Abstract
Primary sclerosing cholangitis (PSC) is a rare, chronic, cholestatic liver disease in which emerging data suggest that oral antibiotics may offer therapeutic effects. We enrolled patients with PSC in a 12-week open-label pilot study to investigate the efficacy and safety of oral rifaximin 550 mg twice daily. The primary endpoint was serum alkaline phosphatase (ALK) at 12 weeks. Secondary endpoints included: i) serum bilirubin, gamma-glutamyl transpeptidase, and Mayo PSC risk score; ii) Fisk Fatigue Impact Scale (FFIS), Chronic Liver Disease Questionnaire (CLDQ), and Short Form Health Survey (SF-36) scores; and iii) adverse effects (AEs). Analyses were performed with nonparametric tests. Sixteen patients were enrolled, among whom the median age was 40 years, 13 (81%) were male, 13 had inflammatory bowel disease, and baseline ALK was 342 IU/mL (interquartile range 275-520). Following 12 weeks of treatment, there were no significant changes in ALK (median increase of 0.9% to 345 IU/mL, p=0.47) or any of the secondary biochemical endpoints (all p>0.05). Similarly, there were no significant changes in FFIS, CLDQ, or SF-36 scores (all p>0.05). Three patients withdrew from the study due to AEs; four others reported mild AEs but completed the study. In conclusion, while some antibiotics may have promise in treating PSC, oral rifaximin, based on the results herein, appears inefficacious for this indication. Future studies are needed to understand how the antimicrobial spectra and other properties of antibiotics might determine their utility in treating PSC. (clinicaltrials.gov NCT01695174)
Keywords: Biliary tract diseases, Antibiotics, Microbiota, Cholestasis, Fibrosis
Introduction
Primary sclerosing cholangitis (PSC) is a chronic, cholestatic liver disease of uncertain etiopathogenesis characterized by biliary inflammation and fibrosis.1-3 PSC generally progresses to end-stage cirrhosis, represents a major risk factor for hepatobiliary and colonic carcinogenesis, and carries a median liver transplant- (LT) free survival of approximately 12 years.3-6 Due to its progressive nature and lack of effective pharmacotherapy despite ongoing research,1, 7 PSC continues to be a major indication for LT in the United States and indeed worldwide.5 Therefore, given the morbidity and mortality of PSC and the pre- and post- operative challenges inherent to LT,1, 8, 9 safe, effective, and minimally-disruptive pharmacotherapies remain critically needed.
A growing body of evidence suggests that a potentially central and readily modifiable component in the etiopathogenesis of PSC is the enteric microbiota.1, 3, 10, 11 In addition to the well-recognized relationship between PSC and enteropathies, most notably inflammatory bowel disease (IBD),12-14 a condition associated with enteric microbial dysbiosis,15, 16 this potential etiopathogenic link is supported by several observations from translational studies. These include the following: i) patients with PSC exhibit portal bacteremia17 and bacterobilia;18-20 ii) the biliary epithelium is lined by immunobiologically active cells that express multiple pathogen recognition receptors, including all known toll-like receptors (TLRs);21 iii) TLR ligands such as lipopolysaccharide (LPS) are present in bioactive form in bile,22, 23 accumulate in and modify signaling in PSC biliary epithelial cells;24-26 iv) PSC cholangiocytes exhibit persistent hypersensitivity to LPS in vitro;27 and lastly, v) animal models of microbial dysbiosis demonstrate various PSC-like hepatobiliary lesions.28, 29 In addition, small case series and pilot studies increasingly suggest that antibiotics may render a number of therapeutic effects in patients with PSC, including improvements in both the serum biomarker alkaline phosphatase (ALK)30, 31 and the Mayo PSC survival estimation model (hereinafter “PSC risk score”).32-37 Collectively, these intriguing translational and clinical observations form the basis of the “leaky gut” and “PSC-microbiota” hypotheses, as summarized schematically in Figure 1.1, 11, 36 To date, however, the number and size of clinical studies have been limited, in part due to the rarity of PSC, and the ideal antibiotic, dose, and regimen remain unknown.37
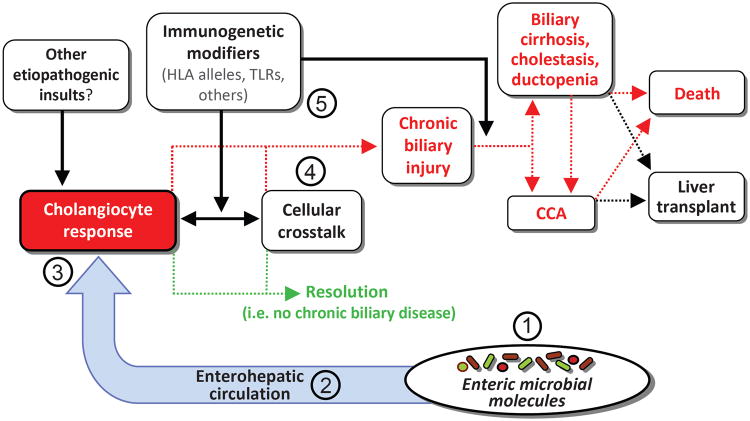
Figure 1. Conceptual model of the “PSC-microbiota” hypothesis and natural history of PSC.
Biliary epithelial cells, i.e. cholangiocytes, exist in an environment with multiple potential etiologic mediators of hepatobiliary injury. A growing body of basic, translational, and clinical evidence suggests that among these, microbially-derived molecules may be central to hepatobiliary injury in and thus the etiopathogenesis of PSC. However, while (1) increased exposure to microbial molecules (e.g. through the enterohepatic circulation, facilitated by compromised intestinal barrier function), (2) alterations in microbial diversity and/or the repertoire of microbial molecules (e.g. due to intestinal microbial dysbiosis), (3) an aberrant or exaggerated cholangiocyte response to microbial molecules (e.g. induction of cholangiocyte senescence and the senescence-associated secretory phenotype), and (4) pro-injurious effects of microbial metabolites or abnormalities on non-cholangiocyte resident and recruited hepatic cells (e.g. leukocytes) have all been proposed, their roles remain incompletely understood. In addition, host immunogenetics likely modulate the impact of any of these variables (5) and thus likely play a role in determining whether hepatobiliary injury resolves or if it persists and results in chronic disease (i.e. PSC). These variables may also determine whether PSC progresses to its associated clinical endpoints, including development of cholangiocarcinoma, liver failure, and death. Oral antibiotics are believed to exert therapeutic effects through diversity of the enteric microbiota (1) and reducing synthesis and enterohepatic circulation (2) of dysbiotic microbial metabolites. Further investigation of the cellular, molecular, and microbial interactions proposed in this figure will undoubtedly be pursued in future research and are likely to advance current understanding of the etiopathogenesis of PSC.
Key: CCA, cholangiocarcinoma; HLA, human leukocyte antigen; primary sclerosing cholangitis, PSC; TLR, toll-like receptor.
Rifaximin is an oral, nonsystemic, broad-spectrum antibacterial with therapeutic indications for several gastrointestinal and hepatic diseases.38-41 In this study, we performed a 12-week, open-label clinical trial of rifaximin to evaluate its efficacy as well as its safety and tolerability and to determine whether it may hold promise as a potential pharmacotherapy for patients with PSC.
Methods
The study was approved by the Mayo Foundation Institutional Review Board and was carried out in accordance with the ethical principles of the current revision of the Declaration of Helsinki. (clinicaltrials.gov registration number: NCT01695174)
Participants
Patients with PSC were enrolled between August 2012 and June 2013 in a 12-week, open-label pilot study of oral rifaximin at a dose of 550 mg twice a day. PSC was diagnosed using the following criteria: ALK >1.5 times the upper limit of normal for ≥6 months, cholangiography demonstrating intrahepatic and/or extrahepatic biliary strictures, beading, or irregularity consistent with PSC, and/or liver histology.7, 34 Exclusion criteria were as follows: treatment with investigational agents within three months prior to the study, history of allergic reactions to rifaximin, decompensated or comorbid liver disease, pregnancy or lactation, illicit drug or alcohol abuse, and age <16 years.
Informed consent was obtained from each patient (i.e. participant). A complete history and physical examination was performed at study entry if one had not been done within the previous year at Mayo Clinic.
Study medication and monitoring
Rifaximin (Salix Pharmaceuticals, Raleigh, NC) is a semi-synthetic, non-systemic antimicrobial drug which inhibits transcription by binding to the β-subunit of bacterial RNA polymerase and has broad-spectrum activity against aerobic and anaerobic gram-positive and gram-negative microorganisms.40, 41 In addition to providing written material containing information about potential adverse effects (AEs) of rifaximin, serum complete blood counts and liver biochemistries were measured at 3 and 12 weeks, and phone calls were made every four weeks to monitor for AEs.
Endpoints and definitions
Study endpoints were assessed at 12 weeks (i.e. study end) and compared to values at study entry, as described previously.34 The primary endpoint was serum ALK, and secondary endpoints were as follows: i) serum aspartate aminotransferase (AST), total bilirubin, gamma-glutamyl transpeptidase, albumin, C-reactive protein, and PSC risk score;35 ii) scores from standardized symptom-based questionnaires, namely the Fisk Fatigue Impact Scale (FFIS),42 5-D itch scale,43 Chronic Liver Disease Questionnaire (CLDQ),44 and Short Form (36) Health Survey (SF-36);45 and iii) frequency and types of AEs.
With respect to the questionnaires used in the present study, the FFIS is a 40-item questionnaire which evaluates the impact of perceived fatigue during the last month; scores range from 0 (no problem) to 4 (extreme problem) for each item. The 5-D itch scale is a multi-dimensional measure of pruritus; scores range from 0 (least problematic) to 5 (most problematic) for each dimension (i.e. item). The CLDQ is a 29-item, liver disease–specific HRQL questionnaire; scores range from 1 (most impairment) to 7 (least impairment) for each item. The SF-36 is a 36-item questionnaire for assessing functional health and well-being over eight distinct domains; scores range from 0 (worst) to 100 (best) for each domain. In summary, higher scores indicate greater (i.e. worse) symptoms on the FFIS and 5-D itch scale, while the opposite is true for the CLDQ and SF-36.
Sample size
In patients with PSC, there is often spontaneous variation in the ALK level within ± 10% of baseline (unpublished data). Therefore, it seemed reasonable to consider a response to therapy in PSC patients as a reduction in ALK levels by at least 10%, and ideally between 40-50% based on prior studies.34, 46 A study with 16 patients would have a power of 0.91, and a study with 12 patients a power of 0.80, to detect a reduction in ALK of 50% with a two-sided alpha level of 0.05; we thus enrolled 16 patients, which would have a power of ≥0.80 to detect a positive response rate of 50% at two-sided alpha level of 0.05 while allowing for drop-out of up to 4 patients.
Data analysis
All data were summarized as median (interquartile range [IQR]) or proportions. Considering the small number of patients, the heterogeneous nature of PSC, and variations in individual responses, statistical analyses were conducted using conservative (i.e. nonparametric) tests, namely Wilcoxon signed-rank and Fisher's exact test for continuous and categorical variables, respectively. Tests of significance were two-sided, with an alpha level of 0.05. Analyses were performed using JMP statistical software (version 8.0, SAS Institute, NC).
Results
Patient characteristics
A total of 16 patients were enrolled in the study. The median sample age was 40 years, 13 (81%) were male, 81% had inflammatory bowel disease, 44% had a history of prior ursodeoxycholic acid (UDCA) use, and baseline serum ALK was 342 IU/mL (IQR 275-520). All patients had stage 1-3 PSC, and none had a history of proctocolectomy, colorectal dysplasia, or cholangiocarcinoma.
Serum biochemical tests and PSC risk score
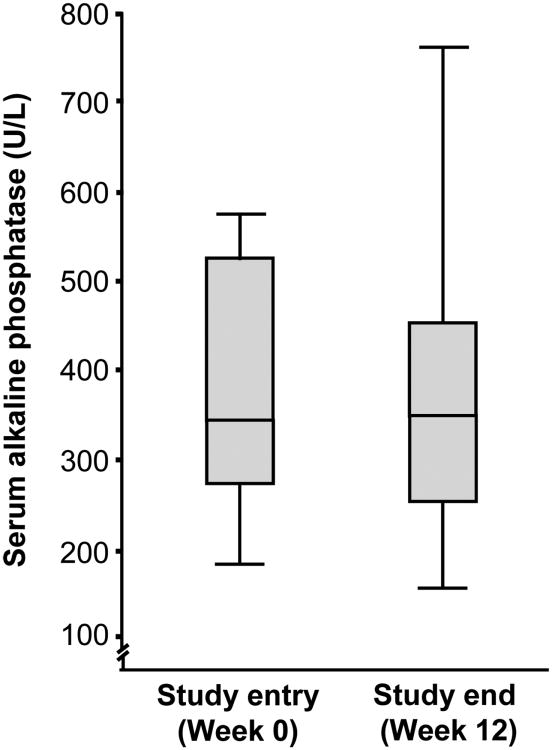
Baseline and 12-week serum liver biochemistry values and PSC risk score are summarized in Table 1. After 12 weeks of rifaximin therapy, no clinically or statistically significant changes were observed in the primary endpoint, ALK (342 IU at study baseline to 345 IU/mL at study end, median increase of 0.9%, p=0.47), as shown in Figure 2. In addition, there were no significant changes in any of the secondary biochemical endpoints (all p>0.05), including PSC risk score, although there was a trend toward significant improvement in γ-glutamyl transpeptidase and C-reactive protein (Table 1). Of note, serum biochemistries measured following 3 weeks of rifaximin were also not significantly changed (data not shown).
Table 1. Serum biochemical tests before and after 12 weeks of rifaximin treatment.
| Study entry | Study end | Change | p-value | |
|---|---|---|---|---|
| Alkaline phosphatase, U/L | 342 (275–520) | 345 (256–451) | 0.8% | 0.47 |
| Aspartate aminotransferase, U/L | 88 (53-129) | 78 (59-114) | -11% | 0.76 |
| Total bilirubin, mg/dL | 0.7 (0.5–1.2) | 1.1 (0.4–1.4) | 57% | 0.57 |
| γ-glutamyl transpeptidase (U/L) | 488 (297-925) | 391 (278-744) | -20% | 0.09 |
| Albumin, g/dL | 4.2 (4-4.5) | 4.2 (3.9-4.4) | 0% | 0.9 |
| C-reactive protein, mg/L | 3.6 (3–5.8) | 1.0 (1.0–1.0) | -72% | 0.10 |
| Mayo PSC risk score | 0.12 (-0.55–0.40) | 0.27 (-0.42–0.80) | 133% | 0.21 |
Values reported as median (interquartile range).
Figure 2. Serum alkaline phosphatase at baseline and after 12 weeks of rifaximin therapy.
Following 12 weeks of rifaximin therapy, there was no clinically or statistically significant change in the primary endpoint, serum alkaline phosphatase (p=0.47). The median serum alkaline phosphatase following 12 weeks of rifaximin therapy among individuals who completed the study (318 U/L) was also not significantly different from baseline value (i.e. per-protocol analysis, p=0.81).
Symptom-based questionnaires
Baseline and 12-week scores from the FFIS, 5-D itch scale, CLDQ, and SF-36 are summarized in Table 2. Of note, sample patients had considerably lower scores in all SF-36 domains compared to historical general population controls.47, 48 FFIS and 5-D itch scale scores were highly heterogeneous, with some patients reporting minimal fatigue and/or pruritus, and others reporting levels similar to or greater than historical disease controls.43, 49
Table 2. Symptom-based questionnaire scores before and after 12 weeks of rifaximin treatment.
| Study entry | Study end | Change | p-value | |
|---|---|---|---|---|
| Fatigue score (FFIS) | 16 (5-27) | 6 (0-11) | -63% | 0.10 |
| Total 5-D itch scale† | 7 (7-11) | 7 (6-8) | 0% | 0.88 |
| Chronic Liver Disease Questionnaire (CLDQ)†* | 161 (140-178) | 170 (164-183) | 6% | 0.31 |
| Short Form Health Survey (SF-36) | ||||
| Physical functioning | 56 (53-58) | 55 (53-57) | -2% | 0.75 |
| Role-physical | 54 (52-57) | 54 (55-57) | 0% | 0.5 |
| Bodily pain | 52 (52-60) | 56 (45-62) | 8% | 1.0 |
| General health | 49 (35-54) | 39 (32-57) | -20% | 0.46 |
| Vitality | 49 (44-54) | 48 (38-58) | -2% | 0.86 |
| Social functioning | 56 (44-58) | 57 (56-58) | 2% | 0.63 |
| Role-emotional | 56 (55-56) | 55 (55-56) | -2% | 1.0 |
| Mental health | 56 (51-59) | 55 (52-58) | -2% | 0.47 |
Values reported as median (interquartile range).
Summated scores.
There were no significant differences in any of the 6 individual CLDQ domains (data not shown).
Although individual responses to therapy were variable across the four questionnaires, overall there were no significant changes overall in any of the scores on these scales (all p>0.05), although there was a trend toward significant improvement in FFIS scores (Table 2). Intriguingly, one patient reported marked improvements in FFIS and SF-36 scores, and another reported similar improvements as well as improved total CLDQ score; the former experienced a 17% increase in serum ALK at 12 weeks, while the latter experienced a 13% decrease.
Adverse effects
Three patients did not complete the study due to AEs, including severe headaches, rapid increase in liver enzymes (by week 3), and need for endoscopic retrograde cholangiography. Four other patients reported AEs; one experienced headache, one dizziness, and two dyspepsia, but all four were able to complete the study. Patients with AEs were followed, and in all cases, AEs resolved after discontinuation of rifaximin.
Discussion
Here, we performed the first open-label clinical trial of rifaximin in patients with PSC and tested its efficacy and safety over 12 weeks of treatment. Overall, there were no significant improvements in the primary endpoint (ALK) or any of the secondary endpoints. In addition, several patients experienced AEs, as a result of which three were unable to complete the study as a result. Therefore, while there was a trend toward significant improvement in two of the secondary biochemical endpoints (gamma-glutamyl transpeptidase and C-reactive protein) as well as symptomatic improvements in a few individual patients, based on the collective results herein, rifaximin does not appear to be a safe and efficacious pharmacologic treatment in a majority of patients at the dose studied in this clinical trial.
Despite the overall negative results of this trial, it should be noted that at least two patients reported marked symptomatic improvements on rifaximin on therapy. The fact that some patients reported symptomatic improvements (e.g. in fatigue, a frequent and problematic symptom which to date has no established treatment2, 12, 14, 50, 51) despite only minimal or no improvements in ALK is intriguing. This observation might suggest at least two questions: first, could rifaximin (or other oral antibiotics) represent a potential therapy for fatigue among a subset of PSC patients even if not associated with a significant decrease in ALK? Considering our study was not powered to detect significant differences in fatigue and the positive treatment biases which can occur with subjective endpoints in open-label studies, further studies will be needed to address this question. Intriguingly, the patients who experienced symptomatic improvements were also those who experienced improvements in C-reactive protein values. The second question is whether ALK is a sufficiently sensitive and specific biomarker for all patients with PSC? This is relevant not only in the context of the question above and the heterogeneity of PSC, but also given the finding from previous studies that approximately 15% of PSC patients with improvements in ALK continue to have disease progression and poor outcomes.6, 52, 53 This emphasizes the need for more accurate, readily-accessible biomarkers and the potential importance of utilizing more than solely the ALK response in determining the utility of emerging therapies, particularly in light of the clinical challenges which remain in PSC management (e.g. fatigue, pruritus).
Although rifaximin may not be a promising pharmacotherapy for patients with PSC, three other prospective clinical trials in the last decade have demonstrated therapeutic effects with oral antibiotics. The first of these, by Farkkila et al.,54 was a randomized study of UDCA plus metronidazole (n=39) compared to UDCA only (n=41); after 36 months of therapy, there was evidence of significant improvement in ALK, PSC risk score, and histologic stage and grade as well as a trend toward less cholangiographic progression in the UDCA plus metronidazole group compared to the UDCA only group. In the second trial, Silveira et al.33 conducted an open-label study wherein 16 patients with PSC were treated with minocycline for one year; although a quarter of patients withdrew from the study (the majority due to AEs), those who continued minocycline treatment experienced a significant reduction in serum ALK and a trend toward a significant reduction in AST and Mayo PSC risk score. Lastly, in the third trial, we conducted a 12-week, phase II, double-blind, randomized study of thirty-five PSC patients treated with one of four regimens: low-dose vancomycin, high-dose vancomycin, low-dose metronidazole, and high-dose metronidazole. We detected a significant improvement in ALK, the primary endpoint, as well as multiple secondary endpoints in both the low- and high- dose vancomycin groups, while metronidazole appeared to be somewhat less efficacious and associated with more AEs. Based on these findings, we recommended further investigation of vancomycin, and in fact a phase III study is now underway (NCT01802073). Until the much anticipated results of this trial become available, vancomycin thus far appears to be the most promising antibacterial pharmacotherapy for PSC.
An even more fundamental question than which antibiotic is superior in treating PSC is that of the mechanism of action. A prevailing hypothesis relates to decreasing the biosynthesis and enterohepatic cirulcation of immunoactive bacterial metabolites, including but not limited to LPS, lipoteichoic acid, and peptidoglycan. Such molecules can be recognized by biliary epithelial and other resident hepatic cells and initiate signaling cascades that induce increased expression of a variety of pro-fibroinflammatory mediators, thus leading to hepatobiliary injury and potentially chronic disease (Figure 1).26, 36 To that effect, it has been postulated that the efficacy of vancomycin in PSC may be related to its selective activity against clostridia, the class of enteric bacteria primarily responsible for bile acid metabolism. With respect to rifaximin, while there may be several reasons why it appears to be inefficacious for treating PSC, we propose that it may be related to its overly broad spectrum of activity; thus, rifaximin may be bactericidal not only against the clostridia (or other pro-fibroinflammatory bacteria), but also commensal, cytoprotective microorganisms. Consequently, this nonselective pressure may result in or exacerbate enteric dysbiosis in patients with PSC and mitigate the putative benefits of rifaximin's known anti-clostridial activity. This notion is supported by the growing observations regarding the role of commensal microorganisms, i.e. symbionts, in protecting against host disease and promoting anti-inflammatory, pro-repair pathways.26, 55-57 Therefore, the therapeutic effects of antibacterials may be mediated through various, including non-antibiotic (and to date poorly understood) mechanisms; indeed, the decrease in C-reactive protein and symptomatic improvements in several individuals in our trial may point to potential anti-inflammatory effects and benefits in a small subset of patients with PSC. With the increasing improvements in molecular biological, metagenomic, and bioinformatic techniques, future research is expected to address this exciting area of investigation.
The present study has several limitations. As commonly seen with pilot studies, group sizes were small, and there was no control arm; this, together with the lack of blinding, allows for several potential biases, and for this reason, we selected an objective primary endpoint, which is less susceptible to bias. The study was not powered to detect small changes in endpoints, for example in γ-glutamyl transpeptidase, C-reactive protein, or fatigue. Similarly, it was not powered to identify subgroups which may benefit preferentially from rifaximin therapy. Treatment duration was relatively short, although not uncommon in early phase studies for PSC and generally sufficient for preliminary assessment of efficacy; moreover, continuing treatment in light of minimal evidence of efficacy would likely have prompted concerns of futility or ethicality.34 Lastly, the FFIS was used to measure fatigue but has not been validated in patients with PSC.42 Even with these limitations, it is unlikely that a larger-scale (e.g. phase 3) randomized trial of rifaximin can be recommended for treatment of PSC, at least at the dose studied herein, although it remains to be seen if rifaximin could be further studied as an experimental therapy for some of the debilitating symptoms associated with PSC such as fatigue.
Based on the outcomes of this open-label clinical trial, rifaximin does not appear to be an efficacious therapy in a majority of patients with PSC. Moreover, nearly 20% of patients were unable to remain on this medication for the duration of this study as a result of AEs. The failure of rifaximin to demonstrate efficacy for PSC might ostensibly be related to its broad-spectrum antibacterial activity and thus disruption of commensal microorganisms. While the enteric microbiota represents a promising therapeutic target for PSC, additional studies are needed to better understand their role in the pathogenesis of PSC and how the antimicrobial spectrum and other (e.g. anti-inflammatory) properties of an antibiotic might determine its utility for this indication.
Acknowledgments
Grant support: Salix Pharmaceuticals and NIH T32DK007198 (fellowship training grant support for JHT).
Footnotes
Other disclosures: The abstract of this manuscript has been selected for an oral presentation at the Cholestatic Liver Disease session on May 4, 2014 at Digestive Disease week in Chicago, IL.
References
- 1.Tabibian JH, Lindor KD. Primary sclerosing cholangitis: a review and update on therapeutic developments. Expert review of gastroenterology & hepatology. 2013;7:103–14. doi: 10.1586/egh.12.80. [DOI] [PubMed] [Google Scholar]
- 2.Gupta A, Bowlus CL. Primary sclerosing cholangitis: etiopathogenesis and clinical management. Frontiers in bioscience. 2012;4:1683–705. doi: 10.2741/e490. [DOI] [PubMed] [Google Scholar]
- 3.Levy C, Lindor KD. Primary sclerosing cholangitis: epidemiology, natural history, and prognosis. Seminars in liver disease. 2006;26:22–30. doi: 10.1055/s-2006-933560. [DOI] [PubMed] [Google Scholar]
- 4.Tabibian JH, Lindor KD. Challenges of Cholangiocarcinoma Detection in Patients with Primary Sclerosing Cholangitis. Journal of Analytical Oncology. 2012;1:50–55. doi: 10.6000/1927-7229.2012.01.01.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karlsen TH, Schrumpf E, Boberg KM. Update on primary sclerosing cholangitis. Digestive and liver disease: official journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver. 2010;42:390–400. doi: 10.1016/j.dld.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 6.Feldstein AE, Perrault J, El-Youssif M, Lindor KD, Freese DK, Angulo P. Primary sclerosing cholangitis in children: a long-term follow-up study. Hepatology. 2003;38:210–7. doi: 10.1053/jhep.2003.50289. [DOI] [PubMed] [Google Scholar]
- 7.Chapman R, Fevery J, Kalloo A, et al. Diagnosis and management of primary sclerosing cholangitis. Hepatology. 2010;51:660–78. doi: 10.1002/hep.23294. [DOI] [PubMed] [Google Scholar]
- 8.Brandsaeter B, Broome U, Isoniemi H, et al. Liver transplantation for primary sclerosing cholangitis in the Nordic countries: outcome after acceptance to the waiting list. Liver transplantation: official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2003;9:961–9. doi: 10.1053/jlts.2003.50169. [DOI] [PubMed] [Google Scholar]
- 9.Ali JM, Bonomo L, Brais R, et al. Outcomes and diagnostic challenges posed by incidental cholangiocarcinoma after liver transplantation. Transplantation. 2011;91:1392–7. doi: 10.1097/TP.0b013e31821aba57. [DOI] [PubMed] [Google Scholar]
- 10.Tabibian JH, O'Hara SP, Larusso NF. Primary sclerosing cholangitis: the gut-liver axis. Clinical gastroenterology and hepatology: the official clinical practice journal of the American Gastroenterological Association. 2012;10:819. doi: 10.1016/j.cgh.2012.01.024. author reply 819-20. [DOI] [PubMed] [Google Scholar]
- 11.Weismuller TJ, Wedemeyer J, Kubicka S, Strassburg CP, Manns MP. The challenges in primary sclerosing cholangitis--aetiopathogenesis, autoimmunity, management and malignancy. Journal of hepatology. 2008;48(1):S38–57. doi: 10.1016/j.jhep.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 12.Wiesner RH, Grambsch PM, Dickson ER, et al. Primary sclerosing cholangitis: natural history, prognostic factors and survival analysis. Hepatology. 1989;10:430–6. doi: 10.1002/hep.1840100406. [DOI] [PubMed] [Google Scholar]
- 13.Lepage P, Hasler R, Spehlmann ME, et al. Twin study indicates loss of interaction between microbiota and mucosa of patients with ulcerative colitis. Gastroenterology. 2011;141:227–36. doi: 10.1053/j.gastro.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 14.Broome U, Olsson R, Loof L, et al. Natural history and prognostic factors in 305 Swedish patients with primary sclerosing cholangitis. Gut. 1996;38:610–5. doi: 10.1136/gut.38.4.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duboc H, Rajca S, Rainteau D, et al. Connecting dysbiosis, bile-acid dysmetabolism and gut inflammation in inflammatory bowel diseases. Gut. 2012 doi: 10.1136/gutjnl-2012-302578. [DOI] [PubMed] [Google Scholar]
- 16.DuPont AW, DuPont HL. The intestinal microbiota and chronic disorders of the gut. Nature reviews Gastroenterology & hepatology. 2011;8:523–31. doi: 10.1038/nrgastro.2011.133. [DOI] [PubMed] [Google Scholar]
- 17.Mistilis SP, Skyring AP, Goulston SJ. Effect of long-term tetracycline therapy, steroid therapy and colectomy in pericholangitis associated with ulcerative colitis. Australasian annals of medicine. 1965;14:286–94. doi: 10.1111/imj.1965.14.4.286. [DOI] [PubMed] [Google Scholar]
- 18.Pohl J, Ring A, Stremmel W, Stiehl A. The role of dominant stenoses in bacterial infections of bile ducts in primary sclerosing cholangitis. European journal of gastroenterology & hepatology. 2006;18:69–74. doi: 10.1097/00042737-200601000-00012. [DOI] [PubMed] [Google Scholar]
- 19.Olsson R, Bjornsson E, Backman L, et al. Bile duct bacterial isolates in primary sclerosing cholangitis: a study of explanted livers. Journal of hepatology. 1998;28:426–32. doi: 10.1016/s0168-8278(98)80316-4. [DOI] [PubMed] [Google Scholar]
- 20.Hiramatsu K, Harada K, Tsuneyama K, et al. Amplification and sequence analysis of partial bacterial 16S ribosomal RNA gene in gallbladder bile from patients with primary biliary cirrhosis. Journal of hepatology. 2000;33:9–18. doi: 10.1016/s0168-8278(00)80153-1. [DOI] [PubMed] [Google Scholar]
- 21.Chen XM, O'Hara SP, Nelson JB, et al. Multiple TLRs are expressed in human cholangiocytes and mediate host epithelial defense responses to Cryptosporidium parvum via activation of NF-kappaB. Journal of immunology. 2005;175:7447–56. doi: 10.4049/jimmunol.175.11.7447. [DOI] [PubMed] [Google Scholar]
- 22.Mimura Y, Sakisaka S, Harada M, Sata M, Tanikawa K. Role of hepatocytes in direct clearance of lipopolysaccharide in rats. Gastroenterology. 1995;109:1969–76. doi: 10.1016/0016-5085(95)90765-3. [DOI] [PubMed] [Google Scholar]
- 23.Osnes T, Sandstad O, Skar V, Osnes M. Lipopolysaccharides and beta-glucuronidase activity in choledochal bile in relation to choledocholithiasis. Digestion. 1997;58:437–43. doi: 10.1159/000201480. [DOI] [PubMed] [Google Scholar]
- 24.Sasatomi K, Noguchi K, Sakisaka S, Sata M, Tanikawa K. Abnormal accumulation of endotoxin in biliary epithelial cells in primary biliary cirrhosis and primary sclerosing cholangitis. Journal of hepatology. 1998;29:409–16. doi: 10.1016/s0168-8278(98)80058-5. [DOI] [PubMed] [Google Scholar]
- 25.Tabibian JH, O'Hara SP, Splinter PL, Trussoni CE, Larusso NF. Cholangiocyte senescence via N-Ras activation is a characteristic of primary sclerosing cholangitis. Hepatology. 2014 doi: 10.1002/hep.26993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O'Hara SP, Tabibian JH, Splinter PL, Larusso NF. The dynamic biliary epithelia: Molecules, pathways, and disease. Journal of hepatology. 2013;58:575–82. doi: 10.1016/j.jhep.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mueller T, Beutler C, Pico AH, et al. Enhanced innate immune responsiveness and intolerance to intestinal endotoxins in human biliary epithelial cells contributes to chronic cholangitis. Liver international: official journal of the International Association for the Study of the Liver. 2011;31:1574–88. doi: 10.1111/j.1478-3231.2011.02635.x. [DOI] [PubMed] [Google Scholar]
- 28.Lichtman SN, Keku J, Clark RL, Schwab JH, Sartor RB. Biliary tract disease in rats with experimental small bowel bacterial overgrowth. Hepatology. 1991;13:766–72. [PubMed] [Google Scholar]
- 29.Yamada S, Ishii M, Liang LS, Yamamoto T, Toyota T. Small duct cholangitis induced by N-formyl L-methionine L-leucine L-tyrosine in rats. Journal of gastroenterology. 1994;29:631–6. doi: 10.1007/BF02365447. [DOI] [PubMed] [Google Scholar]
- 30.Stanich PP, Bjornsson E, Gossard AA, Enders F, Jorgensen R, Lindor KD. Alkaline phosphatase normalization is associated with better prognosis in primary sclerosing cholangitis. Digestive and liver disease: official journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver. 2011;43:309–13. doi: 10.1016/j.dld.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lindstrom L, Hultcrantz R, Boberg KM, Friis-Liby I, Bergquist A. Association between reduced levels of alkaline phosphatase and survival times of patients with primary sclerosing cholangitis. Clinical gastroenterology and hepatology: the official clinical practice journal of the American Gastroenterological Association. 2013;11:841–6. doi: 10.1016/j.cgh.2012.12.032. [DOI] [PubMed] [Google Scholar]
- 32.Davies YK, Cox KM, Abdullah BA, Safta A, Terry AB, Cox KL. Long-term treatment of primary sclerosing cholangitis in children with oral vancomycin: an immunomodulating antibiotic. Journal of pediatric gastroenterology and nutrition. 2008;47:61–7. doi: 10.1097/MPG.0b013e31816fee95. [DOI] [PubMed] [Google Scholar]
- 33.Silveira MG, Torok NJ, Gossard AA, et al. Minocycline in the treatment of patients with primary sclerosing cholangitis: results of a pilot study. The American journal of gastroenterology. 2009;104:83–8. doi: 10.1038/ajg.2008.14. [DOI] [PubMed] [Google Scholar]
- 34.Tabibian JH, Weeding E, Jorgensen RA, et al. Randomised clinical trial: vancomycin or metronidazole in patients with primary sclerosing cholangitis - a pilot study. Alimentary pharmacology & therapeutics. 2013;37:604–12. doi: 10.1111/apt.12232. [DOI] [PubMed] [Google Scholar]
- 35.Kim WR, Therneau TM, Wiesner RH, et al. A revised natural history model for primary sclerosing cholangitis. Mayo Clinic proceedings Mayo Clinic. 2000;75:688–94. doi: 10.4065/75.7.688. [DOI] [PubMed] [Google Scholar]
- 36.Tabibian JH, Talwalkar JA, Lindor KD. Role of the microbiota and antibiotics in primary sclerosing cholangitis. BioMed research international. 2013;2013:389537. doi: 10.1155/2013/389537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Elfaki DA, Lindor KD. Antibiotics for the treatment of primary sclerosing cholangitis. American journal of therapeutics. 2011;18:261–5. doi: 10.1097/MJT.0b013e3181b7b8c0. [DOI] [PubMed] [Google Scholar]
- 38.Prantera C, Lochs H, Grimaldi M, Danese S, Scribano ML, Gionchetti P. Rifaximin-extended intestinal release induces remission in patients with moderately active Crohn's disease. Gastroenterology. 2012;142:473–481. e4. doi: 10.1053/j.gastro.2011.11.032. [DOI] [PubMed] [Google Scholar]
- 39.Mas A, Rodes J, Sunyer L, et al. Comparison of rifaximin and lactitol in the treatment of acute hepatic encephalopathy: results of a randomized, double-blind, double-dummy, controlled clinical trial. Journal of hepatology. 2003;38:51–8. doi: 10.1016/s0168-8278(02)00350-1. [DOI] [PubMed] [Google Scholar]
- 40.Adachi JA, DuPont HL. Rifaximin: a novel nonabsorbed rifamycin for gastrointestinal disorders. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2006;42:541–7. doi: 10.1086/499950. [DOI] [PubMed] [Google Scholar]
- 41.Joseph J, Singhal S, Patel GM, Anand S. Clostridium Difficile Colitis: Review of the Therapeutic Approach. American journal of therapeutics. 2012 doi: 10.1097/MJT.0b013e318245992d. [DOI] [PubMed] [Google Scholar]
- 42.Fisk JD, Ritvo PG, Ross L, Haase DA, Marrie TJ, Schlech WF. Measuring the functional impact of fatigue: initial validation of the fatigue impact scale. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 1994;18(1):S79–83. doi: 10.1093/clinids/18.supplement_1.s79. [DOI] [PubMed] [Google Scholar]
- 43.Elman S, Hynan LS, Gabriel V, Mayo MJ. The 5-D itch scale: a new measure of pruritus. The British journal of dermatology. 2010;162:587–93. doi: 10.1111/j.1365-2133.2009.09586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Younossi ZM, Guyatt G, Kiwi M, Boparai N, King D. Development of a disease specific questionnaire to measure health related quality of life in patients with chronic liver disease. Gut. 1999;45:295–300. doi: 10.1136/gut.45.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Medical care. 1992;30:473–83. [PubMed] [Google Scholar]
- 46.Pares A, Caballeria L, Rodes J. Excellent long-term survival in patients with primary biliary cirrhosis and biochemical response to ursodeoxycholic Acid. Gastroenterology. 2006;130:715–20. doi: 10.1053/j.gastro.2005.12.029. [DOI] [PubMed] [Google Scholar]
- 47.Benito de Valle M, Rahman M, Lindkvist B, Bjornsson E, Chapman R, Kalaitzakis E. Factors that reduce health-related quality of life in patients with primary sclerosing cholangitis. Clinical gastroenterology and hepatology: the official clinical practice journal of the American Gastroenterological Association. 2012;10:769–775. e2. doi: 10.1016/j.cgh.2012.01.025. [DOI] [PubMed] [Google Scholar]
- 48.Wijnands TF, Neijenhuis MK, Kievit W, et al. Evaluating health-related quality of life in patients with polycystic liver disease and determining the impact of symptoms and liver volume. Liver international: official journal of the International Association for the Study of the Liver. 2013 doi: 10.1111/liv.12430. [DOI] [PubMed] [Google Scholar]
- 49.Prince MI, James OF, Holland NP, Jones DE. Validation of a fatigue impact score in primary biliary cirrhosis: towards a standard for clinical and trial use. Journal of hepatology. 2000;32:368–73. doi: 10.1016/s0168-8278(00)80385-2. [DOI] [PubMed] [Google Scholar]
- 50.Alba LM, Angulo P, Lindor KD. Primary sclerosing cholangitis. Minerva gastroenterologica e dietologica. 2002;48:99–113. [PubMed] [Google Scholar]
- 51.ter Borg PC, van Os E, van den Broek WW, Hansen BE, van Buuren HR. Fluvoxamine for fatigue in primary biliary cirrhosis and primary sclerosing cholangitis: a randomised controlled trial [ISRCTN88246634] BMC gastroenterology. 2004;4:13. doi: 10.1186/1471-230X-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Imam MH, Sinakos E, Gossard AA, et al. High-dose ursodeoxycholic acid increases risk of adverse outcomes in patients with early stage primary sclerosing cholangitis. Alimentary pharmacology & therapeutics. 2011;34:1185–92. doi: 10.1111/j.1365-2036.2011.04863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lindor KD. Ursodiol for primary sclerosing cholangitis. Mayo Primary Sclerosing Cholangitis-Ursodeoxycholic Acid Study Group. The New England journal of medicine. 1997;336:691–5. doi: 10.1056/NEJM199703063361003. [DOI] [PubMed] [Google Scholar]
- 54.Farkkila M, Karvonen AL, Nurmi H, et al. Metronidazole and ursodeoxycholic acid for primary sclerosing cholangitis: a randomized placebo-controlled trial. Hepatology. 2004;40:1379–86. doi: 10.1002/hep.20457. [DOI] [PubMed] [Google Scholar]
- 55.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–41. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 56.Backhed F, Manchester JK, Semenkovich CF, Gordon JI. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:979–84. doi: 10.1073/pnas.0605374104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Le Roy T, Llopis M, Lepage P, et al. Intestinal microbiota determines development of non-alcoholic fatty liver disease in mice. Gut. 2013;62:1787–94. doi: 10.1136/gutjnl-2012-303816. [DOI] [PubMed] [Google Scholar]