To the editor,
Information on the natural history of peanut allergic sensitization (PAS) and clinical peanut allergy (PA) remains limited. Most previous studies selected children who were diagnosed with PA, which does not provide the population perspective and probably ignores those with low levels of sensitization.1,2 There are no population based studies on the natural history of PAS or PA. To provide a population perspective, we used the Isle of Wight (IoW) birth cohort (n=1,456) and determined the natural history of PAS and PA, focusing on incidence, persistence and remission. At 1, 2, 4, 10 and 18 years, validated questionnaires were completed to obtain information on allergic symptoms, including peanut allergic reactions. Skin prick test (SPT) were carried out to 14 aero- and food allergens, including peanut at ages 4, 10 and 18 years in all consenting participants and at 1 and 2 years in those with allergic symptoms.3 Allergic sensitization was defined as a mean weal diameter of 3 mm greater than the negative control to an allergen on SPT. The diagnosis of PA was based on a convincing clinical history (one or more recognized allergic symptoms developing within 2 hours of food ingestion) plus evidence of sensitization to peanut on SPT. We used the term PAS, where SPT was positive to peanut irrespective of clinical reactivity.
Details of experimental methods used and statistical analyses are provided in the online supplement of this article. Briefly, prevalence and their 95% confidence intervals were calculated. Means of SPT wheal sizes in those with persistent and remittent sensitization were compared using T test. Figure E1 (see online repository) provides information on data at each assessment. Details of each child sensitized to peanut at age 4, 10 and 18 years is provided in table E1, while table E2 provides a complete picture with imputed data (see online repository).
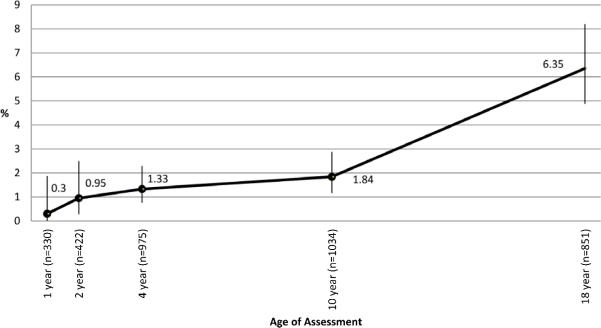
PAS increased gradually in the first decade of life but showed a steeper rise from 10 to 18 years (Fig 1a). At age 4 years, 13 of 976 children (1.3%, 95% confidence intervals (CI): 0.8%-2.3%) were sensitized to peanut. This increased to 19 of 1034 (1.8%, 95% CI: 1.2%-2.9%) at age 10 years with a further increase to 54 out of 851 (6.4%, 95% CI: 4.9-8.2%) at age 18 years. Previous studies have reported a similar prevalence of PAS varying from 1.1% to 8.6%.4,5 We show that this is primarily age dependent and varies from 1.3% in early childhood to 6.8% in adolescence (Fig 1b). At age 1 and 2, only those children reporting allergic symptoms were skin prick tested and hence the prevalence could have been overestimated. However, prevalence figures at age 1 and 2 are similar to those reported previously in another unselected birth cohort on the IoW.6 All peanut sensitized children had positive reactions to at least one aeroallergen with grass pollen sensitization being more common than tree pollen (Table E3). Cross-reactivity of peanut and grass pollen has been reported previously.7 At 4 years, eczema was the major co-morbidity, while at 10 and 18 years, rhinitis was present in the majority of peanut sensitized children.
Figure 1a. Sensitization to peanut on skin prick test from 1 to 18 years of age.
Sensitization was defined as a positive SPT to peanut. Numbers above each point represent % of participants with sensitization at each assessment point.
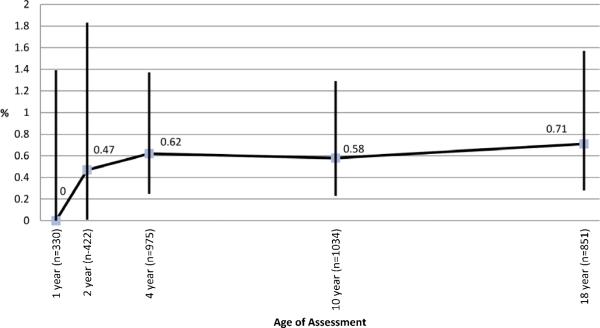
Figure 1b. Clinical allergy to peanut from 1 to 18 years.
Clinical allergy to peanut was defined as appearance of typical type I hypersensitivity symptoms within 2 hours of exposure to peanut in participants with sensitization on SPT. Numbers above each point represent the % of participants with peanut allergy at each assessment.
The most common pattern of peanut sensitization was its development for the first time at 18 years; in 42 of 66 children (63.6%, 95% CI: 51.6%-74.2%) in association with grass pollen sensitivity and allergic rhinitis (Table 1 & E3). The other common pattern was persistent sensitization from age 4 (n=9) or 10 (n=6) years (15 of 66 or 22.7%, 95% CI: 14.3%-34.2%). However, other patterns where sensitization was transient or recurrent were observed in 9 of 66 (13.6%, 95% CI: 73.5%-23.9%) children. The mean difference in wheal size of 1.1 mm between persistence (mean; 4.4, SD: 2.0) and transient group (mean; 3.3, SD: 0.4) failed to reach statistical significance (p=0.06).
Table 1.
Natural history of sensitization to peanut during childhood.
| Sensitized subjects | SPT size at 4 year | SPT size at 10 year | SPT size at 15 year | |
|---|---|---|---|---|
| Pattern of sensitization | N=66 | Median (IQ range 25-75) | Median (IQ range 25-75) | Median (IQ range 25-75) |
| +++ | 9 (13.6%) | 3.5 (3.0-6.0) | 4.5 (3.0-7.5) | 5.50 (4.6-9.3) |
| -++ | 6 (9.1%) | 0 | 3.5 (3.0-5.3) | 3.50 (3.4-4.3) |
| --+ | 42 (63.6%) | 0 | 0 | 4.0 (3.3-4.6) |
| ++- | 2 (3.0%) | 3.3 (3.0-3.5) | 4.50 (3.0-6.0) | 0 |
| +-- | 4 (6.1%) | 3.0 (3.0-3.8) | 0 | 0 |
| +-+ | 1 (1.5%) | 4.0 | 0 | 3.8 |
| -+- | 2 (3.0%) | 0 | 3.0 (3.0-3.0) | 0 |
There was minimal change in the overall prevalence of PA beyond early childhood and it remained around 0.6% (Fig 1b). New onset of PA was uncommon at 10 years (1 of 6) but more common at 18 years (3 of 6). Remission of PA was seen in 1 of 6 (17%) between ages 4 to 10 years and 1 of 4 (25%) from age 10 to 18 years (Table E4). The numbers with PA in our study were small and therefore, these findings should be interpreted with caution. However, the development of natural tolerance in a small proportion is consistent with previous reports.1 We did not observe recurrence of PA, although one patient had recurrence of PAS after initial loss of SPT reactivity (Table 1).
The diagnosis of PA can be questioned as oral challenge was not performed. However, a history of acute clinical reaction and evidence of allergic sensitization is considered adequate for the diagnosis in most cases.8 Moreover, we have previously shown that even a 3mm wheal has about 75% positive predictive value for PA if symptoms are present.9 Another limitation was missing information for SPT, which is inevitable in a long-term study, which spans over 20 years.
Sensitization to peanut in early childhood was more commonly associated with clinical reactivity than during adolescence. Of those with sensitization at 4 year (n=10), 4 (40%) developed PA, while at age 18, only 2 of 42 (4.76%) children with new-onset sensitization developed PA (P=0.009). Thus, the increase in the number of subjects sensitized to peanut during adolescence was largely due to the onset of asymptomatic sensitization.
In summary, in this cohort, PA started early in childhood and persisted in most children until adult life. A few adolescents did have new-onset PA compensating for those who remitted and therefore the overall prevalence remained around 0.6%. The natural history of PAS differed from that of PA in that it increased gradually from early childhood, with a significant peak incidence during adolescence. Most of this new onset sensitization to peanut was asymptomatic and associated with pollen sensitization and allergic rhinitis. The numbers with peanut allergy were small and thus generalizability of these findings is uncertain. Similar assessments in other longitudinally followed birth cohorts will clarify developmental patterns of PAS and PA during childhood.
Supplementary Material
Capsule summary.
In this birth cohort, peanut allergy started in early childhood and persisted until young adult life. Allergic sensitization to peanut rose gradually from early childhood, with a rapid increase during adolescence; the latter being mostly asymptomatic.
Acknowledgments
We would like to acknowledge the help of the participants and their families who helped us with this project over the last two decades. We are grateful to the staff at The David Hide Asthma and Allergy Research Centre in helping with the assessments of 1989 Isle of Wight birth cohort, in particular Mrs Sharon Matthews, Mr Roger Twiselton, Mrs Monica Fenn, Mrs Linda Terry and Mr Stephen Potter.
Funding: The Isle of Wight birth cohort assessments have been supported by the National Institutes of Health USA (Grant no. R01 HL082925) and Asthma UK (Grant no. 364).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
SHA generated the original hypothesis and all authors contributed to study design. SHA, GR and RJK were responsible for all allergy phenotype data collection. CV and TD advised on analysis and interpretation of the data. SHA wrote the first draft of the manuscript, and all authors have seen and approved the final version of the report. SHA will serve as guarantors for its contents.
Conflict of interest statement
None of the authors have any conflicts of interests to declare.
References
- 1.Skolnick HS, Conover-Walker MK, Koerner CB, Sampson HA, Burks W, Wood RA. The natural history of peanut allergy. J Allergy Clin Immunol. 2001;107:367–74. doi: 10.1067/mai.2001.112129. [DOI] [PubMed] [Google Scholar]
- 2.Savage JH, Limb SL, Brereton NH, Wood RA. The natural history of peanut allergy: Extending our knowledge beyond childhood. J Allergy Clin Immunol. 2007;120:717–9. doi: 10.1016/j.jaci.2007.07.027. [DOI] [PubMed] [Google Scholar]
- 3.Roberts G, Zhang H, Karmaus W, Raza A, Scott M, Matthews S, et al. Trends in cutaneous sensitization in the first 18 years of life: results from the 1989 Isle of Wight birth cohort study. Clin Exp Allergy. 2012;42:1501–9. doi: 10.1111/j.1365-2222.2012.04074.x. [DOI] [PubMed] [Google Scholar]
- 4.Arbes SJ, Jr., Gergen PJ, Elliott L, Zeldin DC. Prevalences of positive skin test responses to 10 common allergens in the US population: results from the third National Health and Nutrition Examination Survey. J Allergy Clin Immunol. 2005;116:377–83. doi: 10.1016/j.jaci.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 5.Rona RJ, Keil T, Summers C, Gislason D, Zuidmeer L, Sodergren E, et al. The prevalence of food allergy: a meta-analysis. J Allergy Clin Immunol. 2007;120:638–46. doi: 10.1016/j.jaci.2007.05.026. [DOI] [PubMed] [Google Scholar]
- 6.Dean T, Venter C, Pereira B, Arshad SH, Grundy J, Clayton CB, et al. Patterns of sensitization to food and aeroallergens in the first 3 years of life. J Allergy Clin Immunol. 2007;120:1166–71. doi: 10.1016/j.jaci.2007.06.042. [DOI] [PubMed] [Google Scholar]
- 7.Nicolaou N, Poorafshar M, Murray C, Simpson A, Winell H, Kerry G, et al. Allergy or tolerance in children sensitized to peanut: prevalence and differentiation using component-resolved diagnostics. J Allergy Clin Immunol. 2010;125:191–7. e1–13. doi: 10.1016/j.jaci.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 8.Boyce JA, Assa'ad A, Burks AW, Jones SM, Sampson HA, Wood RA, et al. Guidelines for the Diagnosis and Management of Food Allergy in the United States: Summary of the NIAID-Sponsored Expert Panel Report. J Allergy Clin Immunol. 2010;126:1105–18. doi: 10.1016/j.jaci.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DunnGalvin A, Daly D, Cullinane C, Stenke E, Keeton D, Erlewyn-Lajeunesse M, et al. Highly accurate prediction of food challenge outcome using routinely available clinical data. J Allergy Clin Immunol. 2011;127:633–9. e1–3. doi: 10.1016/j.jaci.2010.12.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.