Abstract
The zebrafish pronephros provides an excellent in vivo system to study the mechanisms of vertebrate nephron development. When and how renal progenitors in the zebrafish embryo undergo tubulogenesis to form nephrons is poorly understood, but is known to involve a mesenchymal to epithelial transition (MET) and the acquisition of polarity. Here, we determined the precise timing of these events in pronephros tubulogenesis. As the ternary polarity complex is an essential regulator of epithelial cell polarity across tissues, we performed gene knockdown studies to assess the roles of the related factors atypical protein kinase C iota and zeta (prkcι, prkcζ). We found that prkcι and prkcζ serve partially redundant functions to establish pronephros tubule epithelium polarity. Further, the loss of prkcι or the combined knockdown of prkcι/ζ disrupted proximal tubule morphogenesis and podocyte migration due to cardiac defects that prevented normal fluid flow to the kidney. Surprisingly, tubule cells in prkcι/ζ morphants displayed ectopic expression of the transcription factor pax2a and the podocyte-associated genes wt1a, wt1b, and podxl, suggesting that prkcι/ζ are needed to maintain renal epithelial identity. Knockdown of genes essential for cardiac contractility and vascular flow to the kidney, such as tnnt2a, or elimination of pronephros fluid output through knockdown of the intraflagellar transport gene ift88, was not associated with ectopic pronephros gene expression, thus suggesting a unique role for prkcι/ζ in maintaining tubule epithelial identity separate from the consequence of disruptions to renal fluid flow. Interestingly, knockdown of pax2a, but not wt1a, was sufficient to rescue ectopic tubule gene expression in prkcι/ζ morphants. These data suggest a model in which the redundant activities of prkcι and prkcζ are essential to establish tubule epithelial polarity and also serve to maintain proper epithelial cell type identity in the tubule by inhibiting pax2a expression. These studies provide a valuable foundation for further analysis of MET during nephrogenesis, and have implications for understanding the pathways that affect nephron epithelial cells during kidney disease and regeneration.
Keywords: kidney, nephrogenesis, tubulogenesis, polarity, mesenchymal to epithelial transition, pronephros, vertebrate, atypical protein kinase C, pax2a
INTRODUCTION
Vertebrate kidney development is a complex process that involves the precisely regulated formation of nephrons—highly specialized epithelial tubes that are constructed from mesenchymal precursors derived from the intermediate mesoderm (IM) (Little, et al., 2010). Nephrons across species consist of conserved epithelial cell types arranged in a common pattern of segment domains, and each segment fulfills particular functions (Dressler, 2006; Reilly, et al., 2007; Wingert and Davidson, 2008). In general, nephrons contain a blood filter, followed by proximal and distal tubule segments that perform solute absorption and secretion to modify the filtrate, followed by collecting ducts that fine-tune salt and water levels. Nephron epithelial identity and functionality are vital for kidney health, and disruptions are associated with conditions like polycystic kidney disease and cancer (Hsu, et al., 2010; Wilson, 2011).
The zebrafish embryonic kidney, termed the pronephros, is a valuable model for studying the genetic and cellular mechanisms of nephrogenesis (Gerlach and Wingert, 2013; Cheng and Wingert, 2014). It is composed of two nephrons that have a comparable segment composition as other vertebrates, including mammals (Wingert, et al., 2007). Further, several recent studies have documented analogous patterns of gene expression and conserved gene functions during nephrogenesis events in zebrafish and mammals (Wingert and Davidson, 2011; O’Brien, et al., 2011; Naylor, et al., 2013; Li, et al., 2014). Zebrafish pronephric nephrons have a common blood filter comprised of podocytes, tubules organized into several proximal and distal segments, and finally have ducts that join with the cloaca (Wingert, et al., 2007). These nephrons originate from bilateral renal progenitor fields that emerge by the tailbud stage (Drummond, et al., 1998; Serluca and Fishman, 2001). During subsequent somitogenesis, the renal progenitors exhibit dynamic subdivisions that culminate with the refinement of segment boundaries and the emergence of discrete epithelial cell types by 24 hours post fertilization (hpf) (Wingert, et al., 2007; Wingert and Davidson, 2011), ultimately undergoing a mesenchymal to epithelial transition (MET) to form nephron epithelial tubules. Over the next day, the nephrons continue to mature based on the expression of additional solute transporter genes (Wingert, et al., 2007) and then begin blood filtration around 48 hpf (Drummond, et al., 1998). During subsequent larval stages, additional nephrons develop and connect to the existing pronephric kidney to begin forming a more elaborate renal structure, the mesonephros, which serves as the adult kidney in zebrafish (Gerlach and Wingert, 2013; McCampbell and Wingert, 2014). While there have been progressive advances in the molecular understanding of zebrafish nephron formation, many of the signaling events and processes remain poorly defined—including the precise timing and mechanisms of tubulogenesis in the pronephros (Fig. 1A).
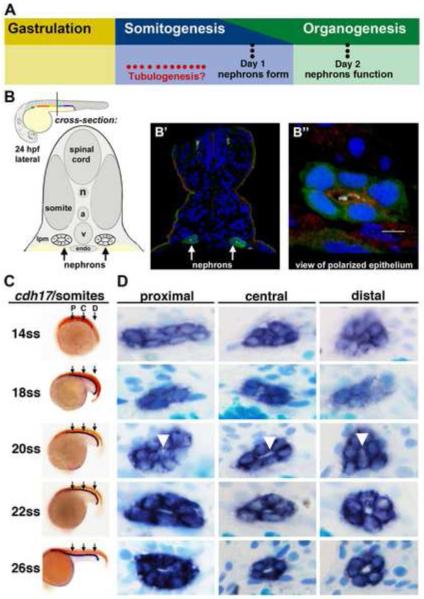
Figure 1. Renal progenitors exhibit a progression of rearrangements during nephrogenesis, and undergo tubulogenesis with lumen formation between the 20-22 ss time points.
(A) Although the timing of tubulogenesis during pronephros development has been unresolved, we hypothesized that it transpires sometime during somitogenesis, as prior studies have demonstrated that nephron patterning is completed by embryonic day 1, and that pronephros function initiates at approximately day 2. (B) The zebrafish embryonic kidney is comprised of two bilateral nephrons that have a lumen at 24 hpf. (B’) Cross section of a zebrafish embryo in which the nephron tubule epithelial cells were labeled by IF to detect GFP (green) in the transgenic strain Tg(cdh17:eGFP), along with acetylated α-tubulin (light blue), Prkcι/ζ (red), and nuclei marked with DAPI (dark blue). (B”) Digital zoom of a single nephron tubule, white bar indicates 5 um. (A-B”) Schematics and images adapted with author rights (Gerlach and Wingert, 2013). (C) Renal progenitors were labeled in the developing pronephros between the 14 and 26 ss by performing WISH on wild-type embryos for the renal marker cdh17 (purple) and a somite marker (red) to confirm embryo stage (the somite marker myod1 was used embryos <18 ss and smyhc1 for embryos >18 ss). Black arrows indicate approximate region where proximal (P), central (C), or distal (D) cross-sections were analyzed. (D) Serial sections of WISH labeled embryos were collected and analyzed at proximal, central, and distal regions of the renal progenitor field. At 14 ss, renal progenitors were found in clusters of approximately ~8 cells. Between 18-22 ss, renal progenitors were found in circumferential clusters of ~6 cells. At the 20 ss, a small lumen was discernible (white arrowheads), and a clear lumen was subsequently visible in all regions of the pronephros at 22 ss that enlarged by 26 ss.
Tubulogenesis is the process of forming a lumen, or a tubule with a hollow center, and is closely intertwined with MET. Epithelial tubes are essential for the structure and function of many organs, which has prompted numerous studies to delineate the mechanisms of tubulogenesis (Datta, et al., 2011). Much of what is currently known about kidney tubule formation has come from in vitro renal culture models that may not fully recapitulate in vivo events (Schlüter and Margolis, 2009; Roignot, et al., 2013). For example, recent studies of mouse kidney development have provided several new insights into the mechanisms of renal tubulogenesis, including how nephrons attach to a collecting duct (Kao, et al., 2012) and how pretubular aggregates undergo a MET to form a nephron tubule (Yang, et al., 2013).
One conserved phenomenon that occurs during MET and tubulogenesis is the acquisition of cell polarity (Schlüter and Margolis, 2012). Polarity is established by complexes of proteins that segregate the cellular membrane into apical and basolateral domains (Pieczynski and Margolis, 2011). Several in vitro studies have shown that changes in protein distribution precede these domains such that apical components are trafficked to the future luminal surface—deliveries which make an apical membrane initiation site (AMIS) (Meder, et al., 2005; Schlüter, et al., 2009; Bryant, et al., 2010; Galvez-Santisteban, et al., 2012). Similar membrane changes have been noted in vivo during nephrogenesis in the developing mouse kidney (Yang, et al., 2013). Ultimately, the generation of a polarized epithelium creates functionally distinct cell surfaces, which allows for proper trafficking and facilitates cell adhesion. In nephron tubule epithelia, the apical membrane faces the lumen and is critical for solute exchange during urine formation. A number of polarity and cell junction complexes prevent the free flow of molecules between cells and help to establish ionic gradients. For example, the Na+/K+ ATPase protein is localized to basolateral sides of nephron epithelia and has roles in ion transport and tight junction assembly (Rajasekaran, et al., 2001; Krupinski, et al., 2009). The mislocalization of Na+/K+ ATPase has been observed in human renal disease and in zebrafish kidney mutants that develop edema (Wilson, 2011; Drummond, et al., 1998). Thus, proper nephron function requires that epithelial cell polarity is both established and stably maintained.
Located at the apical surface of epithelial cells, the ternary polarity complex consists of atypical protein kinase C (aPKC), Par-3, and Par-6 (Chen and Zhang, 2013). In different in vitro and in vivo settings, the abrogation of any single ternary polarity protein can disrupt lumen development and tissue functionality (Chen and Zhang, 2013). In mammals and zebrafish, two aPKC-related proteins, iota (ι) and zeta (ζ), are expressed during embryogenesis (Kovac, et al., 2007; Patten, et al., 2007). Gene targeting studies revealed an immunological defect in aPKCζ deficient mice (Leitges, et al., 2001), while knockout of aPKCι was embryonic lethal (Soloff, et al., 2004). Cloning of the zebrafish heart and soul (has) mutation revealed that it encoded an embryonic lethal recessive allele of the aPKCι gene, which is now known as prkcι (Horne-Badovinac, et al., 2001; Peterson, et al., 2001). Although has disrupts diverse epithelia, and together with the polarity factor nagio oko (nok)/mpp5a is required for cardiac morphogenesis, pronephros formation was normal (Yelon, et al., 1999; Peterson, et al., 2001; Horne-Badovinac, et al., 2001; Rohr, et al., 2006; Serluca, 2008). Subsequent research defined redundant roles for prkcι and the related aPKCζ gene, now known as prkcζ, during zebrafish retina development (Cui, et al., 2007). Interestingly, both aPKC isoforms are expressed in the developing mouse kidney (Kovac, et al., 2007), and podocyte-specific deletion of these aPKC isoforms revealed they have partially redundant functions in the proper distribution of proteins during cell maturation (Hirose, et al., 2009; Huber, et al., 2009; Hartleben, et al., 2013). However, the roles of these aPKC isoforms during mammalian nephron tubulogenesis in vivo have not yet been defined. Given the broad conservation of these genes, along with the similarities in nephron composition and ontogeny across vertebrates, the zebrafish pronephros provides a relevant developmental setting to further explore the functions of these factors.
In this study, we delineated the spatiotemporal sequence of tubulogenesis in the zebrafish pronephros. We determined the timing of lumen formation and found it to be preceded by the formation of an AMIS. Through loss of function analysis, we show that prkcι and prkcζ have partially redundant roles in establishing polarity when renal progenitors undergo MET. Interestingly, the pronephros in prkcι/ζ morphants eventually underwent lumen formation, but this was accompanied with deficient epithelial functions, such as abrogated renal clearance and proximal tubule endocytosis. prkcι/ζ morphants had dramatic defects in pronephros morphogenesis, with the proximal tubule failing to undergo normal convolutions and failure of normal podocyte migration—phenotypes linked to the loss of vascular flow to the pronephros which originates with cardiac defects. Surprisingly, prkcι/ζ deficiency led to a high incidence of ectopic expression of several renal factors in the pronephros which was rescued by knockdown of pax2a, indicating that prkcι/ζ maintain nephron epithelial identity by regulating pax2a expression. Disruptions in other genes that are essential for cardiac contractility (tnnt2a), or fluid propulsion in the nephron (ift88), was not associated with ectopic misexpression in the pronephros, highlighting the role for prkcι/ζ in tubule epithelial maintenance distinct from the impact of fluid flow. Incidentally, knockdown of the polarity gene mpp5a was associated with a low incidence of ectopic renal gene expression, suggesting possible partial redundancy with prkcι/ζ. Taken together, these studies further establish the zebrafish pronephros as a relevant nephrogenesis model and provide an important foundation for future in vivo tubulogenesis research.
MATERIALS AND METHODS
Zebrafish maintenance and ethics statement
Zebrafish were maintained in the Center for Zebrafish Research at the University of Notre Dame Freimann Life Science Center. All studies were performed with approval of the University of Notre Dame Institutional Animal Care and Use Committee (IACUC), Protocol numbers 13-021 and 16-025, and under the supervision of our Veterinarian Dr. Mark Suckrow. Wild-type Tübingen strain and Tg:cdh17-eGFP were raised and staged as described (Westerfield, 1993; Kimmel, et al., 1995). Embryos were incubated in E3 medium (5mM NaCl, 0.17mM KCl, 0.33 mM CaCl2, 0.33 mM MgSO4) at 28°C (Westerfield, 1993).
Whole mount in situ hybridization (WISH)
WISH was performed as described (Wingert, et al., 2007; Lengerke, et al., 2011; Cheng, et al., 2014). Antisense riboprobes were generated to detect transcripts for the following genes with accession numbers: cdh17 (AF428098), podxl (AI641045), myod1 (AF318503), nephrin (NM_001040687), odf3b (formerly annotated shippo) (NM_199958), podocin (NM_001018145), smyhc1 (formerly annotated myosin heavy chain (MHC)) (NM_001020507), pax2a (NM_131184), slc20a1a (NM_213179) wt1a (NM_131046), and wt1b (NM_001039634), as described previously (Kramer-Zucker, et al., 2005; Liu, et al. 2006; Wingert, et al., 2007; O’Brien, et al., 2011). DNA Templates were generated by polymerase chain reaction (PCR), PCR products purified (Qiagen PCR purification kit) and riboprobes generated with t3 RNA polymerase to detect the following gene transcripts with accession numbers as follows: prkcι (BC047164) IMAGE clone 3819020, forward primer (5’-3’) ATGCCCACGCTGCGGGACAGC and reverse primer (5’-3’) AATTAACCCTCACTAAAGGGGCCTGCTCAAAC, and prkcζ (BC163349) IMAGE clone 9038386, forward primer (5’-3’) AATTAACCCTCACTAAAGGGCATGAGATCTCC and reverse primer (5’-3’) AATTAACCCTCACTAAAGGGCATGAGATCTCC. Antisense RNA probes were detected by alkaline phosphatase conjugated anti-digoxigenin and anti-fluorescein antibody (Roche) and NBT/BCIP/INT reagents (Sigma) (Galloway, et al., 2008). Images provided were representative based on at least 10 animals, though typically >15, from at least 3 batches of embryos for each WISH data point, which were found to be consistent in wild-types and prkcι/ζ knockdowns.
Histology
Following WISH to label cdh17 transcripts, zebrafish embryos were fixed in 4% paraformaldehyde in phosphate buffered saline (PBS) overnight at 4°C, dehydrated in increasing concentrations of ethanol, and embedded into JB-4 plasticizing resin (Polysciences, Inc.). Five-micrometer thick sections were cut using glass knives on a Sorvall JB-4 microtome sectioner (Sorvall Inc.). Sections were counterstained with methylene blue (Sigma-Aldrich) and mounted in Polymount (Polysciences, Inc.). Images provided were representative based on at least 10 animals from at least 2 batches of embryos that were serially sectioned for each wild-type stage described.
Immunofluorescence (IF)
Zebrafish embryos were dechorionated and fixed in 4% paraformaldehyde in PBS at 4°C overnight. Adult kidneys were obtained as described and fixed in 4% paraformaldehyde/0.1% dimethylsulfoxide in PBS at 4°C overnight (Gerlach, et al., 2011; McCampbell, et al., 2014). Tissue samples were infiltrated with 5% and 30% sucrose solution and then subjected to a 1:1 solution of 30% sucrose and tissue freezing medium (TFM). Infiltrated samples were embedded in 100% TFM and oriented in Tissue-Tek cryomolds and frozen at −80°C. Sections (20 μm) were taken on a Microm HM 550 Cryostat (Thermo), and rehydrated in PBS. Rehydrated tissue was blocked for 2 hours at room temperature in a humidity chamber. Blocking buffer was composed of 0.05% Tween-20 in PBS with 2% DMSO and 3% fetal calf serum. Primary antibodies were diluted in blocking buffer and applied to the sections in a humidity chamber at 4°C overnight. Sections were then washed 3x in PBS with 0.05% Tween-20. Fluorescent-conjugated secondary antibodies were applied at 1:500 concentration and incubated at room temperature in humidity chambers for 2 hours in PBS with 0.05% Tween-20. Sections were then washed 3x in PBS with 0.05% Tween-20, mounted in Antifade (Vector Laboratories) and sealed with nail polish. Primary antibodies used were: Rabbit anti-aPKC (1:500) (SC-216 Santa Cruz), Sheep anti-GFP (1:500) (Osenses), monoclonal α6F Na+/K+ ATPase (1:35) (Developmental Studies Hybridoma Bank), phosphorylated ezrin(T567) radixin(T564) moesin(T558) (p-ERM) (1:50) (Cell Signaling Technology), and Rabbit anti-Laminin (1:200) (Sigma-Aldrich). Secondary antibodies used were: goat anti-rabbit conjugated to Alexa-Fluor568 (Invitrogen), goat anti-mouse conjugated to Alexa-Fluor 594, (Invitrogen) and donkey anti-sheep conjugated to Alexa-Fluor 488 (Invitrogen). 4,6-diamidino-2-phenylindole, dihydrochloride (DAPI) (Invitrogen) was used to counter stain cell nuclei and actin was labeled with Phalloidin-Rhodamine (1:500). The Na+/K+ ATPase and p-ERM antibodies were applied to embryos fixed in Dent’s solution (80% methanol, 20% DMSO) overnight at 4°C and processed as described above. Images provided were representative based on samples from at least 10 individuals, though typically >15 were examined, from at least 3 batches of embryos, and protein localizations and levels were found to be consistent in wild-types and prkcι/ζ knockdowns.
Morpholinos
Morpholino oligonucleotides (MO) were synthesized (Gene Tools, LLC) and handled according to the manufacturer instructions. MOs were resuspended with distilled water to create 4 mM stocks and stored at −20°C. MO sequences were selected from previous reports for prkcι, prkcζ, ift88, tnnt2a, mpp5a, and wt1a; mismatch MO controls were designed for prkcι and prkcζ (Table S1). For pax2a knockdown studies, a MO was designed to abrogate splicing, and two previously published MOs were also used (Table S1). prkcι/ζ knockdown was further verified by immunofluorescence with anti-aPKC. Please refer to Table S1 for all MO sequences, injection dosages, and references. Triplicates of all knockdown studies (prkci/z, prkci/z + pax2a, mpp5a, ift88, tnnt2a, ift88 + tnnt2a) were analyzed at 72 hpf for expression of one or more renal markers (wt1a, podxl, pax2a). Each replicate experimental group consisted of at least 75 embryos. For each experiment, the embryo cohorts were subjected to WISH, phenotypes counted, and an average incidence of embryos of each phenotype in the grading series (e.g. normal expression (wild-type (WT)), high, low, neck) was calculated for each replicate. For statistical analysis of these categories within each genotype, a standard error was calculated for the triplicates, and then a pair-wise two-tailed Student t-test was performed to compare this value to the appropriate wild-type control.
Cell death analysis
Cell death was assessed using the vital dye acridinium chloride hemi-(zinc chloride) (acridine orange). Morphant and control embryos were incubated in 0.003% N-phenylthiourea/E3 after 24 hpf, then placed in 5 mg/ml acridine orange/E3 for 45 minutes at the desired time point. Embryos were then rinsed in E3 and imaged in 2% methylcellulose/0.02% tricaine.
Dextran clearance assays
Embryos were injected with 40 kDa dextran-FITC (Invitrogen) at 2.5 mg /mL at 48 hpf then assessed for PCT uptake and dextran clearance at subsequent stages (Li, et al., 2014).
Image acquisition
IF images were acquired as z-stacks using a Nikon A1 confocal microscope. WISH images were acquired using a Nikon eclipse Ni with a DS-Fi2 camera. Images were processed using Adobe Photoshop CS5.
RESULTS
Timing of lumen formation by renal progenitors during pronephros development
Studies of zebrafish pronephros ontogeny have demonstrated that segment domains are established by the 28 somite stage (ss) (Wingert, et al., 2007; Wingert and Davidson, 2011; Li, et al., 2014) and that the nephrons have tubular lumens comprised of polarized epithelial cells by approximately this point in development (Fig. 1A, Fig. 1B) (Drummond, et al., 1998; Gerlach and Wingert, 2013). However, the exact timing of tubulogenesis during pronephros formation has been unknown (Fig. 1A). To identify precisely when lumen formation occurs and better understand the cellular rearrangements that occur as the pronephros develops, we characterized renal progenitors over a developmental timecourse with histology. Renal progenitors were labeled by whole mount in situ hybridization (WISH) in wild-type embryos of various ages using a riboprobe to detect cadherin 17 (cdh17) transcripts, which are expressed throughout the pronephros tubule segments and duct (Horsfield, et al., 2002; Wingert, et al., 2007), and then serial cross sections were analyzed along the renal progenitor field at proximal, central, and distal locations (Fig. 1C).
Using this strategy, we observed that renal progenitors underwent rearrangements and subsequent tubulogenesis during the time period between the 14 ss and the 26 ss, concomitant with the developmental events of axial extension and somitogenesis (Fig. 1D). At the 14 ss, proximal renal progenitors were initially distributed across a larger lateral domain, while in central and distal regions they were clustered in a more circumferential arrangement (Fig. 1D). Throughout all regions at 14 ss though, an aggregate of approximately eight renal progenitor cells was present (Fig. 1D). At the 18 ss and 22 ss, the renal progenitor cells were arranged into more circumferential aggregates, typically comprised of six cells by the 22 ss time point (Fig. 1D). The change from average clusters of eight to six cells is consistent with rearrangements of the renal progenitor field that occur through convergence-related movements, which have been previously documented (Fig. 1D) (Wingert, et al., 2007; Lam, et al., 2009). Interestingly, the first time point when a nephron lumen could be detected was the 20 ss, when a small but discrete opening was visualized in proximal, central and distal areas of the renal progenitor field (Fig. 1D). Between the 22 and 26 ss, the lumen in these areas showed a progressively wider diameter (Fig. 1D). These data provide the first documentation for the onset of nephron lumen formation at approximately the 20 ss, and suggest that the mesenchymal renal progenitors transition to become epithelial in nature in a manner that is largely synchronized along the length of the renal progenitor field.
Changes in protein localization accompany lumen formation in the pronephros
To gain insights into the molecular processes that accompany tubulogenesis, we assayed protein localization before, during, and after lumen formation. We used immunofluorescence (IF) to identify specific proteins localized to different domains within the renal progenitor cells using the transgenic strain Tg(cdh17:eGFP) that enabled renal cell labeling based on expression of green fluorescent protein (GFP) (Diep, et al., 2011). Using an antibody that recognizes both Prkcι and Prkcζ, we found that Prkcι/ζ localized to the apical surface of renal progenitor cells at the 18 ss, prior to lumen formation (Fig. 2A). Actin also showed apical membrane localization at 18 ss (Fig. 2A). In contrast, Na+/K+ ATPase surrounded the membrane of most renal progenitor cells at 18 ss in a punctate pattern, and was not excluded from the apical surface (Fig. 2A). The apical localization of Prkcι/ζ before lumen formation implies an AMIS, which has been observed in murine nephrogenesis and cell culture tubulogenesis models (Meder, et al., 2005; Schlüter, et al., 2009; Bryant, et al., 2010; Galvez-Santisteban, et al., 2012).
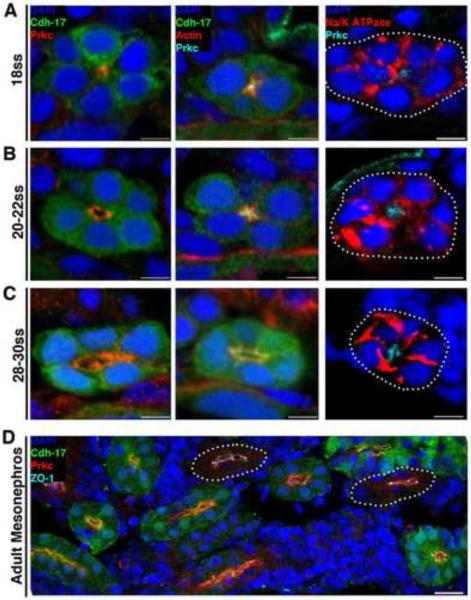
Figure 2. Changes in protein localization precede lumen formation in the pronephros, and similar polarity features characterize embryonic and adult zebrafish nephron tubules.
Localization of proteins during nephrogenesis in wild-type transgenic Tg(cdh17:eGFP) zebrafish embryos and in the uninjured adult kidney. Tubules not labeled with GFP (due to incompatibility of particular antibody-fixative combinations) are outlined with white dots. (A) At the 18 ss: Prkcι/ζ and actin localized to the apical membrane domain prior to the appearance of a lumen, while Na+/K+ ATPase surrounded the cell membrane and was not excluded from the apical surface. (B) When a lumen formed at the 20-22 ss: Prkcι/ζ encompassed the apical membrane domain abutting the lumen, actin was found in a punctate pattern at the apical surface presumably localizing to junctional complexes, and Na+/K+ ATPase still surrounded a majority of the cell membrane but was not present in the region where Prkcι/ζ was localized. (C) At the 28-30 ss: Prkcι/ζ demarcated the apical membrane, actin was more band-like at the apical surface yet still exhibited punctate expression at cell junctions, and Na+/K+ ATPase showed a distinct basolateral membrane localization. (D) In the adult kidney, or mesonephros, Prkcι/ζ was located at the apical surface of tubule cells, and the tight junction marker ZO-1 showed strong labeling at junctional complexes. (A-C) Scale bars, 5 μm. (D) Scale bar, 20 μm.
Subsequent time points were examined to identify the timing of when basolateral membrane identity was established based on the further segregation of epithelial cell proteins. At the 20-22 ss, a small lumen was present, consistent with our prior histological section analysis, and Prkcι/ζ encompassed the apical membrane domain (Fig. 2B). Furthermore, actin was present in a punctate pattern at the apical surface, presumably localizing to the sites of junctional complexes (Fig. 2B). In addition, Na+/K+ ATPase was still present throughout the basolateral membrane, and was now excluded from the apical region (Fig. 2B). This data indicates that the demarcation of respective apical versus basolateral domains occurs by the 20-22 ss, and is coincident with lumen formation. By the 28-30 ss, actin accumulated and became more band-like, yet still exhibited punctate expression consistent with cell junction localization, while Prkcι/ζ and Na+/K+ ATPase occupied apical and basolateral membranes, respectively (Fig. 2C). Of further note, examination of epithelial polarity in mesonephric tubules in the adult zebrafish kidney revealed an analogous apical localization of Prkcι/ζ, and had tight junctions marked by ZO-1 (Fig. 2D; Fig. S1). Taken together, these data reveal insights into the sequence of protein distribution changes that occur during zebrafish pronephros tubulogenesis, and illustrate that similar polarity features characterize embryonic and adult zebrafish nephrons.
prkcι and prkcζ have partially redundant roles in establishing pronephros tubule epithelial polarity
Previous characterization of the zebrafish mutant has, a genetic defect in prkcι, demonstrated that Prkcι was required for proper epithelial polarity in several tissues, including the retina, neural tube, and intestine (Peterson, et al., 2001; Horne-Badovinac, et al., 2001;). However, epithelial polarity in has pronephros tubules was intact (Peterson, et al., 2001; Horne-Badovinac, et al., 2001). Given the functional redundancy subsequently characterized between prkcι/ζ in other tissues such as the retina (Cui, et al., 2007), we hypothesized that they may share overlapping roles in the pronephros. In support of this, we found that transcripts encoding prkcι and prkcζ were located in the developing pronephros between 20-22 ss and were maintained through 72 hpf, though both transcripts were ubiquitously expressed throughout embryos at these stages (Fig. S2). Next, we utilized validated morpholino oligonucleotides (MO) (Cui, et al., 2007; Rohr, et al., 2006) to perform single and double knockdowns of prkcι and prkcζ in order to investigate their individual and redundant roles in pronephros development (Table S1). The embryos that received double prkcι/ζ mismatch control MOs and single prkcζ morphants displayed mostly normal morphology by 72 hpf, with only a few embryos displaying mild morphological differences (Fig. 3A). However, single prkcι morphants showed a range of phenotypes: while approximately 40% had a severe phenotype with pronounced axial curvature, cardiac edema, and patchy eye pigmentation, approximately ~50% had a more mild axial curvature associated with edema (Fig. 3A). By comparison, approximately 90% of double prkcι/ζ morphants showed the severe phenotype (Fig. 3A). Importantly, these observations are consistent with prior findings (Cui, et al., 2007; Rohr, et al., 2006; Serluca, 2008).
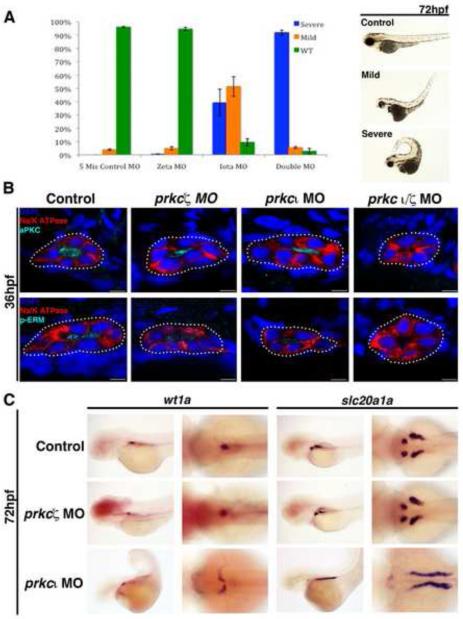
Figure 3. prkcι and prkcζ are partially redundant during tubule polarity establishment, while prkcι is required for normal glomerulus development.
(A) MO injections were performed with wild-type embryos to generate single prkcι, prkcζ, and double prkcι/ζ knockdowns, and morphants were scored at 72 hpf for gross morphology compared to embryos injected with control double prkcι/ζ mismatch MOs. (Left) Percentage distributions of three classes of phenotypes, categorized as wild-type, mild, and severe. These categories were based on curled tail, edema, and patchy eye pigmentation (Right, lateral views of live representative embryos). (B) Confocal imaging of IF analysis during tubulogenesis in wild-type, prkcι, prkcζ and prkcι/ζ morphant nephrons at the 36 hpf stage. Single morphants showed expression and proper localization of Prkcι/ζ and p-ERM to the apical surface, whereas Prkcι/ζ and p-ERM were absent in the double prkcι/ζ morphants. Na+/K+ ATPase was basolateral in wild-type embryos and single prkc morphants, but diffuse in the double prkcι/ζ morphants. Scale bars, 5 μm. (C) WISH analysis of podocyte and PCT development in wild-type control embryos and single prkc morphants using wt1a and slc20a1a, respectively. prkcζ morphants had midline clusters of podocytes, similar to wild-type embryos, while prkcι morphants had scattered clusters of podocytes that failed to migrate to the midline. PCT coiling morphogenesis was normal in prkcζ morphants and wild-type control embryos, while prkcι morphants had disrupted PCT coiling morphogenesis. (Left columns, lateral view; right columns, dorsal view).
After confirming that our knockdown phenotypes were in keeping with published prkc loss of function models, we used IF to examine the pronephros in prkc morphants (Fig. 3B). IF staining for Na+/K+ ATPase revealed normal basolateral distribution in the single prkc morphant embryos at 36 hpf, but Na+/K+ ATPase was not localized properly in double prkcι/ζ morphants (Fig. 3B). Both of the single prkc morphants had normal localization of Prkc at the apical membrane, yet in noticeably reduced amounts compared to wild-type controls (Fig. 3B). Since the available Prkc antibody detects both Prkcι/ζ, our single knockdown data suggests that both Prkc proteins are present at the apical membrane. In keeping with this notion, Prkc proteins were not detected in the pronephros of prkcι/ζ doubly deficient embryos (Fig. 3B). Interestingly, the phosphorylated form of the cytoskeletal membrane-cortex adaptor protein Ezrin, Radixin, and Moesin (p-ERM) was reduced in single prkc morphants compared to wild-type controls, but still apically localized (Fig. 3B). In contrast, double prkcι/ζ morphants lacked p-ERM entirely, suggesting shared roles for Prkcι/ζ in ERM regulation during nephron formation. Taken together, these findings show that Prkcι and Prkcζ have partially redundant roles in establishing tubule epithelial polarity during nephrogenesis, such that only deficiency of both proteins leads to a disruption in the membrane localization of apical and basolateral proteins.
prkcι and prkcζ loss of function is associated with subsequent pronephros morphogenesis defects in both the podocyte population and the tubule
The pronephros undergoes several morphogenesis events during the first several days of embryonic development, including the migration of podocytes to the midline and convolution of the linear proximal tubule into a tightly coiled anatomical structure, the latter of which is driven by collective cell migration of nephron epithelial cells (Drummond, et al., 1998; Vasilyev, et al., 2009; Kroeger and Wingert, 2014). These morphogenesis events fail to transpire in the absence of fluid flow either into the kidney or within the tubules themselves (Vasilyev, et al., 2009). Previous studies have shown that disruption of normal cardiac development prevents the occurrence of embryonic circulation, ultimately precluding fluid input into the pronephros. For example, nok embryos that have a mutation in the polarity gene mpp5a have circulatory failure that originates with defective heart development, and normal podocyte migration fails to occur (Rohr, et al., 2006; Serluca, 2008; Ichimura, et al., 2012). In silent heart/tnnt2 mutant embryos, which have defective production of the cardiac troponin T2a protein, collective cell migration is inhibited and this abrogates proximal tubule coiling (Vasilyev, et al., 2009). has embryos, which have a mutation in prkcι, also have disrupted cardiac morphogenesis and subsequent circulatory system failure (Yelon, et al., 1999; Peterson, et al., 2001; Horne-Badovinac, et al., 2001; Rohr, et al., 2006; Serluca, 2008). Thus, we hypothesized that pronephros morphogenesis may be aberrant in prkcι morphants, and possibly in prkcζ morphants as well.
To evaluate pronephros morphogenesis in the setting of prkcι or prkcζ loss of function, we performed single MO knockdowns and analyzed the morphant kidneys compared to wild-types with WISH using markers that label podocytes or the proximal tubule. Podocytes arise as bilateral clusters that migrate toward the midline and recruit vasculature to establish a single integrated glomerulus structure by 48 hpf (Drummond, et al., 1998; Majumdar and Drummond, 1999; Majumdar and Drummond, 2000; Majumdar, et al., 2000; Serluca and Fishman, 2002). The proximal convoluted tubule (PCT) segment is a linear tube that progressively coils between 48-144 hpf (Drummond, et al., 1998; Wingert, et al., 2007). Here, we surveyed morphogenesis in these populations at the 72 hpf time point, and found that both wild-type embryos and prkcζ morphants had consistently similar podocyte arrangements at the midline along with compact PCT coils, indicating that podocyte migration and PCT convolution were not disrupted (Fig. 3C). In contrast, prkcι morphants with severe morphological phenotypes had disorganized lateral groups of podocytes and the PCT remained linear (Fig. 3C). prkcι morphants with mild phenotypes did not display these defects and instead resembled wild-type embryos (data not shown). These data indicate that the loss of prkcι leads to a disruption in both podocyte migration and tubule morphogenesis, while the loss of prkcζ alone does not lead to phenotypes that impact these renal development processes. Based on the established cardiac formation defects in has mutants, and the prior finding that circulatory failure in mpp5a mutants abrogates podocyte migration, the pronephros morphogenesis defects in prkcι morphants most likely represent a secondary consequence of cardiac system failure that prevents renal fluid flow.
prkcι and prkcζ knockdown disrupts apical-basal protein localization and tubule architecture
Next, to further explore the notion of redundant functions of Prkcι/ζ during tubulogenesis, we characterized when protein distribution and epithelial polarity were disrupted in the pronephros of double prkcι/ζ morphants. Wild-type embryos were co-injected with morpholinos to target prkcι/ζ, then analyzed between the 22 ss to 36 hpf stages using IF (Fig. 4A-C). Antibody staining for Prkc was performed to confirm complete knockdown. As observed previously, wild-type tubules at the 22 ss stage had a small lumen, basolateral Na+/K+ ATPase membrane localization, and apical membrane distributions of both Prkcι/ζ and actin (Fig. 4A). In contrast, double prkcι/ζ morphants had no apparent lumen, lacked exclusive basolateral and apical cell membrane domains based on the broad distribution of Na+/K+ ATPase and actin, and lacked Prkcι/ζ reactivity (Fig. 4A). Furthermore, the actin staining was diffuse in double prkcι/ζ morphants and lacked punctate expression at the site of presumable cell-to-cell contacts compared to controls (Fig. 4A). These findings reveal that the timely, organized formation of the AMIS is disrupted in the absence of Prkcι/ζ activity.
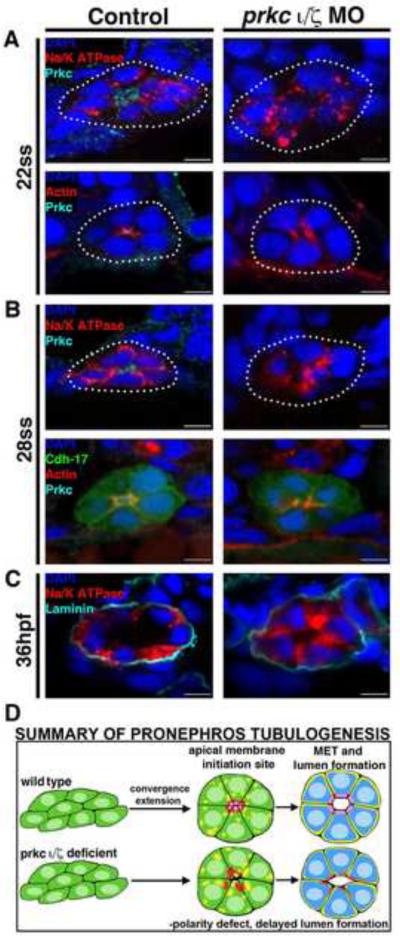
Figure 4. prkcι/ζ loss of function disrupts apical-basal protein localization and prevents proper polarity establishment, though delayed tubulogenesis eventually occurs.
Protein localization during pronephros development between the 22 ss through 36 hpf stages revealed alterations in double prkcι/ζ morphant nephrons compared to wild-type embryo controls. (A) At the 22 ss, double prkcι/ζ morphants had disorganized actin and lack Prkc at the apical domain, while Na+/K+ ATPase was diffusely spread over the entire cell membrane in both wild-types and double morphants. (B) At 28 ss, the Na+/K+ ATPase was still diffusely spread over the cell membrane in the morphants, but in wild-types this protein was restricted to the basolateral cell surface in a mutually exclusive domain to that of Prkcι/ζ and actin, which defined the apical domain. Double prkcι/ζ morphants continued to show disorganized actin and lack Prkcι/ζ at the 28 ss. (C) At 36 hpf, wild-type control and double prkcι/ζ morphant tubules were surrounded by the ECM component laminin. prkcι/ζ morphant embryos had unrestricted Na+/K+ ATPase and a single lumen was present, albeit less pronounced than in wild-type controls. (A-C) Scale bars, 5 μm. (D) Summary of pronephros tubulogenesis. Renal progenitors emerge as groups of mesenchymal cells that undergo rearrangements into circumferential clusters that display differential distribution of apical membrane components prior to lumen formation, at which time mutually exclusive domains of apical and basolateral proteins are established. Deficiency of Prkcι/ζ disrupts timely apical protein localization, delays lumen formation, and discrete apical and basolateral domains are not created.
Similar phenotypes persisted between the 28 ss and 36 hpf stages in double prkcι/ζ morphants compared to wild-type control embryos (Fig. 4B, Fig. 4C, Fig S3). In 28 ss wild-types, Na+/K+ ATPase localized to basolateral nephron membranes, while in prkcι/ζ morphants it was found at the apical surface in a diffuse punctate pattern (Fig. 4B). In 28 ss wild-type embryos, actin was apical, band-like and accumulated at junctional complexes (Fig. 4B, Fig S3). In contrast, actin distribution was diffuse at the apical surface in double prkcι/ζ morphant embryos (Fig. 4B). Interestingly, while the double prkcι/ζ knockdowns failed to form a tubule lumen at the 28 ss time point, by the 36 hpf stage a lumen was distinguished in double prkcι/ζ morphant nephrons (Fig. 4C). Furthermore, although the mutually exclusive domains of the normally apical actin and the basolateral protein Na+/K+ ATPase (Fig. 4C) were disrupted in prkcι/ζ morphants, the extracellular matrix (ECM) component laminin was deposited normally around the tubule periphery at 36 hpf (Fig. 4C). Additionally, double prkcι/ζ morphant kidneys showed expression of the ciliogenesis marker odf3b, suggesting that patterning to form multiciliated cells was intact in the pronephros, and also had luminal IF staining for acetylated α-tubulin (Fig. S4). These data are consistent with the notion that double prkcι/ζ morphants may undergo ciliogenesis, but assessments of cilia number and length at later time points would be needed to further investigate this phenotypic aspect. Taken together, these data indicate that prkcι/ζ are essential for the establishment of proper tubule cell polarity, but that prkcι/ζ loss of function is also associated with the generation of nephrons that are surrounded by an ECM and eventually form a lumen (Fig. 4D).
prkcι/ζ knockdown does not disrupt nephron segmentation, but abrogates proximal tubule morphogenesis and compromises pronephros functionality
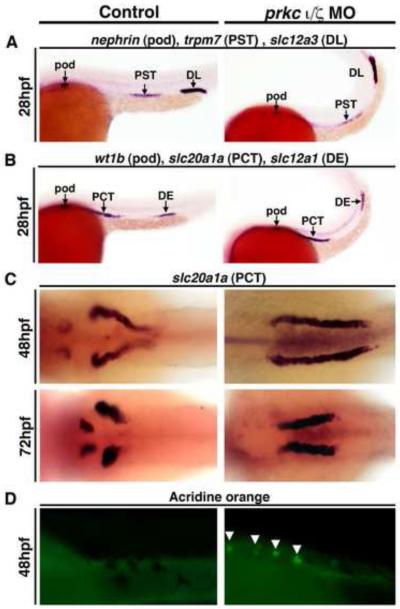
To further investigate epithelial patterning and differentiation in the context of prkcι/ζ loss of function, we assessed pronephros segmentation. Nephrons in the zebrafish pronephros, like their mammalian counterparts, are divided into segments that regulate the absorption and secretion of specific solutes (Wingert, et al., 2007). To determine if the elimination of Prkcι/ζ activity affected epithelial segment patterning, we examined gene expression of relevant markers at 28 hpf (Wingert, et al., 2007). Compared to wild-type controls, double prkcι/ζ morphants had no change in the specification of podocyte cells (wt1b, nephrin) or the PCT (slc20a1a), proximal straight tubule (PST) (trpm7), distal early (DE) (slc12a1) or the distal late (DL) (slc12a3) segments using riboprobe combinations that label alternating segments (Kroeger, et al., 2014) (Fig. 5A, Fig. 5B). At later developmental time points, the expression domains of numerous segments, such as the PCT (Fig. 5C) and both distal segments (DE, DL) were also maintained (Fig. S5). These analyses document that pronephros segment patterning and adoption of epithelial segment identity are not reliant on Prkcι/ζ function.
Figure 5. prkcι/ζ knockdown does not disrupt proximodistal segmentation, but does lead to defects in PCT morphogenesis.
WISH was used to assess epithelial specification and segment pattern using antisense riboprobes (purple) in wild-types (left column) and prkcι/ζ morphants (right column). (A,B) In control and prkc ι/ζ morphants the proximo-distal specification of nephron segments was normal. (Lateral views, anterior to the left) (A) Podocytes were labeled with nephrin, the proximal straight tubule (PST) with trpm7, and distal late tubule (DL) with slc12a3. (B) Podocytes were labeled with wt1b, the proximal convoluted tubule (PCT) with slc20a1a, and the distal early tubule (DE) with slc12a1. (C) In prkcι/ζ morphants the PCT failed to undergo proper morphogenesis and instead remained linear, while the PCT segment in control wild-type embryos at 48 hpf and 72 hpf underwent progressive coiling morphogenesis. (Dorsal views) (D) Acridine orange labeling indicates cell death in the PCT segment of the pronephros (white arrows) in prkcι/ζ morphants, but not wild-types. (Lateral views, anterior to the left)
Since prkcι knockdown alone caused nephron morphogenesis defects, namely in the glomerulus and tubule, we further scrutinized PCT development in double prkcι/ζ morphants (Fig. 5C, Fig. 5D). prkcι/ζ morphants aged 28-72 hpf were examined by WISH using riboprobes for slc20a1a to visualize the PCT. prkcι/ζ knockdown was not associated with changes in the slc20a1a pronephros expression domain between 28-36 hpf (data not shown). However, the PCT in double prkcι/ζ morphants remained linear between the 48-72 hpf stages, when wild-types had progressively coiled PCT segments (Fig. 5C). To assess whether the failure of morphogenesis was also related to changes in cell survival, prkcι/ζ morphants were examined with acridine orange, which is a marker of cell death. Wild-type nephrons did not contain acridine orange positive cells in the PCT, but dying cells were detected in prkcι/ζ morphant PCT segments (Fig. 5D). This indicates that the abrogation of PCT morphogenesis in prkcι/ζ deficient embryos is accompanied by cell death. However, given our analysis of single prkcι knockdown embryos (Fig. 3C), the lack of tubule morphogenesis in double prkcι/ζ morphants is most likely a secondary consequence from cardiac failure that prevents hemodynamic flow to the pronephros.
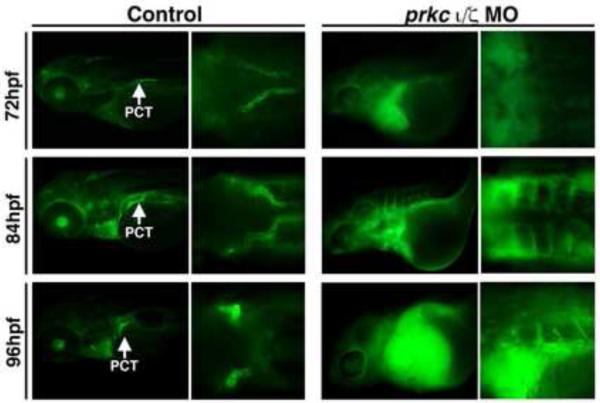
The combination of these overt morphological proximal tubule defects in double prkcι/ζ morphants, along with the severe edema that these embryos develop, suggests that renal-based fluid excretion is eliminated. To evaluate this in prkcι/ζ morphants, we microinjected embryos with 40 kilodalton (kDa) dextran-fluorescein (FITC) to provide a visual label for fluids. If fluid flow is normal within an embryo, the dextran-FITC will move from the circulation into the pronephros, where the PCT epithelial cells will uptake the dextran-FITC through endocytosis (Anzenberger, et al., 2006; Li, et al., 2014). Wild-type embryos injected with 40 kDA dextran-FITC at 48 hpf showed normal PCT uptake between 72 hpf and 96 hpf, with clearly labeled PCT segments (Fig. 6). In contrast, prkcι/ζ morphants showed a failure to clear labeled dextran from the body between 48 and 72 hpf, as evidenced by bright green fluorescence surrounding the heart cavity, in addition to an absence of PCT labeling (Fig. 6). At the 84 hpf and 96 hpf time points, prkcι/ζ morphants maintained strong dextran accumulation in the pericardial cavity, indicating that prkcι/ζ morphants are unable to excrete appreciable fluids, and displayed no dextran uptake in the PCT likely due to the deficiency of fluid flow into the pronephros (Fig. 6).
Figure 6. Renal clearance and PCT endocytosis are abrogated in prkcι/ζ deficient embryos.
Embryos were injected with 40-kDa dextran-FITC at 48 hpf, and imaged at 72, 84, and 96 hpf. Control wild-type embryos displayed renal clearance by diminution of the net fluorescent signal intensity over time, as well as dextran-FITC internalization throughout the PCT (white arrows) during its progressive morphogenesis. Double prkcι/ζ morphants displayed severe fluid accumulation, notably pericardial edema that remained strongly positive for the dextran-FITC conjugate label, and the PCT was not labeled by dextran endocytosis (Left panels, lateral views; right panels, dorsal views, with exception of bottom right which shows a lateral/dorsal angled view).
prkcι/ζ knockdown is associated with alterations in podocyte development and ectopic misexpression of renal transcription factors and podocyte-specific genes
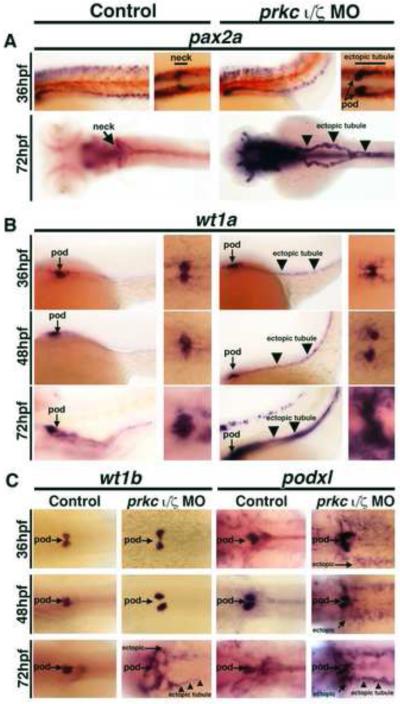
Next, we examined prkcι/ζ morphants using WISH to further evaluate pronephros cell types after the 28 hpf stage of development. Interestingly, the majority of prkcι/ζ morphants showed ectopic expression of the developmental transcription factor pax2a between the 36 hpf and 72 hpf stages (Fig. 7A, Fig. S6). At 36 hpf, prkcι/ζ morphants had an expanded domain of pax2a in the proximal tubule and ectopic pax2a expression in podocytes, while wild-type controls had restricted pax2a expression in the neck segment at this stage (Fig. 7A). Additionally, prkcι/ζ morphant embryos lacked whole mount immunoreactivity for Na+/K+ ATPase in this neck region, while wild-type controls showed continuous Na+/K+ ATPase staining in the tubule (Fig. S7). At 72 hpf, even more dramatic alterations were observed, such that ~90% of double prkcι/ζ morphant embryos showed high levels of ectopic pax2a transcripts throughout the entire pronephros tubule (Fig. 7A, Fig. S6). Wild-type embryos only showed strong pax2a expression in the neck segment, had low transcript levels in scattered tubule cells along the distal nephron and never displayed ectopic pronephros expression (Fig. 7A, Fig. S6). Also at 72 hpf, double prkcι/ζ morphants still had reduced proximal labeling of Na+/K+ ATPase, in contrast to wild-types where labeling was strong throughout the length of each nephron (Fig. S7). These surprising observations suggest that prkcι/ζ deficiency leads to some alterations in tubule cell identity even though many segment region identities are maintained at these later stages (e.g. the PCT, DE, and DL segments, as shown in Fig. 5C, Fig. S5).
Figure 7. prkcι/ζ knockdown disrupts glomerular development and causes ectopic renal progenitor transcription factor expression.
WISH was used to detect gene expression (purple) in wild-types (left column) and prkcι/ζ morphants (right column). (A-B) Left panels, lateral views; right panels, dorsal views; all embryos are shown with anterior to the left. (A) pax2a was restricted to the neck segment in wild-types, but showed ectopic podocyte and tubular expression (black arrowheads) in prkcι/ζ morphants at 36 and 72 hpf. (B) wt1a transcripts marked wild-type podocyte cells, but in prkcι/ζ morphants wt1a transcripts were also found in tubular cells (black arrowheads) and surrounding mesoderm cells. (C) wt1b and podxl were ectopically expressed in in prkcι/ζ morphants, though podxl showed ectopic expression beginning at 48 hpf and wt1b showed ectopic transcripts beginning at 72 hpf. Diffuse ectopic transcript expression (arrows) and ectopic tubule expression (black arrowheads) (Dorsal views, anterior to the left).
Based on the observation that prkcι/ζ morphants showed ectopic pax2a expression in podocytes at 36 hpf, we hypothesized that prkcι and prkcζ may be essential to maintain other aspects of epithelial identity in podocytes as well. When podocytes arise, they express the Wilms tumor suppressor transcription factor paralogs wt1a/1b and pax2a, then downregulate pax2a and transition to the expression of mature markers such as nephrin, podocin, and podocalyxin1-like (podxl) (Drummond, et al., 1998; Majumdar, et al., 2000; Serluca and Fishman, 2002; Bollig, et al., 2006; O’Brien, et al., 2011; Kroeger and Wingert, 2014). Interplay between pax2a and wt1a is essential for normal nephron patterning, such that pax2a is required to inhibit wt1a expression in the neck and proximal tubule, as demonstrated by the finding that zebrafish no isthmus (noi) mutants that have recessive mutations in pax2a have ectopic wt1a expression in the neck regions of the pronephros (Majumdar, et al., 2000). To explore podocyte identity in prkcι/ζ deficient embryos, we surveyed the expression of wt1a and several other podocyte markers in prkcι/ζ morphants between 36-72 hpf of development (Fig. 7B, Fig. 7C, Fig. S8). Wild-type controls and prkcι/ζ morphants had podocytes that expressed wt1a, wt1b, nephrin, podocin and podxl (Fig. 7B, Fig. 7C, Fig. S8). As noted previously, aberrant podocyte migration was detected in the prkcι/ζ knockdowns that had the severe morphological phenotype (Fig. 7B, Fig. 7C, Fig. S8). Surprisingly, prkcι/ζ morphants expressed wt1a in ectopic locations within the nephron tubule, with ~90% displaying ectopic wt1a in the tubule by 72 hpf (Fig. 7B, Fig. S6). In contrast, wild-types never displayed ectopic pronephros wt1a expression (Fig. 7B, Fig. S6), and neither did single prkc knockdowns (Fig. 3C). prkcι/ζ morphants had diffuse ectopic wt1a expression in the vicinity of the podocyte populace, possibly suggestive of ectopic expression in the surrounding mesoderm (Fig. 7B). Compared to wild-type controls, prkcι/ζ morphants showed elevated wt1b transcripts at 72 hpf, with 29% (p < 0.05) of knockdown embryos showing high ectopic expression (Fig. 7C, data not shown). Further, prkcι/ζ morphants showed ectopic expression of podxl beginning at 48 hpf, with >80% affected by 72 hpf (Fig. 7C, Fig. S6). In contrast, podocin and nephrin did not show ectopic expression at these stages (Fig. S8). Overall, these results were unexpected, as similar ectopic transcription factor patterns within the tubule have not been previously described in other kidney mutants (Gerlach and Wingert, 2013). In sum, the discovery that prkcι/ζ loss of function is associated with subsequent ectopic misexpression of pax2a, wt1a, wt1b, and podxl in the pronephros suggests that prkcι/ζ may have roles in the maintenance of epithelial identity in the nephron tubule.
Ectopic expression of renal transcription factors and podocyte genes is not a generalized effect from the loss of fluid flow, but can be partly induced by knockdown of the mpp5a polarity gene
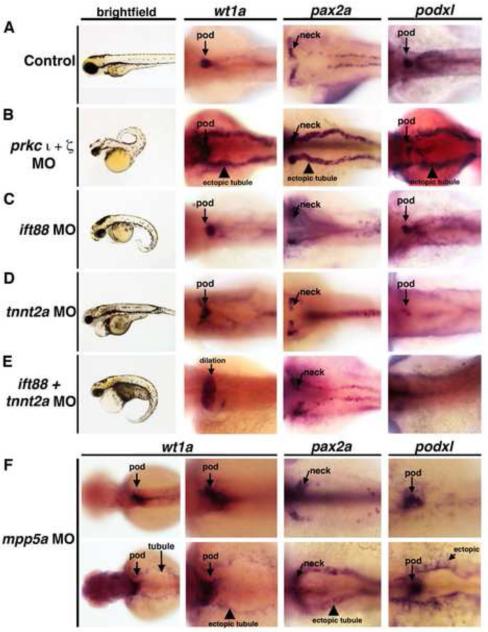
In evaluating the hypothesis that the prkcι/ζ genes may be specifically required to maintain tubule epithelium identity, we wondered if the ectopic pronephros expression phenotypes in prkcι/ζ morphants were related to the absence of fluid flow. To assess whether disruptions in flow within the nephron could result in ectopic pronephros gene expression, we performed knockdown of the intraflagellar transport factor ift88 (Table S1), which is required for fluid propulsion within the tubule (Vasilyev, et al., 2009). Wild-type embryos were injected with a morpholino to knockdown ift88 or prkcι/ζ and analyzed at 72 hpf by WISH alongside wild-type controls (Fig. 8, Fig. S6, Fig. S9). Wild-type controls showed restricted podocyte expression of wt1a and podxl, exclusive of the tubule marker cdh17, and pax2a expression in the neck (Fig 8A, Fig. S6, Fig. S9). In contrast, prkcι/ζ morphants showed high levels of ectopic wt1a, pax2a, podxl, in the pronephros, and wt1a transcripts co-localized with cdh17 transcripts in tubule cells (Fig 8B, Fig. S6, Fig. S9). ift88 morphants showed normal podocyte expression of wt1a, pax2a, and podxl, and none of these transcripts were detected in ectopic locations (Fig 8C, Fig. S6). To further confirm this, double WISH was performed on ift88 morphants, and wt1a transcripts were indeed exclusive to podocytes and restricted from the cdh17-expressing tubule domain (Fig. S9). This indicates that the reduction/elimination of flow within the tubule alone does not generally lead to ectopic pronephros gene expression.
Figure 8. Ectopic expression of podocyte genes and early developmental transcription factors in the pronephros is not a general consequence of embryonic defects in fluid flow.
WISH was used to assess gene expression using antisense riboprobes to detect wt1a, pax2a, or podxl (purple) in (A) wild-types, (B) prkcι/ζ morphants, (C) ift88 morphants, (D) tnnt2a morphants, (E) double ift88/tnnt2a morphants, and (F) mpp5a morphants. (A-E) Knockdown of prkcι/ζ was the only circumstance that led to high levels of ectopic expression of podocyte genes in the pronephros tubule. (F) (Top row) Most mpp5a morphants displayed no ectopic pronephros expression, but (bottom row) a subset (< 10%) displayed low levels of ectopic wt1a, pax2a, or podxl in the tubule and also diffuse ectopic expression.
To address specifically how cardiac failure might impact the state of tubular cells in the pronephros, we performed knockdown of tnnt2a (Table S1) to arrest the embryonic heartbeat (Vasilyev, et al., 2009). tnnt2a morphants were fixed at 72 hpf and gene expression assessed via WISH (Fig 8D, Fig. S6, Fig. S9). As with ift88 morphants, tnnt2a knockdown was associated with normal podocyte expression, and not associated with ectopic expression of wt1a, pax2a or podxl transcripts (Fig 8D, Fig. S6). Further, in double WISH analysis, tnnt2a morphants showed mutually exclusive domains of wt1a and cdh17 in the podocytes and tubule, respectively (Fig. S9). These results indicate that embryonic heartbeat arrest, and subsequent circulatory failure, does not generally lead to ectopic pronephros gene expression.
Next, we assessed whether double knockdown of ift88 and tnnt2a would recapitulate the ectopic pronephros gene expression phenotype observed in prkcι/ζ morphants. The combined knockdown of ift88 and tnnt2a was not associated with ectopic tubule transcripts of wt1, pax2a or podxl, though ift88/tnnt2a morphants had proximal dilations of the pronephros (Fig 8E, Fig. S6). Taken together, these data show that ectopic pronephros expression of factors such as wt1a and podxl is not a general consequence of fluid flow into or within the pronephros tubule, and suggest that the polarity genes prkcι/ζ serve a function in the maintenance of renal epithelial identity in addition to their role in polarity establishment during tubulogenesis.
Given these findings, we postulated whether disruptions in the expression of other genes that modulate epithelial polarity, like mpp5a, would also lead to ectopic pronephros expression. To investigate this idea, we performed knockdown of mpp5a (Table S1) and assessed gene expression by WISH in the embryos at 72 hpf (Fig. 8F, Fig. S6, Fig. S10). Unlike prkcι/ζ knockdown, where the vast majority of morphants displayed high levels of ectopic wt1a transcripts in the pronephros, mpp5a knockdown was associated with normal wt1a, pax2a, and podxl expression in most embryos (Fig. 8F, Fig. S6). A small fraction of mpp5a morphant embryos displayed low, diffuse levels of ectopic wt1a, pax2a, and podxl expression in the embryonic trunk where the pronephros is located (Fig. 8F, Fig. S6). Of these, only the elevated pax2a expression was significant (p < 0.05) based on a pair-wise Student’s t-test (Fig. S6). In double WISH analysis, mpp5a morphants with ectopic wt1a showed partial overlap with cdh17, indicating that some ectopic expression was located in the pronephros (Fig. S10). Further studies would be needed to determine whether mpp5a morphants are delayed in renal tubule development and/or have other tubule alterations, such as polarity or MET defects. In sum, these findings suggest that mpp5a may have independent or partially redundant functions with prkcι/ζ in the maintenance of pronephros tubule epithelial identity, though this mild phenotype may indicate that mpp5a has a minor role in comparison to the prkcι/ζ genes.
Knockdown of pax2a, but not wt1a, is sufficient to rescue ectopic misexpression of renal transcription factors and podocyte-specific genes in the pronephros tubule of prkcι/ζ morphants
Since changes in the transcription factors pax2a and wt1a were among the first discernible evidence of ectopic pronephros tubule gene expression in prkcι/ζ morphants, and both the pax2a and wt1a are required to regulate renal genes in various contexts (Dressler, 2006; Gerlach and Wingert, 2013), we hypothesized that the alteration of these factors may be the initiating event(s) that trigger ectopic expression in prkcι/ζ knockdowns. Therefore, we tested whether concomitant knockdown of pax2a or wt1a in prkcι/ζ morphants would rescue ectopic misexpression within the pronephros tubules.
First, we explored whether wt1a expression in prkcι/ζ morphants was the cause of ectopic misexpression within the pronephros (Table S1, Fig. S11). During normal zebrafish development, expression of wt1a precedes that of wt1b and podxl in the podocyte lineage (O’Brien, et al., 2011; data not shown). Prior studies that examined wt1a loss of function have not shown that wt1a knockdown is associated with ectopic pronephros gene expression suggestive of altered tubule identities (Hsu, et al., 2003; Perner, et al., 2007; O’Brien, et al., 2011). Rather, MO knockdown of wt1a leads to reduced podocyte formation, which is associated with a partial reduction in the expression of numerous podocyte-specific genes, including wt1b, and in biochemical assays demonstrated that Wt1a forms a dynamic multi-factor transcriptional complex that regulates podocyte gene expression (O’Brien, et al., 2011). With this information in mind, it is conceivable that ectopic wt1a expression in the tubule epithelium of prkcι/ζ morphants may be one component responsible for inducing ectopic wt1b and podxl in those cells. Therefore, we examined the outcome of prkcι/ζ knockdown compared to the combined knockdown of wt1a and prkcι/ζ in wild-type embryos (Fig. S11). Knockdown of wt1a along with prkcι/ζ was still associated with variable ectopic tubule misexpression of wt1b, podxl and pax2a transcripts in the majority of embryos (Fig. S11; data not shown). Based on these results, we concluded that a reduction of wt1a expression in double prkcι/ζ morphants is not sufficient to rescue epithelial identity in the pronephric tubule. Of further note, in triple wt1a/prkcι/ζ morphants, the podocyte expression domain of podxl was abrogated in 65% (69/106) of embryos (Fig. S11). This incidentally reveals that wt1a is required for podxl expression in podocytes, which is consistent with the findings wt1a is essential for transcription of podocyte genes (O’Brien, et al., 2011). Overall, these data show that changes in wt1a expression alone do not explain the misexpression of wt1b, pax2a or podxl during prkcι/ζ loss of function, but do not eliminate the possibility that Wt1a may participate, such as through cooperative interactions with other transcription factors.
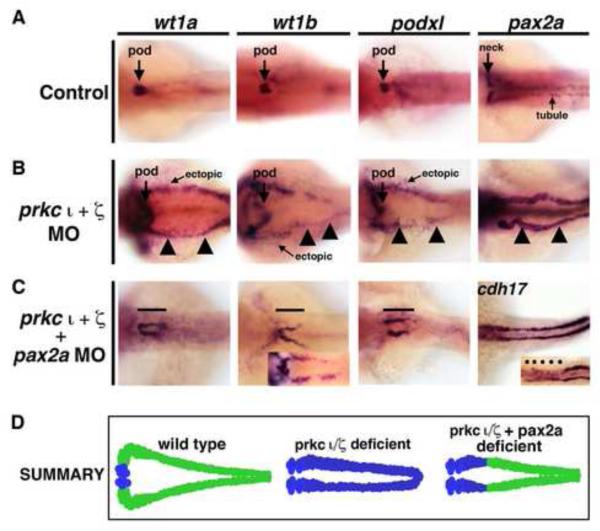
Next, we examined the outcome of pax2a knockdown alone, prkcι/ζ knockdown alone and combined knockdown of pax2a and prkcι/ζ in wild-type embryos (Fig. 9, Fig. S6, Fig. S12, Fig. S13, Fig. S14). Previous gain of function studies have shown that overexpression of pax2a is sufficient to induce ectopic pronephric tissues that express the renal marker cdh17 (Bedell, et al., 2012). Analysis of the no isthmus (noi) mutants, which have a defect in the pax2a gene, has shown that the absence of pax2a leads to an expansion of wt1a expression that is restricted to the neck region (Majumdar, et al., 2000). pax2a MO knockdown in wild-type embryos recapitulated this loss of function phenotype, with expansion of the wt1a and wt1b expression domain to the neck region (Fig. S12). Combined knockdown of pax2a and prkcι/ζ in wild-type embryos similarly caused a short expression domain of wt1a, wt1b, and podxl expression in the neck region, where low levels of cdh17 transcripts were expressed (Fig. 9C, Fig. S6). However, high ectopic misexpression of these transcripts in the remainder of the pronephros tubule was eliminated in triple pax2a/prkcι/ζ morphant embryos (Fig. 9C, Fig. S6). To confirm these findings, two additional pax2a morpholinos (previously published, Bricaud and Collazo, 2006; McCarroll, et al., 2012) were tested to examine if they similarly rescued prkcι/ζ morphant embryos (Table S1, Fig. S13, Fig. S14). Both of these other morpholinos rescued ectopic wt1a and podxl in prkcι/ζ morphant embryos, though pax2a MO4 was quantified as more statistically significant (Fig. S13, Fig. S14). These results indicate that knockdown of pax2a was sufficient to rescue ectopic misexpression in prkcι/ζ morphants. Taken together, these data support the conclusion that prkcι/ζ loss of function leads to ectopic pax2a misexpression in the pronephros, which is sufficient to induce the ectopic misexpression of multiple genes throughout the tubule and thus alters their epithelial cell identity—a condition that can be ameliorated by knockdown of pax2a (Fig. 10).
Figure 9. Ectopic expression of early developmental transcription factors in prkc ι/ζ morphants is abrogated by concomitant knockdown of pax2a.
WISH was used to assess gene expression using antisense riboprobes to detect wt1a, wt1b, podxl, pax2a or cdh17 (purple), in (A) wild-types, (B) prkcι/ζ morphants, or (C) prkcι/ζ morphants with combined pax2a knockdown. Wild-types showed restricted podocyte expression of wt1a, wt1b, and podxl, while pax2a was localized to the neck and intermittent pronephros tubule cells. prkcι/ζ morphants had high ectopic expression of each gene in the pronephros tubule. Knockdown of pax2a in prkcι/ζ morphants was associated with a short proximal pronephros domain of ectopic wt1a, wt1b, and podxl expression and low cdh17 transcript levels, while the tubule segment past this short ectopic segment domain was present and marked by a robust level of cdh17 transcripts. (D) Summary of ectopic misexpression during prkcι/ζ loss of function: wild-type embryos have mutually exclusive domains of podocyte versus tubule gene expression, prkcι/ζ morphants have ectopic misexpression of transcription factors throughout the pronephros, and pax2a knockdown represses this ectopic tubule misexpression in prkcι/ζ morphants, except for the neck region of the pronephros.
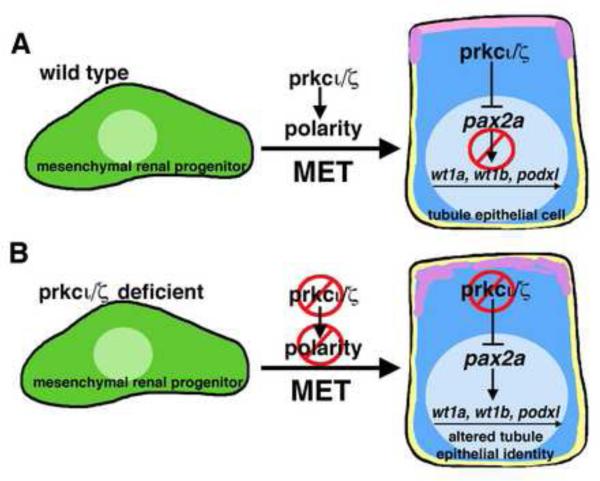
Figure 10. prkcι/ζ are required for the establishment and maintenance of epithelial identity during nephron tubulogenesis in the zebrafish pronephros.
(A) During normal development, prkcι/ζ play an essential function in polarity establishment when mesenchymal renal progenitors undergo a MET and concerted tubulogenesis to become epithelial cells and form the pronephros. When pronephros tubule epithelial identity is established, the activity of prkcι/ζ represses pax2a expression (either directly or indirectly) to maintain normal gene expression. (B) In the absence of prkcι/ζ, polarity is not properly established during MET. Further, pronephros cells in prkcι/ζ deficient embryos ectopically express pax2a, which leads (directly or indirectly) to misexpression of genes that include wt1a, wt1b, and podxl.
DISCUSSION
Tubulogenesis is a fundamental process in development. Despite the great abundance of research on tubulogenesis, very little is known about how this process is regulated while nephron formation occurs during vertebrate kidney ontogeny in different species. The zebrafish has emerged as a valuable model for ongoing basic and translational kidney research based on the conservation of nephrons composition between zebrafish and mammalian nephrons (Poureetezadi, et al., 2013; Marra, et al., 2014). The attributes of the zebrafish embryo pronephros, in particular, have provided a novel opportunity for in vivo analysis of tubulogenesis processes (Mitra, et al., 2012; Jung, et al., 2013) and investigation of the mechanisms involved in the morphogenesis of tubular epithelial cells, such as collective cell migration (Vasilyev, et al., 2009). Our study adds to this work by providing the first spatiotemporal characterization of tubulogenesis in the zebrafish pronephros. We have documented the precise timing of lumen formation at the 20-22 ss, and the prior emergence of apical-basal protein segregation in the tubular epithelium precursors. Knowledge about the developmental time when renal progenitors acquire polarity and undergo MET provides an important foundation for future studies.
Roles of prkcι/ζ in zebrafish pronephros polarity establishment and epithelial identity maintenance
Through gene knockdown experiments we have identified overlapping roles for prkcι and prkcζ during polarity establishment in renal progenitors. Our findings are consistent with a model in which the establishment of tubule epithelial cell polarity is reliant on the partially redundant activities of prkcι/ζ (Fig. 10A). In the absence of prkcι/ζ, the tubular epithelium does not establish proper apical-basal protein localization, and undergoes delayed lumen formation (Fig. 4D). As the ternary polarity complex is broadly appreciated to play roles in the proper segregation of proteins into distinct membrane domains in epithelial cells (Chen, et al., 2013), our data show that this role is conserved during zebrafish pronephros tubulogenesis. Interestingly, prkcι/ζ loss of function was not disruptive to nephron segment patterning, and both proximal and distal segment domains were specified in morphants. This parallels a recent finding in nephrogenesis during mouse metanephros development, where disruption of cell adhesion and polarity due to loss of the Afadin adaptor protein did not abrogate proximo-distal nephron pattern in the elongating nephron (Yang, et al., 2013).
The surprising finding that prkcι/ζ morphant nephrons misexpress several transcription factors and genes associated with the podocyte lineage suggests that prkcι/ζ are required to maintain aspects of the differentiated character of the tubular epithelium (Fig. 10B). Both wt1a and pax2a are expressed by early mesenchymal renal progenitors in the zebrafish embryo (Wingert, et al., 2007). Thus, the ectopic expression of these genes in prkcι/ζ deficient tubules might reflect the absence of a stable epithelial identity, such that the cells retain features of a mesenchymal cell state or perhaps are triggered to re-express pax2a and wt1a in a reversion to mesenchymal state in the absence of proper polarity. Previous research has demonstrated that a transcriptional network that includes wt1a mediates podocyte development in the zebrafish pronephros (O’Brien, et al., 2011; Miceli, et al., 2014). However, based on our wt1a knockdown analysis here, the misexpression of wt1a alone does not appear to explain the appearance of wt1b and podxl transcripts in the context of prkcι/ζ loss of function. Interestingly, pax2a knockdown alone was sufficient to abrogate ectopic expression of wt1a, wt1b, and podxl in the tubule of prkcι/ζ morphants. This reveals that ectopic pax2a activity is sufficient to induce the transcription of these genes, either directly or indirectly, in the context of prkcι/ζ loss of function. The Pax2/8 transcription factors are known to mediate cell fate in renal cells and other tissues (McCarroll, et al., 2012; Boualia, et al., 2013), and our findings suggest that identifying the roles of pax2a in gene regulatory networks in renal tissues will be important to understand how nephron epithelial identity is maintained. Taken together, this study has established that prkcι/ζ are requisite for normal development of polarity in the pronephros epithelium, and revealed a novel genetic pathway that acts to maintain nephron tubule epithelial identity through which prkcι/ζ function acts, again either directly or indirectly, to repress pax2a expression (Fig. 10).
Roles of prkcι/ζ in renal lineage differentiation—the case of podocytes
Here, we observed that knockdown of prkcι alone was sufficient to disrupt podocyte migration in zebrafish and that prkcι/ζ deficiency in zebrafish caused a greater incidence of podocyte migration defects. The pronephros morphogenesis phenotypes in prkcι deficiency correlate with the elimination of vascular flow due to cardiac development abnormalities. Previous studies have demonstrated that the absence of normal vascular flow disrupts podocyte midline migration (Serluca, et al., 2002) and PCT morphogenesis (Vasilyev, et al., 2009). Both prkcι and prkcι/ζ morphants showed expression of podocyte genes, suggesting this lineage was specified correctly.
In the mouse, recent studies have documented crucial roles for Prkcι/ζ during podocyte maturation in the mammalian kidney (Huber, et al., 2009; Hirose, et al., 2009; Hartelben, et al., 2013). Selective depletion of Prkcι in mouse podocytes disrupted their polarity and led to the disassembly of slit diaphragms (Hirose, et al., 2009). Dual Prkcι/ζ knockdown in mouse podocytes disrupts foot processes and arrests glomerular formation (Hartelben, et al., 2013). It remains unclear from our present study whether prkcι/ζ have direct roles in podocyte maturation, i.e. terminal differentiation, in the zebrafish pronephros. While it is tempting to speculate that prkcι/ζ have conserved roles in podocyte differentiation/maturation across vertebrates, especially in light of the similar gene expression profiles of podocytes between zebrafish and mammals, electron microscopy would be necessary to visualize foot process formation and podocyte maturation in prkcι/ζ deficient embryos.
Prkc complexes and identification of their kinase targets in specific tissues
The established functions of Prkc proteins are diverse and can be attributed in part to spatial and temporal differences between interacting protein partners (Pieczynski and Margolis, 2011; Chen and Zhang, 2013). The ternary polarity complex is a crux for protein interactions that moderate cellular signaling. This is achieved through various protein domains that are quintessential for establishing a nexus between the ECM, cell membrane, cell cortex, and the cytoplasm. Understanding the roles of Prkc will entail knowing the targets of Prkc kinase activity. To date, investigations into the ternary polarity complex binding partners and Prkc substrates have for the most part been worked out in vitro. An assessment of these interactions within an in vivo context has not been established, especially in regards to renal development.
To further understand the activities of Prkcι/ζ, it will be essential to identify kinase targets in a cellular context, which can be performed using new in vivo analog-sensitive kinase assays (Uhalte, et al., 2012). Other insights into the mechanisms of polarity loss in Prkcι/ζ morphants could emerge from further analysis as to how reductions or abrogation of p-ERM, Na+/K+ ATPase activity and actin localization affect nephron progenitors. The disruption of Na+/K+ ATPase has been ascribed to different renal disease states (Hsu, et al., 2010; Wilson, 2011). For example, aberrant Na+/K+ ATPase localization occurs in polycystic kidney disease (Hsu, et al., 2010; Wilson, 2011). As changes in epithelial integrity occur in these and other renal disease states, there is significant clinical value to understanding the pathways that affect epithelial polarity in the kidney.
Mesenchymal and epithelial transitions in development and regeneration
Epithelial integrity is essential for normal development of nephrons, and understanding how a tubule maintains cell identity, and how the cells might sense damage and respond to injury, are relevant to understanding clinical conditions like acute kidney injury (AKI). One proposed mechanism of nephron epithelial regeneration after AKI is that tubule damage triggers surviving epithelia to change their molecular repertoire and undergo an epithelial to mesenchymal transition (EMT) (McCampbell and Wingert, 2012; Li and Wingert, 2013). This mesenchymal populace proliferates and migrates to occupy the denuded basement membrane where damage has occurred. Interestingly, in both mammals and zebrafish, the mesenchymal populace that regenerates nephrons after AKI expresses Pax2 (Imgrund, et al., 1999; Cosentino, et al., 2013). Thus, it is intriguing to speculate whether the absence of polarity in the pronephros of prkcι/ζ morphants, and the consequent expression of developmental factors, capitulates aspects of tubular injury. Nevertheless, as both the zebrafish embryo and adult provide conserved models to study nephron epithelial regeneration (Johnson, et al., 2011; McCampbell and Wingert, 2014), further analysis of prkcι/ζ deficiency in these settings, or knockdown of other polarity regulators, may provide relevant insights into how tubule epithelial cells respond to damage.
In summary, this study has demonstrated that prkcι/ζ are crucial components of the molecular machinery of nephron tubulogenesis and tubule maintenance in the zebrafish embryo kidney. These studies further establish the zebrafish pronephros as a relevant model that can bridge the gap between cell culture and mammalian rodent models of nephrogenesis research, and may potentially provide insights into the renal pathophysiology associated with various disease states.
Supplementary Material
Highlights.
- zebrafish renal progenitors undergo synchronized tubulogenesis to form nephrons
- pronephros tubulogenesis involves the formation of an apical membrane initiation site
- prkcι/ζ have partially redundant roles in establishing polarity during nephrogenesis
- prkcι/ζ loss of function leads to ectopic misexpression of renal genes in the tubule
- prkcι/ζ maintain pronephros epithelial identity by inhibiting pax2a expression
ACKNOWLEDGEMENTS
The Wingert lab was supported by the following grants to RAW: NIH-NIDDK grant K01DK083512, NIH New Innovator grant DP2OD008470, March of Dimes Basil O’Connor Starter Scholar research grant award #5-FY12-75, NIH-NIDDK grant R01DK100237, and start up funding from the University of Notre Dame and College of Science. We are especially grateful to Elizabeth and Michael Gallagher for their generous gift to the University of Notre Dame on behalf of their family for the support of developmental biology and stem cell research. The funders had no role in the study design, data collection and analysis, decision to publish, or manuscript preparation. We thank the entire staff of the Department of Biological Sciences, and the Center for Zebrafish Research at Notre Dame for their dedication and care of our zebrafish aquarium. We thank Kristen K. McCampbell for outstanding management of the Wingert lab. We thank Ryne Gorsuch and Manuela Lahne for assistance with adapting various protocols for these renal studies. GFG sends a special thanks to his family and Sally Horne-Badovinac for her teachings. RAW thanks ZM and MD for support during this project. Finally, we thank all members of our wonderful research lab for their comments, discussions and insights about this work.
Abbreviations
- AMIS
apical membrane initiation site
- cdh17
cadherin 17
- DE
distal early
- DL
distal late
- dpf
days post fertilization
- ECM
extracellular matrix
- G
glomerulus
- hpf
hours post fertilization
- has
heart and soul
- IF
immunofluorescence
- ift88
intraflagellar transport 88 homolog
- IM
intermediate mesoderm
- MET
mesenchymal to epithelial transition
- MO
morphant
- mpp5a
membrane protein palmitolyated 5a (MAGUK p55 subfamily member 5a)
- nok
nagie oko
- N
notochord
- PCT
proximal convoluted tubule
- pod
podocytes
- PST
proximal straight tubule
- prkcι
atypical protein kinase C iota
- prkcζ
atypical protein kinase C zeta
- ss
somite stage
- tnnt2a
troponin T2a, cardiac
- WISH
whole mount in situ hybridization
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Anzenberger U, Bit-Avragim N, Rohr S, Rudolph F, Dehmel B, Willnow TE, Abdelilah-Seyfried S. Elucidation of megalin/LRP2-dependent endocytic transport processes in the larval zebrafish pronephros. J Cell Sci. 2006;119:2127–37. doi: 10.1242/jcs.02954. [DOI] [PubMed] [Google Scholar]
- Bedell VM, Person AD, Larson JD, McLoon A, Balciunas D, Clark KJ, Neff KI, Nelson KE, Bill BR, Schimmenti LA, Bieraghi S, Ekker SC. The lineage-specific gene ponzr1 is essential for zebrafish pronephros and pharyngeal arch development. Development. 2012;139:793–804. doi: 10.1242/dev.071720. doi: 10.1242/dev.071720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollig F, Mehringer R, Perner B, Hartung C, Schäfer M, Schartl M, Volff JN, Winkler C, Englert C. Identification and comparative expression analysis of a second wt1 gene in zebrafish. Dev Dyn. 2006;235:554–561. doi: 10.1002/dvdy.20645. [DOI] [PubMed] [Google Scholar]
- Boualia SK, Gaitan Y, Tremblay M, Sharma R, Cardin J, Kania A, Bouchard M. A core transcriptional network composed of Pax2/8, Gata3 and Lim1 regulates key players of pro/mesonephros morphogenesis. Dev Biol. 2013;382:555–566. doi: 10.1016/j.ydbio.2013.07.028. doi: 10.1016/j.ydbio.2013.07.028. [DOI] [PubMed] [Google Scholar]
- Bricaud O, Collazo A. The transcription factor six1 inhibits neuronal and promotes hair cell fate in the developing zebrafish (Danio rerio) inner ear. J Neurosci. 2006;26:10438–10451. doi: 10.1523/JNEUROSCI.1025-06.2006. doi: 10.1523/JNEUROSCI.1025-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant DM, Datta A, Rodríguez-Fraticelli AE, Peränen J, Martín-Belmonte F, Mostov KE. A molecular network for de novo generation of the apical surface and lumen. Nat Cell Biol. 2010;12(11):1035–45. doi: 10.1038/ncb2106. doi: 10.1038/ncb2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Zhang M. The Par3/Par6/aPKC complex and epithelial cell polarity. Exp Cell Res. 2013;319:1357–1364. doi: 10.1016/j.yexcr.2013.03.021. doi: 10.1016/j.yexcr.2013.03.021. [DOI] [PubMed] [Google Scholar]
- Cheng CN, Wingert RA. Chapter 9: Renal system development in the zebrafish: a basic model of the human kidney. In: Carver E, Lessman C, editors. Zebrafish: Topics in Reproduction & Development. Nova Scientific Publishers; 2014. pp. 179–214. [Google Scholar]
- Cheng CN, Li Y, Marra A, Verdun V, Wingert RA. Flat mount preparation for observation and analysis of fixed zebrafish embryo specimens. J Vis Exp. 2014;89:e51604. doi: 10.3791/51604. doi: 10.3791/51604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosentino CC, Skrypnyk NI, Brilli LL, Chiba T, Novitskaya T, Woods C, West J, Korotchenko VN, McDermott L, Day BW, Davidson AJ, Harris RC, de Caestecker MP, Hukriede NA. Histone deacetylase inhibitor enhances recovery after AKI. J Am Soc Nephrol. 2013;24:943–953. doi: 10.1681/ASN.2012111055. doi: 10.1681/ASN.2012111055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui S, Otten C, Rohr S, Abdelilah-Seyfried S, Link BA. Analysis of aPKC lambda and aPKC zeta reveals multiple and redundant functions during vertebrate retinogenesis. Mol Cell Neurosci. 2007;34:431–444. doi: 10.1016/j.mcn.2006.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta A, Bryant DM, Mostov KE. Molecular regulation of lumen morphogenesis. Curr Biol. 2011;21:R126–36. doi: 10.1016/j.cub.2010.12.003. doi: 10.1016/j.cub.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diep CQ, Ma D, Deo RC, Holm TM, Naylor RW, Arora N, Wingert RA, Bollig F, Djordjevic G, Lichman B, Zhu H, Ikenaga T, Ono F, Englert C, Cowan CA, Hukriede NA, Handin RI, Davidson AJ. Identification of adult nephron progenitors capable of kidney regeneration in zebrafish. Nature. 2011;470:95–100. doi: 10.1038/nature09669. doi: 10.1038/nature09669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dressler GR. The cellular basis of kidney development. Annu Rev Cell Dev Biol. 2006;22:509–529. doi: 10.1146/annurev.cellbio.22.010305.104340. [DOI] [PubMed] [Google Scholar]
- Drummond IA, Majumdar A, Hentschel H, Elger M, Solnica-Krezel L, Schier AF, Neuhauss SC, Stemple DL, Zwartkruis F, Rangini Z, Driever W, Fishman MC. Early development of the zebrafish pronephros and analysis of mutations affecting pronephric function. Development. 1998;125:4655–4667. doi: 10.1242/dev.125.23.4655. [DOI] [PubMed] [Google Scholar]
- Galloway JL, Wingert RA, Thisse C, Thisse B, Zon LI. Combinatorial regulation of novel erythroid gene expression in zebrafish. Exp Hematol. 2008;36:424–432. doi: 10.1016/j.exphem.2007.11.015. doi: 10.1016/j.exphem.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvez-Santisteban M, Rodriguez-Fraticelli AE, Bryant DM, Vergarajauregui S, Yasuda T, Banon-Rodriguez I, Bernascone I, Datta A, Spivak N, Young K, Slim CL, Brakeman PR, Fukuda M, Mostov KE, Martin-Belmonte F. Synaptotagmin-like proteins control the formation of a single apical membrane domain in epithelial cells. Nat Cell Biol. 2012;14:838–849. doi: 10.1038/ncb2541. doi: 10.1038/ncb2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlach GF, Schrader LN, Wingert RA. Dissection of the adult zebrafish kidney. J Vis Exp. 2011;54:e2839. doi: 10.3791/2839. doi: 10.3791/2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlach GF, Wingert RA. Kidney organogenesis in the zebrafish: insights into vertebrate nephrogenesis and regeneration. Wiley Interdiscip Rev Dev Biol. 2013;2:559–585. doi: 10.1002/wdev.92. doi: 10.1002/wdev.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartelben B, Widmeier E, Suhm M, Worthmann K, Schell C, Helmstädter M, Wiech T, Walz G, Leitges M, Schiffer M, Huber TB. aPKCl/I and aPKCz contribute to podocyte differentiation and glomerular maturation. J Am Soc Nephrol. 2013;24:253–267. doi: 10.1681/ASN.2012060582. doi: 10.1681/ASN.2012060582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose T, Satoh D, Kurihara H, Kusaka C, Hirose H, Akimoto K, Matsusaka T, Ichikawa I, Noda T, Ohno D. An essential role of the universal polarity protein, aPKC lambda, on the maintenance of podocyte slit diaphragms. PLoS One. 2009;4:e4194. doi: 10.1371/journal.pone.0004194. doi: 10/1371/journal.pone.0004194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne-Badovinac S, Lin D, Waldron S, Schwarz M, Mbamalu G, Pawson T, Jan YN, Stainier DY, Abdelilah-Seyfried S. Positional cloning of heart and soul reveals multiple roles for PKC lambda in zebrafish organogenesis. Curr Biol. 2001;11:1492–1502. doi: 10.1016/s0960-9822(01)00458-4. [DOI] [PubMed] [Google Scholar]
- Horsfield J, Ramachandran A, Reuter K, LaVallie E, Collins-Racie L, Crosier K, Crosier P. Cadherin-17 is required to maintain pronephric duct integrity during zebrafish development. Mech Dev. 2002;115:15–26. doi: 10.1016/s0925-4773(02)00094-1. [DOI] [PubMed] [Google Scholar]
- Huber TB, Hartleben B, Winkelmann K, Schneider L, Becker JU, Leitges M, Walz G, Haller H, Schiffer M. Loss of podocyte aPKC lambda/iota causes polarity defects and nephrotic syndrome. J Am Soc Nephrol. 2009;20:798–806. doi: 10.1681/ASN.2008080871. doi: 10.1681/ASN.2008080871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu YH, Lin WL, Hou YT, Pu YS, Shun CT, Chen CL, Wu YY, Chen JY, Chen TH, Jou TS. Podocalyxin EBP50 ezrin molecular complex enhances the metastatic potential of renal cell carcinoma through recruiting Rac1 guanine nucleotide exchange factor ARHGEF7. Am J Pathol. 2010;176:3050–61. doi: 10.2353/ajpath.2010.090539. doi: 10.2353/ajpath.2010.090539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imgrund M, Grone HJ, Kretzler M, Holzman L, Schlondorff D, Rothenpieler UW. Re-expression of the developmental gene Pax-2 during experimental acute tubular necrosis in mice. Kidney Int. 1999;56:1423–1431. doi: 10.1046/j.1523-1755.1999.00663.x. [DOI] [PubMed] [Google Scholar]
- Johnson CS, Holzemer NF, Wingert RA. Laser ablation of the zebrafish pronephros to study renal epithelial regeneration. J Vis Exp. 2011;54:e2845. doi: 10.3791/2845. doi: 10.3791/2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung JJ, Inamdar SM, Tiwari A, Ye D, Lin F, Choudhury A. Syntaxin 16 regulates lumen formation during epithelial morphogenesis. PLoS One. 2013;8:e61857. doi: 10.1371/journal.pone.0061857. doi: 10.1371/journal/pone.0061857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao RM, Vasilyev A, Miyawaki A, Drummond IA, McMahon AP. Invasion of distal nephron precursors associates with tubular interconnection during nephrogenesis. J Am Soc Nephrol. 2012;23:1682–1690. doi: 10.1681/ASN.2012030283. doi: 10.1681/ASN.2012030283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- Kovac J, Oster H, Leitges M. Expression of the atypical protein kinase C (aPKC) isoforms iota/lambda and zeta during mouse embryogenesis. Gene Expr Patterns. 2007;7:187–96. doi: 10.1016/j.modgep.2006.07.002. doi: 10/1016/j.modgep.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Kramer-Zucker AG, Wiessner S, Jensen AM, Drummond IA. Organization of the pronephric filtration apparatus in zebrafish requires Nephrin, Podocin, and the FERM domain protein Mosaic eyes. Dev Biol. 2005;285:316–329. doi: 10.1016/j.ydbio.2005.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroeger PT, Jr., Poureetezadi SJ, McKee R, Jou J, Miceli R, Wingert RA. Production of haploid zebrafish embryos by in vitro fertilization. J Vis Exp. 2014;89:e51708. doi: 10.3791/51708. doi: 10.3791/51708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroeger PT, Jr., Wingert RA. Using zebrafish to study podocyte genesis during kidney development and regeneration. Genesis. 2014 doi: 10.1002/dvg.22798. doi:10.1002/dvg.22798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupinski T, Beitel GJ. Unexpected roles of the Na-K-ATPase and other ion transporters in cell junctions and tubulogenesis. Physiology (Bethesda) 2009;24:192–201. doi: 10.1152/physiol.00008.2009. doi: 10/1152/physiol.00008.2009. [DOI] [PubMed] [Google Scholar]
- Lam PY, Webb SE, Leclerc C, Moreau M, Miller AL. Inhibition of stored Ca2+ release disrupts convergence-related cell movements in the lateral intermediate mesoderm resulting in abnormal positioning and morphology of the pronephric anlagen in intact zebrafish embryos. Develop Growth Difffer. 2009;51:429–442. doi: 10.1111/j.1440-169X.2009.01106.x. doi: 10.1111/j.1440-169X.2009.01106.x. [DOI] [PubMed] [Google Scholar]
- Lengerke C, Wingert R, Beeretz M, Grauer M, Schmidt AG, Konantz M, Daley GQ, Davidson AJ. Interactions between Cdx genes and retinoic acid modulate early cardiogenesis. Dev Biol. 2011;163:134–142. doi: 10.1016/j.ydbio.2011.03.027. doi: 10.1016/j.ydbio.2011.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitges M, Sanz L, Martin P, Duran A, Braun U, García JF, Camacho F, Diaz-Meco MT, Rennert PD, Moscat J. Targeted disruption of the zeta PKC gene results in the impairment of the NF-kappaB pathway. Mol Cell. 2001;8:771–80. doi: 10.1016/s1097-2765(01)00361-6. [DOI] [PubMed] [Google Scholar]
- Li Y, Wingert RA. Regenerative medicine for the kidney: stem cell prospects and challenges. Clin Trans Med. 2013;2:11. doi: 10.1186/2001-1326-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Cheng CN, Verdun VA, Wingert RA. Zebrafish nephrogenesis is regulated by interactions between retinoic acid, mecom, and Notch signaling. Dev Biol. 2014;386:111–122. doi: 10.1016/j.ydbio.2013.11.021. doi: 10.1016/j.ydbio.2013.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little M, Georgas K, Pennisi D, Wilkinson L. Kidney development: two tales of tubulogenesis. Curr Top Dev Biol. 2010;90:193–229. doi: 10.1016/S0070-2153(10)90005-7. doi: 10.1016/S0070-2153(10)90005-7. [DOI] [PubMed] [Google Scholar]
- Liu Y, Pathak N, Kramer-Zucker A, Drummond IA. Notch signaling controls the differentiation of transporting epithelia and multiciliated cells in the zebrafish pronephros. Development. 2006;134:1111–22. doi: 10.1242/dev.02806. [DOI] [PubMed] [Google Scholar]
- Majumdar A, Drummond IA. Podocyte differentiation in the absence of endothelial cells as revealed in the zebrafish avascular mutant, cloche. Dev Genet. 1999;24:220–9. doi: 10.1002/(SICI)1520-6408(1999)24:3/4<220::AID-DVG5>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Majumdar A, Drummond IA. The zebrafish floating head mutant demonstrates podocytes play an important role in directing glomerular differentiation. Dev Biol. 2000;222:147–157. doi: 10.1006/dbio.2000.9642. [DOI] [PubMed] [Google Scholar]
- Majumdar A, Lun K, Brand M, Drummond IA. Zebrafish no isthmus reveals a role for pax2.1 in tubule differentiation and patterning events in the pronephric primordia. Development. 2000;127:2089–2098. doi: 10.1242/dev.127.10.2089. [DOI] [PubMed] [Google Scholar]
- Marra A, Wingert RA. Roles of Iroquois transcription factors in kidney development. Cell Dev Biol. 2014;3:131. doi: 10.4172/2168-9296.1000131. doi: 10.4172/2168-9296.1000131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCampbell KK, Wingert RA. Renal stem cells: fact or science fiction? Biochem J. 2012;444:153–168. doi: 10.1042/BJ20120176. doi: 10.1042/BJ20120176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCampbell KK, Wingert RA. New tides: using zebrafish to study renal regeneration. Transl Res. 2014;163:109–122. doi: 10.1016/j.trsl.2013.10.003. doi: pii: S1931-5244(13)00346-0.10.1016/j.trsl.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCampbell KK, Springer KN, Wingert RA. Analysis of nephron composition and function in the adult zebrafish kidney. J Vis Exp. 2014;90:e51644. doi: 10.3791/51644. doi: 10.3791/51644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarroll MN, Lewis ZR, Culbettson MD, Martin BL, Kimelman D, Nechiporuk AV. Graded levels of Pax2a and Pax8 regulate differentiation during sensory placode formation. Development. 2012;139:2740–2750. doi: 10.1242/dev.076075. doi: 10.1242/dev.076075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meder D, Shevchenko A, Simons K, Füllekrug J. Gp135/podocalyxin and NHERF-2 participate in the formation of a preapical domain during polarization of MDCK cells. J Cell Biol. 2005;168:303–13. doi: 10.1083/jcb.200407072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miceli R, Kroeger PT, Jr., Wingert RA. Molecular mechanisms of podocyte development revealed by zebrafish kidney research. Cell Dev Biol. 2014;3:138. doi: 10.4172/2168-9296.1000138. doi: 10.4172/2168-9296.1000138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra S, Lukianov S, Ruiz WG, Cosentino CC, Sanker S, Traub LM, Hukriede NA, Apodaca G. Requirement for a uroplakin 3a-like protein in the development of zebrafish pronephric tubule epithelial cell function, morphogenesis, and polarity. PLoS One. 2012;7:e41816. doi: 10.1371/journal.pone.0041816. doi: 10.1371/journal.pone.0041816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naylor RW, Przepiorski A, Ren Q, Yu J, Davidson AJ. HNF1B is essential for nephron segmentation during nephrogenesis. J Am Soc Nephrol. 2013;24:77–87. doi: 10.1681/ASN.2012070756. doi: 10.1681/ASN.2012070756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien LL, Grimaldi M, Kostun Z, Wingert RA, Selleck R, Davidson AJ. Wt1a, Foxc1a, and the Notch mediator Rbpj physically interact and regulate the formation of podocytes in zebrafish. Dev Biol. 2011;358:318–30. doi: 10.1016/j.ydbio.2011.08.005. doi: 10.1016/j.ydbio.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patten SA, Sihra RK, Dhami KS, Coutts CA, Ali DW. Differential expression of PKC isoforms in developing zebrafish. Int J Dev Neuroscience. 2007;25:155–164. doi: 10.1016/j.ijdevneu.2007.02.003. doi: 10.1016/j.ijdevneu. 2007.02.003. [DOI] [PubMed] [Google Scholar]
- Peterson RT, Mably JD, Chen JN, Fishman MC. Convergence of distinct pathways to heart patterning revealed by the small molecule concentramide and the mutation heart-and-soul. Curr Biol. 2001;11:1481–91. doi: 10.1016/s0960-9822(01)00482-1. [DOI] [PubMed] [Google Scholar]
- Pieczynski J, Margolis B. Protein complexes that control renal epithelial polarity. Am J Physiol Renal Physiol. 2011;300:F589–601. doi: 10.1152/ajprenal.00615.2010. doi: 10.1152/ajprenal.00615.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poureetezadi SJ, Wingert RA. Congenital and acute kidney disease: translational research insights from zebrafish chemical genetics. General Med. 2013;1:112. doi: 10.4172/2327-5146.1000112. doi: 10.4172/2327-5146.1000112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajasekaran SA, Palmer LG, Moon SY, Peralta Soler A, Apodaca GL, Harper JF, Zheng Y, Rajasekaran AK. Na,K-ATPase activity is required for formation of tight junctions, desmosomes, and induction of polarity in epithelial cells. Mol Biol Cell. 2001;12:3717–32. doi: 10.1091/mbc.12.12.3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly RF, Bulger RE, Kriz W. Structural-functional relationships in the kidney. Diseases of the Kidney and Urinary Tract. Lippincott Williams & Wilkins; Philadelphia: 2007. pp. 2–53. [Google Scholar]
- Roignot J, Peng X, Mostov K. Polarity in mammalian epithelial morphogenesis. Cold Spring Harbor Perspect Biol. 2013;5:a013789. doi: 10.1101/cshperspect.a013789. doi: 10.1101/cshperspect.a013789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohr S, Bit-Avragim N, Abdelilah-Seyfried S. Heart and soul/PRKCi and nagie oko/Mpp5 regulate myocardial coherence and remodeling during cardiac morphogenesis. Development. 2006;133:107–115. doi: 10.1242/dev.02182. doi: 10.1242/dev.02182. [DOI] [PubMed] [Google Scholar]
- Schlüter MA, Pfarr CS, Pieczynski J, Whiteman EL, Turd TW, Fan S, Liu CJ, Margolis B. Trafficking of Crumbs3 during cytokinesis is crucial for lumen formation. Mol Biol Cell. 2009;20(22):4652–4663. doi: 10.1091/mbc.E09-02-0137. doi: 10.1091/mbc.E09-02-0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlüter MA, Margolis B. Apical lumen formation in renal epithelia. J Am Soc Nephrol. 2009;20:1444–52. doi: 10.1681/ASN.2008090949. doi: 10.1681/ASN.2008090949. [DOI] [PubMed] [Google Scholar]
- Schlüter MA, Margolis B. Apicobasal polarity in the kidney. Exp Cell Res. 2012;318:1033–9. doi: 10.1016/j.yexcr.2012.02.028. doi: 10.1016/j.yexcr.2012.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serluca FC, Fishman MC. Pre-pattern in the pronephric kidney field of zebrafish. Development. 2002;128:2233–2241. doi: 10.1242/dev.128.12.2233. [DOI] [PubMed] [Google Scholar]
- Serluca FC, Drummond IA, Fishman MC. Endothelial signaling in kidney morphogenesis: a role for hemodynamic forces. Curr Biol. 2002;12:492–497. doi: 10.1016/s0960-9822(02)00694-2. [DOI] [PubMed] [Google Scholar]
- Serluca FC. Development of the proepicardial organ in the zebrafish. Dev Biol. 2008;315:18–27. doi: 10.1016/j.ydbio.2007.10.007. doi: 10.1016/j.ydbio.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Soloff RS, Katayama C, Lin MY, Feramisco JR, Hedrick SM. Targeted deletion of protein kinase C lambda reveals a distribution of functions between the two atypical protein kinase C isoforms. J Immunol. 2004;173:3250–60. doi: 10.4049/jimmunol.173.5.3250. [DOI] [PubMed] [Google Scholar]
- Uhalte EC, Kirchner M, Hellwig N, Allen JJ, Donat S, Shokat KM, Selbach M, Abdelilah-Seyfried S. In vivo conditions to identify Prkci phosphorylation targets using the analog-sensitive kinase method in zebrafish. PLoS One. 2012;7:e40000. doi: 10.1371/journal.pone.0040000. doi:10.1371/journal.pone.0040000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasilyev A, Liu Y, Mudumana S, Mangos S, Lam PY, Majumdar A, Zhao J, Poon KL, Kondrychyn I, Korzh V, Drummond IA. Collective cell migration drives morphogenesis of the kidney nephron. PLoS Biol. 2009;7:e9. doi: 10.1371/journal.pbio.1000009. doi: 10.1371/journal.pbio.1000009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerfield M. The zebrafish book. University of Oregon Press; Eugene: 1993. [Google Scholar]
- Wilson PD. Apico-basal polarity in polycystic kidney disease epithelia. Biochim Biophys Acta. 2011;1812:1239–48. doi: 10.1016/j.bbadis.2011.05.008. doi: 10.1016/j.bbadis.2011.05.008. [DOI] [PubMed] [Google Scholar]
- Wingert RA, Selleck R, Yu J, Song HD, Chen Z, Song A, Zhou Y, Thisse B, Thisse C, McMahon AP, Davidson AJ. The cdx genes and retinoic acid control the positioning and segmentation of the zebrafish pronephros. PLoS Genet. 2007;3:1922–1938. doi: 10.1371/journal.pgen.0030189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingert RA, Davidson AJ. The zebrafish pronephros: a model to study nephron segmentation. Kid Int. 2008;73:1120–1127. doi: 10.1038/ki.2008.37. doi: 10.1038/ki.2008.37. [DOI] [PubMed] [Google Scholar]
- Wingert RA, Davidson AJ. Zebrafish nephrogenesis involves dynamic spatiotemporal expression changes in renal progenitors and essential signals from retinoic acid and irx3b. Dev Dyn. 2011;240:2011–2027. doi: 10.1002/dvdy.22691. doi: 10/1002/dvdy.22691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Zimmerman S, Brakeman PR, Beaudoin GM, 3rd, Reichardt LF, Marciano DK. De novo lumen formation and elongation in the developing nephron: a central role for afadin in apical polarity. Development. 2013;140:1774–84. doi: 10.1242/dev.087957. doi: 10.1242/dev.087957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yelon D, Horne SA, Stainier DY. Restricted expression of cardiac myosin genes reveals regulated aspects of heart tube assembly in zebrafish. Dev Biol. 1999;214:23–27. doi: 10.1006/dbio.1999.9406. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.