Abstract
The NAD malic enzyme from Crassula argentea shows a slow reaction transient in the form of a lag before reaching a steady-state rate in assays. This lag, which has a half-time or τ ranging from seconds to many minutes under various conditions, poses problems in the interpretation of kinetic data with this enzyme. The NAD malic enzyme from Kalanchoë daigremontiana has a similar lag.
The lag is greatest in freshly prepared enzyme and diminishes with storage at −70°C, but the activity of the enzyme also diminishes with storage.
The lag is inversely proportional to the concentration of enzyme, both in the assay and in storage prior to assay. The lag is also inversely proportional to the concentration of malate used in the assay, which poses particular problems because the lag with low malate concentrations may be so long that activity begins to be lost before the steady-state rate is reached.
Various buffer ions produce different lags, but the lag with all buffers is longer than in the absence of buffer. The effectors CoA and SO42− in the assay substantially decrease the lag. The lag is shorter with Mn2+ as the required divalent cation than when Mg2+ is used.
The response of enzyme activity to pH shows that the intrinsic activity is greater with magnesium than with manganese, although the rate actually attained is lower with Mg2+ because the pK values for the response to pH are closer together when that cation is used. The enzyme has a higher optimum pH and a broader response to pH when Mn2+ is used. The change in lag with pH follows the general pattern of activity with longer lags at intermediate pH values.
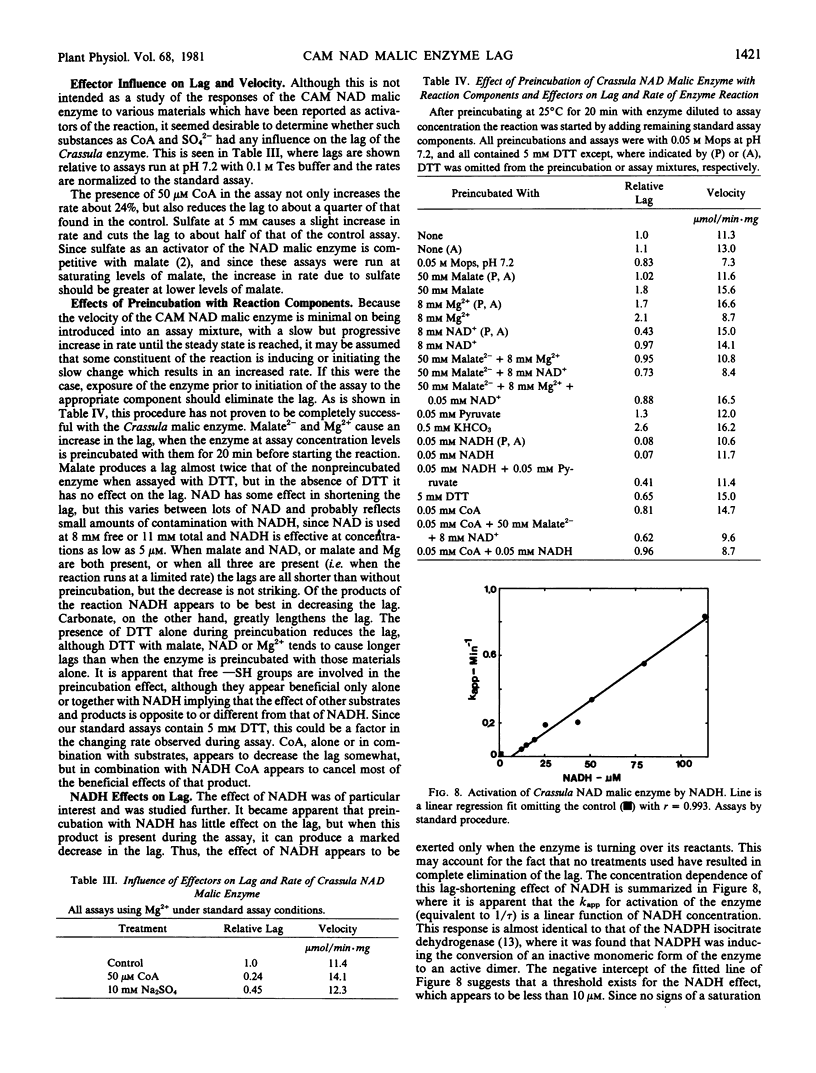
Preincubation of the enzyme with various reaction components and effectors reduces the lag, with NADH being the most effective. The presence of NADH in the assay is much more effective, but none of the treatments tried will completely eliminate the lag of freshly prepared enzyme.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Canellas P. F., Wedding R. T. Substrate and metal ion interactions in the NAD+ malic enzyme from cauliflower. Arch Biochem Biophys. 1980 Jan;199(1):259–264. doi: 10.1016/0003-9861(80)90279-9. [DOI] [PubMed] [Google Scholar]
- Chapman K. S., Hatch M. D. Regulation of mitochondrial NAD-malic enzyme involved in C4 pathway photosynthesis. Arch Biochem Biophys. 1977 Nov;184(1):298–306. doi: 10.1016/0003-9861(77)90354-x. [DOI] [PubMed] [Google Scholar]
- Cleland W. W. Determining the chemical mechanisms of enzyme-catalyzed reactions by kinetic studies. Adv Enzymol Relat Areas Mol Biol. 1977;45:273–387. doi: 10.1002/9780470122907.ch4. [DOI] [PubMed] [Google Scholar]
- Day D. A. Malate Decarboxylation by Kalanchoë daigremontiana Mitochondria and Its Role in Crassulacean Acid Metabolism. Plant Physiol. 1980 Apr;65(4):675–679. doi: 10.1104/pp.65.4.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittrich P. Nicotinamide Adenine Dinucleotide-specific "Malic" Enzyme in Kalanchoë daigremontiana and Other Plants Exhibiting Crassulacean Acid Metabolism. Plant Physiol. 1976 Feb;57(2):310–314. doi: 10.1104/pp.57.2.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grover S. D., Canellas P. F., Wedding R. T. Purification of NAD malic enzyme from potato and investigation of some physical and kinetic properties. Arch Biochem Biophys. 1981 Jul;209(2):396–407. doi: 10.1016/0003-9861(81)90297-6. [DOI] [PubMed] [Google Scholar]
- Hatch M. D., Mau S. L., Kagawa T. Properties of leaf NAD malic enzyme from plants with C4 pathway photosynthesis. Arch Biochem Biophys. 1974 Nov;165(1):188–200. doi: 10.1016/0003-9861(74)90155-6. [DOI] [PubMed] [Google Scholar]
- Kelly J. H., Plaut G. W. Kinetic evidence for the dimerization of the triphosphopyridine nucleotide-dependent isocitrate dehydrogenase from pig heart. J Biol Chem. 1981 Jan 10;256(1):335–342. [PubMed] [Google Scholar]
- Macrae A. R. Isolation and properties of a 'malic' enzyme from cauliflower bud mitochondria. Biochem J. 1971 May;122(4):495–501. doi: 10.1042/bj1220495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milne J. A., Cook R. A. Role of metal cofactors in enzyme regulation. Differences in the regulatory properties of the Escherichia coli nicotinamide adenine dinucleotide specific malic enzyme depending on whether Mg2+ or Mn2+ serves as divalent cation. Biochemistry. 1979 Aug 7;18(16):3604–3610. doi: 10.1021/bi00583a026. [DOI] [PubMed] [Google Scholar]
- Neet K. E., Ainslie G. R., Jr Hysteretic enzymes. Methods Enzymol. 1980;64:192–226. doi: 10.1016/s0076-6879(80)64010-5. [DOI] [PubMed] [Google Scholar]
- Reynolds C. H., Hsu R. Y., Matthews B., Pry T. A., Dalziel K. Transient kinetic studies of malic enzyme. A conformational change associated with substrate inhibition by malate. Arch Biochem Biophys. 1978 Aug;189(2):309–316. doi: 10.1016/0003-9861(78)90217-5. [DOI] [PubMed] [Google Scholar]
- Spalding M. H., Arron G. P., Edwards G. E. Malate decarboxylation in isolated mitochondria from the Crassulacean acid metabolism plant Sedum praealtum. Arch Biochem Biophys. 1980 Feb;199(2):448–456. doi: 10.1016/0003-9861(80)90301-x. [DOI] [PubMed] [Google Scholar]
- Wedding R. T., Black M. K., Pap D. Malate Dehydrogenase and NAD Malic Enzyme in the Oxidation of Malate by Sweet Potato Mitochondria. Plant Physiol. 1976 Dec;58(6):740–743. doi: 10.1104/pp.58.6.740. [DOI] [PMC free article] [PubMed] [Google Scholar]


