Abstract
Methods for the preparation of protoplasts and vacuoles from mesophyll tissues of sweet clover (Melilotus alba Desr.) are described. Vacuoles are obtained using a new procedure which involves lysis of the plasmalemma during a brief centrifugation of protoplasts through a diethylaminoethyl dextran layer. This method combines the release of vacuoles and their purification in one step. The contamination of vacuole preparations was found to be low, as judged by enzymic markers and microscopic inspection. The method described is rapid and gives a good yield of vacuoles without causing changes in osmotic pressure. Several hydrolases were found to be located in vacuoles from sweet clover, which were also examined for their amino acid content.
Full text
PDF
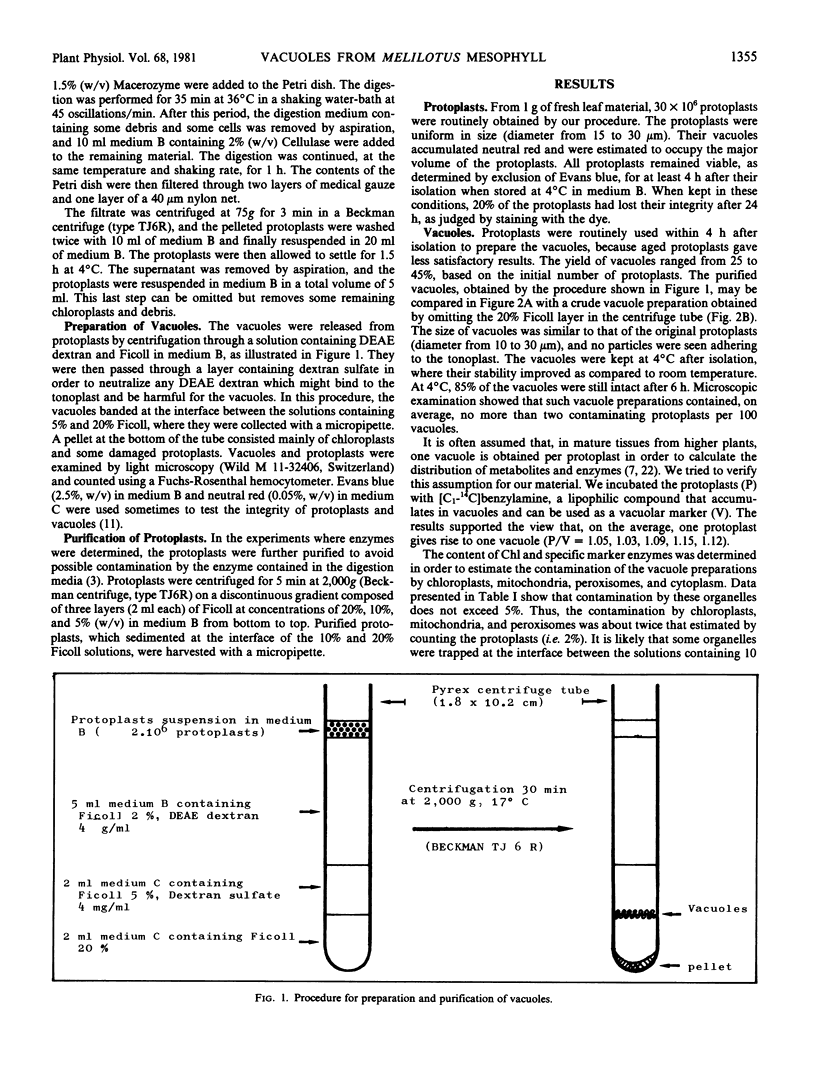


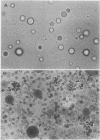
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Admon A., Jacoby B. Assessment of cytoplasmic contaminations in isolated vacuole preparations. Plant Physiol. 1980 Jan;65(1):85–87. doi: 10.1104/pp.65.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRUINSMA J. A comment on the spectrophotometric determination of chlorophyll. Biochim Biophys Acta. 1961 Sep 30;52:576–578. doi: 10.1016/0006-3002(61)90418-8. [DOI] [PubMed] [Google Scholar]
- Boller T., Kende H. Hydrolytic enzymes in the central vacuole of plant cells. Plant Physiol. 1979 Jun;63(6):1123–1132. doi: 10.1104/pp.63.6.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brulfert J., Guerrier D., Queiroz O. Photoperiodism and Enzyme Activity: Balance between Inhibition and Induction of the Crassulacean Acid Metabolism. Plant Physiol. 1973 Jan;51(1):220–222. doi: 10.1104/pp.51.1.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher H. C., Wagner G. J., Siegelman H. W. Localization of Acid hydrolases in protoplasts: examination of the proposed lysosomal function of the mature vacuole. Plant Physiol. 1977 Jun;59(6):1098–1103. doi: 10.1104/pp.59.6.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper T. G., Beevers H. Mitochondria and glyoxysomes from castor bean endosperm. Enzyme constitutents and catalytic capacity. J Biol Chem. 1969 Jul 10;244(13):3507–3513. [PubMed] [Google Scholar]
- Dürr M., Boller T., Wiemken A. Polybase induced lysis of yeast spheroplasts. A new gentle method for preparation of vacuoles. Arch Microbiol. 1975 Nov 7;105(3):319–327. doi: 10.1007/BF00447152. [DOI] [PubMed] [Google Scholar]
- Guy M., Reinhold L., Michaeli D. Direct evidence for a sugar transport mechanism in isolated vacuoles. Plant Physiol. 1979 Jul;64(1):61–64. doi: 10.1104/pp.64.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leigh R. A., Branton D. Isolation of Vacuoles from Root Storage Tissue of Beta vulgaris L. Plant Physiol. 1976 Nov;58(5):656–662. doi: 10.1104/pp.58.5.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinoia E., Heck U., Boller T., Wiemken A., Matile P. Some properties of vacuoles isolated from Neurospora crassa slime variant. Arch Microbiol. 1979 Jan 16;120(1):31–34. doi: 10.1007/BF00413268. [DOI] [PubMed] [Google Scholar]
- Nishimura M., Beevers H. Hydrolases in vacuoles from castor bean endosperm. Plant Physiol. 1978 Jul;62(1):44–48. doi: 10.1104/pp.62.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oba K., Conn E. E., Canut H., Boudet A. M. Subcellular Localization of 2-(beta-d-Glucosyloxy)-Cinnamic Acids and the Related beta-glucosidase in Leaves of Melilotus alba Desr. Plant Physiol. 1981 Dec;68(6):1359–1363. doi: 10.1104/pp.68.6.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders J. A., Conn E. E. Presence of the cyanogenic glucoside dhurrin in isolated vacuoles from sorghum. Plant Physiol. 1978 Feb;61(2):154–157. doi: 10.1104/pp.61.2.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders J. A. Investigations of vacuoles isolated from tobacco: I. Quantitation of nicotine. Plant Physiol. 1979 Jul;64(1):74–78. doi: 10.1104/pp.64.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt R., Poole R. J. Isolation of protoplasts and vacuoles from storage tissue of red beet. Plant Physiol. 1980 Jul;66(1):25–28. doi: 10.1104/pp.66.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner G. J. Content and vacuole/extravacuole distribution of neutral sugars, free amino acids, and anthocyanin in protoplasts. Plant Physiol. 1979 Jul;64(1):88–93. doi: 10.1104/pp.64.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]