Abstract
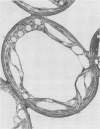
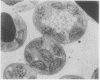
The lipid composition of leaves of wild strawberry (Fragaria virginiana Duchesne) was analyzed throughout an annual growth cycle in the field. Cellular hardiness to temperature stress was assessed concomitantly by a solute leakage technique. Leaves were shown to be very sensitive to an applied temperature of −5°C during the summer months but insensitive to a 35°C treatment. This general pattern was also seen in young overwintering leaves but was reversed after a period of low-temperature hardening of these same leaves. Associated with cold hardening of the overwintering leaves was a twofold increase in the phospholipid content of the leaf membranes with a proportionately smaller increase in free sterols. The large increase in phospholipids presumably is due primarily to the proliferation of a sterol-poor membrane fraction, probably the endoplasmic reticulum. These quantitative changes in membrane material may be important in increasing freezing tolerance in the overwintering leaf cells by enhancing the overall capacity of the cell for plasma membrane and tonoplast extension through vesicle fusion using components from this endomembrane pool. Analysis of electron micrographs of hardened leaf cells showed an increase in vesiculated smooth endoplasmic reticulum and tonoplast membrane over nonhardened leaf cells, the latter resulting in an enhanced tonoplast surface area to vacuolar volume ratio. During this same period, no changes in the fatty acid or free sterol composition were detectable, suggesting that regulation of membrane fluidity via these components is not required for cold acclimation in this species. During aging and senescence of both the overwintering and the summer leaves, the cellular membranes remained functionally intact but became progressively more vulnerable to temperature stress. Free sterol content increased during this time. This feature may be related to the inability of the older leaves to withstand environmental stress. Increasing sensitivity of the cellular membranes to stress may, in turn, be causally related to the actual onset of senescence in these leaves, thus explaining why only the older leaves senesce when the plant is challenged by periodic environmental stress.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunwald C. Sterol distribution in intracellular organelles isolated from tobacco leaves. Plant Physiol. 1970 Jun;45(6):663–666. doi: 10.1104/pp.45.6.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges T. K., Leonard R. T., Bracker C. E., Keenan T. W. Purification of an ion-stimulated adenosine triphosphatase from plant roots: association with plasma membranes. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3307–3311. doi: 10.1073/pnas.69.11.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leopold A. C., Musgrave M. E. Respiratory changes with chilling injury of soybeans. Plant Physiol. 1979 Nov;64(5):702–705. doi: 10.1104/pp.64.5.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElhaney R. N., Souza K. A. The relationship between environmental temperature, cell growth and the fluidity and physical state of the membrane lipids in Bacillus stearothermophilus. Biochim Biophys Acta. 1976 Sep 7;443(3):348–359. doi: 10.1016/0005-2736(76)90455-7. [DOI] [PubMed] [Google Scholar]
- McKersie B. D., Lepock J. R., Kruuv J., Thompson J. E. The effects of cotyledon senescence on the composition and physical properties of membrane lipid. Biochim Biophys Acta. 1978 Apr 4;508(2):197–212. doi: 10.1016/0005-2736(78)90325-5. [DOI] [PubMed] [Google Scholar]
- Palta J. P., Levitt J., Stadelmann E. J. Freezing injury in onion bulb cells: I. Evaluation of the conductivity method and analysis of ion and sugar efflux from injured cells. Plant Physiol. 1977 Sep;60(3):393–397. doi: 10.1104/pp.60.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn P. J., Williams W. P. Plant lipids and their role in membrane function. Prog Biophys Mol Biol. 1978;34(2):109–173. doi: 10.1016/0079-6107(79)90016-6. [DOI] [PubMed] [Google Scholar]
- Raheja R. K., Kaur C., Singh A., Bhatia I. S. New colorimetric method for the quantitative estimation of phospholipids without acid digestion. J Lipid Res. 1973 Nov;14(6):695–697. [PubMed] [Google Scholar]
- Rottem S., Cirillo V. P., de Kruyff B., Shinitzky M., Razin S. Cholesterol in mycoplasma membranes. Correlation of enzymic and transport activities with physical state of lipids in membranes of Mycoplasma mycoides var. capri adapted to grow with low cholesterol concentrations. Biochim Biophys Acta. 1973 Nov 16;323(4):509–519. doi: 10.1016/0005-2736(73)90159-4. [DOI] [PubMed] [Google Scholar]
- Roughan P. G., Batt R. D. Quantitative analysis of sulfolipid (sulfoquinovosyl diglyceride) and galactolipids (monogalactosyl and digalactosyl diglycerides) in plant tissues. Anal Biochem. 1968 Jan;22(1):74–88. doi: 10.1016/0003-2697(68)90261-3. [DOI] [PubMed] [Google Scholar]
- Siminovitch D., Singh J., de la Roche I. A. Studies on membranes in plant cells resistant to extreme freezing. I. Augmentation of phospholipids and membrane substance without changes in unsaturation of fatty acids during hardening of black locust bark. Cryobiology. 1975 Apr;12(2):144–153. doi: 10.1016/s0011-2240(75)80006-x. [DOI] [PubMed] [Google Scholar]
- Willemot C. Stimulation of Phospholipid Biosynthesis during Frost Hardening of Winter Wheat. Plant Physiol. 1975 Feb;55(2):356–359. doi: 10.1104/pp.55.2.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R. J., Hope H. J. The relationship between cell injury and osmotic volume reduction. III. Freezing injury and frost resistance in winter wheat. Cryobiology. 1981 Apr;18(2):133–145. doi: 10.1016/0011-2240(81)90085-7. [DOI] [PubMed] [Google Scholar]