Abstract
The ultraprecise wiring of neurons banks on the instructions provided by guidance cue proteins that steer them to their appropriate target tissue during neuronal development. Semaphorins are one such family of proteins. Semaphorins are known to play major physiological roles during the development of various organs including nervous system, cardiovascular, and immune systems. Their role in different pathologies including cancer remains an intense area of investigation. This review focuses on a novel member of this family of proteins, semaphorin 5A, which is much less explored in comparison to its other affiliates. Recent reports suggest that semaphorins play important roles in the pathology of cancer by affecting angiogenesis, tumor growth and metastasis. We will firstly give a general overview of the semaphorin family and its receptors. Next, we discuss their roles in cellular movements and how that makes them a connecting link between nervous system and cancer. Finally, we focus our discussion on semaphorin 5A to summarize the prevailing knowledge for this molecule in developmental biology and carcinogenesis.
Keywords: Semaphorin, Semaphorin 5A, Developmental biology, Cancer, Neurons, Growth cones, Migration, Plexins and Neuropilins
1. Introduction
The term semaphorin originates from the word semaphore, which is a system of sending signals visually using flags [1]. Semaphorins are a large family of phylogenetically conserved proteins, initially identified as guidance cue ligands that with their cognate receptors regulate axonal and dendritic growth during the development of embryonic nervous system. Later, the expression of semaphorin receptors on various cells including immune cells, endothelial cells and vascular smooth muscle cells suggested their roles in non-neuronal processes. Semaphorins regulate cardiovascular development, vasculogenesis and also the functioning of immune system [2–4]. Recent reports suggest that semaphorins play important roles in the pathology of cancer by affecting angiogenesis, tumor growth and metastasis.
2. Classification and nomenclature
The first identified semaphorin was fasciclin IV in grasshoppers [5]. Since then the family has expanded to thirty proteins having 21 vertebrate and 8 invertebrate members [6]. They were originally named “collapsins” for their roles in the collapse of neuronal growth cones [7]. Later, the term “semaphorin” was introduced. The general rule for naming any member of semaphorin family is “sema” followed by the class number and then an alphabet which represents the member of the class, for e.g. sema5A (lower case in mice) and SEMA5A (upper case in humans) [1].
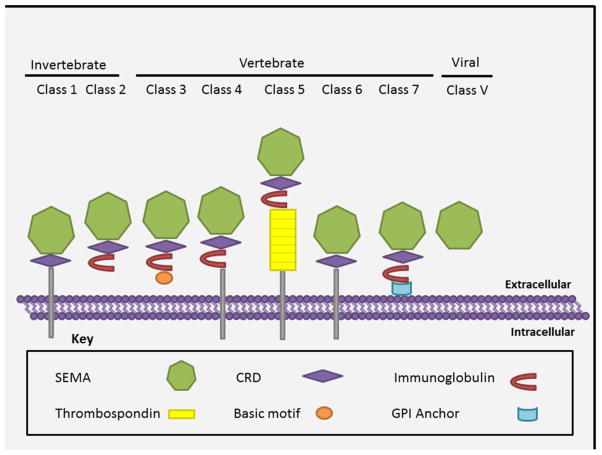
The sub-classification of semaphorins into eight classes is based on their structural similarities, species of origin and presence of class specific carboxy-terminal domains. The members of semaphorin family show a complex multi-domain structure with their sizes varying from 400–1000 amino acids [8]. They are characterized by a conserved sema domain of ~ 500 amino acids present in the N-terminal region, which is necessary for their binding to the cognate receptors (Figure 1) [9]. The characteristic structure of sema domain is a seven blade beta propeller topology with similarity to alpha integrins [8]. The sema domain is also shared by plexin (semaphorin interacting protein), Met and Ron receptor tyrosine kinases [1]. A cysteine rich domain (CRD) that is also referred to as the MET related sequence (MRS as they have homology with Met subfamily of tyrosine-kinase receptor) or PSI domain (as it is found common in plexins, semaphorins and integrins) occurs immediately next to the sema domain at its terminal end in almost all of the semaphorin family members. However, the viral semaphorins (except fowlpox virus) lack the CRD domain (Figure 1) [10]. Though the semaphorin family of proteins, in general, shares sema and PSI domains, they have unique features representing each class. In class 2–5 and class 7 semaphorins, the CRD domain is followed by one immunoglobulin domain that is absent in class 6 semaphorin proteins. More relevant to this review, class 5 semaphorins are unique in having thrombospondin (TSP 1) repeats [11, 12]. It is suggested that this domain aids in ligand receptor binding leading to the effective functioning of the molecule [11, 12]. Based on the mode of expression, semaphorins can also be classified as secreted (class 2, 3, 5 and V) or memebrane bound (class 1, 4–7). Figure 1 decribes structures of various classes of the semaphorin family. The membrane bound semaphorins can be transmembrane having a membrane spanning domain (class1, 4–6) or membrane anchored (class 7) via Glycosylphosphatidyl inositol (GPI) sequences [13]. The diversity in the domain structures of semaphorins account for the distinct roles played by these molecules.
Figure 1. The members of semaphorin family.
This classification is based on the similarity in domain architecture and the species in which it is expressed. All the members of this family share the characteristic Sema domain, which is known to have a seven blade beta propeller topology (represented here as a heptagon). Semaphorins can also be classified based on their property of being trans-membrane, membrane anchored or secreted. Abbreviations, CRD: Cystine rich domain, GPI: Glycophosphotidyl inositol.
3. Receptors and signaling pathways for semaphorins
Semaphorins, in general, have two specific receptors: plexins and neuropilins (NRPs). All membrane bound semaphorins interact with plexins alone whereas secreted members of class 3 are known to bind NRPs which act as co-receptors.
3.1. Plexins
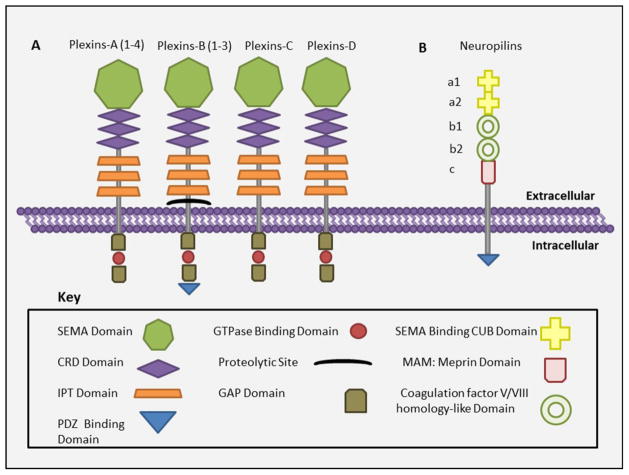
Plexins were initially identified as molecules that mediate cell adhesion [14]. The plexin family from vertebrates comprises four subfamilies named plexin A, B, C and D whereas only two plexin proteins are found in invertebrates. Each of the vertebrate plexin subfamilies has multiple proteins: - subfamily A has four (A 1–4) subfamily B has three (B 1–3), and subfamily C and D have one protein each. Like semaphorins, plexins contain a sema domain in their extracellular region, which, in this case, is known to have an auto-inhibitory effect by preventing activation of the receptor in the absence of the ligand [14]. Figure 2A provides an overview of structural aspects of the plexin family of receptors. The extracellular region has three CRD domains (also referred as MET or PSI domains) [15] and three immunoglobulin like domains called immunoglobulin like plexin transcription factor domains (IPT). The intracellular part consists of two highly conserved domains which show striking similarity to GTPase activating proteins (GAP like domains) and a linker domain that interacts with GTP bound monomeric GTPases of the Rho family, but has no intrinsic GAP activity. Class B plexins, in addition to the above domains; also have a C terminal consensus sequence that interacts with PDZ domains, in particular with that of Rho-GEFs. Plexins can be both membrane bound or secreted (members of plexin-B family are shed by proteolytic cleavage of their ectodomains at convertase-cleavage site), and they can mediate autocrine or paracrine signaling by binding to their respective semaphorin [16]. Plexins can act as ligands (in the case of reverse signaling) as well as receptors for semaphorins [17]. Furthermore, they can either mediate signaling alone or with their co receptors, NRPs.
Figure 2. Structure of plexins and the co receptors neuropilins.
A) The domain architecture of members of plexin family of semaphorin receptors and B) co receptor neuropilins. Abbreviations, CRD: Cystine rich domain, IPT: Immunoglobulin like plexin transcription factor, GAP: GTPase activating protein.
3.2. Neuropilins
NRPs, another binding partners of semaphorins, are known to be expressed in many tumors including breast, colorectal [18], prostate, lung [19], astrocytoma and pancreatic cancers [20]. There are two members in this family of proteins, NRP1 and NRP2. They are membrane bound as well as soluble molecules of 120 to 130 kDa molecular weight and can have multiple isoforms [21, 22]. The structure of NRPs (Figure 2B) contains sema binding CUB a1, a2 domains, two coagulation factor V/VIII homology domains b1 and b2, a meprin domain (MAM or C domain; involved in protein dimerization), a trans-membrane domain and a short cytoplasmic domain [23, 24]. The cytoplasmic region of NRPs contains a PDZ domain that binds to the PDZ domain of NRP interacting proteins to initiate signaling. Due to the absence of a kinase motif and a short cytoplasmic tail, NRPs cannot signal themselves, rather they signal by forming complexes with plexins or VEGF receptors based on whether they interact with semaphorins or VEGFs, respectively [20]. NRPs are known to have bifunctional activity similar to semaphorins depending on the ligands with which they interact i.e. they inhibit angiogenesis and tumor progression by interacting with semaphorins or enhance them by interacting with angiogenic VEGF ligand [20]. More research is required to understand the role of NRPs in semaphorin induced carcinogenesis and angiogenesis.
Both secreted and membrane-bound semaphorins are known to form dimers which are important for their functional activity [25–27]. The semaphorin plexin based–signaling is triggered by interaction between the sema domains in the two molecules [28]. However, Class 3 semaphorins interact first with NRPs, which in turn form complexes with plexins to initiate downstream signaling. Semaphorin dimers bind independently to two plexin molecules and this, in turn, stabilizes the plexin dimerization that is critical for downstream signaling [16, 25].
The diversity of cellular effects mediated by semaphorins can be attributed to the various signaling cascades triggered by these molecules upon binding with their cognate plexin receptors. The intracellular domains of plexins lack kinase activity and are known to interact with different tyrosine kinses such as Met, ERBB2 and Src, which may elicit divergent and sometimes opposite fuctional outcomes in a cell-specific manner [29]. These receptor- as well as cellular- tyrosine kinases associate with plexin receptors and initiate downstream signaling either by phosphorylating themselves (PI(3)K/AKT pathway) or by phosphorylating the tyrosine residues on the cytoplasmic GAP domains of plexins that via different effectors like RRAS and Rho can control integrin activation and cytoskeletal dynamics respectively [29].
4. Semaphorins as connecting link between nervous system and cancer biology
Whether they are neuronal circuits or the channels of blood vessels, network precision is indispensable for the normal physiological functions. The movement of the tips of growing nerves or vessels during embryonic development depends upon signaling cues provided by the microenvironment. Growth cone term refers to a dynamic structure formed at the tip of moving cells and it is present in neurons or endothelial cells. In case of neurons they help in the precise wiring of neurons during the development of nervous system by responding to both attractive and repulsive guidance cue ligands via the receptors present on their cell surface. Angiogenesis is, also, tightly regulated process by such navigation cues that guide the growth cones of endothelial tip cells [30, 31]. The initial command to move ahead by interaction with a guidance cue leads to a series of molecular events, which triggers the onset of a cascade of molecular and cytoskeletal changes, that either make the cell move forward or hinders it. The tips of growth cones have filopodia, which extend out and this is the first moiety to come in contact with the navigation cue. The main steps involved in cell movement are first, adhesion to the surface or extracellular matrix, second, the actin filament assembly and finally the rapid polymerization of microtubules and their forward movement by myosine motors [32]. Likewise, the movement of cells during development and pathological conditions is also mediated by similar cytoskeletal attraction and repulsion [2].
Semaphorin family of proteins are bifunctional guidance cue ligands that help growing neurons follow the right developmental path. They form molecular boundaries either by preventing the neurons to enter certain regions by repelling them or by allowing their advancement via attraction [2]. The biology of cancer progression relies on pathological angiogenesis and metastatic dissemination of cells. A growing body of evidence now supports the roles of various semaphorins in oncogenesis by regulating similar mechanisms. Semaphorins act as cues necessary for angiogenesis, for cellular migration during metastasis [33–36] and for organ specific homing of cancer cells [37]. In this review we will focus on role of class 5 semaphorins as a prototype semaphorin playing major role in neuronal growth, angiogenesis and tumor progression.
5. Class 5 Semaphorins
There are three known class 5 semaphorins SEMA/Sema5 A, B and c [11, 38]. Semaphorin 5A and B are the vertebrate members of this family whereas sema5c is an invertebrate member found in drosophila [38]. Semaphorin 5A and B were originally identified as semF and semG respectively in murine embryos. Later in 1999 the semaphorin nomenclature committee renamed them as Sema5A (mouse)/SEMA5A (human) and Sema5B (mouse)/SEMA5B (human) under the unified nomenclature system [1]. These molecules were unique in having a 408 amino acid (aa) domain consisting of thrombospondin repeats carboxy-terminal to the conserved sema domain in their extracellular region. These two proteins were closely related by being 72% similar and 58% identical to each other. However, their cytoplasmic domains showed lower degree of similarity to each other in comparison to the other parts. They were found to be integral membrane proteins [11]. In this part of the review we will capsulate the current knowledge for semaphorin 5A in developmental biology and cancer.
5.1. Semaphorin 5A
Semaphorin 5A was initially identified as semF, by cloning and characterization of murine embryonic cDNA [11]. It was found to span on chromosome 5p15. Semaphorin 5A can be both transmembrane and secreted. A soluble form of SEMA5A was reported in pancreatic cancer cell supernatants and serum of patients with rheumatoid arthritis [39] ADAM-17, a metalloprotease was recently shown to be essential for the cleavage of SEMA5A from cell surface in Hela cells [39]. The unique feature of this protein is the presence of seven specific repeats carboxy terminal to the semaphorin domain which show high similarity to type 1 repeats found on thrombospondin 1 and 2. Thrombospondin repeats promote attachment of various cells to extracellular matrix substrates and also play growth promoting roles. Thus, due to the presence of this unique domain it was suggested that semaphorin 5A might have an outgrowth promoting effect unlike other members of its family which act as repulsive signals for neuronal growth cones [11, 40]. However, it was found to be a bifunctional molecule, which will be discussed later in this review.
6. Semaphorin 5A in developmental biology
The initial evidence for the role of Sema5A (identified as semF) in embryogenesis was provided by Adams et al. in 1996 who demonstrated the expression of Sema5A in mRNA of murine embryos by northern blot analysis [11]. Later Simmons et al. 1998 showed their tissue specific expression of SEMA5A in human fetal tissues including brain, lung, liver and kidney using similar experiments [41]. Though, there are many evidences now suggestive of their functions in development of various important organs in fetus, still the validation of their precise effects in embryogenesis needs more functional examination.
6.1. Nervous system
Evidence for the role of semaphorin 5A in normal development of embryonic brain came from a cytogenetic study inquiring the etiology of cri du chat syndrome (CDCS), which is also named as chromosome p deletion syndrome as it results from the deletion of a piece of chromosome number 5. The study revealed that haplo insufficiency for SEMA5A leads to CDCS with phenotypic features like developmental delay and severe mental retardation [41]. Furthermore, northern blot analysis of human fetal tissues indicated the expression of SEMA5A in brain. In furtherance of correlating their tissue distribution pattern with the possible roles in developing brain the expression of Sema5A in embryonic mouse brain was examined by in situ hybridization. High expression of Sema5A as observed in ventricular zone (VZ). The VZ of embryonic brain consists of proliferating neuronal precursor/neuroblast cells, which give rise to discrete cell layers of adult cortex. Cortex is the center for motor behavior, perception, vision, emotion and memory. The expression of Sema5A in VZ provides the probable explanation of defects arising in this haplo insufficient syndrome. These findings for the first time illustrated the role of Sema5A in normal development of brain. Also the expression of Sema5A in VZ suggested the possible roles played by them in proliferation and emigration of these neuronal precursor cells [41]. Sema5A expression was also detected in neuroepithelium of developing rat brain where it inhibited the neuronal growth cones [42]. Further evidence to support the role of Sema5A in proper neuronal development is provided by reports of its down-regulated expression in idiopathic autism [43]. These data suggest that Sema5A is key to the normal development of the brain. Furthermore, the role of SEMA5A in lying down of precise neuronal circuits in developing embryo can be inferred from the presence of its transcripts in various human fetal tissues like the lung, liver, kidney and colon [41]. A robust expression of Sema5A was observed differentially in the budding limbs at various stages of early embryonic growth suggesting its role in limb innervation [11].
As opposed to the initial prediction of semaphorin 5A as an attractive cue in neuronal growth, Oster et al. (2003) demonstrated its inhibitory role on neurite outgrowth. Sema5A was detected in neuroepithelial cells of the optic disc along the developing optic nerve in the mouse embryo, which is necessary to prevent retinal axon defasciculation during optic nerve formation. In order to clarify the functional contribution of the sema and thrombospondin domains in this inhibitory effect on growth cones, collapse assays were performed on embryonic retinal explants. The TSP domain alone was found nonfunctional in this growth cone collapse. This study showed that the repulsion was due to the sema domain; however, the TSP domain was necessary to provide the full inhibitory potential. Furthermore, these results suggested the possibility that TSP repeats are important for ligand-receptor binding via the sema domain [27]. Recently, the class 5 semaphorins including Sema5A have been shown to cause retinal lamination by repelling the retinal ganglion cells (RGC) and amacrine cells and thus segregating them within the IPL during the development of retina [44]. Unlike the peripheral nervous system, the central nervous system (CNS) is reported to have least regenerative capacity followed by injury. Goldberg et al. (2004) suggested the role of Sema5A in the regenerative inability of the CNS. They demonstrated the expression of Sema5A by oligodendrocytes (oligo) and oligodendrocyte precursor cells (OPC), which are glial cell type in the CNS. These cells express Sema5A that inhibits the regeneration of retinal ganglion cells (RGC) axon growth after injury in vitro. They suggested a similar effect on regeneration of CNS axons in vivo [45]. Thus, these evidences cumulatively demonstrate the inhibitory roles of Sema5A in both prenatal developmental phase as well as post natal regenerative phases after injury.
Concurrent to the later discovery a study aimed to identify the receptor for Sema5A provided initial evidence that Sema5A can act both as a repulsive or an attractive cue. Sema5A served as an inhibitory cue that induced cellular collapse in cultured fibroblast cells by suppressing integrin-based adhesions. Contrary to this they also demonstrated Sema5A to induce the migration of primary human umbilical vein endothelial cells (HUVEC) cells via plexin-B3 receptor by the activation of Met signaling [26]. In furtherance of providing a possible explanation for this bifunctional activity of Sema5A, Kantor et al. (2005) put forward that the responsiveness to this guidance cue may depend upon the environmental context in which they are encountered. Specifically, Sema5A can act as a permissive or a repulsive cue based on its interaction with specific proteoglycans in the extracellular matrix. They examined the role of Sema5A in the development of fasciculus retroflexus, which is a bundle of nerve fibers connecting the forebrain and the mid brain. Their results demonstrated that by interaction of the TSP repeats with chondroitin sulfate proteoglycan (CSPGs) Sema5A acts as an inhibitory molecule; however, its interaction with axonally expressed heparan sulfate proteoglycans (HSPGs) modifies its function to an attractive cue [46]. A study to evaluate the role of Sema5A in motor neuron guidance led to the conclusion that bi-functionality of Sema5A can be attributed to the presence of two functionally opposite domains. The TSP domain was shown to mediate the motor axon extension via attraction of growth cones whereas the sema domain regulated the inhibitory process by preventing the branching of axons in the surrounding tissues [47].
6.2. Cardiovascular development
The detection of Sema5A transcripts in adult murine cardiac tissues provided evidence for the possible roles of this molecule in heart tissue [11]. In situ hybridization analysis detected Sema5A transcripts in developing heart of mouse embryos. The expression was observed in the atrial septum, endocardial cushions of atrium and ventricles underscoring its importance in cardiogenesis. However, knockout studies using Sema5A null mutant embryos did not show any defects in the development of these heart structures [48]. Whole mount immunohistochemistry of mouse embryos using anti-PECAM antibodies demonstrated that these mice had impaired branching of large vessels in the cranial region. This abnormal vasculogenesis was accounted for the lethality of the Sema5A null mice [48]. Using in situ hybridization studies in chick embryonic heart Jin et al. demonstrated the expression of Sema5A transcripts in cardiac cushion in both epithelial and mesenchymal cells during the period of active cardiac remodeling. However, further studies are required to define their precise roles in cardiac morphogenesis and regeneration [4].
Thus this overview of the functions semaphorin 5A in neuronal and vascular development suggests its role in processes like cell migration, proliferation and vasculogenesis, which are also common to oncogenesis.
7. Semaphorin 5A in cancer
The foremost evidence implicating semaphorin 5A in tumorigenesis was derived from a study designed to identify candidate genes involved in the induction of leiomyomata, which is a benign smooth muscle neoplasm of uterus. The microarray data on patient samples demonstrated that SEMA5A was up regulated in leiomyomata in comparison to the control myometrium [49]. This initial deposition was later corroborated by results derived from a drosophila screening study aimed at the identification of genes important in tumorigenecity and metastasis. Drosophila lethal giant larvae (l(2)gl) is a tumor suppressor gene in drosophila and inactivation of this gene leads to neoplastic growth and lethality at the late larval stages. Furthermore, the l(2)gl null larval tissue on transplantation to adult host can form primary tumors and metastases. D-sema5c is a drosophila homologue of Sema5A. It was observed that P element insertion causing the deletion of sema5c in the l(2)gl larvae abrogated tumor growth and metastasis of the transplanted tissue. Also, this phenotype was reverted back to the cancerous growth upon reconstitution of D-sema5c. This study demonstrated that sema5c has a functional role in l(2)gl cancer phenotype and also suggested that homologues of this gene may operate analogously in higher organisms [50]. Currently there are many reports including our report in pancreatic ductal adenocarcinoma suggesting important roles of SEMA5A in various cancers. In this section we are trying to summarize the existent information about SEMA5A in oncogenesis.
7.1. Pancreatic cancer
Using an in vivo phage display library and integrated computational analysis, we identified semaphorin 5A as putative cell adhesion molecule involved in organ-specific pancreatic cancer metastasis. Our in silico data were validated by performing RT-PCR analysis on nine human pancreatic cancer cell lines, which were derived from either primary tumors or metastases. SEMA5A is expressed in cell lines from metastases whereas cell lines from primary tumors did not expression the gene. These data for the first time demonstrated the expression of SEMA5A in pancreatic cancer and also suggested its role in the metastatic progression of this disease [37]. Furthermore, using computational analysis plexin-B3 was identified as a binding partner for SEMA5A. These results were further validated in various mouse tissues using immunoprecipitation [51]. In order to determine the significance of SEMA5A in pancreatic tumor growth, angiogenesis and metastasis further work was performed. The expression of SEMA5A was found to be higher in moderately or well differentiated human pancreatic tissues than the normal pancreas. Similarly aggressive human pancreatic cancer cell lines having greater tumorigenic and metastatic potential in xenograft assays showed higher expression of SEMA5A. These initial findings were further strengthened by in vitro assays where the ectopic expression of SEMA5A in Panc1, a cell line with no endogenous expression resulted in enhanced cell proliferation and invasion. SEMA5A-Panc1 cells during in vivo studies showed greater tumorigenic and metastatic potential [52].
SEMA5A null mice show defects in patterning of cranial vasculature [47]. This initial evidence laid the foundation for its role in pathological angiogenesis. The angiogenesis cascade has multiple steps, enhanced cell proliferation, decreased apoptosis and increased migration being the key events. Treatment of endothelial cells with recombinant Sema5A- extracellular domain (ECD) enhanced the proliferation via the Akt pathway. In independent experiments, the increased proliferation of endothelial cells was found to be mediated through plexin-B3. However, whether this SEMA5A-plexin-B3 triggered proliferative response is mediated by the Akt pathway still needs further analysis. We observed that inhibition of apoptosis in endothelial cells by Sema5A-ECD is through the induction of anti-apoptotic genes like BCL-2 and survivin. Furthermore, enhanced migration of endothelial cells is through Met tyrosine kinase receptor. It is also up regulated MMP9, which aids in the degradation of the extracellular matrix for proper cellular migration. These in vitro results were supported by in vivo Matrigel plug assays where SEMA5A shows enhanced microvessel density [53]. Overall these data suggests that Sema5A-ECD is involved in majority of the steps involved in physiological angiogenesis.
In our earlier study we reported Sema5A (135kDa) to be a membrane bound molecule in capan1 cells which was in agreement with the established opinion of its being a transmembrane molecule. However, we recently detected a secretable (110 kDa) form of this molecule in culture supernatants of another pancreatic cancer cell line T3M4. Furthermore, Panc1 cells were transfected with extracellular domain (ECD) of SEMA5A to analyze its role in tumor progression and metastasis. They found that Panc1 Sema5A-ECD cells show higher metastatic potentials both in vitro and in vivo. Enhanced angiogenesis was observed in tumors from these cells and supernatants from these cells induced proliferation of endothelial cells. Further these cells secreted proangiogenic factors like VEGF and interleukin-8 (IL-8). However, surprisingly Panc1-Sema5A-ECD cells were not tumorigenic when implanted in nude mice unlike the Panc1-Sema5A cells [50]. These data suggest that Sema5A induced proliferation is an autonomous process which involves the transmembrane and the cytoplasmic domain of the molecule.
7.2. Gastric Cancer
A study of samples from gastric carcinoma patients illustrated that the expression of both SEMA5A and its receptor plexin-B3 were elevated in primary gastric carcinoma and lymph node metastasis in comparison to the non-neoplastic tissue [54]. Likewise, higher expression of SEMA5A was detected in human gastric cancer cell lines. The siRNA mediated knockdown of SEMA5A in a gastric cancer cell line, SGC7901 resulted in decreased proliferation and anchorage independent growth. In addition, SEMA5A knockdown cells showed enhanced apoptosis when evaluated by flow cytometry and cell death detection ELISA assays. Furthermore, an elevated expression of activated caspase-3 was reported in SEMA5A knockdown cells. A recent report by the same research group shows that knocking down of SEMA5A in the SGC7901 cell line inhibited the in vitro invasiveness of these cells. This data supports their earlier observation of higher SEMA5A expression in the lymph node metastasis than the primary tumor and the normal tissues [55]. This increased invasiveness was found to be mediated by the activation of the MEK/ERK pathway leading to the up regulation of MMP9 [56]. These initial reports are suggestive of important roles of SEMA5A in gastric carcinogenesis; however, more work deciphering the molecular pathways is needed.
7.3. Glioblastoma
Markedly reduced expression of SEMA5A was observed in clinical samples of high grade astrocytomas compared to the normal brain. SEMA5A was found to inhibit the invasion of human glioma cells by interacting with the receptor plexin-B3 and disrupting Rac1 activity via Rho GDIα, which causes its inactivation and cytosolic sequestration. Rac1 belongs to the Rho family of GTPases and is known to regulate cellular migration by altering cytoskeleton and focal attachments [57]. Further examination of the mechanistic changes inside the glioma cells revealed that SEMA5A inhibits the motility of glioma cells by reducing actin stress fibers and disruption of vinculin mediated focal adhesions. Furthermore, SEMA5A plexin-B3 interaction triggered PKC mediated fascilin I phosphorylation which leads to process out growth and the re-expression of astrocytic marker glial fibrillary acidic protein (GFAP) causes astrocytic differentiation of glioma cells [58]. Together these findings support the role of SEMA5A as a tumor suppressor in glioma.
7.4. Lung cancer
Recently, in a genome-wide gene expression analysis a lower expression of SEMA5A was reported in non-smoking lung cancer female patients of Taiwan and this down regulation was associated with over all poor survival [59].
7.5. Melanoma and Prostate Cancer
SEMA5A was detected in membrane preparations of melanoma cells [50]. A higher expression of plexin-B3 was observed in metastasis of prostate cancer in comparison to the primary tumor [37]. These initial data suggest that SEMA5A may be a putative biomarker for these cancers. Nevertheless, precise functions of SEMA5A in these cancers are yet to be resolved.
Together these data suggest that semaphorin 5A acts both as a tumor suppressor and promoter in various cancers (Table 1). These contrary functions can be attributed to firstly, interaction with various receptors and stimulation of different pathways in cell specific manner and secondly, the alterations caused by the difference in extracellular matrix components of various tissues. These are discussed in more detail in the next section.
Table 1.
Semaphorin 5A in various cancers.
| Cancer | Comparison to normal tissue | Functions | References |
|---|---|---|---|
| Gastric cancer | Up regulated | Enhances cell proliferation Decreases apoptosis Enhances cell migration and invasion |
[54–56] |
| Glioma | Down regulated | Inhibits motility of cells by remodeling actin cytoskeleton and disruption of focal adhesions Induces astrocytic differentiation |
[57, 58] |
| Lung cancer | Down regulated | Lower expression is associated with poor patient survival | [59] |
| Pancreatic cancer | Up regulated | Enhances tumorigenicity Increases metastasis Induces angiogenesis Acts as a cell adhesion molecule and causes the formation of homotypic aggregates |
[3, 37, 51–53] |
| Melanoma | _ | Expression observed | [50] |
| Prostate Cancer | Up regulated | Enhances metastatic progression | [37] |
8. Immune responses
Sugimoto et al. (2006) identified SEMA5A as immune semaphorin for its role in innate immune responses in mastitis resistance. SEMA5A was shown to induce the expression of tumor necrosis factor-α and IL-8 genes, which have been documented for mediating neutrophil infiltration leading to enhanced antimicrobial activity [60]. Likewise, ectopic expression of SEMA5A in pancreatic cancer cells caused the up regulation of IL-8 expression [3]. A recent study by Gras et al. (2014) demonstrates that recombinant Sema5A increased the in vitro proliferation of NK cells and T cells and induced the secretion of Th1/Th17 cytokines. Furthermore, they detected an increased expression of SEMA5A in serum of rheumatoid arthritis patients [39]. Taken together these findings suggest a role of SEMA5A in immune responses by mediating both cellular responses and cytokine production.
9. A growing crew of semaphorin 5A receptors
Plexin-B3 was identified as a high affinity receptor for Sema5A. Both Sema and TSP1 domains were shown to interact with plexin-B3. Besides, the oligomerization of the Sema domain of Sema5A was shown to be necessary for its functional response via plexin-B3 receptor. The same study reported that Sema5A did not interact with plexin-B2 and plexin-B3. It was also determined that repulsive signaling for growth cones via Sema5A –plexin-B3 interaction does not require NRP1 or NRP2 as co-receptors [26]. However, this observation does not avert the possibility of NRP receptors in mediating the Sema5A signaling. Studies have reported the co expression of NRP2 with Sema5A in many tissues [46, 51]. Recently, it was shown that retinal lamination caused by Sema5A is mediated by plexin-A1 and A3 receptors [44]. Interaction of plexin-A1 and A3 with NRP2 has been demonstrated by co-precipitation experiments [61]. Thus, it can be suggested that though plexin-B3 and Sema5A signaling is independent of NRP2, still NRP2 may mediate its signaling by acting as co-receptor for plexin-A1 and plexin-A3. Table 2 provides a summary of the currently known binding partners of Sema5A.
Table 2.
A summary of putative and confirmed binding partners for semaphorin 5A.
| Binding status | Receptor | Co receptor | Sources | References |
|---|---|---|---|---|
| Confirmed positive | Plexin-A1, A3 and B3 | - | Endothelial cells, COS cells, brain, lung, liver tissues, RGC and amacrine cells | [26, 44, 51] |
| Confirmed negative | Plexin-B1 and B2 | - | COS cells | [26] |
| Putative | - | NRP 2 | Pancreatic cancer cells, lung, brain and spleen tissues | [46, 51] |
10. A compendious analysis of semaphorin 5A signaling pathways
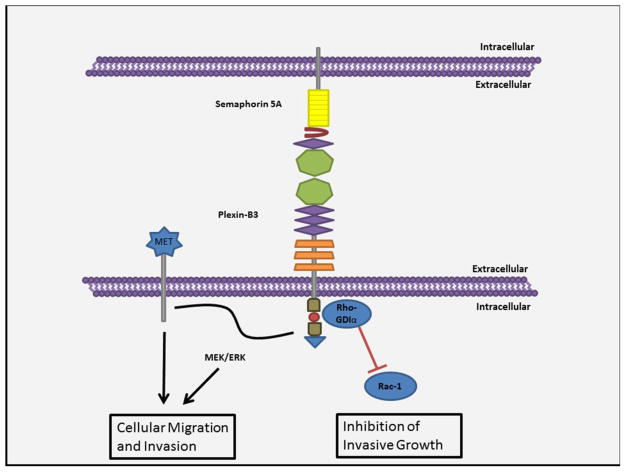
Reports suggest that semaphorin 5A is mainly involved in cell proliferation and migration. It is known to elicit contradicting outcomes in the case of cellular migration by participating in both the migration of cellular growth cones as well as their collapse. The treatment of fibroblast cells with oligomerized sema domain of Sema5A disrupted focal adhesions and inhibited cellular migration [26]. However, contrary to this it induced chemotactic migration in MLP-29 and endothelial cells. Similarly, it was shown that SEMA5A stimulated invasiveness in pancreatic [52] and gastric [54] cancer but has the opposite functions in glioma cell motility [57]. The reason for this discrepancy can be derived from the difference in the signaling pathways triggered by the interaction of Sema5A with plexin-B3. As observed the invasive growth via plexin-B3 receptor involves signaling through the Met receptor tyrosine kinase or the MEK/ERK pathway [56]. However, whether or not there is any cross talk between these two pathways still remains unexplained. In case of glioma cells, semaphorin 5A stimulates plexin-B3 receptor to recruit Rho-GDIα at its cytosolic tail. Rho-GDIα in turn blocks the guanine nucleotide exchange for Rac1 and further sequesters it to the cytoplasm inhibiting its pro-migratory activity [57]. Figure 3 summarizes the semaphorin 5A signaling pathways in cell migration. Although, it is still unidentified as to what causes the activation of two different pathways by the interaction of the same receptor ligand complex two potential reasons can be put forth. Firstly, the alternative interaction with either the repulsive Sema domain or the attractive TSP1 domain may elicit functionally opposite pathways [47]. Secondly, this can arise due to the environmental context i.e. the extracellular matrix components in which these molecules are encountered. As it has been shown that bifunctionality of semaphorin 5A may be due to its interaction with different proteoglycans like HSPG or CSPG in the nervous system [46]. This can also be a possible explanation of contrasting behavior of semaphorin 5A in tumors from different tissues as each organ has its own distinct ECM composition. Semaphorin 5A is a transmembrane protein. Thus, the possibility of it initiating its own downstream pathways via its cytosolic domain cannot be ruled out. The evidence of SEMA5A’s autonomous signaling can be derived from the tumorigenecity study where the Panc1-Sema5A-ECD cells lacking the transmembrane and cytoplasmic domain of SEMA5A were not tumorigenic when implanted in nude mice unlike the Panc1-Sema5A cells expressing the full protein [50].
Figure 3. Semaphorin 5A signaling pathways in cell migration.
Semaphorin 5A can both promote and inhibit the migration of cells based on the intracellular pathways triggered. It is known to enhance cellular invasion via its interaction with MET receptor tyrosine kinase or by the activation of the MEK/ERK pathway. However, contrary to this it can recruit Rho-GDIα at its intracellular domains, which in turn inhibits cellular migration by disrupting the activity of Rac-1.
11. Conclusion
Our current repertoire of knowledge for semaphorin 5A is fragmented. Although, there are many initial findings which give us an idea for the direction of our future work, yet a lot still remains unknown. The importance of its roles during developmental biology can be deduced from the lethality of Sema5A null mouse which can be further explored. Knowledge derived from studies in developmental biology suggesting that this molecule regulates cell proliferation and migration leads us to the assumption that it may have similar roles in cancer progression. Its initial recognition as a tumorigenic molecule in leiomyomata and drosophila has led us to some interesting discoveries in field of cancer during the recent past. Unraveling the mysteries of the bifunctionality of this molecule in regulating cellular motility can be an interesting area for future research endeavors. Further, the discovery of the putative receptor candidates for semaphorin 5A may explain some of the initial perplexing observations regarding its functions. Nevertheless, the muti-domain structure of semaphorin 5A in itself can be a cause of the diverse functional effects. Semaphorin 5A belongs to the family of molecules that acts as a functional bridge connecting nervous system and carcinogenesis. Hence, research focusing on the neuronal complications that arise during cancer progression is also suggested.
The initial inkling evidence suggestive of its possible linkage with immune regulation may take us to some very interesting findings in the future. In cancer progression, semaphorin 5A can be both a modulator and an effector of the microenvironment. A report of the presence of semaphorin 5A receptors on the endothelial cells and its rudimentary relationship with immune response implies to its potential of regulating the tumor milieu. At the same time the regulation of its attractive or repelling activities by proteoglycans provides evidence of the extent to which the microenvironment can alter its functions. The adaption of these presumptions as affirmations implores further experimental examinations. Thus, in conclusion it can be said that though our knowledge for semaphorin 5A may be still in its infancy yet this molecule promises to be an emerging candidate for future explorations in both developmental biology and cancer.
Highlights.
Semaphorin 5A acts as a functional bridge connecting nervous system and carcinogenesis
Semaphorin5A is up-regulated in glioma, melanoma, pancreatic, breast and gastric cancer
Semaphorin5A has three receptors plexin-A1, A2 and B3
Future investigations needed to define Semaphorin5A in developmental biology and cancer
Acknowledgments
This work was supported in part by grants U54CA163120 and Cancer Center Support Grant (P30CA036727) from National Cancer Institute, National Institutes of Health. Abhilasha Purohit is a graduate student supported by University of Nebraska Medical Center fellowship. We would like to thank Michelle Varney and Dr. Vinee Purohit for careful review and critique of this manuscript.
Abbreviations used
- aa
Amino acid(s)
- CRD
cysteine rich domain
- CSPG
Chondriotin sulfate protieoglycans
- ECD
Extracellular domain
- ECM
Extracellular matrix
- GPI
Glycophosphotidyl inositol
- HSPG
Heparan sulfate proteoglycans
- IL-8
Interleukin-8
- RT-PCR
Reverse transcriptase polymerase chain reaction
- Sema5A
Mouse semaphorin 5A
- SEMA5A
Human semaphorin 5A
- TGF
Transforming growth factor
- TSP-1
Thrombospondin repeats
- PSI
plexins, semaphorins and integrin domain
- IPT
immunoglobulin like plexin transcription factor domain
Footnotes
Conflict of Interest: The authors declare no potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference list
- 1.Goodman CS, Kolodkin AL, Luo Y, Püschel AW, Raper JA. Unified Nomenclature for the Semaphorins/Collapsins. Cell. 1999;97:551–552. [Google Scholar]
- 2.Tran TS, Kolodkin AL, Bharadwaj R. Semaphorin regulation of cellular morphology. Annual review of cell and developmental biology. 2007;23:263–292. doi: 10.1146/annurev.cellbio.22.010605.093554. [DOI] [PubMed] [Google Scholar]
- 3.Sadanandam A, Sidhu SS, Wullschleger S, Singh S, Varney ML, Yang CS, Ashour AE, Batra SK, Singh RK. Secreted semaphorin 5A suppressed pancreatic tumour burden but increased metastasis and endothelial cell proliferation. British journal of cancer. 2012;107:501–507. doi: 10.1038/bjc.2012.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jin Z, Chau MD, Bao ZZ. Sema3D, Sema3F, and Sema5A are expressed in overlapping and distinct patterns in chick embryonic heart. Developmental dynamics: an official publication of the American Association of Anatomists. 2006;235:163–169. doi: 10.1002/dvdy.20614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kolodkin AL, Matthes DJ, O’Connor TP, Patel NH, Admon A, Bentley D, Goodman CS. Fasciclin IV: sequence, expression, and function during growth cone guidance in the grasshopper embryo. Neuron. 1992;9:831–845. doi: 10.1016/0896-6273(92)90237-8. [DOI] [PubMed] [Google Scholar]
- 6.Neufeld G, Kessler O. The semaphorins: versatile regulators of tumour progression and tumour angiogenesis. Nature reviews. Cancer. 2008;8:632–645. doi: 10.1038/nrc2404. [DOI] [PubMed] [Google Scholar]
- 7.Luo Y, Raible D, Raper JA. Collapsin: a protein in brain that induces the collapse and paralysis of neuronal growth cones. Cell. 1993;75:217–227. doi: 10.1016/0092-8674(93)80064-l. [DOI] [PubMed] [Google Scholar]
- 8.Gherardi E, Love CA, Esnouf RM, Jones EY. The sema domain. Current opinion in structural biology. 2004;14:669–678. doi: 10.1016/j.sbi.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 9.Kolodkin AL, Matthes DJ, Goodman CS. The semaphorin genes encode a family of transmembrane and secreted growth cone guidance molecules. Cell. 1993;75:1389–1399. doi: 10.1016/0092-8674(93)90625-z. [DOI] [PubMed] [Google Scholar]
- 10.Gherardi E, Love CA, Esnouf RM, Jones EY. The sema domain. Current opinion in structural biology. 2004;14:669–678. doi: 10.1016/j.sbi.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 11.Adams RH, Betz H, Puschel AW. A novel class of murine semaphorins with homology to thrombospondin is differentially expressed during early embryogenesis. Mechanisms of development. 1996;57:33–45. doi: 10.1016/0925-4773(96)00525-4. [DOI] [PubMed] [Google Scholar]
- 12.Adams J, Lawler J. The thrombospondin family. Current Biology. 1993;3:188–190. doi: 10.1016/0960-9822(93)90270-x. [DOI] [PubMed] [Google Scholar]
- 13.Tamagnone L, Comoglio PM. Signalling by semaphorin receptors: cell guidance and beyond. Trends in cell biology. 2000;10:377–383. doi: 10.1016/s0962-8924(00)01816-x. [DOI] [PubMed] [Google Scholar]
- 14.Takahashi T, Strittmatter SM. Plexina1 autoinhibition by the plexin sema domain. Neuron. 2001;29:429–439. doi: 10.1016/s0896-6273(01)00216-1. [DOI] [PubMed] [Google Scholar]
- 15.Kozlov G, Perreault A, Schrag JD, Park M, Cygler M, Gehring K, Ekiel I. Insights into function of PSI domains from structure of the Met receptor PSI domain. Biochemical and biophysical research communications. 2004;321:234–240. doi: 10.1016/j.bbrc.2004.06.132. [DOI] [PubMed] [Google Scholar]
- 16.Kruger RP, Aurandt J, Guan KL. Semaphorins command cells to move. Nature reviews. Molecular cell biology. 2005;6:789–800. doi: 10.1038/nrm1740. [DOI] [PubMed] [Google Scholar]
- 17.Zhou Y, Gunput R-AF, Pasterkamp RJ. Semaphorin signaling: progress made and promises ahead. Trends in biochemical sciences. 2008;33:161–170. doi: 10.1016/j.tibs.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 18.Hansel DE, Wilentz RE, Yeo CJ, Schulick RD, Montgomery E, Maitra A. Expression of neuropilin-1 in high-grade dysplasia, invasive cancer, and metastases of the human gastrointestinal tract. The American journal of surgical pathology. 2004;28:347–356. doi: 10.1097/00000478-200403000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Lantuejoul S, Constantin B, Drabkin H, Brambilla C, Roche J, Brambilla E. Expression of VEGF, semaphorin SEMA3F, and their common receptors neuropilins NP1 and NP2 in preinvasive bronchial lesions, lung tumours, and cell lines. The Journal of pathology. 2003;200:336–347. doi: 10.1002/path.1367. [DOI] [PubMed] [Google Scholar]
- 20.Ellis LM. The role of neuropilins in cancer. Molecular cancer therapeutics. 2006;5:1099–1107. doi: 10.1158/1535-7163.MCT-05-0538. [DOI] [PubMed] [Google Scholar]
- 21.Soker S, Fidder H, Neufeld G, Klagsbrun M. Characterization of novel vascular endothelial growth factor (VEGF) receptors on tumor cells that bind VEGF165 via its exon 7-encoded domain. The Journal of biological chemistry. 1996;271:5761–5767. doi: 10.1074/jbc.271.10.5761. [DOI] [PubMed] [Google Scholar]
- 22.Gluzman-Poltorak Z, Cohen T, Herzog Y, Neufeld G. Neuropilin-2 is a receptor for the vascular endothelial growth factor (VEGF) forms VEGF-145 and VEGF-165 [corrected] The Journal of biological chemistry. 2000;275:18040–18045. doi: 10.1074/jbc.M909259199. [DOI] [PubMed] [Google Scholar]
- 23.Yazdani U, Terman JR. The semaphorins. Genome biology. 2006;7:211. doi: 10.1186/gb-2006-7-3-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neufeld G, Cohen T, Shraga N, Lange T, Kessler O, Herzog Y. The neuropilins: multifunctional semaphorin and VEGF receptors that modulate axon guidance and angiogenesis. Trends in cardiovascular medicine. 2002;12:13–19. doi: 10.1016/s1050-1738(01)00140-2. [DOI] [PubMed] [Google Scholar]
- 25.Janssen BJ, Robinson RA, Perez-Branguli F, Bell CH, Mitchell KJ, Siebold C, Jones EY. Structural basis of semaphorin-plexin signalling. Nature. 2010;467:1118–1122. doi: 10.1038/nature09468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Artigiani S, Conrotto P, Fazzari P, Gilestro GF, Barberis D, Giordano S, Comoglio PM, Tamagnone L. Plexin-B3 is a functional receptor for semaphorin 5A. EMBO reports. 2004;5:710–714. doi: 10.1038/sj.embor.7400189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oster SF, Bodeker MO, He F, Sretavan DW. Invariant Sema5A inhibition serves an ensheathing function during optic nerve development. Development. 2003;130:775–784. doi: 10.1242/dev.00299. [DOI] [PubMed] [Google Scholar]
- 28.Rizzolio S, Tamagnone L. Semaphorin signals on the road to cancer invasion and metastasis. Cell adhesion & migration. 2007;1:62–68. doi: 10.4161/cam.1.2.4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Capparuccia L, Tamagnone L. Semaphorin signaling in cancer cells and in cells of the tumor microenvironment--two sides of a coin. Journal of cell science. 2009;122:1723–1736. doi: 10.1242/jcs.030197. [DOI] [PubMed] [Google Scholar]
- 30.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473:298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eichmann A, Le Noble F, Autiero M, Carmeliet P. Guidance of vascular and neural network formation. Curr Opin Neurobiol. 2005;15:108–115. doi: 10.1016/j.conb.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 32.Vitriol EA, Zheng JQ. Growth cone travel in space and time: the cellular ensemble of cytoskeleton, adhesion, and membrane. Neuron. 2012;73:1068–1081. doi: 10.1016/j.neuron.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tseng CH, Murray KD, Jou MF, Hsu SM, Cheng HJ, Huang PH. Sema3E/plexin-D1 mediated epithelial-to-mesenchymal transition in ovarian endometrioid cancer. PloS one. 2011;6:e19396. doi: 10.1371/journal.pone.0019396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Basile JR, Castilho RM, Williams VP, Gutkind JS. Semaphorin 4D provides a link between axon guidance processes and tumor-induced angiogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:9017–9022. doi: 10.1073/pnas.0508825103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuznetsova EB, Kekeeva TV, Larin SS, Zemliakova VV, Babenko OV, Nemtsova MV, Zaletaev DV, Strel’nikov VV. Novel methylation and expression markers associated with breast cancer. Molekuliarnaia biologiia. 2007;41:624–633. [PubMed] [Google Scholar]
- 36.Konno R. Gene expression profiling of human ovarian epithelial tumors by digo nucleotide microarray. Human cell. 2001;14:261–266. [PubMed] [Google Scholar]
- 37.Sadanandam A, Varney ML, Kinarsky L, Ali H, Mosley RL, Singh RK. Identification of functional cell adhesion molecules with a potential role in metastasis by a combination of in vivo phage display and in silico analysis. Omics: a journal of integrative biology. 2007;11:41–57. doi: 10.1089/omi.2006.0004. [DOI] [PubMed] [Google Scholar]
- 38.Bahri SM, Chia W, Yang X. The Drosophila homolog of human AF10/AF17 leukemia fusion genes (Dalf) encodes a zinc finger/leucine zipper nuclear protein required in the nervous system for maintaining EVE expression and normal growth. Mechanisms of development. 2001;100:291–301. doi: 10.1016/s0925-4773(00)00539-6. [DOI] [PubMed] [Google Scholar]
- 39.Gras C, Eiz-Vesper B, Jaimes Y, Immenschuh S, Jacobs R, Witte T, Blasczyk R, Figueiredo C. Secreted Semaphorin 5A Activates Immune Effector Cells and Is a Biomarker for Rheumatoid Arthritis. Arthritis & Rheumatology. 2014;66:1461–1471. doi: 10.1002/art.38425. [DOI] [PubMed] [Google Scholar]
- 40.Adams J, Lawler J. Extracellular matrix: the thrombospondin family. Current biology: CB. 1993;3:188–190. doi: 10.1016/0960-9822(93)90270-x. [DOI] [PubMed] [Google Scholar]
- 41.Simmons AD, Puschel AW, McPherson JD, Overhauser J, Lovett M. Molecular cloning and mapping of human semaphorin F from the Cri-du-chat candidate interval. Biochemical and biophysical research communications. 1998;242:685–691. doi: 10.1006/bbrc.1997.8027. [DOI] [PubMed] [Google Scholar]
- 42.Skaliora I, Singer W, Betz H, Puschel AW. Differential patterns of semaphorin expression in the developing rat brain. The European journal of neuroscience. 1998;10:1215–1229. doi: 10.1046/j.1460-9568.1998.00128.x. [DOI] [PubMed] [Google Scholar]
- 43.Melin M, Carlsson B, Anckarsater H, Rastam M, Betancur C, Isaksson A, Gillberg C, Dahl N. Constitutional downregulation of SEMA5A expression in autism. Neuropsychobiology. 2006;54:64–69. doi: 10.1159/000096040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Matsuoka RL, Chivatakarn O, Badea TC, Samuels IS, Cahill H, Katayama K, Kumar SR, Suto F, Chedotal A, Peachey NS, Nathans J, Yoshida Y, Giger RJ, Kolodkin AL. Class 5 transmembrane semaphorins control selective Mammalian retinal lamination and function. Neuron. 2011;71:460–473. doi: 10.1016/j.neuron.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goldberg JL, Vargas ME, Wang JT, Mandemakers W, Oster SF, Sretavan DW, Barres BA. An oligodendrocyte lineage-specific semaphorin, Sema5A, inhibits axon growth by retinal ganglion cells. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2004;24:4989–4999. doi: 10.1523/JNEUROSCI.4390-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kantor DB, Chivatakarn O, Peer KL, Oster SF, Inatani M, Hansen MJ, Flanagan JG, Yamaguchi Y, Sretavan DW, Giger RJ, Kolodkin AL. Semaphorin 5A is a bifunctional axon guidance cue regulated by heparan and chondroitin sulfate proteoglycans. Neuron. 2004;44:961–975. doi: 10.1016/j.neuron.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 47.Hilario JD, Rodino-Klapac LR, Wang C, Beattie CE. Semaphorin 5A is a bifunctional axon guidance cue for axial motoneurons in vivo. Developmental biology. 2009;326:190–200. doi: 10.1016/j.ydbio.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 48.Fiore R, Rahim B, Christoffels VM, Moorman AF, Puschel AW. Inactivation of the Sema5a gene results in embryonic lethality and defective remodeling of the cranial vascular system. Molecular and cellular biology. 2005;25:2310–2319. doi: 10.1128/MCB.25.6.2310-2319.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tsibris JC, Segars J, Coppola D, Mane S, Wilbanks GD, O’Brien WF, Spellacy WN. Insights from gene arrays on the development and growth regulation of uterine leiomyomata. Fertility and sterility. 2002;78:114–121. doi: 10.1016/s0015-0282(02)03191-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Woodhouse EC, Fisher A, Bandle RW, Bryant-Greenwood B, Charboneau L, Petricoin EF, 3rd, Liotta LA. Drosophila screening model for metastasis: Semaphorin 5c is required for l(2)gl cancer phenotype. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:11463–11468. doi: 10.1073/pnas.2031202100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sadanandam A, Varney ML, Singh RK. Identification of semaphorin 5A interacting protein by applying apriori knowledge and peptide complementarity related to protein evolution and structure. Genomics, proteomics & bioinformatics. 2008;6:163–174. doi: 10.1016/S1672-0229(09)60004-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sadanandam A, Varney ML, Singh S, Ashour AE, Moniaux N, Deb S, Lele SM, Batra SK, Singh RK. High gene expression of semaphorin 5A in pancreatic cancer is associated with tumor growth, invasion and metastasis. International journal of cancer. Journal international du cancer. 2010;127:1373–1383. doi: 10.1002/ijc.25166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sadanandam A, Rosenbaugh EG, Singh S, Varney M, Singh RK. Semaphorin 5A promotes angiogenesis by increasing endothelial cell proliferation, migration, and decreasing apoptosis. Microvascular research. 2010;79:1–9. doi: 10.1016/j.mvr.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pan GQ, Ren HZ, Zhang SF, Wang XM, Wen JF. Expression of semaphorin 5A and its receptor plexin B3 contributes to invasion and metastasis of gastric carcinoma. World journal of gastroenterology: WJG. 2009;15:2800–2804. doi: 10.3748/wjg.15.2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pan G, Lv H, Ren H, Wang Y, Liu Y, Jiang H, Wen J. Elevated expression of semaphorin 5A in human gastric cancer and its implication in carcinogenesis. Life sciences. 2010;86:139–144. doi: 10.1016/j.lfs.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 56.Pan G, Zhang X, Ren J, Lu J, Li W, Fu H, Zhang S, Li J. Semaphorin 5A, an Axon Guidance Molecule, Enhances the Invasion and Metastasis of Human Gastric Cancer through Activation of MMP9. Pathology oncology research: POR. 2012 doi: 10.1007/s12253-012-9550-8. [DOI] [PubMed] [Google Scholar]
- 57.Li X, Lee AY. Semaphorin 5A and plexin-B3 inhibit human glioma cell motility through RhoGDIalpha-mediated inactivation of Rac1 GTPase. The Journal of biological chemistry. 2010;285:32436–32445. doi: 10.1074/jbc.M110.120451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li X, Law JW, Lee AY. Semaphorin 5A and plexin-B3 regulate human glioma cell motility and morphology through Rac1 and the actin cytoskeleton. Oncogene. 2012;31:595–610. doi: 10.1038/onc.2011.256. [DOI] [PubMed] [Google Scholar]
- 59.Lu TP, Tsai MH, Lee JM, Hsu CP, Chen PC, Lin CW, Shih JY, Yang PC, Hsiao CK, Lai LC, Chuang EY. Identification of a novel biomarker, SEMA5A, for non-small cell lung carcinoma in nonsmoking women. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research cosponsored by the American Society of Preventive Oncology. 2010;19:2590–2597. doi: 10.1158/1055-9965.EPI-10-0332. [DOI] [PubMed] [Google Scholar]
- 60.Sugimoto M, Fujikawa A, Womack JE, Sugimoto Y. Evidence that bovine forebrain embryonic zinc finger-like gene influences immune response associated with mastitis resistance. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:6454–6459. doi: 10.1073/pnas.0601015103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tamagnone L, Artigiani S, Chen H, He Z, Ming GI, Song H, Chedotal A, Winberg ML, Goodman CS, Poo M, Tessier-Lavigne M, Comoglio PM. Plexins are a large family of receptors for transmembrane, secreted, and GPI-anchored semaphorins in vertebrates. Cell. 1999;99:71–80. doi: 10.1016/s0092-8674(00)80063-x. [DOI] [PubMed] [Google Scholar]