Abstract
Evidence that indoleacetic acid (IAA) conjugates are metabolized via enzyme-catalyzed hydrolysis to free IAA and that their biological activities are related to the rates at which they are hydrolyzed by the tissue is presented. These conclusions are based on the following observations. Slow but continuous decarboxylation of the IAA moiety of IAA-l-alanine and IAA-glycine occurs when these conjugates are applied to pea (Pisum sativum L. cv. Alaska) stem segments. Inasmuch as IAA conjugates are protected from peroxidase-catalyzed oxidative decarboxylation, the conjugates are probably hydrolyzed and the freed IAA then further metabolized. Free IAA and IAA-l-alanine are converted, by pea stem tissue, into the same metabolites. The metabolism is enzymic, since conjugates of IAA with the d-isomers of the amino acids are inactive. Ethylene production induced by IAA-l-alanine and by IAA-glycine is correlated with their hydrolysis, as indicated by their decarboxylation and with the appearance or nonappearance of IAA metabolites in the tissues.
Full text
PDF
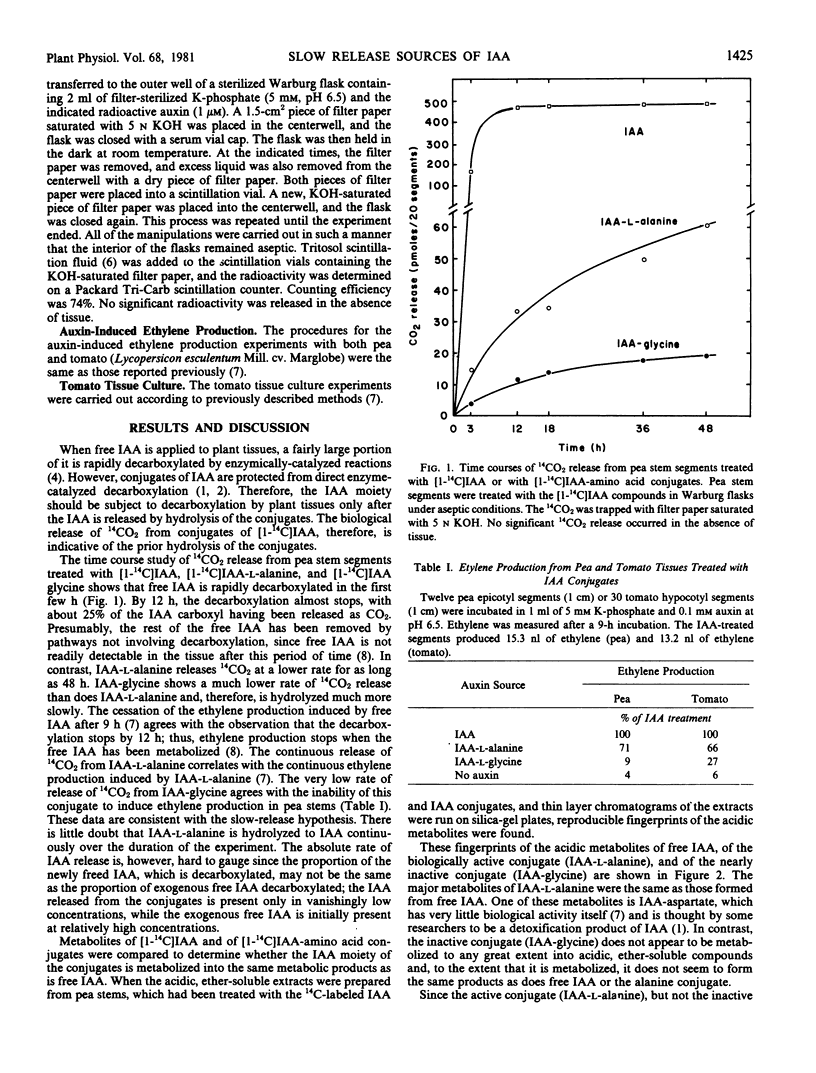
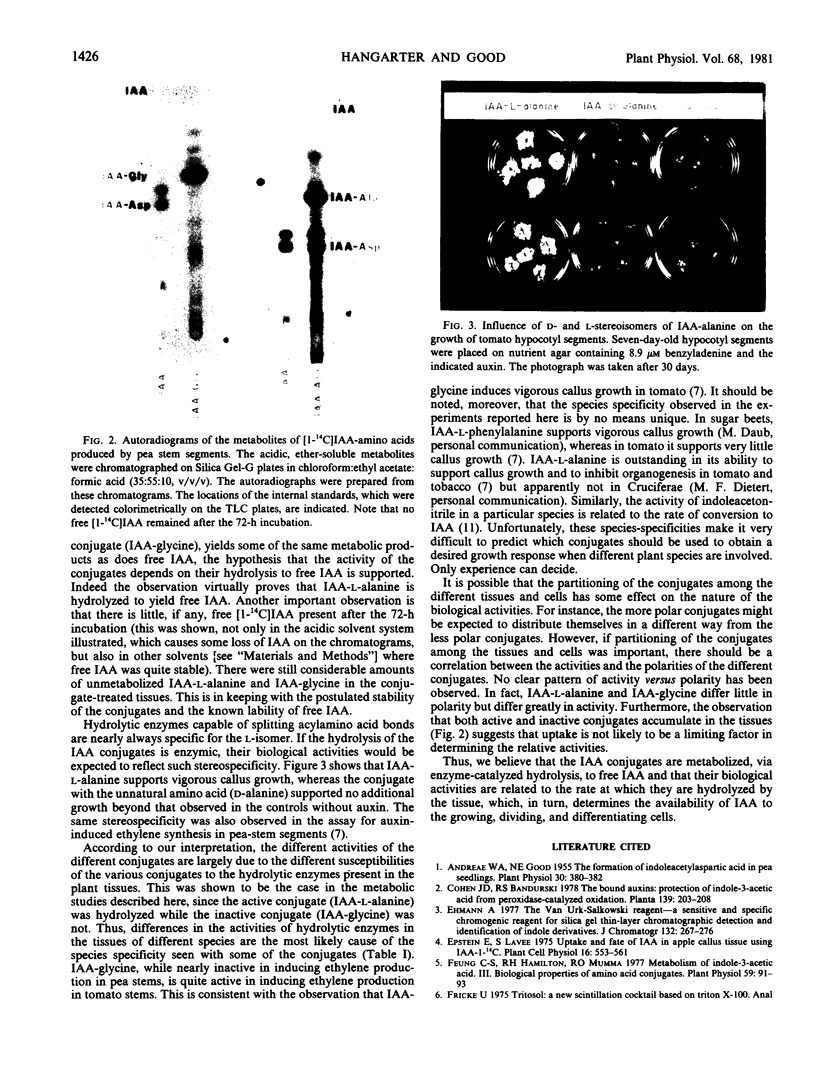

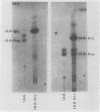
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreae W. A., Good N. E. The Formation of Indoleacetylaspartic Acid in Pea Seedlings. Plant Physiol. 1955 Jul;30(4):380–382. doi: 10.1104/pp.30.4.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehmann A. The van urk-Salkowski reagent--a sensitive and specific chromogenic reagent for silica gel thin-layer chromatographic detection and identification of indole derivatives. J Chromatogr. 1977 Feb 11;132(2):267–276. doi: 10.1016/s0021-9673(00)89300-0. [DOI] [PubMed] [Google Scholar]
- Feung C. S., Hamilton R. H., Mumma R. O. Metabolism of Indole-3-acetic Acid: IV. Biological Properties of Amino Acid Conjugates. Plant Physiol. 1977 Jan;59(1):91–93. doi: 10.1104/pp.59.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hangarter R. P., Peterson M. D., Good N. E. Biological activities of indoleacetylamino acids and their use as auxins in tissue culture. Plant Physiol. 1980 May;65(5):761–767. doi: 10.1104/pp.65.5.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau O. L., Yang S. F. Mechanism of a Synergistic Effect of Kinetin on Auxin-induced Ethylene Production: Suppression of Auxin Conjugation. Plant Physiol. 1973 Jun;51(6):1011–1014. doi: 10.1104/pp.51.6.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THIMANN K. V. Hydrolysis of indoleacetonitrile in plants. Arch Biochem Biophys. 1953 May;44(1):242–243. doi: 10.1016/0003-9861(53)90030-7. [DOI] [PubMed] [Google Scholar]