Abstract
The surge in noninvasive brain stimulation studies investigating cognitive enhancement has neglected the effect of interindividual differences, such as traits, on stimulation outcomes. Using the case of mathematics anxiety in a sample of healthy human participants in a placebo-controlled, double-blind, crossover experiment, we show that identical transcranial direct current stimulation (tDCS) exerts opposite behavioral and physiological effects depending on individual trait levels. Mathematics anxiety is the negative emotional response elicited by numerical tasks, impairing mathematical achievement. tDCS was applied to the dorsolateral prefrontal cortex, a frequent target for modulating emotional regulation. It improved reaction times on simple arithmetic decisions and decreased cortisol concentrations (a biomarker of stress) in high mathematics anxiety individuals. In contrast, tDCS impaired reaction times for low mathematics anxiety individuals and prevented a decrease in cortisol concentration compared with sham stimulation. Both groups showed a tDCS-induced side effect—impaired executive control in a flanker task—a cognitive function subserved by the stimulated region. These behavioral and physiological double dissociations have implications for brain stimulation research by highlighting the role of individual traits in experimental findings. Brain stimulation clearly does not produce uniform benefits, even applied in the same configuration during the same tasks, but may interact with traits to produce markedly opposed outcomes.
Keywords: brain stimulation, cognitive cost, cognitive enhancement, dorsolateral prefrontal cortex, individual differences, mathematics anxiety
Introduction
Transcranial electrical stimulation (tES) produces benefits across the psychological spectrum (Jacobson et al., 2012). However, one relatively neglected line of research is the evaluation of how interindividual differences affect tES outcomes (Krause and Cohen Kadosh, 2014). Indeed, recent evidence has alerted to interindividual variability in responsiveness to stimulation (López-Alonso et al., 2014; Wiethoff et al., 2014). We explored this theme with regard to mathematics anxiety, the fear elicited by numerical tasks (Richardson and Suinn, 1972), which affects performance in varied situations, from examinations to tipping. Mathematics anxiety provides a useful model of trait differences. Similar to conventional traits, it possesses physiological markers, (e.g., activation of brain regions associated with pain perception; Lyons and Beilock, 2012a), cognitive markers (e.g., working memory impairments during complex mathematics tasks; Ashcraft and Kirk, 2001), and behavioral markers (e.g., poorer performance, avoiding careers emphasizing numerical ability; Hembree, 1990).
The premier theory of mathematics anxiety suggests that the automatic perception and interpretation of numerical tasks as negative, and the subsequent emotional response, consume cognitive resources necessary for task completion (Ashcraft and Kirk, 2001). Neuroimaging evidence suggests failure to regulate negative emotions during numerical tasks may be responsible for the poorer performance commonly associated with mathematics anxiety. For instance, Young et al. (2012) found amygdala hyperactivity, implicated in processing fear (Phelps and LeDoux, 2005), in high mathematics anxiety children exposed to mathematical stimuli. The anxious response may be ameliorated by applying transcranial direct current stimulation (tDCS) to the dlPFC, a frequent target for emotional control in stimulation studies (Boggio et al., 2009; see Materials and Methods). Enhancing emotional control should decrease perceptions of numerical tasks as negative/stressful.
Based on findings supporting the reduction of negative emotions through tDCS (Boggio et al., 2009), high mathematics anxiety participants were expected to benefit from stimulation while performing a numerical task. We included a low mathematics anxiety group for comparative purposes to examine how identical stimulation might interact with low levels of the same trait.
Materials and Methods
Participants.
The tES compatibility criteria included absence of psychiatric/neurological disorders, psychoactive medication, and personal/family epilepsy. We screened 165 individuals using the brief version of the Mathematics Anxiety Rating Scale (Suinn and Winston, 2003). The high mathematics anxiety group comprised 25 participants (≥75th percentile; 10 males, mean age = 21.4 years, SD = 3.5, 2 left-handed), and the low mathematics anxiety group comprised 20 participants (≤25th percentile; 13 males, mean age = 23.54 years, SD = 3.11, 1 left-handed). The groups did not differ in age (t(43) = −0.29, p = 0.77) or gender (Fisher's exact test, p = 0.14). Participants gave written informed consent. The Berkshire Research Ethics Committee approved the study (10/H0505/72).
Stimulation site.
Previous research has established the role of the dlPFC in affective control (Lorenz et al., 2003), including in amygdala interactions during affective control (Banks et al., 2007). Furthermore, activity in the frontoparietal network, including the dlPFC, in high mathematics anxiety individuals enhances performance compared with high mathematics anxiety individuals who did not show such activation (Lyons and Beilock, 2012b).
tDCS is the application of a direct-form electrical signal that shifts cortical neuronal resting membrane potentials, with anodal tDCS typically exhibiting excitatory effects through subthresholding neuronal depolarization and cathodal tDCS typically displaying inhibitory effects through hyperpolarization (Fritsch et al., 2010). In sham stimulation, current is applied briefly, generating the same cutaneous sensations as real stimulation, but without altering excitability, therefore serving as a reliable placebo (Gandiga et al., 2006).
We applied bilateral tDCS to the dlPFC to assess performance changes on arithmetic decision tasks for individuals with high and low mathematics anxiety. We also investigated the possibility that tDCS modulates a physiological correlate of anxiety: salivary cortisol, a biomarker of stress (Hellhammer et al., 2009). The specific configuration (anode-F3 and cathode-F4, based on the 10–20 international electroencephalography system) relates to the lateralized properties of the dlPFC: the left-dlPFC has been associated with positive emotional processing and emotional control (Herrington et al., 2005; Wolkenstein et al., 2014), while the right dlPFC has been linked to negative emotional processing, and activation variation in this region correlates with threat-evoked anxiety and avoidance-oriented/withdrawal behavior (Dalton et al., 2005; Shackman et al., 2009). Therefore, it is expected that excitatory (anodal) stimulation to the left dlPFC and inhibitory (cathodal) stimulation to the right dlPFC would produce the greatest reduction in negative emotional reactions. Indeed, Brunoni et al. (2013) confirmed this by comparing all three cases (anodal-F3, cathodal-F4; anodal-F4, canthodal-F3; sham), finding that the anodal-F3, cahtodal-F4 montage exerted the largest decrease in salivary cortisol, hence, our adoption of the same configuration.
tDCS was conveyed at 1 mA via a battery-driven stimulator (NeuroConn) for 30 min (real) and 30 s (sham condition). Rubber electrodes, 5 × 5 cm, were encased in saline-soaked sponges.
Procedure.
We followed a double-blind, placebo-controlled, crossover design. Participants attended two sessions separated by at least 1 week, while receiving real or sham tDCS. Because of scheduling constraints, one participant was tested with a 5 d gap, and another was tested 24 h apart. The results were not affected by their removal from the analysis, so they were retained. Participants were informed beforehand that they would be given a mathematics task. All participants completed a computerized task that combined arithmetic decisions with affective primes (Rubinsten et al., 2012). Participants were first primed with a valenced word (positive or negative), and then decided whether a simple equation was true or false (e.g., 6 + 2 = 16). Every participant provided saliva samples before and after stimulation. To minimize influence of intracircadian variation on cortisol, participants attended both sessions at a similar time of day. To examine the possibility of effects on other cognitive abilities (Iuculano and Cohen Kadosh, 2013), participants completed the attentional networks task (ANT; Fan et al., 2002) in both sessions after the arithmetic decision task. The ANT assesses three variables: orienting, alerting, and executive control. This allowed for an examination of changes in performance in cognitive functions that are reliant on the dlPFC (e.g., executive control). At the beginning of the first session, we also assessed mathematics achievement using the relevant section of the Wechsler Individual Achievement Test (WIAT-II UK; Wechsler, 2005), which assesses performance on two subscales: numerical operations and mathematical reasoning.
In this study, we did not examine changes in working memory capacity because the central theory of mathematics anxiety suggests that working memory is only necessary for more complex arithmetic, such as carryover operations or the maintenance of intermediate steps, and is unlikely to be deployed for simpler processes (Ashcraft and Kirk, 2001; Ashcraft, 2002), such as the one in the present study, which only involved verification of simple arithmetic facts against existing knowledge (see below).
Affective priming and arithmetic decision task.
Similar to Rubinsten et al. (2012), every trial began with a 500 ms fixation cross in the center of the screen, followed by the presentation of the prime for 250 ms. The target equation was then presented for 3000 ms, and the participant had to decide whether it was true or false by pressing the “p” or “q” on the keyboard, respectively. The positive (e.g., “success”) and negative (e.g., “failure”) sets were composed of 10 primes each. One prime preceded each arithmetic decision target. Predetermined prime-target pairs were presented at random.
As in Rubinsten et al. (2012), targets were equations in the format AϕB = C. A and B were numbers between 1 and 9, and C was the solution. ϕ represented a cardinal operation: addition (+), subtraction (−), multiplication (×) and division (:). C was always a positive whole number and each of the four possible ϕ resulted in a different C. Applying these constraints, the following AϕB were usable: 9ϕ3, 8ϕ4, 8ϕ2, 6ϕ3, and 6ϕ2. Each ϕ represented one of four operations, such that 20 “true” targets were derivable from the set. The solutions belonged to one of two conditions: (1) “true,” C was the correct solution to the equation (e.g., 8 × 2 = 16) and (2) “false,” the solution presented was borrowed from another equation, such that C was incorrect. For example, a false solution for 8 × 2 is 3, where 3 is the correct solution for 6:2. In this way, 40 equations were generated, 20 correct and 20 false.
The participant encountered each prime of the 20 primes 32 times, 16 preceding true equations, and 16 preceding false ones, amounting to a total of 640 trials (because of technical errors, one participant completed 320 trials, and one completed 590 trials). Each prime preceded two true and two false equations.
Salivary cortisol.
Participants provided 2 ml of saliva in sterile test tubes. The first sample was provided immediately after giving informed consent. The second sample was collected after completion of the arithmetic decision task. Test tubes were centrifuged at 2500 g for 10 min to remove cellular and exogenous debris, and stored at −20°C before cortisol assays. Analysis was performed using a Salimetrics cortisol ELISA kit following the manufacturer's recommendations. Saliva samples from two high mathematics anxiety participants were discarded due to experimenter error.
Results
Arithmetic decisions
Reaction time
Incorrect responses were removed from the analysis. A 2(stimulation: real/sham) × 2(valence: positive/negative) × 2(mathematics anxiety: high/low) mixed-model ANCOVA was used with stimulation type and valence as the within-subject factors, mathematics anxiety as the between-subjects factor, and stimulation order (sham-real, real-sham) as a covariate.
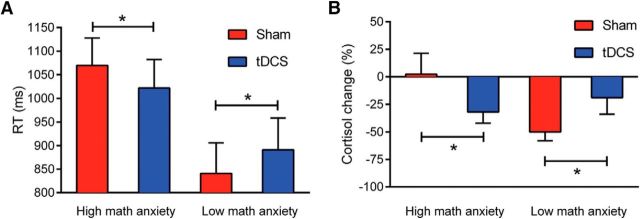
Mathematics anxiety exerted a significant main effect (F(1,42) = 8.5, p = 0.006, ηp2 = 0.17), with the low mathematics anxiety group showing faster reaction times (RTs) than the high mathematics anxiety group. Central to the hypothesis, the main effect of stimulation reached significance (F(1,42) = 249.24, p < 0.001, ηp2 = 0.86), and interacted with mathematics anxiety (F(1,42) = 14.76, p < 0.001, ηp2 = 0.26). In the high mathematics anxiety group, participants performed significantly faster in real than sham stimulation (F(1,23) = 185.06, p < 0.001, ηp2 = 0.89). In contrast, RTs were longer in real compared with sham stimulation for low mathematics anxiety participants (F(1,18) = 76.06, p < 0.001, ηp2 = 0.81; Fig. 1A). There were no significant effects of valence (F(1,42) = 2.68, p = 0.11, ηp2 = 0.06), stimulation × valence and mathematics anxiety × valence (Fs < 1).
Figure 1.
Mathematics anxiety-dependent effects of tDCS. A, Behavioral double dissociation between high and low mathematics anxiety groups. For the high mathematics anxiety group (n = 25), tDCS produced faster arithmetic decisions compared with sham stimulation. For the low mathematics anxiety group (n = 20), tDCS impaired arithmetic decision RTs compared with sham (both ps < 0.001). B, Double dissociation in salivary cortisol concentration between high and low mathematics anxiety. Negative cortisol change indicates lower cortisol concentrations at post-test compared with pre-test. High mathematics anxiety participants showed greater cortisol reductions after real stimulation, while low mathematics anxiety participants showed greater cortisol reductions after sham stimulation (both ps < 0.05). *p < 0.05. Error bars represent 1SEM.
Accuracy
We employed the factorial design used for RT. The main effects of stimulation and mathematics anxiety, and the stimulation type × mathematics anxiety interaction, were not significant (sham stimulation: Mhigh anxiety = 0.91, SD = 0.06; Mlow anxiety = 0.93, SD = 0.03; real stimulation Mhigh anxiety = 0.91, SD = 0.04; Mlow anxiety = 0.92, SD = 0.05; both Fs < 1). The valence × mathematics anxiety interaction was significant (F(1,42) = 5.64, p < 0.022, ηp2 = 0.12), but further analysis of this interaction did not yield any significant effects. Correlations between accuracy and RTs in each stimulation condition were nonsignificant, indicating no speed-accuracy trade-off (sham stimulation, r(43) = −0.21; real stimulation, r(43) = − 0.07, both ps > 0.16).
Salivary cortisol
Participants provided saliva samples before and after each session of the arithmetic decision task, or four samples per participant. To examine tDCS-induced changes in cortisol, data were standardized to account for change from prestimulation concentrations, similar to Brunoni et al. (2013), according to the following equation:
 |
Data were analyzed using a 2(stimulation: sham/real) × 2(mathematics anxiety: high/low) mixed-model ANCOVA with stimulation as the within-subjects factor, mathematics anxiety as the between-subjects factor, and stimulation order as a covariate. There was a significant main effect for stimulation (F(1,40) = 11.59, p = 0.002, ηp2 = 0.22). Critically, the stimulation×mathematics anxiety reached significance (F(1,40) = 6.91, p = 0.012, ηp2 = 0.14). We decomposed the interaction by analyzing high and low mathematics anxiety groups separately. For the high anxiety group, the change was larger during real than sham stimulation (Fig. 1B; F(1,21) = 4.42, p < 0.05, ηp2 = 0.17). Moreover, a one-sample t test showed that cortisol changes differed significantly from zero only during real tDCS (real tDCS: t(22) = −3.22, p < 0.005, d = 1.37; sham tDCS: t(22) = −0.13, p = 0.9, d = −0.05). Analogous to the behavioral results, the low-anxiety group exhibited a significant effect in the opposite direction: a larger change for sham than for real stimulation (F(1,18) = 7.29, p < 0.05, ηp2 = 0.28; Fig. 1B). Notably, only the cortisol changes under sham stimulation significantly differed from zero (t(19) = −5.52, p < 0.001, d = 2.53; real stimulation: t(19) = −1.46, p = 0.16, d = 0.66).
Mediation analysis
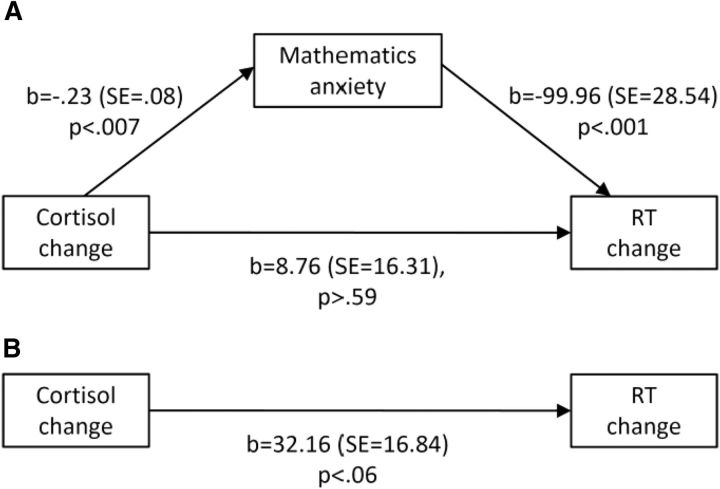
We performed mediation analysis with bootstrapping (10,000 samples; Hayes, 2013) to examine the effect of mathematics anxiety on the tDCS-induced changes in arithmetic decision RT and cortisol concentrations (Fig. 2A,B). The RT and cortisol differences were calculated as sham − real stimulation, with order as a covariate. When we entered cortisol change as a predictor of arithmetic decision RT, mathematics anxiety fully mediated these changes, and the model was significant and effective at accounting for variance (R2 = 0.87, F(3,39) = 93.25, p < 0.00001; 95% Bias Corrected Confidence Intervals for the mediating variable: [8.09–46.45]). We reapplied the model with RT change as a predictor of cortisol change. This model was inferior (R2 = 0.33, F(3,39) = 6.27, p < 0.001). When we removed the mediator, the link between cortisol change and RT change was nonsignificant.
Figure 2.
Mediation of the effect of cortisol change on RT change by mathematics anxiety. A, Mediation analysis suggests that the link between cortisol change and RT change due to tDCS is fully mediated by mathematics anxiety, and the model was highly effective at accounting for variance in performance (R2 = 0.87, p < 0.00001). B, On excluding the mediator, only 8.4% of the variance was explained, and was marginally significant.
Attentional networks test
Median RTs for correct responses on the ANT were transformed into network scores for alerting (reaching and sustaining a state of alertness), orienting (choosing stimuli from an array of perceptual information), and executive control (deciding between conflicting responses; Fan et al., 2002). Network scores were analyzed separately using a 2(stimulation: sham/real) × 2(mathematics anxiety: high/low) mixed-model ANCOVA with stimulation as the within-subjects factor and mathematics anxiety as the between-subjects factor with stimulation order as a covariate. Two participants were unable to complete the task because of time constraints. The only significant finding was the main effect of stimulation on executive control scores (F(1,40) = 10.6, p = 0.002, ηp2 = 0.21), with Msham = 82 (SD = 30.12) and Mreal = 86 (SD = 39.16).
Because executive control scores are calculated as RTs on incongruent trials minus RTs on congruent trials, larger scores indicate poorer executive control. Therefore, it appears that executive control was impaired by real compared with sham tDCS. Similar analysis using mean accuracy, transformed into network scores, did not reveal significant main effects or interactions (all Fs < 1).
We examined the correlation between changes in arithmetic decision RTs and executive control, controlling for order. The difference in RTs and executive control scores was calculated as sham − real stimulation. The relationship was nonsignificant (rp(40) = 0.002, p > 0.99).
Mathematical achievement
Participants' mathematical achievement was assessed using the WIAT-II UK. Data were examined using a 2(WIAT subscale: numerical operations/mathematical reasoning) × 2(mathematics anxiety: high/low) between-subjects ANOVA. There was no main effect for the WIAT subscales (F < 1), nor was the subscale × mathematics anxiety interaction significant (F < 1). However, there was a significant main effect of mathematics anxiety (F(1,42) = 10.39, p = 0.002, ηp2 = 0.20). The low mathematics anxiety group significantly outperformed the high-anxiety group on both subscales (mathematical reasoning: Mhigh anxiety = 106.84, SD = 11.39; Mlow anxiety = 115.85, SD = 7.15, and F(1,43) = 9.48, p = 0.004; numerical operations: Mhigh anxiety = 105.10, SD = 15.49; Mlow anxiety = 116.25, SD = 8.39, and F(1,43) = 8.39, p = 0.006). However, the high mathematics anxiety group was certainly not below the population average, and well above the level required to verify the facts that composed the arithmetic decision task. When we included WIAT subscale scores as covariates to arithmetic decision performance, all terms relating to either of the WIAT subscales were nonsignificant (all Fs < 1), and the reported effects remained significant (all ps < 0.004). This suggests the observed changes are attributable to mathematics anxiety rather than mathematical achievement.
Gender
To examine possible gender effects, we repeated the arithmetic decision RT analysis with gender as a covariate. However, none of the gender-linked terms reached significance (all Fs < 1), and the reported stimulation effects remained significant (all ps < 0.001). Similar results were obtained for the cortisol analysis with gender as a covariate. Again, the gender-related effects were nonsignificant (all Fs < 1), while the reported effects remained significant (all ps < 0.015). These results also reduce concerns that effects may be attributable to factors such as menstrual cycle and contraceptive use.
Discussion
We showed that tDCS to the dlPFC produced mathematics anxiety-dependent changes in behavioral and physiological indices, independent of mathematical achievement or gender. To the best of our knowledge, such a trait-dependent double-dissociation has never been shown before with neuromodulation methods such as intracranial or transcranial stimulation. While tDCS research has shown differences in the extent of benefits for individuals with different characteristics (Tseng et al., 2012), no previous studies have shown that some individuals experience costs and others benefits. The pattern of changes in both RT and cortisol, which corroborate one another, demonstrates that trait differences can play a critical role in determining the outcome of brain stimulation.
Of course, being a correlational result, the direction of causality between RT and cortisol changes cannot be ascertained. The changes in cortisol may have been caused by an improvement in performance speed. However, as there was no difference in accuracy in real or sham stimulation, participants would have had to notice improvements in the order of milliseconds for this to be a viable explanation. Furthermore, the mediation analysis suggests cortisol changes affected RT changes, a link mediated by mathematics anxiety. Admittedly, such an inference derives from a statistical method, and future studies should establish the causal order experimentally.
The double dissociation may be interpreted in terms of an interaction between brain stimulation and trait-activated neural networks. Different mathematics anxiety levels produce different neural states that are task and situation specific. For example, high mathematics anxiety individuals show substantial amygdala activation and limited frontoparietal activity, including in the dlPFC, while low mathematics anxiety individuals do not show such abnormal subcortical activation and exhibit greater frontoparietal activity (Young et al., 2012). Distinct neural signatures may interact with stimulation to produce very different outcomes. In this sense, these results are also consistent with suggestions that neural state at the time of stimulation may influence stimulation effects (Silvanto et al., 2008). Another possibility is that stimulation-induced impairment and improvement effects depended on baseline cortical excitation-inhibition level (Krause et al., 2013), which may vary based on anxiety. This idea is supported by animal studies linking differential excitation-inhibition levels and anxiety (Davis et al., 1994; Wu et al., 2008). At any rate, this explanation by itself might be too simplistic to capture the exact mechanisms involved in the interactions between stimulation outcomes and anxiety in humans, and future research should focus on elucidating these mechanisms.
One might have expected cortisol concentrations to increase for the high mathematics anxiety group during sham stimulation, following the stress of arithmetic performance. However, the lack of change is consistent with extant research that the anticipation of mathematical performance produces the negative emotional state characterizing mathematics anxiety (Lyons and Beilock, 2012a). Because our participants were informed beforehand that the experiment entailed mathematics tasks, we would have expected elevated cortisol concentrations in the high mathematics anxiety participants, representing increased anxiety, before task onset, which may persist into the task and impair performance. It appears that tDCS helps to ameliorate this anxious anticipation and interference, reflected in lower post-test cortisol concentrations in real, but not sham, stimulation. In contrast, low mathematics anxiety participants may have been more successful in monitoring and controlling their arousal and responses during sham stimulation. This is reflected by faster RTs and greater reduction in cortisol concentrations during sham stimulation, processes which may have been disrupted during real tDCS.
Another important finding is that tDCS effects may be associated with cognitive costs in other domains. Poorer executive control in a non-mathematical, nonanxiety-inducing task after real compared with sham stimulation suggests that tDCS, while advantageous in certain contexts, may impair processes dependent on the stimulated region (Iuculano and Cohen Kadosh, 2013).
The central finding is that stimulation may be beneficial to some populations but detrimental to others, rather than merely less effective, depending on the trait. Future studies should carefully consider which characteristics may be associated with their hypotheses and control them accordingly. Traits can therefore serve as a source of information rather than noise. The double dissociation also answers neuroethical concerns over the possibility of increasing natural cognitive disparities using brain stimulation (Cohen Kadosh et al., 2012) by showing that it may not be a likely scenario. By demonstrating possible trait-dependent costs and benefits of tDCS, we hope these results will motivate further research on trait-based effects. Such research is essential given the increasing academic and public enthusiasm over tDCS (Dubljević et al., 2014), including do-it-yourself stimulation (Fitz and Reiner, 2013). These tendencies may overlook the importance of identifying traits and situations that respond to stimulation as costs with no demonstrable gains, as with our low mathematics anxiety participants.
Footnotes
R.C.K. is supported by the Wellcome Trust (WT88378). We thank Philip Burnet and Li Chen for performing the cortisol assays and Anna-Katharine Brem, Andrew Elliot, Kevin Jennison, Beatrix Krause, and Jacqueline Thompson for their comments on this manuscript. We would also like to thank the reviewers for their insightful suggestions.
R.C.K. has filed a patent entitled “Apparatus for Improving and/or Maintaining Numerical Ability” (International Application PCT/GB2011/050211). A.S. and A.D. declare no competing financial interests.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0), which permits unrestricted use, distribution and reproduction in any medium provided that the original work is properly attributed.
References
- Ashcraft MH. Math anxiety: personal, educational, and cognitive consequences. Curr Dir Psychol Sci. 2002;11:181–185. doi: 10.1111/1467-8721.00196. [DOI] [Google Scholar]
- Ashcraft MH, Kirk EP. The relationships among working memory, math anxiety, and performance. J Exp Psychol Gen. 2001;130:224–237. doi: 10.1037/0096-3445.130.2.224. [DOI] [PubMed] [Google Scholar]
- Banks SJ, Eddy KT, Angstadt M, Nathan PJ, Phan KL. Amygdala–frontal connectivity during emotion regulation. Soc Cogn Affect Neurosci. 2007;2:303–312. doi: 10.1093/scan/nsm029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggio PS, Zaghi S, Fregni F. Modulation of emotions associated with images of human pain using anodal transcranial direct current stimulation (tDCS) Neuropsychologia. 2009;47:212–217. doi: 10.1016/j.neuropsychologia.2008.07.022. [DOI] [PubMed] [Google Scholar]
- Brunoni AR, Vanderhasselt MA, Boggio PS, Fregni F, Dantas EM, Mill JG, Lotufo PA, Benseñor IM. Polarity-and valence-dependent effects of prefrontal transcranial direct current stimulation on heart rate variability and salivary cortisol. Psychoneuroendocrinology. 2013;38:58–66. doi: 10.1016/j.psyneuen.2012.04.020. [DOI] [PubMed] [Google Scholar]
- Cohen Kadosh R, Levy N, O'Shea J, Shea N, Savulescu J. The neuroethics of noninvasive brain stimulation. Curr Biol. 2012;22:R108–R111. doi: 10.1016/j.cub.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton KM, Kalin NH, Grist TM, Davidson RJ. Neural-cardiac coupling in threat-evoked anxiety. J Cogn Neurosci. 2005;17:969–980. doi: 10.1162/0898929054021094. [DOI] [PubMed] [Google Scholar]
- Davis M, Rainnie D, Cassell M. Neurotransmission in the rat amygdala related to fear and anxiety. Trends Neurosci. 1994;17:208–214. doi: 10.1016/0166-2236(94)90106-6. [DOI] [PubMed] [Google Scholar]
- Dubljević V, Saigle V, Racine E. The rising tide of tDCS in the media and academic literature. Neuron. 2014;82:731–736. doi: 10.1016/j.neuron.2014.05.003. [DOI] [PubMed] [Google Scholar]
- Fan J, McCandliss BD, Sommer T, Raz A, Posner MI. Testing the efficiency and independence of attentional networks. J Cogn Neurosci. 2002;14:340–347. doi: 10.1162/089892902317361886. [DOI] [PubMed] [Google Scholar]
- Fitz N, Reiner P. The challenge of crafting policy for do-it-yourself brain stimulation. J Med Ethics. 2013 doi: 10.1136/medethics-2013-101458. doi: 10.1136/medethics-2013-101458. Advance online publication. Retrieved Dec. 31, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsch B, Reis J, Martinowich K, Schambra HM, Ji Y, Cohen LG, Lu B. Direct current stimulation promotes BDNF-dependent synaptic plasticity: potential implications for motor learning. Neuron. 2010;66:198–204. doi: 10.1016/j.neuron.2010.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandiga PC, Hummel FC, Cohen LG. Transcranial DC stimulation (tDCS): a tool for double-blind sham-controlled clinical studies in brain stimulation. Clin Neurophysiol. 2006;117:845–850. doi: 10.1016/j.clinph.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Hayes A. F. Introduction to mediation, moderation, and conditional analysis. New York: Guilford; 2013. [Google Scholar]
- Hellhammer DH, Wüst S, Kudielka BM. Salivary cortisol as a biomarker in stress research. Psychoneuroendocrinology. 2009;34:163–171. doi: 10.1016/j.psyneuen.2008.10.026. [DOI] [PubMed] [Google Scholar]
- Hembree R. The nature, effects, and relief of mathematics anxiety. J Res Math Edu. 1990;21:33–46. doi: 10.2307/749455. [DOI] [Google Scholar]
- Herrington JD, Mohanty A, Koven NS, Fisher JE, Stewart JL, Banich MT, Webb AG, Miller GA, Heller W. Emotion-modulated performance and activity in left dorsolateral prefrontal cortex. Emotion. 2005;5:200–207. doi: 10.1037/1528-3542.5.2.200. [DOI] [PubMed] [Google Scholar]
- Iuculano T, Cohen Kadosh R. The mental cost of cognitive enhancement. J Neurosci. 2013;33:4482–4486. doi: 10.1523/JNEUROSCI.4927-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson L, Koslowsky M, Lavidor M. tDCS polarity effects in motor and cognitive domains: a meta-analytical review. Exp Brain Res. 2012;216:1–10. doi: 10.1007/s00221-011-2891-9. [DOI] [PubMed] [Google Scholar]
- Krause B, Cohen Kadosh R. Not all brains are created equal: the relevance of individual differences in responsiveness to transcranial electrical stimulation. Front Syst Neurosci. 2014;8:25. doi: 10.3389/fnsys.2014.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause B, Márquez-Ruiz J, Cohen Kadosh R. The effect of transcranial direct current stimulation: a role for cortical excitation/inhibition balance? Front Hum Neurosci. 2013;7:602. doi: 10.3389/fnhum.2013.00602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Alonso V, Cheeran B, Río-Rodríguez D, Fernandez-Del-Olmo M. Inter -individual variability in response to non-invasive brain stimulation paradigms. Brain Stimul. 2014;7:372–380. doi: 10.1016/j.brs.2014.02.004. [DOI] [PubMed] [Google Scholar]
- Lorenz J, Minoshima S, Casey KL. Keeping pain out of mind: the role of the dorsolateral prefrontal cortex in pain modulation. Brain. 2003;126:1079–1091. doi: 10.1093/brain/awg102. [DOI] [PubMed] [Google Scholar]
- Lyons IM, Beilock SL. When math hurts: math anxiety predicts pain network activation in anticipation of doing math. PLoS One. 2012a;7:e48076. doi: 10.1371/journal.pone.0048076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons IM, Beilock SL. Mathematics anxiety: separating the math from the anxiety. Cereb Cortex. 2012b;22:2102–2110. doi: 10.1093/cercor/bhr289. [DOI] [PubMed] [Google Scholar]
- Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48:175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Richardson FC, Suinn RM. The Mathematics Anxiety Rating Scale: psychometric data. J Couns Psychol. 1972;19:551–554. doi: 10.1037/h0033456. [DOI] [Google Scholar]
- Rubinsten O, Bialik N, Solar Y. Exploring the relationship between math anxiety and gender through implicit measurement. Front Hum Neurosci. 2012;6:279. doi: 10.3389/fnhum.2012.00279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman AJ, McMenamin BW, Maxwell JS, Greischar LL, Davidson RJ. Right dorsolateral prefrontal cortical activity and behavioral inhibition. Psychol Sci. 2009;20:1500–1506. doi: 10.1111/j.1467-9280.2009.02476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvanto J, Muggleton N, Walsh V. State-dependency in brain stimulation studies of perception and cognition. Trends Cogn Sci. 2008;12:447–454. doi: 10.1016/j.tics.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Suinn RM, Winston EH. The mathematics anxiety rating scale, a brief version: psychometric data. Psychol Rep. 2003;92:167–173. doi: 10.2466/pr0.2003.92.1.167. [DOI] [PubMed] [Google Scholar]
- Tseng P, Hsu TY, Chang CF, Tzeng OJ, Hung DL, Muggleton NG, Walsh V, Liang WK, Cheng SK, Juan CH. Unleashing potential: transcranial direct current stimulation over the right posterior parietal cortex improves change detection in low -performing individuals. J Neurosci. 2012;32:10554–10561. doi: 10.1523/JNEUROSCI.0362-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Individual Achievement Test (WIAT-II. London: Psychological Corporation; 2005. [Google Scholar]
- Wiethoff S, Hamada M, Rothwell JC. Variability in response to transcranial direct current stimulation of the motor cortex. Brain Stimul. 2014;7:468–475. doi: 10.1016/j.brs.2014.02.003. [DOI] [PubMed] [Google Scholar]
- Wolkenstein L, Zeiller M, Kanske P, Plewnia C. Induction of a depression-like negativity bias by cathodal transcranial direct current stimulation. Cortex. 2014;59C:103–112. doi: 10.1016/j.cortex.2014.07.011. [DOI] [PubMed] [Google Scholar]
- Wu LJ, Kim SS, Zhuo M. Molecular targets of anxiety: from membrane to nucleus. Neurochem Res. 2008;33:1925–1932. doi: 10.1007/s11064-008-9679-8. [DOI] [PubMed] [Google Scholar]
- Young CB, Wu SS, Menon V. The neurodevelopmental basis of math anxiety. Psychol Sci. 2012;23:492–501. doi: 10.1177/0956797611429134. [DOI] [PMC free article] [PubMed] [Google Scholar]