Abstract
Background
RAG1 deficiency presents a varied spectrum of combined immunodeficiency, ranging from a T−B−NK+type of disease to a T+B+NK+ phenotype.
Objective
To assess the genetic background of common variable immunodeficiency (CVID) patients.
Methods
A patient diagnosed with CVID, who was born in a consanguineous family and thus would be expected to show an autosomal recessive inheritance, was subjected to clinical evaluation, immunological assays, homozygosity gene mapping, exome sequencing, Sanger sequencing and functional analysis.
Results
The 14-year-old patient, who suffered from liver granuloma, extranodal marginal zone B cell lymphoma and autoimmune neutropenia, is presented with a clinical picture resembling CVID. Genetic analysis of this patient showed a homozygous hypomorphic RAG1 mutation (c.1073 G>A, p.C358Y) with a residual functional capacity of 48% of wild-type protein.
Conclusion
Our finding broadens the range of disorders associated with RAG1 mutations and may have important therapeutic implications.
Keywords: Common variable immunodeficiency, Granulomatous lesion, Recombination activation genes, Differential diagnosis, mismanagement
Introduction
The product of Recombinase Activating Gene 1 (RAG1) is part of the complex which is responsible for initiating programmed DNA cleavage for rearrangement of the genes encoding the variable (V), diversity (D) and joining (J) segments of the immunoglobulin and T cell antigen receptors in progenitor lymphocytes1, 2. RAG1 may also have important functions subsequent to the generation of double strand breaks by forming a transient cleaved signal complex with the recombining ends to facilitate DNA end processing and ligation by other non-homologous end joining proteins (NHEJ)3–5.
The spectrum of primary immunodeficiency (PID) associated with RAG1 mutations varies based on the nature of the defect, and its quantitative and qualitative effects on V(D)J recombination. Mutations in RAG1 accounts for approximately 10% of all severe combined immunodeficiency (SCID) patients and nearly 60 cases have been reported to date6, 7. Complete RAG1 deficiency (<1% activity of the wild type protein), resulting from amorphic or null mutations of both alleles, leads to a severe combined immunodeficiency phenotype with lack of T and B lymphocytes (T-B-NK+SCID)8, 9. Patients with this phenotype present with life-threatening infections and failure to thrive in early infancy. NK cell activity is however not affected because of its independency of antigen receptor gene rearrangement10–12.
Patients with hypomorphic RAG1 mutations that allow very low levels of recombination activity may present with different phenotypes, characterized by residual development of T and/or B cells. These forms include Omenn syndrome (hepatosplenomegaly, lymphadenopathy, severe erythroderma, eosinophilia, elevated serum IgE levels and autoantibodies, colitis, and infiltration of oligoclonal populations of T cells)13–15, atypical Omenn syndrome (skin inflammation without T-cell expansion, a low proportion of CD31 cells, and more than 3% B cells)16, and atypical SCID with γδ T cell expansion (autoimmunity, severe cytomegalovirus infection and partially functioning B cells with a limited ability for antibody production)17–19. Finally, a delayed onset form, with generalized granulomatous lesions, severe varicella infection, normal frequencies of T and B cells, progressive hypogammaglobulinemia, defective specific antibody production and autoimmunity, has recently been reported6, 20–22 and is due to hypomorphic mutations that support significant, residual levels of recombination activity. Some of the features reported in these patients overlap with those seen in patients with common variable immunodeficiency (CVID) and may lead to misdiagnosis and insufficient treatment, based on immunoglobulin replacement rather than curative hematopoietic stem cell transplantation. Herein we report a case of RAG1 deficiency with granuloma who was initially diagnosed and treated as a CVID patient.
Materials and Methods
1. Clinical Evaluation
Informed consent for the performed studies was obtained from the patient’s parents, in accordance with the principles of the ethics committee of the Tehran University of Medical Sciences. An evaluation sheet was used to summarize demographic information of patients including name, gender, date of birth, age of onset of symptoms, clinical symptoms, age at diagnosis, family history and consanguinity, previous history of medications and vaccinations, and laboratory data.
2. Immunological Assays
Complete blood count was evaluated by the cell counter using anticoagulated whole blood. Serum levels of IgG, IgA and IgM were measured by turbidimetry (Behring Nephelometer, Behringwerke, Marburg, Germany), and lymphocyte subpopulations of CD3, CD4, CD8 and CD19 were counted by flow cytometry (Partec PAS, Münster, Germany) at the time of the study. Immunoglobulin E levels were measured, using enzyme-linked immunosorbent assay (ELISA, Neuss, Germany).
3. Homozygosity Mapping
Genomic DNA was extracted from whole blood by standard methods. Genome-wide scan was conducted by homozygosity mapping using GENEHUNTER where the patient was genotyped for a panel of 381 microsatellite markers. The Linkage Mapping Set MD-10 (Applied Biosystems) formed the core marker set for the scan.
4. Exome Sequencing
4.1. Library Preparation and Exome Sequencing
Three ug of genomic DNA was randomly fragmented using the Covaris Acoustic System. Adapters were then ligated to both ends of the fragments. The adaptor-ligated DNA templates were purified by Agencourt AM Pure SPRI beads where fragments with an insert size of about 200 bp were excised. Extracted DNA was amplified by ligation-mediated PCR (LM-PCR), purified and hybridized to the Agilent Sure Select Human All Exon 50 Mb kit for the enrichment. The hybridized fragments were subsequently bound to strepavidin beads where non-hybridized fragments were washed off after 24 hours. Captured LM-PCR products were subjected to quantitative PCR by an Agilent 2100 Bioanalyzer to estimate the magnitude of the enrichment. Each captured library was subsequently loaded on the Hiseq2000 Illumina sequencer according to the manufacturer’s protocol and high-throughput sequencing was performed to reach the desired average sequencing depth.
4.2. Read Mapping and Variant Analysis
After the raw image files were processed by Illumina base-calling Software 1.7 for base calling with default parameters, the sequences of each individual were generated as 90bp pair-end reads. The sequenced reads were aligned to the human genome reference (UCSC hg 19 version; build 37.1) using SOAP aligner (soap2.21) software23. Duplicated reads were filtered out and only uniquely mapped reads were kept for subsequent analysis. SOAPsnp (V.1.03) software24 was subsequently used with the default parameters to assemble the consensus sequence and call genotypes in target regions. For SNP quality control, low quality SNPs that meet one of four following criterions were filtered out: (i) Genotype quality less than 20; (ii) Sequencing depth of the site is less than 4; (iii) The estimated copy number is more than 2; (iv) The distance from the adjacent SNPs is less than 5 bp. Small insertions/deletions (Indels) were detected using the Unified Genotype tool from GATK (version v1.0.4705)25 after the high quality reads were aligned to the human reference genome using BWA (version 0.5.9–r16)26.
4.3. Analysis Protocol for Exome Sequencing Results
As the patient was born in a consanguineous family, he would be expected to show an autosomal recessive inheritance pattern. Thus, we first filtered out the synonymous mutations and then eliminated the common variants (frequency >0.5% in dbSNP132, 1000 genomes, HapMap and in-house database). Non-synonymous/splice site (NS/SS) changes and Indels were prioritized according to (a) presence of homozygous or compound heterozygous variants based on a recessive inherited trait; (b) related to PID disease associated genes based on the RAPID website (http://rapid.rcai.riken.jp/RAPID/index_html); (c) predictive software scoring the likelihood for pathogenicity (e.g. Polyphen-2, SIFT, etc.); (d) homozygosity mapping analysis27 was also used to detect the homozygous region to further narrow down candidate genes.
5. Sanger Sequencing
20 ng of genomic DNA was used in PCR employing exon-specific primers (forward primer: CTTCAGCCAAACTTGCAGCTC and reverse primer: CCTCTTTGCAATGGTGTCCAC) with the following conditions: 95°C 3 minutes followed by 94°C 15 seconds, 60°C 30 seconds, 72°C 1 minute for 38 cycles, and a final extension of 72°C for 7 minutes. The PCR products were purified using QIAquick gel extraction kit (Qiagen, Stockholm, Sweden) and subsequently sequenced at the Macrogen Company, South Korea and Eurofins MWG Operon, Germany. The sequences were analysed using the Lasergene software package (DNAStar, Madison, WI, USA).
6. Functional analysis
Recombination activity of the RAG1 mutant protein was analysed using a flow cytometry-based assay as previously described28. Results were expressed as percentage of recombination activity of the wild-type protein.
Results
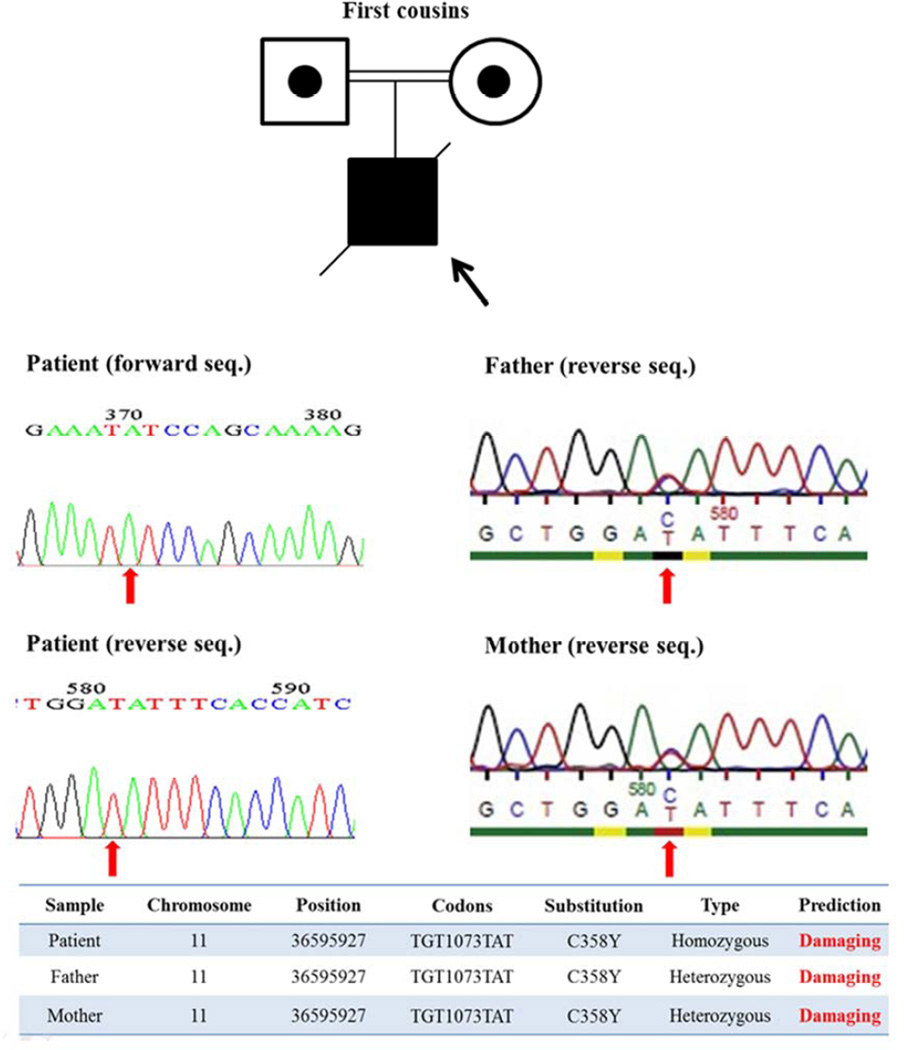
The patient was born to first-degree consanguineous parents in 1989 (Figure 1) with no history of PID in his family. He had a Persian ethnicity and presented at 4 years of age with recurrent pneumonias. He received the regular vaccines included in the Iranian children program (e.g. oral polio and measles, mumps and rubella) without any complicationsHe subsequently developed lymphoproliferative manifestations including retroperitoneal para-aortic lymph node hyperplasia and hepatosplenomegaly (Coombs, Wright, Widal tests were negative and the G6PD level was normal) leading to pancytopenia. Serologic investigations failed to show any direct antiglobulin or antiplatelet antibodies. Leishmandonovani bodies in his bone marrow biopsy were documented and glucantime was administrated (20 mg/kg/day) for 10 days.
Figure 1.
Pedigree and mutation analysis of a RAG1 deficient patient presenting as CVID (shown by arrow)
During the following 6 years, he was admitted to hospital because of severe varicella infection and subsequent pneumonitis, and pulmonary tuberculosis. Despite isolation of Mycobacterium tuberculosis bacillus from the sputum’s samples, his tuberculosis skin test (PPD test) was negative. Liver biopsy was performed because of a long duration of hepatomegaly, refractory to treatment, which revealed a granulomatous hepatitis. The immunologic profile of this patient was evaluated at 10 years of age and demonstrated hypogammaglobulinemia (IgG: 320 mg/dl, IgM: 30 mg/dl, IgA: 15 mg/dl, blood type O yet with an absence of anti-A and anti-B isohemagglutinins), a largely preserved distribution of T and NK cell subsets but with a B cell lymphopenia (CD3: 78.2%, CD4: 55.2%, CD8: 20.0%, CD19: 5.7%, CD20: 3.11%, CD20: 5.7%, CD16: 11.9%, CD56: 6.9%) and normal neutrophil function and complement levels (NBT: 90%, CH50: 96%, C3: 79mg/L, C4: 25 mg/L) (Table 1). According to these data and the clinical and laboratory criteria of PID suggested by the IUIS Expert Committee on PID, a tentative diagnosis of CVID was made, and treatment with intravenous immunoglobulin was initiated.
Table 1.
Immunologic data of a CVID-like patient 438 with RAG1 deficiency
| Parameter | 4y | 7y | 10y | 11y | 12y | 14y | Reference range |
|---|---|---|---|---|---|---|---|
| Leukocytes (cell/ul) | 8,000 | 2,400 | 4,000 | 3,400 | 2,100 | 1,200 | 4,300–11,400 |
| Lymphocytes (% of leukocytes) | 15 | 68 | 48 | 23 | 28 | 26 | 28–67 |
| Neutrophils (% of leukocytes) | 85 | 30 | 45 | 60 | 60 | 64 | 45–70 |
| Haemoglobin (mg/ml) | 8.4 | 8.1 | 9.6 | 9.9 | 10.0 | 10.3 | 12–15 |
| Platelets (cell/ul) | 75,000 | 66,000 | 59,000 | 79,000 | 120,000 | 110,000 | 150,000–500,000 |
| Eosinophils (% of leukocytes) | 2 | 2 | 1 | 1 | 8 | 8 | 0–3 |
| Absolute T cell (cell/ul) | - | - | 1,501 | - | - | 157 | 1,100–4,100 |
| Absolute B cell (cell/ul) | - | - | 109 | - | - | 50 | 200–1,400 |
| Absolute NK cell (cell/ul) | - | - | 228 | - | - | 105 | 70–120 |
| IgG (mg/ml) | - | - | 580* | 260 | 320 | 450 | 600–1,500 |
| IgM (mg/ml) | - | - | <40 | 20 | <40 | <20 | 50–370 |
| IgA (mg/ml) | - | - | <20 | 10 | <20 | <10 | 80–380 |
| IgE (mg/ml) | - | - | 2 | 4 | 5 | 5 | 0–5 |
| Isohemagglutinin titer | - | - | 0 | 0 | 0 | 0 | >1/8 |
The time of start of treatment with intravenous immunoglobulin
Because of early finger clubbing, high resolution computed tomography was performed and showed cylindrical bronchiectasis in the lower lobes of both lungs and atelectasis of the right middle lobe. Pulmonary function tests revealed a mild mixed obstructive/restrictive pattern (FEV1: 63.6%, FVC: 60%, MMEF25–75: 45%). CD19+ CD27+IgD− memory B cells of the patient were 0.0017% of peripheral blood lymphocytes (0.03% of B cells) suggesting a reduced frequency of class switch recombination.
Mutations in all known gene defects associated with CVID, including CD19, CD20, CD21, CD81, ICOS (inducible costimulator), TACI (transmembrane activator, calcium-modulator, and cyclophilin ligand interactor), BAFFR (B-cell activation factor receptor) and LRBA (LPSresponsive beige-like anchor), were ruled out by the exome sequencing data.
Homozygosity mapping suggested one linkage to a 71.68 Mb region on chromosome 11 (11p15.1-11q14.3, p=1.8×10−42, Table 2). As this patient was born in a consanguineous family, he would be expected to show an autosomal recessive inheritance pattern. We first prioritized the exome sequencing results for non-synonymous homozygous mutations, PID associated genes and those being probably damaging as justified by predictive software. We subsequently found a novel, homozygous mutation (c. G1073A, p. C358Y) in the RAG1 gene, which is predicted to be probably damaging by the Polyphen 2 (http://genetics.bwh.harvard.edu/pph2/). This mutation was confirmed by Sanger sequencing in the patient (homozygous form) and his parents (heterozygous form), who were asymptomatic (Figure 1). We furthermore performed a functional test for RAG1 and the results were consistent with a mild phenotype, atypical SCID with granuloma with a residual recombination activity of 48.8±0.6% of wild-type protein, as measured using an artificial green fluorescent protein substrate (Figure 2).
Table 2.
Homozygosity mapping of a common variable immunodeficiency patient with RAG1 deficiency
| Chromosome | Length (Mb) | Start Position | End Position | P value |
|---|---|---|---|---|
| 1 | 19.81 | 69650962 | 89465553 | 2.2×10−12 |
| 1 | 16.85 | 95105523 | 111955492 | 6.1×10−11 |
| 5 | 27.33 | 90383142 | 117710846 | 1.7×10−18 |
| 8 | 16.81 | 107275147 | 124084081 | 6.5×10−13 |
| 10 | 1.30 | 98646501 | 109945399 | 2.9×10−7 |
| 10 | 10.65 | 121492422 | 132145048 | 1.4×10−7 |
| 11* | 71.68 | 20044296 | 91722855 | 1.8×10−42 |
| 16 | 35.4 | 22048426 | 57446002 | 2.9×10−12 |
| 18 | 5.92 | 555760 | 6478249 | 6.1×10−6 |
Where we identified our novel homozygous RAG1 mutation
Figure 2.
A-Mul transformed Rag1−/− murine pro-B cell line was first transduced with a retrovirus carrying a single copy of INV V(D)J substrate containing an inverted GFP flanked by RSS. A clone with a single integrant was selected and transduced with a retrovirus co-expressing either human wild-type (WT) RAG1 and human CD2 (hCD2), mutant C358Y RAG1 and hCD2, or just hCD2. V(D)J recombination activity of the C358Y mutant was calculated by measuring GFP expression, normalized to what observed in the presence of WT RAG1. One representative experiment of three is shown.
Despite regular follow-up and adequate immunoglobulin therapy, the patient progressed clinically with giardiasis and a mucosa-associated lymphoid tissue lymphoma (MALToma) at 11 years of age (refractory to eradicative MALToma therapy), esophageal candidiasis, autoimmune neutropenia (absolute neutrophil count=768/µl, perinuclear pattern anti-neutrophil cytoplasmic antibody=92 IU/ml), atrophic tonsils and lymphopenia (absolute lymphocyte count=312/µl) and moderate hydronephrosis at 13 years old. The patient died at the age of 14 due to combined respiratory and renal failures.
Discussion
To our knowledge, this case is the eighth patient with RAG1 deficiency in the world who presented a phenotype of late onset leaky SCID with granuloma, and the one clinically most similar to CVID. Although the diagnosis of CVID is based on exclusion of other known forms of PID, no case has been reported to have a presentation of a typical CVID as a consequence of RAG1 deficiency. Furthermore, it is not cost-effective to consider RAG1 gene analysis in all patients with a diagnosis of CVID. Therefore, development of some hallmarks and clues may help physicians to select cases for mutation analysis29.
Among the reported patients, most were males (62.5%). The median age of onset was 2 years (range 0.8–5) and the median age of granuloma formation was 6 years (range 2.2–14). The late onset in these patients, which differs from other typical forms of SCID, is due to a gradual reduction in the number and function of B cells, and residual presence (and to some extent, function) of T lymphocytes. This phenomenon leads to a delay in diagnosis of these cases to a median age of 7 years (range 4–14) and clinical mismanagement6, 7, 20–22, 30.
The delayed onset of disease and milder clinical phenotype of these patients are consistent with residual levels of recombination activity of the mutant RAG1 protein (ranging from less than 5% to 50% of wild-type protein)6, 7, 20–22, 30, that is sufficient to support partial preservation of a diversified T cell repertoire. A similar leaky phenotype has also been demonstrated in mice homozygous for the S723C Rag1 mutation3. Recombination activity of the C358Y RAG1 mutant protein was performed using a recently described method, in which flow cytometry-based measurement of GFP expression serves as a rapid read-out of RAG1 function on an intrachromosomal substrate. With this assay, the C358Y RAG1 mutant had 48% recombination activity of wild-type RAG1. This result should not be confused with the condition in heterozygous healthy carriers of null RAG1 mutations who express lower amounts of RAG1 protein, which however is functionally intact, enabling efficient V(D)J recombination and generation of a diversified T and B cell repertoire. By contrast, even 50% reduction of recombination activity at the protein level has been shown to affect generation of a diversified pool of V(D)J rearrangements at the endogenous Igh locus28.
Although most reported patients carried compound heterozygous mutations (Figure 3), the current case is the fourth individual with a homozygous RAG1 mutation. Indeed, efforts in finding a correlation between a given mutation and the clinical outcome have failed. However, there have been some reports in which the same mutation resulted in a different clinical picture of SCID, suggestive of modifying roles of as yet unknown genetic or epigenetic mechanisms7, 16, 31, 32.
Figure 3.
The location of RAG1 mutation in patients with leaky SCID and a granulomatous phenotype by compound heterozygous (P1–P4) and homozygous mutations (P5–P7, New P). CC: catalytic core, NBD: nanomer binding domain, UL: Ubiquitin ligase activity, HD: Homeodomain6, 7, 20–22, 30
Although RAG1 defects are characterized by B cell lymphopenia, 20% of the patients, mostly with consanguineous parents, present a B+phenotype with viral infections and BCG infection7. In the patient reported here, circulating B cells were detectable but markedly reduced in number. Immunoglobulin serum levels in patients with atypical forms of RAG deficiency may be variable, ranging from normal30 to selective IgA deficiency6, 22, or agammaglobulinemia20. One patient with leaky SCID due to compound heterozygosity for RAG2 mutations (p.T77N and C451A) has been reported, who was initially diagnosed with CVID6. A variable number of peripheral B cells with clonotypic restrictions, have also been observed in mice with homozygous hypomorphic Rag1 mutations3, 33.
One of the most important shared features of leaky SCID and CVID in our case was immune dysregulation. Inflammatory and autoimmune manifestations, including autoimmune cytopenia21, 30, vitiligo30 and myasthenia gravis22, have been reported in 57% of known leaky cases of RAG1 mutations associated with granuloma. Impaired receptor editing, and defects of central and peripheral T cell tolerance have been reported in patients and animal models with RAG deficiency, and may account for the autoimmunity of the disease34, 35. Furthermore, inflammatory complications, due to elevated levels of IL-1, a skewed Th2 cytokine profile and oligoclonal expansion of T cell clonotypes36, 37, have been reported in 50% of these patients, causing organomegaly and lymphadenopathy21, 30.
Granulomatous lesions, restricted to the skin or also involving other organs, have been reported in the majority of patients with hypomorphic RAG1 mutations and a delayed-onset presentation6, 7, 20–22, 30, 38. Although skin involvement was not documented in our case, this may still represent an important factor in the differential diagnosis between RAG1 deficiency and CVID, because cutaneous granulomatous lesions are rare in the latter39. Moreover, granulomas in RAG1 deficient patients are mostly necrotizing6, 20, 22, 30, whereas CVID patients more frequently show a non-caseating type40, 41. Nonetheless, the observation that not all patients with leaky RAG1 mutations manifest immune dysregulation indicates that epigenetic and/or environmental factors play important roles in shaping the clinical phenotype7, 34.
Our case developed a MALToma which has not been previously reported in patients with delayed-onset RAG1 deficiency. However, an Epstein–Barr virus (EBV) induced lymphoma has been described in one case6 and the overall clinical pattern of lymphoid malignancies in these patients is compatible with CVID42. The pathophysiology of cancers in RAG1 deficient patients with leaky SCID can be explained by aberrant generation of broken DNA ends and premature release of the post cleavage complex which may engage in interlocus trans-rearrangements and activation of proto-oncogenes, although this has not been clearly demonstrated in CVID patients3, 6.
One of the most important point for differing CVID from RAG1 deficiency is the pattern of infections, where severe systemic viral infections, especially varicella (in 83.3%), is more prevalent in RAG1 deficient patients with granuloma, suggesting a dysregulated response to viral antigens43.
In summary, this case illustrates the need for careful assessment of patients with hypogammaglobulinemia and granulomatous lesions. Establishing a correct differential diagnosis between CVID and RAG deficiency (or other forms of leaky SCID) in such cases is essential to initiation of appropriate treatment and improvement of survival and quality of life.
Clinical Implications.
Patients with a diagnosis of hypogammaglobulinemia, granulomatous lesions and a gradual decrease in the number of B and T cells should be investigated for RAG deficiency. In these patients, it is essential to promptly initiate appropriate treatment with curative hematopoietic stem cell transplantation rather than immunoglobulin replacement.
Acknowledgments
Financial disclosure: The research was funded in part by grants from the Swedish Research Council. This work was also supported by NIH grant U54AI082973 and by a grant from The Jeffrey Modell Foundation (to L.D.N.)
List of abbreviations
- CVID
common variable immunodeficiency
- NHEJ
non-homologous end joining proteins
- PID
primary immunodeficiency
- RAG1
recombination activation genes1
- SCID
severe combined immunodeficiency
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: None
References
- 1.Hesslein DG, Schatz DG. Factors and forces controlling V(D)J recombination. Adv Immunol. 2001;78:169–232. doi: 10.1016/s0065-2776(01)78004-2. [DOI] [PubMed] [Google Scholar]
- 2.Gellert M. V(D)J recombination: RAG proteins, repair factors, and regulation. Annu Rev Biochem. 2002;71:101–132. doi: 10.1146/annurev.biochem.71.090501.150203. [DOI] [PubMed] [Google Scholar]
- 3.Giblin W, Chatterji M, Westfield G, Masud T, Theisen B, Cheng HL, et al. Leaky severe combined immunodeficiency and aberrant DNA rearrangements due to a hypomorphic RAG1 mutation. Blood. 2009;113:2965–2975. doi: 10.1182/blood-2008-07-165167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huye LE, Purugganan MM, Jiang MM, Roth DB. Mutational analysis of all conserved basic amino acids in RAG-1 reveals catalytic, step arrest, and joining-deficient mutants in the V(D)J recombinase. Mol Cell Biol. 2002;22:3460–3473. doi: 10.1128/MCB.22.10.3460-3473.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones JM, Gellert M. Intermediates in V(D)J recombination: a stable RAG1/2 complex sequesters cleaved RSS ends. Proc Natl Acad Sci U S A. 2001;98:12926–12931. doi: 10.1073/pnas.221471198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schuetz C, Huck K, Gudowius S, Megahed M, Feyen O, Hubner B, et al. An immunodeficiency disease with RAG mutations and granulomas. N Engl J Med. 2008;358:2030–2038. doi: 10.1056/NEJMoa073966. [DOI] [PubMed] [Google Scholar]
- 7.Kutukculer N, Gulez N, Karaca NE, Aksu G, Berdeli A. Novel mutations and diverse clinical phenotypes in recombinase-activating gene 1 deficiency. Ital J Pediatr. 2012;38:8. doi: 10.1186/1824-7288-38-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buckley RH, Schiff RI, Schiff SE, Markert ML, Williams LW, Harville TO, et al. Human severe combined immunodeficiency: genetic, phenotypic, and functional diversity in one hundred eight infants. J Pediatr. 1997;130:378–387. doi: 10.1016/s0022-3476(97)70199-9. [DOI] [PubMed] [Google Scholar]
- 9.van der Burg M, Weemaes CM, Preijers F, Brons P, Barendregt BH, van Tol MJ, et al. B-cell recovery after stem cell transplantation of Artemis-deficient SCID requires elimination of autologous bone marrow precursor-B-cells. Haematologica. 2006;91:1705–1709. [PubMed] [Google Scholar]
- 10.Sobacchi C, Marrella V, Rucci F, Vezzoni P, Villa A. RAG-dependent primary immunodeficiencies. Hum Mutat. 2006;27:1174–1184. doi: 10.1002/humu.20408. [DOI] [PubMed] [Google Scholar]
- 11.Asai E, Wada T, Sakakibara Y, Toga A, Toma T, Shimizu T, et al. Analysis of mutations and recombination activity in RAG-deficient patients. Clin Immunol. 2011;138:172–177. doi: 10.1016/j.clim.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 12.Cossu F. Genetics of SCID. Ital J Pediatr. 2010;36:76. doi: 10.1186/1824-7288-36-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Honig M, Schwarz K. Omenn syndrome: a lack of tolerance on the background of deficient lymphocyte development and maturation. Curr Opin Rheumatol. 2006;18:383–388. doi: 10.1097/01.bor.0000231907.50290.6f. [DOI] [PubMed] [Google Scholar]
- 14.Villa A, Notarangelo LD, Roifman CM. Omenn syndrome: inflammation in leaky severe combined immunodeficiency. J Allergy Clin Immunol. 2008;122:1082–1086. doi: 10.1016/j.jaci.2008.09.037. [DOI] [PubMed] [Google Scholar]
- 15.Santagata S, Villa A, Sobacchi C, Cortes P, Vezzoni P. The genetic and biochemical basis of Omenn syndrome. Immunol Rev. 2000;178:64–74. doi: 10.1034/j.1600-065x.2000.17818.x. [DOI] [PubMed] [Google Scholar]
- 16.Villa A, Sobacchi C, Notarangelo LD, Bozzi F, Abinun M, Abrahamsen TG, et al. V(D)J recombination defects in lymphocytes due to RAG mutations: severe immunodeficiency with a spectrum of clinical presentations. Blood. 2001;97:81–88. doi: 10.1182/blood.v97.1.81. [DOI] [PubMed] [Google Scholar]
- 17.de Villartay JP, Lim A, Al-Mousa H, Dupont S, Dechanet-Merville J, Coumau-Gatbois E, et al. A novel immunodeficiency associated with hypomorphic RAG1 mutations and CMV infection. J Clin Invest. 2005;115:3291–3299. doi: 10.1172/JCI25178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ehl S, Schwarz K, Enders A, Duffner U, Pannicke U, Kuhr J, et al. A variant of SCID with specific immune responses and predominance of gamma delta T cells. J Clin Invest. 2005;115:3140–3148. doi: 10.1172/JCI25221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karaca NE, Aksu G, Genel F, Gulez N, Can S, Aydinok Y, et al. Diverse phenotypic and genotypic presentation of RAG1 mutations in two cases with SCID. Clin Exp Med. 2009;9:339–342. doi: 10.1007/s10238-009-0053-1. [DOI] [PubMed] [Google Scholar]
- 20.Sharapova SO, Migas A, Guryanova I, Aleshkevich S, Kletski S, Durandy A, et al. Late-onset combined immune deficiency associated to skin granuloma due to heterozygous compound mutations in RAG1 gene in a 14 years old male. Hum Immunol. 2013;74:18–22. doi: 10.1016/j.humimm.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 21.Schuetz C, Niehues T, Friedrich W, Schwarz K. Autoimmunity, autoinflammation and lymphoma in combined immunodeficiency (CID) Autoimmun Rev. 2010;9:477–482. doi: 10.1016/j.autrev.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 22.De Ravin SS, Cowen EW, Zarember KA, Whiting-Theobald NL, Kuhns DB, Sandler NG, et al. Hypomorphic Rag mutations can cause destructive midline granulomatous disease. Blood. 2010;116:1263–1271. doi: 10.1182/blood-2010-02-267583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li R, Yu C, Li Y, Lam TW, Yiu SM, Kristiansen K, et al. SOAP2: an improved ultrafast tool for short read alignment. Bioinformatics. 2009;25:1966–1967. doi: 10.1093/bioinformatics/btp336. [DOI] [PubMed] [Google Scholar]
- 24.Li R, Li Y, Fang X, Yang H, Wang J, Kristiansen K, et al. SNP detection for massively parallel whole-genome resequencing. Genome Res. 2009;19:1124–1132. doi: 10.1101/gr.088013.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, Kernytsky A, et al. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010;20:1297–1303. doi: 10.1101/gr.107524.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li H, Durbin R. Fast and accurate long-read alignment with Burrows-Wheeler transform. Bioinformatics. 2010;26:589–595. doi: 10.1093/bioinformatics/btp698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lamperti C, Fang M, Invernizzi F, Liu X, Wang H, Zhang Q, et al. A novel homozygous mutation in SUCLA2 gene identified by exome sequencing. Mol Genet Metab. 2012;107:403–408. doi: 10.1016/j.ymgme.2012.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee YN, Frugoni F, Dobbs K, Walter JE, Giliani S, Gennery AR, et al. A systematic analysis of recombination activity and genotype-phenotype correlation in human recombination-activating gene 1 deficiency. J Allergy Clin Immunol. 2013 doi: 10.1016/j.jaci.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liadaki K, Sun J, Hammarstrom L, Pan-Hammarstrom Q. New facets of antibody deficiencies. Curr Opin Immunol. 2013;25:629–638. doi: 10.1016/j.coi.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 30.Reiff A, Bassuk AG, Church JA, Campbell E, Bing X, Ferguson PJ. Exome Sequencing Reveals RAG1 Mutations in a Child with Autoimmunity and Sterile Chronic Multifocal Osteomyelitis Evolving into Disseminated Granulomatous Disease. J Clin Immunol. 2013;33:1289–1292. doi: 10.1007/s10875-013-9953-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xiao Z, Yannone SM, Dunn E, Cowan MJ. A novel missense RAG-1 mutation results in T-B-NK+ SCID in Athabascan-speaking Dine Indians from the Canadian Northwest Territories. Eur J Hum Genet. 2009;17:205–212. doi: 10.1038/ejhg.2008.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gruber TA, Shah AJ, Hernandez M, Crooks GM, Abdel-Azim H, Gupta S, et al. Clinical and genetic heterogeneity in Omenn syndrome and severe combined immune deficiency. Pediatr Transplant. 2009;13:244–250. doi: 10.1111/j.1399-3046.2008.00970.x. [DOI] [PubMed] [Google Scholar]
- 33.Walter JE, Rucci F, Patrizi L, Recher M, Regenass S, Paganini T, et al. Expansion of immunoglobulin-secreting cells and defects in B cell tolerance in Rag-dependent immunodeficiency. J Exp Med. 2010;207:1541–1554. doi: 10.1084/jem.20091927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ijspeert H, Driessen GJ, Moorhouse MJ, Hartwig NG, Wolska-Kusnierz B, Kalwak K, et al. Similar recombination-activating gene (RAG) mutations result in similar immunobiological effects but in different clinical phenotypes. J Allergy Clin Immunol. 2014 doi: 10.1016/j.jaci.2013.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cassani B, Poliani PL, Moratto D, Sobacchi C, Marrella V, Imperatori L, et al. Defect of regulatory T cells in patients with Omenn syndrome. J Allergy Clin Immunol. 2010;125:209–216. doi: 10.1016/j.jaci.2009.10.023. [DOI] [PubMed] [Google Scholar]
- 36.Aksentijevich I, Nowak M, Mallah M, Chae JJ, Watford WT, Hofmann SR, et al. De novo CIAS1 mutations, cytokine activation, and evidence for genetic heterogeneity in patients with neonatal-onset multisystem inflammatory disease (NOMID): a new member of the expanding family of pyrin-associated autoinflammatory diseases. Arthritis Rheum. 2002;46:3340–3348. doi: 10.1002/art.10688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aksentijevich I, Masters SL, Ferguson PJ, Dancey P, Frenkel J, van Royen-Kerkhoff A, et al. An autoinflammatory disease with deficiency of the interleukin-1-receptor antagonist. N Engl J Med. 2009;360:2426–2437. doi: 10.1056/NEJMoa0807865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Felgentreff K, Perez-Becker R, Speckmann C, Schwarz K, Kalwak K, Markelj G, et al. Clinical and immunological manifestations of patients with atypical severe combined immunodeficiency. Clin Immunol. 2011;141:73–82. doi: 10.1016/j.clim.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 39.Aghamohammadi A, Abolhassani H, Rezaei N, Kalantari N, Tamizifar B, Cheraghi T, et al. Cutaneous granulomas in common variable immunodeficiency: case report and review of literature. Acta Dermatovenerol Croat. 2010;18:107–113. [PubMed] [Google Scholar]
- 40.Abdel-Naser MB, Wollina U, El Hefnawi MA, Habib MA, El Okby M. Non-sarcoidal, non-tuberculoid granuloma in common variable immunodeficiency. J Drugs Dermatol. 2006;5:370–372. [PubMed] [Google Scholar]
- 41.Ardeniz O, Cunningham-Rundles C. Granulomatous disease in common variable immunodeficiency. Clin Immunol. 2009;133:198–207. doi: 10.1016/j.clim.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abolhassani H, Aghamohammadi A, Imanzadeh A, Mohammadinejad P, Sadeghi B, Rezaei N. Malignancy phenotype in common variable immunodeficiency. J Investig Allergol Clin Immunol. 2012;22:133–134. [PubMed] [Google Scholar]
- 43.Kyewski B, Klein L. A central role for central tolerance. Annu Rev Immunol. 2006;24:571–606. doi: 10.1146/annurev.immunol.23.021704.115601. [DOI] [PubMed] [Google Scholar]