Abstract
Aims
The association between epicardial adipose tissue (EAT) volume and coronary artery disease (CAD) severity was evaluated, independent of traditional risk factors and coronary artery calcium (CAC) scores, in patients with diabetes type 2 (DM-2) using cardiac computed tomography angiography (CTA).
Methods
A multivariate analysis was utilized to assess for an independent association after calculating EAT volume, CAD severity, and calcium scores in 92 patients with DM-II from the CTRAD study. We graded CAD severity as none (normal coronaries), mild-moderate (<70% stenosis), and severe (70% or greater stenosis).
Results
A total of 39 (42.3 %) asymptomatic patients with diabetes did not have CAD; 30.4% had mild/moderate CAD; and 27.1% had severe CAD. Mean EAT volume was highest in patients with severe CAD (143.14 cm3) as compared to mild/moderate CAD (112.7 cm3), and no CAD (107.5 cm3) (p= 0.003). After adjustment of clinical risk factors, notably, CAC score, multivariate regression analysis showed EAT volume was an independent predictor of CAD severity in this sample (odds ratio 11.2, 95% confidence interval 1.7 –73.8, p =0.01).
Conclusions
Increasing EAT volume in asymptomatic patients with DM-II is associated with presence of severe CAD, independent of BMI and CAC, as well as traditional risk factors.
Keywords: EAT, Pericardial fat, Coronary Artery Disease, Multi-detector Computed Tomography, Computed Tomography Angiography, Diabetes, Metabolic Syndrome
INTRODUCTION
It is well known that Diabetes Mellitus (DM) confers a high risk of cardiovascular disease (CVD) (1, 2). Since myocardial ischemia due to multi-vessel atherosclerosis typically occurs asymptomatically and atypically among those with DM, early risk assessment strategies are paramount to improve identification, medical optimization, and to ultimately decrease morbidity and mortality from CVD.
The relationship between central obesity and cardiovascular disease is well established (3–6). Visceral adiposity increases with obesity and is a key component of insulin resistance (7), and may reflect greater accuracy of attendant CVD risk in those with diabetes compared to traditional markers of obesity, such as BMI. Local visceral adiposity, specifically, human epicardial adipose tissue (EAT), has recently garnered much intrigue as it is located perivascularly along large coronary arteries and expands with obesity (8, 9). Predominately, coronaries encased in EAT have been correlated with atherosclerotic intimal lesions (10, 11). These perivascular adipose deposits have been hypothesized to be potentially a metabolically active paracrine organ able to bi-directionally traffic numerous locally released bioactive and inflammatory cytokines or adipokines to the underlying coronary artery adventitia, thus mediating atherogenesis (12).
Additionally, recent studies have correlated the burden of coronary artery disease (CAD) with radiologic measurement of increasing EAT in among others: populations of post-menopausal women (13), patients presenting with angina (5, 14), and more recently, in asymptomatic subjects with multiple CVD risk factors referred for computed tomography (CT) angiography (15). Further, studies quantifying EAT have utilized various imaging modalities including: echocardiography (16), cardiac magnetic resonance (MR) (17), and multi-detector computed tomography (MDCT) (18).
Although prior studies have examined the correlation between EAT thickness (48) or EAT volume (49,50) and CAD severity, they have done so using Asian participants, who may have different thresholds for visceral adiposity than other ethnic groups. Prior studies have also not looked at the independent association of EAT and CAD severity after adjusting for Coronary Artery Calcium (CAC) score. We assessed EAT volume and severity of CAD using CTA imaging in asymptomatic patients with diabetes; a burgeoning cohort which is notoriously afflicted with obesity and has significant CAD risk. We assessed the independent association between the volume of EAT, as detected by CTA, and the presence and severity of CAD in patients with type 2 diabetes.
SUBJECTS, MATERIALS AND METHODS
Patient Selection
CTA scans were used from the CTRAD study (Cardiac CT’s Role in Asymptomatic Patients with DM-II) in which consecutive asymptomatic patients (n=203) with type 2 diabetes from three community clinics of the University of California, Irvine, were randomly assigned to either undergo 64-slice CT angiography or continue their usual care. We used data from the 92 patients who were randomized to getting a CTA. Patients with type 2 diabetes were identified by ongoing treatment with diabetic medications or having a fasting blood sugar greater than or equal to 126 mg/dL, or hemoglobin A1C (HgbA1c) greater than or equal to 6.5%, or a physician diagnosis of diabetes. Patients were excluded if they had a history of known coronary artery disease, any prior catheterizations with interventions, cardiac bypass surgery, or if they were currently having ongoing chest pain and undergoing a cardiac work up.
Institutional Board Review (IRB) Approval
Our study was approved by the IRB of our institution and was compliant with Health Insurance Portability and Accountability Act (HIPAA) regulations.
CT Scan Parameters and Acquisition
Oral or intravenous metoprolol was used to achieve a heart rate of less than 65 beats per minute (bpm). In addition, sublingual nitroglycerin (0.4–0.8 mg tablet) was given to the patient 1 minute prior to image acquisition unless contraindicated.
Using a prospectively ECG-gated (mid-diastole) technique, pre-contrast 3 mm non-spiral images were obtained from above the level of tracheal bifurcation to the diaphragm to cover the entire heart. Sequential images were obtained without overlap for measurement of EAT volume.
For contrast enhancement, each patient received 64–93 ml (mean 74.9 ± 3.3 ml) of Iohexol (Omnipaque 350 mg/mL, Amersham Health, Cork, Ireland), which was injected at a rate of 4–5 ml/sec, followed by an injection of 50 ml of saline at 5 ml/sec through an 18-gauge catheter. Routine coronary CT angiography (CTA) was performed using a 64-slice MDCT (Toshiba Aquilion, Tustin, CA) with a technique described in previous publications (19). A retrospective ECG-gated volumetric data set was acquired during a single breath-hold. The mean scan time was 9.1 ± 1.4 seconds (range was between 8–13 seconds). Datasets were reconstructed based on a relative-delay strategy at 10% of R-R intervals. Reconstructed images were then transferred to a remote workstation (Vitrea 2, Vital Images Inc., Minnetonka, Minnesota) where the images could be evaluated by two independent and blinded clinicians, who are both level III certified in cardiac CTA interpretation. Calcium score was routinely obtained with the application of Vitrea software and an overall Agaston score was registered for each patient (20). Coronary artery assessment is described in the following section.
CAD Assessment
The CTA datasets were evaluated by two independent, cardiac CTA level III certified clinicians who were blinded to the EAT volume measurements. They used dedicated remote workstations to which all the reconstructed images were transferred (Vitrea 2, Vital Images Inc., Minnetonka, Minnesota). Calcium score was assessed using the Agaston method (20). The presence or absence of coronary plaque was performed using reconstructed data sets, which were evaluated at different ECG-phases for diagnostic image quality and from which the optimal data set was chosen. Plaques were then categorized as none, mild/ moderate or severe if there were no visible atherosclerotic plaques, less than 50%, 50–70%, or greater than 70% luminal narrowing, respectively. CAD was diagnosed if there were moderate or severe plaques present
Volumetric Measurements of EAT
EAT was defined as fat density (attenuation values between −30 to −190 HU) (21, 22) observed within the parietal pericardium boundary (23). Volumetric analysis was performed on all 92 patients by two separate blinded clinicians who had been trained in measuring EAT volume. We utilized the same non-enhanced images used for calcium scoring analysis. Total volume was measured using axial non-contrast data set during the mid-diastolic phase. Starting from the lower margin of the aortic arch and advancing images toward the inferior cardiac border by 6 mm, the area of every observed epicardial fat pocket located between the myocardial surface and the pericardium was manually traced. The sum of measured areas for each slice was multiplied by the slice thickness (3 mm, range 35 to 40 slices per heart)) to achieve the volume of the slice in mm3. Coronary vessels were included in measured areas (24, 25). Figure 1 shows the examples of EAT from our patients, including assessment of EAT in someone with higher volume (Figure 1a) and in someone with lower volume (Figure 1b).
Figure 1.
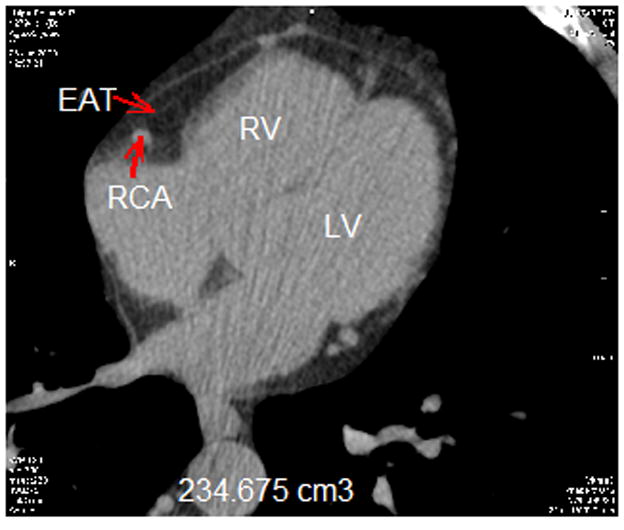
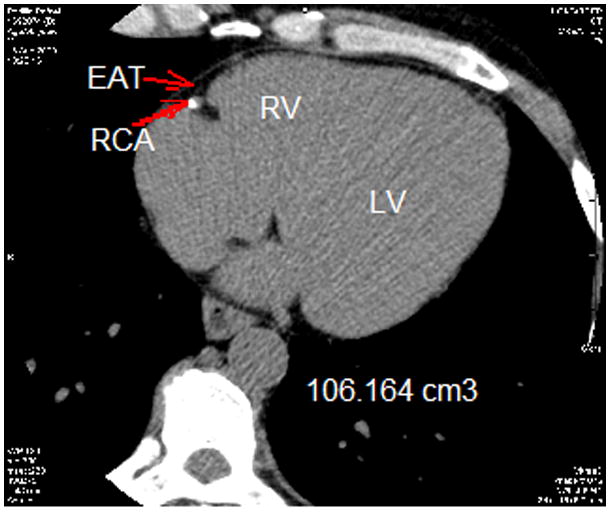
Epicardial adipose tissue (EAT) total volume measurement. Segmentation of EAT was achieved by isolating the EAT by manually tracing the pericardium in the axial view. After segmentation, a threshold of −190 to −30 Hounsfield units was applied to isolate the adipose tissue.
(A) EAT in someone with higher volume
(B) EAT in someone with lower volume
Statistical Analysis
Continuous data were presented as mean values ± SD and categorical variables were reported as percentages. The continuous data such as EAT volume, age and BMI were examined for normality before analyses and were found to be normally distributed. Univariate analyses described the clinical characteristics of the study participants. Bivariate Analysis was performed using chi-square and ANOVA. Chi square for independence was used to explore the association between each of categorical variable and the CAD severity variable with three categories. ANOVA was used to assess the distribution of the continuous variables across the three categories of CAD severity and distribution of EAT volume across the categories of clinical variables such as BMI, Calcium score and CAD severity.
A multivariate logistic analysis was performed to assess the relationship between presence of severe coronary artery disease (CAD) (stenosis of 70% or more in at least one segment of the coronary arteries) and EAT. EAT volume was also dichotomized with EAT value >120 cm3 as a cut off. This cutoff was based on the distribution of our data EAT value of close to 75th percentile. We first ran staged models, with the first model adjusting for age, gender, race, hgbA1c, and use of medications including; anti-hypertensives, statins, and insulins. The second model additionally adjusted for BMI and the third model also adjusted for CAC scores. Statistical significance was defined as p-value < 0.05. All statistical analyses were performed using SAS 9.3. (SAS institute, Cary, North Carolina).
RESULTS
Clinical characteristics of patients
Table 1 lists the clinical characteristics of 92 study participants. The sample included 39% males and 53% females and the mean age of all participants was 56.2 ± 9.4. With respect to race demographics, 63% of the sample were of Hispanic descent. The average Hemoglobin A1C (HgbA1c) was 7.94 % ± 1.7. The mean body mass index (BMI) was 32 ±7.5 and the mean EAT volume was 118.27± 42.24. Sixty-four percent of the participants were hypertensive, 84% had dyslipidemia and only 9% were smokers. Additionally, 19% were on insulin, 54% on statins, 58% on other anti-lipemics and 69% on anti-hypertensives.
Table 1.
Clinical Characteristics of study participants(n=92)
| Characteristics | Overall | Coronary Artery Disease (CAD) severity | |||
|---|---|---|---|---|---|
|
| |||||
| None | Mild & Moderate | Severe | p-value | ||
| Age (years) | 56.2 ± 9.4 | 50.6 ± 6.9 | 59.0± 8.53 | 61.7 ± 9.44 | 0.0004 ||§ |
| Males | 42.4 (39) | 7.6 (7) | 15.2 (14) | 19.6 (18) | < 0.0001||‡ |
| Females | 57.6 (53) | 34.8 (32) | 15.2 (14) | 7.6 (7) | |
|
| |||||
| Race | |||||
| White | 18.5 (17) | 5.4 (5) | 4.3 (4) | 8.7 (8) | |
| Hispanic | 68.5 (63) | 34.8 (32) | 22.8 (21) | 10.9 (10) | 0.02 |
| Asian | 9.8 (9) | 1.1 (1) | 2.2 (2) | 6.5 (6) | |
| Others | 3.3 (3) | 1.1 (1) | 1.1 (1) | 1.1 (1) | |
|
| |||||
| Ethnicity | |||||
| Hispanic | 68.5 (63) | 34.8 (32) | 22.83 (21) | 10.9 (10) | 0.001 ¶ ‡ |
| Non-Hispanic | 31.5 (29) | 7.6 (7) | 7.6 (7) | 16.3 (15) | |
|
| |||||
| A1C | 7.94 ± 1.7 | 8.01 ± 1.7 | 8.15 ± 1.8 | 7.57 ± 1.45 | 0.42 |
| Glucose | 162.74 ± 58.0 | 166.6 ± 65.7 | 164.5 ± 61.1 | 154.7 ± 40.3 | 0.72 |
| BMI numeric | 32.0 ± 7.5 | 32.7 ± 9.0 | 31.6 ± 4.9 | 31.1 ± 7.6 | 0.7 |
| Body Mass Index (kg/m2) | |||||
| Less than 25 | 15.2 (14) | 6.5 (6) | 2.2 (2) | 6.5 (6) | 0.25 ‡ |
| 25 to 29.9 | 27.2 (25) | 12.0 (11) | 9.8 (9) | 5.4 (5) | |
| 30 to 39.9 | 46.7 (43) | 16.3 (15) | 17.4 (16) | 13.0 (12) | |
| Greater than 40 | 10.9 (10) | 7.6 (7) | 1.1 (1) | 2.2 (2) | |
|
| |||||
| Hypertension * | 69.6 (64) | 27.2 (25 | 22 (23.9) | 17 (18.5) | 0.44 ‡ |
| Dyslipidemia † | 91.3 (84) | 37.0 (34) | 29.4 (27) | 25.0 (23) | 0.41 ‡ |
| Smoking | 9.8 (9) | 5.4 (5) | 1.1 (1) | 3.3 (3) | 0.41 ‡ |
| Insulin | 20.7 (19) | 10.9 (10) | 3.3 (3) | 6.5 (6) | 0.29 |
| Statin | 58.7 (54) | 22.8 (21) | 18.5 (17) | 17.4 (16) | 0.7 |
| Anti-lipemic | 63.0 (58) | 25 (23) | 19.6 (18) | 18.5 (17) | 0.77 |
| Anti-hypertensive | 75 (69) | 28.3 (26) | 22.8 (21) | 23.9 (22) | 0.16 |
Values are expressed as mean ± standard deviation for continuous variables or % (n) for dichotomous variables
Defined as a persistent diastolic blood pressure above 80 mm Hg or systolic blood pressure above 130 mm Hg for people with diabetes
For diabetes patients, defined as (Low Density Cholesterol) LDL > 100, (Tri Glycerides)TG > 150, (High Density Cholesterol) HDL <40 for men and <50 for women.
Chi-Square test of Independence for categorical variables
ANOVA for continuous variables
p-value < 0.001
p-value < 0.05
Clinical characteristics of patients for three categories of CAD severity
A total of 39 (42.3%) asymptomatic patients in our sample had normal coronary arteries (Table 1). We found mild/moderate CAD in 28 (30.4%) of these asymptomatic patients and severe CAD in 25(27.1%) asymptomatic patients. As expected mean age was higher across increasing severity of CAD (p<0.001). The mean age for patients with absent CAD was 50.6 ± 6,9, for patients with mild/moderate CAD, mean age was 59± 8.53 and for those with severe CAD, mean age was 61.7 ± 9.44 (p<0.001). Thirty-four percent of asymptomatic female patients with diabetes did not have CAD; compared with only 7.6 % of asymptomatic male patients who had no evidence of CAD; we found a statistically significant difference in the gender distribution across the three categories of CAD severity (p< 0.001). There was a noted difference across stages of CAD severity between Hispanics and Non-Hispanics (p< 0.0001). Notably, there was no significant difference in HgbA1c or mean BMI across the categories of CAD severity (p=0.42 and p=0.70, respectively).
Mean EAT volumes compared across patient’s characteristics
Table 2 shows the relationship between clinical characteristics of patients and mean EAT volume. Men tended to have higher EAT volumes as compared to women (130.3 ± 48.5 vs. 110 ± 36.6; p=0.02). Subjects older than 60 years old had higher EAT volumes (131.1 ± 41.53) as compared to those aged 35–60 (112 ± 42.67; p=0.05). EAT volumes were the highest among Caucasians (136.1 ±37.55), as compared to Asians (135.0 ± 43.80), and Hispanics (113.8 ± 42.57) who had the lowest EAT values (p= 0.03). EAT was higher in patients with the BMI category 30–39 compared to those with less than 25 to 29 BMI, (131.6 ± 44.64 cm3 vs 103.6 ± 37.5 cm3, p=0.01). Patients with higher CAC score ( >400) had higher EAT volume (141.30 ± 42.5 cm3) as compared to those with CAC between 1–100 (121.07 ± 47.09 cm3) (p=0.03), however the relationship between EAT volume and CAC was not linear. The EAT volume was the highest in patients with severe CAD (143.14 ± 46.0) as compared to those with no CAD (107.5 ± 39.4) or mild/moderate CAD (112.7 ± 37.23) (p= 0.003).
Table 2.
Relationship Between Clinical Characteristics of Patients and Mean Epicardial fat Volume
| Characteristics | Mean Epicardial Fat * Volume (cm3) | P - Value |
|---|---|---|
| Overall | 118.57 ± 43.02 | |
|
| ||
| Gender † | ||
| Men | 130.3 ± 48.5 | 0.02 |
| Women | 110.0 ± 36.6 | |
|
| ||
| Age† | ||
| 35–60 | 112.2 ± 42.67 | 0.05 |
| >60 | 131.1 ± 41.53 | |
|
| ||
| Race | ||
| White | 136.1 ± 37.55 | |
| Hispanic | 113.8 ± 42.57 | 0.03 |
| Asian | 135.0 ± 43.80 | |
| Others | 69.4 ± 29.5 | |
|
| ||
| Ethnicity | ||
| Hispanic | 113.8 ± 42.6 | 0.11 |
| Non-Hispanic | 128.9 ± 42.9 | |
|
| ||
| Body Mass Index (kg/m2) ‡ | ||
| Less than 25 | 97.65 ± 38.0 | 0.01 § |
| 25 to 29.9 | 103.6 ± 37.5 | |
| 30 to 39.9 | 131.6 ± 44.64 | |
| Greater than 40 | 129.2 ± 36.49 | |
|
| ||
| A1C† | ||
| < 7.0 | 131 ± 43.51 | |
| >=7.0 and <9 | 112.35 ± 37.33 | 0.12 |
| >=9.0 | 112.08 ± 50.7 | |
|
| ||
| Hypertension † | ||
| Yes | 122.2 ± 39.7 | 0.22 |
| No | 110.4 ± 49.60 | |
|
| ||
| Dyslipidemia † | ||
| Yes | 119.7 ± 41.13 | 0.4 |
| No | 106.4 ± 61.73 | |
|
| ||
| Smoking † | ||
| Yes | 130.7 ± 44.1 | 0.4 |
| No | 117.3 ± 43.0 | |
|
| ||
| Insulin | ||
| Yes | 114.3 ± 52.0 | 0.63 |
| No | 119.7 ± 40.7 | |
|
| ||
| Statin | ||
| Yes | 116.3 ± 37.7 | 0.54 |
| No | 121.8 ± 50.0 | |
|
| ||
| Anti-hypertensive medication | ||
| Yes | 121.5 ± 43.1 | 1.00 |
| No | 109.7 ± 42.60 | |
|
| ||
| Calcium Score ‡ | ||
| Zero | 106.55 ± 38.44 | 0.03 |
| 1–100 | 121.07 ± 47.09 | |
| 101–400 | 118.9 ± 38.90 | |
| > 400 | 141.30 ± 42.5 | |
|
| ||
| CAD Severity ‡ | ||
| None | 107.5 ± 39.4 | 0.003 § |
| Mild/Moderate | 112.7 ± 37.23 | |
| Severe | 143.14 ± 46.0 | |
Values are expressed as mean ± standard deviation
Epicardial fat (Epicardial Adipose Tissue), defined as fat density (attenuation values between −30 to −190 HU) observed within the parietal pericardium boundary. Total volume was measured using axial non-contrast data set during the mid diastolic phase.
t-test for continuous variables
ANOVA for categorical variables
p-value < 0.01
Association between EAT volume and presence of severe CAD
As seen in table 3, all three models showed EAT to be an independent predictor of presence of severe CAD. The odds of severe CAD were increased with higher EAT [odds ratio (OR) 9.2, 95% confidence interval (CI): 1.97 –43.56; p =0.005] after further adjustment for BMI in addition to age, gender, race, hgbA1c, and the use of anti-hypertensives, statins, and insulin. The estimated associated between EAT and severe CAD increased with additional adjustment for calcium score (OR = 11.20, CI: 1.70–73.88; p= 0.01).
Table 3.
Multivariate Logistic Regression analyses showing association of Epicardial Fat (>120cm3) with the presence of Coronary Artery Disease in patients with diabetes
| Age, Gender and Race, A1C, Ant-hypertensives, Statin, Insulin | 6.26 (1.64–23.82) | 0.007 |
| Age, Gender, Race, BMI A1C, Anti-hypertensives, Statin, Insulin | 9.2 (1.97– 43.56) | 0.005 |
| Age, Gender, Race, BMI, A1C, Anti-hypertensives, Statin, Insulin, and Calcium score | 11.20 (1.70– 73.88) | 0.01 ¶ |
Percicardial fat (Epicardial Adipose Tissue), defined as fat density (attenuation values between −30 to −190 HU) observed within the parietal pericardium boundary. Total volume was measured using axial non-contrast data set during the mid diastolic phase.
Defined as a persistent diastolic blood pressure above 80 mm Hg or systolic blood pressure above 130 mm Hg for people with diabetic
For diabetes patients, defined as (Low Density Cholesterol) LDL > 100, (Tri Glycerides)TG > 150, (High Density Cholesteol) HDL <40 for men and <50 for women
Race:Included races such as Caucasian, Hispanic, Asian, African-American
Calcium score categories defined as <=99 and >100
p-value < 0.05
DISCUSSION
Our study found an independent association between EAT volumes with severity of CAD in an asymptomatic cohort of patients with Diabetes type 2 after adjustment of traditional risk factors, BMI, and CAC scores. Although EAT volumes correlated with increasing coronary artery calcium (CAC) scores, an established surrogate marker of CAD (26–28), our study shows that EAT volumes predicted presence and severity of CAD independent of CAC. As such, anatomical identification of EAT represents an objective imaging marker in the assessment of CAD severity in those with diabetes. In tandem, the findings of an association of EAT with CAD independent of CAC scores, which are typically attained with CT, offers powerful prognostic potential for the use of a more practically available imaging modalities with no radiation burden associated with measurement of EAT, such as echocardiography.
Further, our unique cohort represents a diverse population of asymptomatic patients with DM-II in whom CTA is normally not performed, giving insight into the contributors of CAD severity in this group of patients. Prior studies correlating CAD and EAT have done so in a population that was referred for CTA, usually due to symptoms or suspicion of CAD and therefore may have had selection bias.
Additionally, although recent studies have been completed which describe the association of EAT and CAD in DM cohorts (48–50), these studies were completed exclusively in homogenous Asian populations. In contrast, the current analysis occurred in a diverse and heterogeneous cohort reflective of population demographics of the United States (US). Although we did not have sufficient power to perform subanalysis by ethnicity, we found that Hispanics, who represented 63% of our study participants had lower EAT volumes than Causaians or Asians. Future studies could further explore if lower EAT volume could possibly be a contributing mechanism underlying the Hispanic paradox in cardiovascular disease, in which Hispanics despite paradoxically greater prevalence of obesity and diabetes, actually have lower CVD events compared to Cauasians with similar risk factors.
Moreover, we showed that obesity measured using BMI has potential limitations. Although there was an association of EAT with BMI, EAT volume remained a predictor of CAD, independent of conventional cardiac risk factors including, most notably BMI. In fact, there was a non-significant correlation of BMI with severity of CAD, confirming the non-linear relationship of BMI to CAD (29–31). In conjunction with these finding, the long held association between central obesity, a characterization which includes populations with Diabetes, and CVD may be more accurately described by the effects of visceral fat (as measured in this study by EAT) as opposed to BMI, which also measures the less metabolically active subcutaneous fat. The association of EAT and CAD, independent of BMI may legitimize the plausible “local” paracrine metabolism of perivascular adipose tissue, an extension of visceral adiposity.
Although previously thought to have a possible protective role, including allowance for coronary wall expansion in early atherosclerotic plaque generation of up to 40% luminal obstruction; recent studies are implicating EAT as a potent mediator of atherosclerotic disease via a dynamic bi-directional cytokine trafficking system influenced by both endocrine and inflammatory response processes (32–35). Within the local coronary environment increased pathogenic epicardial adipocyte expression of certain deleterious cytokines such as: tumor necrosis factor –alpha, interleukin -6, interleukin-16, visfatin, and leptin, have been shown to be coupled with decreased expression of vaso-protective adipokines such as adiponectin (9,36,37). Further, free molecular trafficking and communication across and through the coronary wall layers may be facilitated by the coronary microcirculation, as the local vaso vasorum exhibits increased neovascularization preceding overt endothelial dysfunction in at risk patient populations (25,38). Further, recent data using animal models have revealed resection of coronary EAT decreased the progression of CAD, suggesting that EAT may exacerbate coronary atherosclerosis (39). These insights have prompted further studies which have attempted to correlate EAT measurements to the extent of CAD (40–44).
Moreover, cohort studies such as the Framingham Heart Study (45, 46) and the Multi-Ethnic Study of Atherosclerosis (MESA) study (8) have reported EAT measurements as an independent predictor of high coronary artery calcium score. However, these studies did not have subgroup analysis of populations with Diabetes nor did they directly visually assess the severity of the coronary artery plaque. The findings of the current study show that the association between EAT and severity of CAD is also present in those with DM; further, this association exists independent of CAC. Incident cardiovascular events in populations associated with EAT, such as the DM population in question, have yet to be established, and represents a topic where future research may be focused.
The advantages of quantifying EAT using CTA as opposed to alternative imaging modalities include higher reproducibility than measurement by echocardiography (14, 47). Nevertheless, as we have reported an association of EAT with CAD, independent of CAC, and since echocardiography is a more utilized imaging modality with no radiation burden to the patient,, assessing EAT thickness via echocardiography maybe a valuable parameter to add to standard reporting if future studies show that CTA and echocardiographic measures of EAT are strongly correlated.
Although our study is the largest to date on assessing EAT and CAD in asymptomatic patients with CAD, due to the relatively smaller sample size and cross sectional nature of this study, the generalizability and causal association of this study is limited. Additionally, the index analysis may have yielded a still more powerful and interesting result with a comparison to control groups, including asymptomatic patients without type 2 diabetes and symptomatic type 2 patients. As such, prospective research is warranted to establish the causal relationship between EAT and development of cardiovascular events in this population.
CONCLUSION
Increasing EAT volume as measured by cardiac 64-slice CTA in patients with DM-II is found be a significant predictor of presence of severe CAD, independent of BMI and CAC, as well as traditional risk factors including: hypertension, dyslipidemia. The anatomical proximity of EAT to the coronary arteries, and the evidence indicating that EAT is an active metabolic fat depot suggest that EAT may contribute to the pathogenesis of diabetic coronary atherosclerosis.
Abbreviations
- BMI
Body Mass Index
- CAC
Coronary Artery Calcium
- CAD
Coronary Artery Disease
- CTA
Computed Tomography Angiography
- DM2
Diabetes Mellitus Type 2
- EAT
Epicardial Adipose Tissue
- ECG
Electrocardiogram
- HDL
High Density Cholesterol
- HgbA1c
Hemoglobin A1c
- HU
Hounsfield units
- LDL
Low Density Cholesterol
- MACE
Myocardial infarction, cardiac death, stroke, late revascuarization
- MDCT
Multi-detector Computed Tomography
- MRI
Magnetic Resonance Imaging
- NCEP-ATP III
National Cholesterol Education Program -Adult Treatment Panel III
- TG
Triglycerides
Footnotes
Disclosures: The authors declare that they have no conflict of interest
ClinicalTrial.gov ID: NCT01564485
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could a3ect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Reaven GM. Insulin resistance and its consequences: non-insulin-dependent diabetes mellitus and coronary heart disease. In: Leroith D, Taylor SI, Olefsky JM, editors. Diabetes Mellitus: A fundamental and Clinical Text. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 509–519. [Google Scholar]
- 2.ADA Consensus Panel. Role of cardiovascular risk factors in prevention and treatment of macrovascular disease in diabetes: American Diabetes Association. Diabetes Care. 1989;12:573–579. doi: 10.2337/diacare.12.8.573. [DOI] [PubMed] [Google Scholar]
- 3.Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, Pi-Sunyer FX, Eckel RH American Heart Association; Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association Scientific Statement on Obesity and Heart. Circulation. 2006;113(6):898–918. doi: 10.1161/CIRCULATIONAHA.106.171016. [DOI] [PubMed] [Google Scholar]
- 4.Van Gaal LF, Mertens IL, De Block CE. Mechanisms linking obesity with cardiovascular disease. Nature. 2006;444 (7121):875–80. doi: 10.1038/nature05487. [DOI] [PubMed] [Google Scholar]
- 5.Gorter PM, de Vos AM, van der Graff Y, Stella PR, Doevendans PA, Meijs MF, Prokop M, Visseren FL. Relation of epicardial and pericoronary fat to coronary atherosclerosis and coronary artery calcium in patients undergoing coronary angiography. Am J Cardiol. 2008;102:380–385. doi: 10.1016/j.amjcard.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 6.Chaowalit N, Somers VK, Pellikka PA, Rihal CS, Lopez-Jimenez F. Subepicardial adipose tissue and the presence and severity of coronary artery disease. Atherosclerosis. 2006;186:354–359. doi: 10.1016/j.atherosclerosis.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 7.Peiris AN, Sothmann MS, Hoffmann RG, Hennes MI, Wilson CR, Gustafson AB, Kissebah AH. Adiposity, fat distribution, and cardiovascular risk. Ann Intern Med. 1989;110:867–872. doi: 10.7326/0003-4819-110-11-867. [DOI] [PubMed] [Google Scholar]
- 8.Ding J, Hsu FC, Harris TB, Liu Y, Kritchevsky SB, Szklo M, Ouyang P, Espeland MA, Lohman KK, Criqui MH, Allison M, Bluemke DA, Carr JJ. The association of pericardial fat with incident coronary heart disease: the Multi-Ethnic Study of Atherosclerosis (MESA) Am J Clin Nutr. 2009;3:499–504. doi: 10.3945/ajcn.2008.27358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iozzo P. Myocardial, perivascular, and epicardial fat. Diabetes Care. 2011 May;34(Suppl 2):S371–9. doi: 10.2337/dc11-s250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sacks HS, Fain JN. Human epicardial adipose tissue: a review. Am Heart J. 2007;153:907–917. doi: 10.1016/j.ahj.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 11.Greif M, Becker A, von ZF, Lebherz C, Lehrke M, Broedl UC, et al. Pericardial adipose tissue determined by dual source CT is a risk factor for coronary atherosclerosis. Arterioscler Thromb Vasc Biol. 2009;29:781–786. doi: 10.1161/ATVBAHA.108.180653. [DOI] [PubMed] [Google Scholar]
- 12.Sacks HS, Fain JN. Human epicardial fat: what is new and what is missing? Clin Exp Pharmacol Physiol. 2011;38:879–87. doi: 10.1111/j.1440-1681.2011.05601.x. [DOI] [PubMed] [Google Scholar]
- 13.De Vos AM, Prokop M, Roos CJ, Meijs MF, Van der Schouw YT, Rutten A, Gorter PM, et al. Peri-coronary epicardial adipose tissue is related to cardiovascular risk factors and coronary artery calcification in post-menopausal women. Eur Heart J. 2008;29(6):777–83. doi: 10.1093/eurheartj/ehm564. [DOI] [PubMed] [Google Scholar]
- 14.Ahn SG, Lim HS, Joe DY, Kang SJ, Choi BJ, Choi SY, Yoon MH, Hwang GS, Tahk SJ, Shin JH. Relationship of epicardial adipose tissue by echocardiography to coronary artery disease. Heart. 2008;94(3):e7. doi: 10.1136/hrt.2007.118471. [DOI] [PubMed] [Google Scholar]
- 15.Bachar GN, Dicker D, Kornowski R, Atar E. Epicardial adipose tissue as a predictor of coronary artery disease in asymptomatic subjects. Am J Cardiol. 2012;110(4):534–8. doi: 10.1016/j.amjcard.2012.04.024. [DOI] [PubMed] [Google Scholar]
- 16.Iacobellis G, Assael F, Ribaudo MC, Zappaterreno A, Alessi G, Di Mario U, Leonetti F. Epicardial fat from echocardiography: a new method for visceral adipose tissue prediction. Obes Res. 2003;11(2):304–10. doi: 10.1038/oby.2003.45. [DOI] [PubMed] [Google Scholar]
- 17.Kessels K, Cramer MJ, Velthuis B. Epicardial adipose tissue imaged by magnetic resonance imaging: an important risk marker of cardiovascular disease. Heart. 2006;92:962. doi: 10.1136/hrt.2005.074872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bandekar An, Baghavi M, Kakadiaris IA. Automated pericardial fat quantification in CT data. Conf Proc IEEE Eng Med Biol Soc. 2006;1:932–5. doi: 10.1109/IEMBS.2006.259259. [DOI] [PubMed] [Google Scholar]
- 19.Saremi F, Channual S, Krishnan S, et al. Bachmann Bundle and its arterial supply: imaging with multidetector CT - implications for interatrial conduction abnormalities and arrhythmias. Radiology. 2008;248:447–57. doi: 10.1148/radiol.2482071908. [DOI] [PubMed] [Google Scholar]
- 20.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–32. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 21.Yoshizumi T, Nakamura T, Yamane M, Islam AH, Menju M, Yamasaki K, Arai T, Kotani K, Funahashi T, Yamashita S, Matsuzawa Y. Abdominal fat: standardized technique for measurement at CT. Radiology. 1999;211:283–6. doi: 10.1148/radiology.211.1.r99ap15283. [DOI] [PubMed] [Google Scholar]
- 22.Baumgartner RN, Heymsfield SB, Roche AF, Bernardino M. Abdominal composition quantified by computed tomography. Am J Clin Nutr. 1988;48:936–45. doi: 10.1093/ajcn/48.4.936. [DOI] [PubMed] [Google Scholar]
- 23.Singh N, Singh H, Khanijoun HK, Iacobellis G. Echocardiographic assessment of epicardial adipose tissue - a marker of visceral adiposity. Mcgill J Med. 2007;10:26–30. [PMC free article] [PubMed] [Google Scholar]
- 24.Djaberi R, Schuijf JD, van Werkhoven JM, Nucifora G, Jukema JW, Bax JJ. Relation of epicardial adipose tissue to coronary atherosclerosis. Am J Cardiol. 2008;102:1602–7. doi: 10.1016/j.amjcard.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 25.Gössl M, Versari D, Mannheim D, Ritman EL, Lerman LO, Lerman A. Increased spatial vasa vasorum density in the proximal LAD in hypercholesterolemia--implications for vulnerable plaque-development. Atherosclerosis. 2007;192(2):246–52. doi: 10.1016/j.atherosclerosis.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 26.Kondos GT, Hoff JA, Sevrukov A, et al. Electron-beam tomography coronary artery calcium and cardiac events: a 37-month follow-up of 5635 initially asymptomatic low to intermediate risk adults. Circulation. 2003;107:2571–2576. doi: 10.1161/01.CIR.0000068341.61180.55. [DOI] [PubMed] [Google Scholar]
- 27.Budoff MJ, Shaw LJ, Liu ST, et al. Long-term prognosis associated with coronary calcification: Observations from a registry of 25,253 patients. J Am Coll Cardiol. 2007;49:1860–1870. doi: 10.1016/j.jacc.2006.10.079. [DOI] [PubMed] [Google Scholar]
- 28.Shaw LJ, Raggi P, Schisterman E, Berman DS, Callister TQ. Prognostic value of cardiac risk factors and coronary artery calcium screening for all-cause mortality. Radiology. 2003;228:826–833. doi: 10.1148/radiol.2283021006. [DOI] [PubMed] [Google Scholar]
- 29.Coutinho T, Goel K, Corrêa de Sá D, Carter RE, Hodge DO, Kragelund C, Kanaya AM, Zeller M, Park JS, Kober L, Torp-Pedersen C, Cottin Y, Lorgis L, Lee SH, Kim YJ, Thomas R, Roger VL, Somers VK, Lopez-Jimenez F. Combining body mass index with measures of central obesity in the assessment of mortality in subjects with coronary disease: role of “normal weight central obesity”. J Am Coll Cardiol. 2013;61(5):553–60. doi: 10.1016/j.jacc.2012.10.035. [DOI] [PubMed] [Google Scholar]
- 30.Hainer V, Aldhoon-Hainerová I. Obesity paradox does exist. Diabetes Care. 2013;36 (Suppl 2):S276–81. doi: 10.2337/dcS13-2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Romero-Corral A, Somers VK, Sierra-Johnson J, Jensen MD, Thomas RJ, Squires RW, Allison TG, Korinek J, Lopez-Jimenez F. Diagnostic performance of body mass index to detect obesity in patients with coronary artery disease. Eur Heart J. 2007;28(17):2087–93. doi: 10.1093/eurheartj/ehm243. [DOI] [PubMed] [Google Scholar]
- 32.Xu A, Wang Y, Lam KS, Vanhoutte PM. Vacular actions of adipoknes molecular mechanisms and therapeutic implications. Adv Pharmacol. 2010;60:229–55. doi: 10.1016/B978-0-12-385061-4.00008-8. [DOI] [PubMed] [Google Scholar]
- 33.Rajsheker S, Manka D, Blomkalns AL, Chatterjee TK, Stoll LL, Weintraub NL. Crosstalk between perivascular adipose tissue and blood vessels. Curr Opin Pharmacol. 2010;10:191–196. doi: 10.1016/j.coph.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ouwens DM, Sell H, Greulich S, Eckel J. The role of epicardial and perivascular adipose tissue in the pathophysiology of cardiovascular disease. J Cell Mol Med. 2010;14(9):2223–34. doi: 10.1111/j.1582-4934.2010.01141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Verhagen SN, Visseren FL. Perivascular adipose tissue as a cause of atherosclerosis. Atherosclerosis. 2011;214(1):3–10. doi: 10.1016/j.atherosclerosis.2010.05.034. [DOI] [PubMed] [Google Scholar]
- 36.Karastergiou K, Evans I, Ogston N, Miheisi N, Nair D, Kaski JC, Jahangiri M, Mohamed-Ali V. Epicardial adipokines in obesity and coronary artery disease induce atherogenic changes in monocytes and endothelial cells. Arterioscler Thromb Vasc Biol. 2010;30(7):1340–6. doi: 10.1161/ATVBAHA.110.204719. [DOI] [PubMed] [Google Scholar]
- 37.Payne GA, Kohr MC, Tune JD. Epicardial perivascular adipose tissue as a therapeutic target in obesity-related coronary artery disease. Br J Pharmacol. 2012;165:659–69. doi: 10.1111/j.1476-5381.2011.01370.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Staub D, Schinkel AF, Coll B, Coli S, van der Steen AF, Reed JD, Krueger C, Thomenius KE, Adam D, Sijbrands EJ, ten Cate FJ, Feinstein SB. Contrast-enhanced ultrasound imaging of the vasa vasorum: from early atherosclerosis to the identification of unstable plaques. JACC Cardiovasc Imaging. 2010;3(7):761–71. doi: 10.1016/j.jcmg.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 39.McKenney ML, Schultz KA, Boyd JH, Byrd JP, Alloosh M, Teague SD, Arce-Esquivel AA, Fain JN, Laughlin MH, Sacks HS, Sturek M. Epicardial adipose excision slows the progression of porcine coronary atherosclerosis. J Cardiothorac Surg. 2014 Jan 3;9(1):2. doi: 10.1186/1749-8090-9-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Verhagen SN, Vink A, van der Graaf Y, Visseren FL. Coronary perivascular adipose tissue characteristics are related to atherosclerotic plaque size and composition. A postmortem study. Atherosclerosis. 2012;225(1):99–104. doi: 10.1016/j.atherosclerosis.2012.08.031. [DOI] [PubMed] [Google Scholar]
- 41.Xu Y, Cheng X, Hong K, Huang C, Wan L. How to interpret epicardial adipose tissue as a cause of coronary artery disease: a meta-analysis. Coron Artery Dis. 2012;23(4):227–33. doi: 10.1097/MCA.0b013e328351ab2c. [DOI] [PubMed] [Google Scholar]
- 42.Gorter PM, van Lindert AS, de Vos AM, Meijs MF, van der Graaf Y, Doevendans PA, Prokop M, Visseren FL. Quantification of epicardial and peri-coronary fat using cardiac computed tomography; reproducibility and relation with obesity and metabolic syndrome in patients suspected of coronary artery disease. Atherosclerosis. 2008;197(2):896–903. doi: 10.1016/j.atherosclerosis.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 43.Sacks HS, Fain JN, Holman B, Cheema P, Chary A, Parks F, Karas J, Optican R, et al. Uncoupling protein-1 and related messenger ribonucleic acids in human epicardial and other adipose tissues: epicardial fat functioning as brown fat. J Clin Endocrinol Metab. 2009;94(9):3611–5. doi: 10.1210/jc.2009-0571. [DOI] [PubMed] [Google Scholar]
- 44.Mazurek T, Zhang L, Zalewski A, Mannion JD, Diehl JT, Arafat H, Sarov-Blat L, O’Brien S, et al. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation. 2003;108(20):2460–6. doi: 10.1161/01.CIR.0000099542.57313.C5. [DOI] [PubMed] [Google Scholar]
- 45.Schlett CL, Massaro JM, Lehman SJ, Bamberg F, O’Donnell CJ, Fox CS, Hoffmann U. Novel measurements of periaortic adipose tissue in comparison to anthropometric measures of obesity, and abdominal adipose tissue. Int J Obes. 2009;33(2):226–32. doi: 10.1038/ijo.2008.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lehman SJ, Massaro JM, Schlett CL, O’Donnell CJ, Hoffmann U, Fox CS. Peri-aortic fat, cardiovascular disease risk factors, and aortic calcification: the Framingham Heart Study. Atherosclerosis. 2010;210 (2):656–61. doi: 10.1016/j.atherosclerosis.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jeong JW, Jeong MH, Yun KH, Oh SK, Park EM, Kim YK, Rhee SJ, Lee EM, Lee J, Yoo NJ, Kim NH, Park JC. Echocardiographic epicardial fat thickness and coronary artery disease. Circ J. 2007;71(4):536–9. doi: 10.1253/circj.71.536. [DOI] [PubMed] [Google Scholar]
- 48.Kim HM, Kim KJ, Lee HJ, Yu HT, Moon JH, Kang ES, et al. Epicardial adipose tissue thickness is an indicator for coronary artery stenosis in asymptomatic type 2 diabetic patients: its assessment by cardiac magnetic resonance. Cardiovasc Diabetol. 2012;11:83. doi: 10.1186/1475-2840-11-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yerramasu A, Dey D, Venuraju S, Anand DV, Atwal S, Corder R, Berman DS, Lahiri A. Increased volume of epicardial fat is an independent risk factor for accelerated progression of sub-clinical coronary atherosclerosis. Atherosclerosis. 2012;220(1):223–30. doi: 10.1016/j.atherosclerosis.2011.09.041. [DOI] [PubMed] [Google Scholar]
- 50.Wang CP, Hsu HL, Hung WC, Yu TH, Chen YH, Chiu CA, Lu LF, Chung FM, Shin SJ, Lee YJ. Increased epicardial adipose tissue (EAT) volume in type 2 diabetes mellitus and association with metabolic syndrome and severity of coronary atherosclerosis. Clin Endocrinol (Oxf) 2009;70 (6):876–82. doi: 10.1111/j.1365-2265.2008.03411.x. [DOI] [PubMed] [Google Scholar]


