Abstract
The epidemiological evidence suggests a strong inverse relationship between dietary intake of cruciferous vegetables and the incidence of cancer. Among other constituents of cruciferous vegetables, isothiocyanates (ITC) are the main bioactive chemicals present. Phenethyl isothiocyanate (PEITC) is present as gluconasturtiin in many cruciferous vegetables with remarkable anti-cancer effects. PEITC is known to not only prevent the initiation phase of carcinogenesis process but also to inhibit the progression of tumorigenesis. PEITC targets multiple proteins to suppress various cancer-promoting mechanisms such as cell proliferation, progression and metastasis. Pre-clinical evidence suggests that combination of PEITC with conventional anti-cancer agents is also highly effective in improving overall efficacy. Based on accumulating evidence, PEITC appears to be a promising agent for cancer therapy and is already under clinical trials for leukemia and lung cancer. This is the first review which provides a comprehensive analysis of known targets and mechanisms along with a critical evaluation of PEITC as a future anti-cancer agent.
Keywords: cruciferous vegetables, isothiocyanates, PEITC, reactive oxygen species, chemoprevention
1. Introduction
Since prehistoric times, nature has been a rich source of many drugs and drug leads, which are currently being used to treat various ailments, including cancer. According to Newman and Cragg, 74.8% of present anticancer agents originated from natural sources, with almost 48.6% being the actual natural compound or a direct derivative [1]. These include several widely used anticancer agents like taxanes, vinca alkaloids and camptothecin class of compounds. Interest in natural products as an alternative to synthetic drugs for cancer treatment is largely due to low cost, established historical use in traditional medicinal systems, easy availability and minimal or no toxicity. Also, the potential for targeting multiple pathways represents an advantage of natural compounds over synthetic agents, the latter being more target-specific.
Cancer is a complex manifestation of genomic instability in cells caused by environmental or genetic factors [2]. Genomic instability leads to disruption of basic biological functions, such as cell division, differentiation, angiogenesis and migration, which promote cancer [2]. The involvement of multiple mechanisms in cancer development raises an urgent need for multi-targeted therapy. The current anti-cancer agents primarily focus on specific targets. The suppression of unique targets usually leads to activation of compensatory mechanisms resulting in failure of therapy or development of resistance to drugs. To circumvent these problems, combination therapy is currently employed by the oncologists. Nonetheless, combination approach has also shown marginal advantage in the overall therapy due to development of resistance or associated toxicity. We have reached a threshold in terms of clinical benefit and tolerance in patients.
Of all the isothiocyanates (ITCs), phenethylisothiocyanate (PEITC) is the only one that has reached the clinical stage of testing. This review provides a critical and comprehensive analysis of the outcomes of studies showing the anti-cancer effects of PEITC.
2. Epidemiological evidence for chemo-preventive effects of dietary intake of cruciferous vegetables
Several epidemiological studies from different parts of the world provide a strong evidence for the reduced risk of cancer with higher intake of cruciferous vegetables [3–7]. Although none of these studies provide a direct correlation between cancer incidence and intake of a specific ITC, many studies do identify a correlation between the total ITC intake in the form of cruciferous vegetables and reduced risk of several types of cancers in lung, breast, gastric, bladder, colorectal, pancreatic, prostate and kidney (Table 1) [5, 8–27]. Interestingly, there are also few studies where the results obtained were opposite to the preventive effects of cruciferous vegetables. For example, depending on the duration of intake and age of subjects, one of the studies has shown differential effects of cruciferous vegetables [20]. In a prospective study, Giovannucci et. al. did not observe any significant correlation between prostate cancer risk and a short-term intake of cruciferous vegetables [20]. However, long-term intake of cruciferous diet showed a stronger inverse correlation with initial stages of prostate cancer and this effect was stronger in men less than 65 years of age [20]. In contrast, a few other studies reported no correlation between the intake of cruciferous vegetables and cancer incidence [27]. The differences in the outcome showing no correlation with dietary intake of cruciferous vegetables could be due to several confounding factors such as, differences in the subject population, duration of intake of cruciferous vegetables in subject’s lifetime, age of subjects, variation in the isothiocyanate content in cruciferous vegetables based on geographic locations and whether the consumed vegetables were raw or cooked. Out of 22 studies performed, one study showed increased risk of thyroid cancer with ITC intake, while three studies (urinary, prostate and breast cancers) showed no correlation of ITC intake with cancer incidence. Interestingly all the studies on stomach, colon and lung cancers showed reduced cancer incidence with ITC intake. A detailed analysis revealed that studies showing no correlation between ITC intake and cancer incidence typically had wider trial durations or greater age differences for the subjects. Interestingly, a study performed in 2001 showed a significant change in peoples food habits and lifestyle over the last decade [28]. Hence, the outcomes from studies performed in the last decade might be affected due to these confounding factors. Furthermore, the majority of the findings were based on questionnaires for the intake of cruciferous vegetables, which can have lot of variability. In most of the studies, questionnaires were not specific in asking whether the vegetables were raw or cooked.
Table 1.
Epidemiological evidence for the anti-cancer effects of ITCs
| Cancer form | Subjects | Year Conducted | Sample Size | Geographical Location | ITC quantitation method | Mechanism Proposed | Outcome | Reference |
|---|---|---|---|---|---|---|---|---|
| Bladder Cancer | Bladder cancer patients | 1980–1998 | 239 Cases | Roswell Park Cancer Institute, USA | Dietary ITC intake based on patient questionnaire | _ | Improved survival | [5] |
| Mixed population with mean ages 63.3 and 62.5 for cases and controls respectively | 1999 | 697 cases & 708 controls | Texas, USA | Dietary cruciferous vegetables intake based on patient questionnaire | GSTM1 and GSTT1 null | Reduced cancer risk | [23] | |
| Cases with ages 25–86 years and controls with ages 21–92 years | 1982–1998 | 275 cases & 825 controls | USA | Dietary cruciferous vegetables intake based on patient questionnaire | - | Reduced cancer risk | [24] | |
| Breast Cancer | Chinese women Age 20–70 years |
1996–2005 | 3035 cases & 3037 controls | Shanghai, China | Dietary ITC intake based on patient questionnaire | GSTP1 polymorphism | Reduced cancer risk | [14] |
| Breast cancer survivors | - | 536 intervention & 620 comparison | USA | Dietary cruciferous vegetables intake based on patient questionnaire | - | Reduced cancer recurrence | [25] | |
| US and Chinese breast cancer survivors | 1990–2006 | 11390 subjects | USA and China | Dietary cruciferous vegetables intake based on patient questionnaire | - | No association with cancer mortality or recurrence | [27] | |
| Colorectal Cancer | Compiled from published literature | - | 24case control studies and 11 prospective studies | - | Dietary intake of cruciferous vegetables | - | Reduced cancer risk | [12] |
| Gastric Cancer | Middle aged men ; mean age ±65 | 1986–1989 | 307 Cases and 911 Control | Shanghai, China | Urinary ITC | GSTM1 and GSTT1 deletion enhanced protective effect | Reduced cancer incidence | [8] |
| Kidney Cancer | Age 20–79 years | 1999–2003 | 1097 cases & 1555 controls | Europe (multicenter) | Dietary ITC intake based on patient questionnaire | GSTM1 and GSTT1 polymorphism | Reduced cancer risk | [15] |
| Non-Asians | 1986–1994 | 1204 cases and 1204 controls | California, USA | Dietary cruciferous vegetables intake based on patient questionnaire | - | Reduced cancer risk | [26] | |
| Lung Cancer | African Americans & Caucasians; Age 40–84 years | 1991–1994 | 311 Cases & 922 Control | Los Angeles, California | Dietary ITC intake based on patient questionnaire | GSTM1 deletion enhanced protective effect | Reduced cancer incidence | [9] |
| >18 year adults | 1990–2005 | 274 Cases & 1089 Control | Maryland, USA | Dietary ITC intake based on patient questionnaire | _ | Reduced cancer incidence | [11] | |
| Chinese women Age 40–70 years |
1997–2009 | 74 914 women | Shanghai, China | Dietary intake of cruciferous vegetables | - | Reduced cancer risk | [13] | |
| Men and women with average ages 62.3 and 60.9 years respectively | - | 503 cases & 465 controls | Texas, USA | Dietary ITC intake based on patient questionnaire | GSTM1 and GSTT1 polymorphism | Reduced cancer risk | [16] | |
| Chinese women | 1996–1998 | 233 cases & 187 controls | Singapore | Dietary ITC intake based on patient questionnaire | GSTM1 and GSTT1 polymorphism | Reduced cancer risk | [17] | |
| Chinese women | 1996–1998 | 303 cases & 765 controls | Singapore | Dietary ITC intake based on patient questionnaire | - | Reduced cancer risk | [18] | |
| Hospital patients | 1992–2000 | 716 cases and 939 controls | Massachusetts, USA | Dietary cruciferous vegetables intake based on patient questionnaire | GSTM1 presence | Reduced cancer risk | [21] | |
| Prostate cancer | Men Age 40–75 years |
1986–2000 | 47365 subjects | USA | Dietary cruciferous vegetables intake based on patient questionnaire | - | Non-significant correlation with cancer risk | [20] |
| Stomach and colorectal cancer | Japanese population | - | 149 cases & 287 controls for stomach cancer and 115 cases and 230 controls for colorectal cancer | Japan | Dietary cruciferous vegetables intake based on patient questionnaire | - | Reduced cancer risk | [19] |
| Thyroid cancer | Melanesian women | 1993–1999 | 293 Cases & 354 Control | New Caledonia | Dietary ITC intake based on patient questionnaire | Low dietary intake of iodine | Increased risk of thyroid cancer | [10] |
| Urinary carcinoma | Scottish Terriers | - | 92 cases & 83 controls | - | Dietary cruciferous vegetables intake based on owner questionnaire | - | Non-significant correlation with cancer risk | [22] |
However, based on the majority of the studied outcomes, we can conclude that an inverse relationship exists between intake of ITCs in the form of cruciferous vegetables and the overall incidence of cancer. But no conclusion can be drawn with respect to any specific isothiocyanate at this time.
3. PEITC source and pharmacokinetics
3.1 Cruciferous vegetables – Source of PEITC
The isothiocyanates (R-N=C=S) (ITC) are known to be the major bioactive compounds present in cruciferous vegetables and responsible for anti-cancer activity. ITCs are released from glucosinolates by the action of the enzyme myrosinase. The enzyme myrosinase can be activated by cutting or chewing the vegetables, but heating can destroy its activity [29]. However, microbial myrosinase from gut can also release ITCs in the stomach after ingestion of cruciferous vegetables [30, 31]. Studies show that myrosinase as well as isothiocyanates are thermolabile [32]. Hence ingestion of only raw vegetables can release ITCs and cooking of the vegetables can reduce ITC content [32]. Although water cress and broccoli are known to be the richest source, PEITC can also be obtained from turnips and radish. PEITC is present as gluconasturtiin in cruciferous plants. Like other ITCs, PEITC can be released from gluconasturtiin by the action of myrosinase [30, 31]. In a study conducted with human volunteers, approximately 2 to 6 mg of PEITC was found to be released by the consumption of one ounce of watercress [33, 34]. Similarly, ingestion of 100g of broccoli and watercress can release up to 200μmol of PEITC in humans [29, 33]. Interestingly, several pre-clinical studies have shown that significant anti-cancer effects can be achieved at micromolar concentrations of PEITC.
3.2 Pharmacokinetics and metabolism
A significant number of studies have shown a positive pharmacokinetic profile for orally administered PEITC (Table 2). PEITC is highly bioavailable after oral administration. A single dose of 10–100 μmol/kg PEITC in rats resulted in bioavailability ranging between 90–114% [35]. The high bioavailability was accompanied by low clearance as well as high protein binding [35]. Increased bioavailability of PEITC was also observed in another study with repeated dosing of PEITC [36]. Furthermore, about 928.5±250nM peak plasma concentration of PEITC was achieved in human subjects, after the consumption of 100g watercress. The time to reach peak plasma concentration was observed to be 2.6h±1.1h with a t1/2 4.9±1.1h [37]. This indicates that oral administration of PEITC can result in effective concentrations in blood plasma. However, long term studies are required to establish the safety profile of PEITC, since regular intake of PEITC can cause its accumulation resulting in cumulative effects, which could be toxic.
Table 2.
Pharmacokinetic parameters of PEITC
| Route of admin istrati on |
Dose | Vehicl e |
Mo del |
Nu mb er of sa mp les (n) |
Org an |
AUC ±SD or (SE) |
Cmax ±SD or (SE) |
Tmax ±SD or (SE) |
T1/2a±S D or (SE) |
T1/2e ±SD or (SE) |
Vd ±SD or (SE) |
F %± SD or (SE) |
R ef |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Oral | 10μmol (50μmol/kg) (single) | Corn Oil | Male F344 rats | 3/time point | Blood | 328.1 nmol ml−1 h (106.93) | 18.77 pmol/μl (2.9) | 2.94h (0.78) | 1.34h (3.15) | α=2.41h (6.96) β=21.7h (25.77) | - | - | [182] |
| Liver | 1277.57 nmol ml−1 h (291.76) | 39.79 pmol/μl (3.29) | 2.5h (0.56) | 0.44h (0.14) | 20.45h (5.84) | - | - | ||||||
| Lungs | 346.321 nmol ml−1 h (6.51) | 9.73 pmol/μl (0.39) | 4.53h (0.4) | 0.97h (0.14) | 21.3h (2.99) | - | - | ||||||
| Kidneys | 987.941 nmol ml−1 h (114.88) | 46.29 pmol/μl (2.06) | 4.31h (0.39) | 1.18h (0.2) | 11.37h (1.99) | - | - | ||||||
| Nasal mucosa | 378.50 pmol mg−1h (341.42) | 20.4 pmol/mg (7.31) | 3.52 (0.66) | 0.93h (1.2) | 10.10 (12.16) | - | - | ||||||
| Esophagus | 140.19 pmol mg−1 h (111.69) | 8.2 pmol/bg (2.14) | 2.77h (1.78) | 0.67h (0.69) | 9.72h (9.89) | - | - | ||||||
| Stomach | 1409.7 pmol mg−1 h (457.62) | 211.19 pmol/mg (26.44) | 1.35h (0.45) | 0.37h (0.24) | 3.57h (1.93) | - | - | ||||||
| Small Intestine | 1691.21 pmol mg−1 h (292.94) | 130.58 pmol/mg (7.64) | 4.07h (0.41) | 1.69h (0.64) | 5.23h (2.22) | - | - | ||||||
| Cecum | 102422.29 pmol mg−1 h (44129.9) | 15.07 pmol/mg (1.78) | 19.09h (11.51) | 465.87h (2061.42) | 2.52h (1.07) | - | - | ||||||
| Colon | 361.39 pmol mg−1 h (47.32) | 16.43 pmol/g (1.22) | 8.08h (1.15) | 5.81h (37.92) | 5.4h (35.71) | - | - | ||||||
| Pancreas | 176.0 pmol mg−1 h (29.04) | 9.73 pmol/mg (0.39) | 4.53h (0.4) | 54.96h (11.78) | 0.97h (0.14) | - | - | ||||||
| Spleen | 57.71 pmol mg−1 h (30.55) | 9.64 pmol/mg (2.0) | 1.2h (0.71) | 3.2h (2.83) | 0.33h (0.37) | - | - | ||||||
| Heart | 94.35 pmol mg−1 h (22.99) | 5.71 pmol/mg (0.51) | 4.36h (0.74) | 7.75h (3.41) | 1.48h (0.61) | - | - | ||||||
| Brain | 53.71 pmol mg−1 h (5.4) | 2.07 pmol/mg (0.1) | 6.46h (0.57) | 12.58h (2.27) | 2.07h (0.41) | - | - | ||||||
| Oral (Gastric-intubation) | 0.5, mg/kg(single) | DMSO | Male Wistar albino rats | 4 | Blood | 1033±174 378 μg L− 1 h | 318±62 μg/L | 0.73±0.15h | 0.2h (Ka=3.52±1.58) | 2.62±1.37h | 1.53±0.81 L/kg | 77±13 | [37] |
| 1.0 mg/kg(single) | 4 | 1646±284 378 μg L− 1 h | 387±73 μg/L | 0.45±10h | 0.13h (Ka=5.19±2.14) | 2.78±0.36h | 1.75±0.29 L/kg | 61±11 | |||||
| 5.0 mg/kg(single) | 4 | 2438±462 μg L−1 h | 769±107 μg/L | 0.78±0.21h | 0.18h (Ka=3.88±0.40) | 3.19±0.37h | 2.15±0.40 | 23±5 | |||||
| 0.5 mg/kg/day (4 days) | 4 | 1117±282 378μg L−1 h | 404±80 μg/L | 0.7±0.24h | 0.16h (Ka=4.26±2.31) | 2.55±0.26h | 1.5±0.14 L/kg | 83±21 | |||||
| 1.0 mg/kg/day (4 days) | 4 | 2311±258 μg L− 1 h | 647±38 μg/L | 0.90±0.12h | 0.3h (Ka=2.26±0.48) | 1.86±0.2 h | 1.05±0.10 L/kg | 86±10 | |||||
| 5.0mg/kg/day (4 days) | 4 | 5796±1004 μg L−1 h | 2139±444 μg/L | 0.95±0.10h | 0.22h (Ka=3.02±2.03) | 1.62±0.44h | 0.88±0.28 L/kg | 43±7 | |||||
| Intravenous | 0.5mg/kg (single) | 15% hydroxypropyl-β-cyclodextrin | 4 | 1346±378 μg L− 1 h | - | - | - | 1.36±0.36h | 0.75±0.06 L/kg | 100 | |||
| Intravenous | 2μmol/kg | 15% hydroxypropyl-β-cyclodextrin | Male Sprague Dawley rats | 3–4 | Blood | 2.96±0.78 μM h | - | - | - | 3.52±0.35h | 1.94±0.42 L/kg | 100 | [36] |
| 10μmol/kg | 17.3±9.3 | - | - | - | 6.92±3.73h | 3.27±2.06 | 100 | ||||||
| 100μmol/kg | 322.1±149.6 | - | - | - | 9.19±0.83h | 2.66±1.22 | 100 | ||||||
| 400μmol/kg | 807.5±66.9 | - | - | - | 13.1±2.0 | 5.72±1.10 | 100 | ||||||
| Oral | 10μmol/kg | - | 19.89±3.27 | 9.2±0.6 μM | 0.44±0.1h | - | - | - | 115 | ||||
| 100μmol/kg | 298±139.4 | 42±11.4 | 2±1 | - | - | - | 93 |
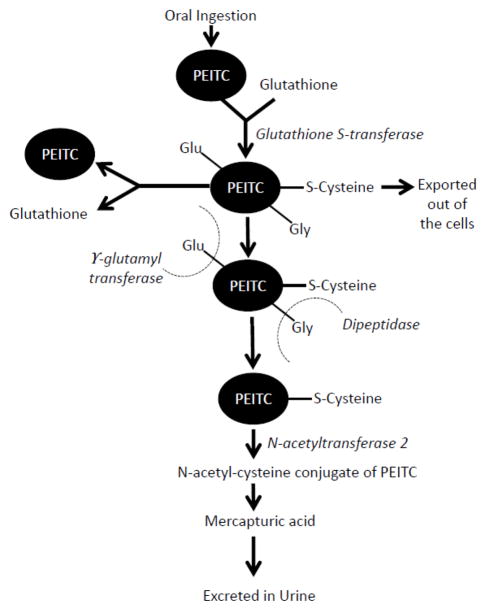
PEITC metabolism has been reviewed in detail by two groups of investigators [38, 39]. PEITC is primarily removed as mercapturic acid from the body (Fig. 2). After oral ingestion, PEITC is converted into glutathione conjugates by the action of enzyme glutathione S-transferases (GST). Several epidemiological as well as pre-clinical studies showed that the anti-cancer effects of ITC can vary significantly with GST polymorphism (Table 3) [15–17, 21, 40–63]. These glutathione conjugates are exported out of the cells by membrane bound transporter proteins and are cleaved by γ-glutamyl transferase and dipeptidase. The conjugation of PEITC with intracellular glutathione and the subsequent removal of the conjugate result in depletion of glutathione and alteration in redox homeostasis leading to oxidative stress, as explained in detail in section 4.3 of this review. Cysteine conjugated PEITC is acetyated by N-acetyl transferase in liver and eliminated from the body as N-acetylcysteine (NAC) conjugates or mercapturic acid (Fig 2) [64]. Interestingly, Tang et al. reported that NAC conjugates are also biologically active and have similar activity to parent compounds, suggesting prolonged effects of ITCs [64].
Figure 2.
General metabolic pathway for PEITC
Table 3A.
Epidemiologic evidence for the effects of GST polymorphism on ITC uptake/metabolism and cancer risk
| Cancer | Design | Populatio n |
Location | Method of analysis |
ITC intake/ levels |
GST studied |
Status | Outcomes | Statistics | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| Adenoma | Case control (459 cases; 507 control) | Mixed | USA | Dietary intake | High | GSTM1 | Null | Reduced risk | P=0.001 for trend and 0.1 for interaction | [42] |
| Breast | Case control (740 cases; 810 control) | Caucasian women | USA | Dietary intake | High | GSTM1/T1 | Null/positive | Reduced risk | OR=0.6 (0.4–1.01) Premenopausal women OR=1.0 (0.7–1.4) Postmenopausal women |
[44] |
| Case control (1052 cases and 1098 control) | Women (<65 years age) | USA | Dietary intake | - | GSTT1 | Null | Increased risk | OR=1.86 (1.12–3.08) | [46] | |
| - | GSTM1 | Null | Increased risk | |||||||
| - | GSTP | Ile/Ile | Increased risk | |||||||
| High/Low | GSTT1/M1/P | Null/Positive | No correlation | - | ||||||
| Colon | Case control (213 cases; 1194 control) | Chinese men and women | Singapore | Dietary intake | High | GSTM1/T1 | Null | Reduced risk | OR=0.43 (0.2–0.96) | [43] |
| Colorectal | Case control (173 cases and 313 controls) | Mixed | USA | Urinary ITC | Detectable | GSTT1 | Null/Positive | No correlation | - | [48] |
| Detectable | GSTM1 | Null/Positive | No correlation | - | ||||||
| Detectable | GSTP1 | AG or GG | Marginally reduced risk | P=0.09 | ||||||
| Case control (322 cases and 1251 controls) | Women 40–70 years | China | Urinary ITC | High | GSTT1 | Null | Reduced risk | P=0.04 | [50] | |
| High | GSTM1 | Null | Reduced risk | P=0.07 | ||||||
| High | GSTT1/M1 | Null | Reduced risk | OR=0.51 (0.27–0.95) | ||||||
| Kidney | Case control (1097 cases and 1555 control) | Mixed | Europe | Dietary intake | Low | GSTT1 | Null | Increased risk | OR=1.86 (1.07–3.23) | [15] |
| Low | GSTM1/T1 | Null | Increased risk | OR=2.49 (1.08–5.77) | ||||||
| Lung | Case control (503 cases; 465 control) | Mixed subjects from Houston | USA | Dietary intake | Low | GSTM1 | Null/Current smoker | Increased risk in | OR=2.22 (1.2–4.1) | [16] |
| Low | GSTT1 | Null/Current smoker | Increased risk | OR=3.19 (1.54–6.62) | ||||||
| Low | GSTM1 and T1 | Null/Current smoker | Increased risk | OR=5.45 (1.72–17.22) | ||||||
| Low | GSTM1 | Null/Former smoker | No correlation | OR=1.14 (0.66–1.96) | ||||||
| Low | GSTT1 | Null/Former smoker | Increased risk | OR=1.79 (0.95–3.37) | ||||||
| Case control (233 cases; 187 control) | Chinese women | - | Dietary intake | High | GSTM1/T1 | Null | Reduced risk | OR=0.54 (0.3–0.95) | [17] | |
| High | GSTM1 | Positive | No effect | 1.07 (0.5–2.29) | ||||||
| Case control (232 cases; 710 control) | Chinese men | China | Dietary intake and urinary ITC analysis | Detectable | GSTM1 | Null | Reduced risk | Relative risk=0.36 (0.2–.63) | [41] | |
| Detectable | GSTM1/T1 | Null | Reduced risk | Relative risk=0.28 (0.13–0.57) | ||||||
| Case control (716 cases; 939 control) | Caucasian women and men | USA | Dietary intake | High | GSTM1 | Positive | Reduced risk | OR=0.61 (0.39–0.95) | [21] | |
| High | GSTM1 | Null | No correlation | OR=1.15 (0.78–1.68) | ||||||
| High | GSTM1 | Null/positive | No correlation | - | ||||||
| High | GSTM1/T1 | Null/positive | No correlation | - | ||||||
| Literature review | 30 studies | - | Dietary intake | High | GSTT1/M1 | Null | Reduced risk | OR=0.41 (0.26–0.65) | [47] | |
| - | Cohort | Chinese (45–74 years age) 111 men, 135 women |
Singapore | Dietary intake and urinary ITC analysis | - | GSTM1 | Null | No correlation | P = 0.61 | [40] |
| - | GSTM1 | Positive | No correlation | |||||||
| - | GSTT1 | Null | Low excretion | P=0.006 | ||||||
| - | GSTT1 | Positive | High excretion | |||||||
| - | GSTP1 | a/a | No correlation | P = 0.77 | ||||||
| - | GSTP1 | a/b | No correlation | |||||||
| - | GSTP1 | b/b | No correlation | |||||||
| Cohort (114 subjects) 18–50 years | Mixed | USA | Urinary ITC and metabolites | Single intake | GSTM1 | Null | High excretion in 62% subjects | ITC (mol/24h)=9.9± 1.4 | [45] | |
| GSTM1 | Positive | High excretion in 39% subjects | ITC (mol/24h)=14.0 ±1.3 (P=0.48) | |||||||
| GSTT1 | Null | Marginally significant | ITC (mol/24h)=9.9± 2.7 | |||||||
| GSTM1 | Positive | Marginally significant | ITC (mol/24h)=11.6 ±0.9 (P=0.05) | |||||||
| GSTP1 | A/A | Non significant | ITC (mol/24h)=9.9± 1.5 | |||||||
| GSTP1 | G/A | Non significant | ITC (mol/24h)=14.8 ±1.4 | |||||||
| Cross-over intervention study | 20 healthy subjects | - | Plasma ITC | - | GSTM1 | Null/Positive | No correlation | - | [49] | |
| - | GSTT1 | Null/Positive | No correlation | - | ||||||
| 48 subjects, 28 GSTT1 and M1 positive and 20 null genotypes | Healthy volunteers | - | Urinary ITC | Watercress juice | GSTT1/M1 | Null/Positive | No correlation | - | [51] |
PEITC and/or glutathione conjugates were found to be substrates of important membrane bound transporter proteins like breast cancer resistance protein (BCRP), multidrug resistance- associated proteins 1 (MRP1) and 2 (MRP2) [65–67]. BCRP plays an important role in absorption and disposition of many drugs. Hence, PEITC being a substrate of BCRP can potentially affect the pharmacokinetics and pharmacodynamics of other BCRP substrates. In addition, PEITC can modulate other important transporter and metabolic proteins like P-glycoprotein, [67] and cytochrome P450 enzymes [68, 69]. PEITC-mediated modulation of transporter proteins could be an important factor for the chemopreventive effects of PEITC. These effects are explained in section 4.1 of this review. Nevertheless, transporter proteins are important determinants of the efficacy and toxicity of many clinical drugs and by modulating these proteins, PEITC can affect the efficacy as well as toxicity of some other drugs.
3.3 GST polymorphism and anticancer activity of PEITC
GST is a polymorphic enzyme which promotes conjugation of glutathione (GSH) with a wide variety of electrophilic xenobiotics including carcinogens. The conjugation of GSH with carcinogens catalyzed by GST and subsequent removal of the conjugate results in reduced carcinogenic effects [70, 71]. Increasing evidence from epidemiological and pre-clinical studies suggest a significant impact of GST polymorphism on the outcomes of dietary ITC intake (Table 3A). A majority of these studies indicates an important effect of GSTT1, GSTM1 and GSTP1 polymorphism in ITC mediated anti-cancer effects. The presence of GSTT1 or GSTM1 or both have been strongly correlated with reduced or no effects of dietary ITCs on cancer risk. Interestingly, pre-clinical studies have shown ITC-mediated induction of GST enzymes, suggesting GST-mediated metabolism and inactivation of ITCs (Table 3B). Interestingly, a study indicates concentration dependent effect of specific GST polymorphism in catalyzing glutathione conjugation with PEITC [56]. The GSTA1-1 enzyme reacts with micromolar concentrations of PEITC, while GSTM1a-1a exhibits a significantly lower affinity for PEITC. On the contrary, Meyer et. al. suggested that GST catalyzes forward as well as reverse conjugation of PEITC with GSH [56]. Studies also showed PEITC mediated tissue specific induction of GST isoforms [55, 57, 60, 63, 72, 73]. Van et. al. showed significant induction of GST by PEITC in esophagus [57]. Furthermore, PEITC mediated induction of GSTA in liver, GSTM in stomach and liver as well as GSTP in colon was observed. PEITC treatment also induced glutathione levels by about 1.6 fold in colon [57]. PEITC-mediated GSTP modulation was observed in few other studies as well [58, 59]. The induction of GST and glutathione appears to be an important pathway for PEITC-mediated chemopreventive effects. In a chemical carcinogen-induced multi-organ cancer model, differential effects of PEITC were observed in pre- and post-tumor phases. PEITC treatment during pre-initiation period caused reduced hyperplasia in esophagus and lungs accompanied with reduction of GSTP in kidney and liver [58]. However, PEITC treatment during post-initiation phase increased liver GSTP positive foci along with increased incidence of urinary bladder hyperplasia and tumors [58]. This suggests preventive effects of PEITC in preinitiation conditions, while during post-initiation, PEITC treatment increases tumor incidence. However, it is important to note that 0.1% PEITC was administered in the diet in this study, whereas, in most of the studies, a pure and higher concentration of PEITC was administered.
Table 3B.
Pre-clinical evidence for effect of GST polymorphism on isothiocyanate efficacy
| Model | Agent used | GST polymorph | Outcome | Reference |
|---|---|---|---|---|
| Male Wistar rats | BITC | Subunit 3 & 2 | Increased | [52] |
| Rat T9 Glioma cells | AITC, BITC | GSTP | Increased marginally | [53] |
| AITC, BITC + dibutyryl cAMP | GSTP | Increased | ||
| Male ACI/N rats | BITC, BTC | GSTP | Reduced | [54] |
| Kinetic study | ITC | GSTP1-1 and M1-1 | Most efficient catalysis | [55] |
| GATM4-4 | Least efficient catalysis | |||
| Kinetic study | PEITC, BITC | GSTA1, A2, P1 | Catalyze forward and reverse reactions in a concentration dependent manner | [56] |
| Male Wistar rats | PEITC | GSTA | Increased in liver | [57] |
| GSTM | Increased in stomach and liver | |||
| GSTP | Increased on colon | |||
| Male F344 rats | PEITC | GSTP | Reduced in liver (Pre-initiation period of tumors) with reduced hyperplasia in esophagus and kidney | [58] |
| Enhanced in liver (Post-initiation period of tumors) with increased urinary bladder tumors | ||||
| RL34 cells (Rat liver epithelial) | BITC, PEITC | GSTP1 | Increased | [59] |
| RL34 cells | ITC | GSTA1/2 | Nrf2 dependent increase | [60] |
| GSTA3 | ||||
| GSTM1 | ||||
| PBMC cells | Watercress/PEITC | GSTM1-0 | Increased GPX and SOD | [61] |
| GSTM1-1 | Not increased GPX and SOD | |||
| GSTT1 | No impact | |||
| HL60 cells | ITC | GSTA (Overexpression) | Reduced efficacy | [62] |
| Male Wistar rats | ITC | GSTM | Increased in liver | [63] |
| GSTA | Increased in Liver | |||
| GSTT | Increased in kidney |
The role of GST in chemoprevention has been reported to be dependent on the polymorphic status of GST as well as the source of induction [74]. This implies that GST polymorphism and its tissue specific differential effects are important determinants to correlate cancer risk and ITC intake. Many studies have also reported the outcomes of ITC intake and cancer risk with no consideration of GST status in the subjects. Hence, outcomes from these studies need to be re-confirmed using better designed studies.
4. Anti-cancer activity of PEITC
Several researchers have demonstrated the anticancer properties of isothiocyanates in various cancer types [75–81]. The anti-cancer activity of PEITC can be divided into chemopreventive and chemotherapeutic effects. Although chemopreventive effects were identified through epidemiological evidence, chemical carcinogenesis models and transgenic mouse models, more extensive mechanistic studies were performed to evaluate its therapeutic potential in various pre-clinical models.
4.1 Chemopreventive effects of PEITC
The diverse epidemiological studies provided the first evidence of anticancer activity of ITCs (Table 1). The preventive effects of ITCs were evaluated in multiple chemically-induced cancer models. For example Morse et. al. and others, demonstrated that administration of ITCs including PEITC prevented the carcinogenic effects of chemical carcinogens in rodents [82–86]. In general, carcinogenesis occurs due to the bio-activation of carcinogens by oxidation, reduction or hydrolysis via phase I drug metabolizing enzymes. Hence, modulation of phase I enzymes can affect the carcinogen activation process. PEITC shows a dual activity on phase I enzymes. For example, PEITC on one hand causes induction of CYP1A1 and CYP1A2; however, it inhibits activity of certain CytP450 enzymes, such as CYP2E1, CYP3A4 and CYP2A3 [68, 87–91]. Detailed studies are required to elucidate the differential effects of PEITC on CYP enzymes and their mechanistic implications in cancer prevention.
Phase II enzymes, which include mainly transferases, play an important role in detoxifying xenobiotics and carcinogens. As reviewed by Cheung et. al., various ITCs including PEITC have been shown to induce phase II detoxification enzymes, which can explain their chemopreventive activity [92]. GSTs play an important role in chemopreventive effects of PEITC, as discussed earlier in section 3.3. PEITC also acts on other phase II enzymes. Saw et. al. demonstrated induction of Nrf2-antioxidant response element by a combination of ITC and phyto-indoles [93]. Although in general, ITCs modulate phase II enzymes, human livers demonstrated a nonidentical response to PEITC [94]. Liver enzymes were found to be most sensitive to PEITC, while lung enzymes were mostly resistant [95]. Hence, based on current evidence, it can be concluded that the effects of PEITC on drug metabolizing enzymes are individual as well as tissue specific.
Continuous high levels of ROS also promote carcinogenesis by causing oxidative DNA damage leading to mutations [96]. Interestingly, PEITC treatment caused a significant increase in the activities of ROS detoxifying enzymes such as glutathione peroxidase1, superoxide dismutase 1 and 2. This was also confirmed in human study where subjects were administered watercress, a major source of PEITC [61].
4.2 Chemotherapeutic effects of PEITC
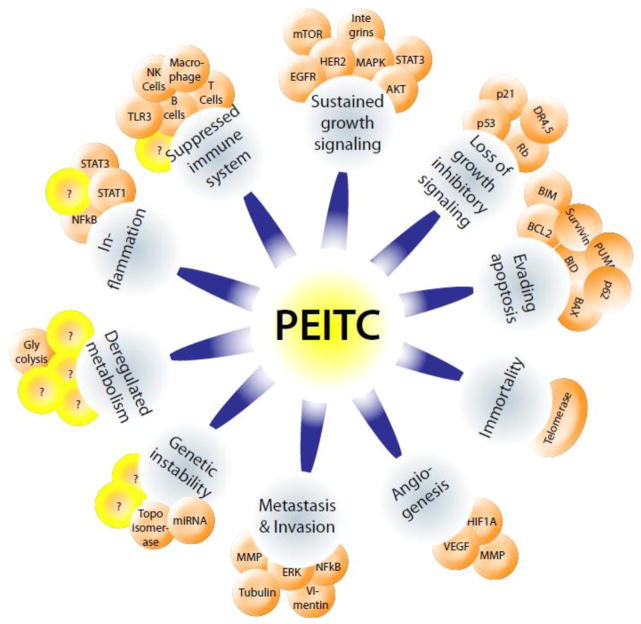
Hanahan and Weinberg have defined ten basic common hallmarks underlying cancer progression [2, 97]. All types of cancers require these basic hallmarks for development and advancement. Hence, effective inhibition of one or more of these hallmarks can potentially enhance the outcomes of current anti-cancer therapeutics. Accumulating evidence suggests that PEITC targets a broad spectrum of proteins to suppress cancer growth and progression (Figure 1). Research group led by Zigang Dong and An-Ng Tony Kong for the first time in 1998 independently demonstrated the apoptosis-inducing effects of PEITC in cultured cancer cells [98–100]. The cytotoxic concentrations of PEITC range from 120 nM to 14 μM in cancer cells [101, 102]. Tseng et. al. have reported that working concentrations of PEITC are similar to chemotherapeutic drug daunomycin in human breast cancer cells as well as in mammary epithelial cells, implying PEITC’s anti-cancer potential comparable to conventional drugs [103].
Figure 1.
PEITC and hallmarks of cancer
Since the discovery of anticancer effects of PEITC, several mechanisms have been proposed for its activity. Two primary mechanisms that have been identified are cell cycle arrest and induction of apoptosis [104–106]. PEITC-mediated generation of reactive oxygen species (ROS) is known to be a general mechanism of action leading to cytotoxic effects, especially specific to cancer cells [107, 108]. Some studies have also shown significant inhibition of other important cancer promoting mechanisms like angiogenesis and metastasis [109–113]. PEITC targets specific regulatory proteins to inhibit cancer-related oncogenes. Mi et. al. have identified more than 30 different biological targets of PEITC [114]. There are about 350 articles available so far on the anti-cancer effects of PEITC. Table 4 summarizes the targets and major mechanisms of PEITC that have been studied in different cancer models by various investigators during recent years (2011–2014).
Table 4.
Molecular targets and major mechanism of action of PEITC in various cancer types
| Cancer type |
Mechanism | Molecular Targets | Outcome | Concentration and duration |
Model used | Reference |
|---|---|---|---|---|---|---|
| - | Epigenetic | CYP2E1 | Reduced activation of carcinogens | 25, 50μM | Escherichia coli MV1304 (c DNA CYP2E1) | [89] |
| - | Thiol modification | Topoisomerase IIα | Apoptosis | 2.5, 5, 10μM (6h) | Top2β+/+; top2β−/− primary MEFs | [175] |
| Breast | Protein suppression | BIM, PUMA, BCL2, BCL-xl, BAX, Bak, | Apoptosis | 2.5, 5μM (24h) | BRI-JM04; MDA-MB-231; MCF-7; HCT- 116; HCT-116 (PUMA −/−) | [136] |
| Synergism with paclitaxel | - | Apoptosis; G2/M cell cycle arrest | 1–10μM (24, 48h) | MCF7; MDA-MB-231 | [190] | |
| Protein suppression | HSP90, HSF1, Cyclin B1, CDK1, Cdc25C, PLK-1, and p21, caspase 3, 9 | Apoptosis; G2/M cell cycle arrest | 0.1, 0.5, 2.5, 5μM (24h) | MCF7; MDA-MB-231 | [192] | |
| Protein suppression | BCL2, BAX, NRF2, GSH, caspase 3, 8 and 9 | Apoptosis; G2/M cell cycle arrest | 20μM (2, 4, 6, 24, 48h) | MCF7; BT549; MDA-MB-231; ZR-75-1; SKBR3; T47D | [171] | |
| Protein suppression | p70S6K, Akt, NDRG1, HSP70, ERK, AMPK, 4E-BP1, mTORC1 | Proliferation inhibition | 0–20μM (3,4h) | MCF-7; PTEN deficient MEF; TSC2 deficient MEF | [194] | |
| Chemoprevention | - | Reduced tumor incidence and increased tumor free survival | 50 μmol/kg or 150 μmol/kg every 2 days (pre-tumor initiation); PEITC continued (18 weeks) | NMU induced breast cancer in Sprague Dalwey rats | [85] | |
| Chemoprevention | - | Angiogenesis inhibition and tumor growth inhibtion | 3 μmol/g diet (29 weeks) | MMTV-neu mice | [86] | |
| Epigenetic changes | PARP-1, BCL-2, Bax, Cdk-1, Cyclin B1, α-tubulin, β-tubulin, acetyl-α-tubulin | Increase in Taxol (10, 100nM) sensitivity | 5, 10μM (24h) | MCF-7; MDA-MB-231 | [191] | |
| Genetic expression | P57, CYP19, BRCA2, IL2, ATF2, p53, Hsp27 | - | 3μM (48h) | MCF-7 | [123] | |
| ROS | HER2, EGFR, Akt, STAT3, BIM, BAX, BCL-XL, XIAP, | Proliferation inhibition; apoptosis; reduced tumor growth | 5, 10, 15μM (24h) | MCF-7; MCF-7 high HER2; MDA-MB-231; MDA-MB-231 high HER2; MDA-MB-231 high HER2 xenograft in athymic nude mice | [102] | |
| Breast and prostate | Protein suppression | Vimentin | Migration inhibition; apoptosis; tumor growth inhibition | 2.5, 5μM (6, 12, 24h); 3 μmol PEITC/g diet (29 weeks) | MDA-MB-231; PC-3; DU145; MMTV-neu mice | [156] |
| Cervical | Protein suppression | DR4, DR5, HO-1, cytochrome c, clusterin, claspin, cIAP1, cIAP2, catalase, Bcl2, Bcl-xl, BAX, BAD, Bid, ERK, JNK, MEK | Apoptosis | 2.5, 5, 10μM (48h) | HEp-2; KB | [141] |
| Cervical, Breast | Synergism with cisplatin | MAPKs, MEK1/2, NFkB, Bcl2, Bcl-xl, BAX, NOXA, PUMA, DR4, DR5, TRAIL | Apoptosis | 5μM (2, 4, 8, 12, 24h) | HeLa; C33A; MCF-7 | [200] |
| Cholangio carcinoma | Increase of free calcium level in cytosol | Cyt c, BCL-XL, BAX, AIF | Apoptosis | 1, 3, 10μM (3, 6h) | KKU-M214; Chang cells (reference) | [133] |
| ROS | Cyt c, BCL2, caspases 3, 8, 9, AIF | Apoptosis | 3, 10μM (3, 6, 24h) | KKU-100 | [135] | |
| Colon | ROS | DDB2 | Tumor regression | 10μM (0.5, 1, 2, 4, 6, 12h) | HCT116; HCT116 expressing control shRNA or DDB2 shRNA xenograft | [139] |
| Colon and blood | Epigenetic (Histone modification and genetic modulations) | NFkB, STAT1, chemokines, MMPs | Proliferation inhibition | 2.5, 5, 10, 15μM (5, 8, 12, 18h) | SW480; HT-29; THP-1 | [189] |
| Gastric | ROS; Rhodamine- 123; Cisplatin resistance reversal | ERCC1, survivin, and Mad2, Akt, NfkB, Glutathione, PGP, MRP1 | Apoptosis; cell cycle arrsest, Cisplatin resistance reversal | 1, 5μM (24h) | SGC7901/DDP | [201] |
| Glioma | ROS; Sensitization to TRAIL | DR5, p53, caspase 3, 8 and 9 | Apoptosis | 2.5μM (24h) | U87MG; A172; U343; T98G | [199] |
| Glioma, prostate, colon, liver, breast | Sensitivity to hypoxia | HIF1α, VEGF, ERK, Akt, MMP2, MMP9, uPA, | Apoptosis | 1, 2.5, 5μM (8, 16h) | U87; DU145; HCT116; HepG2; SKBR3 | [154] |
| Leukemia | Protein suppression | Akt, JNK, XIAP, MCl1, BCL2, BCL-XL, BAD, BAX | Apoptosis; tumor growth suppression | 2, 4, 6, 8μM (0–24h); 50 mg/kg, i.p. (20 days) | U937; HL-60; Jurkat cells | [116] |
| Immune-modulation | CD3, CD19, Mac-3 | Enhanced phagocytic activity of macrophages; Increased NK cell activity; Enhanced T cell proliferation | 80 & 160 mg/kg (2 weeks) | WEHI-3; BALB/c mice | [162] | |
| ROS, nitric oxide | Mitochondrial complexes, peroxiredoxin III (Prx III), superoxide dismutase-2 (SOD-2), Bcl-2, Glutathione peroxidase 4, NDUSF3 | Apoptosis | 10μM (1, 3, 6, 12h) | HL-60; Raji | [169] | |
| Lung | Epigenetic (Covalent protein binding) | Actin, Tubulin, Tropomyosin, HSP’s, Vimentin, ADAM7, thioredoxin etc. | - | 20μM (1h) | A549 | [114] |
| Cytoskeletal changes | F-actin, α-tubulin, | Apoptosis; G2/M cell cycle arrest | 1–10μM (24 & 72h) | A549; H1299 | [126] | |
| Epigenetic | Tubulin | Cell growth inhibition and apoptosis | 5, 10, 20μM (4h) | A549 | [176] | |
| Genetic expression | AP1, JNK, p38 MAPK, p44/42 MAPK, cyclin B1 | Apoptosis; G2/M cell cycle arrest | 12.5, 20μM (24h) | L9981 | [188] | |
| Post translational modification | p53 (mutant) | Apoptosis | 15, 20μM (0–24h) | H596; MDA- MB-231; | [195] | |
| Lung, colon, ovarian | ROS; Sensitization of platinum resistant cells | GSH | Reduced cell survival | 5, 7.5 μM (6, 12, 24h); 25mg/kg (bid, once a week-3 weeks) | A549; A549/CDDP; THC8307; THC8307/L-OHP; HCT116; A2780; A549/CDDP xenografts in BALB/c nude mice | [155] |
| Myeloid leukemia | ROS | Fas, Fas ligand, caspase 9 and 3 | Apoptosis | 5, 10, 15, 20μM (24, 48, 72h) | K562 | [143] |
| Melanoma | ROS, ER stress | CDC25C, CDK1, Chk1, Chk2, cyclin A, Wee1 p53 and p21 (a); Bax, Bcl2 and Bid (b); Apaf- 1, caspase-9 and -3, PARP, AIF and Endo G (c); GRP 78 and GADD153 (d); caspase- 8, Fas and FasL (e); i- NOS, catalase, SOD (Cu/Zn) and SOD (Mn) | Apoptosis; G2/M cell cycle arrest | 5–20μM (24–48h) | A375.S2 | [193] |
| Multiple Myeloma (MM) | Protein suppression | AIF, XIAP, MCL1, Survivin, Hsp70 Hsp90, | Apoptosis; G2/M cell cycle arrest; tumor growth suppression | 1.56–20μM (12, 24, 48h); 60mg/kg (34 days) | RPMI 8226-S; RPMI-Dox40; RPMI-MR20; RPMI-LR5; OPM-1; OPM-2; MM.1S; MM.1R cells; the non-transformed human liver epithelial cell line THLE-3; the human bone marrow stromal cell line HS-55; primary MM cells purified from bone marrow aspirates of MM patients or healthy donors | [122] |
| Myeloma | ROS; Proteasomal activity inhibition | p53, IkBα | Proliferation inhibition | 10, 20, 40μM (0–24h) | U266; RPMI-8226; A549 | [127] |
| Oral | Protein suppression | DR5, caspase 8, BID, p38 | Proliferation inhibition; apoptosis | 7.5, 15, 20μM (48h) | HN22; HSC-2; HSC-4; YD-15; Mc-3 | [140] |
| ROS | p53, p27, p21, CDK2, Cyclin E, p15, Cdc25a, CDK6, Cyclin D, AIF, NFkB, GRP78 | Apoptosis; G0/G1 cell cycle arrest | 0.5, 1, 2, 2.5, and 5 μM (24, 48h) | HSC-3 | [121] | |
| Oral squamous carcinoma | Protein suppression | EGF receptor signaling, MMP2, 9, Akt, ERK, PDK1, PI3K, IkBα, JNK, p38 | Invasion inhibition | 1, 2μM (24h) | OSCC SAS | [157] |
| Ovarian | Protein suppression | EGFR, Akt, mTOR, complex, | Apoptosis; tumor growth inhibition | 2.5–40μM (24h); 12μmol/mouse | SKOV-3; OVCAR-3; TOV-21G | [117] |
| ROS; combination effect with metformin | - | Proliferation inhibition | 5μM (24h) | OVCAR3; CAOV3; SKOV3; PA-1; A2780; A2780cis | [197] | |
| Pancreatic | Protein suppression | Bcl-2 and Bcl-XL, upregulated the proapoptotic protein Bak, and suppressed Notch 1 and 2 levels | Apoptosis; G2/M cell cycle arrest; tumor growth delayed | 2.5, 5, 10μM (24h); 12 μmol/day (5 days/wk – 1wk pre-inoculation) | MIAPaca2; PL- 45; BxPC3; MIAPaca2 xenograft | [134] |
| ROS, miRNA | miR-27a/miR-20a:miR-17-5p, Sp1, Sp3, Sp4, CD1, VEGF, c-Myc | Apoptosis | 10, 20μM (24h) | Panc28; L3.6PL; Panc1; SW480; RKO; | [184] | |
| Prostate | Protein suppression | ITGB1, ITGA2, ITGA6 | Proliferation inhibition; tumor growth inhibition | 0.1, 1, 5μM (24h); 3 μmol/g diet (2 weeks) | LNCaP; xenograft | [179] |
| Protein suppression | P53, Wee1, Cdc25c, caspases 3, 8, 9, FAS, AIF, cytochrome c, Bcl2, BAK, BID, GRP78, GADD153 | Apoptosis; G2/M cell cycle arrest | 10μM (6, 12, 24, 48h) | DU 145 | [124] | |
| Autophagy | p62, LC3 | Apoptosis; Tumor growth suppression | 3 μmol PEITC/g diet (19 weeks) | Mouse model | [148] | |
| DNA Fragmentation | XIAP, Survivin | Proliferation inhibition and apoptosis | 0–5μM (6, 12, 24h) | PC-3; LNCaP | [144] | |
| miRNA and transcriptional activity | Androgen receptor, miR-17, PCAF | Proliferation inhibition | 10–20μM (24–48h) | LNCaP; PC-3; DU145; C4–2B PCa; ALVA31 | [183] | |
| Mitchondrial structure, autophagy | Mitochondria, β-tubulin | Apoptosis | 8μM (4–18h) | LNCaP | [146] | |
| Gene expression | Insulin-like growth factor binding protein 3, fibronectin, thyroxine degradation enzyme, and integrin β6 | Tumor growth suppression | 3μmol/g diet (7 weeks) | LNCaP | [161] | |
| Prostate, head and neck | Protein suppression | TLR3, IRF3, | Inhibition of anchorage independent growth and colony formation; tumor growth inhibition | 2.5, 5, 10μM; 3μmol/g diet (19 weeks) | HEK293; HEK293 derived TLR3 expressing stable cells (Wt11); RL24 cells; HT1080 cells; RAW264.7 cells; SV40 T antigen expressing FVB MEF; PCI15A; LNCaP; C57/BL6 mice | [163] |
| Sarcoma | ROS, DNA damage | Cyclin A, Cyclin B1, Chk1, p53, catalase, iNOS, Mn-SOD, AIF, cytochrome c, caspase 9 and 3 | Apoptosis; G2/M cell cycle arrest | 5, 7.5, 10, 15μM (12, 18, 24h) | U-2 OS | [125] |
4.2.1 Anti-proliferative effects
Sustained proliferative signaling is a major characteristic of cancer cells. Ras activation in cancer by different oncogenes is a common mechanism to sustain proliferation. Ras further activates various pathways like RAF/MEK-MAPK and PI3K/Akt that are crucial for cell proliferation, and their inhibition is known to suppress cancer growth. It was observed that Ras mutation did not correlate with PEITC-mediated inhibition of tumorigenesis, indicating that Ras may not be a target of PEITC [115]. However, PEITC inhibits Akt, a component of Ras signaling to inhibit tumor growth in several cancer types [116–118]. Akt (protein kinase B) is over-activated in many types of cancers and promotes multiple cell survival mechanisms. PEITC is also known to inhibit EGFR and HER2, which are important growth factors and regulators of Akt in different cancer models [102, 117, 119]. Hence, Akt inhibition can be due to the suppression of EGFR or HER2. Overall, it is evident from these examples that even-though PEITC is unable to suppress Ras, tumor cell growth suppression is achieved through an alternative mechanism.
4.2.2 Effects on cell division and cell cycle
Cell proliferation is a tightly regulated process, orchestrated by pro- and anti-proliferative signals. Generally, the pro-proliferative signals are activated when new cells are required to replace damaged cells. At other times, the anti-proliferative signals maintain cells in a quiescent state and also keep cell division under control. However, cancer cells lock in the pro-proliferative signals, while suppressing tumor suppressor genes responsible for anti-proliferative effects. Cells have multiple check points for proliferation, which are regulated by tumor suppressor genes such as p53, Retinoblastoma protein (Rb) and PTEN. PEITC causes activation of Rb protein at the cellular level in prostate cancer cells, leading to attenuation of cell cycle progression [100, 120]. Interestingly, metabolic conjugates of PEITC with N-acetyl cysteine (NAC) also inhibit the phosphorylation of Rb leading to cell cycle arrest [67]. PEITC-mediated activation of another tumor suppressor, p53 was observed in oral squamous cell carcinoma, causing G0/G1 phase arrest in multiple myeloma, osteogenic sarcoma, breast cancer and prostate cancer, along with inhibition of other proteins regulating G2/M phase [93, 121–125]. It was also observed that lung carcinoma cells with wild type p53 were relatively less sensitive to PEITC as compared to cells with mutated p53 [100, 114, 126, 127]. Although, a direct effect on another important tumor suppressor PTEN has not been reported, PEITC inhibits Akt, which is overexpressed when PTEN is mutated.
Cells have a capability to keep track of the number of divisions undergone so that cell death can be initiated after a certain number of divisions. This phenomenon uses the shortening of telomere length with every division. This can be reversed by enzyme telomerase. Often, cancer cells exploit telomerase enzyme to alter the length of telomeres rendering them immortal [128]. PEITC has been shown to inhibit telomerase activity in prostate and cervical cancer cells [129, 130]. Furthermore, it was observed that pre-treatment with PEITC induced apoptosis as well as increased the efficacy of adriyamycin and etoposide by inhibiting protein kinase C and telomerase [130].
4.2.3 Pro-apoptotic effects
Cells usually undergo apoptosis when proliferation cannot be controlled by cell cycle check points. However, cancer cells develop anti-apoptotic mechanisms to enable survival. Mounting evidence suggests strong pro-apoptotic activity of PEITC by diverse mechanisms. Most of these mechanisms are affected by generation of reactive oxygen species (ROS), which also has been shown to be the basis of selectivity of PEITC toward cancer cells leaving normal cells undamaged [107]. A detailed account of PEITC mediated ROS generation is provided in section 4.3. ROS generation by PEITC leads to mitochondrial deregulation and modulation of proteins like Bcl2, BID, BIM and BAX, causing the release of cytochrome c into cytosol leading to apoptosis [102, 121, 124, 131–136]. However, Wu et. al., suggested that cytochrome c does not play a role in PEITC-induced apoptosis [137, 138]. Roy et. al. have shown an interesting tumor regression due to PEITC mediated inhibition of DDB2 through ROS generation [139]. In addition, PEITC also induces apoptosis by activation of extrinsic apoptotic pathway in oral and cervical cancer cells through the induction of death receptors and Fas-mediated apoptosis [140–143]. Some studies also show PEITC-mediated suppression of anti-apoptotic proteins like XIAP and survivin, which are up-regulated in cancer cells [144]. Taken together, a strong cytotoxic potential of PEITC cannot be denied, although differential roles have been described.
4.2.4. Autophagy
Autophagy is a stress response mechanism, which leads to degradation of cellular organelles to preserve cell energy. Autophagy is generally considered as cell survival mechanism. However, it is also a unique form of cell death, occurring under special circumstances [145]. Investigations show that PEITC induces autophagic cell death in cancer cells [146–148]. Interestingly, Mi et. al. showed the formation of aggresomes by PEITC treatment [149], indicating induction of autophagy, since these structures are formed due to proteasome failure and are degraded by autophagic pathway. Bommareddy et. al. showed induction of autophagy by PEITC in prostate cancer cells [147]. Treatment with 3-methyladenine, an autophagy inhibitor, provided evidence that autophagy induced by PEITC was unable to protect the cells from apoptosis. It was observed that PEITC-induced autophagy was mediated by Atg5 [147]. A partial role of the inhibition of mTOR/Akt signaling was also observed in PEITC-induced autophagy. Later PEITC-mediated induction of autophagy was shown in a transgenic mice model of prostate cancer [148]. Taken together, autophagy can be one important anti-cancer mechanisms of PEITC. Nevertheless, further study is required to determine whether or not PEITC induces autophagy in other in vivo cancer models.
4.2.5. Anti-angiogenic effects
Rapidly proliferating cancer cells have increased demand for nutrients and oxygen. Hence, these cells enhance the growth of new blood vessels (angiogenesis) to meet the increasing nutritional demand. Targeting vascular endothelial growth factor (VEGF), a major promoter of angiogenesis, has been an important mechanism of cancer treatment for the past few decades. As reviewed by Loureiro and D’Amore, VEGF is regulated by two major mechanisms: hypoxic regulation and non-hypoxic regulation [150]. Importantly, cancer cells are constantly under hypoxia due to limited oxygen supply. During hypoxia, hypoxia-inducible factor (HIF1α) accumulation stimulates secretion of VEGF leading to angiogenesis [150]. A study by Xiao and Singh provided evidence on the anti-angiogenic effects of PEITC through VEGF suppression, but the exact mechanism of this inhibition was not clear [113]. It was shown later that PEITC directly or indirectly suppresses HIF1α [151–154]. Besides hypoxic regulation, VEGF is also regulated by growth factors released by cancer cells (heregulin, EGF and TGF), hormones (estrogen, testosterone and insulin) as well as oncogenes Wnt and Ras [150]. In addition, VEGF is negatively regulated by tumor suppressor genes such as p53 and p73. Based on the effects of PEITC on proliferation and cell cycle described in sections 4.2.1 and 4.2.2, it is possible that PEITC can block angiogenesis by non-hypoxic mechanisms also.
4.2.6. Anti-metastatic effects
In malignant cancer, tumor cells tend to invade blood circulation to reach other organs of the body leading to spread of cancer. Under normal conditions, non-cancerous cells undergo apoptosis under anchorage-independent conditions due to the absence of extracellular matrix, which provides survival signals. In contrast, cancer cells develop survival mechanisms that allow them to survive during circulation under anchorage-independent conditions and spread to distant organs. This process, referred to as “invasion and metastasis”, is a major reason for the poor prognosis in majority of cancers. It leads to relapse of cancer, which is mostly resistant to conventional chemotherapy. There are few studies which suggest anti-invasive and anti-metastatic effects of PEITC. Various studies with PEITC have shown suppression of invasion through inhibition of matrix metalloproteinases along with anti-metastatic effects caused by suppression of ERK kinase activity and transcriptional activity of NFkB [109, 111]. PEITC was also known to inhibit processes, such as epithelial to mesenchymal transition (EMT), cell invasion and migration, which are essential pre-requisites for metastasis [155–157]. A recent study by Gupta et. al. demonstrated anti-metastatic potential of PEITC in a novel in vivo model of breast cancer metastasis [119]. In this model, when MDA-MB-231 brain seeking luciferase breast cancer cells were injected into the left ventricle of the heart of female athymic nude mice, a small percentage of tumor cells lodge in the brain via blood circulation and grow there as metastatic tumors. Observations from a pretreatment model suggested that oral administration of 10μmol PEITC significantly prevented the migration of breast cancer cell to the brain in vivo. In another experiment, PEITC administration after tumor cell implantation not only suppressed the growth of metastasized tumors in brain but also prolonged the survival of tumor bearing mice [119]. Although these studies reveal the anti-metastatic efficacy of PEITC in a breast cancer model, more evidence is required to establish similar anti-metastatic effects in other cancer models.
4.2.7. Anti-inflammatory effects
The tumor microenvironment is similar to inflammatory lesions and plays a very important role in carcinogenesis. Inflammation promotes cancer cells to grow in a bimodal way. Firstly, pre-existing inflammation promotes carcinogenesis by stimulating cells through several chemokines, cytokines and growth factors. Secondly, the same factors can be secreted by established cancer cells to re-enforce tumor growth and development. Hence, cancer growth can be inhibited by controlling the inflammatory process. Several studies have demonstrated direct modulation of inflammatory process by PEITC. It has been shown that chemically-induced inflammatory responses in mice were suppressed by PEITC [141, 158]. The mechanistic studies have shown suppression of inflammatory mediators like nitric oxide, TNF-α, and IL-10 in LPS-stimulated macrophages, as well as impairment of macrophage migration inhibitory factor (MIF) through covalent modification by PEITC [159, 160]. A few reports also connect the immunomodulatory action with anti-cancer activity of PEITC. For example, PEITC has been shown to protect mouse liver and lung from changes caused by cigarette smoke [153]. A bio-informatic analysis of PEITC treated animal tumors showed modulation of genes involved in inflammation and extracellular matrix pathways [161]. Taken together, these findings suggest anti-inflammatory effect as one of the anticancer effects of PEITC. However, further comprehensive studies between cancer and inflammation are still required to elucidate the anti-cancer mechanisms of PEITC.
4.2.8. Immunomodulatory effects
The human body’s integral defense mechanisms serve as a major host for future therapeutic opportunities and can help in the fight against cancer. The immune system is equipped with several check points to scan and selectively eliminate malignant cells. Interestingly, cancer cells develop sophisticated mechanisms to evade the immune system. There is a need for extensive and rigorous studies to discover agents that can prevent cancer cells from evading the immune system. Tsou et. al. demonstrated the immunomodulatory activities of PEITC [162]. PEITC treatment caused activation of macrophages and NK cells in a mouse leukemia model. PEITC treatment also promoted the differentiation of B cells but the proliferation remained unaffected. Conversely, it was observed that PEITC increased the proliferation of T cells while inhibiting the differentiation of the precursor cells of T cells and macrophages. PEITC treatment caused reduction in weight of the spleen and liver of leukemic mice, although no reduction in the overall weight of mice was observed [162]. The reduction of the size of the spleen and liver is more likely a result of killing of the leukemia cells in those organs, with a concomitant reduction in weight of those organs, rather than a detrimental effect on the immune system. Another study reported in vitro and ex vivo effects of PEITC on toll-like receptor 3 (TLR3). TLR’s are considered to be an important component of innate immune system. Studies showed that PEITC treatment inhibited TLR3 signaling [163]. PEITC treatment also inhibited the dimerization of TLR3 receptors leading to inhibition of IRF3 signaling. These effects were accompanied by inhibition of anchorage-independent growth and colony formation of cancer cells. Of note, PEITC also inhibits lipopolysaccharide induced TLR/IRF3 signaling in leukemia cells [164]. Thus, while theoretically the inhibition of TLR3 might be considered a negative effect on the immune system, the resulting inhibition of IRF3 signaling inhibits at least two mechanisms of metastatic tumor development. Further detailed studies are required to elucidate the complete effects of PEITC on immune system.
4.2.9. Effect of PEITC on cancer cell energetics and metabolism
Cancer cells have a high demand of ATP, which is fulfilled by higher glycolysis rates, a phenomenon known as Warburg’s effect. The discovery of Warburg’s effect in cancer cells has given new insight into cancer cell-specific biological pathways along with the possibility of specific novel targets. Agents that can inhibit or reverse this metabolic switch can be pivotal in the advancement of cancer therapeutics with reduced side effects and improved efficacy. A recent study showed reduced rates of glycolysis in PEITC-treated cells and depletion of ATP lead to death in prostate cancer cells [165]. The study showed increased glycolysis and lactic acid production in cancer cells, as measured through estimation of oxygen consumption rate and extracellular acidification rate, respectively. PEITC (5μM) treatment suppressed glycolysis in the cancer cells, but no changes were observed in normal cells. In addition, reduced concentration of ATP was also observed in cancer cells. Although this study provides an in vitro evidence of PEITC mediated inhibition of glycolysis, it is important to confirm these observations in animal models as well as in other cancer types.
4.3 Reactive Oxygen Species, a key mechanism of PEITC’s anti-cancer effects
Reactive oxygen species (ROS) have a dual role inside cells. In normal cells, ROS production causes DNA damage that drives cells toward apoptosis. Conversely, in cancer cells, ROS promotes cell survival by inducing several survival pathways responsible for cell proliferation, apoptosis suppression, cell migration and invasion, as well as suppression of the immune system. Cancer cells generate higher ROS levels due to increased oxidative metabolism and shortage of nutrient supply. However, increasingly high ROS levels can be toxic to cancer cells and can induce cell death. The levels and duration of ROS determine the final outcome (survival or death) of ROS present in cancer cells. In other words, ROS has to cross a threshold to induce apoptosis. The threshold is usually low in cancer cells as compared to normal cells, probably due to consistently high ROS levels. This difference provides an opportunity to target cancer cells by increasing ROS generation to a level that becomes toxic to cancer cells.
Interestingly, isothiocyanates were originally identified as natural antioxidants that can reduce ROS levels to serve as a chemo-preventive component of the diet [166–168]. The antioxidant effect is achieved at very low ITC levels in normal cells as shown in various animal models. At higher concentrations, ITCs may generate ROS by depleting antioxidant levels. PEITC is known to cause ROS generation, which is the major mechanism of toxicity in cancer cells [107, 119, 138]. Several mechanisms have been explained for PEITC-induced ROS. There is a continuous leakage of electrons from the electron transport chain (ETC), which is major source of ROS production. PEITC causes generation of endogenous ROS by disrupting mitochondrial respiratory chain and PEITC-mediated degradation of NADH dehydrogenase Fe-S subunit 3 inhibits complex I functioning [116, 169]. In addition, PEITC also inhibits mitochondrial complex III activity and reduces the oxygen consumption rate in prostate cancer cells [165]. Another study showed that PEITC treatment induced the growth factor adaptor protein, p66Shc, as a mechanism of ROS generation in cancer cells [170]. These outcomes were observed in the in vitro study using prostate cancer cells. However, no studies have yet been performed to confirm these effects in vivo.
Another established mechanism to induce ROS toxicity is by the inhibition of ROS detoxification of the cell. Each cell has well-developed mechanisms to protect against ROS-induced damage. Primary ROS scavenging mechanisms include dismutation of superoxide anion to oxygen and H2O2. Further, hydrogen peroxide is converted into water by glutathione peroxidase (GPX), or to oxygen and water by the enzymatic action of catalase. Glutathione (GSH) is a substrate for GPX. Levels of GSSG (oxidized form) and GSH (reduced form) reflect the redox status of cells. PEITC binds to GSH and causes its depletion in cancer cells leading to ROS-induced cell damage [107, 165, 169]. Interestingly, the sensitivity towards PEITC correlates with constitutive GSH levels present in the cells [171, 172]. The cells with higher levels of GSH were relatively more sensitive to PEITC, which may also explain the selectivity toward cancer cells compared with normal cells, since cancer cells have higher levels of GSH in normal cells.
Based on the available data, it is well-established that PEITC acts as a pro-oxidant to initiate ROS generation, leading to apoptosis in cell culture models. However, according to a recently published article, resultant modulation of ROS from an in vitro study can vary significantly from an in vivo study with the same agent [173]. Thus, outcomes based on the in vitro studies may be debatable. Hence, it is crucial to confirm ROS generation by PEITC and its manifestations in an in vivo model, along with the mechanisms involved. In another study, capsaicin treatment was shown to increase ROS levels in implanted pancreatic tumors, suggesting the role of ROS in tumor suppression. This action, along with a decrease in SOD activity and an increase in GSSG/GSH ratio in the tumors, correlated with the overall tumor growth suppression [174].
4.4 Direct protein modification by PEITC
Isothiocyanates are chemically characterized by N=C=S, which imparts electrophilic properties to these compounds. As reviewed by Mi et. al., the electrophilic property of PEITC leads to covalent interaction with nucleophilic entities like DNA, RNA, or critical amino acids of proteins and peptides [114]. Although, DNA and RNA were not the direct targets, PEITC was found to bind directly to proteins [120, 175]. PEITC modifies the functionality of several proteins by covalently binding with their nucleophilic amino acids. Some of the most important target proteins/peptides of PEITC include glutathione via sulfhydryl group, Cyp450 and tubulin via cysteine and macrophage migration inhibitory factor via proline [120, 176–178]. The modulation of proteins undoubtedly affects several cellular processes leading to cell growth suppression.
4.5 Cancer Specific Biomarkers
Cancer occurs due to genetic abnormalities. Several identified cancer-specific genetic changes are individual-specific. These genetic signatures demand individualized treatment strategies for patients, resulting in better therapeutic outcomes. For example, mutations in BRCA1 and/or BRCA2 account for 5–10% of breast cancer and 10–15% of ovarian cancer. Another major gene mutation known to enhance risk of any cancer significantly is p53. Interestingly, PEITC is beneficial in these cancers and has been shown to increase the expression of BRCA2 along with induction of tumor suppressors, such as p53 and p57 [123]. In addition, aberrant expression of several other genes is known to be responsible for poor prognosis in cancer. An example of this phenomenon is HER2 and EGFR overexpression, which leads to poor prognosis in lung, breast and ovarian cancers. PEITC works exceptionally well in suppressing EGFR, HER2 and their oncogenic manifestations [102, 117, 119, 141]. PEITC has been shown to induce apoptosis in diverse cancer cell lines with varying levels of HER2. Integrins have recently been identified as important therapeutic targets in various cancer types. PEITC was found to inhibit major integrins, such as ITGB1, ITGA2 and ITGA6 in prostate cancer cells [179]. Based on the available data on PEITC on cancer specific biomarkers, its application can be streamlined for personalized treatment options.
4.6 miRNAs
miRNAs are small single-stranded RNA molecules that can regulate the function and expression of various genes. miRNAs have recently been identified to be of significant importance due to their role in tumorigenesis. miRNAs commonly act as tumor suppressors, but a few miRNAs that are overexpressed and cause tumorigenesis also have been identified. An overall loss of miRNAs has been detected in human cancers, suggesting mostly tumor suppressor effects. To date, very few studies have been performed to test miRNA modulation by PEITC. Izzotti et. al. demonstrated the chemopreventive effects of PEITC, using environmental cigarette smoke (ECS)-induced miRNA modulations in rat lungs and liver [153, 180, 181]. Interestingly, it was observed that miRNAs were more susceptible to changes induced by ECS as compared to proteins. In another study, 484 miRNAs were analyzed in rat lungs exposed with ECS. About 25 miRNAs were modulated significantly by ECS. However, combination treatment with PEITC and indole 3-carbinol reversed the effects of ECS on most of these miRNAs [181]. PEITC alone or in combination with indole 3-carbinol reversed the effects of ECS in rat lungs, but the combination had a much stronger effect. The combination reversed the majority of miRNAs down-regulated by ECS, which were involved in multiple processes related to cancer progression, such as angiogenesis, cell proliferation and stress response. A similar study was performed on mouse lungs and liver, where 576 miRNAs were analyzed after exposure to ECS [180]. The effect of PEITC on miRNAs modulated by ECS was more pronounced in liver as compared to lungs. A plausible reason for this phenomenon could be the first pass effect of liver, as suggested by the authors. Conaway et. al. showed significantly higher concentrations of PEITC achieved in liver as compared to lungs, also suggesting enhanced effect in liver than lungs [182]. Furthermore, another group observed that PEITC caused miR-17 mediated suppression of p300/CBP-associated factor (PCAF), a co-regulator for androgen receptors (AR) leading to inhibition AR in prostate cancer cells [183]. Jutooru et. al. showed the modulation of miR-27a, miR-20a and miR-17-5p by PEITC in pancreatic cancer cells, leading to apoptosis [184]. One result of reduction in some tumor suppressor miRNAs is reduction in ROS (Croce, personal communication). Taken together these observations confirm that a number of miRNAs are modulated by PEITC. However, additional animal studies are required to delineate the implications of these changes in chemoprevention or chemotherapeutics.
4.7 Stem cells
Cancer stem cells (CSC) are considered to be the central element in the process of tumorigenesis. Furthermore, stem cells are resistant to many agents used to kill cancer cells. Although intensive research in this field is ongoing, in-depth knowledge on CSCs is still limited. Based on increasing evidence, the role of CSCs as therapeutic targets cannot be ignored. There is still lack of evidence for direct activity of PEITC on cancer stem cells [109, 111]. This probably can be due to lack of precise techniques for isolation and characterization of cancer stem cells. Indirect studies indicate the effects of PEITC on stem cells. Wu et. al. demonstrated reversal of resistance by PEITC in platinum resistant cells, which are known to have stem cell properties [155]. Furthermore, PEITC inhibits developmental genes in the embryonic stem cells, suggesting its effects on stem cells development. However, this also raises a concern of potential toxicity during embryonic development [185]. The clear mechanistic evidence for effects of PEITC on the growth and proliferation of CSCs is still lacking.
4.8 Epigenetics
Epigenetic modulations have recently gained a significant importance in cancer therapy. Many new agents are being identified that can alter gene expression without causing changes in gene sequence. Although gene array data showed that PEITC caused modulation of gene expression, it is now well known for epigenetic modulations in cancer cells [123, 186–188]. As discussed in sections 4.4, PEITC has the capability to bind directly with proteins to alter their function. Moreover, PEITC also affects a number of miRNAs which can play a critical role in cancer progression (section 4.6). As reviewed by Wang et. al., PEITC acts as an inhibitor of CpG methylation and histone deacetylation to manipulate gene expression [187]. Liu et. al. demonstrated that epigenetic changes like histone tail modifications occur at low non-cytotoxic concentrations of PEITC, which can play an important role in chemoprevention [189]. Furthermore, two other studies show synergism of PEITC with paclitaxel through epigenetic mechanisms [190, 191]. The hyperacetylation of α-tubulin induced by the combination of PEITC and paclitaxel was identified as the major mechanism of synergism in cancer cells. PEITC also modulates proteins like heat shock proteins (HSP) and proteases, which play a pivotal role in protein maturation and folding. For example, inhibition of HSP90, 70, 60, GRP78, ADAM 17 or topoisomerase IIα by PEITC results in epigenetic modifications [114, 121, 122, 124, 175, 192–194]. In line with this, PEITC-induced post-translational modification was observed in p53 mutant lung cancer cells, but the precise mechanism was not clear [195].
5.0 Combination therapy with PEITC
Drug resistance is a major obstacle in successful cancer therapeutics leading to poor clinical outcome. The therapy failure can occur due to different mechanisms, including reduced drug uptake, increased efflux of drug, induction of anti-apoptotic mechanism, inactivation of the drug through metabolic enzymes or reversal of DNA damage by induction of DNA repair pathways. As discussed earlier in this article, PEITC has been observed to modify several of these mechanisms. The outcomes of the studies where PEITC was used to potentiate the effects of conventional anti-cancer drugs or new anti-cancer agents have been detailed in our previous review [196]. The proposed mechanisms by which PEITC enhances the effects of other agents or drugs are: reduction in the availability of GSH for conjugation leading to efflux and inactivation, inhibition of drug transporter proteins like MRP and PgP, hence improving drug availability to the cells and induction of pro-apoptotic pathways to counteract anti-apoptotic mechanisms. Using pre-clinical studies, improved outcomes were observed when the conventional agents, such as docetaxel, metformin, vinblastine, doxorubicin and HDAC inhibitors were combined with PEITC [102, 165, 197, 198] (Table 4). PEITC treatment sensitized glioma cells to TRAIL via ROS generation [199]. Interestingly, cisplatin also showed synergism with PEITC in cervical and breast cancer cells, which was mediated by modulation of MAPkinases, NFkB and death receptors [200]. PEITC treatment also reversed resistance to cisplatin in gastric cancer cells by inhibition of transporter proteins PgP and MRP1[201]. Considering the above facts, it can be concluded that PEITC can potentiate the effects of classical chemotherapeutic agents and therefore could be beneficial for drug-resistant cancers.
6.0 PEITC and tobacco smoke induced changes
Most of the initial studies used chemicals present in tobacco smoke induced tumor models to demonstrate efficacy of PEITC against lung tumors. Studies have shown that dietary administration of PEITC significantly reduces tumor incidence induced by 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) and benzoypyrene (BaP) in mouse models [202–204]. Importantly, these agents are the major carcinogens of the tobacco smoke. Interestingly, studies showed PEITC mediated inhibition of the metabolic activation of these carcinogens leading to reduced tumorigenesis [205]. Furthermore, studies also showed that PEITC inhibits the formation of 4-hydroxy-1-(3-pyridyl)-1-butanone (HPB) releasing DNA adducts of NNK and BaP [206–209]. The inhibition of adduct formation was observed in whole lung tissue and in lung cells except macrophages [209]. Recently, various studies also showed stronger chemopreventive effects of PEITC in combination with myoinositol or aspirin, in NNK and BaP-induced lung tumor models [210–212]. Interestingly, PEITC suppressed cell proliferation in cigarette smoke treated human bronchial epithelial cells (HBEC) as well as lung cancer A549 cells but not in DMSO treated HBEC cells [210]. Furthermore, administration of dietary PEITC also reduced the DNA damage and molecular changes caused by cigarette smoke, along with reduction in tumor incidence in lung tumor model [213–215]. Taken together, these observations demonstrate potentially strong anti-cancer effects of PEITC for smokers and tobacco consumers.
7.0 Clinical advancement
Copious evidence from preclinical studies indicates efficacy of PEITC against cancer and supports its further development for clinical applications. Interestingly, four clinical studies for human testing of PEITC were initiated, out of which one study (NCT00968461) was withdrawn for unexplained reasons. Out of three, one study (NCT00005883) has been completed with a goal of multi-dose testing of PEITC in lung cancer patients. Although this phase I study is completed, the results have not been published yet. Another Phase I clinical study (NCT01790204) is planned at Georgetown University, with the goal of testing the beneficial effects of PEITC in oral carcinoma with mutant p53. A Phase I study (NCI CN-55120) has confirmed that after oral intake of 200mg PEITC by human volunteers, 10μM PEITC can be achieved in plasma, as mentioned by Mi et. al. [216]. Interestingly, many studies have shown the anti-cancer efficacy of PEITC at concentrations less than 10μM. The Masonic Cancer Center at the University of Minnesota, in collaboration with National Cancer Institute, is currently conducting a phase II study with PEITC in lung cancer patients. The results of these studies will provide a clear idea of the efficacy and toxicity of PEITC in humans and will be instrumental in designing plans for further development of PEITC.
8.0 Summary
PEITC is a multifaceted agent that targets multiple processes required for cancer growth and development. It modulates many proteins to suppress survival and proliferation of cancer cells. Identification of specific targets paved ways to develop individualized patient treatment strategies. In addition, modification of proteins through covalent binding with PEITC opens avenues to develop this compound based on its affinity or selectivity towards specific cellular proteins. Based on the mechanisms of action of PEITC, such as ROS generation and inhibition of cell growth with mutant p53, there is significant evidence to demonstrate selectivity of PEITC towards cancer cells. Organ selective effects of PEITC are also evident by studies showing enhanced modulation of enzymes, protein targets and miRNAs observed in liver as compared to lungs, probably explained by the fact that PEITC levels achieved in liver were significantly higher than in lungs. PEITC exhibits strong anti-migratory and anti-invasive effects in different cancer forms, suggesting its anti-metastatic potential. Current evidence on these aspects relies mainly on in vitro studies. Due to the lack of ideal animal models, in vivo evidence is limited to breast cancer. Combination therapeutics is a common mode of intervention to circumvent problems due to drug resistance. Although, some evidence exists for increased efficacy of conventional drugs when used in combination with PEITC, major advancement is still awaited in this area. Moreover, being a natural compound, PEITC is not expected to present any severe toxicity, unlike many traditional anti-cancer agents. However, due to the likelihood of drug interactions as a result of its effects on drug metabolizing and detoxifying enzymes, more evidence is required for its practical clinical application in patients. Nonetheless, phase I and phase II clinical trials are in process. At the same time, missing information in the areas mentioned above is needed to fill in the gaps in our knowledge. Nonetheless, broad spectrum of PEITC justifies the rationale for its clinical development.
Acknowledgments
The work was supported in part by R01 grants CA106953 and CA129038 (to S.K.S.) awarded by the National Cancer Institute, NIH and MRC grant 2007-0054931 (to S.H.K.). Dr. Sanjay K. Srivastava is currently an International Scholar at Kyung Hee University, Seoul, South Korea.
Abbreviations
- BaP
Benzo(a)pyrene
- BCRP
Breast Cancer Resistance Protein
- CSC
Cancer Stem cells
- ECS
Environmental Cigarette Smoke
- GPX
Glutathione peroxidase
- GSH
Glutathione
- GST
Glutathione S-transferase
- HBEC
Human Bronchial Epithelial Cells
- HBP
4-hydroxy-1-(3-pyridyl)-1-butanone
- ITC
Isothiocyanate
- NAC
N-acetyl cysteine
- NNK
4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone
- PEITC
Phenethylisothiocyanate
- Rb
Retinoblastoma protein
- ROS
Reactive oxygen species
- DDB2
DNA-damage binding protein 2
Footnotes
Conflict of Interest
Authors declare that there are no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Newman DJ, Cragg GM. Natural products as sources of new drugs over the 30 years from 1981 to 2010. Journal of Natural Products. 2012;75:311–335. doi: 10.1021/np200906s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 3.Higdon JV, Delage B, Williams DE, Dashwood RH. Cruciferous vegetables and human cancer risk: epidemiologic evidence and mechanistic basis. Pharmacological Research. 2007;55:224–236. doi: 10.1016/j.phrs.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boggs DA, Palmer JR, Wise LA, Spiegelman D, Stampfer MJ, Adams-Campbell LL, Rosenberg L. Fruit and vegetable intake in relation to risk of breast cancer in the Black Women’s Health Study. American Journal of Epidemiology. 2010;172:1268–1279. doi: 10.1093/aje/kwq293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tang L, Zirpoli GR, Guru K, Moysich KB, Zhang Y, Ambrosone CB, McCann SE. Intake of cruciferous vegetables modifies bladder cancer survival. Cancer Epidemiology, Biomarkers & Prevention. 2010;19:1806–1811. doi: 10.1158/1055-9965.EPI-10-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Palmer S. Diet, nutrition, and cancer. Progress in Food & Nutrition Science. 1985;9:283–341. [PubMed] [Google Scholar]
- 7.Verhoeven DT, Goldbohm RA, van Poppel G, Verhagen H, van den Brandt PA. Epidemiological studies on brassica vegetables and cancer risk. Cancer Epidemiology, Biomarkers & Prevention. 1996;5:733–748. [PubMed] [Google Scholar]
- 8.Moy KA, Yuan JM, Chung FL, Wang XL, Van Den Berg D, Wang R, Gao YT, Yu MC. Isothiocyanates, glutathione S-transferase M1 and T1 polymorphisms and gastric cancer risk: a prospective study of men in Shanghai, China. International Journal of Cancer. 2009;125:2652–2659. doi: 10.1002/ijc.24583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carpenter CL, Yu MC, London SJ. Dietary isothiocyanates, glutathione S-transferase M1 (GSTM1), and lung cancer risk in African Americans and Caucasians from Los Angeles County, California. Nutrition and Cancer. 2009;61:492–499. doi: 10.1080/01635580902752270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Truong T, Baron-Dubourdieu D, Rougier Y, Guenel P. Role of dietary iodine and cruciferous vegetables in thyroid cancer: a countrywide case-control study in New Caledonia. Cancer Causes & Control. 2010;21:1183–1192. doi: 10.1007/s10552-010-9545-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lam TK, Ruczinski I, Helzlsouer KJ, Shugart YY, Caulfield LE, Alberg AJ. Cruciferous vegetable intake and lung cancer risk: a nested case-control study matched on cigarette smoking. Cancer Epidemiology, Biomarkers & Prevention. 2010;19:2534–2540. doi: 10.1158/1055-9965.EPI-10-0475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu QJ, Yang Y, Vogtmann E, Wang J, Han LH, Li HL, Xiang YB. Cruciferous vegetables intake and the risk of colorectal cancer: a meta-analysis of observational studies. Annals of Oncology. 2013;24:1079–1087. doi: 10.1093/annonc/mds601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu QJ, Xie L, Zheng W, Vogtmann E, Li HL, Yang G, Ji BT, Gao YT, Shu XO, Xiang YB. Cruciferous vegetables consumption and the risk of female lung cancer: a prospective study and a meta-analysis. Annals of Oncology. 2013;24:1918–1924. doi: 10.1093/annonc/mdt119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee SA, Fowke JH, Lu W, Ye C, Zheng Y, Cai Q, Gu K, Gao YT, Shu XO, Zheng W. Cruciferous vegetables, the GSTP1 Ile105Val genetic polymorphism, and breast cancer risk. The American Journal of Clinical Nutrition. 2008;87:753–760. doi: 10.1093/ajcn/87.3.753. [DOI] [PubMed] [Google Scholar]
- 15.Moore LE, Brennan P, Karami S, Hung RJ, Hsu C, Boffetta P, Toro J, Zaridze D, Janout V, Bencko V, Navratilova M, Szeszenia-Dabrowska N, Mates D, Mukeria A, Holcatova I, Welch R, Chanock S, Rothman N, Chow WH. Glutathione S-transferase polymorphisms, cruciferous vegetable intake and cancer risk in the Central and Eastern European Kidney Cancer Study. Carcinogenesis. 2007;28:1960–1964. doi: 10.1093/carcin/bgm151. [DOI] [PubMed] [Google Scholar]
- 16.Spitz MR, Duphorne CM, Detry MA, Pillow PC, Amos CI, Lei L, de Andrade M, Gu X, Hong WK, Wu X. Dietary intake of isothiocyanates: evidence of a joint effect with glutathione S-transferase polymorphisms in lung cancer risk. Cancer Epidemiology, Biomarkers & Prevention. 2000;9:1017–1020. [PubMed] [Google Scholar]
- 17.Zhao B, Seow A, Lee EJ, Poh WT, Teh M, Eng P, Wang YT, Tan WC, Yu MC, Lee HP. Dietary isothiocyanates, glutathione S-transferase -M1, -T1 polymorphisms and lung cancer risk among Chinese women in Singapore. Cancer Epidemiology, Biomarkers & Prevention. 2001;10:1063–1067. [PubMed] [Google Scholar]
- 18.Seow A, Poh WT, Teh M, Eng P, Wang YT, Tan WC, Chia KS, Yu MC, Lee HP. Diet, reproductive factors and lung cancer risk among Chinese women in Singapore: evidence for a protective effect of soy in nonsmokers. International Journal of Cancer. 2002;97:365–371. doi: 10.1002/ijc.1615. [DOI] [PubMed] [Google Scholar]
- 19.Hara M, Hanaoka T, Kobayashi M, Otani T, Adachi HY, Montani A, Natsukawa S, Shaura K, Koizumi Y, Kasuga Y, Matsuzawa T, Ikekawa T, Sasaki S, Tsugane S. Cruciferous vegetables, mushrooms, and gastrointestinal cancer risks in a multicenter, hospital-based case-control study in Japan. Nutrition and Cancer. 2003;46:138–147. doi: 10.1207/S15327914NC4602_06. [DOI] [PubMed] [Google Scholar]
- 20.Giovannucci E, Rimm EB, Liu Y, Stampfer MJ, Willett WC. A prospective study of cruciferous vegetables and prostate cancer. Cancer Epidemiology, Biomarkers & Prevention. 2003;12:1403–1409. [PubMed] [Google Scholar]
- 21.Wang LI, Giovannucci EL, Hunter D, Neuberg D, Su L, Christiani DC. Dietary intake of Cruciferous vegetables, Glutathione S-transferase (GST) polymorphisms and lung cancer risk in a Caucasian population. Cancer Causes & Control. 2004;15:977–985. doi: 10.1007/s10552-004-1093-1. [DOI] [PubMed] [Google Scholar]
- 22.Raghavan M, Knapp DW, Bonney PL, Dawson MH, Glickman LT. Evaluation of the effect of dietary vegetable consumption on reducing risk of transitional cell carcinoma of the urinary bladder in Scottish Terriers. Journal of the American Veterinary Medical Association. 2005;227:94–100. doi: 10.2460/javma.2005.227.94. [DOI] [PubMed] [Google Scholar]
- 23.Zhao H, Lin J, Grossman HB, Hernandez LM, Dinney CP, Wu X. Dietary isothiocyanates, GSTM1, GSTT1, NAT2 polymorphisms and bladder cancer risk. International Journal of Cancer. 2007;120:2208–2213. doi: 10.1002/ijc.22549. [DOI] [PubMed] [Google Scholar]
- 24.Tang L, Zirpoli GR, Guru K, Moysich KB, Zhang Y, Ambrosone CB, McCann SE. Consumption of raw cruciferous vegetables is inversely associated with bladder cancer risk. Cancer Epidemiology Biomarkers & Prevention. 2008;17:938–944. doi: 10.1158/1055-9965.EPI-07-2502. [DOI] [PubMed] [Google Scholar]
- 25.Thomson CA, Rock CL, Caan BJ, Flatt SW, Al-Delaimy WA, Newman VA, Hajek RA, Chilton JA, Pierce JP. Increase in cruciferous vegetable intake in women previously treated for breast cancer participating in a dietary intervention trial. Nutrition and Cancer. 2007;57:11–19. doi: 10.1080/01635580701267875. [DOI] [PubMed] [Google Scholar]
- 26.Yuan JM, Gago-Dominguez M, Castelao JE, Hankin JH, Ross RK, Yu MC. Cruciferous vegetables in relation to renal cell carcinoma. International Journal of Cancer. 1998;77:211–216. doi: 10.1002/(sici)1097-0215(19980717)77:2<211::aid-ijc7>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 27.Nechuta S, Caan BJ, Chen WY, Kwan ML, Lu W, Cai H, Poole EM, Flatt SW, Zheng W, Pierce JP, Shu XO. Postdiagnosis cruciferous vegetable consumption and breast cancer outcomes: a report from the After Breast Cancer Pooling Project. Cancer Epidemiology, Biomarkers & Prevention. 2013;22:1451–1456. doi: 10.1158/1055-9965.EPI-13-0446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Health and Behavior: The Interplay of Biological, Behavioral, and Societal Influences. Washington (DC): 2001. [PubMed] [Google Scholar]
- 29.Shapiro TA, Fahey JW, Wade KL, Stephenson KK, Talalay P. Chemoprotective glucosinolates and isothiocyanates of broccoli sprouts: metabolism and excretion in humans. Cancer Epidemiology, Biomarkers & Prevention. 2001;10:501–508. [PubMed] [Google Scholar]
- 30.Shapiro TA, Fahey JW, Wade KL, Stephenson KK, Talalay P. Human metabolism and excretion of cancer chemoprotective glucosinolates and isothiocyanates of cruciferous vegetables. Cancer Epidemiology, Biomarkers & Prevention. 1998;7:1091–1100. [PubMed] [Google Scholar]
- 31.Getahun SM, Chung FL. Conversion of glucosinolates to isothiocyanates in humans after ingestion of cooked watercress. Cancer Epidemiology, Biomarkers & Prevention. 1999;8:447–451. [PubMed] [Google Scholar]
- 32.Van Eylen D, Oey I, Hendrickx M, Van Loey A. Kinetics of the stability of broccoli (Brassica oleracea Cv. Italica) myrosinase and isothiocyanates in broccoli juice during pressure/temperature treatments. Journal of Agricultural and Food Chemistry. 2007;55:2163–2170. doi: 10.1021/jf062630b. [DOI] [PubMed] [Google Scholar]
- 33.Chung FL, Morse MA, Eklind KI, Lewis J. Quantitation of human uptake of the anticarcinogen phenethyl isothiocyanate after a watercress meal. Cancer Epidemiology, Biomarkers & Prevention. 1992;1:383–388. [PubMed] [Google Scholar]
- 34.Hecht SS, Chung FL, Richie JP, Jr, Akerkar SA, Borukhova A, Skowronski L, Carmella SG. Effects of watercress consumption on metabolism of a tobacco-specific lung carcinogen in smokers. Cancer Epidemiology, Biomarkers & Prevention. 1995;4:877–884. [PubMed] [Google Scholar]
- 35.Ji Y, Kuo Y, Morris ME. Pharmacokinetics of dietary phenethyl isothiocyanate in rats. Pharmaceutical Research. 2005;22:1658–1666. doi: 10.1007/s11095-005-7097-z. [DOI] [PubMed] [Google Scholar]
- 36.Konsue N, Kirkpatrick J, Kuhnert N, King LJ, Ioannides C. Repeated oral administration modulates the pharmacokinetic behavior of the chemopreventive agent phenethyl isothiocyanate in rats. Molecular Nutrition & Food Research. 2010;54:426–432. doi: 10.1002/mnfr.200900090. [DOI] [PubMed] [Google Scholar]
- 37.Ji Y, Morris ME. Determination of phenethyl isothiocyanate in human plasma and urine by ammonia derivatization and liquid chromatography-tandem mass spectrometry. Analytical Biochemistry. 2003;323:39–47. doi: 10.1016/j.ab.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 38.Wu X, Zhou QH, Xu K. Are isothiocyanates potential anti-cancer drugs? Acta Pharmacologica Sinica. 2009;30:501–512. doi: 10.1038/aps.2009.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morris ME, Dave RA. Pharmacokinetics and Pharmacodynamics of Phenethyl Isothiocyanate: Implications in Breast Cancer Prevention. The AAPS Journal. 2014 doi: 10.1208/s12248-014-9610-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seow A, Shi CY, Chung FL, Jiao D, Hankin JH, Lee HP, Coetzee GA, Yu MC. Urinary total isothiocyanate (ITC) in a population-based sample of middle-aged and older Chinese in Singapore: relationship with dietary total ITC and glutathione S-transferase M1/T1/P1 genotypes. Cancer Epidemiology, Biomarkers & Prevention. 1998;7:775–781. [PubMed] [Google Scholar]
- 41.London SJ, Yuan JM, Chung FL, Gao YT, Coetzee GA, Ross RK, Yu MC. Isothiocyanates, glutathione S-transferase M1 and T1 polymorphisms, and lung-cancer risk: a prospective study of men in Shanghai, China. Lancet. 2000;356:724–729. doi: 10.1016/S0140-6736(00)02631-3. [DOI] [PubMed] [Google Scholar]
- 42.Lin HJ, Probst-Hensch NM, Louie AD, Kau IH, Witte JS, Ingles SA, Frankl HD, Lee ER, Haile RW. Glutathione transferase null genotype, broccoli, and lower prevalence of colorectal adenomas. Cancer Epidemiology, Biomarkers & Prevention. 1998;7:647–652. [PubMed] [Google Scholar]
- 43.Seow A, Yuan JM, Sun CL, Van Den Berg D, Lee HP, Yu MC. Dietary isothiocyanates, glutathione S-transferase polymorphisms and colorectal cancer risk in the Singapore Chinese Health Study. Carcinogenesis. 2002;23:2055–2061. doi: 10.1093/carcin/23.12.2055. [DOI] [PubMed] [Google Scholar]
- 44.Ambrosone CB, McCann SE, Freudenheim JL, Marshall JR, Zhang Y, Shields PG. Breast cancer risk in premenopausal women is inversely associated with consumption of broccoli, a source of isothiocyanates, but is not modified by GST genotype. The Journal of Nutrition. 2004;134:1134–1138. doi: 10.1093/jn/134.5.1134. [DOI] [PubMed] [Google Scholar]
- 45.Steck SE, Gammon MD, Hebert JR, Wall DE, Zeisel SH. GSTM1, GSTT1, GSTP1, and GSTA1 polymorphisms and urinary isothiocyanate metabolites following broccoli consumption in humans. The Journal of Nutrition. 2007;137:904–909. doi: 10.1093/jn/137.4.904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Steck SE, Gaudet MM, Britton JA, Teitelbaum SL, Terry MB, Neugut AI, Santella RM, Gammon MD. Interactions among GSTM1, GSTT1 and GSTP1 polymorphisms, cruciferous vegetable intake and breast cancer risk. Carcinogenesis. 2007;28:1954–1959. doi: 10.1093/carcin/bgm141. [DOI] [PubMed] [Google Scholar]
- 47.Lam TK, Gallicchio L, Lindsley K, Shiels M, Hammond E, Tao XG, Chen L, Robinson KA, Caulfield LE, Herman JG, Guallar E, Alberg AJ. Cruciferous vegetable consumption and lung cancer risk: a systematic review. Cancer Epidemiology, Biomarkers & Prevention. 2009;18:184–195. doi: 10.1158/1055-9965.EPI-08-0710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Epplein M, Wilkens LR, Tiirikainen M, Dyba M, Chung FL, Goodman MT, Murphy SP, Henderson BE, Kolonel LN, Le Marchand L. Urinary isothiocyanates; glutathione S-transferase M1, T1, and P1 polymorphisms; and risk of colorectal cancer: the Multiethnic Cohort Study. Cancer Epidemiology, Biomarkers & Prevention. 2009;18:314–320. doi: 10.1158/1055-9965.EPI-08-0627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Riso P, Brusamolino A, Moro M, Porrini M. Absorption of bioactive compounds from steamed broccoli and their effect on plasma glutathione S-transferase activity. International Journal of Food Sciences and Nutrition. 2009;60(Suppl 1):56–71. doi: 10.1080/09637480802089751. [DOI] [PubMed] [Google Scholar]
- 50.Yang G, Gao YT, Shu XO, Cai Q, Li GL, Li HL, Ji BT, Rothman N, Dyba M, Xiang YB, Chung FL, Chow WH, Zheng W. Isothiocyanate exposure, glutathione S-transferase polymorphisms, and colorectal cancer risk. The American Journal of Clinical Nutrition. 2010;91:704–711. doi: 10.3945/ajcn.2009.28683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dyba M, Wang A, Noone AM, Goerlitz D, Shields P, Zheng YL, Rivlin R, Chung FL. Metabolism of isothiocyanates in individuals with positive and null GSTT1 and M1 genotypes after drinking watercress juice. Clinical Nutrition. 2010;29:813–818. doi: 10.1016/j.clnu.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vos RM, Snoek MC, van Berkel WJ, Muller F, van Bladeren PJ. Differential induction of rat hepatic glutathione S-transferase isoenzymes by hexachlorobenzene and benzyl isothiocyanate. Comparison with induction by phenobarbital and 3-methylcholanthrene. Biochemical Pharmacology. 1988;37:1077–1082. doi: 10.1016/0006-2952(88)90513-8. [DOI] [PubMed] [Google Scholar]
- 53.Hara A, Sakai N, Yamada H, Tanaka T, Kato K, Mori H, Sato K. Induction of glutathione S-transferase, placental type in T9 glioma cells by dibutyryladenosine 3′,5′-cyclic monophosphate and modification of its expression by naturally occurring isothiocyanates. Acta Neuropathologica. 1989;79:144–148. doi: 10.1007/BF00294371. [DOI] [PubMed] [Google Scholar]
- 54.Sugie S, Okumura A, Tanaka T, Mori H. Inhibitory effects of benzyl isothiocyanate and benzyl thiocyanate on diethylnitrosamine-induced hepatocarcinogenesis in rats. Japanese Journal of Cancer Research. 1993;84:865–870. doi: 10.1111/j.1349-7006.1993.tb02059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kolm RH, Danielson UH, Zhang Y, Talalay P, Mannervik B. Isothiocyanates as substrates for human glutathione transferases: structure-activity studies. The Biochemical Journal. 1995;311(Pt 2):453–459. doi: 10.1042/bj3110453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Meyer DJ, Crease DJ, Ketterer B. Forward and reverse catalysis and product sequestration by human glutathione S-transferases in the reaction of GSH with dietary aralkyl isothiocyanates. The Biochemical Journal. 1995;306(Pt 2):565–569. doi: 10.1042/bj3060565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van Lieshout EM, Peters WH, Jansen JB. Effect of oltipraz, alpha-tocopherol, beta-carotene and phenethylisothiocyanate on rat oesophageal, gastric, colonic and hepatic glutathione, glutathione S-transferase and peroxidase. Carcinogenesis. 1996;17:1439–1445. doi: 10.1093/carcin/17.7.1439. [DOI] [PubMed] [Google Scholar]
- 58.Ogawa K, Futakuchi M, Hirose M, Boonyaphiphat P, Mizoguchi Y, Miki T, Shirai T. Stage and organ dependent effects of 1-O-hexyl-2,3,5-trimethylhydroquinone, ascorbic acid derivatives, n-heptadecane-8-10-dione and phenylethyl isothiocyanate in a rat multiorgan carcinogenesis model. International Journal of Cancer. 1998;76:851–856. doi: 10.1002/(sici)1097-0215(19980610)76:6<851::aid-ijc14>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 59.Nakamura Y, Morimitsu Y, Uzu T, Ohigashi H, Murakami A, Naito Y, Nakagawa Y, Osawa T, Uchida K. A glutathione S-transferase inducer from papaya: rapid screening, identification and structure-activity relationship of isothiocyanates. Cancer Letters. 2000;157:193–200. doi: 10.1016/s0304-3835(00)00487-0. [DOI] [PubMed] [Google Scholar]
- 60.McWalter GK, Higgins LG, McLellan LI, Henderson CJ, Song L, Thornalley PJ, Itoh K, Yamamoto M, Hayes JD. Transcription factor Nrf2 is essential for induction of NAD(P)H:quinone oxidoreductase 1, glutathione S-transferases, and glutamate cysteine ligase by broccoli seeds and isothiocyanates. The Journal of Nutrition. 2004;134:3499S–3506S. doi: 10.1093/jn/134.12.3499S. [DOI] [PubMed] [Google Scholar]
- 61.Hofmann T, Kuhnert A, Schubert A, Gill C, Rowland IR, Pool-Zobel BL, Glei M. Modulation of detoxification enzymes by watercress: in vitro and in vivo investigations in human peripheral blood cells. European Journal of Nutrition. 2009;48:483–491. doi: 10.1007/s00394-009-0039-5. [DOI] [PubMed] [Google Scholar]
- 62.Sharma R, Ellis B, Sharma A. Role of alpha class glutathione transferases (GSTs) in chemoprevention: GSTA1 and A4 overexpressing human leukemia (HL60) cells resist sulforaphane and curcumin induced toxicity. Phytotherapy Research. 2011;25:563–568. doi: 10.1002/ptr.3297. [DOI] [PubMed] [Google Scholar]
- 63.Krajka-Kuzniak V, Szaefer H, Bartoszek A, Baer-Dubowska W. Modulation of rat hepatic and kidney phase II enzymes by cabbage juices: comparison with the effects of indole-3-carbinol and phenethyl isothiocyanate. The British Journal of Nutrition. 2011;105:816–826. doi: 10.1017/S0007114510004526. [DOI] [PubMed] [Google Scholar]
- 64.Tang L, Li G, Song L, Zhang Y. The principal urinary metabolites of dietary isothiocyanates, N-acetylcysteine conjugates, elicit the same anti-proliferative response as their parent compounds in human bladder cancer cells. Anti-cancer Drugs. 2006;17:297–305. doi: 10.1097/00001813-200603000-00008. [DOI] [PubMed] [Google Scholar]
- 65.Ji Y, Morris ME. Effect of organic isothiocyanates on breast cancer resistance protein (ABCG2)-mediated transport. Pharmaceutical Research. 2004;21:2261–2269. doi: 10.1007/s11095-004-7679-1. [DOI] [PubMed] [Google Scholar]
- 66.Ji Y, Morris ME. Membrane transport of dietary phenethyl isothiocyanate by ABCG2 (breast cancer resistance protein) Molecular Pharmaceutics. 2005;2:414–419. doi: 10.1021/mp050029f. [DOI] [PubMed] [Google Scholar]
- 67.Chiao JW, Wu H, Ramaswamy G, Conaway CC, Chung FL, Wang L, Liu D. Ingestion of an isothiocyanate metabolite from cruciferous vegetables inhibits growth of human prostate cancer cell xenografts by apoptosis and cell cycle arrest. Carcinogenesis. 2004;25:1403–1408. doi: 10.1093/carcin/bgh136. [DOI] [PubMed] [Google Scholar]
- 68.Nakajima M, Yoshida R, Shimada N, Yamazaki H, Yokoi T. Inhibition and inactivation of human cytochrome P450 isoforms by phenethyl isothiocyanate. Drug Metabolism and Disposition. 2001;29:1110–1113. [PubMed] [Google Scholar]
- 69.Hecht SS, Carmella SG, Murphy SE. Effects of watercress consumption on urinary metabolites of nicotine in smokers. Cancer Epidemiology, Biomarkers & Prevention. 1999;8:907–913. [PubMed] [Google Scholar]
- 70.Srivastava SK, Xia H, Pal A, Hu X, Guo J, Singh SV. Potentiation of benzo[a]pyrene-induced pulmonary and forestomach tumorigenesis in mice by D,L-buthionine-S,R-sulfoximine-mediated tissue glutathione depletion. Cancer Letters. 2000;153:35–39. doi: 10.1016/s0304-3835(00)00333-5. [DOI] [PubMed] [Google Scholar]
- 71.Srivastava SK, Watkins SC, Schuetz E, Singh SV. Role of glutathione conjugate efflux in cellular protection against benzo[a]pyrene-7,8-diol-9,10-epoxide-induced DNA damage. Molecular Carcinogenesis. 2002;33:156–162. [PubMed] [Google Scholar]
- 72.Navarro SL, Chang JL, Peterson S, Chen C, King IB, Schwarz Y, Li SS, Li L, Potter JD, Lampe JW. Modulation of human serum glutathione S-transferase A1/2 concentration by cruciferous vegetables in a controlled feeding study is influenced by GSTM1 and GSTT1 genotypes. Cancer Epidemiology, Biomarkers & Prevention. 2009;18:2974–2978. doi: 10.1158/1055-9965.EPI-09-0701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gum SI, Cho MK. Differential hepatic GSTA2 expression of arylalkyl isothiocyanates in vivo and in vitro: the molecular mechanism of gene induction by phenethyl isothiocyanate. Molecular Nutrition & Food Research. 2013;57:2223–2232. doi: 10.1002/mnfr.201300259. [DOI] [PubMed] [Google Scholar]
- 74.Guo J, Pal A, Srivastava SK, Orchard JL, Singh SV. Differential expression of glutathione S-transferase isoenzymes in murine small intestine and colon, Comparative Biochemistry and Physiology. Part B. Biochemistry & Molecular Biology. 2002;131:443–452. doi: 10.1016/s1096-4959(01)00515-2. [DOI] [PubMed] [Google Scholar]
- 75.Murillo G, Mehta RG. Cruciferous vegetables and cancer prevention. Nutrition and Cancer. 2001;41:17–28. doi: 10.1080/01635581.2001.9680607. [DOI] [PubMed] [Google Scholar]
- 76.Hecht SS. Inhibition of carcinogenesis by isothiocyanates. Drug Metabolism Reviews. 2000;32:395–411. doi: 10.1081/dmr-100102342. [DOI] [PubMed] [Google Scholar]
- 77.Xiao D, Srivastava SK, Lew KL, Zeng Y, Hershberger P, Johnson CS, Trump DL, Singh SV. Allyl isothiocyanate, a constituent of cruciferous vegetables, inhibits proliferation of human prostate cancer cells by causing G2/M arrest and inducing apoptosis. Carcinogenesis. 2003;24:891–897. doi: 10.1093/carcin/bgg023. [DOI] [PubMed] [Google Scholar]
- 78.Talalay P, Fahey JW. Phytochemicals from cruciferous plants protect against cancer by modulating carcinogen metabolism. The Journal of Nutrition. 2001;131:3027S–3033S. doi: 10.1093/jn/131.11.3027S. [DOI] [PubMed] [Google Scholar]
- 79.Boreddy SR, Pramanik KC, Srivastava SK. Pancreatic tumor suppression by benzyl isothiocyanate is associated with inhibition of PI3K/AKT/FOXO pathway. Clinical Cancer Research. 2011;17:1784–1795. doi: 10.1158/1078-0432.CCR-10-1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sahu RP, Srivastava SK. The role of STAT-3 in the induction of apoptosis in pancreatic cancer cells by benzyl isothiocyanate. Journal of the National Cancer Institute. 2009;101:176–193. doi: 10.1093/jnci/djn470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kandala PK, Srivastava SK. Diindolylmethane-mediated Gli1 protein suppression induces anoikis in ovarian cancer cells in vitro and blocks tumor formation ability in vivo. The Journal of Biological Chemistry. 2012;287:28745–28754. doi: 10.1074/jbc.M112.351379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Morse MA, Amin SG, Hecht SS, Chung FL. Effects of aromatic isothiocyanates on tumorigenicity, O6-methylguanine formation, and metabolism of the tobacco-specific nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in A/J mouse lung. Cancer Research. 1989;49:2894–2897. [PubMed] [Google Scholar]
- 83.Wattenberg LW. Inhibition of carcinogenic effects of polycyclic hydrocarbons by benzyl isothiocyanate and related compounds. Journal of the National Cancer Institute. 1977;58:395–398. doi: 10.1093/jnci/58.2.395. [DOI] [PubMed] [Google Scholar]
- 84.Adam-Rodwell G, Morse MA, Stoner GD. The effects of phenethyl isothiocyanate on benzo[a]pyrene-induced tumors and DNA adducts in A/J mouse lung. Cancer Letters. 1993;71:35–42. doi: 10.1016/0304-3835(93)90094-p. [DOI] [PubMed] [Google Scholar]
- 85.Aras U, Gandhi YA, Masso-Welch PA, Morris ME. Chemopreventive and anti-angiogenic effects of dietary phenethyl isothiocyanate in an N-methyl nitrosourea-induced breast cancer animal model. Biopharmaceutics & Drug Disposition. 2013;34:98–106. doi: 10.1002/bdd.1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Singh SV, Kim SH, Sehrawat A, Arlotti JA, Hahm ER, Sakao K, Beumer JH, Jankowitz RC, Chandra-Kuntal K, Lee J, Powolny AA, Dhir R. Biomarkers of phenethyl isothiocyanate-mediated mammary cancer chemoprevention in a clinically relevant mouse model. Journal of the National Cancer Institute. 2012;104:1228–1239. doi: 10.1093/jnci/djs321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fimognari C, Lenzi M, Hrelia P. Interaction of the isothiocyanate sulforaphane with drug disposition and metabolism: pharmacological and toxicological implications. Current Drug Metabolism. 2008;9:668–678. doi: 10.2174/138920008785821675. [DOI] [PubMed] [Google Scholar]
- 88.Gross-Steinmeyer K, Stapleton PL, Liu F, Tracy JH, Bammler TK, Quigley SD, Farin FM, Buhler DR, Safe SH, Strom SC, Eaton DL. Phytochemical-induced changes in gene expression of carcinogen-metabolizing enzymes in cultured human primary hepatocytes. Xenobiotica. 2004;34:619–632. doi: 10.1080/00498250412331285481. [DOI] [PubMed] [Google Scholar]
- 89.Yoshigae Y, Sridar C, Kent UM, Hollenberg PF. The Inactivation of Human CYP2E1 by Phenethyl Isothiocyanate, a Naturally Occurring Chemopreventive Agent, and Its Oxidative Bioactivation. Drug Metabolism and Disposition. 2013;41:858–869. doi: 10.1124/dmd.112.050609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gross-Steinmeyer K, Stapleton PL, Tracy JH, Bammler TK, Strom SC, Eaton DL. Sulforaphane- and phenethyl isothiocyanate-induced inhibition of aflatoxin B1-mediated genotoxicity in human hepatocytes: role of GSTM1 genotype and CYP3A4 gene expression. Toxicological Sciences. 2010;116:422–432. doi: 10.1093/toxsci/kfq135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Morris CR, Chen SC, Zhou L, Schopfer LM, Ding X, Mirvish SS. Inhibition by allyl sulfides and phenethyl isothiocyanate of methyl-n-pentylnitrosamine depentylation by rat esophageal microsomes, human and rat CYP2E1, and Rat CYP2A3. Nutrition and Cancer. 2004;48:54–63. doi: 10.1207/s15327914nc4801_8. [DOI] [PubMed] [Google Scholar]
- 92.Cheung KL, Kong AN. Molecular targets of dietary phenethyl isothiocyanate and sulforaphane for cancer chemoprevention. The AAPS Journal. 2010;12:87–97. doi: 10.1208/s12248-009-9162-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Saw CL, Cintron M, Wu TY, Guo Y, Huang Y, Jeong WS, Kong AN. Pharmacodynamics of dietary phytochemical indoles I3C and DIM: Induction of Nrf2-mediated phase II drug metabolizing and antioxidant genes and synergism with isothiocyanates. Biopharmaceutics & Drug Disposition. 2011;32:289–300. doi: 10.1002/bdd.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Konsue N, Ioannides C. Differential response of four human livers to modulation of phase II enzyme systems by the chemopreventive phytochemical phenethyl isothiocyanate. Molecular Nutrition & Food Research. 2010;54:1477–1485. doi: 10.1002/mnfr.200900598. [DOI] [PubMed] [Google Scholar]
- 95.Konsue N, Ioannides C. Tissue differences in the modulation of rat cytochromes P450 and phase II conjugation systems by dietary doses of phenethyl isothiocyanate. Food and Chemical Toxicology. 2008;46:3677–3683. doi: 10.1016/j.fct.2008.09.046. [DOI] [PubMed] [Google Scholar]
- 96.Waris G, Ahsan H. Reactive oxygen species: role in the development of cancer and various chronic conditions. Journal of Carcinogenesis. 2006;5:14. doi: 10.1186/1477-3163-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 98.Yu R, Jiao JJ, Duh JL, Tan TH, Kong AN. Phenethyl isothiocyanate, a natural chemopreventive agent, activates c-Jun N-terminal kinase 1. Cancer Res. 1996;56:2954–2959. [PubMed] [Google Scholar]
- 99.Yu R, Mandlekar S, Harvey KJ, Ucker DS, Kong AN. Chemopreventive isothiocyanates induce apoptosis and caspase-3-like protease activity. Cancer Res. 1998;58:402–408. [PubMed] [Google Scholar]
- 100.Huang C, Ma WY, Li J, Hecht SS, Dong Z. Essential role of p53 in phenethyl isothiocyanate-induced apoptosis. Cancer Research. 1998;58:4102–4106. [PubMed] [Google Scholar]
- 101.Guo Z, Smith TJ, Wang E, Eklind KI, Chung FL, Yang CS. Structure-activity relationships of arylalkyl isothiocyanates for the inhibition of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone metabolism and the modulation of xenobiotic-metabolizing enzymes in rats and mice. Carcinogenesis. 1993;14:1167–1173. doi: 10.1093/carcin/14.6.1167. [DOI] [PubMed] [Google Scholar]
- 102.Gupta P, Srivastava SK. Antitumor activity of phenethyl isothiocyanate in HER2-positive breast cancer models. BMC Medicine. 2012;10:80. doi: 10.1186/1741-7015-10-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tseng E, Scott-Ramsay EA, Morris ME. Dietary organic isothiocyanates are cytotoxic in human breast cancer MCF-7 and mammary epithelial MCF-12A cell lines. Experimental Biology and Medicine. 2004;229:835–842. doi: 10.1177/153537020422900817. [DOI] [PubMed] [Google Scholar]
- 104.Keum YS, Jeong WS, Kong AN. Chemoprevention by isothiocyanates and their underlying molecular signaling mechanisms. Mutation Research. 2004;555:191–202. doi: 10.1016/j.mrfmmm.2004.05.024. [DOI] [PubMed] [Google Scholar]
- 105.Chen YR, Wang W, Kong AN, Tan TH. Molecular mechanisms of c-Jun N-terminal kinase-mediated apoptosis induced by anticarcinogenic isothiocyanates. The Journal of Biological Chemistry. 1998;273:1769–1775. doi: 10.1074/jbc.273.3.1769. [DOI] [PubMed] [Google Scholar]
- 106.Yang YM, Conaway CC, Chiao JW, Wang CX, Amin S, Whysner J, Dai W, Reinhardt J, Chung FL. Inhibition of benzo(a)pyrene-induced lung tumorigenesis in A/J mice by dietary N-acetylcysteine conjugates of benzyl and phenethyl isothiocyanates during the postinitiation phase is associated with activation of mitogen-activated protein kinases and p53 activity and induction of apoptosis. Cancer Research. 2002;62:2–7. [PubMed] [Google Scholar]
- 107.Trachootham D, Zhou Y, Zhang H, Demizu Y, Chen Z, Pelicano H, Chiao PJ, Achanta G, Arlinghaus RB, Liu J, Huang P. Selective killing of oncogenically transformed cells through a ROS-mediated mechanism by beta-phenylethyl isothiocyanate. Cancer Cell. 2006;10:241–252. doi: 10.1016/j.ccr.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 108.Zhang Y, Talalay P. Mechanism of differential potencies of isothiocyanates as inducers of anticarcinogenic Phase 2 enzymes. Cancer Research. 1998;58:4632–4639. [PubMed] [Google Scholar]
- 109.Lai KC, Hsu SC, Kuo CL, Ip SW, Yang JS, Hsu YM, Huang HY, Wu SH, Chung JG. Phenethyl Isothiocyanate Inhibited Tumor Migration and Invasion via Suppressing Multiple Signal Transduction Pathways in Human Colon Cancer HT29 Cells. Journal of Agricultural and Food Chemistry. 2010 doi: 10.1021/jf102384n. [DOI] [PubMed] [Google Scholar]
- 110.Wu X, Zhu Y, Yan H, Liu B, Li Y, Zhou Q, Xu K. Isothiocyanates induce oxidative stress and suppress the metastasis potential of human non-small cell lung cancer cells. BMC Cancer. 2010;10:269. doi: 10.1186/1471-2407-10-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hwang ES, Lee HJ. Phenylethyl isothiocyanate and its N-acetylcysteine conjugate suppress the metastasis of SK-Hep1 human hepatoma cells. The Journal of Nutritional Biochemistry. 2006;17:837–846. doi: 10.1016/j.jnutbio.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 112.Wang XH, Cavell BE, Syed Alwi SS, Packham G. Inhibition of hypoxia inducible factor by phenethyl isothiocyanate. Biochemical Pharmacology. 2009;78:261–272. doi: 10.1016/j.bcp.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 113.Xiao D, Singh SV. Phenethyl isothiocyanate inhibits angiogenesis in vitro and ex vivo. Cancer Research. 2007;67:2239–2246. doi: 10.1158/0008-5472.CAN-06-3645. [DOI] [PubMed] [Google Scholar]
- 114.Mi L, Hood BL, Stewart NA, Xiao Z, Govind S, Wang X, Conrads TP, Veenstra TD, Chung FL. Identification of potential protein targets of isothiocyanates by proteomics. Chemical Research in Toxicology. 2011;24:1735–1743. doi: 10.1021/tx2002806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Matzinger SA, Crist KA, Stoner GD, Anderson MW, Pereira MA, Steele VE, Kelloff GJ, Lubet RA, You M. K-ras mutations in lung tumors from A/J and A/J x TSG-p53 F1 mice treated with 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone and phenethyl isothiocyanate. Carcinogenesis. 1995;16:2487–2492. doi: 10.1093/carcin/16.10.2487. [DOI] [PubMed] [Google Scholar]
- 116.Gao N, Budhraja A, Cheng S, Liu EH, Chen J, Yang Z, Chen D, Zhang Z, Shi X. Phenethyl isothiocyanate exhibits antileukemic activity in vitro and in vivo by inactivation of Akt and activation of JNK pathways. Cell Death & Disease. 2011;2:e140. doi: 10.1038/cddis.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Loganathan S, Kandala PK, Gupta P, Srivastava SK. Inhibition of EGFR-AKT axis results in the suppression of ovarian tumors in vitro and in preclinical mouse model. PLoS One. 2012;7:e43577. doi: 10.1371/journal.pone.0043577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Okubo T, Washida K, Murakami A. Phenethyl isothiocyanate suppresses nitric oxide production via inhibition of phosphoinositide 3-kinase/Akt-induced IFN-gamma secretion in LPS-activated peritoneal macrophages. Molecular Nutrition & Food Research. 2010;54:1351–1360. doi: 10.1002/mnfr.200900318. [DOI] [PubMed] [Google Scholar]
- 119.Gupta P, Adkins C, Lockman P, Srivastava S. Metastasis of breast tumor cells to brain is suppressed by phenethyl isothiocyanate in a novel in vivo metastasis model. PLoS One. 2013;8 doi: 10.1371/journal.pone.0067278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Beklemisheva AA, Feng J, Yeh YA, Wang LG, Chiao JW. Modulating testosterone stimulated prostate growth by phenethyl isothiocyanate via Sp1 and androgen receptor down-regulation. The Prostate. 2007;67:863–870. doi: 10.1002/pros.20472. [DOI] [PubMed] [Google Scholar]
- 121.Chen PY, Lin KC, Lin JP, Tang NY, Yang JS, Lu KW, Chung JG. Phenethyl Isothiocyanate (PEITC) Inhibits the Growth of Human Oral Squamous Carcinoma HSC-3 Cells through G(0)/G(1) Phase Arrest and Mitochondria-Mediated Apoptotic Cell Death. Evidence-based Complementary and Alternative Medicine. 2012;2012:718320. doi: 10.1155/2012/718320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jakubikova J, Cervi D, Ooi M, Kim K, Nahar S, Klippel S, Cholujova D, Leiba M, Daley JF, Delmore J, Negri J, Blotta S, McMillin DW, Hideshima T, Richardson PG, Sedlak J, Anderson KC, Mitsiades CS. Anti-tumor activity and signaling events triggered by the isothiocyanates, sulforaphane and phenethyl isothiocyanate, in multiple myeloma. Haematologica. 2011;96:1170–1179. doi: 10.3324/haematol.2010.029363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Moon YJ, Brazeau DA, Morris ME. Dietary phenethyl isothiocyanate alters gene expression in human breast cancer cells. Evidence-based Complementary and Alternative Medicine. 2011;2011 doi: 10.1155/2011/462525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tang NY, Huang YT, Yu CS, Ko YC, Wu SH, Ji BC, Yang JS, Yang JL, Hsia TC, Chen YY, Chung JG. Phenethyl isothiocyanate (PEITC) promotes G2/M phase arrest via p53 expression and induces apoptosis through caspase- and mitochondria-dependent signaling pathways in human prostate cancer DU 145 cells. Anticancer Research. 2011;31:1691–1702. [PubMed] [Google Scholar]
- 125.Wu CL, Huang AC, Yang JS, Liao CL, Lu HF, Chou ST, Ma CY, Hsia TC, Ko YC, Chung JG. Benzyl isothiocyanate (BITC) and phenethyl isothiocyanate (PEITC)-mediated generation of reactive oxygen species causes cell cycle arrest and induces apoptosis via activation of caspase-3, mitochondria dysfunction and nitric oxide (NO) in human osteogenic sarcoma U-2 OS cells. Journal of Orthopaedic Research. 2011;29:1199–1209. doi: 10.1002/jor.21350. [DOI] [PubMed] [Google Scholar]
- 126.Pawlik A, Szczepanski MA, Klimaszewska A, Gackowska L, Zuryn A, Grzanka A. Phenethyl isothiocyanate-induced cytoskeletal changes and cell death in lung cancer cells. Food and Chemical Toxicology. 2012;50:3577–3594. doi: 10.1016/j.fct.2012.07.043. [DOI] [PubMed] [Google Scholar]
- 127.Mi L, Gan N, Chung FL. Isothiocyanates inhibit proteasome activity and proliferation of multiple myeloma cells. Carcinogenesis. 2011;32:216–223. doi: 10.1093/carcin/bgq242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, Ho PL, Coviello GM, Wright WE, Weinrich SL, Shay JW. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994;266:2011–2015. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- 129.Mukherjee S, Bhattacharya RK, Roy M. Targeting protein kinase C (PKC) and telomerase by phenethyl isothiocyanate (PEITC) sensitizes PC-3 cells towards chemotherapeutic drug-induced apoptosis. Journal of Environmental Pathology, Toxicology and Oncology. 2009;28:269–282. doi: 10.1615/jenvironpatholtoxicoloncol.v28.i4.30. [DOI] [PubMed] [Google Scholar]
- 130.Mukherjee S, Dey S, Bhattacharya RK, Roy M. Isothiocyanates sensitize the effect of chemotherapeutic drugs via modulation of protein kinase C and telomerase in cervical cancer cells. Molecular and Cellular Biochemistry. 2009;330:9–22. doi: 10.1007/s11010-009-0095-4. [DOI] [PubMed] [Google Scholar]
- 131.Lee JW, Cho MK. Phenethyl isothiocyanate induced apoptosis via down regulation of Bcl-2/XIAP and triggering of the mitochondrial pathway in MCF-7 cells. Archives of Pharmacal Research. 2008;31:1604–1612. doi: 10.1007/s12272-001-2158-2. [DOI] [PubMed] [Google Scholar]
- 132.Xiao D, Lew KL, Zeng Y, Xiao H, Marynowski SW, Dhir R, Singh SV. Phenethyl isothiocyanate-induced apoptosis in PC-3 human prostate cancer cells is mediated by reactive oxygen species-dependent disruption of the mitochondrial membrane potential. Carcinogenesis. 2006;27:2223–2234. doi: 10.1093/carcin/bgl087. [DOI] [PubMed] [Google Scholar]
- 133.Tusskorn O, Senggunprai L, Prawan A, Kukongviriyapan U, Kukongviriyapan V. Phenethyl isothiocyanate induces calcium mobilization and mitochondrial cell death pathway in cholangiocarcinoma KKU-M214 cells. BMC Cancer. 2013;13:571. doi: 10.1186/1471-2407-13-571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Stan SD, Singh SV, Whitcomb DC, Brand RE. Phenethyl isothiocyanate inhibits proliferation and induces apoptosis in pancreatic cancer cells in vitro and in a MIAPaca2 xenograft animal model. Nutrition and Cancer. 2014;66:747–755. doi: 10.1080/01635581.2013.795979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tusskorn O, Prawan A, Senggunprai L, Kukongviriyapan U, Kukongviriyapan V. Phenethyl isothiocyanate induces apoptosis of cholangiocarcinoma cells through interruption of glutathione and mitochondrial pathway. Naunyn-Schmiedeberg’s Archives of Pharmacology. 2013;386:1009–1016. doi: 10.1007/s00210-013-0906-8. [DOI] [PubMed] [Google Scholar]
- 136.Hahm ER, Singh SV. Bim contributes to phenethyl isothiocyanate-induced apoptosis in breast cancer cells. Molecular Carcinogenesis. 2012;51:465–474. doi: 10.1002/mc.20811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wu X, Kassie F, Mersch-Sundermann V. Induction of apoptosis in tumor cells by naturally occurring sulfur-containing compounds. Mutation Research. 2005;589:81–102. doi: 10.1016/j.mrrev.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 138.Wu XJ, Hua X. Targeting ROS: selective killing of cancer cells by a cruciferous vegetable derived pro-oxidant compound. Cancer Biology & Therapy. 2007;6:646–647. doi: 10.4161/cbt.6.5.4092. [DOI] [PubMed] [Google Scholar]
- 139.Roy N, Elangovan I, Kopanja D, Bagchi S, Raychaudhuri P. Tumor regression by phenethyl isothiocyanate involves DDB2. Cancer Biology & Therapy. 2013;14:108–116. doi: 10.4161/cbt.22631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Huong LD, Shin JA, Choi ES, Cho NP, Kim HM, Leem DH, Cho SD. beta-Phenethyl isothiocyanate induces death receptor 5 to induce apoptosis in human oral cancer cells via p38. Oral Diseases. 2012;18:513–519. doi: 10.1111/j.1601-0825.2012.01905.x. [DOI] [PubMed] [Google Scholar]
- 141.Huong le D, Shim JH, Choi KH, Shin JA, Choi ES, Kim HS, Lee SJ, Kim SJ, Cho NP, Cho SD. Effect of beta-phenylethyl isothiocyanate from cruciferous vegetables on growth inhibition and apoptosis of cervical cancer cells through the induction of death receptors 4 and 5. Journal of Agricultural and Food Chemistry. 2011;59:8124–8131. doi: 10.1021/jf2006358. [DOI] [PubMed] [Google Scholar]
- 142.Pullar JM, Thomson SJ, King MJ, Turnbull CI, Midwinter RG, Hampton MB. The chemopreventive agent phenethyl isothiocyanate sensitizes cells to Fas-mediated apoptosis. Carcinogenesis. 2004;25:765–772. doi: 10.1093/carcin/bgh063. [DOI] [PubMed] [Google Scholar]
- 143.Wang Y, Wei S, Wang J, Fang Q, Chai Q. Phenethyl isothiocyanate inhibits growth of human chronic myeloid leukemia K562 cells via reactive oxygen species generation and caspases. Molecular Medicine Reports. 2014;10:543–549. doi: 10.3892/mmr.2014.2167. [DOI] [PubMed] [Google Scholar]
- 144.Sakao K, Desineni S, Hahm ER, Singh SV. Phenethyl isothiocyanate suppresses inhibitor of apoptosis family protein expression in prostate cancer cells in culture and in vivo. The Prostate. 2012;72:1104–1116. doi: 10.1002/pros.22457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Glick D, Barth S, Macleod KF. Autophagy: cellular and molecular mechanisms. The Journal of Pathology. 2010;221:3–12. doi: 10.1002/path.2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Xue C, Pasolli HA, Piscopo I, Gros DJ, Liu C, Chen Y, Chiao JW. Mitochondrial structure alteration in human prostate cancer cells upon initial interaction with a chemopreventive agent phenethyl isothiocyanate. Cancer Cell International. 2014;14:30. doi: 10.1186/1475-2867-14-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Bommareddy A, Hahm ER, Xiao D, Powolny AA, Fisher AL, Jiang Y, Singh SV. Atg5 regulates phenethyl isothiocyanate-induced autophagic and apoptotic cell death in human prostate cancer cells. Cancer Research. 2009;69:3704–3712. doi: 10.1158/0008-5472.CAN-08-4344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Powolny AA, Bommareddy A, Hahm ER, Normolle DP, Beumer JH, Nelson JB, Singh SV. Chemopreventative potential of the cruciferous vegetable constituent phenethyl isothiocyanate in a mouse model of prostate cancer. Journal of the National Cancer Institute. 2011;103:571–584. doi: 10.1093/jnci/djr029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Mi L, Gan N, Chung FL. Aggresome-like structure induced by isothiocyanates is novel proteasome-dependent degradation machinery. Biochemical and Biophysical Research Communications. 2009;388:456–462. doi: 10.1016/j.bbrc.2009.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Loureiro RM, D’Amore PA. Transcriptional regulation of vascular endothelial growth factor in cancer. Cytokine & Growth Factor Reviews. 2005;16:77–89. doi: 10.1016/j.cytogfr.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 151.Kang L, Wang ZY. Breast cancer cell growth inhibition by phenethyl isothiocyanate is associated with down-regulation of oestrogen receptor-alpha36. Journal of Cellular and Molecular Medicine. 2010;14:1485–1493. doi: 10.1111/j.1582-4934.2009.00877.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Syed Alwi SS, Cavell BE, Telang U, Morris ME, Parry BM, Packham G. In vivo modulation of 4E binding protein 1 (4E-BP1) phosphorylation by watercress: a pilot study. The British Journal of Nutrition. 2010;104:1288–1296. doi: 10.1017/S0007114510002217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Izzotti A, Larghero P, Balansky R, Pfeffer U, Steele VE, De Flora S. Interplay between histopathological alterations, cigarette smoke and chemopreventive agents in defining microRNA profiles in mouse lung. Mutation Research. 2011;717:17–24. doi: 10.1016/j.mrfmmm.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 154.Gupta B, Chiang L, Chae K, Lee DH. Phenethyl isothiocyanate inhibits hypoxia-induced accumulation of HIF-1alpha and VEGF expression in human glioma cells. Food Chemistry. 2013;141:1841–1846. doi: 10.1016/j.foodchem.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 155.Wu WJ, Zhang Y, Zeng ZL, Li XB, Hu KS, Luo HY, Yang J, Huang P, Xu RH. beta-phenylethyl isothiocyanate reverses platinum resistance by a GSH-dependent mechanism in cancer cells with epithelial-mesenchymal transition phenotype. Biochemical Pharmacology. 2013;85:486–496. doi: 10.1016/j.bcp.2012.11.017. [DOI] [PubMed] [Google Scholar]
- 156.Sakao K, Hahm ER, Singh SV. In vitro and in vivo effects of phenethyl isothiocyanate treatment on vimentin protein expression in cancer cells. Nutrition and Cancer. 2013;65(Suppl 1):61–67. doi: 10.1080/01635581.2013.785002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Chen HJ, Lin CM, Lee CY, Shih NC, Amagaya S, Lin YC, Yang JS. Phenethyl isothiocyanate suppresses EGF-stimulated SAS human oral squamous carcinoma cell invasion by targeting EGF receptor signaling. International Journal of Oncology. 2013;43:629–637. doi: 10.3892/ijo.2013.1977. [DOI] [PubMed] [Google Scholar]
- 158.Dey M, Kuhn P, Ribnicky D, Premkumar V, Reuhl K, Raskin I. Dietary phenethylisothiocyanate attenuates bowel inflammation in mice. BMC Chemical Biology. 2010;10:4. doi: 10.1186/1472-6769-10-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Tsai JT, Liu HC, Chen YH. Suppression of inflammatory mediators by cruciferous vegetable-derived indole-3-carbinol and phenylethyl isothiocyanate in lipopolysaccharide-activated macrophages. Mediators of Inflammation. 2010;2010:293642. doi: 10.1155/2010/293642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Brown KK, Blaikie FH, Smith RA, Tyndall JD, Lue H, Bernhagen J, Winterbourn CC, Hampton MB. Direct modification of the proinflammatory cytokine macrophage migration inhibitory factor by dietary isothiocyanates. The Journal of Biological Chemistry. 2009;284:32425–32433. doi: 10.1074/jbc.M109.047092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Li RW, Li C, Wang TT. Transcriptomic alterations in human prostate cancer cell LNCaP tumor xenograft modulated by dietary phenethyl isothiocyanate. Molecular Carcinogenesis. 2013;52:426–437. doi: 10.1002/mc.21873. [DOI] [PubMed] [Google Scholar]
- 162.Tsou MF, Tien N, Lu CC, Chiang JH, Yang JS, Lin JP, Fan MJ, Lu JJ, Yeh SP, Chung JG. Phenethyl isothiocyanate promotes immune responses in normal BALB/c mice, inhibits murine leukemia WEHI-3 cells, and stimulates immunomodulations in vivo. Environmental Toxicology. 2011 doi: 10.1002/tox.20705. [DOI] [PubMed] [Google Scholar]
- 163.Zhu J, Ghosh A, Coyle EM, Lee J, Hahm ER, Singh SV, Sarkar SN. Differential effects of phenethyl isothiocyanate and D,L-sulforaphane on TLR3 signaling. Journal of Immunology. 2013;190:4400–4407. doi: 10.4049/jimmunol.1202093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Park HJ, Kim SJ, Park SJ, Eom SH, Gu GJ, Kim SH, Youn HS. Phenethyl isothiocyanate regulates inflammation through suppression of the TRIF-dependent signaling pathway of Toll-like receptors. Life Sciences. 2013;92:793–798. doi: 10.1016/j.lfs.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 165.Xiao D, Singh SV. Phenethyl isothiocyanate sensitizes androgen-independent human prostate cancer cells to docetaxel-induced apoptosis in vitro and in vivo. Pharmaceutical Research. 2010;27:722–731. doi: 10.1007/s11095-010-0079-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Barrera LN, Cassidy A, Johnson IT, Bao Y, Belshaw NJ. Epigenetic and antioxidant effects of dietary isothiocyanates and selenium: potential implications for cancer chemoprevention. The Proceedings of the Nutrition Society. 2012;71:237–245. doi: 10.1017/S002966511200016X. [DOI] [PubMed] [Google Scholar]
- 167.Manesh C, Kuttan G. Anti-tumour and anti-oxidant activity of naturally occurring isothiocyanates. Journal of Experimental & Clinical Cancer Research. 2003;22:193–199. [PubMed] [Google Scholar]
- 168.Prawan A, Keum YS, Khor TO, Yu S, Nair S, Li W, Hu L, Kong AN. Structural influence of isothiocyanates on the antioxidant response element (ARE)-mediated heme oxygenase-1 (HO-1) expression. Pharmaceutical Research. 2008;25:836–844. doi: 10.1007/s11095-007-9370-9. [DOI] [PubMed] [Google Scholar]
- 169.Chen G, Chen Z, Hu Y, Huang P. Inhibition of mitochondrial respiration and rapid depletion of mitochondrial glutathione by beta-phenethyl isothiocyanate: mechanisms for anti-leukemia activity. Antioxidants & Redox Signaling. 2011;15:2911–2921. doi: 10.1089/ars.2011.4170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Xiao D, Singh SV. p66Shc is indispensable for phenethyl isothiocyanate-induced apoptosis in human prostate cancer cells. Cancer Research. 2010;70:3150–3158. doi: 10.1158/0008-5472.CAN-09-4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Syed Alwi SS, Cavell BE, Donlevy A, Packham G. Differential induction of apoptosis in human breast cancer cell lines by phenethyl isothiocyanate, a glutathione depleting agent. Cell Stress & Chaperones. 2012;17:529–538. doi: 10.1007/s12192-012-0329-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Powolny AA, Singh SV. Differential response of normal (PrEC) and cancerous human prostate cells (PC-3) to phenethyl isothiocyanate-mediated changes in expression of antioxidant defense genes. Pharmaceutical Research. 2010;27:2766–2775. doi: 10.1007/s11095-010-0278-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Kojo S. Oxygen is the key factor associated with the difference between in vivo and in vitro effects of antioxidants. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:E2028. doi: 10.1073/pnas.1205916109. author reply E2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Pramanik KC, Srivastava SK. Apoptosis signal-regulating kinase 1-thioredoxin complex dissociation by capsaicin causes pancreatic tumor growth suppression by inducing apoptosis. Antioxidants & Redox Signaling. 2012;17:1417–1432. doi: 10.1089/ars.2011.4369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lin RK, Zhou N, Lyu YL, Tsai YC, Lu CH, Kerrigan J, Chen YT, Guan Z, Hsieh TS, Liu LF. Dietary isothiocyanate-induced apoptosis via thiol modification of DNA topoisomerase IIalpha. The Journal of Biological Chemistry. 2011;286:33591–33600. doi: 10.1074/jbc.M111.258137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Xiao Z, Mi L, Chung FL, Veenstra TD. Proteomic analysis of covalent modifications of tubulins by isothiocyanates. The Journal of Nutrition. 2012;142:1377S–1381S. doi: 10.3945/jn.111.152041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Cheung KL, Khor TO, Yu S, Kong AN. PEITC induces G1 cell cycle arrest on HT-29 cells through the activation of p38 MAPK signaling pathway. The AAPS Journal. 2008;10:277–281. doi: 10.1208/s12248-008-9032-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Hollenberg PF, Kent UM, Bumpus NN. Mechanism-based inactivation of human cytochromes p450s: experimental characterization, reactive intermediates, and clinical implications. Chemical Research in Toxicology. 2008;21:189–205. doi: 10.1021/tx7002504. [DOI] [PubMed] [Google Scholar]
- 179.Hudson TS, Perkins SN, Hursting SD, Young HA, Kim YS, Wang TC, Wang TT. Inhibition of androgen-responsive LNCaP prostate cancer cell tumor xenograft growth by dietary phenethyl isothiocyanate correlates with decreased angiogenesis and inhibition of cell attachment. International Journal of Oncology. 2012;40:1113–1121. doi: 10.3892/ijo.2012.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Izzotti A, Larghero P, Cartiglia C, Longobardi M, Pfeffer U, Steele VE, De Flora S. Modulation of microRNA expression by budesonide, phenethyl isothiocyanate and cigarette smoke in mouse liver and lung. Carcinogenesis. 2010;31:894–901. doi: 10.1093/carcin/bgq037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Izzotti A, Calin GA, Steele VE, Cartiglia C, Longobardi M, Croce CM, De Flora S. Chemoprevention of cigarette smoke-induced alterations of MicroRNA expression in rat lungs. Cancer Prevention Research. 2010;3:62–72. doi: 10.1158/1940-6207.CAPR-09-0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Conaway CC, Jiao D, Kohri T, Liebes L, Chung FL. Disposition and pharmacokinetics of phenethyl isothiocyanate and 6-phenylhexyl isothiocyanate in F344 rats. Drug Metabolism and Disposition. 1999;27:13–20. [PubMed] [Google Scholar]
- 183.Yu C, Gong AY, Chen D, Solelo Leon D, Young CY, Chen XM. Phenethyl isothiocyanate inhibits androgen receptor-regulated transcriptional activity in prostate cancer cells through suppressing PCAF. Molecular Nutrition & Food Research. 2013;57:1825–1833. doi: 10.1002/mnfr.201200810. [DOI] [PubMed] [Google Scholar]
- 184.Jutooru I, Guthrie AS, Chadalapaka G, Pathi S, Kim K, Burghardt R, Jin UH, Safe S. Mechanism of action of phenethylisothiocyanate and other reactive oxygen species-inducing anticancer agents. Molecular and Cellular Biology. 2014;34:2382–2395. doi: 10.1128/MCB.01602-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Yu Z, Xu H. Effect of phenethyl isothiocyanate on expression of genes during differentiation process of mouse embryonic stem cells in vitro. Wei Sheng Yan Jiu. 2008;37:693–696. [PubMed] [Google Scholar]
- 186.Gerhauser C. Epigenetic impact of dietary isothiocyanates in cancer chemoprevention. Current Opinion in Clinical Nutrition and Metabolic Care. 2013;16:405–410. doi: 10.1097/MCO.0b013e328362014e. [DOI] [PubMed] [Google Scholar]
- 187.Wang LG, Chiao JW. Prostate cancer chemopreventive activity of phenethyl isothiocyanate through epigenetic regulation (review) International Journal of Oncology. 2010;37:533–539. doi: 10.3892/ijo_00000702. [DOI] [PubMed] [Google Scholar]
- 188.Yan H, Zhu Y, Liu B, Wu H, Li Y, Wu X, Zhou Q, Xu K. Mitogen-activated protein kinase mediates the apoptosis of highly metastatic human non-small cell lung cancer cells induced by isothiocyanates. The British Journal of Nutrition. 2011;106:1779–1791. doi: 10.1017/S0007114511002315. [DOI] [PubMed] [Google Scholar]
- 189.Liu Y, Chakravarty S, Dey M. Phenethylisothiocyanate alters site- and promoter-specific histone tail modifications in cancer cells. PLoS One. 2013;8:e64535. doi: 10.1371/journal.pone.0064535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Liu K, Cang S, Ma Y, Chiao JW. Synergistic effect of paclitaxel and epigenetic agent phenethyl isothiocyanate on growth inhibition, cell cycle arrest and apoptosis in breast cancer cells. Cancer Cell International. 2013;13:10. doi: 10.1186/1475-2867-13-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Cang S, Ma Y, Chiao JW, Liu D. Phenethyl isothiocyanate and paclitaxel synergistically enhanced apoptosis and alpha-tubulin hyperacetylation in breast cancer cells. Experimental Hematology & Oncology. 2014;3:5. doi: 10.1186/2162-3619-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Sarkars R, Mukherjee S, Roy M. Targeting heat shock proteins by phenethyl isothiocyanate results in cell-cycle arrest and apoptosis of human breast cancer cells. Nutrition and Cancer. 2013;65:480–493. doi: 10.1080/01635581.2013.767366. [DOI] [PubMed] [Google Scholar]
- 193.Huang SH, Hsu MH, Hsu SC, Yang JS, Huang WW, Huang AC, Hsiao YP, Yu CC, Chung JG. Phenethyl isothiocyanate triggers apoptosis in human malignant melanoma A375.S2 cells through reactive oxygen species and the mitochondria-dependent pathways. Human & Experimental Toxicology. 2014;33:270–283. doi: 10.1177/0960327113491508. [DOI] [PubMed] [Google Scholar]
- 194.Cavell BE, Syed Alwi SS, Donlevy AM, Proud CG, Packham G. Natural product-derived antitumor compound phenethyl isothiocyanate inhibits mTORC1 activity via TSC2. Journal of Natural Products. 2012;75:1051–1057. doi: 10.1021/np300049b. [DOI] [PubMed] [Google Scholar]
- 195.Wang X, Di Pasqua AJ, Govind S, McCracken E, Hong C, Mi L, Mao Y, Wu JY, Tomita Y, Woodrick JC, Fine RL, Chung FL. Selective depletion of mutant p53 by cancer chemopreventive isothiocyanates and their structure-activity relationships. Journal of Medicinal Chemistry. 2011;54:809–816. doi: 10.1021/jm101199t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Gupta P, Kim B, Kim SH, Srivastava SK. Molecular targets of isothiocyanates in cancer: Recent advances. Molecular Nutrition & Food Research. 2014 doi: 10.1002/mnfr.201300684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Chan DK, Miskimins WK. Metformin and phenethyl isothiocyanate combined treatment in vitro is cytotoxic to ovarian cancer cultures. Journal of Ovarian Research. 2012;5:19. doi: 10.1186/1757-2215-5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Tseng E, Kamath A, Morris ME. Effect of organic isothiocyanates on the P-glycoprotein- and MRP1-mediated transport of daunomycin and vinblastine. Pharmaceutical Research. 2002;19:1509–1515. doi: 10.1023/a:1020460700877. [DOI] [PubMed] [Google Scholar]
- 199.Lee DH, Kim DW, Lee HC, Lee JH, Lee TH. Phenethyl isothiocyanate sensitizes glioma cells to TRAIL-induced apoptosis. Biochemical and Biophysical Research Communications. 2014;446:815–821. doi: 10.1016/j.bbrc.2014.01.112. [DOI] [PubMed] [Google Scholar]
- 200.Wang X, Govind S, Sajankila SP, Mi L, Roy R, Chung FL. Phenethyl isothiocyanate sensitizes human cervical cancer cells to apoptosis induced by cisplatin. Molecular Nutrition & Food Research. 2011;55:1572–1581. doi: 10.1002/mnfr.201000560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Tang T, Song X, Liu YF, Wang WY. PEITC reverse multi-drug resistance of human gastric cancer SGC7901/DDP cell line. Cell Biology International. 2014;38:502–510. doi: 10.1002/cbin.10169. [DOI] [PubMed] [Google Scholar]
- 202.Hecht SS, Kenney PM, Wang M, Trushin N, Upadhyaya P. Effects of phenethyl isothiocyanate and benzyl isothiocyanate, individually and in combination, on lung tumorigenesis induced in A/J mice by benzo[a]pyrene and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone. Cancer Letters. 2000;150:49–56. doi: 10.1016/s0304-3835(99)00373-0. [DOI] [PubMed] [Google Scholar]
- 203.Hecht SS, Isaacs S, Trushin N. Lung tumor induction in A/J mice by the tobacco smoke carcinogens 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone and benzo[a]pyrene: a potentially useful model for evaluation of chemopreventive agents. Carcinogenesis. 1994;15:2721–2725. doi: 10.1093/carcin/15.12.2721. [DOI] [PubMed] [Google Scholar]
- 204.Conaway CC, Wang CX, Pittman B, Yang YM, Schwartz JE, Tian D, McIntee EJ, Hecht SS, Chung FL. Phenethyl isothiocyanate and sulforaphane and their N-acetylcysteine conjugates inhibit malignant progression of lung adenomas induced by tobacco carcinogens in A/J mice. Cancer Research. 2005;65:8548–8557. doi: 10.1158/0008-5472.CAN-05-0237. [DOI] [PubMed] [Google Scholar]
- 205.Staretz ME, Koenig LA, Hecht SS. Effects of long term dietary phenethyl isothiocyanate on the microsomal metabolism of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol in F344 rats. Carcinogenesis. 1997;18:1715–1722. doi: 10.1093/carcin/18.9.1715. [DOI] [PubMed] [Google Scholar]
- 206.Sticha KR, Kenney PM, Boysen G, Liang H, Su X, Wang M, Upadhyaya P, Hecht SS. Effects of benzyl isothiocyanate and phenethyl isothiocyanate on DNA adduct formation by a mixture of benzo[a]pyrene and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in A/J mouse lung. Carcinogenesis. 2002;23:1433–1439. doi: 10.1093/carcin/23.9.1433. [DOI] [PubMed] [Google Scholar]
- 207.Izzotti A, Balansky RM, Dagostini F, Bennicelli C, Myers SR, Grubbs CJ, Lubet RA, Kelloff GJ, De Flora S. Modulation of biomarkers by chemopreventive agents in smoke-exposed rats. Cancer Research. 2001;61:2472–2479. [PubMed] [Google Scholar]
- 208.Boysen G, Kenney PM, Upadhyaya P, Wang M, Hecht SS. Effects of benzyl isothiocyanate and 2-phenethyl isothiocyanate on benzo[a]pyrene and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone metabolism in F-344 rats. Carcinogenesis. 2003;24:517–525. doi: 10.1093/carcin/24.3.517. [DOI] [PubMed] [Google Scholar]
- 209.Staretz ME, Foiles PG, Miglietta LM, Hecht SS. Evidence for an important role of DNA pyridyloxobutylation in rat lung carcinogenesis by 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone: effects of dose and phenethyl isothiocyanate. Cancer Research. 1997;57:259–266. [PubMed] [Google Scholar]
- 210.Kassie F, Melkamu T, Endalew A, Upadhyaya P, Luo X, Hecht SS. Inhibition of lung carcinogenesis and critical cancer-related signaling pathways by N-acetyl-S-(N-2-phenethylthiocarbamoyl)-l-cysteine, indole-3-carbinol and myo-inositol, alone and in combination. Carcinogenesis. 2010;31:1634–1641. doi: 10.1093/carcin/bgq139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Kassie F, Matise I, Negia M, Lahti D, Pan Y, Scherber R, Upadhyaya P, Hecht SS. Combinations of N-Acetyl-S-(N-2-Phenethylthiocarbamoyl)-L-Cysteine and myo-inositol inhibit tobacco carcinogen-induced lung adenocarcinoma in mice. Cancer Prevention Research. 2008;1:285–297. doi: 10.1158/1940-6207.CAPR-08-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Ye B, Zhang YX, Yang F, Chen HL, Xia D, Liu MQ, Lai BT. Induction of lung lesions in Wistar rats by 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone and its inhibition by aspirin and phenethyl isothiocyanate. BMC Cancer. 2007;7:90. doi: 10.1186/1471-2407-7-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Balansky R, Ganchev G, Iltcheva M, Steele VE, De Flora S. Prevention of cigarette smoke-induced lung tumors in mice by budesonide, phenethyl isothiocyanate, and N-acetylcysteine. International Journal of Cancer. 2010;126:1047–1054. doi: 10.1002/ijc.24942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.D’Agostini F, Mastracci L, Izzotti A, Balansky R, Pennisi TM, Steele VE, De Flora S. Modulation by phenethyl isothiocyanate and budesonide of molecular and histopathologic alterations induced by environmental cigarette smoke in mice. Cancer Prevention Research. 2009;2:546–556. doi: 10.1158/1940-6207.CAPR-08-0235. [DOI] [PubMed] [Google Scholar]
- 215.Micale RT, D’Agostini F, Steele VE, La Maestra S, De Flora S. Budesonide and phenethyl isothiocyanate attenuate DNA damage in bronchoalveolar lavage cells of mice exposed to environmental cigarette smoke. Current Cancer Drug Targets. 2008;8:703–708. doi: 10.2174/156800908786733423. [DOI] [PubMed] [Google Scholar]
- 216.Mi L, Xiao Z, Hood BL, Dakshanamurthy S, Wang X, Govind S, Conrads TP, Veenstra TD, Chung FL. Covalent binding to tubulin by isothiocyanates. A mechanism of cell growth arrest and apoptosis. The Journal of Biological Chemistry. 2008;283:22136–22146. doi: 10.1074/jbc.M802330200. [DOI] [PMC free article] [PubMed] [Google Scholar]