Abstract
In the accepted model of T-cell activation, parallel signal transduction pathways activate the transcription factors NF-κB, NFAT and AP-1 to drive clonal expansion of T cells in response to antigen. Genome-wide transcriptional profiling following antigen-induced CD8+ T-cell activation in C57BL/6 mouse T cells revealed that genes regulated by NFAT were also reduced in the absence of NF-κB p50 and cRel subunits. Importantly, p50−/−cRel−/− CD8+ T cells had significantly diminished NFAT and AP-1 activation compared with WT or PKCθ−/− CD8+ T cells. Attenuated NFAT activation after TCR engagement was associated with reduced calcium influx, PLCγ and Zap70 activation. Interestingly, pharmacological bypass of PLCγ regulated pathways largely rescued p50−/−cRel−/− T-cell proliferative defects. These results indicate a crucial and unexpected requirement for NF-κB p50 and cRel subunits in proximal TCR signaling and calcium responses. They further suggest that key defects in T cells in the absence of NF-κB pathway components may be due to impaired proximal T-cell signaling.
Keywords: T-cell activation, transcription factors, signal transduction, gene expression, NF-κB
Introduction
T-cell activation is initiated by TCR binding to cognate peptide/MHC complexes on professional APCs. This interaction initiates a signaling cascade requiring several key kinases, phosphatases, and scaffolding proteins that together drive T-cell activation [1–3]. In the accepted model, proximal T-cell signaling mediators such as Zap70 and PLCγ drive activation of downstream transcription factors. A key event is phosphorylation-induced activation of PLCγ, which then cleaves PIP3 into IP3 and DAG [2]. IP3 is crucial for calcium (Ca2+) influx which in turn is necessary to activate the NFAT transcription factor [2]. On the other hand, DAG activates PKCs which are thought to be important for activation of the transcription factors AP-1 and NF-κB [3, 4]. These seemingly parallel cascades of transcription factors lead to induction of key genes necessary for T-cell development, proliferation, survival and cytokine production [2, 5, 6]. While investigating signaling defects in PKCθ−/− and NF-κB p50−/−cRel−/− CD8+ T cells, we found an unexpected role for NF-κB in regulating proximal T-cell signaling that is crucial for NFAT activation. Therefore, the downstream NF-κB transcription factor can influence proximal T-cell signaling in a manner that results in more robust T-cell responses.
Results and Discussion
NF-κB p50−/−cRel−/− CD8+ T cells exhibit pronounced clonal expansion defects in vitro and in vivo
Previously, we found that absence of PKCθ or p50+cRel in T cells impairs GVHD in allogeneic recipients [7]. To better understand the roles of these proteins in regulating T-cell function in response to antigen, we crossed PKCθ−/− and p50−/−cRel−/− mice to OVA257-264 (SIINFEKL: referred as OVA) specific OT-1 TCR Tg mice. Stimulation of CD8+ T cells from these mice with OVA-pulsed dendritic cells (DCs) showed that while PKCθ−/− T-cell proliferation was reduced compared with WT T cells, the most severe defect in proliferation was in p50−/−cRel−/− T cells (Figure 1A). In addition, IL-2 mRNA expression was most significantly reduced in p50−/−cRel−/− T cells (Figure 1B). Therefore, p50−/−cRel−/− T cells have greater defects in in vitro proliferation and IL-2 expression compared with PKCθ−/− T cells.
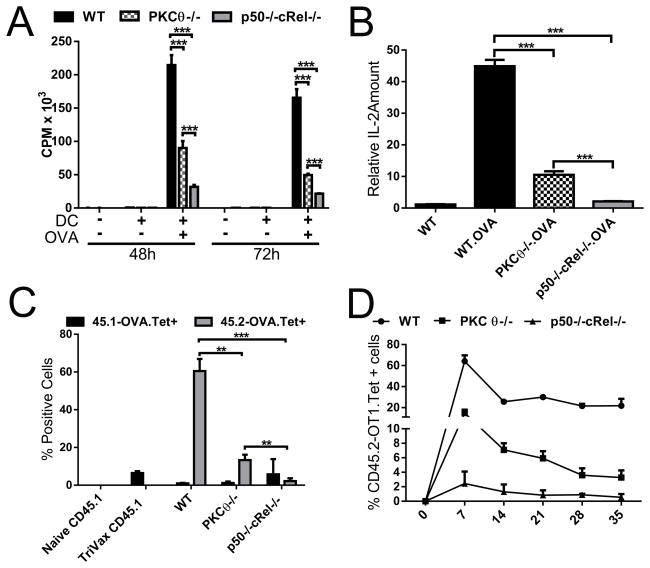
Figure 1. NF-κB p50−/−cRel−/− CD8+ T cells exhibit pronounced clonal expansion defects in vitro and in vivo.
(A) In vitro proliferation of OT-1 WT, PKCθ−/− and p50−/−cRel−/− CD8+ T cells cultured alone or in the presence of DC + OVA. CPM values represent 3H-thymidine incorporation. (B) Relative amount of IL-2 mRNA present in WT OT-1, PKCθ−/− and p50−/−cRel−/− CD8+ T cells after 18 h culture without or with DC + OVA, determined by microarray and normalized to 18S ribosomal RNA. (C) Clonal expansion of transplanted OT-1 WT, PKCθ−/− and p50−/−cRel−/− CD8+ T cells was determined in peripheral blood 7 days post TriVax administration by flow cytometry; values represent % of endogenous (45.1) and transplanted (45.2) OVA-Tetramer+ CD8+ T cells/viable (DAPI-) PBMCs. (D) Percentage of transplanted (CD45.2) OVA-Tetramer+ CD8+ T cells/PBMC over 5 weeks, determined by flow cytometry. ((A–D) Data are shown as mean + SEM (n=4 mice per genotype) and are representative of two independent experiments. Statistical significance is indicated as *p<0.05, **p<0.01, ***p<0.001, Student’s t-test.
To determine the in vivo effect of T-cell-specific absence of p50+cRel and PKCθ, we adoptively transferred CD45.2/OT-1 WT, PKCθ−/− or p50−/−cRel−/− CD8+ T cells into congenic WT CD45.1 hosts. 24 h later, CD45.1 recipients, along with un-injected mice, received TriVax, a vaccine formulation with OVA peptide, anti-CD40, and Poly (I:C) [8]. Every 7 days, thereafter, peripheral blood samples were analyzed for clonal expansion. As expected, WT T cells expanded vigorously, and by day 7, 60% of peripheral WBCs were the transplanted CD45.2 OVA-specific T cells. Mirroring in vitro findings, PKCθ−/− T-cell expansion was significantly reduced; however, the greatest impairment in expansion was in p50−/−cRel−/− T cells (Figure 1C), which was evident at all time-points tested (Figure 1D). Injection of BiVax (100 μg OVA and 50 μg Poly (I:C) vaccine 38 days after initial TriVax treatment of WT OT-1 transplanted mice resulted in death of all 4 injected mice in 3 days. The PKCθ−/− group survived somewhat longer to day 6–7; in contrast, mice receiving p50−/−cRel−/− T-cell were completely protected from the lethal effect of T-cell restimulation. These results indicate that absence of p50+cRel results in substantially more pronounced impairment in T-cell activation than PKCθ absence.
p50−/−cRel−/− T cells have defects in NFAT and AP-1 activation and calcium influx
To better understand consequences of absence p50−/−cRel−/− and PKCθ−/−, we performed genome-wide analysis of gene expression (Supporting Information Table 1). A modest level of overlap in PKCθ and p50+cRel regulated genes was seen (34 probe sets; 22 genes), which included previously defined targets such as IL-2 (Supporting Information Table 1). Notable amongst p50+cRel target genes were several key cytokines (IL-17A, IL-21, IL-23A, IL-3, and IFN-γ), signal transducers (STAT5A, STAT5B, E2F1 and PRKCA), and cell surface receptors (CTLA-4, CD2, TNFRSF4 and IL-12RB2) (Supporting Information Table 1). Interestingly, in addition to IL-2, several of these genes are known to be regulated by calcium/NFAT signaling, including IL-3, STAT5B, IFN-γ and CTLA-4 [9–11]. These results are suggestive of potentially broader defects in absence of p50+cRel and, in particular, warranted a closer examination of NFAT/calcium signaling in p50−/−cRel−/− T cells.
We next determined activation of NFAT, AP-1 and NF-κB in CD8+ T cells from all three genotypes after stimulation with OVA-pulsed DCs. WT T cells showed strong nuclear translocation of all three transcription factors (Figure 2A). Consistent with our previous results [7], NF-κB levels were not substantially impacted in PKCθ−/− T cells; however, a more substantial reduction in NFAT and AP-1 was seen in these T cells (Figure 2A). As expected, p50−/−cRel−/− T cells showed significant reduction in NF-κB activity. Remarkably, both NFAT and AP-1 nuclear levels were also greatly reduced in p50−/−cRel−/− T cells (Figure 2A). A similar trend was also seen in polyclonal CD8+ T cells from WT, PKCθ−/−, and p50−/−cRel−/− mice that were stimulated with anti-CD3/CD28 (Figure 2B). Recently, we found redundancy in function of PKCθ and PKCα in GVHD induction [12]. However, p50−/−cRel−/− T cells also showed more significant reduction in activation of these transcription factors than PKCθ−/−PKCα−/− T cells (Figure 2B). In addition, western blotting of nuclear extracts showed no increase in nuclear presence of NFATc1 in p50−/−cRel−/− T cells after anti-CD3/CD28 stimulation (Figure 2C and Supporting Information Fig. 1A). These results suggest that the substantially reduced responsiveness of p50−/−cRel−/− T cells compared with PKCθ−/− T cells may be due to more significant impairment in activation of multiple transcription factors in the former.
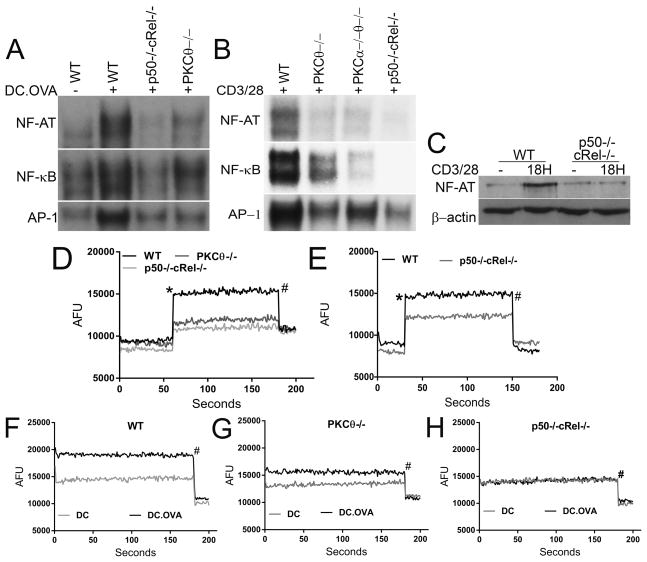
Figure 2. p50−/−cRel−/− T cells have defects in NFAT and AP-1 activation and calcium influx.
(A) Unstimulated and stimulated OT-1 WT, PKCθ−/− and p50−/−cRel−/− CD8+ T cells after 18 h culture with DC and OVA were used to prepare nuclear lysates and EMSA was performed to detect NF-κB, NFAT and AP-1. (B) Same as (A) except polyclonal WT, PKCθ−/−, PKCα−/−PKCθ−/− and p50−/−cRel−/− CD8+ T cells were used, and stimulated with 1 μg/mL anti-CD3/CD28 for 18 h. (C) Western blotting to determine NFATc1 in nuclear fraction of WT and p50−/−cRel−/− CD8+ T cells unstimulated or stimulated with 1 μg/mL CD3/CD28 for 18 h. (D) WT, PKCθ−/−, and p50−/−cRel−/− polyclonal CD8+ T cells were analyzed for the ability to influx calcium: the first 60 s represent a baseline after which the following components were added to the culture: (*)10 μg/mL CD3/CD28 and (#) 9 mM EGTA. Points are Arbitrary Fluorescent Units (AFU) and lines represent one of three repeats within each assay. (E) Same as (D) except polyclonal CD8+ cells were cultured for 48 h with 1 μg/mL CD3/CD28, rested for 2 h, and then assayed for calcium after anti-CD3/CD28 treatment. (F–H) Same as (D) except OT-1 T cells were used and graphs compare calcium influx based on T-cell:DC interaction ± OVA. (A–H) Data are from one experiment (n=4 mice/genotype/pooled and spread across treatments), representative of three independent experiments.
As NFAT activation requires calcium signals [13], we next determined calcium influx in T cells after stimulation. WT T cells showed rapid increase in calcium influx when stimulated with anti-CD3/CD28 (Fig. 2D). Importantly, calcium influx was reduced in PKCθ−/− but the reduction was most severe in p50−/−cRel−/− T cells. Calcium signaling is also important for continued T-cell proliferation and cytokine expression [2, 14]. Importantly, calcium influx was also substantially reduced in p50−/−cRel−/− T cells stimulated for 48 h (Fig. 2E), although less severely than in naïve T cells (Fig. 2D). We next determined calcium influx in T cells after stimulation with unpulsed or OVA-pulsed DCs. WT T cells stimulated with OVA-pulsed DC showed substantially enhanced calcium influx compared with T cells with unpulsed DCs (Fig. 2F). This difference was substantially reduced in PKCθ−/− T cells (Fig. 2G). Strikingly, no difference in calcium influx was seen in p50−/−cRel−/− T cells stimulated with unpulsed versus OVA-pulsed DCs (Fig. 2H). Together, our results indicate that impaired NFAT activation in p50−/−cRel−/− is likely due to defects in calcium influx.
Reduced PLCγ and Zap70 activation in p50−/−cRel−/− CD8+ T cells
Upon TCR engagement, PLCγ cleaves PIP2 into IP3 (leading to ER calcium release followed by store-operated calcium influx and NFAT activation) and DAG (leading to PKC and AP-1/NF-κB activation) [2]. Since p50−/−cRel−/− T cells have defects in both NFAT and AP-1 activation, we next determined whether this was due to impaired PLCγ activation. While total PLCγ levels were equivalent, a decrease in activation-induced phosphorylation (Y783) of PLCγ was seen in p50−/−cRel−/− CD8+ T cells (Fig. 3A and Supporting Information Fig. 1B). To gain broader insight into additional proteins that displayed a reduction of phosphorylation in p50−/−cRel−/− CD8+ T cells, we detected pan-tyrosine phosphorylation using the 4G10 antibody. These resulted pointed to reduction in phosphorylation of proteins in the p50−/−cRel−/− T cells (Supporting Information Figure 2) that may correspond to molecular weight range of PLCγ, Zap70/Slp76/Itk, and LAT. We next specifically determined Zap70 and LAT phosphorylation as they are in a direct pathway leading to PLCγ activation.
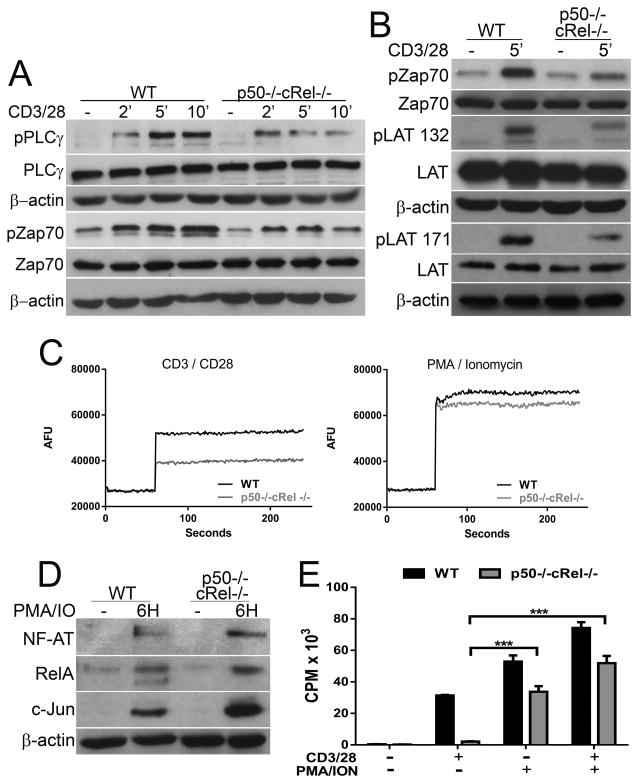
Figure 3. Reduced PLCγ and Zap70 activation in p50−/−cRel−/− CD8++ T cells.
(A) WT and p50−/−cRel−/− CD8+ T cells untreated or stimulated with 10 μg/ml anti-CD3/CD28 for 2, 5 or 10 min after which lysates were made and western blotting was performed to detect pPLCγ, total PLCγ, pZap70 and total Zap70. (B) Same as (A), but only the 5 min time-point was used for detection of pZap70, total Zap70, pLAT (Y132), pLAT (Y171), and total LAT. (C) WT and p50−/−cRel−/− CD8+ T cells were assayed for the ability to influx calcium as in Figure 2C, in unstimulated or either 10 μg/mL CD3/CD28 or 50 ng PMA and 500 ng ionomycin stimulated cells. (D) WT and p50−/−cRel−/− CD8+ + T cells were cultured alone or with 50 ng PMA and 500 ng ionomycin for 6 h; nuclear lysates were made and western blotting was performed to detect NFAT, RelA and c-Jun. (E) In vitro proliferation of WT and p50−/−cRel−/− CD8+ T cells cultured alone or with 1 μg/mL anti-CD3/CD28 and/or 50 ng PMA and 500 ng ionomycin for 48 h; values represent CPM of 3H-thymidine incorporation and are given as mean + SD. (A–E) Data are from one experiment (n=2–4 mice/genotype), representative of three independent experiments. Statistical significance is indicated as *p<0.05, **p<0.01, ***p<0.001, Student’s t-test.
Upon TCR engagement, the tyrosine kinase Zap70 plays a key role in initiating proximal signaling required for T-cell activation [1]. Interestingly, Zap70 phosphorylation (Y319) was also reduced in p50−/−cRel−/− T cells compared with WT T cells (Fig. 3A and Supporting Information Fig. 1C). Moreover, LAT Y132 and Y171 phosphorylation was also reduced in the p50−/−cRel−/− T cells (Fig. 3B and Supporting Information 1C). These two sites are Zap70 targets and potential docking sites for PLCγ. This indicates that the reduction in Zap70 phosphorylation in the p50−/−cRel−/− T cells translates to reduced activation of this protein, and points to a less robust LAT signalisome as a crucial step in the subsequent reduction in activation of PLCγ. Furthermore, these results suggest that defects in proximal T-cell signaling in p50−/−cRel−/− T cells may be responsible for defects in calcium/NFAT and AP-1 activation. Since calcium/NFAT and AP-1 also contribute to T-cell activation and proliferation, we hypothesized that observed defects in p50−/−cRel−/− T cells may also be due to impaired PLCγ signaling rather than p50+cRel absence per se. To test this possibility, we used two agents that bypass the need for activated PLCγ mediated IP3 and DAG generation: ionomycin (IO; calcium ionophore) and PMA (PKC inducer). Intriguingly, IO+PMA largely rescued calcium influx defects (Fig. 3C), the nuclear translocation of NF-AT, RelA/p65, and c-Jun (Fig. 3D and Supporting Information Fig. 1D), and the proliferative response in p50−/−cRel−/− T cells (Fig. 3E). Therefore, a significant component of defects in proliferation of p50−/−cRel−/− T cells stem from impaired proximal T-cell signaling leading to suboptimal activation of PLCγ. Importantly, these results further indicate that key defects in T-cell responses in absence of NF-κB subunits can be due to defects in proximal T-cell signaling.
Our results show an unexpected function of NF-κB in calcium influx and NFAT activation. We further show that this function is at least partly controlled through optimal activation of the key mediators of T-cell signaling: Zap70 and PLCγ. Pharmacological rescue of critical activation components in p50−/−cRel−/− T cells further showed that a substantial component of the defects in these cells are due to impaired PLCγ activation. However, we do not believe that p50−/−cRel−/− T cells are universally incapable of activation-induced signaling: first, expression of 96.5% genes was similar between WT and p50−/−cRel−/− T cells (only 3.5% were changed) (see Supporting Information Table 1), indicating that the majority of activation-induced genes are not impacted. Second, rescue by IO+PMA indicates that p50−/−cRel−/− T cells are fully capable of robust proliferative responses if stimulated in a manner that bypasses specific functional defects. Consistent with impairment in activation of transcription factors regulating IL-2 expression, p50−/−cRel−/− T-cell proliferation was also partially rescued by addition of exogenous IL-2 (Supporting Information Fig. 3). Our results also indicate the novel function for NF-κB described here appear to be a redundantly controlled by p50 and cRel, since CD8+ T cells lacking either p50 or cRel showed only a slight reduction in calcium influx and proliferation (Supporting Information Fig. 4A and B). Preliminary results also indicate that p50−/−cRel−/− CD4+ cells exhibit defective calcium influx (not shown).
A key question is how PLCγ activation and Ca2+/NFAT signaling is regulated by NF-κB. Given the fact that calcium influx was measured minutes after stimulation, it is unlikely that this is due to defective gene induction by activation-induced NF-κB. Instead, regulation of calcium influx likely represents a function for constitutive NF-κB activity. We believe that similar, albeit less severe, defects in PKCθ−/− T cells seen here as well as previous PKCθ studies may suggest some possible answers [3, 15–19]. Both NF-κB subunits and PKCθ associate with the IKK complex [3], which is comprised of IKKα/β/γ. Importantly, IKKβ/γ can localize to the immune synapse (IS) where key proximal signaling events are initiated [20, 21]. It is possible that absence of either PKCθ or NF-κB subunits can somehow impact IKK activation, localization or stability. While IS presence of IKKβ is thought to be important for NF-κB activation, it is interesting to speculate that IKKβ regulates additional events in TCR signaling, which are adversely impacted by PKCθ or p50+cRel absence.
Concluding Remarks
Our results demonstrate a vital function for NF-κB p50 and cRel subunits in calcium/NFAT signaling that is mediated through optimal activation of proximal TCR signaling mediators. Previous studies have defined a crucial role of the NF-κB pathway in promoting T-cell survival [6, 22, 23]. The results shown here indicate that in addition to promoting survival, NF-κB is also crucial for early T-cell activation signaling important for regulating NFAT and AP-1 transcription factors.
Materials and Methods
Mice and cells
PKCθ−/−, p50−/−cRel−/− and OT-1 transgenic mice were described previously [7]. All mice were maintained under specific pathogen-free conditions, and all experiments using mice were carried out in accordance with institutional guidelines. T-cell culture and proliferation were performed as previously described [7]. BM-derived dendritic cells (DCs) were generated as described [24]. OT-1 T cells and polyclonal T cells were stimulated with either 1 or 10 μg anti-CD3/CD28 (ebioscience) and/or 50 ng Phorbol 12-Myristate 13-Acetate (PMA) and 500 ng Ionomycin (both Fisher).
In vivo T-cell expansion and FACS analysis
OT-1 CD8+ T cells (in C57BL/6 background) were isolated from the spleens of CD45.2 mice and 1 × 106 cells were transplanted into C57BL/6 CD45.1 hosts. 24 h later, transplanted and control mice were injected i.p. with 100 μg OVA, 100 μg anti-CD40, and 50 μg Poly (I:C). Peripheral blood was acquired through submandibular bleed every 7 days thereafter. Peripheral blood was RBC lysed (ACK Buffer, Fisher) and Fc-blocked (eBioscience) and WBCs were stained with CD45.1, CD45.2, and CD8 (eBioscience) and KB-OVA tetramer (MLB International) in PBS supplemented with 0.5% Bovine Serum Albumin for 30 min on ice. Cells were washed twice and data was collected on an LSRII (Beckman Dickinson) and analyzed with FlowJo 9.6 (TreeStar). Cells were gated on strict forward and side scatter parameters, to ensure single cell analysis. Additionally, the DAPI- population was used for viable cells. Finally, cells were separated by CD45.1 or CD45.2 positivity, and assessed for CD8/OVA-tetramer expression.
Calcium Assay
T cells were suspended at 1 × 106/mL in Calcium Buffer [No-Phenol Red RPMI (Fisher) with 2% FBS and 10% Probenecid (Thermo-Scientific)] and stained with 4 μM Fluo-4 (Invitrogen) for 45 min at 37°C. Cells were washed 3 times in Calcium Buffer and replated at 2 × 105/well in a 96-well flat-bottom plate. Cells were then rested for 20 min at RT, and incubated at 37°C for 10 min immediately preceding the assay. Where indicated, cells received the following: 1 μg/mL anti-CD3/CD28 (mouse & human-ebioscience) and 3 mM EGTA. Samples were read on a Wallac EnVision 2102 Multilabel Reader (Perkin Elmer).
Microarray, EMSA and Western blotting
Microarray analysis was performed as described in [25] (also see Supporting Information Table 1). EMSA on T cells has been described [23]. Western Blotting was performed using anti-NFATc1 (H-110) and anti-RelA/p65 (372) both from Santa Cruz; anti-pLAT Y132 (4476) & anti-pLAT Y171 (73205) both from Abcam; anti-pZap70 (2717), anti-Zap70 (2705), anti-pPLCγ (2821), anti-LAT (9166), anti-c-Jun (9165) all from Cell Signaling and anti-PLCγ (610027 – BD Biosciences).
Statistical analyses
All statistical analyses were performed using the Student t-test; a p value <0.05 was considered significant.
Supplementary Material
Acknowledgments
We thank Adam Mailloux and acknowledge help provided by Flow Cytometry, Molecular Genomics and mouse core facilities at the Moffitt Cancer Center. This work was supported by NIH R01 AI0802685
Footnotes
Conflict of interest
The authors declare no commercial or financial conflict of interest.
References
- 1.Wang H, Kadlecek TA, Au-Yeung BB, Goodfellow HE, Hsu LY, Freedman TS, Weiss A. ZAP-70: an essential kinase in T-cell signaling. Cold Spring Harb Perspect Biol. 2010;2:a002279. doi: 10.1101/cshperspect.a002279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oh-hora M, Rao A. Calcium signaling in lymphocytes. Curr Opin Immunol. 2008;20:250–258. doi: 10.1016/j.coi.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zanin-Zhorov A, Dustin ML, Blazar BR. PKC-theta function at the immunological synapse: prospects for therapeutic targeting. Trends in immunology. 2011;32:358–363. doi: 10.1016/j.it.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Isakov N, Altman A. Protein kinase C(theta) in T-cell activation. Annu Rev Immunol. 2002;20:761–794. doi: 10.1146/annurev.immunol.20.100301.064807. [DOI] [PubMed] [Google Scholar]
- 5.Oh H, Ghosh S. NF-kappaB: roles and regulation in different CD4(+) T-cell subsets. Immunol Rev. 2013;252:41–51. doi: 10.1111/imr.12033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gerondakis S, Fulford TS, Messina NL, Grumont RJ. NF-kappaB control of T-cell development. Nat Immunol. 2014;15:15–25. doi: 10.1038/ni.2785. [DOI] [PubMed] [Google Scholar]
- 7.Valenzuela JO, Iclozan C, Hossain MS, Prlic M, Hopewell E, Bronk CC, Wang J, Celis E, Engelman RW, Blazar BR, Bevan MJ, Waller EK, Yu XZ, Beg AA. PKCtheta is required for alloreactivity and GVHD but not for immune responses toward leukemia and infection in mice. The Journal of clinical investigation. 2009;119:3774–3786. doi: 10.1172/JCI39692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cho HI, Celis E. Optimized peptide vaccines eliciting extensive CD8+ T-cell responses with therapeutic antitumor effects. Cancer research. 2009;69:9012–9019. doi: 10.1158/0008-5472.CAN-09-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sica A, Dorman L, Viggiano V, Cippitelli M, Ghosh P, Rice N, Young HA. Interaction of NF-kappaB and NFAT with the interferon-gamma promoter. J Biol Chem. 1997;272:30412–30420. doi: 10.1074/jbc.272.48.30412. [DOI] [PubMed] [Google Scholar]
- 10.Macian F. NFAT proteins: key regulators of T-cell development and function. Nat Rev Immunol. 2005;5:472–484. doi: 10.1038/nri1632. [DOI] [PubMed] [Google Scholar]
- 11.Gibson HM, Hedgcock CJ, Aufiero BM, Wilson AJ, Hafner MS, Tsokos GC, Wong HK. Induction of the CTLA-4 gene in human lymphocytes is dependent on NFAT binding the proximal promoter. J Immunol. 2007;179:3831–3840. doi: 10.4049/jimmunol.179.6.3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haarberg KM, Li J, Heinrichs J, Wang D, Liu C, Bronk CC, Kaosaard K, Owyang AM, Holland S, Masuda E, Tso K, Blazar BR, Anasetti C, Beg AA, Yu XZ. Pharmacologic inhibition of PKCalpha and PKCtheta prevents GVHD while preserving GVL activity in mice. Blood. 2013;122:2500–2511. doi: 10.1182/blood-2012-12-471938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hogan PG, Chen L, Nardone J, Rao A. Transcriptional regulation by calcium, calcineurin, and NFAT. Genes Dev. 2003;17:2205–2232. doi: 10.1101/gad.1102703. [DOI] [PubMed] [Google Scholar]
- 14.Oh-Hora M, Yamashita M, Hogan PG, Sharma S, Lamperti E, Chung W, Prakriya M, Feske S, Rao A. Dual functions for the endoplasmic reticulum calcium sensors STIM1 and STIM2 in T-cell activation and tolerance. Nat Immunol. 2008;9:432–443. doi: 10.1038/ni1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zanin-Zhorov A, Ding Y, Kumari S, Attur M, Hippen KL, Brown M, Blazar BR, Abramson SB, Lafaille JJ, Dustin ML. Protein kinase C-theta mediates negative feedback on regulatory T-cell function. Science. 2010;328:372–376. doi: 10.1126/science.1186068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pfeifhofer C, Kofler K, Gruber T, Tabrizi NG, Lutz C, Maly K, Leitges M, Baier G. Protein kinase C theta affects Ca2+ mobilization and NFAT-cell activation in primary mouse T cells. J Exp Med. 2003;197:1525–1535. doi: 10.1084/jem.20020234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sims TN, Soos TJ, Xenias HS, Dubin-Thaler B, Hofman JM, Waite JC, Cameron TO, Thomas VK, Varma R, Wiggins CH, Sheetz MP, Littman DR, Dustin ML. Opposing effects of PKCtheta and WASp on symmetry breaking and relocation of the immunological synapse. Cell. 2007;129:773–785. doi: 10.1016/j.cell.2007.03.037. [DOI] [PubMed] [Google Scholar]
- 18.Manicassamy S, Sadim M, Ye RD, Sun Z. Differential roles of PKC-theta in the regulation of intracellular calcium concentration in primary T cells. J Mol Biol. 2006;355:347–359. doi: 10.1016/j.jmb.2005.10.043. [DOI] [PubMed] [Google Scholar]
- 19.Kong KF, Yokosuka T, Canonigo-Balancio AJ, Isakov N, Saito T, Altman A. A motif in the V3 domain of the kinase PKC-theta determines its localization in the immunological synapse and functions in T cells via association with CD28. Nat Immunol. 2011;12:1105–1112. doi: 10.1038/ni.2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hara H, Bakal C, Wada T, Bouchard D, Rottapel R, Saito T, Penninger JM. The molecular adapter Carma1 controls entry of IkappaB kinase into the central immune synapse. J Exp Med. 2004;200:1167–1177. doi: 10.1084/jem.20032246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Egawa T, Albrecht B, Favier B, Sunshine MJ, Mirchandani K, O’Brien W, Thome M, Littman DR. Requirement for CARMA1 in antigen receptor-induced NF-kappa B activation and lymphocyte proliferation. Curr Biol. 2003;13:1252–1258. doi: 10.1016/s0960-9822(03)00491-3. [DOI] [PubMed] [Google Scholar]
- 22.Vallabhapurapu S, Karin M. Regulation and function of NF-kappaB transcription factors in the immune system. Annu Rev Immunol. 2009;27:693–733. doi: 10.1146/annurev.immunol.021908.132641. [DOI] [PubMed] [Google Scholar]
- 23.Zheng Y, Vig M, Lyons J, Van Parijs L, Beg AA. Combined deficiency of p50 and cRel in CD4+ T cells reveals an essential requirement for nuclear factor kappaB in regulating mature T-cell survival and in vivo function. The Journal of experimental medicine. 2003;197:861–874. doi: 10.1084/jem.20021610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang X, Hussain S, Wang EJ, Wang X, Li MO, Garcia-Sastre A, Beg AA. Lack of essential role of NF-kappa B p50, RelA, and cRel subunits in virus-induced type 1 IFN expression. J Immunol. 2007;178:6770–6776. doi: 10.4049/jimmunol.178.11.6770. [DOI] [PubMed] [Google Scholar]
- 25.Hopewell EL, Zhao W, Fulp WJ, Bronk CC, Lopez AS, Massengill M, Antonia S, Celis E, Haura EB, Enkemann SA, Chen DT, Beg AA. Lung tumor NF-kappaB signaling promotes T-cell-mediated immune surveillance. The Journal of clinical investigation. 2013;123:2509–2522. doi: 10.1172/JCI67250. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.