Abstract
Iron, an essential nutrient for cellular growth and proliferation, enters cells via clathrin-mediated endocytosis (CME). The clathrin assembly lymphoid myeloid (CALM) protein plays an essential role in the cellular import of iron by CME. CALM-AF10 leukemias harbor a single copy of the normal CALM gene, and may therefore be more sensitive to the growth inhibitory effect of iron restriction compared with normal hematopoietic cells. We found that Calm heterozygous (CalmHET) murine fibroblasts exhibit signs of iron deficiency, with increased surface transferrin receptor (sTfR) levels and reduced growth rates. CalmHET hematopoietic cells are more sensitive in vitro to iron chelators than their wild type counterparts. Iron chelation also displayed toxicity towards cultured CalmHET CALM-AF10 leukemia cells and this effect was additive to that of chemotherapy. In mice transplanted with CalmHET CALM-AF10 leukemia, we found that dietary iron restriction reduces tumor burden in the spleen. However, dietary iron restriction, used alone or in conjunction with chemotherapy, did not increase survival of mice with CalmHET CALM-AF10 leukemia. In summary, while Calm heterozygosity results in iron deficiency and increased sensitivity to iron chelation in vitro, our data in mice do not suggest that iron depletion strategies would be beneficial for the therapy of CALM-AF10 leukemia patients.
Keywords: CALM-AF10, leukemia, chelation, iron, endocytosis
INTRODUCTION
Iron is an essential cellular nutrient. It is required for cell growth and proliferation, and participates in a wide variety of cellular processes including respiration, DNA synthesis, and macromolecule biosynthesis. Neoplastic cells have an increased requirement for iron, due to increased cellular growth and proliferation. There has been substantial interest in taking advantage of the dependence of neoplastic cells on iron as a therapeutic modality. Iron chelation has been studied as an adjunct to therapy both in vitro and in vivo for a variety of human malignancies, with mixed results [1].
Iron is taken up into individual cells primarily through the process of clathrin mediated endocytosis (CME) [2]. CME is a well-orchestrated process that involves the formation of a clathrin-coated pit at the plasma membrane. This pit forms an endocytic vesicle with the assistance of multiple adaptor proteins [3]. One of these key proteins is phosphatidylinositol clathrin associated lymphoid myeloid protein (PICALM/CALM, hereafter referred to as CALM), which stabilizes the clathrin scaffold around the endocytic pit, and is required for proper CME [4, 5].
CALM participates in leukemogenesis as a fusion partner with either the mixed lineage leukemia protein (MLL) or AF10, a putative transcription factor [6, 7]. The more commonly found CALM-AF10 fusion gene arises from a t(10;11)(p13;q14) translocation and was originally identified in the U937 cell line derived from a patient with diffuse histiocytic lymphoma [6, 8]. CALM-AF10 translocations give rise to a variety of hematologic malignancies in humans, including T acute lymphoblastic leukemia (T-ALL), acute myeloid leukemia (AML) and undifferentiated leukemias [6, 9]. Because one copy of CALM is involved in the translocation, CALM-AF10 leukemias are heterozygous for the normal CALM gene. However, the biologic ramifications of CALM heterozygosity are largely unknown.
Here, we examined the effect of Calm heterozygosity on cellular sensitivity to iron deprivation using cells from a genetically modified mouse model. We determined that Calm heterozygosity is associated with increased sensitivity to the cytotoxic effects of iron chelation in vitro. However, iron depletion of mice transplanted with Calm heterozygous (CalmHET) CALM-AF10 leukemia cells did not provide a survival benefit.
MATERIALS AND METHODS
Cell culture
Calm/Picalm knockout (CalmKO), heterozygous (CalmHET), and wildtype (CalmWT) murine embryonic fibroblasts (MEFs) were generated from Picalmfit1-5R E14 embryos as previously described [10]. Five day growth assays were performed on primary, unimmortalized MEFs in Dulbecco’s modified eagle medium (Gibco, Waltham, MA) with 10% fetal bovine serum (FBS), non-essential amino acids, penicillin (Invitrogen, Carlsbad, CA), streptomycin (Invitrogen), Fungizone® (Life Technologies, Carlsbad CA) and glutamine (referred to from here as MEF media), with or without supplemental iron in the form of ferric ammonium citrate (FAC, Sigma, St. Louis, MO) at a concentration of 50 μM.
Primary fetal livers were harvested from E14 Picalmfit1-5R mice and single cell suspensions were obtained by drawing the cells into a syringe through a 25-gauge needle. The fetal liver cells were grown in 1 ml of RPMI media supplemented with 20% FBS, glutamine, penicillin, streptomycin, IL-3 (10 ng/ml), IL-6 (10 ng/ml) and stem cell factor (50 ng/ml) for 2 days in a 24 well plate. Cells were counted and 8 × 104 cells were seeded into 0.2 ml of RPMI media supplemented with 20% FBS, glutamine, penicillin, streptomycin and IL-3 (10 ng/ml) with or without DFO (5 μM, Sigma) or DFX (5 μM, Novartis, Basel, Switzerland) in triplicate wells (in a 96 well plate). Viable cells were counted after 3 days by flow cytometry (AccuriC6 equipped with an automated C•Sampler, BD Biosciences, Franklin Lakes, NJ).
Cell lines from CalmHET CALM-AF10 leukemias and from CalmWT HOXA9-MEIS1 leukemias were obtained by culturing bone marrow cells from diseased mice in RPMI media supplemented with 10% FBS, glutamine, penicillin, streptomycin, and IL-3 (10 ng/ml). Leukemia cells were cultured for three days in the presence or absence of varying concentrations of deferoxamine to determine cell sensitivity to iron chelation, with or without varying concentrations of cytarabine (Hospira, Lake Forest, IL). Viable cell counts were measured by flow cytometry. The number of viable cells was plotted as a function of drug concentration to generate isobolograms [11].
Generation of CalmWT, CalmHET or CalmKO CALM-AF10 leukemias
B6(Cg)-TyrC-2J/J (B6 albino) mice were obtained from Jackson Laboratories, then housed and bred in Duke Animal Facilities. All experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals of the National Institute of Health and executed in compliance with the Duke University Institutional Animal Care and Use Committee. To generate CALM-AF10 leukemias, CalmWT, CalmHET or CalmKO fetal liver cells from Picalmfit1-5R E14 grown in RPMI media with 20% FBS, glutamine, penicillin, streptomycin, IL-3 (10 ng/ml), IL-6 (10 ng/ml) and stem cell factor (50 ng/ml) were retrovirally transduced and transplanted into lethally irradiated wild type syngeneic hosts as previously described [12].
Western blotting
Cell lysates were boiled in Laemmli buffer and proteins separated on a 10-15% SDS-PAGE electrophoresis gel. Proteins were transferred to PVDF-F membrane, blocked, and blotted for CALM (HPA019053, Sigma, St. Louis, MO), ferritin (Abcam, Cambridge, England), or actin (Sigma). Results were analyzed and quantified using an Odyssey infrared scanner and Odyssey software, version 3 (Licor, Lincoln, NB).
Surface Transferrin Receptor (TfR) expression
Primary MEFs were immortalized by retroviral transduction of SV40. They were grown in culture with MEF media, and were harvested in log phase growth by trypsinization with 0.05% trypsin/EDTA (Invitrogen). Cells were washed with Hank’s balanced salt solution (Gibco) with 10% FBS and 4 μL/mL of sodium azide (referred to from here as HFN). Samples were blocked with rat IgG (1 μg/mL), and incubated with PE-conjugated anti-CD71 antibody for 20 minutes on ice. Samples were rinsed with and resuspended in HFN, and analyzed for fluorescence by flow cytometry (C6 Accuri, BD Biosciences, Franklin Lakes, NJ).
Analysis of the effect of iron deprivation on leukemic mice
Iron deficient chow was purchased from Harlan Laboratories (TD.99397, Indianapolis, IN) and administered to mice for eight weeks prior to transplantation. Murine serum iron measurements were made using a colorimetric assay from Thermo Scientific (Waltham, MA), which uses ferene as the complexing chromogen. Recipient B6-albino mice were sub-lethally irradiated with 5 Gy on the day of transplantation using an X-Rad 320 irradiator. They were then injected with 2×105 primary CALM-AF10 leukemia cells, and followed for the onset and progression of leukemia with biweekly blood draws from the facial vein. CALM-AF10 leukemia cells co-expressing GFP were detected in the peripheral blood by flow cytometry. Mice were maintained on their pre-transplant diet, either low iron or iron replete, throughout the study. Mice in the chemotherapy experimental groups were treated with cytarabine at 33 mg/kg/day delivered intraperitoneally (IP) on days 8 – 12 after transplantation, and doxorubicin at 1 mg/kg IP on days 8-10 post-transplant. Mice in the DFX experimental groups received DFX 33 mg/kg/day on 5 of 7 days per week, beginning 2 weeks prior to transplantation and continuing until sacrificed. Mice were monitored daily for signs of distress or disease and sacrificed by CO2 euthanasia when terminally ill. After sacrifice, spleens were removed and weighed, and the bone marrow harvested.
RESULTS
Iron homeostasis is altered in CalmHET fibroblasts
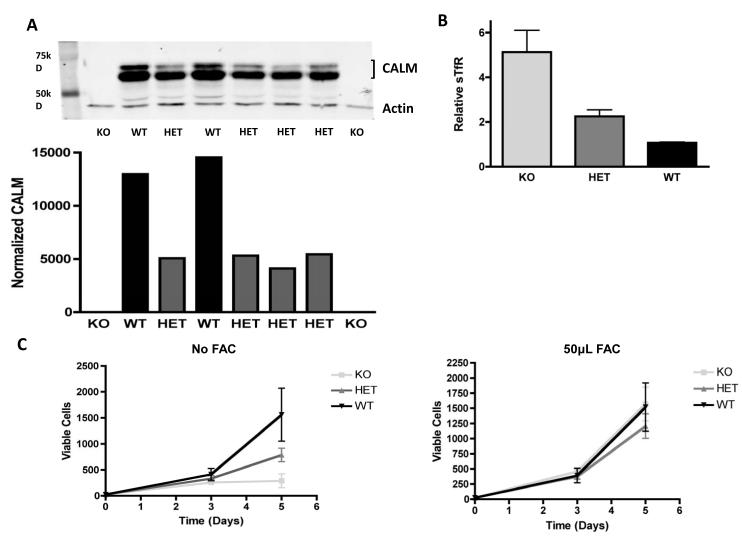
Iron import into cells is primarily mediated by the transferrin receptor, which is internalized by CME. We and others have shown that the CALM protein participates in endocytosis of iron laden transferrin (Tf) bound to its receptor (TfR) [2, 10, 13]. Calm knockout (KO – CalmKO) murine embryonic fibroblasts (MEFs) exhibit iron deficiency, as measured by increased amounts of surface transferrin receptor (sTfR), and a decrease in the labile iron pool [10]. We sought to determine whether iron homeostasis is also perturbed in CalmHET fibroblasts. We first measured Calm protein levels in heterozygous fibroblasts by Western blot (Figure 1A) and confirmed that Calm protein expression is reduced by approximately 50% in these cells compared with wild type (WT) controls.
Figure 1. Calm genotype affects CALM protein levels and iron status.
(A) Upper panel: western blot analysis of primary murine embryonic fibroblasts (MEFs) that are Calm deficient (KO), Calm heterozygous (HET) or Calm wildtype (WT). CALM protein is detected as a doublet due to alternative splicing . Lower panel: Quantification of CALM protein bands normalized to actin using densitometry; the combined measurement of both isoforms of CALM is used. The difference between the CalmWT and CalmHET MEFs is significant (p<0.05) (B) Levels of surface transferrin receptor (sTfR) measured by flow cytometry in CalmKO (KO), CalmHET(HET) and CalmWT (WT) MEFs. The difference in sTfR in the CalmHET cells is significant compared with WT control (p<0.05). (C) Growth curves of primary CalmKO, CalmHET, and CalmWT MEFs cultured in growth media without (left panel) or with (right panel) additional iron in the form of 50 μM ferric ammonium chloride (FAC). Two independent CalmKO, four independent CalmHet, and two independent CalmWT primary MEF cell lines were used for this experiment.
To assess whether CalmHET cells display signs of iron deficiency, we measured sTfR levels and found an increase of sTfR in CalmHET MEFs compared with WT controls (Figure 1B). Levels of sTfR in heterozygous MEFs were intermediate to those seen in CalmKO and CalmWT cells, consistent with the reduction in Calm protein seen in heterozygous MEFs. In order to confirm the presence of iron deficiency, the proliferation rates of Calm heterozygous and WT MEFs were compared. CalmHET cells proliferated more slowly, displaying a rate of increase in cell number over a 5-day period that was approximately half of that seen in CalmWT cells (Figure 1C). This growth disadvantage was overcome by adding supplemental iron to the culture media in the form of ferric ammonium citrate, suggesting that the absence of iron limits the growth of CalmHET cells (Figure 1C).
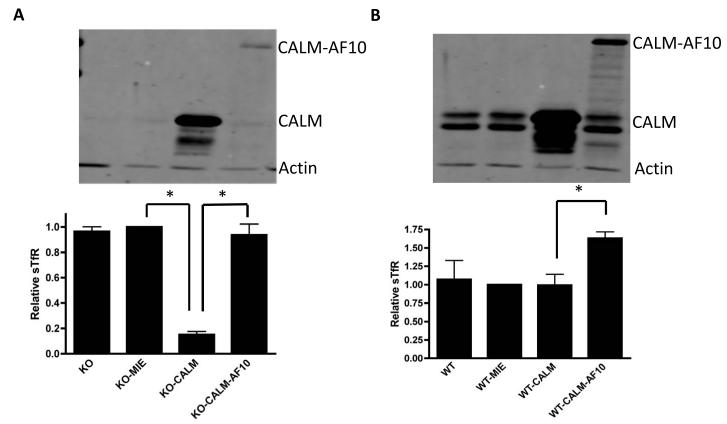
To test whether presence of the CALM-AF10 fusion protein could compensate for the lack of Calm with regards to iron homeostasis, CalmKO MEFs were retrovirally transfected with CALM-AF10. In contrast to the marked decrease in levels of sTfR seen in CalmKO MEFs retrovirally transfected with CALM, CALM-AF10 expression was not associated with a reduction in sTfR levels (Figure 2A). Moreover, CALM-AF10 appeared to act in a dominant negative fashion upon expression in CalmWT MEFs, causing an increase in sTfR expression (Figure 2B). This suggests that the CALM-AF10 fusion protein is able to sequester or otherwise interfere with the ability of the normal CALM protein to participate in CME, resulting in a relative iron deficiency.
Figure 2. CALM-AF10 expression affects iron status of MEFs.
CalmKO (KO) or CalmWT (WT) MEFs were retrovirally transduced with an empty vector (MIE), MIE-CALM (CALM), or MIE-CALM-AF10 (CALM-AF10). Upper panels show western blots with bands for CALM, CALM-AF10 and Actin. Note that endogenous CALM has two forms of slightly different molecular weight due to alternative splicing, and the transduced CALM corresponds to the larger form of the endogenous CALM doublet. Lower panels show flow cytometric analysis of surface transferrin receptor (sTfR) expression, which reflects cellular iron levels. (A) CalmKO CALM-AF10 MEFs have similar levels of sTfR when compared with CalmKO MIE (vector) MEFs. Rescue of CalmKO MEFs with MIE-CALM significantly decreases sTfR (*, p< 0.005). (B) The presence of the CALM-AF10 protein in CalmWT MEFs causes a significant increase in sTfR level compared with CalmWT MEFs transduced with MIE or MIE-CALM (*, p<0.005).
CalmHET hematopoietic cells display increased sensitivity to iron chelators
To determine whether Calm heterozygosity alters iron homeostasis in hematopoietic cells and increases their sensitivity to iron chelation, we studied primary fetal liver cells from CalmWT, CalmHET or CalmKO E14 embryos. Cells isolated from individual embryos were cultured with interleukin-3 (IL-3) in the presence or absence of deferasirox (DFX, 5 μM) or deferoxamine (DFO, 5 μM), two well-established iron chelators, and the number of viable cells was determined after 3 days. CalmKO hematopoietic cells displayed the highest sensitivity to iron chelation, with a 40% or 70% drop in the number of viable cells in the presence of DFX or DFO, respectively. CalmHET cells exhibited a less pronounced, but significant, reduction in viability in the presence of DFX or DFO, compared with WT cells (Figure 3).
Figure 3. Calm heterozygosity sensitizes hematopoietic fetal liver cells to iron chelators.
Primary CalmKO, CalmHET or CalmWT fetal liver (FL) cells were cultured with iron chelators. Viable cells were counted by flow cytometry after 3 days of chelator exposure. (A) Cells were cultured in the presence of deferasirox (DFX, 5 μM), and compared with untreated cells after 3 days of growth. (B) Cells were cultured in the presence of deferoxamine (DFO, 5 μM), and compared with untreated cells after 3 days of growth. In the presence of either iron chelator, the ratio of viable treated cells to untreated cells is decreased in the CalmHET fetal liver (FL) cells compared with CalmWT controls (*, p<0.05; **, p<0.005).
Iron chelation and chemotherapy have an additive effect in vitro
To determine the sensitivity of CALM-AF10 leukemia cells to iron chelators, CalmHET CALM-AF10 leukemia lines were established from the bone marrow of mice that developed leukemia following transplantation of CalmHET fetal liver retrovirally transduced by CALM-AF10. Cells were cultured with various concentrations of DFO and the number of viable cells was determined after 3 days. A dose-dependent cytotoxic effect of DFO was seen in all CALM-AF10 leukemia cell lines studied (Figure 4A). We then assessed whether iron chelation has an additive or synergistic effect when used in combination with traditional chemotherapy. CALM-AF10 leukemia lines were exposed to varying concentrations of DFO with or without varying concentrations of cytarabine, a commonly used agent in the treatment of acute myeloid leukemia. Isobolograms were generated to assess the interaction of these two agents [11]. In all cell lines tested, DFO and cytarabine displayed an additive effect (Figure 4B and Supplemental Figure 1).
Figure 4. CALM-AF10 leukemia cells are sensitive to deferoxamine (DFO) and cytarabine.
(A) CalmHET CALM-AF10 leukemia cell lines (1, 2, 3) were exposed to varying concentrations DFO and the number of viable cells was measured by flow cytometry after 3 days. Four independent experiments were performed for CALM-AF10 #3, three independent experiments were performed for CALM-AF10 #1, and 2 independent experiments were performed for CALM-AF10 #2. Each experiment was performed in triplicate. (B) CalmHET CALM-AF10 cells were exposed to varying concentrations of DFO in conjunction with varying concentrations of cytarabine. Viable cells were counted by flow cytometry after three days. The combined effect of DFO and cytarabine followed the line of additivity by isobolometric analysis. One representative cell line (CALM-AF10 #3) of two total tested is shown. (Please see supplementary data for isobolometric analysis of CALM-AF10 #3).
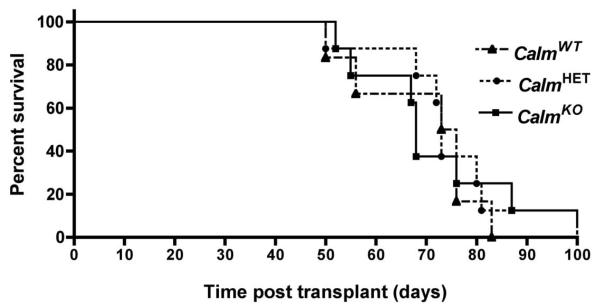
Calm genotype does not affect the latency of CALM-AF10 leukemias in mice
Since Calm deficiency caused a reduction in cell proliferation in vitro (Figure 1C and ref [10]), we questioned whether Calm levels would also affect the rate of cell expansion in vivo. We transplanted CalmWT, CalmHET or CalmKO fetal liver progenitors transduced with CALM-AF10 into irradiated syngeneic mice. All mice developed acute myeloid leukemias as described previously in mice inoculated with CALM-AF10 transduced bone marrow cells [14]. Surprisingly, the survival curves were unaffected by Calm genotype (Figure 5), suggesting that, unlike the proliferation defect observed in CalmKO immortalized fibroblasts in vitro, Calm deficiency does not impair the proliferative potential of leukemia cells in vivo. We reasoned that this could reflect the abundance of physiologically available iron in the leukemia cell microenvironment and sought to test whether inducing a state of iron deficiency in these mice could impair the progression of CalmHET CALM-AF10 leukemias.
Figure 5. Latency of primary CALM-AF10 leukemia is not affected by Calm genotype.
Lethally irradiated B6(Cg)-TyrC-2J/J mice were injected with CalmKO, CalmHET, and CalmWT fetal liver cells retrovirally transduced with CALM-AF10 to generate primary leukemias (n=8, 8, and 6 respectively). Mice were sacrificed when terminally ill. Kaplan-Meier plot demonstrates no significant difference among the three Calm genotypes.
Iron deprivation reduces splenomegaly, but does not prolong survival of CALM-AF10 leukemia mice
We induced iron deficiency in wildtype mice by feeding the animals a low iron diet over a period of eight weeks. This caused a significant decrease in serum iron, hemoglobin, and red blood cell mean corpuscular volume (MCV) (Figure 6A). The red cell distribution width (RDW) was increased (Figure 6A), as would be expected with a mild to moderate iron deficiency. Mice were then exposed to sublethal irradiation (5 Gy) and transplanted with CalmHET CALM-AF10 leukemia cells obtained from a diseased donor mouse. Mice were monitored for disease progression; ill mice were sacrificed and the spleen weighed as a measure of tumor burden. In addition, relative ferritin levels were examined in bone marrow blasts by western blot to assess their iron status. These studies confirmed that bone marrow blasts from mice fed a low iron diet displayed significantly lower ferritin stores, compared with blasts from mice fed a normal diet (Figure 6B).
Figure 6. Effects of iron deprivation on hematopoietic parameters.
(A) B6(Cg)-TyrC-2J/J mice fed a low iron diet for eight weeks displayed significant decreases in serum iron (Fe), hemoglobin and mean corpuscular volume (MCV) compared with control mice fed a normal diet (n=8 per group; *, p<0.05), and a significant increase in red cell distribution width (RDW; *, p<0.05). (B) Western blot of ferritin and actin expression in leukemic blasts from mice fed a normal diet (Fe+) or a low iron diet (Fe−) from eight weeks prior to transplantation through the time of sacrifice (left panel). Right panel shows densitometric analysis of the western blot, and reveals decreased ferritin levels, normalized to actin, in mice fed a low iron diet compared with controls (*, p<0.05). (C) B6(Cg)-TyrC-2J/J mice on a low iron diet were treated with intraperitoneal injections of deferoxamine (DFX, 33 mg/kg/day, 5 days/week) for eight weeks (n=8). Control mice were also fed a low iron diet but injected with a vehicle control (n=8). After 8 weeks of treatment, the chelated mice displayed significantly lower hemoglobin compared with controls (*, p<0.05).
Iron deficient mice typically have enlarged spleens compared with iron replete controls, as the spleen is a site of extramedullary hematopoiesis [15]. In contrast, the spleen size of iron deficient leukemic mice was significantly decreased (Figure 7A), suggesting that iron deficiency was associated with a lower tumor burden. However, this did not translate into an overall survival benefit. The time to onset and progression of leukemic disease, as measured by assessment of peripheral blood was not different between the two groups. In addition, a difference in the total number of circulating blasts was not consistently observed (data not shown). The survival of mice rendered iron deficient via a low iron diet was unchanged compared with that of age and gender matched controls (Figure 7B).
Figure 7. Iron depletion reduces splenomegaly but does not extend survival of CALM-AF10 leukemia mice.
(A) Spleen weight of mice transplanted with CalmHET CALM-AF10 leukemia cells fed either a low iron diet (Fe−, n=10) or normal diet (Fe+, n=10) for eight weeks. Mice were sacrificed when terminally ill, and their spleens were removed and weighed as a measure of tumor burden. The iron-deprived mice had significantly decreased spleen weight (*, p<0.05). (B) Survival curves of mice transplanted with CalmHET CALM-AF10 leukemia cells. Cohorts of mice (n=5) were treated with either vehicle (Veh) or chemotherapy (cytarabine and doxorubicin IP). Survival curves are shown for mice treated with and without chemotherapy, alone and in combination with iron deprivation (**, p<0.005). (C) The survival of leukemic mice fed an iron deficient diet and treated with deferasirox was compared to that of control leukemic mice fed normal chow and treated with vehicle (n=10 per group) (**, p<0.005).
Iron depletion does not improve the therapeutic benefit induced by chemotherapy
Given the additive effect of iron chelation with cytarabine seen in vitro (Figure 4B), we examined whether iron deficiency could augment the therapeutic efficacy of chemotherapy in vivo. We adapted a 5-day regimen of doxorubicin and cytarabine [16] that was tolerated by mice receiving sublethal irradiation and fed an iron deficient diet. Although chemotherapy significantly extended the survival of leukemic mice, combination with a low iron diet failed to show further improvement in survival (Figure 7B).
Finally, to examine whether a greater degree of iron deficiency was required to extend the survival of leukemic mice, we administered oral DFX to mice receiving a low iron diet; this combination approach did result in lower levels of serum hemoglobin than an iron deficient diet alone (Figure 6C). Mice subjected to an iron deficient diet and oral chelation (DFX, 33 mg/kg/day, 5 days a week) for 8 weeks were irradiated and injected with CALM-AF10 leukemia cells. Treatment with DFX was then continued for the length of the experiment. Survival was compared with age- and gender-matched controls treated with vehicle and fed an iron replete diet. While DFX and iron deficient chow was tolerated in healthy mice (treated for 8 weeks, data not shown), it resulted in toxicity and a shortened overall survival in our cohort of leukemic mice (Figure 7C).
DISCUSSION
Iron is an essential cellular nutrient. It plays a critical role in a number of intracellular processes, including those involved in cell growth and proliferation. It has previously been shown that neoplastic cells have an increased requirement for iron as a consequence of increased proliferation rates [17]. As a result, iron chelation has been studied as an adjunct to standard chemotherapy in several human malignancies; the addition of chelation therapy has had variable therapeutic effects [1]. Iron chelation therapy has notably been studied in hematopoietic malignancies, both in vitro and in vivo [18-20]. In addition to promising pre-clinical studies, there have been a few case reports using various forms of iron chelation in human myelodysplastic syndromes and acute leukemias, with some evidence of therapeutic efficacy [21, 22]. Iron chelation has also been studied in solid tumors, such as neuroblastoma [23-25], prostate cancer [26] and breast cancer [27], with mixed results. Some of the difficulties in using iron chelation as an adjunct to cancer therapy have stemmed from the reality that iron plays a critical role in normal host cellular functions; this limits the therapeutic index of iron chelators.
We hypothesized that CALM-AF10 leukemias would be an excellent candidate for iron deprivation therapy, since they are heterozygous for the normal CALM gene necessary for optimal iron import and thus more dependent on extracellular iron. We showed that Calm heterozygosity results in haploinsufficiency for Calm: CalmHET cells express significantly less Calm protein than their CalmWT counterparts and are relatively iron deficient, as evidenced by increased levels of sTfR, compared with CalmWT cells (Figure 1A,B). Total TfR protein expression levels are regulated by intracellular iron abundance and measurement of sTfR can be used to evaluate iron deficiency. Calm haploinsufficiency is also manifested by a reduced rate of cellular proliferation; primary CalmHET MEFs proliferate more slowly than WT controls (Figure 1C). Rescue of this growth deficiency by supplementing the medium with iron suggests that iron availability limits the expansion of CalmHET MEFs. Importantly, the relative iron deficient state of CalmHET cells translates into heightened sensitivity to iron deprivation; we found that CalmHET hematopoietic cells are significantly more susceptible to the cytotoxic effects of iron chelation by deferasirox or deferoxamine, compared with CalmWT control cells (Figure 3).
In addition to CALM-AF10 leukemia cells being heterozygous for CALM, the presence of the aberrant CALM-AF10 fusion protein could further perturb iron homeostasis. Our observation that CALM-AF10 expression in wild type MEFs is associated with an increase in sTfR (Figure 2) suggests that CALM-AF10 could act as a dominant negative mutant that interferes with CALM’s role in iron internalization.
Despite the slower growth of Calm deficient MEFs in vitro (Figure 1 and ref.[10]), we did not see any difference in latency of CalmKO or CalmHET CALM-AF10 leukemias compared with CalmWT CALM-AF10 leukemias (Figure 5). This suggests that iron levels in mice may be sufficiently high to compensate for the perturbed iron/transferrin import into Calm deficient leukemia cells. We reasoned that reduced iron availability, as a result of dietary restriction or chelation, could nevertheless have an anti-leukemic effect in CalmHET CALM-AF10 leukemias.
To study the feasibility of using iron chelators as adjunct therapy in combination with chemotherapy in CALM-AF10 leukemias, we first tested their cytotoxicity towards CalmHET CALM-AF10 leukemia cells in vitro. Iron chelation with deferoxamine was found to be cytotoxic to multiple CALM-AF10 leukemia cell lines, with an IC50 between 15–50 μM. In combination with traditional chemotherapy (cytarabine), iron chelation showed an additive effect in all CALM-AF10 leukemia lines tested in vitro (Figure 4). Based on these results, we tested the effects of iron deprivation both with and without chemotherapy in a murine model of CalmHET CALM-AF10 leukemia.
A moderate iron deficiency was induced in vivo by feeding mice a low iron diet, as measured by decreases in serum iron and hemoglobin (Figure 6). Mice fed low iron chow and transplanted with CALM-AF10 leukemias appeared to have a decreased tumor burden as measured by spleen weight, compared with control mice fed a normal diet. However, the degree of iron deficiency induced by the low iron diet did not result in a prolonged survival, either alone or in combination with chemotherapy (Figure 7A,B). To achieve a more intense degree of iron deficiency we combined the iron deficient diet with deferasirox treatment. However, this therapy was not tolerable to leukemic mice and resulted in shortened overall survival (Figure 7C).
We hypothesized that CALM-AF10 leukemias would be particularly sensitive to iron chelation due to the relative iron deficiency induced by CALM haploinsufficiency and the presence of the fusion protein acting in a dominant negative fashion. While leukemia cells did show a response to iron chelation in vitro, this did not translate into an in vivo survival benefit in our mouse model of CALM-AF10 leukemia. The lack of therapeutic efficacy of iron deprivation in certain leukemia models has been previously reported. For example, while in vitro studies of iron chelation have often shown differentiating or growth inhibitory effects in acute myeloid leukemia [18, 28-30], in vivo studies of these tumors showed mixed results [22, 31-33]. A discrepancy between in vitro and in vivo results has been seen with other tumor types, including non-Hodgkin lymphoma, where studies showed encouraging responses in vitro [34, 35], but mixed or partial responses in human trials [36]. It is possible that a tumor’s ability to adapt to a changing microenvironment overrides the effects of iron deprivation in vivo, or that the complex interplay of metabolism, proliferation and nutrient availability allows for variable tumor responses to iron accessibility. In addition, there likely exists a fine line between the systemic toxicity and anti-leukemic effects of moderate to severe iron deficiency.
One possible explanation for the inability of iron deprivation to extend survival in our mouse model of CalmHET CALM-AF10 leukemia is that the level of iron deficiency did not reach a threshold to impede leukemia progression. Cell toxicity in vitro required relatively high concentrations of iron chelators; however, when used in conjunction with iron-deprived chow, doses of iron chelators that efficiently reduced iron levels in mice proved to be toxic in leukemic animals. It is also possible that the oncogenic properties of the CALM-AF10 fusion protein override any sensitivity that a Calm haploinsufficient state would otherwise induce. This may be related to a direct proliferative advantage conferred by the oncogene or other metabolic changes induced in the leukemic cells.
In summary, iron is essential for neoplastic cell proliferation. The CALM protein is necessary for cellular import of iron, and CALM heterozygosity leads to iron deficiency and an increased susceptibility to the cytotoxic effects of iron deprivation. CALM-AF10 leukemias are haploinsufficient for CALM, and are sensitive to the cytotoxic effects of iron chelation in vitro. However, this in vitro susceptibility does not translate into an in vivo survival benefit in a murine model of Calm+/− CALM-AF10 leukemia possibly because the therapeutic window between potential efficacy and toxicity is too narrow. Iron deprivation with potent chelators could be feasible in the future if it were possible to rescue the organism from toxicity without disrupting the anti-leukemic effect.
Supplementary Figure 1: (A) CalmHET CALM-AF10 cells were exposed to varying concentrations of DFO in conjunction with varying concentrations of cytarabine. Viable cells were counted by flow cytometry after three days. The combined effect of DFO and cytarabine followed the line of additivity by isobolometric analysis. Cell line CALM-AF10 #3 is shown. (B) An identical experiment was carried out with CalmWT HoxA9-Meis cells. An additive effect of DFO and cytarabine is observed.
Highlights.
Cells that are heterozygous for the CALM protein (CALMHET) are iron deficient.
CALMHET cells are sensitive to the growth inhibitory effects of iron depletion in vitro.
Iron depletion combined with chemotherapy shows an additive effect in inhibiting the growth of CALM-AF10 leukemias in vitro.
There is no survival benefit of iron deprivation in a mouse model of CALM-AF10 leukemia, possibly owing to compensatory mechanisms.
ACKNOWLEDGEMENTS
The authors acknowledge financial support from The St. Baldrick’s Foundation (JLH), the American Society of Hematology (JLH), Hyundai Hope on Wheels (JLH) and an Applebee’s/V Foundation grant (DSW). This work was partially funded through NCI RO1 CA109281 (DSW). The authors would like to thank Novartis for supplying deferasirox.
Footnotes
COMPETING INTERESTS The authors declare that they have no competing interests.
AUTHOR CONTRIBUTIONS JLH and CPL designed, performed, and analyzed the experiments, and wrote the paper. JMW performed and analyzed the experiments of Figure 4. DSW designed the research and wrote the paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Heath JL, Weiss JM, Lavau CP, Wechsler DS. Iron deprivation in cancer--potential therapeutic implications. Nutrients. 2013;5:2836–2859. doi: 10.3390/nu5082836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Hanover JA, Willingham MC, Pastan I. Kinetics of transit of transferrin and epidermal growth factor through clathrin-coated membranes. Cell. 1984;39:283–293. doi: 10.1016/0092-8674(84)90006-0. [DOI] [PubMed] [Google Scholar]
- [3].Ungewickell E, Branton D. Assembly units of clathrin coats. Nature. 1981;289:420–422. doi: 10.1038/289420a0. [DOI] [PubMed] [Google Scholar]
- [4].Tebar F, Bohlander SK, Sorkin A. Clathrin assembly lymphoid myeloid leukemia (CALM) protein: localization in endocytic-coated pits, interactions with clathrin, and the impact of overexpression on clathrin-mediated traffic. Mol Biol Cell. 1999;10:2687–2702. doi: 10.1091/mbc.10.8.2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Meyerholz A, Hinrichsen L, Groos S, Esk PC, Brandes G, Ungewickell EJ. Effect of clathrin assembly lymphoid myeloid leukemia protein depletion on clathrin coat formation. Traffic. 2005;6:1225–1234. doi: 10.1111/j.1600-0854.2005.00355.x. [DOI] [PubMed] [Google Scholar]
- [6].Dreyling MH, Martinez-Climent JA, Zheng M, Mao J, Rowley JD, Bohlander SK. The t(10;11)(p13;q14) in the U937 cell line results in the fusion of the AF10 gene and CALM, encoding a new member of the AP-3 clathrin assembly protein family. Proc Natl Acad Sci U S A. 1996;93:4804–4809. doi: 10.1073/pnas.93.10.4804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wechsler DS, Engstrom LD, Alexander BM, Motto DG, Roulston D. A novel chromosomal inversion at 11q23 in infant acute myeloid leukemia fuses MLL to CALM, a gene that encodes a clathrin assembly protein. Genes Chromosomes Cancer. 2003;36:26–36. doi: 10.1002/gcc.10136. [DOI] [PubMed] [Google Scholar]
- [8].Sundstrom C, Nilsson K. Establishment and characterization of a human histiocytic lymphoma cell line (U-937) Int J Cancer. 1976;17:565–577. doi: 10.1002/ijc.2910170504. [DOI] [PubMed] [Google Scholar]
- [9].Dreyling MH, Schrader K, Fonatsch C, et al. MLL and CALM are fused to AF10 in morphologically distinct subsets of acute leukemia with translocation t(10;11): both rearrangements are associated with a poor prognosis. Blood. 1998;91:4662–4667. [PubMed] [Google Scholar]
- [10].Scotland PB, Heath JL, Conway AE, et al. The PICALM protein plays a key role in iron homeostasis and cell proliferation. PLoS One. 2012;7:e44252. doi: 10.1371/journal.pone.0044252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Tallarida RJ. Combination analysis. Adv Exp Med Biol. 2010;678:133–137. doi: 10.1007/978-1-4419-6306-2_17. [DOI] [PubMed] [Google Scholar]
- [12].Conway AE, Scotland PB, Lavau CP, Wechsler DS. A CALM-derived nuclear export signal is essential for CALM-AF10-mediated leukemogenesis. Blood. 2013;121:4758–4768. doi: 10.1182/blood-2012-06-435792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Suzuki M, Tanaka H, Tanimura A, et al. The clathrin assembly protein PICALM is required for erythroid maturation and transferrin internalization in mice. PLoS One. 2012;7:e31854. doi: 10.1371/journal.pone.0031854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Deshpande AJ, Cusan M, Rawat VP, et al. Acute myeloid leukemia is propagated by a leukemic stem cell with lymphoid characteristics in a mouse model of CALM/AF10-positive leukemia. Cancer Cell. 2006;10:363–374. doi: 10.1016/j.ccr.2006.08.023. [DOI] [PubMed] [Google Scholar]
- [15].Gibson JN, Jellen LC, Unger EL, et al. Genetic analysis of iron-deficiency effects on the mouse spleen. Mamm Genome. 2011;22:556–562. doi: 10.1007/s00335-011-9344-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Zuber J, Radtke I, Pardee TS, et al. Mouse models of human AML accurately predict chemotherapy response. Genes Dev. 2009;23:877–889. doi: 10.1101/gad.1771409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Elford HL, Freese M, Passamani E, Morris HP. Ribonucleotide reductase and cell proliferation. I. Variations of ribonucleotide reductase activity with tumor growth rate in a series of rat hepatomas. J Biol Chem. 1970;245:5228–5233. [PubMed] [Google Scholar]
- [18].Noulsri E, Richardson DR, Lerdwana S, et al. Antitumor activity and mechanism of action of the iron chelator, Dp44mT, against leukemic cells. Am J Hematol. 2009;84:170–176. doi: 10.1002/ajh.21350. [DOI] [PubMed] [Google Scholar]
- [19].Kim JL, Kang HN, Kang MH, Yoo YA, Kim JS, Choi CW. The oral iron chelator deferasirox induces apoptosis in myeloid leukemia cells by targeting caspase. Acta Haematol. 2011;126:241–245. doi: 10.1159/000330608. [DOI] [PubMed] [Google Scholar]
- [20].Becton DL, Roberts B. Antileukemic effects of deferoxamine on human myeloid leukemia cell lines. Cancer Res. 1989;49:4809–4812. [PubMed] [Google Scholar]
- [21].Fukushima T, Kawabata H, Nakamura T, et al. Iron chelation therapy with deferasirox induced complete remission in a patient with chemotherapy-resistant acute monocytic leukemia. Anticancer Res. 2011;31:1741–1744. [PubMed] [Google Scholar]
- [22].Estrov Z, Tawa A, Wang XH, et al. In vitro and in vivo effects of deferoxamine in neonatal acute leukemia. Blood. 1987;69:757–761. [PubMed] [Google Scholar]
- [23].Blatt J. Deferoxamine in children with recurrent neuroblastoma. Anticancer Res. 1994;14:2109–2112. [PubMed] [Google Scholar]
- [24].Donfrancesco A, Deb G, Dominici C, Pileggi D, Castello MA, Helson L. Effects of a single course of deferoxamine in neuroblastoma patients. Cancer Res. 1990;50:4929–4930. [PubMed] [Google Scholar]
- [25].Donfrancesco A, De Bernardi B, Carli M, et al. Deferoxamine followed by cyclophosphamide, etoposide, carboplatin, thiotepa, induction regimen in advanced neuroblastoma: preliminary results. Italian Neuroblastoma Cooperative Group. Eur J Cancer. 1995;31A:612–615. doi: 10.1016/0959-8049(95)00068-t. [DOI] [PubMed] [Google Scholar]
- [26].Dixon KM, Lui GY, Kovacevic Z, et al. Dp44mT targets the AKT, TGF-beta and ERK pathways via the metastasis suppressor NDRG1 in normal prostate epithelial cells and prostate cancer cells. Br J Cancer. 2013;108:409–419. doi: 10.1038/bjc.2012.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Rao VA, Klein SR, Agama KK, et al. The iron chelator Dp44mT causes DNA damage and selective inhibition of topoisomerase IIalpha in breast cancer cells. Cancer Res. 2009;69:948–957. doi: 10.1158/0008-5472.CAN-08-1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Callens C, Coulon S, Naudin J, et al. Targeting iron homeostasis induces cellular differentiation and synergizes with differentiating agents in acute myeloid leukemia. J Exp Med. 2010;207:731–750. doi: 10.1084/jem.20091488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Eberhard Y, McDermott SP, Wang X, et al. Chelation of intracellular iron with the antifungal agent ciclopirox olamine induces cell death in leukemia and myeloma cells. Blood. 2009;114:3064–3073. doi: 10.1182/blood-2009-03-209965. [DOI] [PubMed] [Google Scholar]
- [30].Roth M, Will B, Simkin G, et al. Eltrombopag inhibits the proliferation of leukemia cells via reduction of intracellular iron and induction of differentiation. Blood. 2012;120:386–394. doi: 10.1182/blood-2011-12-399667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Yee KW, Cortes J, Ferrajoli A, et al. Triapine and cytarabine is an active combination in patients with acute leukemia or myelodysplastic syndrome. Leuk Res. 2006;30:813–822. doi: 10.1016/j.leukres.2005.12.013. [DOI] [PubMed] [Google Scholar]
- [32].Giles FJ, Fracasso PM, Kantarjian HM, et al. Phase I and pharmacodynamic study of Triapine, a novel ribonucleotide reductase inhibitor, in patients with advanced leukemia. Leuk Res. 2003;27:1077–1083. doi: 10.1016/s0145-2126(03)00118-8. [DOI] [PubMed] [Google Scholar]
- [33].DeConti RC, Toftness BR, Agrawal KC, et al. Clinical and pharmacological studies with 5-hydroxy-2-formylpyridine thiosemicarbazone. Cancer Res. 1972;32:1455–1462. [PubMed] [Google Scholar]
- [34].Choi JG, Kim JL, Park J, et al. Effects of oral iron chelator deferasirox on human malignant lymphoma cells. Korean J Hematol. 2012;47:194–201. doi: 10.5045/kjh.2012.47.3.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Vazana-Barad L, Granot G, Mor-Tzuntz R, et al. Mechanism of the antitumoral activity of deferasirox, an iron chelation agent, on mantle cell lymphoma. Leuk Lymphoma. 2013;54:851–859. doi: 10.3109/10428194.2012.734614. [DOI] [PubMed] [Google Scholar]
- [36].Voest EE, Neijt JP, Keunen JE, et al. Phase I study using desferrioxamine and iron sorbitol citrate in an attempt to modulate the iron status of tumor cells to enhance doxorubicin activity. Cancer Chemother Pharmacol. 1993;31:357–362. doi: 10.1007/BF00686148. [DOI] [PubMed] [Google Scholar]