. Addressing the challenges of adherence. J Acquir Immune Defic Syndr. 2002;29:S2–S10. doi: 10.1097/00126334-200202011-00002. [DOI] [PubMed] [Google Scholar]
- 45.Bender BG. Overcoming barriers to nonadherence in asthma treatment. J Allergy Clin Immunol. 2002;109:S554–S559. doi: 10.1067/mai.2002.124570. [DOI] [PubMed] [Google Scholar]
- 46.World Health Organization. Adherence to long-term therapies - evidence for action 2003. 2003 Available at: http://apps.who.int/medicinedocs/pdf/s4883e/s4883e.pdf.
- 47.Vlasnik JJ, Aliotta SL, DeLor B. Medication adherence: factors influencing compliance with prescribed medication plans. The Case Manager. 2005;16:47–51. doi: 10.1016/j.casemgr.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 48.Moher D, Hopewell S, Schulz KF, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ (Clinical research ed) 2010;340:c869. doi: 10.1136/bmj.c869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tolstoy L. Anna Karenina. (Garnett, C., Trans. Kent, L.J. & Berberova, N., Eds.). New York: Modern Library. (Original work published 1877).
- 50.Choinier M, Melzack R, Rondeau J, et al. The pain of burns: characteristics and correlates. J Trauma Acute Care Surg. 1989;29:1531–1539. doi: 10.1097/00005373-198911000-00013. [DOI] [PubMed] [Google Scholar]
- 51.Scadding JW, Wall PD, Wynn Parry CB, et al. Clinical trial of propranolol in post-traumatic neuralgia. Pain. 1982;14:283–292. doi: 10.1016/0304-3959(82)90135-X. [DOI] [PubMed] [Google Scholar]
- 52.Tchivileva IE, Lim PF, Kasravi P, et al. Propranolol in temporomandibular joint disorder treatment. American Pain Society; San Diego, CA: 2009. [Google Scholar]
- 53.Orrey DC, Bortsov AV, Hoskins JM, et al. Catechol-O-methyltransferase genotype predicts pain severity in hospitalized burn patients. J Burn Care Res. 2012;33:518–523. doi: 10.1097/BCR.0b013e31823746ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.National Burn Repository 2011 Report. 2011 Available at: http://www.ameriburn.org/2011NBRAnnualReport.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.