Abstract
Background
Some investigators find a deficiency in interferon (IFN) production from airway epithelial cells infected with human rhinovirus in asthma, but whether this abnormality occurs with other respiratory viruses is uncertain.
Objective
To assess the effect of influenza A virus (IAV) and respiratory syncytial virus (RSV) infection on IFN production and viral level in human bronchial epithelial cells (hBECs) from subjects with and without asthma.
Methods
Primary-culture hBECs from subjects with mild to severe asthma (n=11) and controls without asthma (hBECs; n=7) were infected with live or UV-inactivated IAV (WS/33 strain), RSV (Long strain), or RSV (A/2001/2-20 strain) with MOI 0.01-1. Levels of virus along with IFN-β and IFN-λ and IFN-stimulated gene expression (tracked by OAS1 and MX1 mRNA) were determined up to 72 hours post-inoculation.
Results
After IAV infection, viral levels were increased 2-fold in hBECs from asthmatic compared to non-asthmatic control subjects (p<0.05) and this increase occurred in concert with increased IFN-λ1 levels and no significant difference in IFNB1, OAS1 or MX1 mRNA levels. After RSV infections, viral levels were not significantly increased in hBECs from asthmatic versus nonasthmatic subjects, and the only significant difference between groups was a decrease in IFN-λ levels (p<0.05) that correlated with decrease in viral titer. All of these differences were found only at isolated time points and were not sustained throughout the 72-hour infection period.
Conclusions
The results indicate that IAV and RSV control and IFN response to these viruses in airway epithelial cells is remarkably similar between asthmatic and non-asthmatic subjects.
Keywords: Asthma, Interferon, Influenza A virus, Respiratory syncytial virus, Primary-culture airway epithelial cells
INTRODUCTION
The interferon (IFN) production and signaling pathway is critical for defense against viral infections since it is required for controlling viral replication 1, 2. Accordingly, there is considerable interest in whether variation in the IFN system might account for susceptibility to viral infection. In that regard, it has been recognized that individuals with asthma may be more susceptible to respiratory viral infections and more likely to exhibit slower viral clearance and worse respiratory symptoms, including exacerbations of their underlying disease 3-7. In fact, respiratory viral infections are perhaps the most common trigger of asthma exacerbations 5, 7-10. Moreover, infection with some types of viruses, e.g., respiratory syncytial virus (RSV) and human rhinovirus (HRV) are also implicated in the development of asthma in early childhood 11-21. Thus, it is natural to consider that any possible increase in the incidence or severity of viral infection in asthma might be due to a deficiency in antiviral defense, and in particular a suboptimal pathway for IFN production or signaling 6.
A further extension of the proposal for IFN deficiency in asthma derives from the role of the airway epithelial cell in host defense. For example, the IFN response of airway epithelial cells appears to be required for adequate defense against respiratory viruses in experimental mouse models 22. However, the concept remains to be formally proven in experimental systems and the data for IFN deficiency in asthma remains controversial. Some reports indicate that human bronchial epithelial cells (hBECs) isolated from asthmatic subjects and then placed in culture will produce lower levels of IFN-β and IFN-λ in response to infection with HRV-A16 and that this deficiency will lead to increases in viral level 23-25. These observations have led to clinical trials of IFN treatment to prevent and/or attenuate the impact of viral infections in asthma. By contrast, a series of other reports show no significant difference in IFN production or signaling in response to HRV-A16 infection in hBECs isolated and cultured from asthmatic compared to non-asthmatic subjects 26-28.
There are many possible reasons for the difference in study outcomes, but one particular concern is the nature of the cell culture system used to assess the IFN system. In particular, the use of undifferentiated cells cultured under submerged conditions versus well-differentiated cells maintained under air-liquid interface conditions might have profound effects on the susceptibility to viral infection. It is uncertain whether well-differentiated cell cultures (that mimic physiologic conditions) or undifferentiated cultures (that might mimic epithelial damage in pathologic conditions) are more relevant to natural infection in vivo. The evidence for IFN deficiency in cells from asthma was found in undifferentiated cultures 24 and at least one group was unable to confirm this abnormality despite similar culture conditions 28. However, in both cases, the studies include only a limited number of conditions to optimize viral yield, and the main endpoint of viral titer can be difficult to monitor if viral replication rates are low. Moreover, HRV replication varies significantly with temperature and strain 29, adding additional complexity to defining any differences in HRV control. For example, studies reporting a defect in asthma used the HRV-A16 strain 23, 24 whereas one finding no difference used HRV-A1 28. Furthermore, the previous studies aimed at HRV but did not address other types of viruses despite experimental evidence that paramyxovirus and influenza virus replicate at high efficiencies in the lower airways and in airway epithelial cell cultures and may cause more severe and longer lasting airway disease in experimental models studied in vivo 30, 31 and perhaps in the setting of clinical infections as well 16, 21, 32, 33.
Based on these uncertainties, we reasoned it would be useful to re-examine viral level and IFN response in airway epithelial cells under comprehensive conditions that are optimized for virus-induced IFN production. In addition, given the difficulties in reaching firm conclusions for HRV infection, we aimed to assess other types of respiratory viruses that are also sensitive to IFN actions and are implicated in asthma pathogenesis. We included RSV, which has not yet been assessed in this type of system despite its association with asthma. We also assessed influenza A virus (IAV) which was also implicated in asthma exacerbations and was found to disproportionately impact asthmatics in the latest U.S. epidemic of influenza 32, 33. An initial study of IAV (using the H3N2 A/Bangkok/1/79 strain) found no significant difference in viral clearance and no loss of IFN production in hBECs from asthmatic compared to nonasthmatic subjects 34, However, this study used well-differentiated hBEC cultures so the results cannot be compared directly to the original reports of IFN deficiency in asthma. It therefore remains uncertain whether reports of RSV and IAV link to asthma in humans might be a consequence of IFN deficiency and/or higher viral levels and/or more severe disease.
Here we assess viral level and IFN production and action after inoculation with RSV and IAV in primary-culture hBECs obtained from asthmatic and non-asthmatic subjects and cultured under submerged conditions. We chose these culture conditions to best capture the difference in viral level and IFN production found in previous reports and to avoid inter-subject differences in the degree of airway epithelial cell differentiation that might itself influence viral infection and antiviral response. We utilize well-characterized strains of RSV (RSV Long and RSV A/2001/2-20) and IAV (IAV WS/33), and we establish conditions for high-level viral replication and monitor both type I IFN (marked by IFN-β) and type III IFN (marked by IFN-λ) production as well as interferon-stimulated gene (ISG) expression over a full 72-hour time course of infection. Despite this comprehensive approach, we find a remarkable similarity rather than a significant difference between airway epithelial cells from asthmatic versus nonasthmatic individuals, suggesting that innate epithelial control over two key respiratory pathogens (RSV and IAV) is preserved in this disease state.
METHODS
Study subjects
Eighteen subjects (11 asthmatics and 7 nonasthmatic controls) were recruited for study and were characterized as summarized in Table I (for all asthmatics) and detailed in Table S1 (for each asthmatic severity subset). For asthmatic subjects, the diagnosis and severity of asthma were based on NAEPP guidelines, 35 symptoms of asthma were reported within the past 12 months, and reversible airway obstruction (defined as ≥12% and 200 ml increase in FEV1 with inhaled bronchodilator) and bronchial hyperreactivity (defined as a provocative concentration of methacholine causing a decline in FEV1 of 20% or ≤16 mg/ml) were present on pulmonary function testing. The clinical characteristics of asthmatic and nonasthmatic subjects for each group of experiments were not significantly different from the group as a whole. For nonasthmatic control subjects, participants were required to be in good overall health with no history of asthma, allergy, or nasal or sinus disease. For all subjects, atopic status was defined as one or more positive allergy skin tests to a panel of 14 prevalent US-wide aeroallergens, or alternatively, a positive result to allergen-specific IgE (8 types) by ImmunoCAP assay if skin testing could not be performed. None of the study participants had significant bronchospasm, history of respiratory failure requiring intubation, bronchial or upper respiratory tract infection (including sinus infection) within the month before assessment and had no history of tobacco smoking in the year before assessment. All subjects provided informed consent under a study protocol that was approved by the Human Studies Committee of the Washington University Institutional Review Board.
Table I.
Clinical characteristics of asthmatic and nonasthmatic subjects.
| Characteristic | Nonasthmatic | Asthmatic | p Value |
|---|---|---|---|
| n | 7 | 11 | |
| Age (yr) | 26.3 ± 4.0 | 31.8 ± 2.4 | 0.222 |
| Gender (%M\%F) | 43\57 | 36\64 | 0.335 |
| Atopy (%, n) | 71, 5 | 82, 9 | 1.0 |
| IgE (lu/ml) | 65.8 ± 41.4 | 402.5 ± 111.8 | 0.011 |
| FEV1 (L) | 3.9 ± 0.35 | 3.0 ± 0.26 | 0.061 |
| FEV1 (%pred) | 102.6 ± 3.7 | 85.1 ± 3.8 | 0.007 |
| Max. FEV1 post-BD | 4.0 ± 0.4 | 3.4 ± 0.3 | 0.190 |
| %change FEV1 post-BD | 3.5 ± 1.3 | 14.3 ± 3.4 | 0.011 |
| PC20(mg/ml) | >16 | 1.6 ± 0.5 | < 0.0001 |
| % ICS (%, n) | 0, 0 | 45, 5 |
Values are presented as means ± SEM, and p values are based on Fisher's exact test for gender and atopy comparisons or unpaired t-test for other comparisons. The characteristics for the total group of nonasthmatics and asthmatics was not significantly different for the subsets of nonasthmatics (n=6) and asthmatics (n=8 and 9) used for analysis of RSV inoculations.
BD: Bronchodilator.
FEV1: Forced Expiratory Volume in one second; ICS, Inhaled corticosteroid; PC20: Provocative concentration of methacholine that causes a 20% decrease in FEV1.
Cell isolation and culture
Primary-culture human tracheal epithelial cells (hTECs) were isolated as described previously 36 from samples obtained from lung transplant donors with no evidence of underlying lung disease (including asthma) based on standardized guidelines 37. For the present study, hTEC cultures were maintained on collagen-coated tissue culture plates in BEGM under submerged conditions to prevent further cell differentiation as described previously 38. Primary-culture human bronchial epithelial cells (hBECs) were obtained from endobronchial brushings of six separate segmental or subsegmental airways in the lower lobes performed with an epithelial cytology brush (Medical Engineering Lab, Inc., Shelby, NC) guided by flexible fiberoptic bronchoscopy. The cells were rinsed from the brush into DMEM/F12 media and were kept on ice for transport. The harvested cells were disaggregated with a vortex mixer, pelleted by centrifugation, resuspended in Ham's F12 media, and treated with pronase for 15-30 min at 25 °C before transfer to bronchial epithelial growth medium (BEGM, Lonza, Walkersville, MD) and culture on collagen-coated 12-well tissue culture plates. Cells were passaged once and then stored under liquid nitrogen in medium containing 10% DMSO and 20% FBS. For viral infection experiments, vials of frozen cells were thawed, cultured for 3-6 days to achieve confluence, and then trypsinized and plated on collagen-coated 12-well plates for study at passage 3 under submerged culture conditions.
Virus infection conditions
Infection conditions were optimized using primary-culture human tracheal epithelial cells (hTECs) that were cultured on collagen-coated 12-well tissue culture plates in BEGM at a density of 5 × 104 cells/well. Cultured cells were infected with IAV A/WS/33 lab strain or RSV Long lab strain (MOI of 0, 0.01, 0.1 or 1.0) or RSV A/2001/2-20 clinical isolate strain 39 (MOI of 0, 0.01, or 0.10) in BEGM for 1 h and then washed twice with DPBS. At 8, 24, 48, and 72 h after viral inoculation, cell lysates and supernatants were collected to determine levels of virus as well as IFNB1 and IFNL1,2/3 mRNA and/or IFN-β and IFN-λ1,2/3 protein using real-time qPCR assay and ELISA.
Based on optimization in hTECs as described above, hBEC cultures were inoculated with IAV A/WS/33 (MOI 0.01), RSV Long (MOI 1), or RSV A/2001/2-20 (MOI 1) or an equivalent amount of UV-inactivated virus in BEGM for 1 h and then washed with DPBS. Triplicate samples were assessed for each experimental condition. At 8-72 after inoculation, cell supernatants were collected, and adherent cells were lysed using Trizol (Life Technologies, Carlsbad, CA). Cell supernatants and lysates were stored at −80 °C and then used to purify RNA using the 96-well viral RNA and 96-well Quick RNA kits (Zymo Research Corporation, Irvine, CA), respectively.
Assessment of cytopathic effect
The LDH cytoxicity kit (Roche Applied Sciences, Indianapolis, IN) was used according to the manufacturer's instructions. For the present experiments, 50 μl of LDH solution was incubated with 50 μl of cell supernatant for 30 min at 25 °C, and absorbance was determined at 490 nm and compared to background at 700 nm in flat-bottom clear 96-well plates on a Synergy 4 plate reader (BioTek, Winooski, VT).
Assays of RNA levels
To quantify released viral level, RNA from cell supernatants was subjected directly to real-time qPCR reactions using the TaqMan Fast Virus 1-Step master mix (Life Technologies). To quantify cellular viral level and host gene expression, cellular RNA was used to generate cDNA using the High Capacity cDNA kit (Life Technologies) and then subjected to real-time qPCR assay in accordance with the Minimum Information for Publication of Quantitative Real-Time PCR Experiments (MIQE) guidelines 40, 41. Assays were performed in duplicate in 384-well plates using 3-μl reactions and a LightCycler480 thermocycler (Roche Applied Sciences). OAS1, and MX1 levels were assessed using qPCR assay primers, probes and standards as described previously 42, 43. IAV level was monitored using a qPCR assay for the PA gene (segment 3) with forward and reverse primers and probe 5’-ggccgactacactctcgatga-3’, 5’- tgtcttatggtgaatagcctggttt-3’, and 5’- agcagggctaggatc-3’, respectively, and a plasmid containing the PA gene as a standard. RSV level was monitored using a qPCR assay for the L gene using forward and reverse primers and probe 5’-tccctacggttgtgatcgataga-3’, 5’-tgatgggaagtagtagtgtaaagttggt-3’, and 5’-aggtaatacagccaaatc-3’, respectively, and a plasmid containing the L gene as a standard. Levels of IFNL1, IFNL2/3, and IFNB1 mRNA were determined using the Hs00601677_g1, Hs00820125_g1, and Hs01077958_s1 TaqMan assays (Life Technologies), respectively. Levels of IFNB1 mRNA copy number were quantified using a plasmid standard, and relative levels of IFNL1 and IFNL2/3 mRNA were determined using dilutions of RNA from hTECs infected with IAV (MOI 1) as a semi-quantitative standard. All values were normalized to level of OAZ1 mRNA (1 × 103 copies per sample) as described previously 42.
Assays of IFN levels
To quantify IFN-λ levels, cell supernatants were analyzed with the human IFN-λ1/3 DuoSet ELISA kit (R&D Systems, Minneapolis, MN) according to the manufacturer's instructions. To quantify IFN-β levels, cell supernatants were incubated in high-binding white half-area plates overnight at 4 °C. Plates were then washed with PBS and incubated with mouse-anti-human IFN-β antibody (clone MMHB-3, PBL Interferon Source, Piscataway, NJ) for 2 h at 25 °C, and then with HRP-conjugated goat-anti-mouse conjugated secondary antibody for 1 h followed by Glo chemiluminescent substrate (R&D Systems) Luminescence was determined using a Synergy 4 plate reader. Assay sensitivity was 30 pg/ml.
Statistical analysis
Statistical analysis was performed with Graphpad Prism software (version 6, Graphpad Software, La Jolla, CA). Unpaired parametric t-test and Fisher's exact test were used to compare clinical characteristics. Welch's correction for t-test was used for comparison of data sets with unequal variances. Two-way ANOVA with Bonferroni adjustment for multiple comparisons was used to detect any differences in the values for viral level or effect in cells from asthmatic and nonasthmatic subjects inoculated with live and inactivated virus. Thus, comparisons were made for the set of values after live and UV-inactivated virus in asthmatic and nonasthmatic subjects at each post-inoculation time point. Two-way ANOVA was also used to detect any difference in the values after live virus inoculation in asthmatic versus nonasthmatic subjects over the time course of the experiment. Data are represented as mean ± SEM, and a p value of less than 0.05 was considered as significant.
RESULTS
IFN and ISG levels after IAV infection
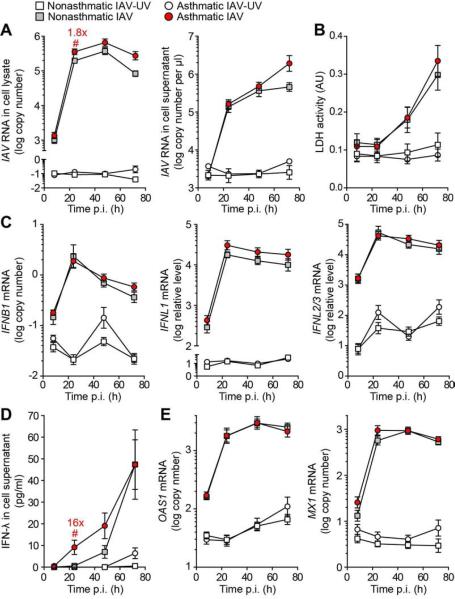
Screening experiments in hTEC cultures (that were available in greater numbers than hBECs) examined a broad range of viral inoculation levels to establish conditions for viral replication and IFN production. Initial experiments indicated that hTEC and hBEC cultures exhibited similar levels of viral replication and consequent viral titer based on inoculation with IAV (strain A/WS/33) (Figure E1). For this viral strain, an MOI of 0.01 resulted in maximal IFNB1 and IFNL1,2/3 mRNA and IFN-β and IFN-λ protein levels (Figure E2 and data not shown). Similarly, inoculation with IAV (MOI 0.01) resulted in a marked increase in viral RNA levels in hBECs cultured from asthmatic and healthy nonasthmatic subjects (Figure 1A). In both types of subjects, the levels of virus in cell lysates and supernatants increased >1000-fold with a similar time course over 72 h. Levels of viral RNA in cell lysates from asthmatic subjects were approximately twice the level compared with nonasthmatic subjects at 24, 48 and 72 h after inoculation, but these differences were significant only at 24 h. Similarly, there was no significant difference in virus-induced cytopathic effect (based on LDH release) between hBECs from asthmatic and nonasthmatic subjects at any time point after inoculation (Figure 1B).
FIG 1.
Viral level and IFN response for IAV (A/WS/33 strain, MOI 0.01) infection in hBEC cultures from asthmatic (n=11) and nonasthmatic (n=7) subjects. A, IAV RNA levels in cell lysate and supernatant at indicated times post-inoculation (p.i.). B, IAV-induced cell toxicity marked by LDH release in cell supernatant. C, IFNB1, IFNL1, and IFNL2/3 mRNA levels. D, IFN-λ protein levels in cell supernatant. E, OAS1 and MX1 mRNA levels. ### p<0.001, ## p<0.01, # p<0.05 for the set of values for IAV and UV-inactivated IAV (IAV-UV) in asthmatics and nonasthmatics at each time point..*** p<0.001, ** p<0.01, * p<0.05 for the values for IAV in asthmatic versus nonasthmatic subjects over the time course of the experiment. The numbers in red indicate the fold-difference in the value for IAV in asthmatic versus nonasthmatic subjects at time points of statistical significance.
In concert with IAV replication, IFNB1 mRNA levels increased approximately 100-fold while IFNL1 mRNA levels increased by 1000-fold and IFNL2/3 mRNA levels by 500-fold after inoculation with IAV compared to UV-inactivated IAV in hBECs from asthmatic and nonasthmatic subjects (Figure 1C). None of the increases in IFN mRNA levels were significantly different between hBECs from asthmatic and nonasthmatic subjects. IFN-β protein levels in cell supernatants were below the limit of detection for all samples (data not shown) but IFN-λ levels progressively increased to a maximum of approximately 50 pg/ml after IAV inoculation (Figure 1D). IFN-λ levels were increased by 16-fold in hBECs from asthmatic compared to nonasthmatic subjects at 24 h post-inoculation, but levels were not significantly different between the two groups at later time points.
In addition to increases in viral and IFN levels, we also found induction of representative IFN-stimulated gene (ISG) expression marked by OAS1 and MX1 mRNA levels after IAV inoculation (Figure 1E). Both viral level and ISG expression were increased 2 to 3-fold at selected time points in hBECs from severe asthmatic subjects compared to nonasthmatic subjects after IAV inoculation but in general each group exhibited similar levels of IAV RNA and ISG expression (Figure E3). Moreover, similar to the case for IAV and IFN levels, there were nearly identical levels of ISG expression in hBECs from asthmatic versus nonasthmatic subjects (Figure 1E). Taken together, the results provide evidence of a relatively minor and transient delay in control of IAV replication but otherwise intact IFN production, ISG expression, and IAV control in hBECs from asthmatic compared to nonasthmatic subjects.
IFN and ISG levels after RSV infection
Screening experiments in hTEC cultures showed that RSV (Long strain) at MOI 0.01-1.0 resulted in maximal IFNB1 and IFNL1,2/3 mRNA and IFN-λ protein levels at MOI 1 (Figure E4), so this inoculum was used for hBEC experiments. Inoculation with RSV-Long (MOI 1) resulted in marked increases in viral RNA levels in hBECs cultured from asthmatic and nonasthmatic subjects (Figure 2A). In both types of subjects, the levels of virus in cell supernatant and lysate increased (~1 × 106-fold) with a similar time course over 72 h. Levels of viral RNA in cell lysates and supernatants were decreased slightly (1.4-2-fold) in hBECs from asthmatic compared to nonasthmatic subjects at 48 and 72 h after inoculation but were no different at all other time points. Similarly, there was no significant difference in virus-induced cytopathic effect between hBECs from asthmatic and nonasthmatic subjects at any time point after inoculation (Figure 2B).
FIG 2.
Viral level and IFN response for RSV (Long strain, MOI 1) infection in hBEC cultures from asthmatic (n=9) and nonasthmatic subjects (n=6). A, RSV RNA levels in cell lysate and supernatant. B, RSV-induced cell toxicity marked by LDH release in cell supernatant. C, IFNB1, IFNL1, and IFNL2/3 mRNA levels. D, IFN-λ protein levels in cell supernatant. E, OAS1 and MX1 mRNA levels. ### p<0.001, ## p<0.01, # p<0.05 for the set of values for RSV and RSV-UV in asthmatics and nonasthmatics at each time point. *** p<0.001, ** p<0.01, * p<0.05 for the values for RSV in asthmatic versus nonasthmatic subjects over the time course of the experiment. The numbers in red indicate the fold-difference in the value for RSV in asthmatic versus nonasthmatic subjects at time points of statistical significance.
In concert with RSV-Long replication, IFNB1 mRNA levels increased 10-100-fold while IFNL1 and IFNL2/3 mRNA levels increased by 1 × 105-fold after inoculation with RSV-Long compared to UV-inactivated RSV-Long in hBECs from asthmatic and nonasthmatic subjects (Figure 1C). Levels of IFNB1 mRNA were no different between asthmatic and nonasthmatic subjects, and IFNL1 and IFNL2/3 mRNA levels were decreased only slightly (1.7-2.8-fold) at only 4 of 8 time points (IFNL1 at 8, 24, and 48 h and IFNL2/3 at 24 h after inoculation) in hBECs from asthmatic compared to nonasthmatic subjects (Figure 2C). However, IFN-λ protein levels in cell supernatants were no different at any time points between asthmatic and nonasthmatic subjects (Figure 2D). IFN-β protein levels were again below the detection limit of the assay (data not shown). Induction of representative ISG expression marked by OAS1 and MX1 mRNA levels was no different in hBECs from asthmatic versus nonasthmatic subjects (Figure 2E). Moreover, we found very few significant differences in the levels of RSV RNA, IFN mRNA and protein, or ISG expression in hBECs across the subgroups of mild, moderate, and severe asthmatic as well as nonasthmatic subjects (Figure E5).
To validate results with a second RSV strain, we also assessed the effects of RSV A/2001/2-20 infection on IFN production and ISG expression in hBECs again at MOI 1 based on screening experiments in hTEC cultures. Inoculation with RSV A/2001/2-20 (MOI 1) resulted in less than 10-fold increases in viral RNA levels in cell lysates and ~100-fold increases in viral RNA cell supernatants from hBECs cultured from asthmatic and nonasthmatic subjects (Figure 3A). Levels of viral RNA in cell lysates were decreased 2.4-5.7-fold and in cell supernatants by 2.2-fold in asthmatic compared to nonasthmatic subjects, but no differences in cytopathic effect were found between groups (Figure 3B). The lack of a difference is consistent with the similar values for RSV in cell supernatants where the majority of RSV is found (viral RNA levels were 7 × 106-fold greater in cell supernatant than lysate). Similarly, levels of IFNB1, IFNL1, and IFNL2/3 mRNA and IFN-λ protein as well as OAS1 and MX1 mRNA were generally no different in hBECs from asthmatic versus nonasthmatic subjects (Figure 3C-E). Thus, the slight (2.2-2.5-fold) decrease in IFNL mRNA levels in hBECs from asthmatic subjects at 8 h after inoculation was no longer found at later time points and was not reflected in any significant decrease in IFN-λ protein level in hBECs from asthmatic versus nonasthmatic subjects (Figure 3C,D). Moreover, we found no significant and consistent differences in the levels of RSV RNA, IFN mRNA and protein, or ISG expression in hBECs across the subgroups of mild, moderate, and severe asthmatic as well as nonasthmatic subjects (Figure E6). We again found a significant decrease in RSV RNA levels in cell lysates from mild and severe asthmatic compared to nonasthmatic subjects, but no comparable difference in moderate asthmatics versus nonasthmatics and no corresponding difference in RSV RNA levels in cell supernatants in any subset of asthmatics versus nonasthmatics.
FIG 3.
Viral level and IFN response for RSV (A/2001/2-20 strain, MOI 1) in hBEC cultures from asthmatic (n=8) and nonasthmatic subjects (n=6). A, RSV RNA levels in cell lysate and supernatant. B, RSV-induced cell toxicity marked by LDH release in cell supernatant. C, IFNB1, IFNL1, and IFNL2/3 mRNA levels. D, IFN-λ protein levels in cell supernatant. E, OAS1 and MX1 mRNA levels. ### p<0.001, ## p<0.01, # p<0.05 for the set of values for RSV and RSV-UV in asthmatics and nonasthmatics at each time point. *** p<0.001, ** p<0.01, * p<0.05 for the values for RSV in asthmatic versus nonasthmatic subjects over the time course of the experiment. The numbers in red indicate the fold-difference in the value for RSV in asthmatic versus nonasthmatic subjects at time points of statistical significance.
Together, these results with RSV Long and RSV A/2001/2-20 also indicate that IFN production, ISG expression, and control of viral level in hBECs is remarkably similar between asthmatic and nonasthmatic subjects. Indeed, an analysis of IFN response (marked by IFNL1,2/3 and OAS1 mRNA expression) across a wide range of experimental conditions reveals a remarkably consistent and positive correlation between viral level and IFN response. (Figure 4). The data therefore indicate that increased viral levels and the associated increase in disease severity are likely to be accompanied by increased levels of IFN production (particularly IFNL) and ISG expression (e.g., OAS1) across asthmatic and nonasthmatic subject groups, and this relationship is not disrupted by a genetic or acquired deficiency of IFN production or signaling.
FIG 4.
Correlations between levels of virus in cell supernatants versus levels of IFNB1, IFNL1, IFNL2/3, and OAS1 mRNA for hBECs obtained from all of the asthmatic and nonasthmatic subjects and inoculated with IAV, RSV Long, or RSV A2001/2-20. Lines represent fits to a linear regression of log values. Values for viral levels represent mRNA copy number per μl of cell supernatant.
DISCUSSION
Our study shows that the IFN response to two key respiratory viral pathogens, IAV and RSV, are remarkably similar in airway epithelial cells obtained from asthmatic and nonasthmatic subjects. This conclusion is based on data from the use of well-characterized systems for isolation and primary-culture of human airway epithelial cells, infection with commonly used strains of IAV (A/WS/33 strain) and RSV (Long strain and A/2001/2-20 strain) and quantification of released and cell-associated viral level as well as IFN production (marked by type I IFN-β and type III IFN-λ) and downstream ISG expression (marked by OAS1 and MX1 mRNA level). In addition, our study included a full range of experimental conditions to optimize viral replication and virus-induced IFN production and to assess a full time course for viral and host response and of subjects with varying degrees of asthma severity. It is possible that other conditions for viral inoculation could reveal a difference between cells from asthmatics versus nonasthmatic controls, but a significant difference appears unlikely given the broad range of time points and consequent levels of virus and IFN production that were assessed in our study. Together, the results support the presence of a vigorous antiviral response of the IFN production and signaling cascade in airway epithelial cells from asthmatic and nonasthmatic subjects in response to IAV and RSV. The findings imply that other aspects of the antiviral response or other aspects of host defense might underlie any alteration in the clinical illness associated with IAV or RSV infection in the susceptible asthmatic population.
We recognize that there has been disagreement on the state of the antiviral IFN response in asthma. As noted in the Introduction, some studies report a deficiency in IFN production (type I and III) in airway epithelial cells isolated from asthmatic subjects and then challenged with human rhinovirus 23, 24 whereas other studies find no difference in the IFN response to this virus or other viruses 28, 34, 44 or to other TLR-dependent stimuli of IFN production 45. Here we discuss three major reasons for this discrepancy and the implications for asthma pathogenesis and treatment.
First, the difference in antiviral IFN response among studies may be related to the type of virus that is being studied and the possibility that different viruses might elicit distinct types of IFN responses from the host cell. In that regard, RNA viruses are subject to rapid mutation and consequent alterations in their capacity to elicit and/or subvert the host response. Thus, it is difficult to fully exclude subtle variations in viruses between and even within laboratories that might influence host response. However, both the previous studies of HRV and the present analysis of IAV and RSV find predominant induction of IFN-λs and lesser production of IFN-β as a rather stereotyped response of airway epithelial cells 46, 47. These findings suggest the development of a similar antiviral IFN response to each of these related RNA viruses. We recognize that we limited our analysis to a small subset of a complex IFN response but the same subset was used in studies that did find a deficiency 23, 24. Similarly, others find that the kinetics of the pathogen recognition can influence the antiviral response 48, but we were careful to include measurements over a relatively long time course in our study. Nonetheless, we cannot fully rule out the possibility that other IFN sub-types (e.g., IFN-α and IFN-κ) and/or their target ISGs are deficient in cells from asthmatic subjects, but it appears unlikely that any differences between studies would reflect divergence in stimulation or endpoints of the antiviral IFN response.
Second, the difference in antiviral IFN response among studies could depend on the types of asthmatic subjects selected for study. In particular, it is possible that asthma severity might influence antiviral response given the differences in immune characteristics among mild, moderate, and severe asthma subsets. Here we found no effect of disease severity on the antiviral IFN response or viral level, although our study was not powered to fully analyze this issue. We also could not assess the separate influence of atopy, given the high prevalence of atopic reactivity in our population of asthmatic and nonasthmatic subjects and in the endogenous population at large. As introduced above, we and others have reported an influence of atopic status on the antiviral IFN response of immune cells 49, 50 and airway epithelial cells 51. Thus, while we found no clear difference in antiviral IFN response or viral level in relation to asthma severity or concomitant atopy, this issue may deserve further study with extension to mechanism if any difference is found.
Third, the difference in antiviral IFN response among studies could be related to airway epithelial cell culture conditions. Our observation of an intact IFN response to IAV-A/WS/33 infection is corroborated by a study of IAV-A/Bangkok/1/79 infection that also found unimpaired induction of IFN-β and ISG mRNA in hBECs isolated from asthmatic subjects and studied under air-liquid interface conditions that allow for epithelial cell differentiation 34. Similarly, others found no difference in HRV levels in hBECs cultured under air-liquid interface conditions from asthmatic and nonasthmatic subjects, although they did not assess IFN response 26. Thus, it appears unlikely that epithelial differentiation under these culture conditions is likely to explain any difference between asthmatic and nonasthmatic subjects. However, we also recognize that our lab and others report decreased IFN production in immune cells isolated from asthmatic subjects 52, 53 as well as subjects at risk for asthma 50, and even passaged epithelial cell cultures can contain immune cells that are carried through the preparative stages of these culture systems 54, 55. Indeed, immune cell carryover may be more prominent if cells are isolated from airways involved by inflammatory disease. In that regard, decreased IFN responses have also been detected in epithelial cells cultured from other inflammatory airway conditions, such as COPD and cystic fibrosis 56, 57. Thus, study of cell cultures that are not carefully screened for immune cell contamination may mistakenly attribute an alteration in IFN production to properties of airway epithelial cells.
Despite all of these issues, our results still appear to rule against an airway epithelial cell deficiency in IFN production or signaling and instead suggest adequate control of viral replication and clearance at the level of the airway epithelial cell. It is still possible that other aspects of the antiviral epithelial response are altered in asthma and thereby explain different outcomes from viral infection in these types of patients. For example, there are increased numbers of airway mucous cells in asthma and this subset of airway epithelial cells may have inherent differences in susceptibility to viral infection 58, 59 as well as a distinct influence on innate and adaptive immune responses that indirectly impact viral clearance 60, 61. However, these aspects of epithelial cell biology would not be expected to respond to an approach aimed at correcting IFN deficiency, e.g., administration of inhaled IFN formulations. Instead, correction of the underlying alteration in airway epithelial remodeling 36 may represent a more rational strategy to decrease the severity of respiratory viral infections.
Our study was designed to take advantage of in vitro conditions that isolate the airway epithelial cells and thereby provide better control over the complex immune response that is found in vivo. Nonetheless, our findings provide some insight into previous observations in asthmatic and healthy subjects with natural and experimental infections with respiratory viruses and either monitored for IFN production or subject to IFN treatment. Indeed, this diagnostic and therapeutic approach began shortly after the discovery of IFN 62. Since that time, some studies showed that IFN induction or administration might reduce respiratory viral infection 63-66 whereas other studies found no effect in otherwise healthy subjects 67 or in those with chronic respiratory disease 68. Presumably, this data on efficacy and associated side effects underlies the fact that IFN treatment is not generally used for antiviral therapy and fits with the idea that endogenous IFN production is already optimal in most individuals. In that regard, some reports suggest that increasing IFN levels may be harmful in asthmatics with viral infections 69-71. We also observed transient increases in IFN production and ISG expression in samples from asthmatic compared to nonasthmatic subjects, but the relationship of these changes in vitro to outcomes in vivo still needs to be defined. Ongoing studies aimed at enhancing the physiologic reserve in the IFN signaling system 42, 43, 72 should help to address this issue and perhaps provide a means to decrease the severity of respiratory viral infection and the consequent postviral induction and/or exacerbation of chronic obstructive lung disease that has been found experimentally and clinically 38, 73.
Supplementary Material
CLINICAL IMPLICATIONS.
The airway epithelial response to two major respiratory pathogens (IAV and RSV) appears preserved in asthma, suggesting that a separate mechanism accounts for more severe infections with these viruses in asthma.
CAPSULE SUMMARY.
Airway epithelial cells activate a potent IFN-dependent system to control respiratory viral infection, and this capability appears to be preserved in asthma based on the epithelial cell response to influenza and respiratory syncytial viruses.
ACKNOWLEDGEMENTS
We thank the Pulmonary Epithelial Cell Core for support in isolating and maintaining cells from endobronchial brushings and lung transplant donors.
Declaration of Funding. This work was supported by grants from the NIH (NIAID AADCRC U19-AI070489 to MJH and U19-AI000000 to RSP, U10-HL109257 to MC, and CTSA UL1 TR000448), and Roche Postdoctoral Fellowship awards to DAP and HJK.
ABBREVIATIONS
- hBEC
human bronchial epithelial cells
- hTECs
human tracheal epithelial cells
- IAV
Influenza A Virus
- IFN
Interferon
- ISG
IFN-Stimulated Gene
- MX1
Myxovirus (Influenza virus) Resistance 1
- OAS1
2’-5’-Oligoadenylate Synthetase 1
- PRR
Pattern Recognition Receptor
- RSV
Respiratory Syncytial Virus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Chapgier A, Kong XF, Boisson-Dupuis S, Jouanguy E, Averbuch D, Feinberg J, et al. A partial form of recessive STAT1 deficiency in humans. J. Clin. Invest. 2009;119:1502–14. doi: 10.1172/JCI37083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dupuis S, Jouanguy E, Al-Hajjar S, Fieschi C, Al-Mohsen IZ, Al-Jumaah S, et al. Impaired response to interferon-α/β and lethal viral disease in human STAT1 deficiency. Nat. Genet. 2003;33:388–91. doi: 10.1038/ng1097. [DOI] [PubMed] [Google Scholar]
- 3.Corne JM, Marshall C, Smith S, Schreiber J, Sanderson G, Holgate ST, et al. Frequency, severity, and duration of rhinovirus infections in asthmatic and non-asthmatic individuals: a longitudinal cohort study. Lancet. 2002;359:831–4. doi: 10.1016/S0140-6736(02)07953-9. [DOI] [PubMed] [Google Scholar]
- 4.Message SD, Laza-Stanca V, Mallia P, Parker HL, Zhu J, Kebadze T, et al. Rhinovirus-induced lower respiratory illness is increased in asthma and related to virus load and Th1/2 cytokine and IL-10 production. Proc. Natl. Acad. Sci. U. S. A. 2008;105:13562–7. doi: 10.1073/pnas.0804181105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Busse WW, Lemanske RF, Jr., Gern JE. Role of viral respiratory infections in asthma and asthma exacerbations. Lancet. 2010;376:826–34. doi: 10.1016/S0140-6736(10)61380-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dulek DE, Peebles RS., Jr. Viruses and asthma. Biochim Biophys Acta. 2011;1810:1080–90. doi: 10.1016/j.bbagen.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jackson DJ, Johnston SL. The role of viruses in acute exacerbations of asthma. J Allergy Clin Immunol. 2010;125:1178–87. doi: 10.1016/j.jaci.2010.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nicholson KG, Kent J, Ireland DC. Respiratory viruses and exacerbations of asthma in adults. BMJ. 1993;307:982–6. doi: 10.1136/bmj.307.6910.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnston SL, Pattemore PK, Sanderson G, Smith S, Lampe F, Josephs L, et al. Community study of role of viral infections in exacerbations of asthma in 9-11 year old children. BMJ. 1995;310:1225–9. doi: 10.1136/bmj.310.6989.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heymann PW, Carper HT, Murphy DD, Platts-Mills TA, Patrie J, McLaughlin AP, et al. Viral infections in relation to age, atopy, and season of admission among children hospitalized for wheezing. J Allergy Clin Immunol. 2004;114:239–47. doi: 10.1016/j.jaci.2004.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sigurs N, Bjarnason R, Sigurbergsson F, Kjellman B. Respiratory syncytial virus bronchiolitis in infancy is an important risk factor for asthma and allergy at age 7. Am J Respir Crit Care Med. 2000;161:1501–7. doi: 10.1164/ajrccm.161.5.9906076. [DOI] [PubMed] [Google Scholar]
- 12.Wu P, Dupont WD, Griffin MR, Carroll KN, Mitchel EF, Gebretsadik T, et al. Evidence of a causal role of winter virus infection during infancy in early childhood asthma. Am J Respir Crit Care Med. 2008;178:1123–9. doi: 10.1164/rccm.200804-579OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jackson D, Gangnon R, Evans M, Roberg K, Anderson E, Pappas T, et al. Wheezing rhinovirus illnesses in early life predict asthma development in high-risk children. Am. J. Respir. Crit. Care Med. 2008;178:667–72. doi: 10.1164/rccm.200802-309OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kusel MM, de Klerk NH, Kebadze T, Vohma V, Holt PG, Johnston SL, et al. Early-life respiratory viral infections, atopic sensitization and risk of subsequent development of persistent asthma. J. Allergy Clin. Immunol. 2007;119:1105–10. doi: 10.1016/j.jaci.2006.12.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henderson J, Hilliard TN, Sherriff A, Stalker D, Al Shammari N, Thomas HM. Hospitalization for RSV bronchiolitis before 12 months of age and subsequent asthma, atopy and wheeze: a longitudinal birth cohort study. Pediatr Allergy Immunol. 2005;16:386–92. doi: 10.1111/j.1399-3038.2005.00298.x. [DOI] [PubMed] [Google Scholar]
- 16.Sigurs N, Aljassim F, Kjellman B, Robinson PD, Sigurbergsson F, Bjarnason R, et al. Asthma and allergy patterns over 18 years after severe RSV bronchiolitis in the first year of life. Thorax. 2010;65:1045–52. doi: 10.1136/thx.2009.121582. [DOI] [PubMed] [Google Scholar]
- 17.James KM, Gebretsadik T, Escobar GJ, Wu P, Carroll KN, Li SX, et al. Risk of childhood asthma following infant bronchiolitis during the respiratory syncytial virus season. J Allergy Clin Immunol. 2013;132:227–9. doi: 10.1016/j.jaci.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Szabo SM, Levy AR, Gooch KL, Bradt P, Wijaya H, Mitchell I. Elevated risk of asthma after hospitalization for respiratory syncytial virus infection in infancy. Paediatr Respir Rev. 2013;13(Suppl 2):S9–15. doi: 10.1016/S1526-0542(12)70161-6. [DOI] [PubMed] [Google Scholar]
- 19.Wu P, Hartert TV. Evidence for a causal relationship between respiratory syncytial virus infection and asthma. Expert Rev Anti Infect Ther. 2011;9:731–45. doi: 10.1586/eri.11.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jartti T, Gern JE. Rhinovirus-associated wheeze during infancy and asthma development. Curr Respir Med Rev. 2011;7:160–6. doi: 10.2174/157339811795589423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bacharier LB, Cohen R, Schweiger T, Yin-Declue H, Christie C, Zheng J, et al. Determinants of asthma after severe respiratory syncytial virus bronchiolitis. J Allergy Clin Immunol. 2012;130:91–100. doi: 10.1016/j.jaci.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shornick LP, Wells AG, Zhang Y, Patel AC, Huang G, Takami K, et al. Airway epithelial versus immune cell Stat1 function for innate defense against respiratory viral infection. J. Immunol. 2008;180:3319–28. doi: 10.4049/jimmunol.180.5.3319. [DOI] [PubMed] [Google Scholar]
- 23.Contoli M, Message SD, Laza-Stanca V, Edwards MR, Wark PAB, Barlett NW, et al. Role of deficient type III interferon-l production in asthma exacerbations. Nat. Med. 2006;12:1023–6. doi: 10.1038/nm1462. [DOI] [PubMed] [Google Scholar]
- 24.Wark PA, Johnston SL, Bucchieri F, Powell R, Puddicombe S, Laza-Stanca V, et al. Asthmatic bronchial epithelial cells have a deficient innate immune response to infection with rhinovirus. J. Exp. Med. 2005;201:937–47. doi: 10.1084/jem.20041901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wark PA, Grissell T, Davies B, See H, Gibson PG. Diversity in the bronchial epithelial cell response to infection with different rhinovirus strains. Respirology. 2009;14:180–6. doi: 10.1111/j.1440-1843.2009.01480.x. [DOI] [PubMed] [Google Scholar]
- 26.Lopez-Souza N, Favoreto S, Wong H, Ward T, Yagi S, Schnurr D, et al. In vitro susceptibility to rhinovirus infection is greater for bronchial than for nasal airway epithelial cells in human subjects. J. Allergy Clin. Immunol. 2009;123:1384–90. doi: 10.1016/j.jaci.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fleming HE, Little FF, Schnurr D, Avila PC, Wong H, Liu J, et al. Rhinovirus-16 colds in healthy and in asthmatic subjects: similar changes in upper and lower airways. Am J Respir Crit Care Med. 1999;160:100–8. doi: 10.1164/ajrccm.160.1.9808074. [DOI] [PubMed] [Google Scholar]
- 28.Bochkov YA, Hanson KM, Keles S, Brockman-Schneider RA, Narjour NN, Gern JE. Rhinovirus-induced modulation of gene expression in bronchial epithelial cells from subjects with asthma. Mucosal Immunol. 2010;3:69–80. doi: 10.1038/mi.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schroth MK, Grimm E, Frindt P, Galagan DM, Konno S, Love R, et al. Rhinovirus replication causes RANTES production in primary bronchial epithelial cells. Am. J. Respir. Cell Mol. Biol. 1999;20:1220–8. doi: 10.1165/ajrcmb.20.6.3261. [DOI] [PubMed] [Google Scholar]
- 30.Walter MJ, Morton JD, Kajiwara N, Agapov E, Holtzman MJ. Viral induction of a chronic asthma phenotype and genetic segregation from the acute response. J. Clin. Invest. 2002;110:165–75. doi: 10.1172/JCI14345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hinojosa ME, Agapov E, Tidwell R, Swanson S, Patel AC, Paessler S, et al. Long-term viral replication and inflammatory disease after influenza A virus infection. Am. J. Respir. Crit. Care Med. 2013;186:A1330. [Google Scholar]
- 32.Nguyen-Van-Tam JS, Openshaw PJ, Hashim A, Gadd EM, Lim WS, Semple MG, et al. Risk factors for hospitalisation and poor outcome with pandemic A/H1N1 influenza: United Kingdom first wave (May-September 2009). Thorax. 2010;65:645–51. doi: 10.1136/thx.2010.135210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kloepfer KM, Olenec JP, Lee WM, Liu G, Vrtis RF, Roberg KA, et al. Increased H1N1 infection rate in children with asthma. Am. J. Respir. Crit. Care Med. 2012;185:1275–9. doi: 10.1164/rccm.201109-1635OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bauer RN, Brighton LE, Mueller L, Xiang Z, Rager JE, Fry RC, et al. Influenza enhances caspase-1 in bronchial epithelial cells from asthmatic volunteers and is associated with pathogenesis. J Allergy Clin Immunol. 2012;130:958–67. doi: 10.1016/j.jaci.2012.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.National Heart L, and Blood Institute National Asthma Education and Prevention Program. Expert panel report 3: Guidelines for the diagnosis and management of asthma-summary report 2007. J. Allergy Clin. Immunol. 2007;120:S94–S138. doi: 10.1016/j.jaci.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 36.Alevy Y, Patel AC, Romero AG, Patel DA, Tucker J, Roswit WT, et al. IL-13–induced airway mucus production is attenuated by MAPK13 inhibition. J. Clin. Invest. 2012;122:4555–68. doi: 10.1172/JCI64896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Orens JB, Boehler A, de Perrot M, Estenne M, Glanville AR, Keshavjee S, et al. A review of lung transplant donor acceptability criteria. J. Heart Lung Transplant. 2003;22:1183–200. doi: 10.1016/s1053-2498(03)00096-2. [DOI] [PubMed] [Google Scholar]
- 38.Byers DE, Alexander-Brett J, Patel AC, Agapov E, Dang-Vu G, Jin X, et al. Long-term IL-33-producing epithelial progenitor cells in chronic obstructive lung disease. J. Clin. Invest. 2013;123:3967–82. doi: 10.1172/JCI65570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stokes KL, Chi MH, Sakamoto K, Newcomb DC, Currier MG, Huckabee MM, et al. Differential pathogenesis of respiratory syncytial virus clinical isolates in BALB/c mice. J Virol. 2011;85:5782–93. doi: 10.1128/JVI.01693-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, et al. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem. 2009;55:611–22. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
- 41.Guenin S, Mauriat M, Pelloux J, Van Wuytswinkel O, Bellini C, Gutierrez L. Normalization of qRTPCR data: the necessity of adopting a systematic, experimental conditions-specific, validation of references. J Exp Bot. 2009;60:487–93. doi: 10.1093/jxb/ern305. [DOI] [PubMed] [Google Scholar]
- 42.Patel DA, Patel AC, Nolan WC, Zhang Y, Holtzman MJ. High throughput screening for small molecule enhancers of the interferon signaling pathway to drive next-generation antiviral drug discovery. PloS ONE. 2012;7:e36594. doi: 10.1371/journal.pone.0036594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patel DA, Patel AC, Nolan WC, Huang G, Romero AG, Charlton N, et al. High-throughput screening normalized to biological response: application to antiviral drug discovery. J. Biomol. Screen. 2014;19:119–30. doi: 10.1177/1087057113496848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sykes A, Macintyre J, Edwards MR, Del Rosario A, Haas J, Gielen V, et al. Rhinovirus-induced interferon production is not deficient in well controlled asthma. Thorax. 2013 doi: 10.1136/thoraxjnl-2012-202909. in press. [DOI] [PubMed] [Google Scholar]
- 45.Sykes A, Edwards MR, Macintyre J, Del Rosario A, Gielen V, Haas J, et al. TLR3, TLR4 and TLRs7-9 Induced Interferons Are Not Impaired in Airway and Blood Cells in Well Controlled Asthma. PLoS One. 2013;8:e65921. doi: 10.1371/journal.pone.0065921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Okabayashi T, Kojima T, Masaki T, Yokota S, Imaizumi T, Tsutsumi H, et al. Type-III interferon, not type-I, is the predominant interferon induced by respiratory viruses in nasal epithelial cells. Virus Res. 2011;160:360–6. doi: 10.1016/j.virusres.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 47.Khaitov MR, Laza-Stanca V, Edwards MR, Walton RP, Rohde G, Contoli M, et al. Respiratory virus induction of alpha-, beta- and lambda-interferons in bronchial epithelial cells and peripheral blood mononuclear cells. Allergy. 2009;64:375–86. doi: 10.1111/j.1398-9995.2008.01826.x. [DOI] [PubMed] [Google Scholar]
- 48.Slater L, Bartlett NW, Haas JJ, Zhu J, Message SD, Walton RP, et al. Co-ordinated role of TLR3, RIG-I and MDA5 in the innate response to rhinovirus in bronchial epithelium. PLoS Pathog. 2010;6:e1001178. doi: 10.1371/journal.ppat.1001178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bufe A, Gehlhar K, Grage-Griebenow E, Ernst M. Atopic phenotype in children is associated with decreased virus-induced interferon-alpha release. Int. Arch. Allergy Immunol. 2002;127:82–8. doi: 10.1159/000048173. [DOI] [PubMed] [Google Scholar]
- 50.Sumino K, Tucker J, Shahab M, Jaffee KF, Visness CM, Gern JE, et al. Antiviral interferon-γ responses of monocytes at birth predict respiratory illness in the first year of life. J. Allergy Clin. Immunol. 2012;129:1267–73. doi: 10.1016/j.jaci.2012.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Baraldo S, Contoli M, Bazzan E, Turato G, Padovani A, Marku B, et al. Deficient antiviral immune responses in childhood: distinct roles of atopy and asthma. J Allergy Clin Immunol. 2012;130:1307–14. doi: 10.1016/j.jaci.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 52.Gehlhar K, Billitewski C, Reinitz R, K., Rhode G, Bufe A. Impaired virus-induced interferon-alpha2 release in adult asthmatic patients. Clin. Exp. Allergy. 2006;36:331–7. doi: 10.1111/j.1365-2222.2006.02450.x. [DOI] [PubMed] [Google Scholar]
- 53.Iikura K, Katsunuma T, Saika S, Saito S, Ichinohe S, Ida H, et al. Peripheral blood mononuclear cells from patients with bronchial asthma show impaired innate immune responses to rhinovirus in vitro. Int. Arch. Allergy Immunol. 2011;155:27–33. doi: 10.1159/000327262. [DOI] [PubMed] [Google Scholar]
- 54.Hackett TL, Shaheen F, Johnson A, Wadsworth S, Pechkovsky DV, Jackoby DB, et al. Stem Cells. Characterization of side population cells from human airway epithelium. 2008;26:2576–85. doi: 10.1634/stemcells.2008-0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Forrest IA, Murphy DM, Ward C, Jones D, Johnson GE, Archer L, et al. Primary airway epithelial cell culture from lung transplant recipients. Eur. Respir. J. 2005;26:1080–5. doi: 10.1183/09031936.05.00141404. [DOI] [PubMed] [Google Scholar]
- 56.Parker D, Cohen TS, Alhede M, Harfenist BS, Martin FJ, Prince A. Induction of type I interferon signaling by Pseudomonas aeruginosa is diminished in cystic fibrosis epithelial cells. Am J Respir Cell Mol Biol. 2012;46:6–13. doi: 10.1165/rcmb.2011-0080OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vareille M, Kieninger E, Alves MP, Kopf BS, Moller A, Geiser T, et al. Impaired type I and type III interferon induction and rhinovirus control in human cystic fibrosis airway epithelial cells. Thorax. 2012;67:517–25. doi: 10.1136/thoraxjnl-2011-200405. [DOI] [PubMed] [Google Scholar]
- 58.Lachowicz-Scroggins ME, Boushey HA, Finkbeiner WE, Widdicombe JH. Interleukin-13 induced mucous metaplasia increases susceptibility of human airway epithelium to rhinovirus infection. Am. J. Respir. Cell Mol. Biol. 2010;43:652–61. doi: 10.1165/rcmb.2009-0244OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jakiela B, Gielicz A, Plutecka H, Hubalewska-Mazgaj M, Mastalerz L, Bochenek G, et al. Th2-type cytokine induced mucous metaplasia decreases susceptibility of human bronchial epithelium to rhinovirus infection. Am. J. Respir. Cell Mol. Biol. 2014;50 doi: 10.1165/rcmb.2013-0395OC. in press. [DOI] [PubMed] [Google Scholar]
- 60.McDole JR, Wheeler LW, McDonald KG, Wang B, Konjufca V, Knoop KA, et al. Goblet cells deliver luminal antigen to CD103+ dendritic cells in the small intestine. Nature. 2012;483:345–9. doi: 10.1038/nature10863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shan M, Gentile M, Yeiser JR, Walland AC, Bornstein VU, Chen K, et al. Mucus enhances gut homeostasis and oral tolerance by delivering immunoregulatory signals. Science. 2013;342:447–53. doi: 10.1126/science.1237910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Isaacs A, Lindenmann J. Viral interference. I. The interferon. Proc R Soc Lond B Biol Sci. 1957;147:268–73. [PubMed] [Google Scholar]
- 63.Douglas RM, Moore BW, Miles HB, Davies LM, Graha NM, Ryan P, et al. Prophylactic efficacy of intranasal alpha 2-interferon against rhinovirus infections in the family setting. N Engl J Med. 1986;314:65–70. doi: 10.1056/NEJM198601093140201. [DOI] [PubMed] [Google Scholar]
- 64.Hayden FG, Albrecht JK, Kaiser DL, Gwaltney JMJ. Prevention of natural colds by contact prophylaxis with intranasal alpha 2-interferon. N Engl J Med. 1986;314:71–5. doi: 10.1056/NEJM198601093140202. [DOI] [PubMed] [Google Scholar]
- 65.Monto AS, Shope TC, Schwartz SA, Albrecht JK. Intranasal interferon-alpha 2b for seasonal prophylaxis of respiratory infection. J. Infect. Dis. 1986;154:128–33. doi: 10.1093/infdis/154.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gao L, Yu S, Chen Q, Duan Z, Zhou J, Mao C, et al. A randomized controlled trial of low-dose recombinant interferons alpha-2b nasal spray to prevent acut viral respiratory infections in military recruits. Vaccine. 2010;28:4445–51. doi: 10.1016/j.vaccine.2010.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hayden FG, Gwaltney JMJ, Johns ME. Prophylactic efficacy and tolerance of low-dose intranasal interferon-alpha 2 in natural respiratory viral infections. Antiviral Res. 1985;5:111–6. doi: 10.1016/0166-3542(85)90037-3. [DOI] [PubMed] [Google Scholar]
- 68.Wiselka MJ, Nicholson KG, Kent J, Cookson JB, Tyrell DA. Prophylactic intranasal alpha 2 interferon and viral exacerbations of chronic respiratory disease. Thorax. 1991;46:706–11. doi: 10.1136/thx.46.10.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bini EJ, Weinshel EH. Severe exacerbation of asthma: a new side effect of interferon-alpha in patients with asthma and chronic hepatitis C. Mayo Clin Proc. 1999;74:367–70. doi: 10.4065/74.4.367. [DOI] [PubMed] [Google Scholar]
- 70.Grayson MH, Cheung D, Rohlfing MM, Kitchens R, Spiegel DE, Tucker J, et al. Induction of high-affinity IgE receptor on lung dendritic cells during viral infection leads to mucous cell metaplasia. J. Exp. Med. 2007;204:2759–69. doi: 10.1084/jem.20070360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Miller EK, Hernandez JZ, Wimmenauer V, Shepherd BE, Hijano D, Libster R, et al. A mechanistic role for type III IFN-λ1 in asthma exacerbations mediated by human rhinoviruses. Am. J. Respir. Crit. Care Med. 2012;185:508–16. doi: 10.1164/rccm.201108-1462OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang Y, Takami K, Lo MS, Huang G, Yu Q, Roswit WT, et al. Modification of the Stat1 SH2 domain broadly improves interferon efficacy in proportion to p300/CREB-binding protein coactivator recruitment. J. Biol. Chem. 2005;280:34306–15. doi: 10.1074/jbc.M503263200. [DOI] [PubMed] [Google Scholar]
- 73.Holtzman MJ. Asthma as a chronic disease of the innate and adaptive immune systems responding to viruses and allergens. J. Clin. Invest. 2012;122:2741–8. doi: 10.1172/JCI60325. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.