Abstract
The recently identified host restriction factor tetherin (BST-2, CD317) potently inhibits the release of nascent retrovirus particles from infected cells. Recently, we reported the identification and characterization of tetherin as a novel feline retroviral restriction factor. Based on homology to human tetherin we identified a putative tetherin gene in the genome of the domestic cat (Felis catus) which was found to be expressed in different feline cell lines both prior to and post treatment with either type I or type II interferon (IFN). The predicted structure of feline tetherin (feTHN) was that of a type II single-pass transmembrane protein encoding an N-terminal transmembrane anchor, central predicted coiled-coil bearing extracellular domain to promote dimerization, and a C-terminal GPI-anchor, consistent with conservation of structure between human and feline tetherin. FeTHN displayed potent inhibition of feline immunodeficiency virus (FIV) and human immunodeficiency virus type 1 (HIV-1) particle release in single-cycle replication assays. Notably, feTHN activity was resistant to antagonism by HIV-1 Vpu. However, stable ectopic expression of feTHN mRNA in different feline cell lines had no inhibitory effect on the growth of diverse primary or cell culture-adapted strains of FIV. Hence, whereas feline tetherin efficiently blocks viral particle release in single-cycle replication assays, it might not prevent dissemination of feline retroviruses in vivo.
1. Introduction
Feline immunodeficiency virus (FIV) is an important global lentiviral pathogen that infects both domestic and nondomestic felids (Brown et al., 1993, 1994; Carpenter et al., 1996; Hofmann-Lehmann et al., 1996; Troyer et al., 2004, 2005). FIV infection of domestic cats (Felis catus) results in a fatal immunodeficiency syndrome similar to AIDS in humans infected with human immunodeficiency virus (HIV) (Pedersen et al., 1987, 1989; Yamamoto et al., 1988; Pedersen, 1993; Bendinelli et al., 1995). The virus-induced gradual immunological deterioration leads to common clinical signs such as recurrent gingivitis and stomatitis, lymphoma, loss of condition (cachexia/wasting), neurological disorders and high mortality in infected cats (Pedersen et al., 1987; Hosie et al., 1989; Sparger et al., 1989; Yamamoto et al., 1989; Ackley et al, 1990; Torten et al., 1991; Callanan et al., 1992, 1996; Pedersen, 1993). Because of the high degree of similarity between the genomic organization, the mode of transmission and the pathology of HIV and FIV infections, the domestic cat has been established as the smallest natural animal model for studying the development of AIDS in humans and for evaluating potential intervention strategies (Willett et al,, 1997; Miller et al., 2000; Troyer et al., 2004).
The ability of retroviruses to initiate a complex array of interactions with host cell proteins and other factors is a critical determinant of cell tropism, successful replication and persistence within the host. The majority of these host-virus interactions are beneficial for the virus (Malim, 2009). In recent years, however, a group of intracellular proteins has been identified that specifically evolved to interfere with viral replication. These proteins are collectively called restriction factors and form a separate branch of the innate immunity termed intrinsic immunity (Bieniasz, 2004; Goff, 2004). Restriction factors affect almost all stages of the viral lifecycle (Bieniasz, 2004), such as uncoating, reverse transcription, nuclear entry and egress, and their cell-type and species-specific expression and activity control the viral host spectrum and may impose a barrier to cross-species transmission events (Troyer et al., 2008). In order to efficiently replicate and to evade immune surveillance, retroviruses have to overcome this line of defense and, thus, have evolved proteins that antagonize the actions of restriction factors or mechanisms to avoid them.
A better understanding of the interactions between host restriction factors and their viral antagonists will help to improve animal models for infection and to facilitate the identification of potential targets for antiviral therapies as well as retroviral gene delivery.
2. Restriction factors to retroviral replication
The longest (alpha) isoform of TRIM5, a member of the tripartite interaction motif family of proteins (Reymond et al., 2001, Stremlau et al., 2004), and APOBEC3 (apolipoprotein B mRNA-editing catalytic polypeptide 3) proteins, a family of cellular polynucleotide cysteine deaminases (Teng et al., 1993; Sheehy et al., 2002; Mangeat et al., 2003; Zhang et al., 2003), constitute the so-called early post-entry blocks to retroviral infection and have been well characterized in humans, non-human primates and domestic cats.
TRIM5α binds to the incoming retroviral capsid (CA) in the cytoplasm via its C-terminal PRY/SPRY (B30.2) domain (Mische et al., 2005; Sebastian and Luban, 2005; Stremlau et al., 2006; Langelier et al., 2008) and the resulting capsid/TRIM5α complex is incapable of completing reverse transcription (Keckesova et al., 2004; Stremlau et al., 2004). Instead, the N-terminal RBCC (RING, B-box and coiled coil) domain of TRIM5α possesses E3 ubiquitin ligase activity (RING) (Yamauchi et al., 2008) and ubiquination of the complex targets it for proteosome-mediated degradation (Diaz-Griffero et al., 2006; Towers, 2007). It has been proposed that TRIM5α may accelerate or abrogate viral uncoating (Stremlau et al., 2006) which not only inhibits reverse transcription but also nuclear import of viral cDNA (Berthoux et al., 2004; Wu et al, 2006). Previously, we reported that the TRIM5 transcript in cat cells possesses a truncation in the B30.2 capsid binding domain, which ablates its restrictive function (McEwan et al., 2009).
The antiviral activity of APOBEC3 proteins was discovered through the study of the HIV-1 accessory protein Vif (viral infectivity factor) (Wolf and Goff, 2008) which was shown to be dispensable for viral replication in certain permissive cell lines such as CEM-SS and SupT1, but absolutely required in non-permissive cells such as primary CD4+ T cells, monocyte-derived macrophages, and some T cell leukemia lines such as CEM (Fisher et al., 1987; Strebel et al., 1987; Gabuzda et al., 1992; Sakai et al., 1993; Sova and Volsky, 1993). The human APOBEC3G protein (A3G; initially called CEM-15) was identified as the responsible cellular factor whose expression renders human cells non-permissive for infection by HIV-1 strains devoid of the Vif gene, but not by Vif-proficient HIV-1 strains (Sheehy et al., 2002). A3G belongs to a large family of cytosine deaminases (reviewed in Harris and Liddament, 2004; Conticello et al., 2007; Holmes et al., 2007; Aguiar and Peterlin, 2008; Conticello, 2008; Goila-Gaur and Strebel, 2008) that catalyze the hydrolysis of cytosines to uracils. In order to carry out its anti-viral activity, A3G has to be packaged into Vif-deficient virions as they are formed in producer cells (Sheehy et al., 2002; Harris et al., 2003; Lecossier et al., 2003; Mangeat et al., 2003; Zhang et al., 2003). A3G is then carried to the target cell, where it, upon initiation of reverse transcription, deaminates cytosine residues in nascent retroviral minus-strand cDNA to uracils. Subsequently, the uracils function as a template for the incorporation of plus-strand adenines resulting in guanine to adenine hypermutations in the viral genome that critically affect viability and infectivity of the virus (Harris et al., 2003; Mangeat et al., 2003; Zhang et al., 2003; Bishop et al., 2004; Liddament et al., 2004; Zheng et al., 2004). Recent studies propose that, in addition to deamination, deamination-independent mechanisms of A3G to inhibit viral replication exist (Shindo et al., 2003; Newman et al., 2005; Guo et al., 2006, 2007; Iwatani et al., 2006, 2007; Opi et al., 2006; Bishop et al., 2006; Holmes et al., 2007; Li et al., 2007; Yang et al., 2007). These affect multiple stages of the reverse transcription and collectively impair the accumulation of reverse transcription products (Mangeat et al., 2003, Guo et al., 2006, 2007; Iwatani et al., 2007; Li et al., 2007; Luo et al., 2007; Mbisa et al., 2007).
The primary role of Vif is to prevent A3G incorporation into virions. It targets A3G for proteasome-mediated degradation (Conticello et al., 2003; Marin et al., 2003; Sheehy et al., 2003; Stopak et al., 2003; Liu et al., 2004, 2005; Mehle et al., 2004a, 2004b) by bridging an interaction between A3G and a ubiquitin E3 ligase complex consisting of elongins B and C, cullin 5 and ring-box-1 (Yu et al., 2003; Yu et al., 2004; Mehle et al., 2004b, Bergeron, 2010). The interaction between A3G and Vif is species-specific and partly determines the host range of a virus (Hatziioannou et al., 2006).
Several APOBEC3 genes have recently been identified and characterized in the genome of domestic cats (Münk et al., 2008). The A3 gene locus encodes three highly similar A3C (A3Z2) genes and an A3H (A3Z3) gene. Additionally, a fifth transcript, which is generated by read-through alternative splicing, encodes the protein A3CH (A3Z2-Z3) (Münk et al., 2008; Zielonka et al., 2010). The feline A3 proteins display different degrees of activity against feline retroviruses. Feline A3C proteins inhibit the replication of Bet-deficient feline foamy virus (FeFV) but do not restrict Vif-deficient FIV or feline leukemia virus (FeLV). In contrast, feline A3H and A3CH proteins are active against Vifdeficient FIV as well as FeLV but not against Bet-deficient FeFV (Löchelt et al., 2005; Münk et al., 2008). Feline A3 proteins are overcome by the FIV Vif and the FeFV Bet protein (Löchelt et al., 2005; Münk et al., 2008; Stern et al., 2010; Zielonka et al., 2010).
In addition to the early post-entry blocks, restriction factors such as tetherin contribute to a late block to retroviral replication in that they prevent the release of mature enveloped viral particles from the membranes of infected cells. Tetherin (also called HM1.24/BST-2/CD317) was originally identified as a bone marrow stromal cell surface antigen selectively expressed on terminally differentiated normal and neoplastic human B cells and corresponding cell lines (Goto et al., 1994, Ishikawa et al., 1995). Several studies have shown that tetherins are novel type II transmembrane proteins with a molecular weight of 30-36 kDa (Ishikawa et al., 1995; Ohtomo et al., 1999, Kupzig et al., 2003). They harbour an N-terminal cytoplasmic tail, followed by a transmembrane domain, an extracellular parallel, dimeric, alpha-helical coiled coil domain and a C-terminal glycosyl-phosphatidylinositol (GPI) anchor (Ishikawa et al., 1995; Ohtomo et al., 1999; Kupzig et al., 2003, Rollason et al., 2007; Hinz et al., 2010). Two potential N-linked glycosylation sites and three conserved cysteine residues are present in the extracellular domain (Ishikawa et al., 1995; Ohtomo et al., 1999; Kupzig et al., 2003). Heterogeneous glycosylation of tetherin has been shown to be essential for efficient secretion and folding (Andrew et al., 2009; Goffinet et al., 2009; Kaletsky et al., 2009; McNatt et al., 2009; Miyagi et al., 2009; Perez-Caballero et al., 2009). The cysteines take part in intra- and intermolecular disulfide bond formation and enable the homodimerization of tetherins (Ohtomo et al., 1999, Kupzig et al., 2003; Perez-Caballero et al., 2009). The GPI-modification causes tetherin to partition into and cross-link cholesterol- and sphingolipid-rich microdomains in the plasma membrane (Simons and Ikonen, 2000; Simons and Toomre, 2000, Kupzig et al., 2003). Tetherin cycles between the lipid rafts on the cell surface and an intracellular pool where it localizes predominantly to the Golgi apparatus, the trans-Golgi network (TGN) and recycling endosomes (Kupzig et al., 2003). Internalization from the plasma membrane is mediated by clathrin-dependent endocytosis (Rollason et al., 2007; Masuyama et al., 2009).
The antiviral activity of tetherin was not discovered until 2008, when it was noted that its cell-type specific expression matched closely the dependency of HIV-1 on the accessory protein Vpu (viral protein U) for virus release from certain human cell lines (Strebel et al., 1989; Terwilliger et al., 1989; Klimkait et al., 1990; Varthakavi et al., 2003; Neil et al., 2008; Van Damme et al., 2008). Tetherin is constitutively expressed in human cell lines such HeLa cells (Gottinger et al., 1993), several cancer cell lines (Ohtomo et al., 1999), B cells, T cells, monocytes, macrophages and plasmacytoid dendritic cells (Vidal-Laliena et al., 2005; Blasius et al., 2006; Miyagi et al., 2009) and its expression is induced or enhanced by type I and type II interferons (IFN) in cell lines such as HOS, 293T, HT1080 cells (Neil et al., 2006, 2007, 2008; Van Damme et al., 2008; Miyagi et al., 2009). Interferon treatment renders cell lines that do not normally require Vpu for efficient virus release Vpu-dependent (Neil et al., 2007).
Tetherin causes the retention of fully formed, mature virions on the surface of cells infected with Vpu-deficient HIV-1 (Neil et al., 2008; Van Damme et al., 2008). At the expense of particle release, virions accumulate at the cell surface and a fraction of them are endocytosed via a clathrin-dependent mechanism and degraded (Neil et al., 2006, 2007, Miyakawa et al., 2009). Current models predict that tetherin is present at sites of particle assembly in the cell membrane and is incorporated into virions (Perez-Caballero et al., 2009; Fitzpatrick et al., 2010). Presumably, one end of tetherin embeds in the lipid bilayer of the cell and the other in that of the virion, so that cell-surface tetherin homodimerizes with virion-associated tetherin via disulfide bonds or via coiled-coil regions in the extracellular domain (Fitzpatrick et al., 2010). Thus, virions remain bound to the cell surface and are cross-linked to each other by tetherin.
HIV-1 Vpu is an integral class I membrane phosphoprotein (Cohen et al., 1988) that promotes virion release from HIV-1 infected human cells that express tetherin (Klimkait et al., 1990; Neil et al., 2006; Neil et al., 2008; Van Damme et al., 2008). It has been shown to colocalize with tetherin (Neil et al., 2008; Van Damme et al., 2008) and to reduce its cell-surface expression by targeting it for degradation (Van Damme et al., 2008; Miyagi et al., 2009; Douglas et al., 2009; Goffinet et al., 2009; Mitchell et al., 2009). A well-studied role of Vpu is to mediate the proteasomal degradation of the HIV-1 receptor CD4 in the ER through the recruitment of the β-transducin repeat-containing protein (βTrcP) subunit of the Skp1-cullin1-F-box (SCF) ubiquitin ligase complex (Bour et al., 1995; Margottin et al., 1998; Willey et al., 1992). βTrCP is also involved in the antagonism of tetherin because disruption of the interaction between βTrCP and the βTrCP binding motif in the cytoplasmic domain of Vpu reduces the capacity of Vpu to promote virus release (Mitchell et al., 2009; Mangeat et al., 2009; Douglas et al., 2009). Vpu serves as an adapter between βTrCP and tetherin. Tetherin and Vpu bind to each other through their transmembrane domains (Rong et al., 2009; Iwabu et al, 2009). It seems that Vpu sequesters tetherin within the endolysosomal system either within the TGN after it has been synthesized or within recycling endosomes after natural endocytosis of tetherin from the cell surface has occurred (Mitchell et al., 2009; Dube et al., 2010). This intracellular sequestration is followed by partial lysosomal degradation of both tetherin and Vpu.
Vpu is only encoded by a unique lineage of primate lentiviruses that include HIV-1 and the simian immunodeficiency viruses (SIVs) of chimpanzees (Pan troglodytes) (Cohen et al., 1988), Mona monkeys (Cercopithecus mona), Mustached monkeys (C. cephus) and greater spot-nosed monkeys (C. nictitans), SIVcpz, SIVmon, SIVmus and SIVgsn, respectively (Courgnaud et al., 2003). SIVmon, SIVmus and SIVgsn Vpu counteract tetherins of their respective host species as well as macaque tetherins, but, with the exception of SIVgsn, not human tetherin (huTHN). Accordingly, non-human, non-chimpanzee tetherins are usually insensitive to antagonism by HIV-1 Vpu (Goffinet et al., 2009; Gupta et al., 2009b; Jia et al., 2009; McNatt et al., 2009; Sauter et al., 2009; Zhang et al., 2009). SIVcpz is the immediate precursor of HIV-1 and its Vpu shares a common ancestry with SIVmon/mus/gsn Vpu (Sauter et al., 2009). However, SIVcpz Vpu is non-functional against both chimpanzee tetherin (cpzTHN) and huTHN. Instead, in SIVcpz the accessory protein Nef has adopted a Vpu-like function. It is likely that, after cross-species transmission from chimpanzees to humans, HIV-1 Vpu has adapted to counteract huTHN, because huTHN is resistant to Nef due to a deletion in the cytoplasmic tail of huTHN (Sauter et al., 2009; Zhang et al., 2009). Species-specific tetherin antagonism by Nef is also conserved in SIVs of sooty mangabeys/rhesus macaques and African green monkeys, SIVsmm/mac and SIVagm, respectively. Like Vpu, Nef also induces cell-surface downregulation of monkey tetherins (Jia et al., 2009). Additionally to Vpu and Nef, the HIV-2 and SIVagmTan (SIVagm of the Tantalus monkey, Chlorocebus tantalus) envelope glycoproteins (Envs) possess anti-tetherin activities (Abada et al., 2005; Gupta et al., 2009a; Le Tortorec, 2009).
Interestingly, in addition to lentiviruses, tetherin blocks the virion release from members of the alpha-, beta-, deltaretrovirus, spumaretrovirus, arenavirus (Lassa) and filovirus (Ebola, Marburg) families (Sakuma et al., 2009; Jouvenet et al., 2009; Kaletsky et al., 2009).
3. Significance of tetherin in felids
Retroviruses have invaded members of the Felidae on multiple occasions. Of the 37 known species of felids, 21 species such as the African lion (Panthera leo), the North American puma (Puma concolor) or the domestic cat have been shown to harbour antibodies reactive to FIV and many of these species harbour viral sequences consistent with species-specific strains (VandeWoude and Apetrei, 2006; Troyer et al., 2008). In addition to FIV, domestic cats harbour gamma retroviruses such as exogenous and endogenous feline leukemia viruses (FeLVs) or RD114 and the spumaretrovirus FeFV (Reeves and O’Brien, 1984). In contrast to the high prevalence of FIV in different felid species, gamma retroviruses are, with the exception of sporadic cross-species transmission events, restricted to domestic cats (Benveniste and Todaro, 1975; Reeves and O’Brien, 1984), which suggests that they entered the domestic cat lineage after it had evolved 10,000 years ago (Vigne et al., 2004). The abundance of different retroviruses in cats necessitates the presence of potent and broadly specific host restriction factors. However, as mentioned above, cats express a truncated and non-functional TRIM5 protein (McEwan et al., 2009) and their A3 proteins are counteracted by wild-type FIV and FeFV (Löchelt et al., 2005; Münk et al., 2008; Stern et al., 2010; Zielonka et al., 2010). Therefore, their ability to suppress retroviral replication may critically depend on the activity of a feline homologue of tetherin.
4. Identification of a feline homologue of BST-2/tetherin
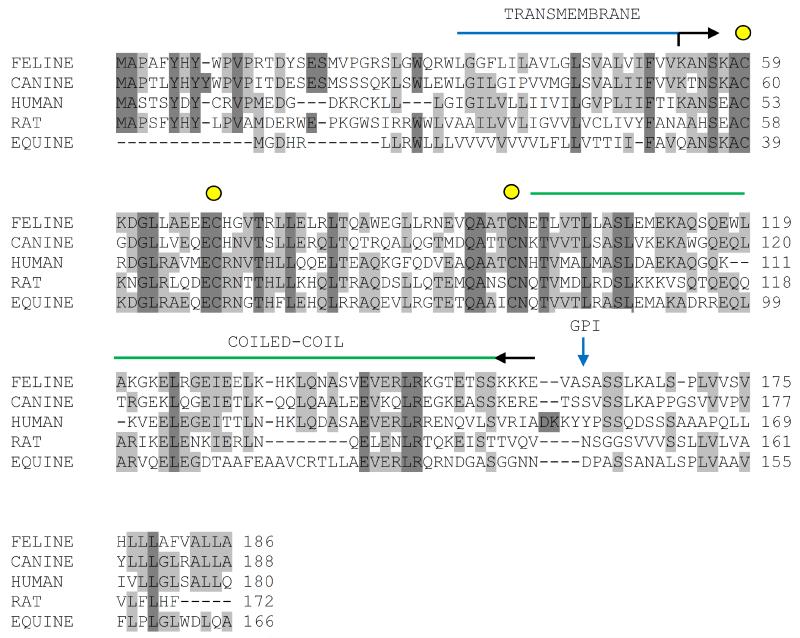
Blast searches of the feline genome using known primate, rodent and canine tetherin sequences identified a candidate gene for a feline homologue of tetherin. The transcript was amplified from interferon-ω stimulated feline IL2-dependent T cell (MYA-1) cDNA. The nucleotide sequence (Genbank accession HM461970) was analyzed and revealed 59% nucleic acid and 44% amino acid identity between cat tetherin (hereafter referred to as feTHN) and its human homologue and 77% nucleic acid and 60% amino acid identity to canine tetherin, transcript variant 2 (XM860510) (Figure 1). Tetherin configuration rather than its amino acid sequence has been shown to be critical for its antiviral activity (Perez-Caballero et al., 2009). Thus, we asked whether feTHN would adopt the same typical protein topology described for other tetherins (Ishikawa et al., 1995; Ohtomo et al., 1999, Kupzig et al., 2003). A hydropathy plot and secondary structure predictions of the feTHN amino acid sequence confirmed the presence of an N-terminal transmembrane domain, which is followed by an alpha-helical region and a coiled-coil domain (Figure 1). The alpha-helical region contains three conserved cysteines (C59, C69, C97). Additionally, feTHN was predicted to contain a C-terminal GPI anchor signal sequence and the potential GPI anchor attachment site has been mapped to S161. Thus, both amino acid sequence and topology described for different tetherins are conserved in feTHN.
Figure 1.
Amino acid sequence alignment of tetherins. The amino acid sequences of feline, canine (transcript variant 2), human, rat and horse tetherin are compared. Amino acids conserved between all tetherin orthologs are highlighted in dark grey, those conserved between at least three sequences in light grey. The positions of predicted protein domains are indicated. The position of the transmembrane domain is marked by a blue bar and the position of the coiled-coil domain, which contains the three conserved cysteine residues, by a green bar. The length of the extracellular domain is indicated by black arrows. The position of the potential GPI anchor attachment site (ω-site) is marked by a blue arrow.
The expression levels of feTHN in feline T cell (MYA-1), fibroblast (AH927), kidney epithelioid (CrFK) and fetal embryo fibroblast-like (FEA) cell lines and the effect of treatment with type I interferons and IFN-γ (1000 U/ml) on its expression were examined by qRT-PCR. All cell lines showed a basal feTHN expression with FEA cells expressing approximately 10-fold lower levels compared to the other cell lines tested. Tetherin expression was inducible by type I IFN (α, ω) in all four cell lines, whereas treatment with IFN-γ had little effect on tetherin expression in MYA cells but up-regulated tetherin expression markedly in AH927, CrFK and FEA cells. In conclusion, feTHN shares the expression profile of huTHN.
5. Antiviral activity of feline tetherin
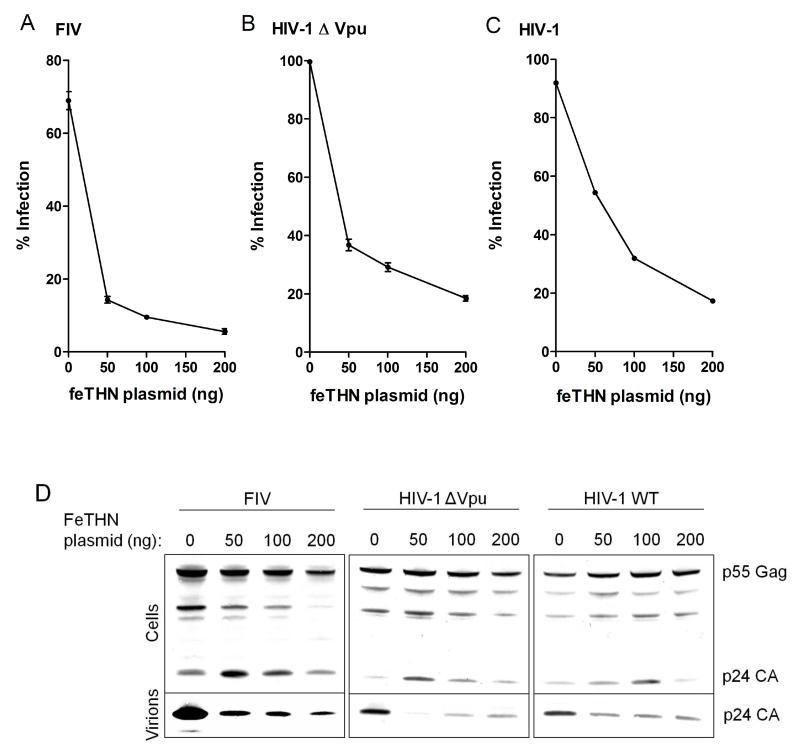
In order to assess the potency of feline tetherin to inhibit viral release, single-cycle viral replication assays were performed. FIV(VSV-G)-GFP pseudotypes were produced by transfecting 293T cells with the FIV-based vectors FP93 (Gagpol) and pGinSin (GFP) (Poeschla et al., 1998) and the vesicular stomatitis virus G glycoprotein (VSV-G)-encoding vector pMDG (Yee et al., 1994) in the presence or absence of feTHN. The pseudotypes were used to transduce CrFK cells and the viral titre was determined by flow cytometry. FeTHN caused a marked and dose-dependent reduction of the FIV(VSV-G)-GFP titer (Figure 2A). Inhibition of viral release was confirmed by immunoblotting against viral p24 in the culture supernatants (Figure 2D). In contrast to viral release, virus production was unaffected by the expression of feTHN. HIV-1 wild-type pseudotypes were produced as described above using the HIV-1-derived vector p8.2 (Gagpol) ans CSGW (GFP) (Naldini et al., 1996) and pMDG. Pseudotypes of Vpu-deficient HIV-1 (HIV-1 Δ Vpu) were generated using p8.91 (Gagpol) (Naldini et al., 1996), CSGW and pMDG. Feline tetherin was equally effective in blocking HIV-1 ΔVpu and HIV-1 wild-type viral release (Figures 2B,C and 2D), suggesting that its activity was not counteracted by HIV-1 Vpu. This finding underlines the concept of species-specificity of the tetherin-Vpu interaction (Yang et al., 2010).
Figure 2.
Feline tetherin restricts FIV and HIV-1 particle release and is not overcome by the HIV-1 accessory protein Vpu. (A) 293T cells were co-transfected with the FIV expression plasmids FP93 (Gagpol), pGinSin (GFP), pMDG (VSV-G) and indicated amounts of feline tetherin (feTHN) plasmid DNA. Infectious virus yield (expressed as percentage of infection) was determined by transducing CrFK cells with the pseudotype-containing culture supernatants of the producer cells and by quantifying the percentage of GFP-expressing cells using flow cytometry (± s.d., n=3). (B) 293T cells were co-transfected with the HIV-1 ΔVpu expression plasmids p8.91 (Gagpol), CSGW (GFP) and pMDG and indicated amounts of feTHN plasmid DNA. The infectious virus yield was determined as described for (A). (C) 293T cells were co-transfected with the HIV-1 wild-type expression plasmids p8.2 (Gagpol), CSGW (GFP) and pMDG and indicated amounts of feTHN plasmid DNA. The infectious virus yield was determined as described for (A). (D) Western blot analysis (anti-p24 capsid) of 293T cell lysates and virions after co-transfection of FIV, HIV-1 ΔVpu or HIV-1 wild-type expression plasmids and varying amounts of feTHN plasmid DNA.
In contrast to the well-defined role of tetherin in preventing viral release, information on its potency to block viral replication and spread is sparse. To this end, CrFK cells were stably transduced with a feTHN expression construct and infected with low or high inputs of CrFK-tropic strains of FIV-Pco (CoLV) or FIV-Fca (Petaluma F14) and virus production monitored by RT assay. Surprisingly and in contrast to the marked inhibitory effect of tetherin on lentiviral pseudotype production, ectopic expression of tetherin did not inhibit virus production from FIV-infected CrFK cells. Instead, syncytium formation was enhanced in the tetherin-expressing cells compared with control cells as virions are trapped at the cell surface promoting cell-cell fusion. As FIV-Pco and FIV-Fca (Petaluma F14) are cell culture-adapted viral strains, we generated CrFK cells and CrFK-feTHN cells stably expressing the viral primary receptor CD134 (Shimojima et al., 2004) and studied the effect of feTHN on replication of the primary strains of FIV, GL8 and PPR. Again, feTHN did not influence the viral growth rate. In summary, these findings suggest that feTHN is unable to prevent replication of cell-culture adapted and primary strains of FIV.
6. Conclusion and future directions
Overall, feline tetherin resembles human tetherin in amino acid sequence, protein topology and anti-viral activity. It is expressed in different feline cells in to a basic level and its expression can be significantly enhanced by treatment with type I or type II IFN. FeTHN exhibited a potent, dose-dependent block to retroviral particle release, which was not relieved by the HIV-1 accessory protein Vpu. In stark contrast to particle release, stable expression of feTHN had no effect on FIV replication and even increased the likelihood of cell-cell fusion events thus possibly promoting viral cell-to-cell spread. Given the fact that feTHN was expressed from a CMV promoter in both the transiently and stably transfected cells, these findings suggest that the number of tetherin molecules on the cell surface might be limited and that feTHN therefore has only a saturable capacity to prevent viral particle release from productively infected cells. In single-cycle replication assays, however, the amount of virus particles to be retained at the cell surface might be lower so that virus release can be controlled by tetherin.
Indeed, there is evidence that Vpu-deficient HIV-1 can replicate in tissue culture with the same kinetics as wild-type virus (Strebel et al., 1988; Terwilliger et al., 1989; Klimkait et al., 1990) by shifting from a cell-free to a cell-to-cell mode of replication. As a consequence of this shift, viral replication was, in contrast to viral release, not inhibited. Further, it was recently shown that in T cells infected with Vpu-defective HIV-1, but not wild-type HIV-1, virus envelope proteins accumulated on the cell surface due to the action of tetherin, which promoted formation of virological synapses (VS) and direct cell-to-cell spread of virions (Jolly et al., 2010).
Future research should focus on the role of tetherin as a regulator of innate immunity. Tetherin has been shown to be a specific marker of type I IFN-producing cells (IPCs) or plasmacytoid dendritic cells (pDCs) (Blasius et al., 2006). These cells circulate through the blood and infiltrate lymph nodes that drain sites of infection. Viruses trigger Toll-like receptor (TLR) 7/9-induced production of large amounts of type I IFN and proinflammatory cytokines that activate anti-viral intrinsic, innate and adaptive immune responses (Colonna et al., 2004; Liu, 2005). A chronic activation of pDCs and continuous IFN production caused by lentivirus infection leads to immune dysregulation, T cell anergy and apoptosis (Tompkins and Tompkins, 2008). Tetherin has been shown to interact with the orphan receptor immunoglobin-like transcript (ILT7), which is expressed exclusively on pDCs (Cao et al., 2006). This interaction induces a negative feedback loop on the production of type I IFN and proinflammatory cytokine production and adjusts the magnitude of immune activation upon viral infection. Additionally, tetherin incorporation into the lipid envelopes of viral particles could enhance their uptake into professional antigen presenting cells (APCs).
The elucidation of the role of feline tetherin in controlling replication of feline retroviruses in vivo by balancing immune responses will help to develop promising new approaches for the prevention and treatment of infections.
Acknowledgements
The authors would like to thank G. Towers for many helpful discussions. This work was supported by funding from The Wellcome Trust to B.J.W. and M.J.H.
Footnotes
Conflict of interest
None of the authors has a financial or personal relationship with other people or organizations that could inappropriately influence or bias the paper.
References
- 1.Abada P, Noble B, Cannon PM. Functional domains within the human immunodeficiency virus type 2 envelope protein required to enhance virus production. J. Virol. 2005;79:3627–3638. doi: 10.1128/JVI.79.6.3627-3638.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ackley CD, Yamamoto JK, Levy N, Pedersen NC, Cooper MD. Immunologic abnormalities in pathogen-free cats experimentally infected with feline immunodeficiency virus. J. Virol. 1990;64:5652–5655. doi: 10.1128/jvi.64.11.5652-5655.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aguiar RS, Peterlin BM. APOBEC3 proteins and reverse transcription. Virus Res. 2008;134:74–85. doi: 10.1016/j.virusres.2007.12.022. [DOI] [PubMed] [Google Scholar]
- 4.Andrew AJ, Miyagi E, Kao S, Strebel K. The formation of cysteine-linked dimers of BST-2/tetherin is important for inhibition of HIV-1 virus release but not for sensitivity to Vpu. Retrovirology. 2009;6:80. doi: 10.1186/1742-4690-6-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bendinelli M, Pistello M, Lombardi S, Poli A, Garzelli C, Matteucci D, Ceccherini-Nelli L, Malvaldi G, Tozzini F. Feline immunodeficiency virus: an interesting model for AIDS studies and an important cat pathogen. Clin. Microbiol. Rev. 1995;8:87–112. doi: 10.1128/cmr.8.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benveniste RE, Todaro GJ. Segregation of RD-114 AND FeLV-related sequences in crosses between domestic cat and leopard cat. Nature. 1975;257:506–508. doi: 10.1038/257506a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bergeron JR, Huthoff H, Veselkov DA, Beavil RL, Simpson PJ, Matthews SJ, Malim MH, Sanderson MR. The SOCS-box of HIV-1 Vif interacts with ElonginBC by induced-folding to recruit its Cul5-containing ubiquitin ligase complex. PLoS Path. 2010;6:e1000925. doi: 10.1371/journal.ppat.1000925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berthoux L, Sebastian S, Sokolskaja E, Luban J. Lv1 inhibition of human immunodeficiency virus type 1 is counteracted by factors that stimulate synthesis or nuclear translocation of viral cDNA. J. Virol. 2004;78:11739–11750. doi: 10.1128/JVI.78.21.11739-11750.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bieniasz PD. Intrinsic immunity: a front-line defense against viral attack. Nat. Immunol. 2004;5:1109–1115. doi: 10.1038/ni1125. [DOI] [PubMed] [Google Scholar]
- 10.Bishop KN, Holmes RK, Sheehy AM, Davidson NO, Cho SJ, Malim MH. Cytidine deamination of retroviral DNA by diverse APOBEC proteins. Curr. Biol. 2004;14:1392–1396. doi: 10.1016/j.cub.2004.06.057. [DOI] [PubMed] [Google Scholar]
- 11.Bishop KN, Holmes RK, Malim MH. Antiviral potency of APOBEC proteins does not correlate with cytidine deamination. J. Virol. 2006;80:8450–8458. doi: 10.1128/JVI.00839-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blasius AL, Giurisato E, Cella M, Schreiber RD, Shaw AS, Colonna M. Bone marrow stromal cell antigen 2 is a specific marker of type I IFN-producing cells in the naive mouse, but a promiscuous cell surface antigen following IFN stimulation. J. Immunol. 2006;177:3260–3265. doi: 10.4049/jimmunol.177.5.3260. [DOI] [PubMed] [Google Scholar]
- 13.Bour S, Schubert U, Strebel K. The human immunodeficiency virus type 1 Vpu protein specifically binds to the cytoplasmic domain of CD4: implications for the mechanism of degradation. J. Virol. 1995;69:1510–1520. doi: 10.1128/jvi.69.3.1510-1520.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown EW, Miththapala S, O’Brien SJ. Prevalence of exposure to feline immunodeficiency virus in exotic felid species. J. Zoo Wildl. Med. 1993;24:357–364. [Google Scholar]
- 15.Brown EW, Yuhki N, Packer C, O’Brien SJ. A lion lentivirus related to feline immunodeficiency virus: epidemiologic and phylogenetic aspects. J. Virol. 1994;68:5953–5968. doi: 10.1128/jvi.68.9.5953-5968.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Callanan JJ, Thompson H, Toth SR, O’Neil B, Lawrence CE, Willett BJ, Jarrett O. Clinical and pathological findings in feline immunodeficiency virus experimental infection. Vet. Immunol. Immunopathol. 1992;35:3–13. doi: 10.1016/0165-2427(92)90116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Callanan JJ, Jones BA, Irvine J, Willett BJ, McCandlish IAP, Jarrett O. Histological classification and immunophenotype of lymphosarcomas in cats with naturally and experimentally acquired feline immunodeficiency virus infections. Vet. Pathol. 1996;33:264–272. doi: 10.1177/030098589603300302. [DOI] [PubMed] [Google Scholar]
- 18.Cao W, Boyer L, Cho M, Wen X, Hanabuchi S, Bao M, Rosen DB, Wang YH, Shaw JL, Du Q, Li C, Arai N, Yao Z, Lanier LL, Liu YJ. Regulation of TLR7/9 responses in plasmacytoid dendritic cells by BST2 and ILT7 receptor interaction. J. Exp. Med. 2009;206:1603–1614. doi: 10.1084/jem.20090547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carpenter MA, Brown EW, Culver M, Johnson WE, Pecon-Slattery J, Brousset D, O’Brien SJ. Genetic and phylogenetic divergence of feline immunodeficiency virus in the puma (Puma concolor) J. Virol. 1996;70:6682–6693. doi: 10.1128/jvi.70.10.6682-6693.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cohen EA, Terwilliger EF, Sodroski JG, Haseltine WA. Identification of a protein encoded by the vpu gene of HIV-1. Nature. 1988;334:532–534. doi: 10.1038/334532a0. [DOI] [PubMed] [Google Scholar]
- 21.Colonna M, Trinchieri G, Liu YL. Plasmacytoid dendritic cells in immunity. Nat. Immunol. 2004;5:1219–1226. doi: 10.1038/ni1141. [DOI] [PubMed] [Google Scholar]
- 22.Conticello SG, Harris RS, Neuberger MS. The Vif protein of HIV triggers degradation of the human antiretroviral DNA deaminase APOBEC3G. Curr. Biol. 2003;13:2009–2013. doi: 10.1016/j.cub.2003.10.034. [DOI] [PubMed] [Google Scholar]
- 23.Conticello SG, Langlois MA, Yang Z, Neuberger MS. DNA deamination in immunity: AID in the context of its APOBEC relatives. Adv. Immunol. 2007;94:37–73. doi: 10.1016/S0065-2776(06)94002-4. [DOI] [PubMed] [Google Scholar]
- 24.Conticello SG. The AID/APOBEC family of nucleic acid mutators. Genome Biol. 2008;9:229. doi: 10.1186/gb-2008-9-6-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Courgnaud V, Abela B, Pourrut X, Mpoudi-Ngole E, Loul S, Delaporte E, Peeters M. Identification of a new simian immunodeficiency virus lineage with a vpu gene present among different cercopithecus monkeys (C. mona, C. cephus, and C. nictitans) from Cameroon. J. Virol. 2003;77:12523–12534. doi: 10.1128/JVI.77.23.12523-12534.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Diaz-Griffero F, Li X, Javanbakht H, Song B, Welikala S, Stremlau M, Sodroski J. Rapid turnover and polyubiquitylation of the retroviral restriction factor TRIM5. Virology. 2006;349:300–315. doi: 10.1016/j.virol.2005.12.040. [DOI] [PubMed] [Google Scholar]
- 27.Douglas JL, Viswanathan K, McCarroll MN, Gustin JK, Früh K, Moses AV. Vpu directs the degradation of the human immunodeficiency virus restriction factor BST-2/Tetherin via a {beta}TrCP-dependent mechanism. J Virol. 2009;83:7931–7947. doi: 10.1128/JVI.00242-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dube M, Roy BB, Guiot-Guillain P, Mercier J, Binette J, Leung G, Cohen EA. Suppression of tetherin-restricting activity upon human immunodeficiency virus type 1 particle release correlates with localization of Vpu in the trans-Golgi network. J. Virol. 2009;83:4574–4590. doi: 10.1128/JVI.01800-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fisher AG, Ensoli B, Ivanoff L, Chamberlain M, Petteway S, Ratner L, Gallo RC, Wong-Staal F. The sor gene of HIV-1 is required for efficient virus transmission in vitro. Science. 1987;237:888–893. doi: 10.1126/science.3497453. [DOI] [PubMed] [Google Scholar]
- 30.Fitzpatrick K, Skasko M, Deerinck TJ, Crum J, Ellisman MH, Guatelli J. Direct restriction of virus release and incorporation of the interferon-induced protein BST-2 into HIV-1 particles. PLoS Pathog. 2010;6:e1000701. doi: 10.1371/journal.ppat.1000701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gabuzda DH, Lawrence K, Langhoff E, Terwilliger E, Dorfman T, Haseltine WA, Sodroski J. Role of vif in replication of human immunodeficiency virus type 1 in CD4+ T lymphocytes. J. Virol. 1992;66:6489–6495. doi: 10.1128/jvi.66.11.6489-6495.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goff SP. Genetic control of retrovirus susceptibility in mammalian cells. Annu. Rev. Genet. 2004;38:61–85. doi: 10.1146/annurev.genet.38.072902.094136. [DOI] [PubMed] [Google Scholar]
- 33.Goffinet C, Allespach I, Homann S, Tervo HM, Habermann A, Rupp D, Oberbremer L, Kern C, Tibroni N, Welsch S, Krijnse-Locker J, Banting G, Kräusslich HG, Fackler OT, Keppler OT. HIV-1 antagonism of CD317 is species specific and involves Vpu-mediated proteasomal degradation of the restriction factor. Cell Host Microbe. 2009;5:285–297. doi: 10.1016/j.chom.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 34.Goila-Gaur R, Strebel K. HIV-1 Vif, APOBEC, and intrinsic immunity. Retrovirology. 2008;5:51. doi: 10.1186/1742-4690-5-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gupta RK, Mlcochova P, Pelchen-Matthews A, Petit SJ, Mattiuzzo G, Pillay D, Takeuchi Y, Marsh M, Towers GJ. Simian immunodeficiency virus envelope glycoprotein counteracts tetherin/BST-2/CD317 by intracellular sequestration. Proc. Natl. Acad. Sci. U S A. 2009a;106:20889–20894. doi: 10.1073/pnas.0907075106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gupta RK, Hué S, Schaller T, Verschoor E, Pillay D, Towers GJ. Mutation of a single residue renders human tetherin resistant to HIV-1 Vpu-mediated depletion. PLoS Pathog. 2009b;5:e1000443. doi: 10.1371/journal.ppat.1000443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goto T, Kennel SJ, Abe M, Takishita M, Kosaka M, Solomon A, Saito S. A novel membrane antigen selectively expressed on terminally differentiated human B cells. Blood. 1994;84:1922–1930. [PubMed] [Google Scholar]
- 38.Guo F, Cen S, Niu M, Saadatmand J, Kleiman L. Inhibition of tRNA3Lys-primed reverse transcription by human APOBEC3G during human immunodeficiency virus type 1 replication. J. Virol. 2006;80:11710–11722. doi: 10.1128/JVI.01038-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guo X, Ma J, Sun J, Gao G. The zinc-finger antiviral protein recruits the RNA processing exosome to degrade the target mRNA. Proc. Natl. Acad. Sci. U S A. 2007;104:151–156. doi: 10.1073/pnas.0607063104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harris RS, Bishop KN, Sheehy AM, Craig HM, Petersen-Mahrt SK, Watt IN, Neuberger MS, Malim MH. DNA deamination mediates innate immunity to retroviral infection. Cell. 2003;113:803–809. doi: 10.1016/s0092-8674(03)00423-9. [DOI] [PubMed] [Google Scholar]
- 41.Harris RS, Liddament MT. Retroviral restriction by APOBEC proteins. Nature Rev. Immunol. 2004;4:868–877. doi: 10.1038/nri1489. [DOI] [PubMed] [Google Scholar]
- 42.Hatziioannou T, Princiotta M, Piatak M, Jr., Yuan F, Zhang F, Lifson JD, Bieniasz PD. Generation of simian-tropic HIV-1 by restriction factor evasion. Science. 2006;314:95. doi: 10.1126/science.1130994. [DOI] [PubMed] [Google Scholar]
- 43.Hinz A, Miguet N, Natrajan G, Usami Y, Yamanaka H, Renesto P, Hartlieb B, McCarthy AA, Simorre JP, Göttlinger H, Weissenhorn W. Structural basis of HIV-1 tethering to membranes by the BST-2/tetherin ectodomain. Cell Host Microbe. 2010;7:314–323. doi: 10.1016/j.chom.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hofmann-Lehmann R, Fehr D, Grob M, Elgizoli M, Packer C, Martenson JS, O’Brien SJ, Lutz H. Prevalence of antibodies to feline parvovirus, calicivirus, herpesvirus, coronavirus, and immunodeficiency virus and of feline leukemia virus antigen and the interrelationship of these viral infections in free-ranging lions in east Africa. Clin. Diagn. Lab. Immunol. 1996;3:554–562. doi: 10.1128/cdli.3.5.554-562.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Holmes RK, Koning FA, Bishop KN, Malim MH. APOBEC3F can inhibit the accumulation of HIV-1 reverse transcription products in the absence of hypermutation. Comparisons with APOBEC3G. J. Biol. Chem. 2007;282:2587–2595. doi: 10.1074/jbc.M607298200. [DOI] [PubMed] [Google Scholar]
- 46.Hosie MJ, Robertson C, Jarrett O. Prevalence of feline leukaemia virus and antibodies to feline immunodeficiency virus in cats in the United Kingdom. Vet. Rec. 1989;12:293–297. doi: 10.1136/vr.125.11.293. [DOI] [PubMed] [Google Scholar]
- 47.Ishikawa J, Kaisho T, Tomizawa H, Lee BO, Kobune Y, Inazaka J, Oritani K, Itoh M, Ochi T, Ishihara K. Molecular cloning and chromosomal mapping of a bone marrow stromal cell surface gene, BST2, that may be involved in pre-B-cell growth. Genomics. 1995;26:527–534. doi: 10.1016/0888-7543(95)80171-h. [DOI] [PubMed] [Google Scholar]
- 48.Iwabu Y, Fujita H, Kinomoto M, Kaneko K, Ishizaka Y, Tanaka Y, Sata T, Tokunaga K. HIV-1 accessory protein Vpu internalizes cell-surface BST-2/tetherin through transmembrane interactions leading to lysosomes. J. Biol. Chem. 2009;284:35060–35072. doi: 10.1074/jbc.M109.058305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Iwatani Y, Takeuchi H, Strebel K, Levin JG. Biochemical activities of highly purified, catalytically active human APOBEC3G: correlation with antiviral effect. J. Virol. 2006;80:5992–6002. doi: 10.1128/JVI.02680-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Iwatani Y, Chan DS, Wang F, Maynard KS, Sugiura W, Gronenborn AM, Rouzina I, Williams MC, Musier-Forsyth K, Levin JG. Deaminase-independent inhibition of HIV-1 reverse transcription by APOBEC3G. Nucleic Acids Res. 2007;35:7096–7108. doi: 10.1093/nar/gkm750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jia B, Serra-Moreno R, Neidermyer W, Rahmberg A, Mackey J, Fofana IB, Johnson WE, Westmoreland S, Evans DT. Species-specific activity of SIV Nef and HIV-1 Vpu in overcoming restriction by tetherin/BST2. PLoS Pathog. 2009;5:e1000429. doi: 10.1371/journal.ppat.1000429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jolly C, Booth NJ, Neil SJD. Cell-cell spread of human immunodeficiency virus type 1 overcomes tetherin/BST-2 mediated restriction in T cells. J. Virol. 2010;84:12185–12199. doi: 10.1128/JVI.01447-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jouvenet N, Neil SJ, Zhadina M, Zang T, Kratovac Z, Lee Y, McNatt M, Hatziioannou T, Bieniasz PD. Broad-spectrum inhibition of retroviral and filoviral particle release by tetherin. J. Virol. 2009;83:1837–1844. doi: 10.1128/JVI.02211-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kaletsky RL, Francica JR, Agrawal-Gamse C, Bates P. Tetherin-mediated restriction of filovirus budding is antagonized by the Ebola glycoprotein. Proc. Natl. Acad. Sci. U. S. A. 2009;106:2886–2891. doi: 10.1073/pnas.0811014106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Keckesova Z, Ylinen LM, Towers GJ. The human and African green monkey TRIM5alpha genes encode Ref1 and Lv1 retroviral restriction factor activities. Proc. Natl. Acad. Sci. U.S.A. 2004;101:10780–10785. doi: 10.1073/pnas.0402474101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Klimkait T, Strebel K, Hoggan MD, Martin MA, Orenstein JM. The human immunodeficiency virus type 1-specific protein vpu is required for efficient virus maturation and release. J. Virol. 1990;64:621–629. doi: 10.1128/jvi.64.2.621-629.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kupzig S, Korolchuk V, Rollason R, Sudgen A, Wilde A, Banting G. Bst-2/HM1.24 is a raft-associated apical membrane protein with an unusual topology. Traffic. 2003;4:694–709. doi: 10.1034/j.1600-0854.2003.00129.x. [DOI] [PubMed] [Google Scholar]
- 58.Langelier CR, Sandrin V, Eckert DM, Christensen DE, Chandrasekaran V, Alam SL, Aiken C, Olsen JC, Kar AK, Sodroski JG, Sundquist WI. Biochemical characterization of a recombinant TRIM5α protein that restricts human immunodeficiency virus type 1 replication. J. Virol. 2008;82:11682–11694. doi: 10.1128/JVI.01562-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lecossier D, Bouchonnet F, Clavel F, Hance AJ. Hypermutation of HIV-1 DNA in the absence of the Vif protein. Science. 2003;300:1112. doi: 10.1126/science.1083338. [DOI] [PubMed] [Google Scholar]
- 60.Le Tortorec A, Neil SJ. Antagonism to and intracellular sequestration of human tetherin by the human immunodeficiency virus type 2 envelope glycoprotein. J. Virol. 2009;83:11966–11978. doi: 10.1128/JVI.01515-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li XY, Guo F, Zhang L, Kleiman L, Cen S. APOBEC3G inhibits DNA strand transfer during HIV-1 reverse transcription. J. Biol. Chem. 2007;282:32065–32074. doi: 10.1074/jbc.M703423200. [DOI] [PubMed] [Google Scholar]
- 62.Liddament MT, Brown WL, Schumacher AJ, Harris RS. APOBEC3F properties and hypermutation preferences indicate activity against HIV-1 in vivo. Curr. Biol. 2004;14:1385–1391. doi: 10.1016/j.cub.2004.06.050. [DOI] [PubMed] [Google Scholar]
- 63.Liu B, Yu X, Luo K, Yu Y, Yu XF. Influence of primate lentiviral Vif and proteasome inhibitors on human immunodeficiency virus type 1 virion packaging of APOBEC3G. J. Virol. 2004;78:2072–2081. doi: 10.1128/JVI.78.4.2072-2081.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu YJ. IPC: professional type I interferon producing cells and plasmacytoid dendritic cell precursors. Annu. Rev. Immunol. 2005;23:275–306. doi: 10.1146/annurev.immunol.23.021704.115633. [DOI] [PubMed] [Google Scholar]
- 65.Löchelt M, Romen F, Bastone P, Muckenfuss H, Kirchner N, Kim YB, Truyen U, Rosler U, Battenberg M, Saib A, Flory E, Cichutek K, Münk C. The antiretroviral activity of APOBEC3 is inhibited by the foamy virus accessory Bet protein. Proc. Natl. Acad. Sci. U.S.A. 2005;102:7982–7987. doi: 10.1073/pnas.0501445102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Luo K, Wang T, Liu B, Tian C, Xiao Z, Kappes J, Yu XF. Cytidine deaminases APOBEC3G and APOBEC3F interact with human immunodeficiency virus type 1 integrase and inhibit proviral DNA formation. J. Virol. 2007;81:7238–7248. doi: 10.1128/JVI.02584-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mariani R, Chen D, Schrofelbauer B, Navarro F, Konig R, Bollman B, Münk C, Nymark-McMahon H, Landau NR. Species-specific exclusion of APOBEC3G from HIV-1 virions by Vif. Cell. 2003;114:21–31. doi: 10.1016/s0092-8674(03)00515-4. [DOI] [PubMed] [Google Scholar]
- 68.Malim MH. APOBEC proteins and intrinsic resistance to HIV-1 infection. Phil. Trans. R. Soc. B. 2009;364:675–687. doi: 10.1098/rstb.2008.0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mangeat B, Turelli P, Caron G, Friedli M, Perrin L, Trono D. Broad antiretroviral defence by human APOBEC3G through lethal editing of nascent reverse transcripts. Nature. 2003;424:99–103. doi: 10.1038/nature01709. [DOI] [PubMed] [Google Scholar]
- 70.Mangeat B, Gers-Huber G, Lehmann M, Zufferey M, Luban J, Piguet V. HIV-1 Vpu neutralizes the antiviral factor tetherin/BST-2 by binding it and directing its beta-TrCP2-dependent degradation. PLoS Pathog. 2009;5:e1000574. doi: 10.1371/journal.ppat.1000574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Margottin F, Bour SP, Durand H, Selig L, Benichou S, Richard V, Thomas D, Strebel K, Benarous R. A novel human WD protein, h-beta TrCp, that interacts with HIV-1 Vpu connects CD4 to the ER degradation pathway through an F-box motif. Mol. Cell. 1998;1:565–574. doi: 10.1016/s1097-2765(00)80056-8. [DOI] [PubMed] [Google Scholar]
- 72.Marin M, Rose KM, Kozak SL, Kabat D. HIV-1Vif protein binds the editing enzyme APOBEC3G and induces its degradation. Nat. Med. 2003;9:1398–1403. doi: 10.1038/nm946. [DOI] [PubMed] [Google Scholar]
- 73.Masuyama N, Kuronita T, Tanaka R, Muto T, Hirota Y, Takigawa A, Fujita H, Aso Y, Amano J, Tanaka Y. HM1.24 is internalized from lipid rafts by clathrin-mediated endocytosis through interaction with alpha-adaptin. J Biol Chem. 2009;284:15927–41. doi: 10.1074/jbc.M109.005124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mbisa JL, Barr R, Thomas JA, Vandegraaff N, Dorweiler IJ, Svarovskaia ES, Brown WL, Mansky LM, Gorelick RJ, Harris RS, Engelman A, Pathak VK. Human immunodeficiency virus type 1 cDNAs produced in the presence of APOBEC3G exhibit defects in plus-strand DNA transfer and integration. J. Virol. 2007;81:7099–7110. doi: 10.1128/JVI.00272-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.McEwan WA, Schaller T, Ylinen LM, Hosie MJ, Towers GJ, Willett BJ. Truncation of TRIM5 in Feliformia explains the absence of retroviral restriction in cells of the domestic cat. J. Virol. 2009;83:8270–8275. doi: 10.1128/JVI.00670-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McNatt MW, McNatt MW, Zang T, Hatziioannou T, Bartlett M, Fofana IB, Johnson WE, Neil SJ, Bieniasz PD. Species-specific activity of HIV-1 Vpu and positive selection of tetherin transmembrane domain variants. PLoS Pathog. 2009;5:e1000300. doi: 10.1371/journal.ppat.1000300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mehle A, Strack B, Ancuta P, Zhang C, McPike M, Gabuzda D. Vif overcomes the innate antiviral activity of APOBEC3G by promoting its degradation in the ubiquitin-proteasome pathway. J. Biol. Chem. 2004a;279:7792–7798. doi: 10.1074/jbc.M313093200. [DOI] [PubMed] [Google Scholar]
- 78.Mehle A, Goncalves J, Santa-Marta M, McPike M, Gabuzda D. Phosphorylation of a novel SOCS-box regulates assembly of the HIV-1 Vif-Cul5 complex that promotes APOBEC3G degradation. Genes Dev. 2004b;18:2861–2866. doi: 10.1101/gad.1249904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Miller RJ, Cairns JS, Bridges S, Sarver N. Human immunodeficiency virus and AIDS: insights from animal lentiviruses. J. Virol. 2000;74:7187–7195. doi: 10.1128/jvi.74.16.7187-7195.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mische CC, Javanbakht H, Song B, Diaz-Griffero F, Stremlau M, Strack B, Si Z, Sodroski J. Retroviral restriction factor TRIM5alpha is a trimer. J. Virol. 2005;79:14446–14450. doi: 10.1128/JVI.79.22.14446-14450.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mitchell RS, Katsura C, Skasko MA, Fitzpatrick K, Lau D, Ruiz A, Stephens EB, Margottin-Goguet F, Benarous R, Guatelli JC. Vpu antagonizes BST-2-mediated restriction of HIV-1 release via b-TrCP and endo-lysosomal trafficking. PLoS Pathog. 2009;5:e1000450. doi: 10.1371/journal.ppat.1000450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Miyagi E, Andrew AJ, Kao S, Strebel K. Vpu enhances HIV-1 virus release in the absence of Bst-2 cell surface down-modulation and intracellular depletion. Proc. Natl. Acad. Sci. U. S. A. 2009;106:2868–2873. doi: 10.1073/pnas.0813223106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Miyakawa K, Ryo A, Murakami T, Ohba K, Yamaoka S, Fukuda M, Guatelli J, Yamamoto N. BCA2/Rabring7 promotes tetherin-dependent HIV-1 restriction. PLoS Pathog. 2009;5:e1000700. doi: 10.1371/journal.ppat.1000700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Münk C, Beck T, Zielonka J, Hotz-Wagenblatt A, Chareza S, Battenberg M, Thielebein J, Cichutek K, Bravo IG, O’Brien SJ, Löchelt M, Yuhki N. Functions, structure, and read-through alternative splicing of feline APOBEC3 genes. Genome Biol. 2008;9:R48. doi: 10.1186/gb-2008-9-3-r48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Naldini L, Blomer U, Gallay P, Ory D, Mulligan R, Gage FH, Verma IM, Trono D. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996;272:263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- 86.Neil SJ, Eastman SW, Jouvenet N, Bieniasz PD. HIV-1 Vpu promotes release and prevents endocytosis of nascent retrovirus particles from the plasma membrane. PLoS Pathog. 2006;2:e39. doi: 10.1371/journal.ppat.0020039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Neil SJ, Sandrin V, Sundquist WI, Bieniasz PD. An interferon-alpha induced tethering mechanism inhibits HIV-1 and Ebola virus particle release but is counteracted by the HIV-1 Vpu protein. Cell Host Microbe. 2007;2:193–203. doi: 10.1016/j.chom.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Neil SJ, Zang T, Bieniasz PD. Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature. 2008;451:425–430. doi: 10.1038/nature06553. [DOI] [PubMed] [Google Scholar]
- 89.Newman EN, Holmes RK, Craig HM, Klein KC, Lingappa JR, Malim MH, Sheehy AM. Antiviral function of APOBEC3G can be dissociated from cytidine deaminase activity. Curr. Biol. 2005;15:166–170. doi: 10.1016/j.cub.2004.12.068. [DOI] [PubMed] [Google Scholar]
- 90.Ohtomo T, Sugamata Y, Ozaki Y, Ono K, Yoshimura Y, Kawai S, Koishihara Y, Ozaki S, Kosaka M, Hirano T, Tsuchiya M. Molecular cloning and characterization of a surface antigen preferentially overexpressed on multiple myeloma cells. Biochem. Biophys. Res. Commun. 1999;258:583–591. doi: 10.1006/bbrc.1999.0683. [DOI] [PubMed] [Google Scholar]
- 91.Opi S, Takeuchi H, Kao S, Khan MA, Miyagi E, Goila-Gaur R, Iwatani Y, Levin JG, Strebel K. Monomeric APOBEC3G is catalytically active and has antiviral activity. J. Virol. 2006;80:4673–4682. doi: 10.1128/JVI.80.10.4673-4682.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pedersen NC, Ho EW, Brown ML, Yamamoto JK. Isolation of a T-lymphotropic virus from domestic cats with an immunodeficiency syndrome. Science. 1987;235:790–793. doi: 10.1126/science.3643650. [DOI] [PubMed] [Google Scholar]
- 93.Pedersen NC, Yamamoto JK, Ishida T, Hansen H. Feline immunodeficiency virus infection. Vet. Immunol. Immunopathol. 1989;21:111–129. doi: 10.1016/0165-2427(89)90134-7. [DOI] [PubMed] [Google Scholar]
- 94.Pedersen NC. The feline immunodeficiency virus. In: Levy JA, editor. The retroviridae. Vol. 2. Plenum Press; New York, NY: 1993. pp. 181–228. [Google Scholar]
- 95.Poeschla EM, Wong-Staal F, Looney DJ. Efficient transduction of nondividing human cells by feline immunodeficiency virus lentiviral vectors. Nat. Med. 1998;4:354–357. doi: 10.1038/nm0398-354. [DOI] [PubMed] [Google Scholar]
- 95.Perez-Caballero D, Zang T,, Ebrahimi A, McNatt MW, Gregory DA, Johnson MC, Bieniasz PD. Tetherin inhibits HIV-1 release by directly tethering virions to cells. Cell. 2009;139:499–511. doi: 10.1016/j.cell.2009.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Reeves RH, O’Brien SJ. Molecular genetic characterization of the RD-114 gene family of endogenous feline retroviral sequences. J. Virol. 1984;52:164–171. doi: 10.1128/jvi.52.1.164-171.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Reymond A, Meroni G, Fantozzi A, Merla G, Cairo S, Luzi L, Riganelli D, Zanaria E, Messali S, Cainarca S, Guffanti A, Minucci S, Pelicci PG, Ballabio A. The tripartite motif family identifies cell compartments. Embo J. 2001;20:2140–2151. doi: 10.1093/emboj/20.9.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rollason R, Korolchuk V, Hamilton C, Schu P, Banting G. Clathrin-mediated endocytosis of a lipid-raft associated protein is mediated through a dual tyrosine motif. J. Cell Sci. 2007;120:3850–3858. doi: 10.1242/jcs.003343. [DOI] [PubMed] [Google Scholar]
- 99.Rong L, Zhang J, Lu J, Pan Q, Lorgeoux RP, Aloysius C, Guo F, Liu SL, Wainberg MA, Liang C. The transmembrane domain of BST-2 determines its sensitivity to down-modulation by human immunodeficiency virus type 1 Vpu. J. Virol. 2009;83:7536–7546. doi: 10.1128/JVI.00620-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sakai H, Shibata R, Sakuragi J, Sakuragi S, Kawamura M, Adachi A. Cell-dependent requirement of human immunodeficiency virus type 1 Vif protein for maturation of virus particles. J. Virol. 1993;67:1663–1666. doi: 10.1128/jvi.67.3.1663-1666.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sakuma T, Noda T, Urata S, Kawaoka Y, Yasuda J. Inhibition of Lassa and Marburg virus production by tetherin. J. Virol. 2009;83:2382–2385. doi: 10.1128/JVI.01607-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sauter D, Schindler M, Specht A, Landford WN, Münch J, Kim KA, Votteler J, Schubert U, Bibollet-Ruche F, Keele BF, Takehisa J, Ogando Y, Ochsenbauer C, Kappes JC, Ayouba A, Peeters M, Learn GH, Shaw G, Sharp PM, Bieniasz P, Hahn BH, Hatziioannou T, Kirchhoff F. Tetherin-driven adaptation of Vpu and Nef function and the evolution of pandemic and nonpandemic HIV-1 strains. Cell Host Microbe. 2009;6:409–421. doi: 10.1016/j.chom.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sebastian S, Luban J. TRIM5alpha selectively binds a restriction-sensitive retroviral capsid. Retrovirology. 2005;2:40. doi: 10.1186/1742-4690-2-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sheehy AM, Gaddis NC, Choi JD, Malim MH. Isolation of a human gene that inhibits HIV-1 infection and is suppressed by the viral Vif protein. Nature. 2002;418:646–650. doi: 10.1038/nature00939. [DOI] [PubMed] [Google Scholar]
- 105.Sheehy AM, Gaddis NC, Malim MH. The antiretroviral enzyme APOBEC3G is degraded by the proteasome in response to HIV-1 Vif. Nat. Med. 2003;9:1404–1407. doi: 10.1038/nm945. [DOI] [PubMed] [Google Scholar]
- 106.Shimojima M, Miyazawa T, Ikeda Y, McMonagle EL, Haining H, Akashi H, Takeuchi Y, Hosie MJ, Willett BJ. Use of CD134 as a primary receptor by the feline immunodeficiency virus. Science. 2004;303:1192–1195. doi: 10.1126/science.1092124. [DOI] [PubMed] [Google Scholar]
- 107.Shindo K, Takaori-Kondo A, Kobayashi M, Abudu A, Fukunaga K, Uchiyama T. The enzymatic activity of CEM15/Apobec-3G is essential for the regulation of the infectivity of HIV-1 virion but not a sole determinant of its antiviral activity. J. Biol. Chem. 2003;278:44412–44416. doi: 10.1074/jbc.C300376200. [DOI] [PubMed] [Google Scholar]
- 108.Simons K,, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 109.Simons K, Toomre D. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- 110.Sova P, Volsky DJ. Efficiency of viral DNA synthesis during infection of permissive and nonpermissive cells with vif-negative human immunodeficiency virus type 1. J. Virol. 1993;67:6322–26. doi: 10.1128/jvi.67.10.6322-6326.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sparger EE, Luciw PA, Elder JH, Yamamoto JK, Lowenstine LJ, Pedersen NC. Feline immunodeficiency virus is a lentivirus associated with an AIDS-like disease in cats. AIDS. 1989;3(Suppl. 1):S43–S49. doi: 10.1097/00002030-198901001-00006. [DOI] [PubMed] [Google Scholar]
- 112.Stern MA, Hu C, Saenz DT, Fadel HJ, Sims O, Peretz M, Poeschla EM. Productive replication of Vif-chimeric HIV-1 in feline cells. J. Virol. 2010;84:7378–7395. doi: 10.1128/JVI.00584-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Stopak K, de Noronha C, Yonemoto W, Greene WC. HIV-1 Vif blocks the antiviral activity of APOBEC3G by impairing both its translation and intracellular stability. Mol. Cell. 2003;12:591–601. doi: 10.1016/s1097-2765(03)00353-8. [DOI] [PubMed] [Google Scholar]
- 114.Strebel K, Daugherty D, Clouse K, Cohen D, Folks T, Martin MA. The HIV ‘A’ (sor) gene product is essential for virus infectivity. Nature. 1987;328:728–730. doi: 10.1038/328728a0. [DOI] [PubMed] [Google Scholar]
- 115.Strebel K, Klimkait T, Maldarelli F, Martin MA. Molecular and biochemical analyses of human immunodeficiency virus type 1 Vpu protein. J. Virol. 1989;63:3784–3791. doi: 10.1128/jvi.63.9.3784-3791.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Stremlau M, Owens CM, Perron MG, Kiessling M, Autissier P, Sodroski J. The cytoplasmic body component TRIM5 alpha restricts HIV-1 infection in Old World monkeys. Nature. 2004;427:848–853. doi: 10.1038/nature02343. [DOI] [PubMed] [Google Scholar]
- 117.Stremlau M, Perron M, Lee M, Li Y, Song B, Javanbakht H, Diaz-Griffero F, Anderson DJ, Sundquist WI, Sodroski J. Specific recognition and accelerated uncoating of retroviral capsids by the TRIM5alpha restriction factor. Proc. Natl. Acad. Sci. U S A. 2006;103:5514–5519. doi: 10.1073/pnas.0509996103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Teng B, Burant CF, Davidson NO. Molecular cloning of an apolipoprotein B messenger RNA editing protein. Science. 1993;260:1816–1819. doi: 10.1126/science.8511591. [DOI] [PubMed] [Google Scholar]
- 119.Terwilliger EF, Cohen EA, Lu YC, Sodroski JG, Haseltine WA. Functional role of human immunodeficiency virus type 1 vpu. Proc. Natl. Acad. Sci. U S A. 1989;86:5163–5167. doi: 10.1073/pnas.86.13.5163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Torten M, Franchini M, Barlough JE, George JW, Mozes E, Lutz H, Pedersen NC. Progressive immune dysfunction in cats experimentally infected with feline immunodeficiency virus. J. Virol. 1991;65:2225–2230. doi: 10.1128/jvi.65.5.2225-2230.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tompkins MB, Tompkins WA. Lentivirus-induced immune dysregulation. Vet. Immunol. Immunopathol. 2008;123:45–55. doi: 10.1016/j.vetimm.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Towers GJ. The control of viral infection by tripartite motif proteins and cyclophilin A. Retrovirology. 2007;4:40. doi: 10.1186/1742-4690-4-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Troyer JL, Pecon-Slattery J, Roelke ME, Black L, Packer C, O’Brien SJ. Patterns of feline immunodeficiency virus multiple infection and genome divergence in a free-ranging population of African lions. J. Virol. 2004;78:3777–3791. doi: 10.1128/JVI.78.7.3777-3791.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Troyer JL, Pecon-Slattery J, Roelke ME, Johnson W, VandeWoude S, Vazquez-Salat N, Brown M, Frank L, Woodroffe R, Winterbach C, Winterbach H, Hemson G, Bush M, Alexander KA, Revilla E, O’Brien SJ. Seroprevalence and genomic divergence of circulating strains of feline immunodeficiency virus among Felidae and Hyaenidae species. J. Virol. 2005;79:8282–8294. doi: 10.1128/JVI.79.13.8282-8294.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Troyer JL, Vandewoude S, Pecon-Slattery J, McIntosh C, Franklin S, Antunes A, Johnson W, O’Brien SJ. FIV cross-species transmission: an evolutionary prospective. Vet. Immunol. Immunopathol. 2008;123:159–166. doi: 10.1016/j.vetimm.2008.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Van Damme N, Goff D, Katsura C, Jorgenson RL, Mitchell R, Johnson MC, Stephens EB, Guatelli J. The interferon-induced protein BST-2 restricts HIV-1 release and is downregulated from the cell surface by the viral Vpu protein. Cell Host Microbe. 2008;3:245–252. doi: 10.1016/j.chom.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.VandeWoude S, Apetrei C. Going wild: lessons from naturally occurring T-lymphotropic lentiviruses. Clin. Microbiol. Rev. 2006;19:728–762. doi: 10.1128/CMR.00009-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Varthakavi V, Smith RM, Bour SP, Strebel K, Spearman P. Viral protein U counteracts a human host cell restriction that inhibits HIV-1 particle production. Proc. Natl. Acad. Sci. U S A. 2003;100:15154–15159. doi: 10.1073/pnas.2433165100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Vidal-Laliena M, Romero X, March S, Requena V, Petriz J, Engel P. Characterization of antibodies submitted to the B cell section of the 8th Human leukocyte differentiation antigens workshop by flow cytometry and immunohistochemistry. Cell Immunol. 2005;236:6–16. doi: 10.1016/j.cellimm.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 130.Vigne JD, Guilaine J, Debue K, Haye L, Gerard P. Early taming of the cat in Cyprus. Science. 2004;304:259. doi: 10.1126/science.1095335. [DOI] [PubMed] [Google Scholar]
- 131.Willett BJ, Flynn JN, Hosie MJ. FIV infection of the domestic cat: an animal model for AIDS. Immunol. Today. 1997;18:182–189. doi: 10.1016/s0167-5699(97)84665-8. [DOI] [PubMed] [Google Scholar]
- 132.Willey RL, Maldarelli F, Martin MA, Strebel K. Human immunodeficiency virus type 1 Vpu protein induces rapid degradation of CD4. J. Virol. 1992;66:7193–7200. doi: 10.1128/jvi.66.12.7193-7200.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wolf D, Goff SP. Host restriction factors blocking retroviral replication. Annu. Rev. Genet. 2008;42:143–63. doi: 10.1146/annurev.genet.42.110807.091704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wu X, Anderson JL, Campbell EM, Joseph AM, Hope TJ. Proteasome inhibitors uncouple rhesus TRIM5alpha restriction of HIV-1 reverse transcription and infection. Proc. Natl. Acad. Sci. U.S.A. 2006;103:7465–7470. doi: 10.1073/pnas.0510483103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yamamoto JK, Sparger E, No EW, Andersen PR, O’Connor TP, Handell CP, Lowenstine L, Munn R, Pedersen NC. Pathogenesis of experimentally induced feline immunodeficiency virus infection in cats. Am. J. Vet. Res. 1988;49:1246–1258. [PubMed] [Google Scholar]
- 136.Yamamoto JK, Hansen H, Ho EW, Morishita TY, Okuda T, Saura TR, Nakamura RM, Pedersen NC. Epidemiologic and clinical aspects of FIV infection in cats from the continental U.S. and Canada and possible mode of transmission. J. Am. Vet. Med. Assoc. 1989;194:213–220. [PubMed] [Google Scholar]
- 137.Yamauchi K, Wada K, Tanji K, Tanaka M, Kamitani T. Ubiquitination of E3 ubiquitin ligase TRIM5α and its potential role. FEBS J. 2008;275:1540–1555. doi: 10.1111/j.1742-4658.2008.06313.x. [DOI] [PubMed] [Google Scholar]
- 138.Yang Y, Guo F, Cen S, Kleiman L. Inhibition of initiation of reverse transcription in HIV-1 by human APOBEC3F. Virology. 2007;365:92–100. doi: 10.1016/j.virol.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 139.Yang SY, Lopez LA, Hauser H, Exline CM, Haworth KG, Cannon PM. Anti-tetherin activities in Vpu-expressing primate lentiviruses. Retrovirology. 2010;7:13. doi: 10.1186/1742-4690-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Yee JK, Miyanohara A, La Porte P, Bouic K, Burns JC, Friedmann T. A general method for the generation of high-titer, pantropic retroviral vectors: highly efficient infection of primary hepatocytes. Proc. Natl. Acad. Sci. U.S.A. 1994;91:9564–9568. doi: 10.1073/pnas.91.20.9564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yu X, Yu Y, Liu B, Luo K, Kong W, Mao P, Yu XF. Induction of APOBEC3G ubiquitination and degradation by an HIV-1 Vif-Cul5-SCF complex. Science. 2003;302:1056–1060. doi: 10.1126/science.1089591. [DOI] [PubMed] [Google Scholar]
- 142.Yu Y, Xiao Z, Ehrlich ES, Yu X, Yu XF. Selective assembly of HIV-1 Vif-Cul5-ElonginB-ElonginC E3 ubiquitin ligase complex through a novel SOCS box and upstream cysteines. Genes Dev. 2004;18:2867–2872. doi: 10.1101/gad.1250204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zhang H, Yang B, Pomerantz RJ, Zhang C, Arunachalam SC, Gao L. The cytidine deaminase CEM15 induces hypermutation in newly synthesized HIV-1 DNA. Nature. 2003;424:94–98. doi: 10.1038/nature01707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zhang F, Wilson SJ, Landford WC, Virgen B, Gregory D, Johnson MC, Munch J, Kirchhoff F, Bieniasz PD, Hatziioannou T. Nef proteins from simian immunodeficiency viruses are tetherin antagonists. Cell Host Microbe. 2009;6:54–67. doi: 10.1016/j.chom.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zheng YH, Irwin D, Kurosu T, Tokunaga K, Sata T, Peterlin BM. Human APOBEC3F is another host factor that blocks human immunodeficiency virus type 1 replication. J. Virol. 2004;78:6073–6076. doi: 10.1128/JVI.78.11.6073-6076.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zielonka J, Marino D, Hofmann H, Yuhki N, Löchelt M, Münk C. Vif of feline immunodeficiency virus from domestic cats protects against APOBEC3 restriction factors from many felids. J. Virol. 2010;84:7312–7324. doi: 10.1128/JVI.00209-10. [DOI] [PMC free article] [PubMed] [Google Scholar]