Abstract
Background
Soft tissue sarcomas (STS) are a heterogeneous group of mesenchymal malignancies that occur throughout the lifespan. The impact of age on disease features and outcome is unclear.
Methods
We analyzed the clinical features and outcome of all STS cases registered between 1973 and 2006 in the SEER database.
Results
There were 48,012 cases that met the selection criteria. Individuals less than 20 years of age represented 5.6%, with rhabdomyosarcoma being the most common subtype. In adults, the most common types were Kaposi sarcoma, fibrohistiocytic tumors, and leiomyosarcoma. Rhabdomyosarcoma was the only entity with a median age < 20 years. Male predominance (male:female of 1.5:1) was noticed for almost all types of STS, except for alveolar soft part sarcoma and leiomyosarcoma. Tumor stage was similar across different age groups. Younger patients (<50 years) had significantly better survival than older patients (88.8%±0.2% vs. 40%±0.3%, P<0.001), but for most histologies the survival decline with advancing age was gradual and did not occur abruptly at the onset of adulthood. The decline in survival with advancing age was particularly significant for rhabdomyosarcoma.
Conclusion
With few exceptions, the clinical features of STS are similar in children and adults. However, individuals over 50 years of age have an inferior survival.
Keywords: Soft tissue sarcoma, age, prognosis, SEER, epidemiology
INTRODUCTION
Soft tissue sarcomas (STS) are a heterogeneous group of mesenchymal extraskeletal malignant tumors, classified on the basis of their differentiation according to the adult tissue they resemble. Whereas benign neoplasms of soft tissues (i.e., lipoma, fibroma, leiomyoma, hemangioma) are relatively frequent and out number malignant cases by a factor of 50 (incidence of about 300 new cases per 100,000 persons), malignant soft tissue tumors are rare, with an annual incidence of about 6 per 100,000 persons. STS occur throughout childhood and adulthood; they account for about 1.5% of all malignant tumors in adults (and 2% of all cancer-related deaths), but represent about 7.4% of all pediatric malignancies (although their absolute number is lower than in adults).[1]
It is well known that the pattern of STS histotypes differs significantly between adults and children: rhabdomyosarcoma is characteristic in childhood, liposarcoma and leiomyosarcoma are seen typically in adults, and certain subtypes such as synovial sarcoma and alveolar soft part sarcoma straddle both age groups. It is less clear whether the clinical behaviour of a given STS is independent of the age of the patient and thus whether adult and pediatric patients with the same disease should be treated with the same or different therapeutic strategies. Few studies have compared the clinical features, treatment approaches, and survival rates of STS in children and adults, and studies addressing differences in the biology of STS in various age groups are scarce.
To better characterize the natural history of the different STS histotypes across the age spectrum, and to investigate the impact of age on disease features and outcome, we performed an analysis of all STS cases registered in the U.S. Surveillance, Epidemiology, and End Results (SEER) public-access database between 1973 and 2006.
PATIENTS AND METHODS
Data were obtained from the SEER registries (http://seer.cancer.gov/data/). [2] The SEER Program of the National Cancer Institute (NCI) currently covers approximately 26 percent of the US population. The rate session of the SEER*Stat program was used to calculate frequencies, percentages and incidence rates for soft tissue sarcomas and all other malignancies by using the original 9 registries as well as the 2000 US standard population. [3]
We used the Case listing session of the SEER*Stat 6.5.2 program to generate a matrix of all individuals reported with a diagnosis of soft tissue sarcoma from January 1973 to December 2006. A selection query was designed to retrieve soft tissue sarcomas (STS) based on the International Classification of Childhood Cancer, version 3 (ICCC-3). The query was designed to exclude patients with autopsy/death certificate only and patients with no microscopic confirmation of diagnosis. The resulting matrix from SEER*Stat was transferred to SAS software program (SAS 9.1 version, SAS Institute Inc., Cary, NC, USA) for descriptive analyses.
Tumors were described using a modified ICCC-3 classification: extraosseous Ewing tumor, Askin tumor of soft tissue and peripheral primitive neuroectodermal tumor (pPNET) of soft tissue were included in one category (Ewing-family tumors); also, miscellaneous and unspecified soft tissue sarcomas together with fibromatous neoplasms were included under one category (Table I). Disease stage was classified using the SEER staging system. Localized stage refers to an invasive neoplasm confined entirely to the organ of origin; regional stage refers to a neoplasm that has extended beyond the limits of the organ of origin directly into surrounding organs or tissues, has spread to regional lymph nodes by way of the lymphatic system, or both; and distant stage refers to a neoplasm that has spread to parts of the body remote from the primary tumor either by direct extension or by discontinuous metastasis to distant organs and tissues or via the lymphatic system to distant lymph nodes. Patients were divided into eight age categories based on age at diagnosis in decades. We arbitrarily defined pediatric patients (children and adolescents) as cases younger than 20 years of age, and adults whose age was 20 years or older.
Table I.
Characteristics of STS patients in different historic subtypes
| Age at diagnosis | All pediatric (0–19 yrs) | ||||
|---|---|---|---|---|---|
|
| |||||
| Histology | N | Median (years) | Range | N | % |
| Rhabdomyosarcoma | 2831 | 15 | 0 to 85+ | 1668 | 58.9 |
| Fibroblastic & myofibroblastic tumors | 3037 | 54 | 0 to 85+ | 286 | 9.4 |
| Fibrohistiocytic tumors | 14599 | 57 | 0 to 85+ | 547 | 3.7 |
| Malignant peripheral nerve sheath tumors | 2186 | 46 | 0 to 85+ | 217 | 9.9 |
| Kaposi sarcoma | 15241 | 39 | 0 to 85+ | 15 | 0.1 |
| Ewing family tumors | 589 | 24 | 0 to 85+ | 233 | 39.6 |
| Extrarenal rhabdoid tumor | 109 | 39.5 | 0 to 85+ | 44 | 40.4 |
| Liposarcomas | 7419 | 60 | 1 to 85+ | 87 | 1.2 |
| Leiomyosarcomas | 13135 | 59 | 0 to 85+ | 112 | 0.9 |
| Synovial sarcomas | 1859 | 35 | 0 to 85+ | 328 | 17.6 |
| Blood vessel tumors | 2742 | 65 | 0 to 85+ | 57 | 2.1 |
| Osseous and chondromatous neoplasms of soft tissue | 680 | 55 | 0 to 85+ | 26 | 3.8 |
| Alveolar soft parts sarcoma | 164 | 25 | 0 to 84 | 47 | 28.7 |
| Miscellaneous/unspecified including other fibromatous neoplasms | 8381 | 61 | 0 to 85+ | 480 | 5.7 |
The survival session of the software was used to calculate the 5-, 10-, and 15-year relative survival percentages overall and by 10-year age category. Unlike event free and overall survival, relative survival represents cancer survival in the absence of other causes of death, defined as the proportion of observed survivors to the proportion of expected survivors in a comparable set of cancer free individuals. [3]The Kaplan-Meier method with cancer-specific survival as the outcome was used to compare mortality by age categories.
RESULTS
Incidence
Data from the SEER Program [2] original 9 registries were used to calculate frequencies, percentages and incidence rates for soft tissue sarcomas (STS) and all other malignancies by 10 year age groups. Results are shown in Supplemental Table I. Of the 3,113,812 malignancies reported, 48,012 (1.5%) were STS. Among all of the STS reported, 2,679 (5.6%) were among persons younger than age 20 years at diagnosis. The proportion of total malignancies that were STS was highest in individuals younger than age 30 at diagnosis. The incidence of STS from 1973 to 2006 was 5.9 per 100,000 persons. Annual incidence rates increased with age, rising from 0.9/100,000 in children younger than age 10 years to 18.2 for individuals older than age 70. The most dramatic rise in incidence occurred after the age of 30 years. This rise was driven by increased numbers of Kaposi sarcoma in young adults and by increased numbers of fibrohistiocytic tumors, liposarcoma and leiomyosarcoma in the elderly.
Demographic and clinical characteristics of STS reported to the SEER registry 1973–2006
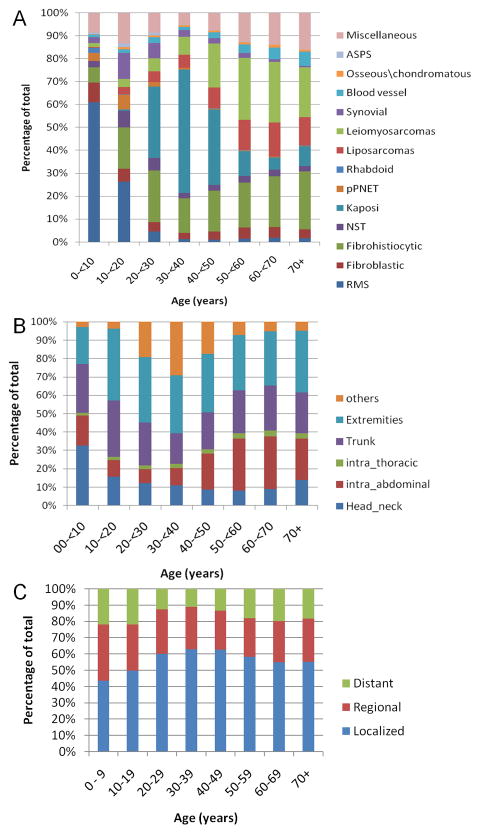
Using data from the SEER Program (SEER 2008) original 9 (1973–2006) and expanded 17 (2000–2006) registries, we identified 72,972 individuals with STS to include in our descriptive analysis. Table 1 shows the median age at diagnosis by histologic subtype. Rhabdomyosarcoma was the only neoplasm that had a median age younger than 20 years (15 years), whereas the median age was younger than 30 years for Ewing sarcoma family of tumors (24 years) and alveolar soft part sarcoma (25 years). Median age was younger than 40 years for both synovial sarcoma (35 years) and extrarenal rhabdoid tumor (39.5 years). Some subtypes were exceedingly rare in children/adolescents: i.e., only 0.1% of Kaposi sarcoma and 0.9% of leiomyosarcoma cases were reported in this age group. Figure 1A shows the great variability in the frequency of each subtype by 10 year age group. In children/adolescents, the most common types were rhabdomyosarcoma (40.2%), fibrohistiocytic tumors (13.2%), miscellaneous/unspecified including other fibromatous neoplasms (11.6%) and synovial sarcoma (7.9%). The most common types in adults were Kaposi sarcoma (22.1%), fibrohistiocytic tumors (20.4%), and leiomyosarcoma (18.9%). Using chi-square test, the distribution of different subtypes was significantly different between children/adolescents and adults (P<0.001)
Figure 1.
(A) Distribution of histologic subtypes by 10-year age groups, (B)distribution of primary tumor sites by 10-year age groups, (C) distribution of tumor stage by 10-year age groups; abbreviations: RMS: rhabdomyosarcomas; Fibroblastic: fibroblastic and myofibroblastic tumors; Fibrohistiocytic: Fibrohistiocytic tumors; NST: malignant peripheral nerve sheath tumors; Kaposi: Kaposi sarcoma; pPNET: Ewing family tumors; Rhabdoid: extraneral rhabdoid tumor; Liposarcoma: liposarcomas; Synovial: Synovial sarcomas; Blood vessel: blood vessel tumors; ASPS: alveolar soft parts sarcoma; Miscellaneous: Miscellaneous/unspecified soft tissue sarcomas including other fibromatous neoplasms.
Extremities (31.9%) were the most common site of origin for the entire STS series, followed by trunk (21.8%) and intra-abdominal (19.7%) sites. These three sites accounted for more than 70% of all reported locations. The distribution of primary tumor sites differed significantly (P<0.001) by age group, with children younger than 10 years of age most likely to have tumors in the head and neck region (32.7%), and individuals older than age 40 more likely to have tumors originating in the abdomen (Figure 1B). Using the SEER historic staging system, 11.7% (n=8543) of all reported STS had distant metastases. No differences in tumor stage distribution were observed among the different age groups (Figure 1C).
Table II summarizes the gender, primary site and stage distributions by histology with separate panels for pediatric and adult patients. Males made up more than half of the cohort for most tumor types among both pediatric and adult malignancies. A female predominance was noted for adult leiomyosarcoma and alveolar soft part sarcoma subtypes. Overall, extremities were the most frequent primary site, but some exceptions existed. Childhood rhabdomyosarcomas and extrarenal rhabdoid tumors were most common in the head and neck region, childhood Ewing family tumors most common in the trunk; and childhood leiomyosarcoma most common in the intra-abdominal region. Adult Ewing tumors, blood vessel, and miscellaneous STS were most common in the trunk, and adult extrarenal rhabdoid tumor and leiomyosarcomas were most common in the intraabdominal region. Extrarenal rhabdoid tumor had different site distributions among the pediatric and adult groups. Among children, the head and neck region was the most frequently reported, and among adults, the abdominal region was the most frequently reported site of origin. Distant metastases were more commonly reported among pediatric patients (19.6%) than among adults (11.2%; p<0.001). Distant stage occurred in fewer than 10% of pediatric and adult patients with fibroblastic sarcomas, fibrohistiocytic sarcomas, and liposarcoma.
Table II.
Characteristics of pediatric and adult patients with soft tissue sarcoma
| Pediatric (< 20 years) | Gender | Primary site | Staging | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| Histology | M | F | H&N | Ab | Intra-T | Trunk | Extre | Others | L | R | D | U | |
|
| |||||||||||||
| Total N | Row % | Row % | Row % | Row % | Row % | Row % | Row % | Row % | Row % | Row % | Row % | Row % | |
| RMS | 1668 | 58.8 | 41.2 | 35.1 | 18.0 | 1.0 | 29.1 | 13.4 | 3.4 | 31.1 | 31.8 | 27.4 | 9.7 |
| Fibroblastic | 286 | 49.3 | 50.7 | 18.9 | 6.6 | 2.4 | 25.5 | 44.8 | 1.7 | 55.6 | 23.1 | 7.0 | 14.3 |
| Fibrohistiocytic | 547 | 47.5 | 52.5 | 15.4 | 0.4 | 0.7 | 34.9 | 47.3 | 1.3 | 58.3 | 30.2 | 2.6 | 9.0 |
| MPNST | 217 | 52.5 | 47.5 | 17.5 | 13.8 | 1.8 | 27.2 | 33.6 | 6.0 | 48.8 | 24.0 | 14.7 | 12.4 |
| Kaposi | 15 | 80.0 | 20.0 | 13.3 | 0.0 | 0.0 | 13.3 | 26.7 | 46.7 | 0.0 | 0.0 | 0.0 | 100.0 |
| Ewing family tumors | 233 | 56.7 | 43.3 | 11.6 | 7.3 | 3.0 | 49.4 | 24.9 | 3.9 | 30.0 | 32.6 | 26.6 | 10.7 |
| Rhabdoid | 44 | 56.8 | 43.2 | 29.5 | 9.1 | 6.8 | 27.3 | 20.5 | 6.8 | 29.5 | 27.3 | 31.8 | 11.4 |
| Liposarcomas | 87 | 48.3 | 51.7 | 8.0 | 4.6 | 3.4 | 18.4 | 63.2 | 2.3 | 67.8 | 19.5 | 3.4 | 9.2 |
| Leiomyosarcomas | 112 | 48.2 | 51.8 | 14.3 | 33.0 | 1.8 | 22.3 | 27.7 | 0.9 | 50.9 | 24.1 | 15.2 | 9.8 |
| Synovial | 328 | 55.2 | 44.8 | 5.8 | 1.5 | 1.2 | 15.5 | 75.3 | 0.6 | 61.6 | 23.2 | 9.5 | 5.8 |
| Blood vessel | 57 | 54.4 | 45.6 | 12.3 | 12.3 | 8.8 | 15.8 | 36.8 | 14.0 | 42.1 | 10.5 | 28.1 | 19.3 |
| Osseous\chondromatous | 26 | 57.7 | 42.3 | 7.7 | 0.0 | 0.0 | 26.9 | 61.5 | 3.8 | 26.9 | 38.5 | 26.9 | 7.7 |
| ASPS | 47 | 38.3 | 61.7 | 27.7 | 2.1 | 0.0 | 14.9 | 55.3 | 0.0 | 55.3 | 17.0 | 23.4 | 4.3 |
| Miscellaneous | 480 | 53.1 | 46.9 | 15.8 | 14.4 | 2.5 | 30.2 | 31.5 | 5.6 | 40.0 | 18.5 | 26.7 | 14.8 |
| Adult (20 + years) | Gender | Primary site | Staging | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| Histology | M | F | H&N | Ab | Intra-T | Trunk | Extre | Others | L | R | D | U | |
|
| |||||||||||||
| Total N | Row % | Row % | Row % | Row % | Row % | Row % | Row % | Row % | Row % | Row % | Row % | Row % | |
| RMS | 1163 | 54.6 | 45.4 | 19.5 | 20.9 | 3.8 | 24.7 | 25.5 | 5.6 | 31.6 | 25.9 | 29.0 | 13.5 |
| Fibroblastic | 2751 | 49.7 | 50.3 | 16.5 | 11.0 | 6.8 | 29.4 | 33.4 | 2.8 | 53.8 | 18.5 | 9.5 | 18.3 |
| Fibrohistiocytic | 14052 | 54.6 | 45.4 | 17.0 | 4.1 | 1.2 | 30.5 | 45.8 | 1.4 | 57.5 | 26.5 | 6.0 | 10.0 |
| MPNST | 1969 | 53.7 | 46.3 | 18.2 | 13.6 | 3.1 | 27.6 | 31.5 | 6.0 | 46.4 | 23.4 | 12.0 | 18.2 |
| Kaposi | 15226 | 94.8 | 5.2 | 10.7 | 2.9 | 1.4 | 5.9 | 31.8 | 47.2 | 0.0 | 0.0 | 0.0 | 100.0 |
| Ewing family tumors | 356 | 53.1 | 46.9 | 10.1 | 13.2 | 8.4 | 39.3 | 25.3 | 3.7 | 30.3 | 27.5 | 30.3 | 11.8 |
| Rhabdoid | 65 | 47.7 | 52.3 | 7.7 | 44.6 | 6.2 | 21.5 | 12.3 | 7.7 | 21.5 | 24.6 | 38.5 | 15.4 |
| Liposarcomas | 7332 | 58.9 | 41.1 | 3.5 | 22.6 | 0.8 | 28.1 | 43.7 | 1.3 | 65.7 | 20.7 | 6.3 | 7.3 |
| Leiomyosarcomas | 13023 | 35.2 | 64.8 | 4.1 | 60.9 | 1.5 | 17.1 | 13.4 | 3.0 | 50.1 | 19.3 | 20.2 | 10.5 |
| Synovial | 1531 | 51.9 | 48.1 | 6.3 | 1.8 | 5.3 | 15.7 | 68.7 | 2.2 | 54.3 | 23.1 | 13.8 | 8.8 |
| Blood vessel | 2685 | 45.6 | 54.4 | 24.6 | 14.2 | 7.3 | 31.8 | 15.5 | 6.6 | 38.8 | 24.6 | 20.2 | 16.5 |
| Osseous\chondromatous | 654 | 59.9 | 40.1 | 8.0 | 0.3 | 0.9 | 36.2 | 52.3 | 2.3 | 47.1 | 32.7 | 12.7 | 7.5 |
| ASPS | 117 | 46.2 | 53.8 | 1.7 | 10.3 | 2.6 | 18.8 | 63.2 | 3.4 | 32.5 | 9.4 | 46.2 | 12.0 |
| Miscellaneous | 7901 | 49.2 | 50.8 | 9.3 | 25.0 | 7.6 | 26.8 | 24.2 | 7.2 | 34.8 | 21.0 | 24.6 | 19.5 |
Abbreviation: RMS: rhabdomyosarcomas; MPNST: malignant peripheral nerve sheath tumors; ASPS: alveolar soft parts sarcoma; Miscellaneous: Miscellaneous/unspecified soft tissue sarcomas including other fibromatous neoplasms. M: males; F: females; H&N: primary site of head and neck; Ab: primary site of intra-abdomen; Intra-T: primary site of intra-thorax; Trunk: primary site of trunk; Extre: primary site of extremities; L: localized; R: regional; D: distant; U: unstaged.
Survival
Overall survival of all persons diagnosed with STS was 51.5% ± 0.2% at five years, 37.7% ± 0.3% at ten years, and 30% ± 0.3% at fifteen years. Relative survival estimates were 62.1% ± 0.2%, 55.3% ± 0.3% and 51.2% ± 0.3% at five, 10 and 15 years, respectively. Survival among individuals with STS differed as a function of age and histology (Supplemental Figure 1 and Table III). Patients younger than 50 years at diagnosis had better cancer-specific survival (88.8% ± 0.2%) than older patients (40% ± 0.3%; log-rank test, P<0.001). A particularly notable progressive decline in survival with advancing age was noted for rhabdomyosarcoma. Kaposi sarcoma was the only STS in which older patients (> 60 years) had better survival rates when compared to younger individuals. Size cut-offs of 5 and 10 cm were associated with significantly increased probability for cancer-specific death in all age groups (P<0.001). Supplemental Table II shows five year relative survival rates by histology and tumor stage, comparing specifically children/adolescents to adults. Children and adolescents with STS had better five year estimated survival than adults when patients with same stage at diagnosis were compared, the only significant exception being localized malignant peripheral nerve sheath tumors.
Table III.
Five-year relative survival rates for the soft tissue sarcomas by histology and age groups
| Age (yrs) | Interval | Overall | RMS | Fibrobl | Fibrohis | MPNST | Ka | EW | RH | Lipo | Leio | Syn | BV | Osse | ASPS | Mis |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0- 9 | 5 yrs | 75 (0.3) | 70 (1.6) | 89 (2.8) | 97 (1.6) | 64 (7.9) | 50 | 74 (6.0) | 45 (8.6) | 89 | 82 (7.5) | 92 (4.3) | 43 | * | 100 | 57 (4.3) |
| 10–19 | 5 yrs | 75 (0.3) | 50 (2.1) | 89 (2.8) | 95 (1.2) | 51 (4.1) | 55 | 63 (4.2) | * | 81 (4.8) | 87 (3.9) | 77 (2.8) | 71 (7.5) | 64 | 91 (5.5) | 59 (3.0) |
| 20–29 | 5 yrs | 79 (0.2) | 38 (3.4) | 84 (2.6) | 97 (0.6) | 50 (3.1) | 20 (1.0) | 43 (5.4) | 67 | 88 (2.2) | 74 (2.7) | 68 (2.7) | 50 (4.6) | 71 (7.6) | 56 (6.6) | 58 (2.4) |
| 30–39 | 5 yrs | 74 (0.1) | 32 (4.1) | 78 (2.5) | 94 (0.6) | 49 (3.1) | 23 (0.6) | 38 (5.9) | 43 | 85 (1.5) | 69 (1.6) | 63 (3.0) | 53 (3.8) | 76 (5.1) | 54 | 52 (2.2) |
| 40–49 | 5 yrs | 69 (0.1) | 42 (4.9) | 78 (2.3) | 88 (0.8) | 57 (3.0) | 27 (0.8) | 38 (7.8) | 36 | 84 (1.3) | 63 (1.1) | 58 (3.5) | 51 (3.1) | 82 (4.2) | 24 | 48 (1.8) |
| 50–59 | 5 yrs | 64 (0.1) | 32 (4.2) | 73 (2.3) | 78 (1.1) | 57 (3.3) | 37 (1.6) | 27 (8.4) | 55 | 82 (1.3) | 47 (1.1) | 55 (3.9) | 41 (2.7) | 74 (5.1) | 26 | 42 (1.5) |
| 60–69 | 5 yrs | 60 (0.1) | 28 (4.0) | 59 (2.7) | 70 (1.2) | 54 (3.6) | 64 (2.6) | 41 (9.5) | 43 | 78 (1.4) | 44 (1.1) | 52 (5.2) | 31 (2.4) | 67 (5.4) | 0 | 33 (1.5) |
| 70+ | 5 yrs | 56 (0.1) | 18 (3.0) | 56 (3.0) | 63 (1.2) | 47 (3.6) | 79 (2.2) | 30 | * | 71 (1.7) | 41 (1.1) | 38 (6.0) | 30 (2.0) | 57 (6.3) | 0 | 29 (1.2) |
The Kaplan-Meier method could not be calculated because of not enough intervals to produce rate. The data shows 5, 10, and 15 yrs estimated relative survival rate including standrad error denoted with brackets. Abbreviation: RMS: rhabdomyosarcomas; Fibrobl: fibroblastic and myofibroblastic tumors; Fibrohis: Fibrohistiocytic tumors; MPNST: malignant peripheral nerve sheath tumors; Ka: Kaposi sarcoma; EW: Ewing family tumors; RH: extraneral rhabdoid tumor; Lipo: liposarcomas; Leio: leoimyosarcomas; Syn: Synovial sarcomas; BV: blood vessel tumors; Osse: Osseous and chondromatous neoplasm of soft tissue; ASPS: alveolar soft parts sarcoma; Mis: Miscellaneous/unspecified soft tissue sarcomas including other fibromatous neoplasms. STS: overall survival rate for the soft tissue sarcomas.
DISCUSSION
Our analysis of SEER registry data over recent decades indicates that age influences the incidence, distribution of histologic subtypes, and survival of patients with STS. Annual incidence rose steadily with age, from 0.9/100,000 children younger than 10 years of age to 18.2/100,000 adults over 70 years of age. The most dramatic rises in incidence occurred at 30 years of age and at 70 years of age. These were driven by increased numbers of Kaposi sarcoma cases in young adults [4–5] and by increased numbers of fibrohistiocytic tumors, liposarcoma, and leiomyosarcoma in the elderly. Despite a rise in incidence with advancing age, STS comprised a significantly higher proportion of all cancers in younger patients. STS accounted for more than 6% of cancers in individuals under 40 years of age, but fewer than 3% of all cancers in individuals over 40 years of age. Rhabdomyosarcoma was the only neoplasm with a median age of onset below 20 years (15 years); only liposarcoma, blood vessel tumors, and miscellaneous tumors had a median age of onset above 60 years.
Our study found that STS are more common in males than in females (P<0.001), in both adults and children. Exceptions to this rule are leiomyosarcoma, alveolar soft part sarcoma, and in adults, blood vessel sarcomas. The preponderance of adult females with leiomyosarcoma is likely due to uterine primary tumors, which account for about 25% of leiomyosarcoma cases in adults.[6] In childhood, where uterine leiomyosarcoma is distinctly rare, the gender balance is nearly equal.
The distribution of STS anatomic sites in our series differed between adults and children. Extremity sites were the most common in all age groups except individuals under 10 years of age. The predominance of head and neck sites in these youngest patients may be explained by the high incidence of rhabdomyosarcoma in this age group since this tumor presents in head and neck locations in 35% of cases.[7] Intra-abdominal tumors were more common in individuals over 40 years of age, likely reflecting patients with retroperitoneal sarcomas such as leiomyosarcoma and liposarcoma.
Observed differences in presenting clinical features between adults and children may reflect underlying differences in biology. For example, the high proportion of head and neck rhabdomyosarcoma cases in children reflects the fact that the most common histologic subtype in this age group, embryonal histology, has a predilection for head and neck sites. [7] Embryonal histology is considerably less common in adults with rhabdomyosarcoma, who more frequently have pleomorphic histology tumors that arise in non-head-and-neck sites.[7] Another tumor that appears to have different biologic underpinnings in adults and children is rhabdoid tumor. The observation that head and neck rhabdoid tumors were nearly four times more common in children than in adults may reflect the biology associated with early onset disease: germline rather than sporadic INI1 mutation.[8]
Previous studies in both children and adults suggest that age is a predictor of outcome in STS. In pediatric rhabdomyosarcoma, younger age (1–10 years) is a favorable prognostic factor and age is currently being used in risk-based treatment stratification approaches.[9–10] Rhabdomyosarcoma occurring in adults is characterized by a more aggressive course and worse prognosis.[11–12] Older age has also emerged as an unfavourable factor in pediatric STS other than rhabdomyosarcoma.[13–14] In adult STS, age has been identified as an independent prognostic indicator of survival [15–17], and it is included as a continuous variable (together with tumor size and depth, site, histology and tumor grade) in the post-operative nomogram developed by the Memorial Sloan-Kettering Cancer Center for prediction of sarcoma-specific death.[18–21]
Our analysis confirmed these observations in a large population-based series, demonstrating significantly inferior survival for patients over 50 years of age. Children and adolescents had better outcome than adults when patients with same histotypes and same stage were compared; the only exception was localized malignant peripheral nerve sheath tumors, probably due to the preponderance of Neurofibromatosis type 1 (associated with poor outcome) in younger cases. [22]
The explanation for the decreasing outcome with age is probably multifactorial. For example, adults with RMS more commonly have non-embryonal histology and tumors that arise in unfavorable primary sites.[7] Other differences in tumor or host biology are possible, although they remain the object of speculative hypotheses [23]. Finally, the impact of the type and intensity of treatment on outcome cannot be ignored. Older patients may receive less aggressive local and systemic treatment, either by prescription or due to lower tolerance. In rhabdomyosarcoma, for example, retrospective single institution analyses have shown that, although outcome for adults overall is poorer than for children, those cases treated according to standard guidelines for pediatric rhabdomyosarcoma had a better outcome, in some cases comparable to that expected for pediatric patients. [12,24–26], Unfortunately, the SEER database does not provide adequate data on treatment, and therefore our study cannot explore the impact of treatment on outcome.
Furthermore, our study has a number of important limitations, similar to other population-based registry studies. The lack of data on tumor grade is problematic in any study of STS, since histologic grade is among the strongest predictors of outcome and would be of interest in trying to explain differences in outcome between pediatric and adult patients. The lack of central pathologic review to confirm the histologic diagnosis also limits to some degree the reliability of our findings. The diagnostic criteria for STS have changed significantly over the years, [27] and errors in the diagnosis of STS are well known to occur, particularly in non-specialized centers. [28–30]. Another limitation of this study is the lack of data regarding treatment-related mortality. As for other population-based analyses, these limitations must be considered in the context of the very large number of cases analyzed.
In conclusion, our study indicates that age influences the incidence, distribution of histologic subtypes, and survival of individuals with STS. Additional studies are needed to better define the biology of these tumors and investigate whether differences between age groups exist. Although outcome was inferior overall for adults in our analysis, we observed a gradual decline in survival with advancing age for most histologic subtypes rather than an abrupt decline at the attainment of adulthood (20 years). The notable exception was rhabdomyosarcoma, which had a substantially better outcome in pediatric patients. Our findings suggest that, except in rare cases where tumor biology is known to be different in pediatric and adult cohorts (e.g., infantile fibrosarcoma), there are no significant epidemiologic reasons why patients with STS should be treated differently based on their age at presentation. Thus, collaboration between pediatric and medical oncologists should be encouraged to expedite the development of cooperative trials that integrate uniform treatment approaches.
Supplementary Material
Five-year relative survival rate trends in soft tissue sarcoma patients in different subtypes among different age groups; abbreviation: RMS: rhabdomyosarcomas; Fibrobl: fibroblastic and myofibroblastic tumors; Fibrohis: Fibrohistiocytic tumors; MPNST: malignant peripheral nerve sheath tumors; EW: Ewing family tumors; Syn: Synovial sarcomas; Leio: leiomyosarcoma; ASPS: alveolar soft parts sarcoma.
Footnotes
The authors have no financial interest or conflict of interest to declare.
References
- 1.Weiss S, Goldblum J. General considerations. In: Weiss S, Goldblum J, editors. Enzinger and Weiss’s Soft Tissue Tumors. St Louis, Missouri: CV Mosby; 2001. pp. 1–19. [Google Scholar]
- 2.National Cancer Institute. Surveillance, Epidemiology, and End Results (SEER) Program Public-Use Data (1973–2006) National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch; released April 2009, based on the November 2008 submission. www.seer.cancer.gov. [Google Scholar]
- 3.Surveillance, Epidemiology, and End Results (SEER) Program. National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch; SEER*Stat Database: Incidence - SEER 9 Regs Limited-Use, Nov 2008 Sub (1973–2006) <Katrina/Rita Population Adjustment> - Linked To County Attributes - Total U.S., 1969–2006 Counties. ( www.seer.cancer.gov) released April 2009, based on the November 2008 submission. [Google Scholar]
- 4.Levi F, Randimbison L, Te VC, et al. Kaposi’s sarcoma in Vaud and Neuchatel, Switzerland, 1978–2002. Eur J Cancer. 2004;40(10):1630–1633. doi: 10.1016/j.ejca.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 5.Rouhani P, Fletcher CD, Devesa SS, et al. Cutaneous soft tissue sarcoma incidence patterns in the U.S. : an analysis of 12,114 cases. Cancer. 2008;113(3):616–627. doi: 10.1002/cncr.23571. [DOI] [PubMed] [Google Scholar]
- 6.Toro JR, Travis LB, Wu HJ, et al. Incidence patterns of soft tissue sarcomas, regardless of primary site, in the surveillance, epidemiology and end results program, 1978–2001: An analysis of 26,758 cases. Int J Cancer. 2006;119(12):2922–2930. doi: 10.1002/ijc.22239. [DOI] [PubMed] [Google Scholar]
- 7.Sultan I, Qaddoumi I, Yaser S, et al. Comparing adult and pediatric rhabdomyosarcoma in the surveillance, epidemiology and end results program, 1973 to 2005: an analysis of 2,600 patients. J Clin Oncol. 2009;27(20):3391–3397. doi: 10.1200/JCO.2008.19.7483. [DOI] [PubMed] [Google Scholar]
- 8.Biegel JA, Zhou JY, Rorke LB, et al. Germ-line and acquired mutations of INI1 in atypical teratoid and rhabdoid tumors. Cancer Res. 1999;59(1):74–79. [PubMed] [Google Scholar]
- 9.Ferrari A, Casanova M. Current chemotherapeutic strategies for rhabdomyosarcoma. Expert Rev Anticancer Ther. 2005;5(2):283–294. doi: 10.1586/14737140.5.2.283. [DOI] [PubMed] [Google Scholar]
- 10.Joshi D, Anderson JR, Paidas C, et al. Age is an independent prognostic factor in rhabdomyosarcoma: a report from the Soft Tissue Sarcoma Committee of the Children’s Oncology Group. Pediatr Blood Cancer. 2004;42(1):64–73. doi: 10.1002/pbc.10441. [DOI] [PubMed] [Google Scholar]
- 11.Sultan I, Qaddoumi I, Yaser S, et al. Comparing Adult and Pediatric Rhabdomyosarcoma in the Surveillance, Epidemiology and End Results Program, 1973–2005: An Analysis of 2600 Cases. Journal of Clinical Oncology. 2009 doi: 10.1200/JCO.2008.19.7483. In press. [DOI] [PubMed] [Google Scholar]
- 12.Ferrari A, Dileo P, Casanova M, et al. Rhabdomyosarcoma in adults. A retrospective analysis of 171 patients treated at a single institution. Cancer. 2003;98(3):571–580. doi: 10.1002/cncr.11550. [DOI] [PubMed] [Google Scholar]
- 13.Hayes-Jordan AA, Spunt SL, Poquette CA, et al. Nonrhabdomyosarcoma soft tissue sarcomas in children: is age at diagnosis an important variable? J Pediatr Surg. 2000;35(6):948–953. doi: 10.1053/jpsu.2000.6934. discussion 953–944. [DOI] [PubMed] [Google Scholar]
- 14.Ferrari A, Casanova M, Collini P, et al. Adult-type soft tissue sarcomas in pediatric-age patients: experience at the Istituto Nazionale Tumori in Milan. J Clin Oncol. 2005;23(18):4021–4030. doi: 10.1200/JCO.2005.02.053. [DOI] [PubMed] [Google Scholar]
- 15.Pisters PW, Leung DH, Woodruff J, et al. Analysis of prognostic factors in 1,041 patients with localized soft tissue sarcomas of the extremities. J Clin Oncol. 1996;14(5):1679–1689. doi: 10.1200/JCO.1996.14.5.1679. [DOI] [PubMed] [Google Scholar]
- 16.Koea JB, Leung D, Lewis JJ, et al. Histopathologic type: an independent prognostic factor in primary soft tissue sarcoma of the extremity? Ann Surg Oncol. 2003;10(4):432–440. doi: 10.1245/aso.2003.05.014. [DOI] [PubMed] [Google Scholar]
- 17.Van Glabbeke M, van Oosterom AT, Oosterhuis JW, et al. Prognostic factors for the outcome of chemotherapy in advanced soft tissue sarcoma: an analysis of 2,185 patients treated with anthracycline-containing first-line regimens--a European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group Study. J Clin Oncol. 1999;17(1):150–157. doi: 10.1200/JCO.1999.17.1.150. [DOI] [PubMed] [Google Scholar]
- 18.Kattan MW, Leung DH, Brennan MF. Postoperative nomogram for 12-year sarcoma-specific death. J Clin Oncol. 2002;20(3):791–796. doi: 10.1200/JCO.2002.20.3.791. [DOI] [PubMed] [Google Scholar]
- 19.Eilber FC, Brennan MF, Eilber FR, et al. Validation of the postoperative nomogram for 12-year sarcoma-specific mortality. Cancer. 2004;101(10):2270–2275. doi: 10.1002/cncr.20570. [DOI] [PubMed] [Google Scholar]
- 20.Ferrari A, Miceli R, Casanova M, et al. Adult-type soft tissue sarcomas in paediatric age: A nomogram-based prognostic comparison with adult sarcoma. Eur J Cancer. 2007;43(18):2691–2697. doi: 10.1016/j.ejca.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 21.Mariani L, Miceli R, Kattan MW, et al. Validation and adaptation of a nomogram for predicting the survival of patients with extremity soft tissue sarcoma using a three-grade system. Cancer. 2005;103(2):402–408. doi: 10.1002/cncr.20778. [DOI] [PubMed] [Google Scholar]
- 22.Carli M, Ferrari A, Mattke A, et al. Pediatric malignant peripheral nerve sheath tumor: the Italian and German soft tissue sarcoma cooperative group. J Clin Oncol. 2005;23(33):8422–8430. doi: 10.1200/JCO.2005.01.4886. [DOI] [PubMed] [Google Scholar]
- 23.Slater O, Shipley J. Clinical relevance of molecular genetics to paediatric sarcomas. J Clin Pathol. 2007;60(11):1187–1194. doi: 10.1136/jcp.2006.040113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferrari A, Gronchi A, Casanova M, et al. Synovial sarcoma: a retrospective analysis of 271 patients of all ages treated at a single institution. Cancer. 2004;101(3):627–634. doi: 10.1002/cncr.20386. [DOI] [PubMed] [Google Scholar]
- 25.Hawkins WG, Hoos A, Antonescu CR, et al. Clinicopathologic analysis of patients with adult rhabdomyosarcoma. Cancer. 2001;91(4):794–803. [PubMed] [Google Scholar]
- 26.Little DJ, Ballo MT, Zagars GK, et al. Adult rhabdomyosarcoma: outcome following multimodality treatment. Cancer. 2002;95(2):377–388. doi: 10.1002/cncr.10669. [DOI] [PubMed] [Google Scholar]
- 27.Fletcher CD. The evolving classification of soft tissue tumours: an update based on the new WHO classification. Histopathology. 2006;48(1):3–12. doi: 10.1111/j.1365-2559.2005.02284.x. [DOI] [PubMed] [Google Scholar]
- 28.Mankin HJ, Mankin CJ, Simon MA. The hazards of the biopsy, revisited. Members of the Musculoskeletal Tumor Society. J Bone Joint Surg Am. 1996;78(5):656–663. doi: 10.2106/00004623-199605000-00004. [DOI] [PubMed] [Google Scholar]
- 29.Lehnhardt M, Daigeler A, Hauser J, et al. The value of expert second opinion in diagnosis of soft tissue sarcomas. J Surg Oncol. 2008;97(1):40–43. doi: 10.1002/jso.20897. [DOI] [PubMed] [Google Scholar]
- 30.Presant CA, Russell WO, Alexander RW, et al. Soft-tissue and bone sarcoma histopathology peer review: the frequency of disagreement in diagnosis and the need for second pathology opinions. The Southeastern Cancer Study Group experience. J Clin Oncol. 1986;4(11):1658–1661. doi: 10.1200/JCO.1986.4.11.1658. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Five-year relative survival rate trends in soft tissue sarcoma patients in different subtypes among different age groups; abbreviation: RMS: rhabdomyosarcomas; Fibrobl: fibroblastic and myofibroblastic tumors; Fibrohis: Fibrohistiocytic tumors; MPNST: malignant peripheral nerve sheath tumors; EW: Ewing family tumors; Syn: Synovial sarcomas; Leio: leiomyosarcoma; ASPS: alveolar soft parts sarcoma.