Abstract
Transient receptor potential (TRP) proteins are non-selective cation channel proteins that are expressed throughout the body. Previous studies demonstrated the expression of TRP Vanilloid 1 (TRPV1), capsaicin (CAP) receptor, in sensory neurons. Recently, we reported TRPV1 expression in mouse motor nerve terminals [MNTs; (Thyagarajan et al., 2009)], where we observed that CAP protected MNTs from botulinum neurotoxin A (BoNT/A). Phrenic nerve diaphragm nerve muscle preparations (NMP) isolated from isoflurane anesthetized adult mice were analyzed for twitch tension, spontaneous (mEPCs) and nerve stimulus evoked (EPCs) acetylcholine release. When acutely applied to isolated NMP, CAP produced a concentration-dependent decline of twitch tension and produced a significant decline in the amplitude of EPCs and quantal content without any effect on the mEPCs. The suppression of nerve stimulus evoked acetylcholine release by CAP was antagonized by capsazepine (CPZ), a TRPV1 antagonist. CAP did not suppress phrenic nerve stimulus evoked acetylcholine release in TRPV1 knockout mice. Also, CAP treatment, in vitro, interfered with the localization of adapter protein 2 in cholinergic Neuro 2a cells. Wortmannin, (WMN; non-selective phosphoinositol kinase inhibitor), mimicked the effects of CAP by inhibiting the acetylcholine exocytosis. Our data suggest that TRPV1 proteins expressed at the MNT are coupled to the exo-endocytic mechanisms to regulate neuromuscular functions.
Keywords: CAP, capsazepine, TRPV1, motor nerve terminal, acetylcholine release, twitch tension, end plate currents, spontaneous release, clathrin, PIP2, endocytosis, exocytosis
1.0 Introduction
Transient receptor potential vanilloid 1 (TRPV1), also known as CAP receptor, is a member of the TRP superfamily. TRPV1 is activated by various exogenous (capsaicin, resiniferatoxin, etc) and endogenous (pH, temperature, endovanilloids, etc) ligands (Caterina et al., 1997). Although reported to be exclusively expressed in the dorsal root ganglion neurons, recent studies have shown a widespread expression of TRPV1 in brain (Toth et al., 2005) spinal cord and in peripheral nociceptive neurons (Cui et al., 2006). At the peripheral terminals of primary nociceptors, TRPV1-mediated Ca2+ influx triggers the release of neuropeptides, which contribute to the development of neurogenic inflammation (Caterina and Julius, 2001). Capsaicin (CAP), a pungent principle from hot peppers of the capsicum family and resiniferatoxin are naturally occurring agonists that activate TRPV1 (Holzer, 1991; Szallasi and Blumberg, 1991). CAP activates the TRPV1 ion channel resulting in depolarization and excitation (Marsh et al., 1987) and the effects of CAP are blocked competitively by the antagonist capsazepine [CPZ; (Bevan et al., 1992)].
As early as in 1982, the presence of CAP-sensitive afferent fibers in the skeletal muscles was recognized and it was shown that stimulation of these group IV afferents (nonmyelinated C fibers) by CAP caused reflex cardiovascular effects (Kaufman et al., 1982). At the frog neuromuscular junction, it was shown that cannabinoid and vanilloid receptor agonist arachidonyl-2-chloroethylamide (ACEA) and CAP increased the quantal content of stimulus evoked acetylcholine release and this effect was blocked by CPZ (Silveira et al., 2010). In a recent study, expression of TRPV1 receptor was demonstrated for the mouse MNT and that the receptor was sensitive to CAP (Thyagarajan et al., 2009). Although the existence of TRPV1 receptors in the mammalian motor nerve terminal (MNT) has been shown, the role it plays in the physiology of acetylcholine release has not been investigated. This work fills this knowledge gap by investigating the effect of CAP on the mouse MNT and analyzes the role of TRPV1 activation in the modulation of acetylcholine release at the MNT.
2.0. Material and methods
2.1. Animals
All animal experimental protocols were approved by the Institutional Animal Care and Use Committee. Wild type C57BL/6 and TRPV1−/− mice were obtained from Jackson Laboratories, CT, USA. Animals were housed in the research animal facility located in the School of Pharmacy and were given food and water ad libitum.
2.2. Muscle twitch tension
Diaphragm nerve muscle preparations (NMP) were isolated from adult male wild type or TRPV1 knockout (TRPV1−/−) mice anesthetized with isoflurane. After assuring full anesthesia, the mice were killed by cervical dislocation. The procedure for removal of diaphragm muscle with the phrenic nerve, mounting in the tissue bath and recording nerve-elicited muscle twitches was according to that described earlier (Thyagarajan et al., 2009; Thyagarajan et al., 2010). The diaphragm with attached phrenic nerve was mounted in a glass chamber (Rodnoti Glass Technology, Inc., USA) filled with oxygenated (95% O2 - 5% CO2) normal Ringer solution (physiological solution; pH 7.4, 37 °C) containing (mM) NaCl (135), KCl (5), MgCl2 (1), CaCl2 (2), Na2HPO4 (1), NaHCO3 (15) and glucose (5.5). The phrenic nerve was drawn into a suction electrode for indirect activation (1 Hz) of muscle twitches. One tendon of the muscle was tied to a Grass force transducer connected to a Digidata 1440A (Molecular Devices Inc., USA) for acquisition of the mechanical response (grams of tension developed) in response to the nerve stimulation. Muscles were stretched to optimal length for force generation and equilibrated for 30 min prior to nerve stimulation at 1 Hz for data acquisition. CAP and CPZ were added to the physiological solution and concentrations were expressed as final bath concentration (µM). Data collected at 120 min. period following the addition of different cumulative concentrations CAP was analyzed with pCLAMP 10 software. In experiment where CAP dose response was studied, no more than two concentrations of CAP were studied in one experiment (n = 4 diaphragms (8 hemidiaphragms) obtained from 4 mice for each experiment).
2.3. EPC and mEPC measurement using Two Electrode Voltage Clamp
Diaphragm NMP were dissected from isoflurane-anesthetized mice after cervical dislocation as described above. The muscles were pinned to a Sylgard-lined plexiglass chamber, and bathed in HEPES Ringer Solution (HRS; 22–25 °C) containing (mM): NaCl (135), KCl (5), MgCl2 (1), CaCl2 (2), Na2HPO4 (1), HEPES (10, pH 7.4) and glucose (5.5). Spontaneous transmitter release [miniature endplate currents (mEPCs)] and nerve stimulus evoked endplate currents (EPCs) were recorded from the endplate region (−75 mV holding potential) using two-electrode voltage clamp technique as described previously (Thyagarajan et al., 2009; Thyagarajan et al., 2010). Two electrodes were inserted into the endplate membrane at interelectrode distance of approximately 50 µm. One electrode filled with 4 M potassium acetate served to deliver current while the other electrode filled with 3 M KCl recorded membrane voltage. The phrenic nerve was drawn into a suction electrode and stimulated supramaximally at 1 Hz or 20 Hz. The recordings of mEPCs and EPCs were obtained using Axoclamp 900A (Molecular Devices, Sunnyvale, CA) and analyzed offline for the EPC amplitude and mEPC amplitude and frequency. Prior to EPC recordings, 0.75 µM Conotoxin GIIIB was added to the HRS solution to block muscle contractions during the EPC recordings. Control recordings were made before application of CAP or CPZ. Collected data were analyzed with pCLAMP 10 software. Quantal content was calculated as ratio of the mean EPC amplitude to mean mEPC amplitude and represented as mean ± S.E.M.
2.4 Cell Culture and transfection
Mouse cholinergic neuroblastoma (Neuro 2a) cells were cultured in Dulbecco’s modified Eagle’s F12 medium, pH 7.4, supplemented with 10% fetal bovine serum and antibiotics. Human embryonic kidney 293 (HEK293) cells were maintained in minimal essential medium supplemented with 10% fetal bovine serum and penicillin/streptomycin. The rat TRPV1 tagged with the myc epitope on the N terminus in pCDNA3 vector was transfected using the Effectene reagent (Qiagen, Chatsworth, CA) in HEK293 cells or Neuro 2a cells. Transfected cells were exposed to Dulbecco’s modified Eagle’s medium (DMEM) containing 1 mg/ml G418 on day 3 and stable clones were obtained on day 21. Cells that stably expressed TRPV1 were maintained in G410 (250 µg/ml) and the medium was changed to normal DMEM (no G418) 48 hours before the experiments.
2.5 Confocal microscopy and immunoblotting
In order to detect if TRPV1 is expressed at the mouse MNT, immunoblotting was performed on the phrenic nerve diaphragm NMP. HEK293 cells expressing TRPV1 channel protein and wild type HEK293 cells served as positive and negative controls, respectively. Diaphragm NMPs were used for immunoblotting experiments to detect the extent of TRPV1 expression. Endplate-rich regions from the muscle NMP were stained for acetylcholine esterase (AChE) by a previously described method (Koelle and Horn, 1968) and modified by Gautron (Gautron, 1982). Acetylthiocholine was the substrate for the staining reaction. AChE labeling was performed at room temperature. After a 30-min reaction period, NMP were washed with HRS. Endplate regions were cut under a stereo zoom microscope and flash frozen in liquid nitrogen. Tissues were homogenized in phosphate-buffered saline (PBS) containing 1% Nonidet 40 and complete protease inhibitor cocktail (GE Healthcare, Little Chalfont, Buckinghamshire, UK). The homogenate was then centrifuged at 20817 g for 10 min. at 4 °C, The crude lysate (100 µg) was resolved via SDS-polyacrylamide gel electrophoresis after boiling in 1 X Laemmli. HEK293 cells that stably express TRPV1 were homogenized and resolved by SDS-PAGE similarly.
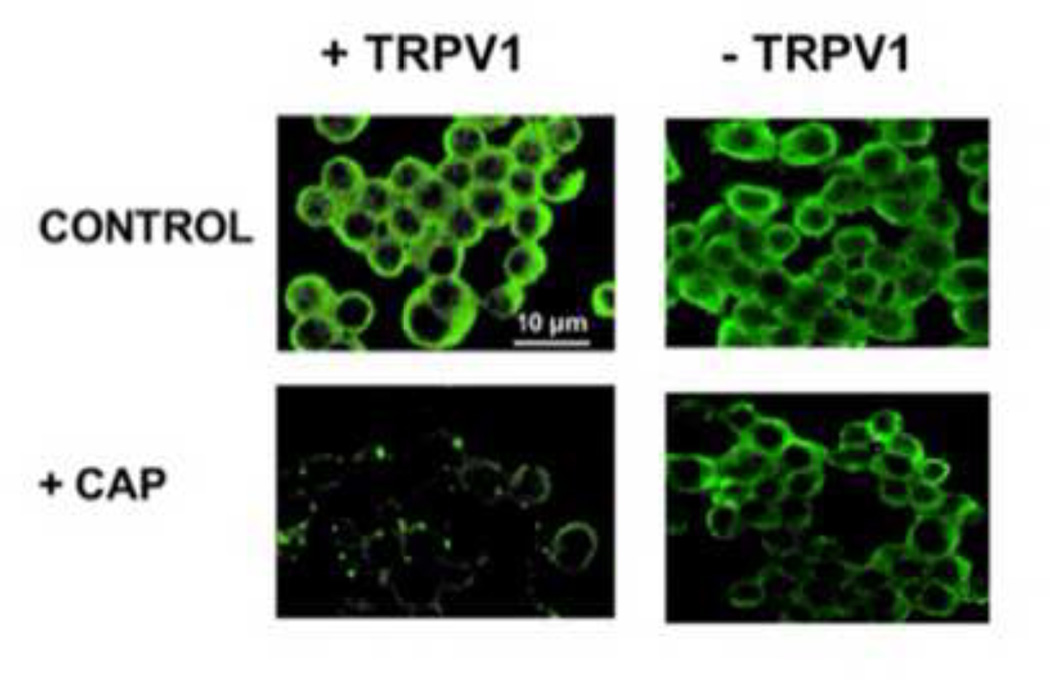
To demonstrate that CAP pretreatment modifies the subcellular distribution of AP-2 (adapter protein-2), TRPV1 stably expressing Neuro 2a cells were used. Two groups of TRPV1 expressing Neuro 2a cells were seeded on poly (L-lysine)-coated cover slips. The experimental group was incubated (at 37 °C) in HRS containing 1 µM CAP (See Fig. 6 legend) for 60 min and washed. The control group was incubated at 37 °C with vehicle only. After washing the coverslips with HEPES, cells were immunostained with AP-2µ-1 antibody (Santa Cruz Biotech, Inc, USA) followed by FITC (secondary antibody) and mounted with Vectashield on a slide and kept frozen at −20°C before imaging with an LSM 710 confocal microscope. Images were saved and represented as TIFF files and analyzed with Image J software (National Institutes of Health, Bethesda, MD).
Figure 6. CAP pretreatment alters the subcellular disposition of AP-2 in Neuro 2a cells.
Confocal micrographs represent the immunostaining for AP-2 µ1 in control and CAP treated wild type Neuro 2a cells and TRPV1 expressing Neuro 2a cells.
2.6 Chemicals and drugs
µ-Conotoxin GIIIB was obtained from Alamone Labs, Israel. CAP, capsazepine, wortmannin, and all other chemicals and drugs were obtained from Sigma, USA.
2.7 Data analyses
Data are expressed as mean ± S.E.M. The number of mice used for each experiment and the number of muscles/endplates from which recordings were made are shown in the figure legends. Student's t test and ANOVA evaluated the statistical significance of population means. Figures from analyzed data were plotted using Microcal Origin 6.0 software.
3.0 Results
3.1. Expression of TRPV1 (CAP receptor) in mouse MNT
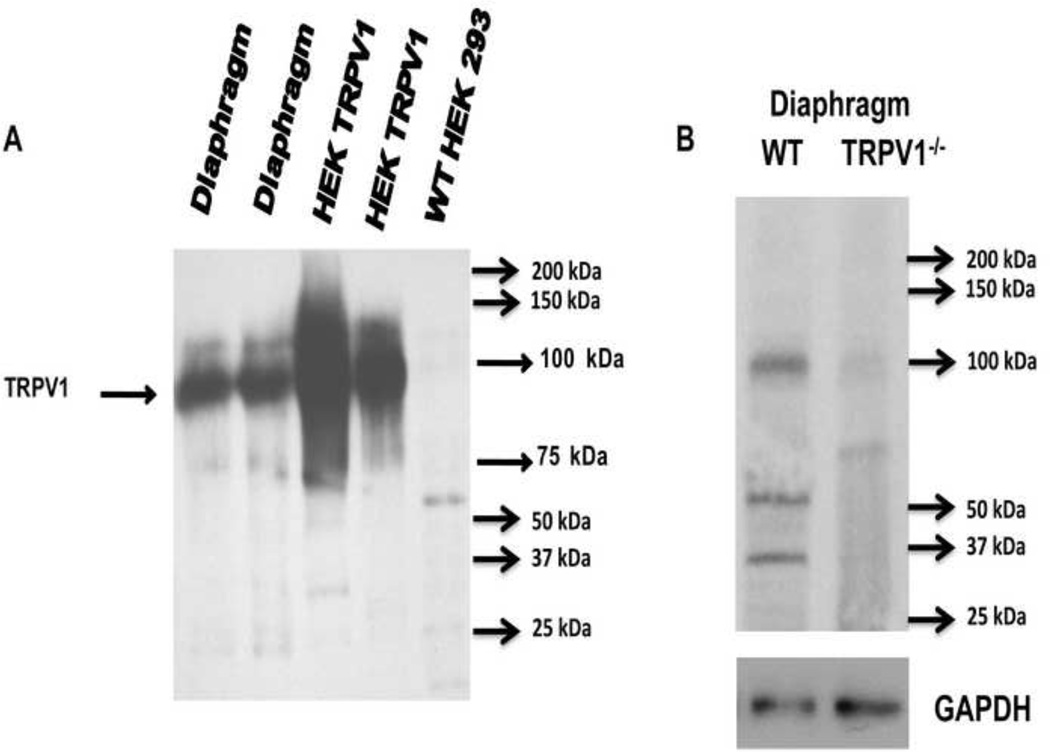
We first wanted to confirm the presence of TRPV1 receptor at the mouse MNT. The results of western blot showing TRPV1 in lysates of mouse diaphragm NMP and HEK293 cells transfected with TRPV1 are shown in Fig.1A. TRPV1 expression (band at ~100 kDa) was seen in the diaphragm NMP in both lane 1 and 2 (n = 6) as well as in HEK 293 cell transfected with TRPV1 (n = 6). The expression of this protein was undetectable in wild type HEK293 cells. Fig.1B illustrates the western blotting for TRPV1 in the diaphragm NMP of the wild type and TRPV1−/− mice. These findings confirmed previous reports showing the expression of TRPV1 in the mouse MNT (Thyagarajan et al., 2009) and in frog neuromuscular junctions (Silveira et al., 2010).
Figure 1. Mouse NMJ expresses TRPV1.
A. Western blot showing TRPV1 in lysates of mouse diaphragm and HEK293 cells transfected with TRPV1. TRPV1 expression is undetectable in wild type HEK293 cells. B. Representative western blot (n = 3 independent experiments) for TRPV1 in wild type and TRPV1−/− diaphragms. GAPDH was used as loading control.
3.2. Effects of CAP on twitch tensions of diaphragm
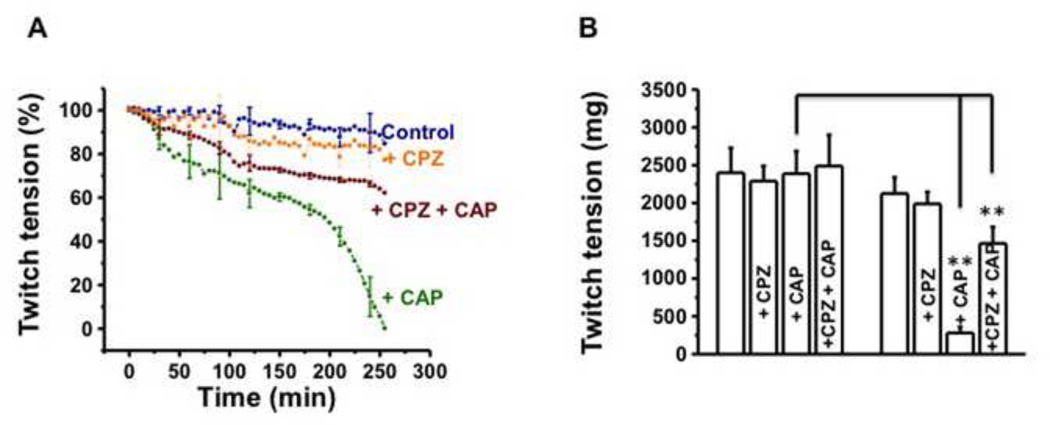
Since CAP activates TRPV1 and CPZ is a competitive antagonist of CAP, we decided to study the effect of these drugs on the mouse NMJ in vitro. The time course of twitch tensions recorded in response to 1 Hz phrenic nerve stimulation and the mean twitch tensions before (30 min equilibration period), soon after CAP (1 µM) or 30 min. pretreatment of CPZ (10 µM) and at the end of 250 min are shown in Fig. 2A and B respectively. In control preparations exposed to only physiological solution, there was approximately 10–15 % decline in the twitch tension by 250 min (2487 ± 122 mg at 0 time to 2188 ± 109 mg at 250 min; Fig. 2B). This decline was within the expected limits for muscles perfused in vitro for 4 hr. duration since in situ conditions cannot be duplicated in vitro CAP produced a progressive decline of the twitch tension over 250 min. (Fig.2A and 2B). To study the effect of TRPV1 antagonist CPZ, the muscles were pretreated with CPZ, 30 min prior to CAP addition, and both compounds were present throughout the duration of experiment. At 250 min. the combination of two drugs produced only about 40% drop in twitch tension (Fig. 2B; 1466 ± 44 mg with CAP + CPZ versus 258 ± 14 mg in CAP only, P<0.001). CPZ alone did not cause any inhibition of stimulus twitch tension as shown in Fig. 2A (orange trace) and B.
Figure 2. CAP (CAP) inhibits stimulus evoked twitch tension of hemidiaphragm nerve muscle preparations and capsazepine (CPZ) antagonizes the effects of CAP.
A. Time course of stimulus evoked twitch tension (%) of control, + CAP (1 µM) and + CPZ (10 µM) + CAP treated hemidiaphragm nerve muscle preparations. B. Representative twitch tensions of control, + CAP and + CPZ + CAP (1 µM) preparations at 0 min (before any treatment) and at 250 min. after treatment with CAP alone or + CPZ + CAP. The data are from 8–12 nerve-muscle preparations obtained from 12 mice. ** represents statistical significance for P<0.001.
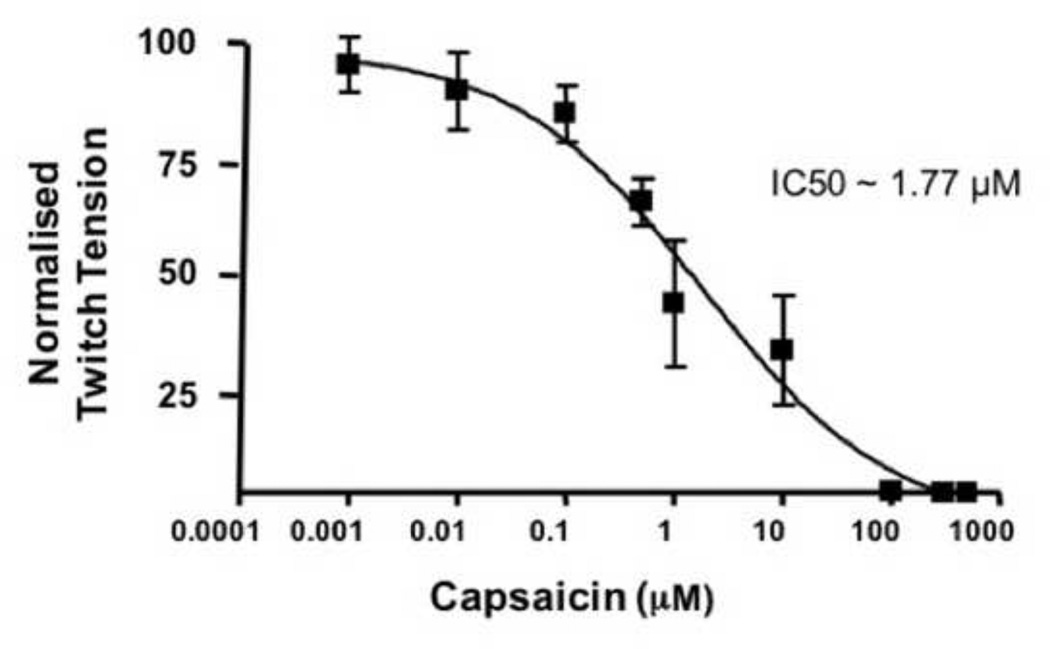
A concentration- response study with CAP in concentrations ranging from 1 nM to 800 µM was performed. The data demonstrated that as little as 10 nM produced a decrease in the normalized muscle tension significantly (Fig. 3; 5 ± 2 %). IC50 for the inhibition of twitch tension by CAP was calculated using the concentration-response curve. The theoretical IC50 for stimulus evoked twitch tension by CAP was calculated to be 1.77 µM. For the electrophysiology studies, we have used 1 µM CAP, which is lower than IC50 dose but was found to be sufficient to block neuromuscular transmission. Also, 1 µM CAP significantly activates TRPV1 and we used this concentration of CAP for the entire study.
Figure 3. CAP concentration-response curve in vitro.
A. IC50 of CAP determined by measuring the concentration-dependent inhibitory effects of CAP on nerve stimulus evoked twitch tensions of diaphragm NMP in vitro. Data collected at 90 min. period following the addition of different concentrations CAP were shown. No more than two concentrations of CAP were studied in one experiment.
3.3. Effects of CAP on EPCs and mEPCs
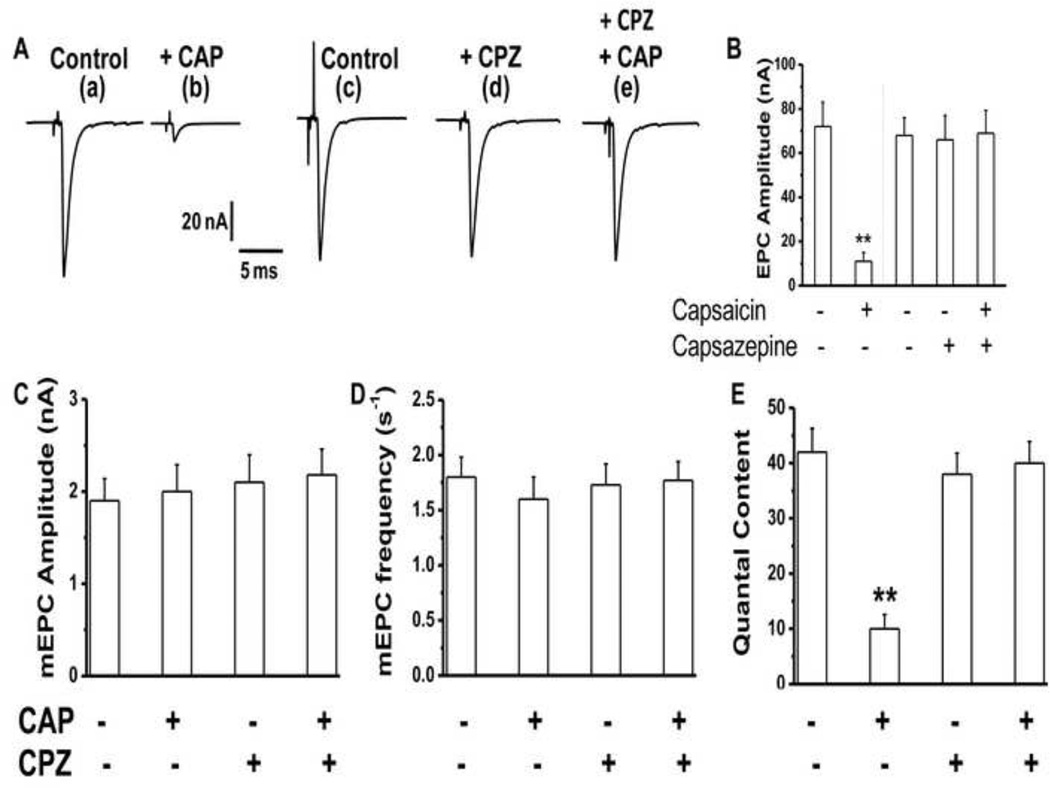
We characterized the nature of nerve stimulus evoked endplate currents (EPCs) and spontaneous transmitter (miniature; mEPCs) following in vitro application of CAP (1 µM). Muscle contractions were eliminated with µ-conotoxin GIIIB (100 nM; for 60 min). As shown in Fig. 4A EPCs were almost completely inhibited at 120 min after 1 µM CAP and this inhibition was prevented by CPZ (10 µM; Fig. 4A), which was added to the bath 30 min. before the application of CAP and was present for the entire duration of the experiment. CPZ alone had neither an activating nor an inhibiting effect on acetylcholine release (Fig. 4A d). During 120 min. exposure to both drugs, alone or in combination, the mEPC amplitude and frequency (Fig. 4C and D) were unaltered indicating that the effect of CAP was primarily on the stimulus evoked acetylcholine release. The data for EPC amplitude and quantal content (Fig. 4 B and E) showed that CAP drastically reduced EPC amplitude and quantal content after 120 min exposure (mean EPC amplitude of 12 ± 5 nA with CAP vs 72 ± 9 in control, P<0.001 and quantal content of 10 ± 3 with CAP versus 42 ± 6 in control, P<0.001). CPZ (10 µM) prevented CAP–induced inhibition of EPCs (quantal content for NMP treated with CPZ + CAP was 38 ± 4; Fig. 4E). These data clearly indicate that CAP blocked nerve stimulus evoked acetylcholine release at the mouse MNT, an effect that was prevented by CPZ and secondly CAP did not alter spontaneous acetylcholine release.
Figure 4. CAP inhibits stimulus evoked neurotransmitter release and capsazepine (CPZ; 10 µM) antagonizes the effects of CAP.
A. Representative traces of 1 Hz stimulus evoked end plate currents (EPC) of control (a and c), + CAP (b), + CPZ (d), and + CPZ + CAP (e) treated nerve muscle preparations (n = 4–6, 16–22) at the end of 120 min. after the application of either vehicle alone or the drug. CPZ was pretreated and was present throughout the experiments (d and e). B. EPC amplitudes ± S.E.M of control (a and c), + CAP (b), + CPZ (d), and + CPZ + CAP (e) treated nerve muscle preparations (n = 4–6, 16–22). C, D and E. Represent the spontaneous miniature end plate current (mEPC) amplitude, frequency and quantal content of control, + CAP, + CPZ + CAP, and + CPZ treated preparations. Numbers in parentheses represent the numbers of nerve muscle preparations and fibers respectively obtained from six mice for each condition. (Quantal content = ratio of EPC amplitude to mEPC amplitude). ** represents statistical significance for P<0.001.
3.4. Lack of effect of CAP on EPCs in diaphragm NMP of TRPV1−/− mice
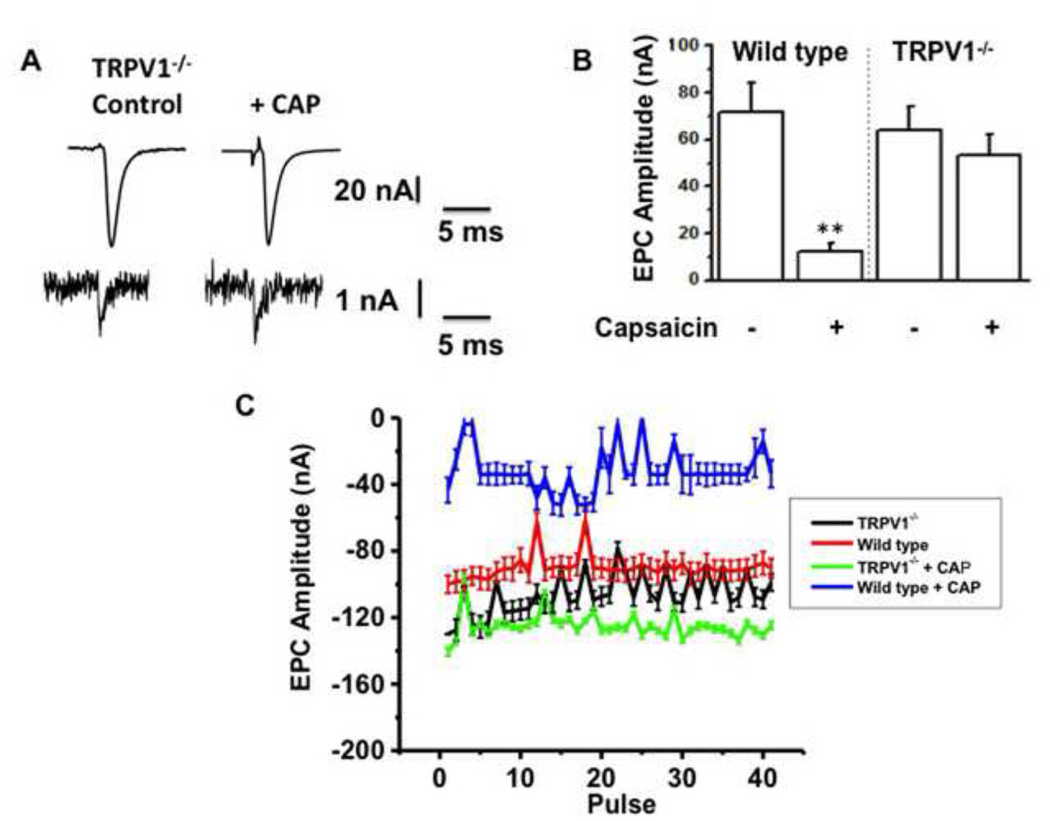
If CAP activation of TRPV1 at the MNT causes a decline in EPC amplitude over the course of 120 min, similar experiments performed in the mouse NMP removed from mice lacking TRPV1 (TRPV1−/−) should show EPC amplitude comparable to that exhibited in controls. The data from EPC recordings in MNTs of TRPV1−/− mice are shown in Fig. 5. Representative EPC and mEPC amplitudes for control and CAP treated diaphragm NMP obtained from TRPV1−/− mice are shown in Fig. 5A. Fig. 5B shows the mean EPC amplitude in diaphragm MNTs from wild type mice was significantly decreased by 120 min. of 1 µM CAP exposure (P<0.001; 13 ± 3 nA and 73 ± 8 nA with CAP and control respectively). In contrast, no significant difference in amplitude was seen in EPC amplitude for TRPV1−/− NMPs exposed to CAP as compared to EPCs recorded from NMPs exposed to CAP in WT mice. Also, there was no significant difference between control EPC amplitudes (before the addition of CAP) recorded from endplates of diaphragm NMP of wild type and TRPV1−/− mice indicating that the neuromuscular physiology in TRPV1−/− MNTs was comparable to that shown in wild type mice. Consistent to this, CAP did not have effects on diaphragm NMPs of TRPV1−/− at 20 Hz stimulation, while significantly inhibited the EPCs of wild type NMPs (Fig. 5C).
Figure 5. CAP inhibits EPC of wild type but not TRPV1−/−.
A. Representative EPC (top) and mEPC (bottom) traces for control and CAP treated diaphragm from TRPV1−/− mice. B. Bar graph represent the mean EPC ± S.E.M. for the diaphragm obtained from wild type and TRPV1−/− animals (n = 6–8 animals, 18–24 endplates). C. EPC amplitudes for control and CAP-treated wild type and TRPV1−/− diaphragm at 120 min. vehicle or CAP application. ** represents statistical significance for P<0.001.
3.5. Interference with the expression/localization of adapter protein 2 (AP2 -µ1) in Neuro 2a cells by CAP pretreatment
In order to determine if CAP interfered with the endocytic mechanisms that couple to exocytosis, experiments were conducted with TRPV1 expressing Neuro 2a cholinergic cells. The cells were preincubated with CAP (1 µM) for 60 min. and immunostained with AP2 -µ1 antibody followed by FITC labeled secondary antibody (see methods for details). As shown in Fig. 6, AP2 -µ1 was localized near the plasma membrane (periphery of the cell) in the control condition. CAP treatment delocalized AP2 -µ1 in TRPV1 expressing Neuro 2a cells. Conversely, CAP treatment did not affect AP-2 in wild type Neuro 2a cells that did not express TRPV1 (Fig. 6; upper and lower panels on right; four independent experiments; n = 288 for AP2 -µ1).
3.6. Effect of wortmannin on EPCs and mEPCs
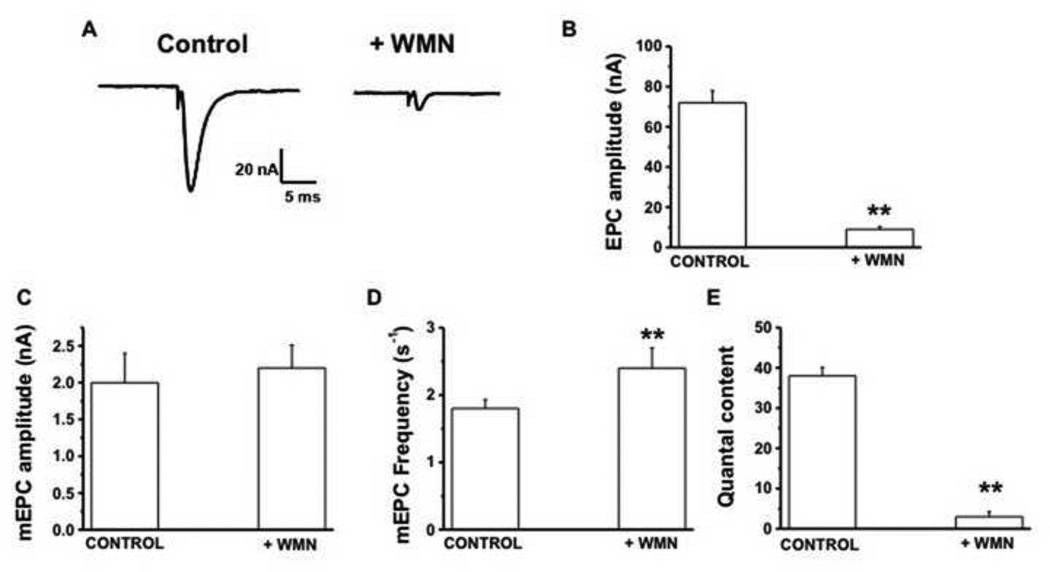
Membrane bound phosphatidyl inositol (4,5) bisphosphate (PIP2) is an important regulator of exo-endocytosis coupling at the synapses and is modulated by neuronal activity (Koch and Holt, 2012; Micheva et al., 2001). Furthermore, previous research demonstrates that CAP-induced Ca2+ influx via TRPV1 causes a decrease in membrane PIP2 via PLC dependent mechanisms (Lukacs et al., 2007; Rohacs et al., 2008; Rosenbaum and Simon, 2007). Next, we tested if wortmannin (WMN), a PI-3 and PI-4 Kinase inhibitor (Hong and Chang, 1999; Winks et al., 2005), which decreases PIP2 synthesis (Halet et al., 2002), will inhibit EPCs and mEPCs at the mouse MNT. The logic for the use of WMN was to examine a TRPV1-independent mechanism, which would decrease PIP2 levels in the nerve terminal by blocking its synthesis. WMN suppresses PIP2 by inhibiting PI Kinases (Halet et al., 2002; Winks et al., 2005). It is known that clathrin coated pit (CCP) mediated endocytosis requires PIP2 (Nakano-Kobayashi et al., 2007) and WMN sensitive PI kinases are involved in clathrin-dependent endocytosis (Haucke, 2005; Nakano-Kobayashi et al., 2007; Wenk et al., 2001). Since, a tight balance between synaptic vesicle exocytosis and endocytosis is important for maintaining synaptic structure and function, inhibiting either of the component will affect this balance and suppress the other. That is, inhibiting endocytic process will have an inhibiting effect on exocytosis. Previous research work demonstrates that inhibition of PI3 kinase slows down endocytosis (Richards et al., 2004). Further, WMN treatment has been shown to inhibit clathrin-dependent endocytosis of neurotoxin by delocalizing endocytic proteins (Potian et al., 2010). If the endocytic mechanisms couple to exocytosis, then WMN treatment should suppress exocytosis. These background literatures provide a rationale for testing the effects of WMN (20 µM) on isolated NMPs. If WMN decreases membrane PIP2 dependent exocytosis, then inhibiting the synthesis of PIP2 by WMN will suppress stimulus evoked acetylcholine release. Consistent to this notion, WMN treatment for 120 min. significantly suppressed the EPCs (Fig. 7A). Also, washing of NMPs even up to 240 min. did not restore the suppressive effect of WMN. The mean EPC amplitude recorded from several NMP showed a significant decrease from the controls (71 ± 6 in control versus 9 ± 1.1 with WMN; P<0.01; Fig. 7B). The quantal content decreased from 38 ± 2 in controls to 4 ± 0.9 with WMN (Fig. 7E). However, WMN did not alter mEPC amplitude while slightly increased the mEPC the frequency (P< 0.05; Fig. 7 C and D).
Figure 7. Wortmannin (WMN; 20 µM) inhibits stimulus evoked neurotransmitter release.
A. Representative traces of 1 Hz stimulus evoked end plate currents (EPC) of control and WMN treated nerve muscle preparations (n = 4–6 muscles and 16–22 neuromuscular junctions) at the end of 120 min. after the application of either vehicle alone or WMN (120 min. treatment). B. EPC amplitudes ± S.E.M of control and WMN treated NMP (n = 3–5, 12–26). C, D and E. Represent the spontaneous miniature end plate current (mEPC) amplitude, frequency, and quantal content of control and WMN treated preparations. Numbers in parentheses represent the numbers of nerve muscle preparations and fibers respectively. ** represents statistical significance for P<0.001.
3.7. WMN inhibits EPCs in TRPV1−/− mouse MNT
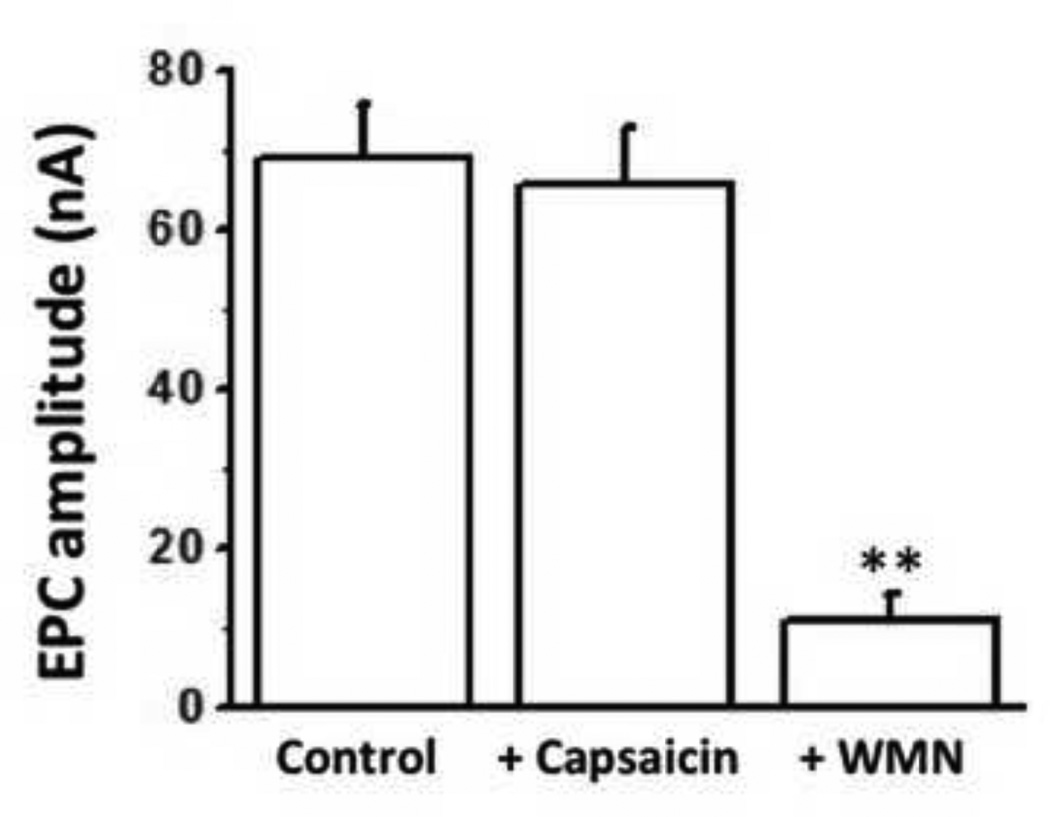
We tested WMN on the EPCs recorded from diaphragm MNTs in TRPV1−/− mice. As seen in Fig. 8, CAP did not change EPC amplitudes whereas a 120 min. exposure of diaphragm NMP to 20 µM WMN inhibited the EPC amplitude significantly (P<0.01) indicating that the effects of WMN were independent of TRPV1.
Figure 8. WMN but not CAP inhibits stimulus evoked neurotransmitter release in TRPV1−/− NMP.
A. Mean amplitudes (nA) in response to 1 Hz stimulus evoked end plate currents (EPC) of control, CAP (1 µM) or WMN (20 µM) treated NMP (n = 4–6 muscles and 14–20 neuromuscular junction, from 6 mice) at the end of 120 min. after the application of either vehicle alone (first bar) or CAP or WMN (second and third bars respectively). ** represents statistical significance for P<0.001.
4.0. Discussion
The molecular machinery underlying nerve stimulus evoked and spontaneous acetylcholine release at the mammalian MNT has been intensely studied in recent years. Although the role of voltage gated Ca2+ channels and intracellular Ca2+ sensors that regulate the fusion and release of acetylcholine containing vesicles at the presynaptic nerve terminal have been recognized for number of years, the precise role of Ca2+-dependent mechanism/s involved in the exo-endocytic processes at the myoneural junction is still under investigation. Also, our understanding of how endocytosis at the motor nerve endings couples to acetylcholine exocytosis still remains unclear.
Nonselective cation channels, including TRP proteins are expressed in the motor nerve endings (Silveira et al., 2010; Thyagarajan et al., 2009). Understanding the role of TRPV1 in MNT may provide new understanding of the relation between exo-endocytotic processes. In the present study, we have used CAP, in vitro, as a tool to elucidate the molecular events involved in endocytosis and its connection to the exocytosis of acetylcholine release. Using immunoblotting technique, we have shown that the CAP receptor, TRPV1 is expressed at the mouse MNT (Fig. 1). However, the precise role of TRPV1 in the endoexocytic processes at the neuromuscular junction has not been studied until now. Recently, we have shown that CAP pretreatment prevented botulinum neurotoxin A (BoNT/A) uptake at the MNT. and accelerated recovery of the muscles from the toxin-induced paralysis (Thyagarajan et al., 2009). The interference of CAP in BoNT/A uptake suggests that CAP affects endocytic mechanisms. Our data demonstrate that activation of TRPV1 by CAP leads to suppression of acetylcholine release at the MNT.
CAP produced a concentration-dependent decline of twitch tension in response to the phrenic nerve stimulation (Fig. 2). The effect of CAP was indeed due to activation of TRPV1 channels because CPZ (a TRPV1 antagonist) was able to antagonize CAP - induced depression of twitch response. Furthermore, results from intracellular recordings of EPCs and mEPCs from MNTs revealed that 1 µM CAP inhibited EPCs (reduced amplitude and quantal content) within 120 min and the inhibitory effect was antagonized by CPZ. Washing CAP did not reverse its suppressing effect on acetylcholine release. However, CAP did not alter the amplitude or frequency of mEPCs in MNTs of diaphragms from WT mice (Fig. 4). Further, CAP did not depress EPCs recorded from MNTs of TRPV1−/− mouse diaphragms (Fig. 4 and 5). Taken together, these findings demonstrate that at the mouse MNT, in vitro application of CAP preferentially causes inhibition of neuromuscular transmission by decreasing the quantal content without any change in the spontaneous (mEPC) release of acetylcholine vesicles.
Previously, we have shown that a local injection of CAP near the innervations of mouse extensor digitorum longus (EDL) muscle (3 µl of 1 mM stock solution) did not cause any overt neuromuscular changes in the EDL muscle 24 hr. after injection. The measurements included compound muscle action potential, twitch tension, and EPC amplitude (Thyagarajan et al., 2009). The same investigation also showed that preinjection of CAP significantly protected mouse EDL from the neuroparalytic effects of BoNT/A. This was a seminal finding that also indicated that CAP prevented the endocytosis of the neurotoxin into MNT. The lack of inhibitory effects of CAP injection, near the EDL region in vivo, on the neuromuscular functions, is suggestive of a disconnection between the in vitro and in vivo effects of CAP.
Experiments performed with Neuro 2a cells that stably express TRPV1 demonstrate that CAP caused delocalization of adapter protein-2 µ1 (AP2-µ1). We used cholinergic Neuro 2a cells for these experiments since the spatial and tempora resolution of MNT is not readily amenable to immunohistochemical manipulations to analyze the subcellular disposition of AP2 -µ1. As shown in Fig. 6, CAP treatment significantly perturbed the subcellular distribution of AP2-µ1 in TRPV1 expressing Neuro cells. Furthermore, in wild type Neuro 2a cells (that lack TRPV1), CAP treatment did not alter the cellular localization of AP2 -µ1 (Fig. 6, right panel top and bottom). These data suggest that CAP treatment interferes with an endocytic process, in vitro, which is dependent on TRPV1 channel protein. Connecting our observations on the suppressive effects of CAP on acetylcholine in wild type NMP, delocalizing effects of CAP on AP2-µ1 in TRPV1 expressing Neuro 2a cells and the lack of effects of CAP on TRPV1−/− NMP and wild type Neuro 2a cells, it is reasonable to hypothesize that CAP downregulates acetylcholine release by disrupting endocytosis-dependent exocytosis mechanism.
One of the important regulator of endocytosis is membrane bound PIP2. Increasing evidence indicates that membrane bound PIP2 is an important regulator of endocytic mechanisms. PIP2 is concentrated at the synaptic membrane and has been shown to interact with the components of the endocytotic machinery during clathrin coated pit-mediated endocytosis (Haucke, 2005). Also, PIP2 depletion has been shown to affect clathin coat formation (Di Paolo et al., 2002; Zoncu et al., 2007). Moreover, disruption of PI3Kinase activity inhibits clathrin-dependent as well as -independent endocytic processes (Anas et al., 2009; Gommerman et al., 1997; Sato et al., 1996). These data demonstrate that interfering with phosphinositides synthesis have profound regulatory effects on exo-endocytic mechanisms and that at the synapses, PIP2 plays a pivotal role in exo-endocytosis coupling (Koch and Holt, 2012).
At the presynaptic nerve terminals, Ca2+ channels have also been shown to couple exocytosis to endocytosis (Xue et al., 2012). Though such a coupling was shown previously through voltage- gated Ca2+ channels, the role of TRPV1 in this process cannot be ignored. If Ca2+ influx via TRPV1 can alter membrane PIP2 levels, then it would also affect PIP2-dependent endo-exocytic coupling. Consistent to this, previous study demonstrate that CAP -stimulated Ca2+ influx via TRPV1 channels caused hydrolysis of PIP2 to form inositol trisphosphate (IP3) and diacylglycerol (DAG) via catalytic action of phospholipase C [PLC; (Lukacs et al., 2007; Prescott and Julius, 2003). Therefore, CAP treatment can cause similar effects at the MNT to decrease membrane PIP2 level, which will affect endocytosis and exocytosis. Our data presented in Fig. 2, 3, 4, 5, and 6 support this notion. The delocalizing effect of CAP on AP2-µ1 in TRPV1 expressing Neuro 2a cells (Fig. 6) reinforces our hypothesis that endocytic process was indeed perturbed by CAP due to its ability to decrease membrane PIP2 levels via TRPV1-PLC dependent mechanisms. Our observation that CAP did not suppress acetylcholine release immediately following its application but had a time lag of about 120 min. is suggestive of a signaling mechanism that precedes the inhibition of acetylcholine release. One possibility is that the increase of presynaptic Ca2+ caused by activation of TRPV1 by CAP could stimulate PLC-dependent PIP2 hydrolysis. This could interfere with the subcellular disposition of AP2 -µ1, which in turn reduces endocytosis and thereby suppresses endocytosis-dependent exocytosis of acetylcholine.
Although we did not perform direct measurements of PIP-dependent vesicle recycling, we evaluated the effects of wortmannin (WMN), which cause a decrease in membrane PIP2 levels by a TRPV1-independent (PI3K dependent; WMN) mechanism. WMN suppresses PIP2 by inhibiting PI Kinase enzyme activity. WMN treatment caused delocalization of clathrin heavy chain ((Potian et al., 2010) and suppressed the EPC and quantal content of diaphragm NMP (Fig. 7). These data support our hypothesis that perturbation of membrane bound PIP2 levels affects endocytosis as well as exocytosis.
The results of this study raise the following question: Does AP2-µ1-dependent endocytosis couple to acetylcholine exocytosis at the MNT? Answering this question would provide an insight into exo-endocytic coupling at the NMJ. Future research work should address this directly and evaluate the role of TRPV1 channel proteins in PIP2-dependent endo-exocytic coupling at the myoneural junction.
Our study suggests that TRPV1 channels are coupled to acetylcholine release. However, the mechanism whereby TRPV1 regulates exocytosis via Ca2+-dependent mechanisms still remains to be investigated. Our observation that CAP fails to suppress EPC in NMP of TRPV1−/− animals provide compelling evidence for the role of TRPV1 in exocytosis. The inhibitory effects of WMN on EPC of TRPV1−/− NMP illustrate the effects of WMN that are independent of TRPV1. Nonetheless, these data suggest an equivocal role of PIP2 in exocytosis. That is, even in the absence of TRPV1, WMN can inhibit membrane PIP2 levels and inhibit EPC.
5.0. Conclusions
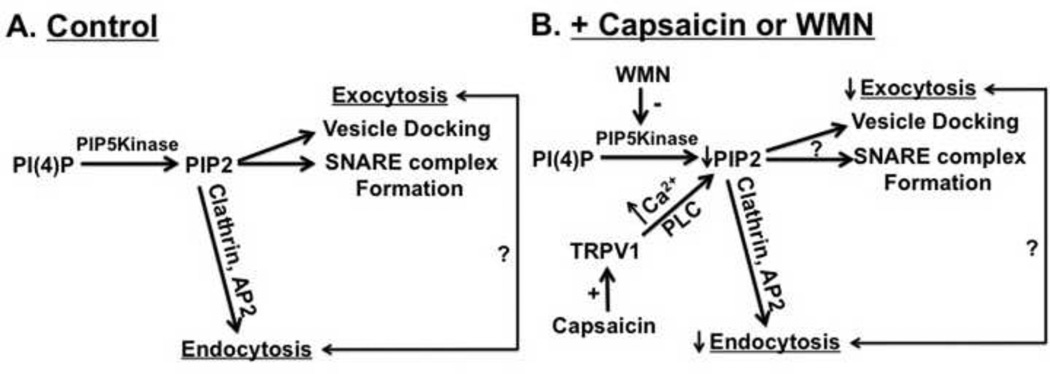
Collectively, our data demonstrate a regulatory role of TRPV1 activation in vesicular acetylcholine release. CAP significantly decreased acetylcholine release in the wild type but not in TRPV1−/− NMP. Further, CAP treatment interfered with the subcellular disposition of endocytic adapter protein. We hypothesize that the reduction of endocytosis and exocytosis occurred via intracellular Ca2+ dependent mechanisms. Moreover, WMN inhibited acetylcholine release and mimicked the effects of CAP. These data support our hypothesis that membrane PIP2 is a determinant of both endocytosis and exocytosis and that perturbation to PIP2 affects the exo-endocytic processes in vitro. Fig. 9 illustrates the possible mechanism involved in the delocalizing effect of CAP or WMN on endocytic protein machinery coupled to exocytosis. CAP may affect exo-endocytic processes by decreasing membrane PIP2 levels. The primary findings that we present in this study warrants future research to specifically address the regulatory role of TRPV1 in the mechanisms by which endocytosis couples to exocytosis at the synapses.
Figure 9. Model describing the effect of CAP or WMN on PIP2 dependent exo-endocytic mechanism.
PIP2 regulates exocytosis by facilitating SNARE complex formation and synaptic vesicle docking, fusion and release. PIP2 also participates in the formation and nucleation of endocytic machinery. The mechanism of how PIP2 regulation of endocytosis couples to exocytosis still remains unclear. CAP activates TRPV1 and causes decrease in PIP2 via Ca2+-PLC dependent mechanisms. WMN inhibits PI Kinase and decreases PIP2 levels. The reduction in PIP2 level down-regulates endocytosis as well as exocytosis. + sign denotes activation. ? Denotes unknown mechanism by which endocytosis and exocytosis couple with each other. Upward and downward arrows represent increase and decrease respectively.
Acknowledgment
This work was supported by the pilot project support from National Institute of General Medical Sciences of the National Institutes of Health under Award Number 8P20GM103432-12 and American Association of Colleges of Pharmacy New Investigator Award to BT and Kirby Foundation Award to JJM.
Abbreviations
- AP-2
adapter protein 2
- TRP
transient receptor potential
- TRPV1
transient receptor potential vanilloid 1
- TRPV1−/−
TRPV1 knockout
- MNT
motor nerve terminal
- BoNT/A
botulinum neurotoxin type A
- HRS
HEPES Ringer Solution
- mEPC
miniature endplate currents
- EPC
endplate current
- CAP
capsaicin
- CPZ
capsazepine
- EDL
extensor digitorum longus
- NMP
nerve muscle preparation
- WMN
wortmannin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interests
None.
References
- Anas A, Okuda T, Kawashima N, Nakayama K, Itoh T, Ishikawa M, Biju V. Clathrin-mediated endocytosis of quantum dot-peptide conjugates in living cells. ACS Nano. 2009;3:2419–2429. doi: 10.1021/nn900663r. [DOI] [PubMed] [Google Scholar]
- Bevan S, Hothi S, Hughes G, James IF, Rang HP, Shah K, Walpole CS, Yeats JC. Capsazepine: a competitive antagonist of the sensory neurone excitant CAP. Br J Pharmacol. 1992;107:544–552. doi: 10.1111/j.1476-5381.1992.tb12781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caterina MJ, Julius D. The vanilloid receptor: a molecular gateway to the pain pathway. Annu Rev Neurosci. 2001;24:487–517. doi: 10.1146/annurev.neuro.24.1.487. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The CAP receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Cui JG, Zhang X, Zhao YH, Chen C, Bazan N. Allodynia and hyperalgesia suppression by a novel analgesic in experimental neuropathic pain. Biochem Biophys Res Commun. 2006;350:358–363. doi: 10.1016/j.bbrc.2006.09.055. [DOI] [PubMed] [Google Scholar]
- Di Paolo G, Pellegrini L, Letinic K, Cestra G, Zoncu R, Voronov S, Chang S, Guo J, Wenk MR, De Camilli P. Recruitment and regulation of phosphatidylinositol phosphate kinase type 1 gamma by the FERM domain of talin. Nature. 2002;420:85–89. doi: 10.1038/nature01147. [DOI] [PubMed] [Google Scholar]
- Gautron J. Ultrastructural localization of acetylcholinesterase. A direct method for light and electron microscopy. Histochemistry. 1982;76:469–478. doi: 10.1007/BF00489902. [DOI] [PubMed] [Google Scholar]
- Gommerman JL, Rottapel R, Berger SA. Phosphatidylinositol 3-kinase and Ca2+ influx dependence for ligand-stimulated internalization of the c-Kit receptor. J Biol Chem. 1997;272:30519–30525. doi: 10.1074/jbc.272.48.30519. [DOI] [PubMed] [Google Scholar]
- Halet G, Tunwell R, Balla T, Swann K, Carroll J. The dynamics of plasma membrane PtdIns(4,5)P(2) at fertilization of mouse eggs. J Cell Sci. 2002;115:2139–2149. doi: 10.1242/jcs.115.10.2139. [DOI] [PubMed] [Google Scholar]
- Haucke V. Phosphoinositide regulation of clathrin-mediated endocytosis. Biochem Soc Trans. 2005;33:1285–1289. doi: 10.1042/BST0331285. [DOI] [PubMed] [Google Scholar]
- Holzer P. CAP: cellular targets, mechanisms of action, and selectivity for thin sensory neurons. Pharmacol Rev. 1991;43:143–201. [PubMed] [Google Scholar]
- Hong SJ, Chang CC. Inhibition of quantal release from motor nerve by wortmannin. Br J Pharmacol. 1999;128:142–148. doi: 10.1038/sj.bjp.0702754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman MP, Iwamoto GA, Longhurst JC, Mitchell JH. Effects of CAP and bradykinin on afferent fibers with ending in skeletal muscle. Circ Res. 1982;50:133–139. doi: 10.1161/01.res.50.1.133. [DOI] [PubMed] [Google Scholar]
- Koch M, Holt M. Coupling exo- and endocytosis: an essential role for PIP(2) at the synapse. Biochim Biophys Acta. 2012;1821:1114–1132. doi: 10.1016/j.bbalip.2012.02.008. [DOI] [PubMed] [Google Scholar]
- Koelle GB, Horn RS. Acetyl disulfide, (CH3COS)2, a major active component in the thiolacetic acid histochemical method for acetylcholinesterase. J Histochem Cytochem. 1968;16:743–753. doi: 10.1177/16.12.743. [DOI] [PubMed] [Google Scholar]
- Lukacs V, Thyagarajan B, Varnai P, Balla A, Balla T, Rohacs T. Dual regulation of TRPV1 by phosphoinositides. J Neurosci. 2007;27:7070–7080. doi: 10.1523/JNEUROSCI.1866-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh SJ, Stansfeld CE, Brown DA, Davey R, McCarthy D. The mechanism of action of CAP on sensory C-type neurons and their axons in vitro. Neuroscience. 1987;23:275–289. doi: 10.1016/0306-4522(87)90289-2. [DOI] [PubMed] [Google Scholar]
- Micheva KD, Holz RW, Smith SJ. Regulation of presynaptic phosphatidylinositol 4,5-biphosphate by neuronal activity. J Cell Biol. 2001;154:355–368. doi: 10.1083/jcb.200102098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano-Kobayashi A, Yamazaki M, Unoki T, Hongu T, Murata C, Taguchi R, Katada T, Frohman MA, Yokozeki T, Kanaho Y. Role of activation of PIP5Kgamma661 by AP-2 complex in synaptic vesicle endocytosis. Embo J. 2007;26:1105–1116. doi: 10.1038/sj.emboj.7601573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potian JG, Patel V, McArdle JJ, Thyagarajan B. The inveterate botulinum neurotoxin A ushers in exo-endocytic crypts. The Botulinum J. 2010;1:418–430. [Google Scholar]
- Prescott ED, Julius D. A modular PIP2 binding site as a determinant of CAP receptor sensitivity. Science. 2003;300:1284–1288. doi: 10.1126/science.1083646. [DOI] [PubMed] [Google Scholar]
- Richards DA, Rizzoli SO, Betz WJ. Effects of wortmannin and latrunculin A on slow endocytosis at the frog neuromuscular junction. J Physiol. 2004;557:77–91. doi: 10.1113/jphysiol.2004.062158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohacs T, Thyagarajan B, Lukacs V. Phospholipase C mediated modulation of TRPV1 channels. Mol Neurobiol. 2008;37:153–163. doi: 10.1007/s12035-008-8027-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum T, Simon SA. TRPV1 Receptors and Signal Transduction. In: Liedtke WB, Heller S, editors. TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades,capsaicin. Boca Raton (FL): 2007. [Google Scholar]
- Sato SB, Taguchi T, Yamashina S, Toyama S. Wortmannin and Li+ specifically inhibit clathrin-independent endocytic internalization of bulk fluid. J Biochem. 1996;119:887–897. doi: 10.1093/oxfordjournals.jbchem.a021326. [DOI] [PubMed] [Google Scholar]
- Silveira PE, Silveira NA, Morini Vde C, Kushmerick C, Naves LA. Opposing effects of cannabinoids and vanilloids on evoked quantal release at the frog neuromuscular junction. Neurosci Lett. 2010;473:97–101. doi: 10.1016/j.neulet.2010.02.026. [DOI] [PubMed] [Google Scholar]
- Szallasi A, Blumberg PM. Characterization of vanilloid receptors in the dorsal horn of pig spinal cord. Brain Res. 1991;547:335–338. doi: 10.1016/0006-8993(91)90982-2. [DOI] [PubMed] [Google Scholar]
- Thyagarajan B, Krivitskaya N, Potian JG, Hognason K, Garcia CC, McArdle JJ. CAP protects mouse neuromuscular junctions from the neuroparalytic effects of botulinum neurotoxin a. J Pharmacol Exp Ther. 2009;331:361–371. doi: 10.1124/jpet.109.156901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thyagarajan B, Potian JG, Garcia CC, Hognason K, Capkova K, Moe ST, Jacobson AR, Janda KD, McArdle JJ. Effects of hydroxamate metalloendoprotease inhibitors on botulinum neurotoxin A poisoned mouse neuromuscular junctions. Neuropharmacology. 2010;58:1189–1198. doi: 10.1016/j.neuropharm.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth A, Boczan J, Kedei N, Lizanecz E, Bagi Z, Papp Z, Edes I, Csiba L, Blumberg PM. Expression and distribution of vanilloid receptor 1 (TRPV1) in the adult rat brain. Brain Res Mol Brain Res. 2005;135:162–168. doi: 10.1016/j.molbrainres.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Wenk MR, Pellegrini L, Klenchin VA, Di Paolo G, Chang S, Daniell L, Arioka M, Martin TF, De Camilli P. PIP kinase Igamma is the major PI(4,5)P(2) synthesizing enzyme at the synapse. Neuron. 2001;32:79–88. doi: 10.1016/s0896-6273(01)00456-1. [DOI] [PubMed] [Google Scholar]
- Winks JS, Hughes S, Filippov AK, Tatulian L, Abogadie FC, Brown DA, Marsh SJ. Relationship between membrane phosphatidylinositol-4,5-bisphosphate and receptor-mediated inhibition of native neuronal M channels. J Neurosci. 2005;25:3400–3413. doi: 10.1523/JNEUROSCI.3231-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue L, Zhang Z, McNeil BD, Luo F, Wu XS, Sheng J, Shin W, Wu LG. Voltage-dependent calcium channels at the plasma membrane, but not vesicular channels, couple exocytosis to endocytosis. Cell Rep. 2012;1:632–638. doi: 10.1016/j.celrep.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoncu R, Perera RM, Sebastian R, Nakatsu F, Chen H, Balla T, Ayala G, Toomre D, De Camilli PV. Loss of endocytic clathrin-coated pits upon acute depletion of phosphatidylinositol 4,5-bisphosphate. Proc Natl Acad Sci U S A. 2007;104:3793–3798. doi: 10.1073/pnas.0611733104. [DOI] [PMC free article] [PubMed] [Google Scholar]