Abstract
Background
Periostin, a secreted extracellular matrix protein, has been localized to deposits of subepithelial fibrosis in asthma, and periostin levels have been linked to elevation of IL-13.
Objective
We hypothesized that periostin is required for airway inflammatory responses to a physiologic aeroallergen, house dust mite (HDM).
Methods
We studied F4-F6 B6;129-Postntm1Jmol/J wild-type (+/+) and null (−/−) mice as well as C57BL/6 mice treated with either IgM or OC-20 periostin neutralizing antibody. Mice were exposed to five doses of HDM intranasally over a 16 day period.
Results
HDM increased airways responsiveness in Postn wild-type but not null mice. In addition, HDM-treated C57BL/6 mice injected with OC-20 had a lower airways responsiveness than HDM-treated mice injected with IgM. Compared to Postn wild-type mice, Postn null mice showed decreases in HDM-induced inflammation and mucous metaplasia, as well as reduced IL-4, IL-25, CD68, Gob5 and periostin mRNA expression. OC-20 antibody gave similar results. HDM exposure increased periostin expression in the airway epithelium, subepithelium, smooth muscle and inflammatory cells. OC-20 blocked the HDM-induced IgE response, and T cells incubated with dendritic cells (DCs) from Postn null mice or treated with OC-20 showed deficient DNA synthesis and IL-13 responses compared to T cells incubated with wild-type DCs. Finally, adoptive transfer of bone marrow-derived DCs from periostin wild-type mice was sufficient to promote allergic responses in F6 periostin null littermates.
Conclusions
In mice, periostin is required for maximal airways hyperresponsiveness and inflammation following HDM sensitization and challenge. Periostin is required for maximal HDM-induced T cell responses.
Keywords: periostin, asthma, mouse models, inflammation, matricellular protein, house dust mite, integrin, CD11b
Introduction
Matricellular proteins are non-structural proteins that are secreted and sequestered in the extracellular matrix where they interact with integrins, growth factors, proteases, cytokines and other extracellular matrix proteins. One matricellular protein, periostin, is deposited in the airway subepithelium of patients with asthma 1. Periostin mRNA expression is also increased in the airway epithelium, and expression level correlates with disease severity 2. Treatment with the anti-IL-13 antibody lebrikizumab improves lung function in patients with asthma, and patients with high pretreatment levels of serum periostin had greater improvement in lung function than patients with low periostin levels 3. In patients with persistent symptoms despite inhaled corticosteroids, serum periostin is the single best predictor of airway eosinophilia 4. Periostin is a marker of omalizumab responsiveness in patients with uncontrolled severe persistent allergic asthma 5. Recently, it was shown in patients with asthma that high serum periostin levels (≥ 95 ng/mL) were associated with a greater decline in FEV1 per year 6. These data are consistent with the notion that periostin plays a deleterious role in asthma pathogenesis.
The periostin gene encodes a 93 kD glycoprotein comprised of an N-terminal secretory signal sequence and 4 fasciclin domains. Periostin also undergoes alternative splicing in its C-terminal region 7, leading to smaller isoforms. Periostin interacts with other extracellular matrix proteins and is a ligand for αvβ3, αvβ5, α4β6 and αMβ2 (CD11b) integrins. For example, eosinophils adhere to periostin via the αMβ2 integrin CD11b 8 and periostin increases eosinophil adhesion to fibronectin 9. Periostin also regulates TGF-β signaling 10 and promotes myofibroblast differentiation of mesenchymal cells 11-13. Because periostin has the potential to regulate both adhesion of lung inflammatory cells and mesenchymal cell differentiation, we hypothesized that periostin is required for maximal allergic airways disease in mice.
Methods
Mice
C57BL/6 and F2 B6;129-Postntm1Jmol/J mice 14 were purchased from Jackson Laboratory (Bar Harbor, ME). Homozygous Postn (−/−) mice were backcrossed into the C57BL/6 strain for 2-4 additional generations (F4-F6). Most experiments compared F4 or F6 homozygous Postn (−/−) mice with their homozygous Postn (+/+) littermates. The remainder of the experiments, examining the effects of an anti-periostin neutralizing antibody (see below), were conducted in C57BL/6 mice. Genotyping was performed by Transnetyx (Cordova, TN) and verified using specific primers and qPCR assays.
Models of allergic airways disease
We exposed 8-12 week old C57BL/6 and F4-F6 B6;129 Postn wild-type (+/+) and null (−/−) mice to 100 μg D. pteronyssinus house dust mite (HDM) extract in 50 μl PBS (Greer Labs, Lenoir, NC) by intranasal installation on days 0, 7, 14, 15, and 16. Mice were anesthetized with isoflurane for each treatment. Animals were studied on day 17. Alternatively, mice were exposed to LPS-free ovalbumin (OVA, Pierce, Rockford, IL), as described 15. Briefly, mice received intraperitoneal injections of 20 g OVA in 2 mg alum on days 0 and 7, and 100 g intranasal OVA on days 14 through 19. Mice were euthanized on day 21. Changes in airways resistance to nebulized methacholine were assessed in anesthetized tracheotomized mice using a Buxco FinePointe plethysmograph (Wilmington, NC) 16.
Periostin neutralization
Mice were injected intraperitoneally with 200 μg OC-20 mouse monoclonal anti-periostin (Sirius-Biotech, Genoa, IT) on days 7 and 14 of HDM exposure. OC-20 blocks periostin's interaction with integrins αvβ3 and αvβ5 13, 17.
Analysis of airway inflammation
Lungs sections were stained with hematoxylin and eosin or periodic acid-Schiff reagent to detect mucins. Bronchoalveolar lavage (BAL) leukocyte differential counts were performed as previously described 18.
Harvesting of lung tissue for flow cytometry, qPCR and immunostaining
For flow cytometry, cell pellets were resuspended in serum-containing medium with bovine serum albumin, anti-mouse CD16/32 (Biolegend, San Diego, CA) and fluorescent antibody or matched isotype control 19, 20. Cells were analyzed on a FACSCanto 2 (BD Biosciences, San Jose, CA) using FACSDiva (BD Biosciences) and FlowJo software (Tree Star, Ashland, OR). Up to 105 cells were analyzed per sample. CD45, CD11b, CD11c, F4/80 (Biolegend), Siglec-F (eBioscience, San Diego, CA), and Gr1 (R&D Systems, Minneapolis, MN) were monitored.
Aliquots were also taken for RNA extraction using Trizol (Invitrogen, Grand Island, NY). Poly A RNA was purified (RNeasy Plus Mini kit, Qiagen, Valencia, CA) and first-strand cDNA was produced for quantitative two-step real time PCR (Eppendorf Realplex2, Westbury, NY). Primer sequences used are shown in Table 1. Results were normalized against GAPDH.
Table 1.
Primer sequences used for qPCR.
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| IL4 | 5′--TGC ACC ATG AAT GAG TCC AA--3′ | 5′ -GGT GGC TCA GTA CTA CGA GTA ATC--3′ |
| Il13 | 5′--GGT CTT GTG TGA TGT TGC TCA--3′ | 5′--CCT GGC TCT TGC TTG CCT T--3′ |
| Il10 | 5’--GCT CTT ACT GAC TGG CAT GAG--3’ | 5’--CGC AGC TCT AGG AGC ATG TG--3’ |
| Il17a | 5′--GCC TGA GAG CTG CCC CTT CAC--3′ | 5′--GGC TGC CTG GCG GAC AAT CG--3′ |
| Il25 | 5’- -ACA GGG ACT TGA ATC GGG TC- -3’ | 5’--TGG TAA AGT GGG ACG GAG TTG--3’ |
| Cd68 | 5′--TCC CAA GAG CCC CCT TGG ACC --3′ | 5′--TCC ACT GTT GGC CCT CAC CCT--3′ |
| Gob5 | 5′--CTG TCT TCC TCT TGA TCC TCC A --3′ | 5′--CGT GGT CTA TGG CGA TGA CG --3′ |
| Muc5b | 5’--GAG CAG TGG CTA TGT GAA AAT CAG--3’ | 5’- -CAG GGC GCT GTC TTC TTC AT— |
| Postn | 5′--GCC TTA GCG ACC TCT ACA AT--3′ | 5′--TAG CCG TCC GAT ACA CAA--3′ |
| Cyr61 | 5’--CCA TGG CCA GAA ATG CAT CG--3’ | 5’--AAC TCG TGT GGA GAT GCC AG--3’ |
| Gapdh | 5′-- GTC GGT GTG AAC GGA TTT G--3′ | 5′-- GTC GTT GAT GGC AAC AAT CTC--3′ |
For fluorescence microscopy, sections were probed with fluorescent labeled mouse anti-α-smooth muscle actin (clone 1A4, Sigma-Aldrich, St. Louis, MO), polyclonal rabbit anti-periostin (Abcam, Cambridge, MA), anti-I-A/I-E (mouse MHC class II, Biolegend) or specific IgG or IgM isotype controls. For immunohistochemistry, sections were probed with rabbit anti-periostin and stained using a biotinylated anti-rabbit IgG-avidin horseradish peroxidase and diaminobenzidine detection system (Vector Labs, Burlingame, CA).
Measurement of serum IgE
IgE was assayed by ELISA (Biolegend, San Diego, CA).
Requirement of periostin for dendritic cell activation
To determine whether periostin is required for dendritic cell (DC) activation, we employed an in vitro assay examining the response of bone marrow-derived DCs to HDM in vitro, using T cell IL-13 expression and bromodeoxyuridine (BrdU) incorporation as outputs 21. Bone marrow was harvested from two F4 or F6 periostin null mice and two (+/+) littermates. For differentiation, DCs were cultured for 7 days with 10% FBS, 40 ng/ml GM-CSF and 15 ng/ml IL-4. On day 7, cells were pulsed with PBS or 100 μg/mL HDM in PBS with either 100 μg/mL mouse IgM or OC-20 anti-periostin antibody. For selected experiments, 1×105 allogenic T cells, purified from Balb/cJ mouse spleen using the MACS T isolation kit (Miltenyi Biotec, Auburn, CA), were added on day 8 of the protocol. On day 10, cells were harvested, stained for dead cells with a Pacific Orange viability dye, incubated with Pacific Blue conjugated anti- CD83, FITC-conjugated anti-CD80, AlexaFluor 555-conjugated anti-IA-IE (a mouse MHC2 protein), PECy5-conjugated anti-CD45, APC-conjugated anti-CD11c, and APC-Cy7 conjugated anti-CD86, and subjected to flow cytometry. To assess IL-13 production, cells were stimulated with brefeldin A, ionomycin and phorbol myristate acetate for 4 h. Cells were incubated with fluorescent anti-TCRβ and anti-CD45 (Biolegend), fixed, permeabilized, incubated with labeled anti-IL13 (eBioscience) and harvested for flow cytometry. IL-13 in cell supernatants was assessed by ELISA (eBioscience). To measure T cell DNA synthesis, 1 μM BrdU (Sigma-Aldrich) was added 4 h before harvest on day 10 and cells were labeled with labeled anti-BrdU (Biolegend).
Adoptive transfer
F6 Postn (+/+) and (−/−) littermate bone marrow cells were differentiated and matured with HDM as above. Next, 1 × 106 labeled cells in 50 μL PBS were given intratracheally to anesthetized Postn −/− mice. On day 11, sensitization and challenge with HDM or PBS was begun. On day 28, lungs were harvested and processed for PAS staining.
Results
Periostin is required for allergen-induced airways inflammation and mucous metaplasia
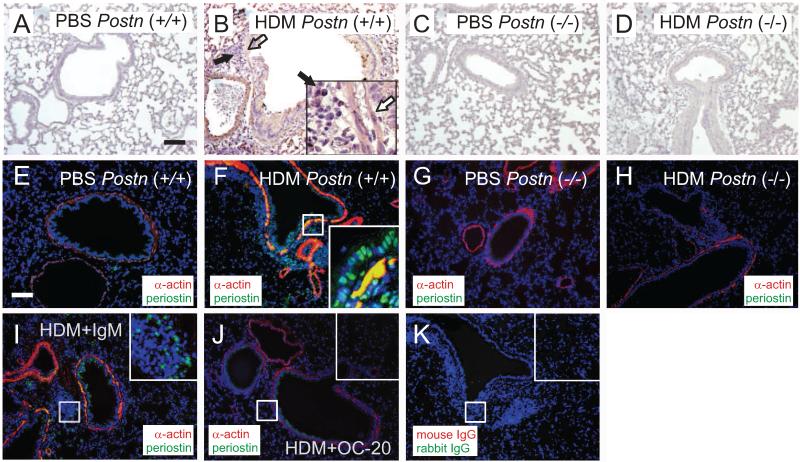
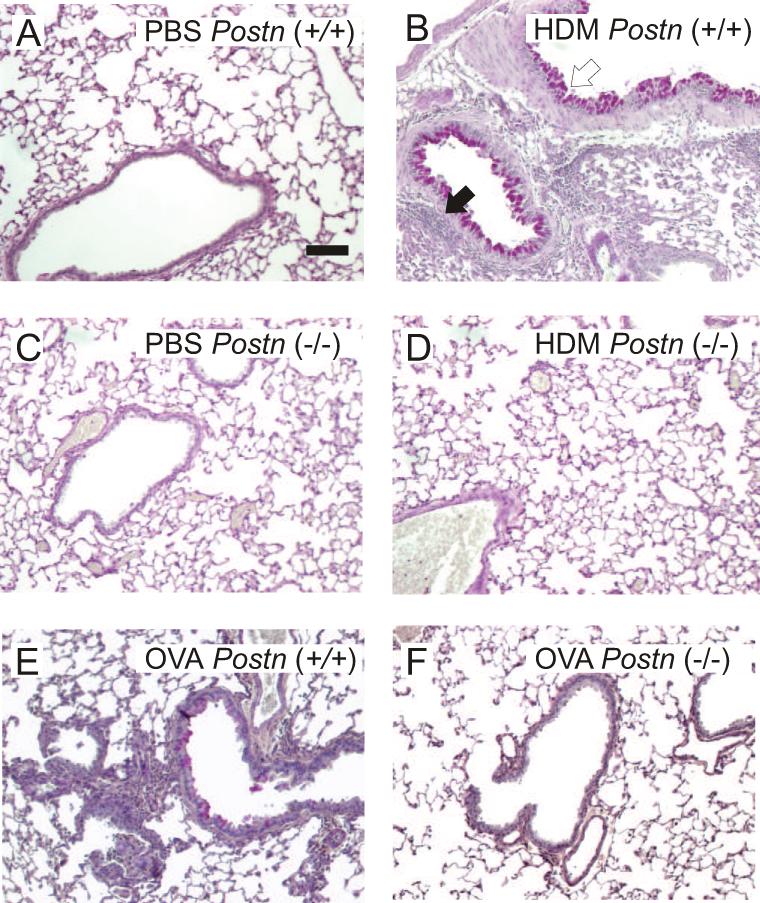
F4 B6;129 Postn (+/+) and (−/−) null mice 14 were exposed to five intranasal exposures of HDM over a 17 d period. Immunohistochemical staining showed that HDM exposure increased periostin expression in the airway epithelium, subepithelium and inflammatory cell infiltrates (Figure 1, panels A-D). Periostin expression was absent in Postn null mice. Fluorescence microscopy showed colocalization of periostin and α-smooth muscle actin in the airway smooth muscle layer (panels E-H). Finally, immunofluorescence showed reduced periostin expression in OC-20-treated mice (panels I-K). Sections were stained with hematoxylin and eosin or periodic acid-Schiff reagent to detect mucus. HDM-exposed (+/+) mice showed substantial peribronchial inflammation and mucous metaplasia (Figure 2A, 2B). Compared to Postn (+/+) mice, Postn (−/−) mice showed decreased inflammation and mucous metaplasia (Figure 2C, 2D).
Figure 1. Periostin localization in the airways of HDM-treated mice.
Panels A-D. Periostin staining in the lungs of PBS- (panels A, C) and HDM-treated (B, D) mice. Lungs from F4 Postn (+/+) (A, B) and null (−/−) mice (C, D). Panel B shows periostin-positive airway smooth muscle (white arrow) and inflammatory cells (black arrow). Images are 100X except for 400X inset. Bars are 100 m. Panels E-K. Immunofluorescence for α-actin (red) and periostin (green), with colocalization in yellow and nuclei in blue. E. PBS-treated Postn (+/+) mouse. F. HDM-treated (+/+) mouse. G. PBS-treated Postn null (−/−) mouse. H. HDM-treated null (−/−) mouse. I. C57BL/6 mouse treated with HDM and IgM. J. C57BL/6 mouse treated with HDM and OC-20. K. Control slide stained with mouse IgG and rabbit IgG. These figures are representative of three separate experiments.
Figure 2. HDM treatment increases mucus production and airway inflammation in Postn (+/+) mice but not Postn null mice.
Lung sections from F4 B6;129 Postn (+/+) (A, B) or (−/−) mice (C, D) treated with PBS (A, C) or HDM (B, D) were stained with PAS and hematoxylin B. Note magenta PAS-staining (open arrow) and hematoxylin-stained inflammatory cells (black arrow). Lung sections from F6 B6;129 Postn (+/+) (E) or null mice (F) treated with OVA. Original magnification, 100X, bar is 100 μm. These figures are representative of three separate experiments.
We also exposed F6 B6;129 Postn (+/+) and (−/−) littermates to OVA sensitization and challenge. OVA-exposed (+/+) mice showed peribronchial inflammation and mucous metaplasia, though less than HDM-treated mice. Postn (−/−) littermates showed decreased inflammation and mucous metaplasia (Figure 2E, 2F).
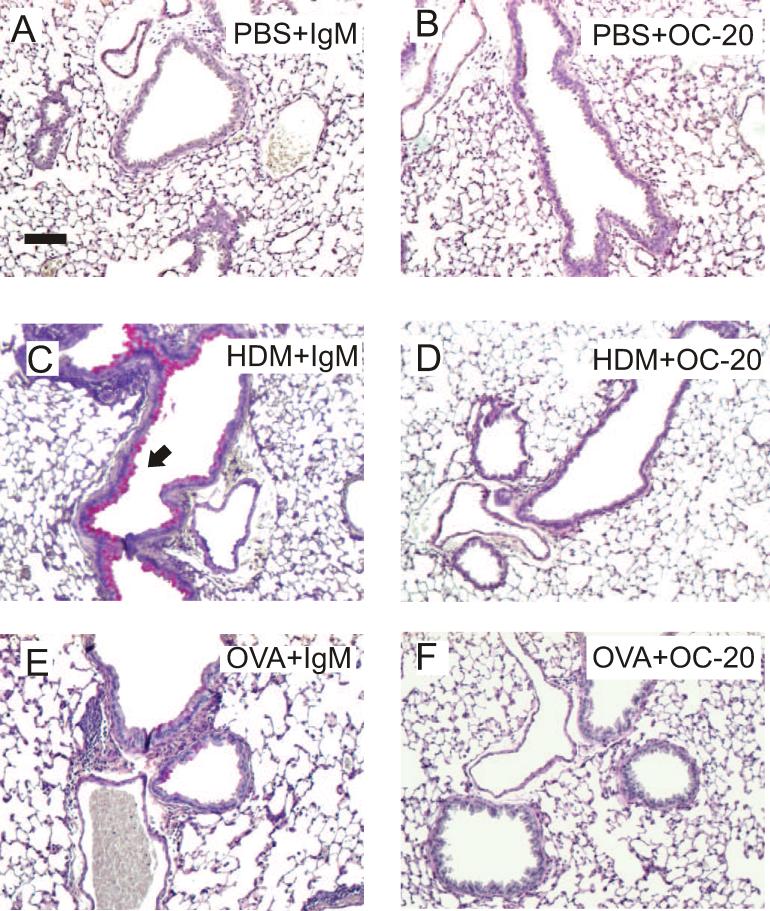
We also examined the requirement for periostin in HDM-exposed C57BL/6 mice using a mouse monoclonal neutralizing antibody (OC-20) 13, 17. OC-20 blocked HDM-induced inflammation and mucous metaplasia (Figure 3A-D).
Figure 3. A periostin-neutralizing monoclonal antibody, OC-20, blocks allergen-induced mucus production and airway inflammation in C57BL/6 mice.
PAS-stained sections from the lungs of PBS (panels A,B)- or HDM (panels C,D)-exposed C57BL/6 mice treated on days 7 and 14 with 200 μg IgM (panels A,C) or 200 μg OC-20 (panels B,D). Mouse lung sections from OVA-exposed C57BL/6 mice treated with IgM (E) or OC-20 (F). These figures are representative of three separate experiments.
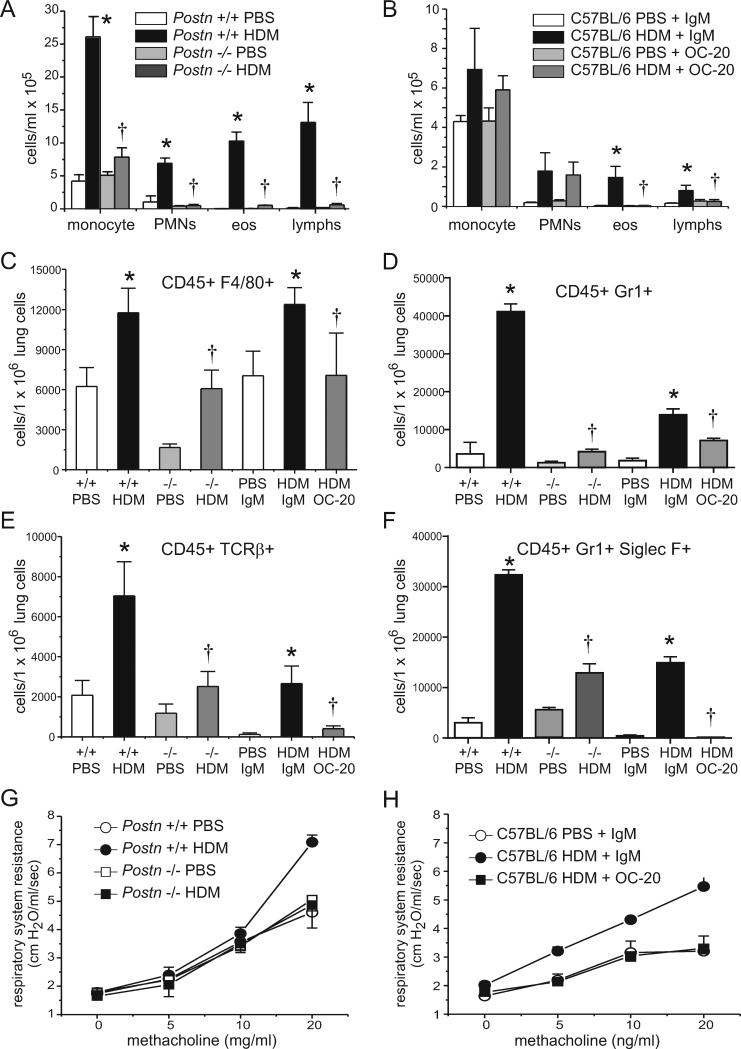
We examined the effects of periostin deficiency on inflammatory cells in the BAL and lung. HDM exposure increased the number of neutrophils, macrophage/monocytes, lymphocytes and eosinophils in the BAL. Compared to (+/+) mice, Postn null mice showed reductions in each cell type (Figure 4A). IgM-treated, HDM-exposed C57BL/6 mice also showed elevations in BAL inflammatory cells, though the changes were less marked (Figure 4B). OC-20 treated mice showed significant reductions in BAL eosinophils. Next, we examined lung inflammatory cells by flow cytometry. Compared to HDM-exposed wild-type Postn mice, Postn null mice showed fewer lung Gr1+ neutrophils, TCR-β+ T cells and Gr1+, Siglec-F+ eosinophils (Figure 4C-F). Compared to HDM-exposed IgM-treated C57BL/6 mice, OC-20 treated mice showed fewer lung T cells and neutrophils (the reduction in eosinophils was not statistically significant).
Figure 4. Periostin is required for HDM-induced airways inflammation and hyperresponsiveness.
A. BAL cells in HDM-treated F4 B6;129 Postn (+/+) mice (open bars) and Postn null (−/−) littermates (black bars). (N=4, *different from Postn (+/+) PBS, †different from Postn (+/+) HDM, p<0.05, one-way ANOVA.) B. HDM-exposed C57BL/6 mice were injected with IgM or OC20 (N=4, *different from PBS+IgM, †different from HDM+IgM, p<0.05, one-way ANOVA). C-F. Lung cells were interacted with antibodies against CD45, F4/80 (C), TCRβ (D), Gr1 (E) or both Gr1 and SiglecF (F). (N=6-12, *different from Postn (+/+) PBS mice or C57BL/6 PBS+IgM mice, as appropriate, p<0.05, †different from Postn (+/+) HDM mice or C57BL/6 HDM+IgM mice, p<0.05, one-way ANOVA.) G. Airways responsiveness was higher for HDM-exposed F4 B6;129 Postn (+/+) mice than (−/−) littermates (N=6, *p<0.05, two way ANOVA). H. Airways responsiveness in HDM-exposed C57BL/6 mice injected with IgM or OC-20 (N=4, *p<0.05, two-way ANOVA).
Periostin is required for HDM-induced airways hyperresponsiveness
Changes in airways cholinergic responsiveness were assessed as described above. HDM increased responsiveness in F4 B6;129 Postn wild-type (+/+) mice (Figure 4G). In contrast, the airways responsiveness of HDM-exposed littermate Postn null mice was not different from PBS controls. We also examined the requirement for periostin in HDM-exposed, IgM- and OC-20-treated C57BL/6 mice. HDM-treated C57BL/6 mice injected intraperitoneally with OC20 had a significantly lower methacholine response than HDM-treated controls injected with IgM (Figure 4H).
Periostin-deficient mice show lower HDM-induced lung cytokine mRNA expression
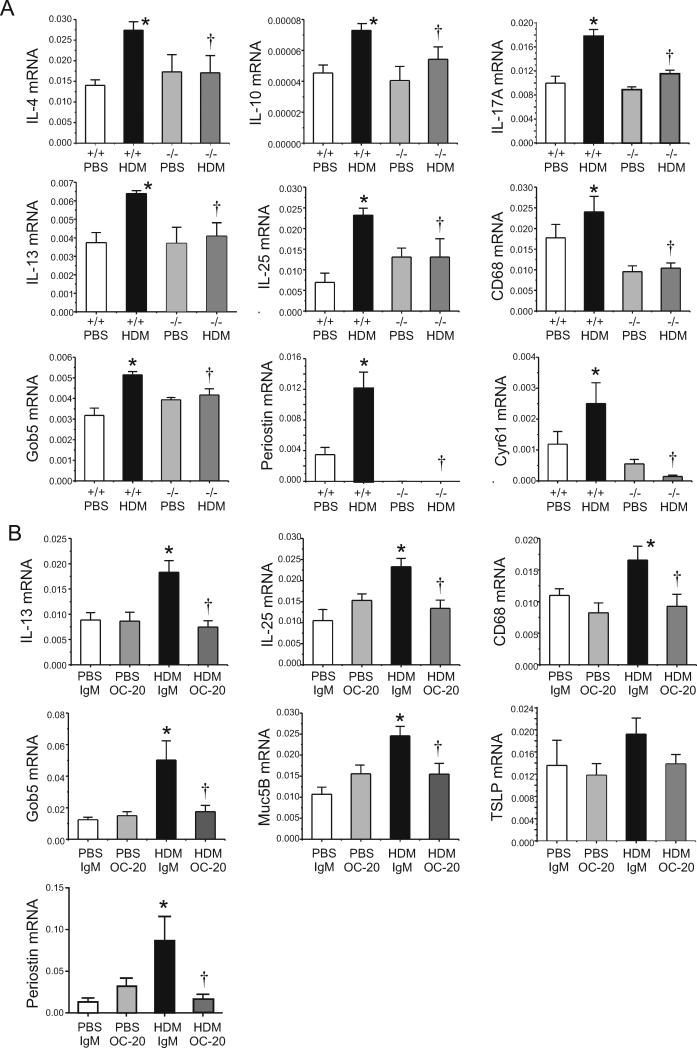
Lung cytokine mRNA was monitored by qPCR. Compared to HDM-treated F4 B6;129 Postn (+/+) mice, Postn null mice showed significant reductions in mRNAs encoding IL-4, IL-10, IL13, IL-17a, IL-25, CD68 (a macrophage marker), Gob5 (a chloride channel which regulates mucus production), Cyr61, another matricellular protein and, as expected, periostin (Figure 5A). Compared with C57BL/6 mice treated with HDM and IgM, mice treated with HDM and the neutralizing anti-periostin monoclonal OC-20 showed reductions in IL-13, IL-25, CD68, Gob5 and Muc5B and periostin (Figure 5B). Changes in TSLP were not statistically significant.
Figure 5. Periostin null and OC-20-treated mice show decreased HDM-induced mRNA expression.
A. mRNA expression was measured by qPCR (N=4, *different from Postn (+/+) PBS, p<0.05, †different from Postn (+/+) HDM, p<0.05, one-way ANOVA). B. C57BL/6 mice were treated with HDM or PBS without or with isotypic IgM or OC-20 (N=4, *different from PBS+IgM, p<0.05, †different from HDM+IgM, p<0.05, one-way ANOVA).
A neutralizing antibody to periostin lowers the serum IgE response to HDM sensitization
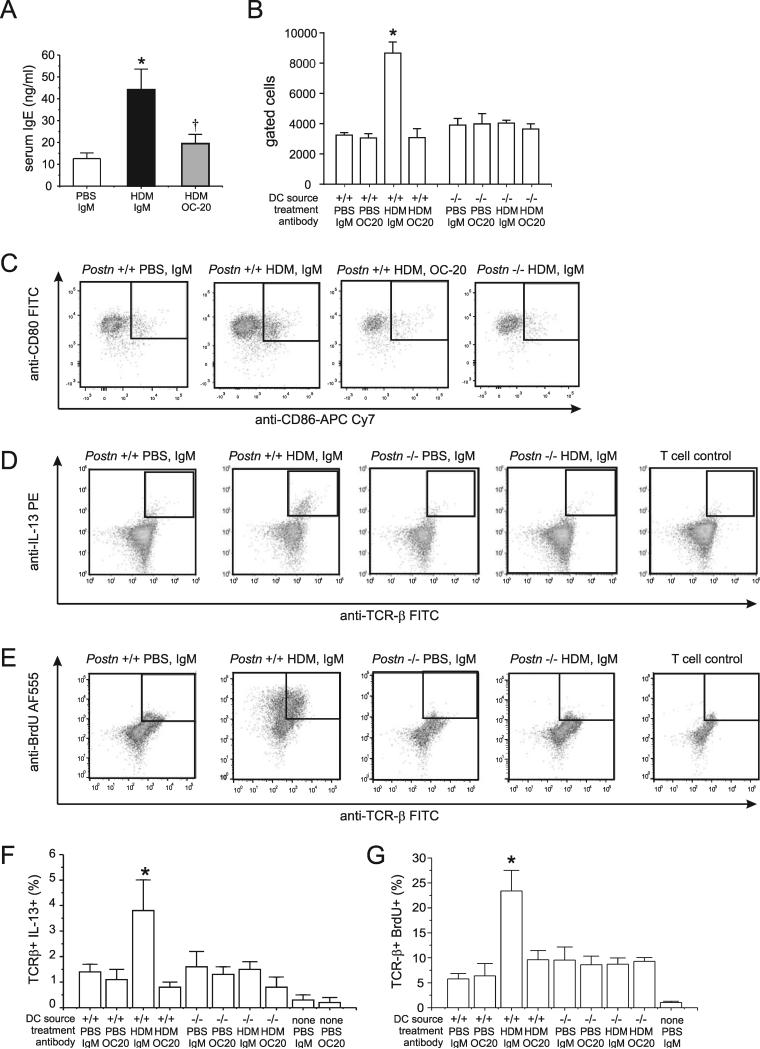
We sensitized C57BL6 mice to HDM in the presence of OC-20 or an IgM control. HDM exposure increased serum IgE and OC-20 blocked the IgE response (Figure 6A). These data suggest that periostin is required for maximal HDM-induced type 2 immune responses.
Figure 6. Periostin is required for IgE production, dendritic cell (DC) CD86 expression and allogenic T cell IL-13 production and DNA synthesis.
A. Serum IgE measured by ELISA (N=11, *different from PBS+IgM, p<0.05, †different from HDM+IgM, p<0.05, one-way ANOVA). B-G. Mice were sensitized to HDM and bone marrow cells from F4 or F6 Postn (+/+) and (−/−) littermates were differentiated to DCs, pulsed with PBS or HDM and incubated with IgM or OC-20. For selected experiments, allogenic T cells were added. B. Group mean data are shown for CD80(+) CD86(+) expression by DCs. C. CD80 and CD86 expression by live, CD45(+), CD83(+), IA-IE(+), CD11c(+) DCs. D-H. DC activation of allogenic T-cell IL-13 production and BrdU incorporation. D. TCR-β and IL-13 were assessed by flow cytometry. E. Group mean data for live, CD45(+), TCR-β (+), IL-13(+). F. DCs and allogenic T cells were prepared as above, and BrdU was added before harvest on day 10. G. Group mean data for the TCR-β (+), BrdU(+) cells. Group mean data for B, E, and G are mean±SEM, N=3, *different from all other groups, p<0.05, one-way ANOVA.
Perisotin-deficient mice show incomplete DC maturation and deficient T cell activation
We hypothesized that CD11b+ DCs require interactions with periostin to facilitate T cell activation and Th2 differentiation. To determine whether periostin is required for maximal DC function, we employed an in vitro assay examining the response of bone marrow-derived DCs to HDM in vitro using T cell IL-13 expression as an output 21.
First, we sorted live, CD45(+), CD83(+), CD11c(+), IA-IE(+) cells for CD80 and CD86 (Figure 6B,6C). Only Postn (+/+) DCs incubated without OC-20 expressed both CD80 and CD86. Next, we examined the number of IL-13-expressing T cells. When DCs derived from Postn (+/+) bone marrow were used, the HDM pulse increased the number of IL-13+, TCR-β+ cells (Figure 6D, 6F). There was no increase when DCs from periostin knockout mice were used. Similarly, incubation with OC-20 but not IgM blocked DC activation of T cell IL-13 production. When we measured IL-13 by ELISA, we only detected IL-13 production by wild-type allogenic T cells incubated with HDM-pulsed DCs (45.1±7.6 pg/ml, n=3). On the other hand, knockout and OC-20 treated DCs failed to activate T cell IL-13 production. Finally, HDM-pulsed DCs from Postn +/+ mice stimulated BrdU incorporation into allogenic T cells, whereas HDM-pulsed DCs from Postn −/− mice did not (Figure 6E, 6G). OC-20 blocked this effect.
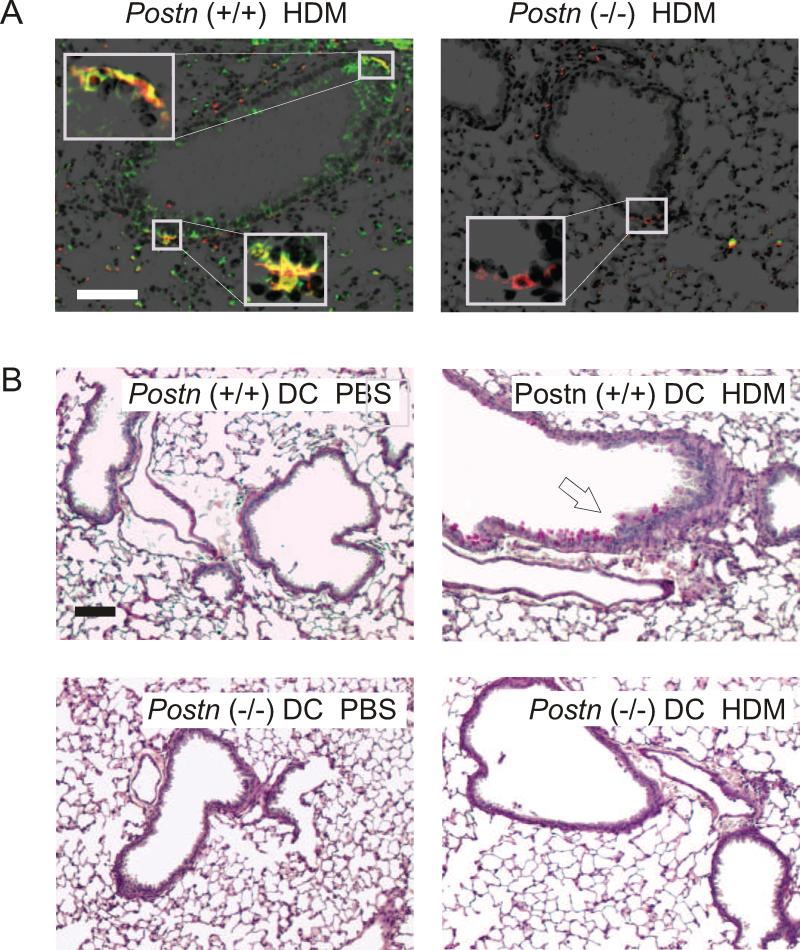
To further examine the role of DC periostin expression, we examined the lungs of HDM-exposed Postn (+/+) and Postn (−/−) mice for I-A/I-E (mouse MHC class II) and periostin (Figure 7A). I-A/I-E+ cells from Postn (+/+) mice expressed periostin. Next, we tested the sufficiency of Postn (+/+) DCs for HDM-induced inflammation by transferring HDM-matured differentiated bone marrow-derived DCs from Postn (+/+) to Postn (−/−) mice, and then challenging with HDM in vivo. Mice treated with HDM-matured Postn (+/+) but not (−/−) DCs showed a greater sensitivity to HDM challenge, as assessed by PAS hematoxylin staining (Figure 7C). These data suggest that periostin-expressing DCs are sufficient for HDM-induced allergic inflammation.
Figure 7.
DCs from Postn (+/+) mice are sufficient for allergic airway inflammation. A, B. Lungs from HDM-exposed F6 B6;129 Postn (+/+) mice (A) and Postn (−/−) littermates (B) were immunostained for periostin (green) and I-A/I-E (red), a mouse DC marker (original magnification 100X, inset 800X). C-F. HDM-matured differentiated bone marrow-derived DCs from Postn (+/+) mice or Postn (−/−) mice were transferred to Postn (−/−) mice intratracheally and challenged with PBS or HDM. PAS-stained lung sections from PBS- (C, E) and HDM-treated mice (D, F) treated with Postn (+/+) DCs (C, D) or Postn (−/−) DCs (E, F). (100x magnification, bar is 100 μm.) These figures are representative of three separate experiments.
Discussion
We examined the requirement of periostin for HDM-induced airways disease. We found deposition of periostin in airway epithelial, subepithelial, smooth muscle and inflammatory cells following HDM exposure. HDM sensitization and challenge increased airways cholinergic responsiveness in Postn wild-type but not null mice. Similarly, HDM-treated C57BL/6 mice injected with OC-20 had a lower methacholine response than HDM-treated controls injected with IgM. Compared to Postn wild-type mice, Postn null mice showed decreases in HDM-induced inflammation and mucous metaplasia. Exposure to OVA instead of HDM, and periostin blockade with a periostin neutralizing antibody, each gave comparable results. OC-20 blocked the HDM-induced IgE response, and DCs isolated from Postn null mice or treated with OC-20 showed an absence of CD86 expression following HDM exposure. In addition, T cells incubated with DCs isolated from Postn null mice or treated with OC-20 showed deficient DNA synthesis and IL-13 responses compared to T cells incubated with wild-type IgM-treated DCs. Finally, transfer of periostin-expressing DCs to periostin null mice restored HDM responsiveness in vivo. Together, these results indicate that periostin has a pro-inflammatory effect in the context of allergic airways disease.
As noted above, periostin has been shown to be deposited in the airway tissues of patients with asthma and expression level correlates with disease severity 1, 2. Patients with high pretreatment levels of serum periostin show greater improvement in lung function with lebrikizumab 3 or omalizumab 5. In patients with uncontrolled asthma, serum periostin level is the best predictor of airway eosinophilia 4. Finally, it was recently shown that polymorphisms of the periostin gene are related to higher serum periostin levels and a more rapid decline in FEV1 6. These new data are consistent with the notion that periostin is not simply a marker of asthma severity, but instead plays a contributing role in asthma pathogenesis. Our data, which show that inhibition of periostin expression/function attenuates allergen-induced airways disease, agree with this concept. Our data are also consistent with previous work using the Postn null mouse showing that F2 B6;129 Postn knockout mice show reduced eosinophil recruitment to the lungs following A. fumigatus sensitization and challenge 9. Periostin has also been shown to promote inflammation in other models of allergic disease including HDM-induced atopic dermatitis 22, OVA-induced allergic rhinitis 23 and A. fumigatus-induced eosinophilic esophagitis 9.
Our data starkly contrast with two studies in B6;129 periostin-deficient (perilacZ) mice 24 showing a protective effect of periostin on allergen-induced airways hyperresponsiveness 15, 25. While it is conceivable that extracellular matrix deposition prevents smooth muscle shortening in asthma, these discrepant results may relate to differences in backcrossing or the mode of allergen administration. Another possibility is that perilacZ knockout mice experience a subtle change in airway wall development which increases airway collapsibility: while Postn null homozygotes are slightly smaller than wild-type and heterozygous animals (not shown), perilacZ mice exhibit dwarfism, enamel defects and early onset periodontal disease 24.
To address potential issues of knockout mouse strain and backcrossing on the results, we employed a periostin neutralizing antibody as a second method of periostin inhibition. This antibody inhibits ovarian cancer growth 26 and bleomycin-induced inflammation and fibrosis in mice 13. Our studies indicate not only indicate that OC-20 decreases HDM-induced periostin accumulation, but also that OC-20 attenuates HDM-induced airways responsiveness and inflammation. These data corroborate our findings in F4 Postn wild-type and null mice. Interestingly, OC-20 appeared to decrease periostin mRNA and protein expression. It is possible that neutralization of periostin blocks expression by interrupting an autocrine periostin loop, as has been shown in pancreatic stellate cells and aortic abdominal aneurysm tissue 27, 28.
We have not completed backcrossing of the Postn mice. F6 B6;129-Postn knockout mice should contain over 98% of C57BL/6 DNA, with <2% 129S genetic background of varying composition. However, it is possible that, even with heterozygote mating, adjacent genes could be responsible for the effects we observed. Finally, the response of F6 B6;129-Postn mice to antigen challenge is unlikely to be due to 129S genetic elements, as these mice are capable of developing lung allergic inflammatory responses 29.
Despite human data suggesting that periostin may play a role in asthma pathogenesis, the mechanisms by which periostin may promote asthmatic airways inflammation and hyperresponsiveness are almost completely unknown. Possibilities include: 1) periostin, a ligand for αMβ2 (CD11b), augments CD11b+ DC activation and Th2 cell differentiation; 2) periostin serves as a cell-binding matrix, thereby increasing infiltration of inflammatory cells in the airways; and 3) periostin promotes airway smooth muscle hypertrophy and contractile protein expression, increasing contractility and airways responsiveness. Based on our results showing reduced IgE in periostin-depleted OC-20 treated mice, we hypothesized that CD11b+ DCs require interactions with periostin to facilitate T cell activation and Th2 differentiation. CD11bhigh DCs are responsible for CD4+ Th2 responses to ovalbumin 30 and HDM 31. We found that DCs from periostin null mice failed to express CD86 after HDM exposure which, upon engagement with CD28, provides CD4+ T cells with the costimulatory signal that lower the activation threshold and allows activation.33 In addition, splenic T cells incubated with DCs isolated from Postn null mice or treated with OC-20 each showed deficient HDM-induced IL-13 responses compared to T cells incubated with wild-type or IgM-treated DCs. Further, transfer of bone marrow-derived DCs from Postn +/+_mice were sufficient to promote allergic responses in HDM-exposed Postn null mice. Together, these results suggest that periostin is required for maximal DC activation. Periostin has been shown to activate NF-κB in keratinocytes 22, 32 and it is possible that NF-κB is involved in DC activation.
In conclusion, we have shown that, in mice, periostin is required for maximal allergen-induced airway responses. Results were similar in periostin null mice and mice treated with a neutralizing antibody. Thus, periostin may represent a promising therapeutic target in asthma.
Key Messages.
Periostin has been shown to be a marker for severe allergic asthma, but its role in asthma pathogenesis is not clear.
Using knockout mice and a neutralizing antibody, we show that periostin is required for maximal allergic airways responses in mice, and that periostin-positive dendritic cells are sufficient to promote allergic airways disease in periostin null mice.
These data indicate that periostin may represent a promising therapeutic target in asthma.
Capsule Summary.
House dust mite-induced airway responsiveness, airway inflammation, IgE production and T cell responses are attenuated in periostin knockout mice and mice treated with anti-periostin antibody. Periostin deposition may promote the development of asthma.
Acknowledgments
This work was supported by the Michigan Institute for Clinical and Health Research/NIH UL1TR000433 (J.K.B.), HL115618 (B.B.M) and HL0079339 (M.B.H.)
Abbreviations
- BrdU
bromodeoxyuridine
- DC
dendritic cells
- HDM
house dust mite
- OVA
ovalbumin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Takayama G, Arima K, Kanaji T, Toda S, Tanaka H, Shoji S, et al. Periostin: A novel component of subepithelial fibrosis of bronchial asthma downstream of IL-4 and IL-13 signals. J Allergy Clin Immunol. 2006;118:98–104. doi: 10.1016/j.jaci.2006.02.046. [DOI] [PubMed] [Google Scholar]
- 2.Woodruff PG, Boushey HA, Dolganov GM, Barker CS, Yang YH, Donnelly S, et al. Genome-wide profiling identifies epithelial cell genes associated with asthma and with treatment response to corticosteroids. Proc Natl Acad Sci USA. 2007;104:15858–63. doi: 10.1073/pnas.0707413104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Corren J, Lemanske RF, Hanania NA, Korenblat PE, Parsey MV, Arron JR, et al. Lebrikizumab Treatment in Adults with Asthma. N Engl J Med. 2011;365:1088–98. doi: 10.1056/NEJMoa1106469. [DOI] [PubMed] [Google Scholar]
- 4.Jia G, Erickson RW, Choy DF, Mosesova S, Wu LC, Solberg OD, et al. Periostin is a systemic biomarker of eosinophilic airway inflammation in asthmatic patients. J Allergy Clin Immunol. 2012;130:647–54. e10. doi: 10.1016/j.jaci.2012.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hanania NA, Wenzel S, Rosén K, Hsieh H-J, Mosesova S, Choy DF, et al. Exploring the Effects of omalizumab in allergic asthma: an analysis of biomarkers in the EXTRA study. Am J Respir Crit Care Med. 2013 doi: 10.1164/rccm.201208-1414OC. [DOI] [PubMed] [Google Scholar]
- 6.Kanemitsu Y, Matsumoto H, Izuhara K, Tohda Y, Kita H, Horiguchi T, et al. Increased periostin associates with greater airflow limitation in patients receiving inhaled corticosteroids. J Allergy Clin Immunol. 2013;132:305–12. e3. doi: 10.1016/j.jaci.2013.04.050. [DOI] [PubMed] [Google Scholar]
- 7.Hoersch S, Andrade-Navarro M. Periostin shows increased evolutionary plasticity in its alternatively spliced region. BMC Evol Biol. 2010;10:30. doi: 10.1186/1471-2148-10-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johansson MW, Annis DS, Mosher DF. αMβ2 integrin-mediated adhesion and motility of interleukin-5-stimulated eosinophils on periostin. Am J Respir Cell Mol Biol. 2013;48:503–10. doi: 10.1165/rcmb.2012-0150OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blanchard C, Mingler MK, McBride M, Putnam PE, Collins MH, Chang G, et al. Periostin facilitates eosinophil tissue infiltration in allergic lung and esophageal responses. Mucosal Immunol. 2008;1:289–96. doi: 10.1038/mi.2008.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Snider P, Hinton RB, Moreno-Rodriguez RA, Wang J, Rogers R, Lindsley A, et al. Periostin is required for maturation and extracellular matrix stabilization of noncardiomyocyte lineages of the heart. Circ Res. 2008;102:752–60. doi: 10.1161/CIRCRESAHA.107.159517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vi L, Feng L, Zhu RD, Wu Y, Satish L, Gan BS, et al. Periostin differentially induces proliferation, contraction and apoptosis of primary Dupuytren's disease and adjacent palmar fascia cells. Exp Cell Res. 2009;315:3574–86. doi: 10.1016/j.yexcr.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bozyk PD, Bentley JK, Popova AP, Anyanwu AC, Linn MD, Goldsmith AM, et al. Neonatal periostin knockout mice are protected from hyperoxia-induced alveolar simplification. PLoS One. 2012;7:e31336. doi: 10.1371/journal.pone.0031336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Naik PK, Bozyk PD, Bentley JK, Popova AP, Birch CM, Wilke CA, et al. Periostin promotes fibrosis and predicts progression in patients with idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2012;303:L1046–L56. doi: 10.1152/ajplung.00139.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oka T, Xu J, Kaiser RA, Melendez J, Hambleton M, Sargent MA, et al. Genetic Manipulation of periostin expression reveals a role in cardiac hypertrophy and ventricular remodeling. Circ Res. 2007;101:313–21. doi: 10.1161/CIRCRESAHA.107.149047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sehra S, Yao W, Nguyen ET, Ahyi A-NN, Barbé Tuana FM, Ahlfeld SK, et al. Periostin regulates goblet cell metaplasia in a model of allergic airway inflammation. J Immunol. 2011;186:4959–66. doi: 10.4049/jimmunol.1002359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Newcomb DC, Sajjan US, Nagarkar DR, Wang Q, Nanua S, Zhou Y, et al. Human rhinovirus 1B exposure induces phosphatidylinositol 3-kinase-dependent airway inflammation in mice. Am J Respir Crit Care Med. 2008;177:1111–21. doi: 10.1164/rccm.200708-1243OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Orecchia P, Conte R, Balza E, Castellani P, Borsi L, Zardi L, et al. Identification of a novel cell binding site of periostin involved in tumour growth. Eur J Cancer. 2011;47:2221–9. doi: 10.1016/j.ejca.2011.04.026. [DOI] [PubMed] [Google Scholar]
- 18.Schneider D, Hong JY, Bowman ER, Chung Y, Nagarkar DR, McHenry CL, et al. Macrophage/epithelial cell CCL2 contributes to rhinovirus-induced hyperresponsiveness and inflammation in a mouse model of allergic airways disease. Am J Physiol Lung Cell Mol Physiol. 2013;304:L162–9. doi: 10.1152/ajplung.00182.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Osterholzer JJ, Chen G-H, Olszewski MA, Curtis JL, Huffnagle GB, Toews GB. Accumulation of CD11b+ lung dendritic cells in response to fungal infection results from the CCR2-mediated recruitment and differentiation of Ly-6Chigh monocytes. J Immunol. 2009;183:8044–53. doi: 10.4049/jimmunol.0902823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schneider D, Hong JY, Popova AP, Bowman ER, Linn MJ, McLean AM, et al. Neonatal rhinovirus infection induces persistent mucous metaplasia and airways hyperresponsiveness. J. Immunol. 2012;188:2894–904. doi: 10.4049/jimmunol.1101391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lewkowich IP, Lajoie S, Clark JR, Herman NS, Sproles AA, Wills-Karp M. Allergen uptake, activation, and IL-23 production by pulmonary myeloid DCs drives airway hyperresponsiveness in asthma-susceptible mice. PLoS One. 2008;3:e3879. doi: 10.1371/journal.pone.0003879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Masuoka M, Shiraishi H, Ohta S, Suzuki S, Arima K, Aoki S, et al. Periostin promotes chronic allergic inflammation in response to Th2 cytokines. J Clin Invest. 2012;122:2590–600. doi: 10.1172/JCI58978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hur DG, Khalmuratova R, Ahn S-K, Ha Y-S, Min Y-G. Roles of periostin in symptom manifestation and airway remodeling in a murine model of allergic rhinitis. Allergy Asthma Immunol Res. 2012;4:222–30. doi: 10.4168/aair.2012.4.4.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rios H, Koushik SV, Wang H, Wang J, Zhou H-M, Lindsley A, et al. Periostin null mice exhibit dwarfism, incisor enamel defects, and an early-onset periodontal disease-like phenotype. Mol Cell Biol. 2005;25:11131–44. doi: 10.1128/MCB.25.24.11131-11144.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gordon ED, Sidhu SS, Wang ZE, Woodruff PG, Yuan S, Solon MC, et al. A protective role for periostin and TGF-β in IgE-mediated allergy and airway hyperresponsiveness. Clin Exp Allergy. 2012;42:144–55. doi: 10.1111/j.1365-2222.2011.03840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hart CE, Forstrom JW, Kelly JD, Seifert RA, Smith RA, Ross R, et al. Two classes of PDGF receptor recognize different isoforms of PDGF. Science. 1988;240:1529–31. doi: 10.1126/science.2836952. [DOI] [PubMed] [Google Scholar]
- 27.Erkan M, Kleeff J, Gorbachevski A, Reiser C, Mitkus T, Esposito I, et al. Periostin creates a tumor-supportive microenvironment in the pancreas by sustaining fibrogenic stellate cell activity. Gastroenterology. 2007;132:1447–64. doi: 10.1053/j.gastro.2007.01.031. [DOI] [PubMed] [Google Scholar]
- 28.Yamashita O, Yoshimura K, Nagasawa A, Ueda K, Morikage N, Ikeda Y, et al. Periostin links mechanical strain to inflammation in abdominal aortic aneurysm. PLoS ONE. 2013;8:e79753. doi: 10.1371/journal.pone.0079753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oh S-Y, Zheng T, Bailey ML, Barber DL, Schroeder JT, Kim Y-K, et al. Src homology 2 domain–containing inositol 5-phosphatase 1 deficiency leads to a spontaneous allergic inflammation in the murine lung. J Allergy Clinical Immunol. 2007;119:123–31. doi: 10.1016/j.jaci.2006.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kinjo Y, Wu D, Kim G, Xing G-W, Poles MA, Ho DD, et al. Recognition of bacterial glycosphingolipids by natural killer T cells. Nature. 2005;434:520–5. doi: 10.1038/nature03407. [DOI] [PubMed] [Google Scholar]
- 31.Mesnil C, Sabatel CM, Marichal T, Toussaint M, Cataldo D, Drion P-V, et al. Resident CD11b+Ly6C− lung dendritic cells are responsible for allergic airway sensitization to house dust mite in mice. PLoS One. 2012;7:e53242. doi: 10.1371/journal.pone.0053242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nishiyama T, Kii I, Kashima TG, Kikuchi Y, Ohazama A, Shimazaki M, et al. Delayed re-epithelialization in periostin-deficient mice during cutaneous wound healing. PLoS One. 2011;6:e18410. doi: 10.1371/journal.pone.0018410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lenschow DJ, Walunas TL, Bluestone JA. CD28/B7 system of T cell costimulation. Annu Rev Immunol. 1996;14:233–258. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]