Abstract
ERα plays an important role in breast cancer. Up-regulation of HIF-1α in ERα-positive cancers suggests that HIF-1α may cooperate with ERα to promote breast cancer progression and consequently affect breast cancer treatment. Here we show the histone demethylase JMJD2B is regulated by both ERα and HIF-1α, and drives breast cancer cell proliferation in normoxia and hypoxia, and epigenetically regulates the expression of cell cycle genes such as CCND1, CCNA1 and WEE1. We also demonstrate that JMJD2B and the hypoxia marker CA9 together stratify a subclass of breast cancer patients and predicts a worse outcome of these breast cancers. Our findings provide a biological rationale to support the therapeutic targeting of histone demethylases in breast cancer patients.
Keywords: Hypoxia, ERα, HIF-1α, Histone demethylase, JMJD2B, breast cancer
Introduction
Estrogen plays an essential role in the establishment and progression of breast cancer. The biological actions of estrogens are mediated by two members of the nuclear receptor family, estrogen receptors α (ERα) and β (ERβ) (1, 2). ERα is critical to mammary epithelial cell division and breast cancer progression while ERβ suppresses transcriptional activity of ERα (2, 3). Hypoxia-inducible factor 1 is a master regulator of oxygen homeostasis, functioning by transactivating hundreds of genes that are involved in angiogenesis, cell survival, metabolism and invasion or metastasis (4-6). In response to physiological or pathological hypoxic stress, the subunit HIF-1α is stabilized and heterodimerizes with HIF-1β, forming a functional HIF-1 transactivator which binds the hypoxia response element (HER) to initiate gene expression. In normoxia, the HIF-1α subunit is post-translationally hydroxylated by a class of enzymes called prolyl hydroxylases (PHDs), resulting in VHL-mediated proteasomal degradation, thus maintaining the activity of HIF-1 at low levels (7, 8). HIF-1α is upregulated in many solid tumors as a consequence of hypoxia. Nevertheless, HIF-1α may also be upregulated in normoxia by oncogenic stimulation, mitochondrial metabolic dysfunction or loss of the tumor suppressor VHL (9).
HIF-1α has been associated with an aggressive phenotype of breast cancer, with large tumor size, high grade, high proliferation and lymph node metastasis (10). Increased HIF-1α expression is also associated with ERα positivity (10) and hypoxia can trigger ligand-independent ERα activation (11), possibly by direct HIF-1α and ERα interaction. Two studies suggest that hypoxia and estrogen can cooperate to regulate gene expression in T47D breast cancer cells (12) or increase transcriptional activity of ERE (13), but whether HIF-1α is involved in this process is unknown. Hypoxia is an important determinant of tumor resistance to chemotherapy and radiotherapy. Tamoxifen and aromatase inhibitors are important antagonists or suppressors of estrogen activity in breast cancer and our recent clinical study has indicated that HIF-1α is significantly associated with endocrine therapy resistance in ERα positive breast cancers (14).
Collectively, these observations suggest that an unidentified mechanism exists by which interaction of the HIF-1α and ERα signaling pathways could coordinate to promote breast cancer progression. However, the molecular mechanism of coordinated gene regulation by HIF-1α *and ERα in breast cancer has not been elucidated. In this study, we found that HIF-1α and ERα regulate a common signaling pathway engaged by the histone lysine demethylase JMJD2B. JMJD2B is upregulated in hypoxia in a HIF-1α-dependent manner. ERα is critical for JMJD2B induction in hypoxia in ER-positive breast cancer cells. JMJD2B is functional in hypoxia and regulates histone demethylation on the promoters of its target genes which include multiple signaling pathways and biological processes that are involved in cancer. JMJD2B is critical to breast cancer cell survival in normoxia and hypoxia, partly via regulation of cell cycle progression, is highly expressed in ERα positive primary breast cancers and is an adverse prognostic factor in hypoxic breast cancers.
Materials and Methods
Cell Culture and Reagents
Breast cancer cell lines MCF7, T47D, MDA-MB-361, MDA-MB-468, and MDA-MB-231 were cultured in Dulbecco’s modified Eagle’s medium (DMEM). All cell culture medium were supplemented with 10% fetal bovine serum, penicillin (50 IU/ml) and streptomycin sulphate (50 μg/ml). For hypoxia incubation, cells were exposed to hypoxic conditions (1% O2, 5% CO2 and 94% N2) in a Heto-Holten CellHouse 170 incubator (RS Biotech, Irvine, Scotland). 17β-estradiol (E2) was purchased from Calbiochem. ICI 182780 was purchased from Tocris Bioscience. 4-hydroxy-tamoxifen and Desferrioxamine (DFX) were purchased from Sigma.
siRNA Transfection
siRNAs were transfected into subconfluent cells using HiPerfect transfection reagent (Qiagen) according to the manufacturer’s instructions. The target sequences for HIF-1α and HIF-2α were synthesized by Eurogentec (Eurogentec, Southampton, UK) were as follows: HIF-1α, 5-UCAAGUUGCUGGUCAUCAGdTdT-3; HIF-2α, 5-ACUGCUAUCAAAGAUGCUGdTdT-3. The siRNA oligos for JMJD2B were purchased from Dharmacon with sequences as follows: JMJD2B#1, 5-GCGCAGAAUCUACCAACUU-3, JMJD2B#2, 5-CAAAUACGUGGCCUACAUA-3. The SMARTpool siRNA oligos for ERα were purchased from Dharmacon. The siRNA non-targeting control was purchased from Dharmacon. For the cell viability assay, long- term transfection protocol was used according to the Qiagen manufacturer’s instructions.
Plasmids and Transfection
HA-JMJD2B plasmid was described previously (15). HA-HIF-1α was introduced into pCMVβ vector. To transfect plasmids into cells, FuGene6 tranfection reagent (Roche) was used according to the manufacture’s protocol.
Western Blotting and Immunoprecipitation
Methods and Antibodies were listed in Supplemental table.
Chromatin Immunoprecipitation
Chromatin immunoprecipitation assays was performed according to the manufacturer’s protocol (EZ-CHIP, Upstate, Millipore). Briefly, MCF cells were crosslinked with 1% paraformaldehyde for 10 minutes, and quenched for 5 minutes with 125 mM of glycine. After sonification, cell lysates were spin down and 100 μl of supernatant was diluted to 1ml for immunopreciptation. Samples were pre-cleared with 60 μl of protein G beads. Appropriate concentrations of antibodies were used for immunopreciptation of the cross-linked DNA-protein complexes. After a serial washing, DNA-protein crosslink was reversed and DNA was extracted for polymerase chain reaction (PCR). PCR primers and antibodies used for ChIP were listed in Supplemental Data
Subcellular Fractionation
Cellular cytoplasmic and nuclear fractions were prepared using the NE-PER kit (Perbio) according to the manufacturer’s protocol.
Cell Viability and Colony Formation Assays
Cell viability and colony formation were assessed as described in Supplemental Methods.
qRT-PCR
Quantitative RT-PCR was performed as previously described (16). Detailed methods and primers were described in supplemental data.
Immunofluorescence
Cells were washed three times with PBS and fixed in 4.0% paraformaldehyde in PBS for 1 hour at room temperature. Cells were permeabilized for 10 min using Triton X-100 (0.5%), and then incubated with 1% BSA for 20 min. Cells were incubated overnight with primary antibodies with a dilution of 1:1000 in 1% BSA. After three consecutive 5 min washes with PBS, cells were incubated with secondary antibodies for 1 hour before being washed with PBS and mounted in a mounting medium (Dako) containing 4,6-diamidino-2-phenylindole dihydrochloride (DAPI). Imaging of the cells was carried out using a Zeiss immunofluorescence microscope.
GSEA
Enrichment analysis of ERα target genes was performed as previously described (17) using the ERα gene signature (18) from molecular signature database (GSEA v2.0 at http://www.broad.mit.edu/gsea) and gene expression profiles of HIF-1α knockdown in MCF7 cells (19).
Gene Expression Profiling
RNA was extracted from MCF7 cells using RNeasy mini kit (Qiagen). Labeling and hybridization of samples to Illumina gene expression chip were performed using standard methodology. Data analysis was performed using GenePattern (20).
Clinical data
Patients and tissue samples were described in detail in the Supplemental data.
Immunohistochemistry
Immunohistochemistry was performed as described previously for CA9 (Cancer Res 2001;61:6394-9. Virology 1992;187:620-6.). Methods are described in brief for JMJD2B. Antigen retrieval was performed by incubating slides in Tris-EDTA, pH 9.0, under 15 psi pressure for 2 minutes using a Decloaking Chamber (Biocare Medical, Concord, CA). Sections were incubated for 1 hour at room temperature with a primary anti-JMJD2B (Bethyl Laboratories, A301-478A) polyclonal rabbit antibody at 0.4 μg/mL. Bound antibody was labelled with anti-rabbit/mouse Envision (DAKO, Carpinteria, CA). Antibody complexes were visualized using 2,3-diaminobenzidine chromogen, and sections were counterstained with Mayer’s haematoxylin.
Intensity of JMJD2B expression was scored separately in the cytoplasm and nuclei of neoplastic cells as either positive or negative. The highest intensity score among replicate cores was used as the score for each patient.
Statistics
The χ2-test was used to evaluate associations between categorical variables. Details are described in the supplemental data.
Results
Identification of an Interaction Between the ERα and HIF-1α Pathways
We found a functional association of HIF-1α and ERα using molecular concept (www.oncomine.com) analysis (Fig. S1A). To further understand the interaction between the HIF-1α and ERα signaling pathways, we used Gene Set Enrichment Analysis (GSEA) (21) to analyze the association of genes differentially regulated by HIF-1α in MCF7 cells from a recent publication (19) with an ERα-positivity gene signature (18). Using this method, we found that ERα–regulated genes such as CA12 and CCNG2 were enriched in the HIF-1α target gene signature (Fig. S1B,C), suggesting an interaction between HIF-1α and ERα. Among the genes that are regulated by both ERα and HIF-1α (Fig. S1A, B), we focused on JMJD2B gene in this study for several reasons. JMJD2B encodes a histone demethylase that has been recently shown to be a HIF-1α target gene by us and others (22-25). However, the functions of the most histone demethylases including JMJD2B are not clear (especially in hypoxia) and how histone demethylase epigenetically regulates gene expression in hypoxia has not well been demonstrated. Importantly, elucidation of the function of JMJD2B may help to find novel therapeutic targets for breast cancers.
ERα-Dependent Regulation of JMJD2B
The histone demethylase JMJD2 family has potential oncogenic functions (26). However, the molecular mechanism by which JMJD2s promote cancer progression is unknown. In addition, how JMJD2 oncogenes respond to proliferative signals (such as hormone stimulation) and antagonize environmental stress (such as hypoxia) has not been reported. To address the role of JMJD2B, we analyzed JMJD2B expression in primary breast cancers and found that JMJD2B is significantly expressed in ER-positive breast cancers (Fig. S1D). To test these observations in human breast cancer cell lines, we examined JMJD2B expression in ER positive (T47D and MCF7) and ER negative (MDA-MB-231, MDA-MB-468) breast cancer cells. Real-time PCR and western blotting showed that JMJD2B was highly expressed in ER-positive cells in comparison to ER-negative cells (Fig. 1A, and data not shown), further indicating that JMJD2B expression is correlated with ERα status.
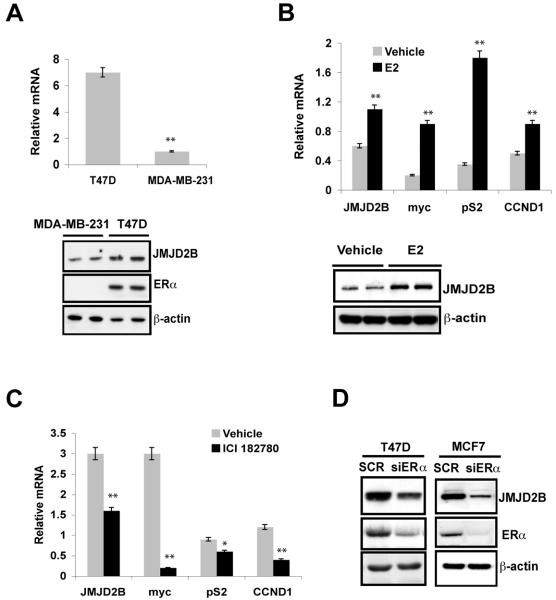
Figure 1. ERα-dependent regulation of JMJD2B.
A, Quantitative RT-PCR (top panel) and immunoblotting (bottom panel) show JMJD2B mRNA and protein expression, respectively, in T47D and MDA-MB-231 cells.
B, After 3 days hormonal starvation, T47D cells were treated with 10 nM of E2 for 16 hours. Quantitative RT-PCR was performed to assess the expression of the indicated genes (top panel). Immunoblotting shows JMJD2B expression from above treatment (bottom panel)
C, T47D cells in normal medium containing serum and phenol red i.e. estrogen present, were treated with 1 μM of ICI182780 for 16 hours. Quantitative RT-PCR was performed to assess the expression of the indicated genes.
D, T47D and MCF7 cells were transfected with smartpool siRNA oligos against ERα. Immunoblotting was used to assess the JMJD2B expression.
(*P<0.05; **P<0.01)
To examine whether ERα regulates JMJD2B expression in a ligand-dependent manner, we treated estrogen deprived T47D and MCF7 cells with 17β-estradiol (E2) for 16 hours and assessed the expression of JMJD2B. E2 treatment caused an increase in JMJD2B expression at mRNA levels and protein levels in T47D cells (Fig. 1B) and in known ERα target genes c-Myc, CCND1 and pS2 (TFF1). Similarly, E2 treatment induced JMJD2B expression in MCF7 cells, though to a lesser extent at the 16 hour time point (Fig. S2A,B). To further examine the role of ERα in regulation of JMJD2B expression, T47D cells were treated with the ERα inhibitor ICI182780. This inhibitor suppressed expression of ERα (Fig. S3A) and c-Myc, CCND1 and pS2 (TFF1) (Fig. 1C). Importantly, ICI182780 inhibited JMJD2B expression at both mRNA and protein levels (Fig. 1C, S3A). Similarly, ICI182780 treatment led to JMJD2B downregulation in MCF7 cells (Fig. S2C, D). To further confirm ERα-dependent expression of JMJD2B, small interfering RNA (siRNA) was used to knock down the expression of ERα in T47D and MCF7 cells. Loss of ERα in T47D and MCF7 cells resulted in a great reduction in JMJD2B expression (Fig. 1D). To determine whether ERα directly binds to JMJD2B gene, we analyzed JMJD2B gene locus and found an ERα binding site in the first intron of JMJD2B gene (Fig. S3B). Our ChIP-PCR assay revealed that ERα bound this region in vivo (Fig. S3C), suggestive of a functional ERα binding element. In agreement with our findings, a genome-wide analysis of ERα binding sites also showed an ER binding in the first intron of JMJD2B (27). In addition, another global analysis of ERα binding showed that JMJD2B is an ER-regulated gene (28)
Collectively, these data indicate that JMJD2B is a bona fide target of ERα and its expression in ER-positive breast cancer cells is mainly dependent on ERα.
ERα Is Important for JMJD2B Expression in Hypoxia
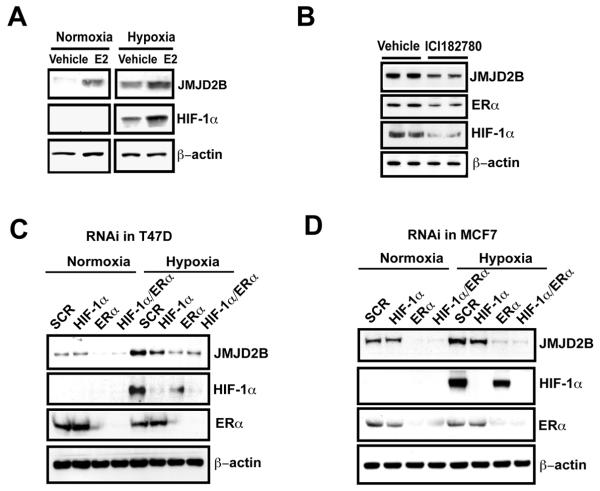
We and others have shown that JMJD2B can be upregulated in hypoxia in a HIF-1α-dependent manner (Fig. S4A-F) (22-25), raising the question that whether ERα is still required for JMJD2B induction in hypoxia. To address this, we treated estrogen deprived T47D cells with E2 in normoxia and hypoxia. E2 treatment increased JMJD2B expression in both normoxia and hypoxia (Fig. 2A). Suppression of ERα, by either treating cells with ICI182780 or RNAi, not only reduced JMJD2B expression in normoxia but significantly downregulated JMJD2B expression in hypoxia in ER-positive breast cancer cells (Fig. 2B-D), indicating that ERα* still functions to regulate *JMJD2B** expression in hypoxia. The ER depletion in hypoxia was more effective at inhibiting JMJD2B induction than the HIF siRNA. Of note, suppression of ERα also reduced HIF-1α expression (Fig. 2B-D), suggesting that ERα is important for HIF-1α induction or protein stabilization.
Figure 2. ERα is critical for JMJD2B induction in hypoxia.
A, T47D cells were treated with 10 nM of E2 in normoxia and hypoxia for 16 hours. Immunoblotting was used to assess the JMJD2B expression.
B, T47D cells were treated with 1 μM of ICI182780 in hypoxia for 16 hours. Immunoblotting was used to assess the proteins with indicated antibodies.
C, T47D cells were transfected with siRNA oligos against ERα or HIF-1α. After 36 hours transfection, cells were incubated in normoxia and hypoxia for 16 hours. Immunoblot was used to assess the JMJD2B expression.
D, MCF7 cells were treated the same as in (C).
JMJD2B Regulates Multiple Biological Processes Including the Cell Cycle
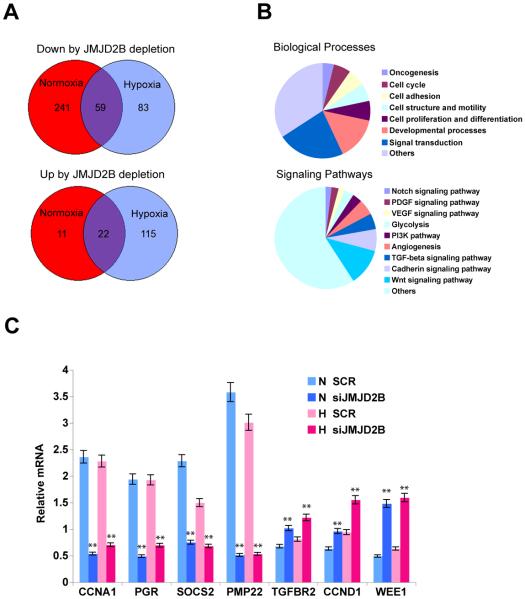
To analyze the functions of JMJD2B, we performed genome-wide expression profiling to identify JMJD2B target genes in MCF7 cells. Depletion of JMJD2B downregulated 300 genes in normoxia but only 142 genes in hypoxia, 59 of which were commonly downregulated in normoxia and hypoxia (Fig. 3A). Intriguingly, depletion of JMJD2B only upregulated 33 genes in normoxia, but 137 genes in hypoxia, 22 of which were commonly upregulated (Fig. 3A). These data have several implications: (1) JMJD2B can regulate a hypoxia-specific transcriptional response; (29) JMJD2B predominantly regulates transcriptional activation in normoxia since loss of JMJD2B causes downregulation of gene expression, though this transcriptional competence of JMJD2B is compromised in hypoxia; (3) transcriptionally repressive and active functions of JMJD2B co-exist in hypoxia since depletion of JMJD2B causes differential expression approximately in a 1:1 ratio; (4) JMJD2B maintains a basal activity in normoxia and regulates expression of a substantial number of genes, preserves this ability under hypoxia stress, as evidenced by a set of common genes such as CCNA1 and SOCS2 that are regulated by JMJD2B in both normoxia and hypoxia (Fig. 3C).
Figure 3. JMJD2B regulates multiple biological processes and signaling pathways including cell cycle.
A, After 48 hour depletion of JMJD2B in MCF7 cells, global gene expression profiling reveals that JMJD2B can both positively (top) and negatively (bottom) regulates gene expression in normoxia and hypoxia.
B, Gene ontology analysis with Panther program shows that genes regulated by JMJD2B are involved in multiple biological processes and signaling pathways.
C, Quantitative RT-PCR was performed to validate gene expression regulated by JMJD2B.
(**P<0.01)
Gene ontology analysis revealed that JMJD2B regulates multiple signaling pathways and biological processes (Fig. 3B). For example, JMJD2B regulates Wnt, TGFβ, Notch, VEGF, PI3K pathways and angiogenesis, all of which play important roles in tumorigenesis or cancer progression. Biological processes engaged by JMJD2B target genes include signal transduction, cell proliferation and differentiation, cell adhesion and oncogenesis. Importantly, JMJD2B regulates cell cycle genes such as CCND1, CCNA1 and WEE1 (Fig. 3C), indicating that JMJD2B is important for regulation of breast cancer cell proliferation. CCND1 encodes cyclin D1, which complexes with cyclin-dependent kinases CDK4/CDK6 to regulate G1-phase progression. CCNA1 encodes cyclin A1, which can activate CDK2 or CDK1 and may play a role in driving cells to enter S phase and G2/M phase transition. Wee1 is a CDK-inhibitory kinase that phosphorylates CDK1, thereby suppressing G2/M phase transition. In this study we found that JMJD2B positively regulates CCNA1 expression and negatively regulates WEE1 expression (Fig. 3C), suggesting that JMJD2B can promote G2/M phase transition. Interestingly, JMJD2B negatively regulates CCND1 expression, suggesting that JMJD2B might be also involved in G1 phase regulation to coordinate cell cycle progression. PGR, which encodes progesterone receptor PR, is also positively regulated by JMJD2B (Fig. 3C). PR has been involved in cell cycle regulation of breast cancer cells (30) and, JMJD2B might also regulate breast cancer cell proliferation via PR.
JMJD2B Is Important for Breast Cancer Cell Proliferation
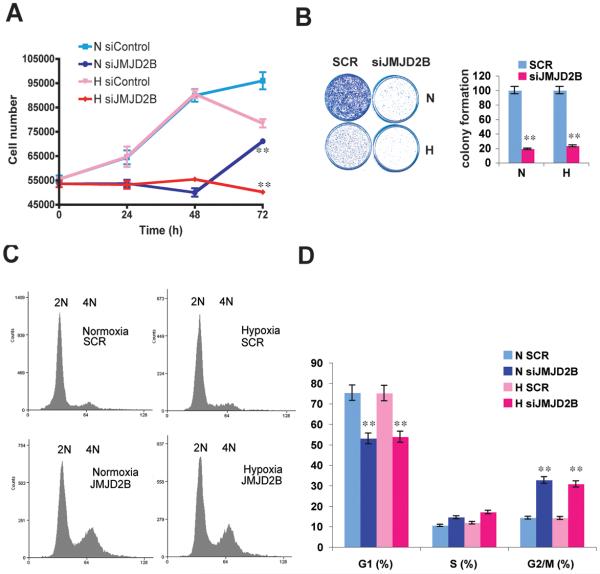
Uncontrolled cell proliferation is the hallmark of cancer, and tumor cells have typically acquired alterations to genes that directly regulate their cell cycles (31, 32). The evidence that JMJD2B gene is amplified in medulloblastoma (33) and herein that it regulates expression of cell cycle genes, indicate that JMJD2B may play a role in oncogenesis by promoting cell proliferation. We therefore analysed the role of JMJD2B in cells grown with full serum and phenol red-containing medium, i.e. in presence of estrogen and other growth factors. Loss of JMJD2B caused a significant decrease in cell proliferation, as assessed by counting cell numbers (Fig. 4A), by cell viability assay (Fig. S5A), and by colony formation assay (Fig. 4B) in the presence of estrogen. FACS analysis revealed that depletion of JMJD2B caused a significant G2/M phase arrest in normoxia and hypoxia (Fig. 4C,D), which is consistent with the western blotting results that loss of JMJD2B upregulated WEE1, Cyclin B1, and Cyclin D1 (Fig. S5B). These data indicate that JMJD2B drives breast cancer cell proliferation in an estrogenic environment through direct or indirect coordination of the G2/M phase and G1 phases of the cell cycle.
Figure 4. JMJD2B is essential for breast cancer cell proliferation.
A, After 48 hour depletion of JMJD2B, the MCF7 cell proliferation was measured by cell number counting.
B, Colony formation assay was used to assess cell proliferation after depletion of JMJD2B in MCF7 cells (left) and was quantified (right).
C, Cell cycle profile analysis after JMJD2B depletion in normoxia and hypoxia.
D, Percentage analysis of DNA histogram data (C) were derived from mathematical analysis of (C) with software called Summit Version 4.3 by gating for single-cell population with FS/Pulse Width, and analyse with “counts vs area of laser”.
(**P<0.01)
Histone Demethylase Function of JMJD2B in Hypoxia
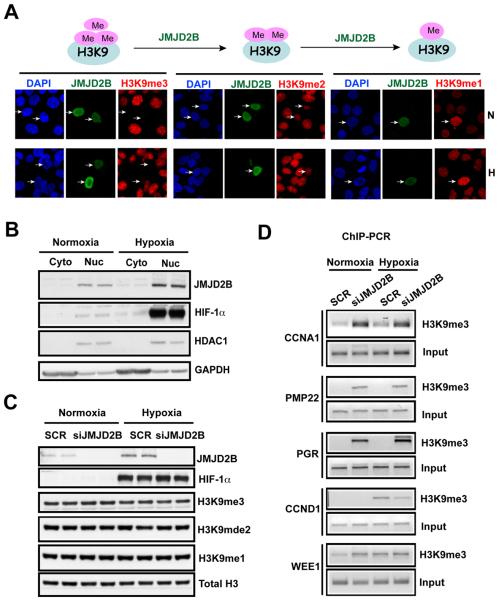
JMJD2B is a JmjC-domain containing histone lysine demethylase, which belongs to the dioxygenase family, whose activities require iron, α-ketoglutarate and oxygen (34). Thus, the induction of JMJD2B by hypoxia raises an important question: does JMJD2B exert an enzyme-independent function in hypoxia or use limited oxygen to catalyze the reaction? To address these questions, we assessed the enzymatic activity of JMJD2B by overexpressing HA-tagged JMJD2B in MCF7 cells. JMJD2B is a specific H3K9me3/me2 demethylase. Therefore we focused on studies of H3K9 methylation. JMJD2B erased the H3K9me3 mark in normoxia (Fig. 5A). Interestingly, this function was similar under 1% O2 (Fig. 5A). H3K9me2 levels were also reduced by JMJD2B, though to a much lesser extent (Fig. 5A). H3K9me1 levels were increased presumably due to the conversion of H3K9me3/me2 to H2K9me1 (Fig. 5A). Immunostaining and subcellular fractionation results revealed that endogenous JMJD2B was mainly localized in the nucleus in normoxia and hypoxia in MCF7 cells (Fig. 5B and data not shown), consistent with its role as a histone demethylase and transcriptional regulator. Nevertheless, neither induction of endogenous JMJD2B in hypoxia nor depletion of JMJD2B caused a significant global change in H3K9me3/me2 levels (Fig. 5C), suggesting that physiological JMJD2B acts locally to control specific gene expression or to maintain the chromatin environment in specific regions.
Figure 5. JMJD2B is functional in hypoxia to exert its histone demethylase activity.
A, Immunofluoresence shows a global loss of H3K9me3 marks in normoxia (N) and hypoxia (H) after overexpression of HA-tagged JMJD2B in MCF7 cells.
B, Subcellular localization experiment demonstrates that JMJD2B is a nuclear protein in normoxia and hypoxia in MCF7 cells. (cyto =cytoplasm; Nuc = nucleus).
C, JMJD2B was depleted in MCF7 cells using siRNA knockdown. The indicated histone methyl marks were analyzed by immunoblot.
D, ChIP-PCR shows that JMJD2B regulates H3K9me3 mark on promoters of its target genes in normoxia and hypoxia in MCF7 cells.
To directly examine the histone demethylase functions of JMJD2B we assessed H3K9me3 levels on its target gene promoters using ChIP-PCR (Fig. 5D). We chose the genes including CCNA1, PGR, PMP22, CCND1 and WEE1 that were positively or negatively regulated by JMJD2B. We found that, after depletion of JMJD2B either in normoxia or in hypoxia, H3K9me3 levels were significantly enriched on those gene promoters that are positively regulated by JMJD2B such as CCNA1, PGR and PMP22, indicating that JMJD2B can directly bind its target gene promoters and erase silencing marks from these promoters, thereby activating gene transcription in response to hypoxic stress. However, for the genes that are negatively regulated by JMJD2B such as WEE1 and CCND1, the H3K9me3 marks were not consistent with this pattern after JMJD2B depletion. We found that the H3K9me3 mark on CCND1 promoter in normoxia was below detection but was significantly enriched in hypoxia. Loss of JMJD2B reduced the H3K9me3 mark on the CCND1 promoter in hypoxia, suggesting that for some genes the increase in JMJD2 expression in hypoxia can result not only in maintenance of activity but also increased activity. However the effects on the suppressed promoters in hypoxia were minimal compared to those upregulated.
Clinical Assessment of JMJD2B Protein Induction in Breast Cancer
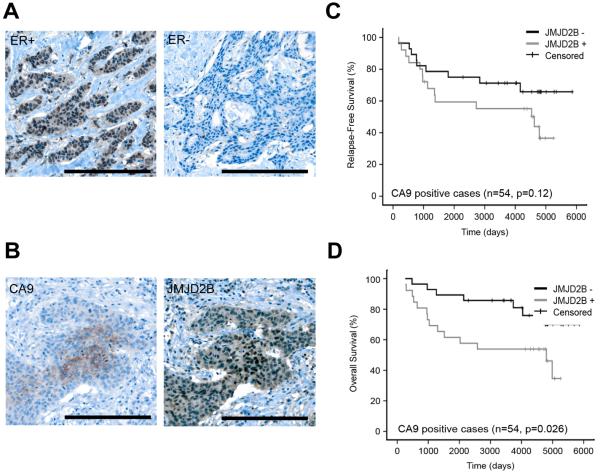
To further confirm the role of JMJD2B in breast cancer, we performed immunohistochemistry on tissue microarrays (TMAs) to observe the in situ expression of JMJD2B protein. Since JMJD2B is an ERα target gene, we assessed the JMJD2B expression according to the ER status. JMJD2B was significantly associated with ERα when it was predominantly localized in the nucleus (P<0.001, Fig. 6A, and Table S1). However, we also oberved that JMJD2B was localized in the cytoplasm of cancer cells in a fraction of tumors (data not shown); cytoplasmic expression of JMJD2B was not associated with ERα* status (P=0.69, Table S1), suggesting that JMJD2B function might also be regulated by an ER-dependent mechanism which can regulate the subcellular localization of JMJD2B. To study the association of JMJD2B and hypoxia, we used CA9 as a hypoxia marker. Nuclear JMJD2B and membranous/cytoplasmic CA9 colocalized in serially cut whole sections (Fig. 6B). Nuclear JMJD2B was highly expressed in perinecrotic hypoxic regions of breast cancer, where there was often strong membranous CA9 staining. Of the 54 CA9 positive cases, 32 were ER positive. Of those 32 CA9+/ER + cases, 19 were positive for JMJD2B nuclear staining.
Figure 6. Clinical assessment of JMJD2B.
A, Immunohistochemistry shows JMJD2B expression in ER-positive and ER-negative breast cancer
B, Immunohistochemistry shows JMJD2B and CA9 expression in a serial sections
C, Kaplan-Meier analysis of relapse-free survival of CA9+ breast cancer with or without JMJD2B expression.
D, Kaplan-Meier analysis of overall survival of CA9+ breast cancer with or without JMJD2B expression.
After correcting for multiple testing, cytoplasmic JMJD2B expression was not statistically significantly associated with clinicopathological variables or CA9 expression (P=0.71) (Table S1). Nuclear JMJD2B expression was independent of other clinicopathological variables such as tumor grade, nodal status or HER2 status (Table S1). We analyzed whether JMJD2B alone could predict clinical outcomes. Again, cytoplasmic JMJD2B expression was not significantly associated with relapse-free or overall survival. Nuclear JMJD2B expression was not significantly associated with relapse-free or overall survival. In multivariate analyses (including all statistically significant clinical and pathological variables by univariate analysis), neither cytoplasmic nor nuclear JMJD2B are significantly associated with relapse-free (Table S2) or overall survival (Table S3). The significance of JMJD2B expression in these multivariate survival analyses is not altered if chemotherapy, radiotherapy or hormonal therapy received is included in the models (data not shown). ERα positivity usually predicts a better survival as we have shown here (Table S2, S3). However, hypoxia or HIF-1α is usually associated with worse survival and resistance to therapy. As JMJD2B expression may be regulated by both ERα and HIF-1α, we combined JMJD2B expression and CA9 expression as a prognosis prediction marker. We found that those patients who are JMJD2B positive and CA9 positive showed a significantly worse outcome in overall survival than CA9 positive JMJD2B negative groups (Fig. 6C, P=0.026) and a worse tendency in relapse-free survival (Fig. 6D, P=0.12), suggesting that JMJD2B plays an important role in breast cancer progression mediated by both ERα and HIF-1α. CA9 was chosen as a marker of HIF-1α function because of its much greater stability and extensive validation in prognosis.
Discussion
Aberrant histone methylation has been linked to cancer (34). A recent study has shown that JMJD2 family of histone demethylases have an oncogenic function and are highly expressed in human cancers (15). The involvement of the JMJD2 proteins in tumorigenesis has been supported further by recent studies that JMJD2C works as an essential coactivator of androgen receptor (AR)-induced transcription and cellular growth via a functional interaction between JMJD2C and the AR in prostate carcinomas (35). JMJD2B and JMJD2C are also amplified in medulloblastomas (36). Nevertheless, how JMJD2 is regulated by upstream oncogenic signals and the nature of its downstream effectors has not been elucidated (15, 22, 23).
In this study, we have shown that JMJD2B is highly expressed in ER-positive primary breast cancers and breast cancer cell lines whose expression can be driven by E2 treatment. The correlation of JMJD2B gene expression with ERα *in* primary breast cancers and the dependence on ERα/E2 for enhanced expression of JMJD2B in breast cancer cells and ChIP analysis demonstrate that JMJD2B is a bona fide target of ERα.
Hypoxia can cause chromatin alterations such as histone methylation (37). Although JMJD2B requires oxygen for its enzymatic activity as a dioxygenase, 1% O2 does not affect its enzymatic function in our model systems. Overexpression of JMJD2B in hypoxia erases global H3K9me3 marks and loss of JMJD2B results in an increase in H3K9me3 levels on its local target gene promoters such as CCNA1 and PGR in hypoxia, suggesting that JMJD2B is able to function under relatively low oxygen levels. Nevertheless, regulation of JMJD2B by HIF-1 does not affect the importance of ERα in hypoxia in ER-positive breast cancer cells, as loss of ERα significantly attenuated (or nearly abolished) JMJD2B expression in hypoxia, suggesting that HIF-1 and ER synergistically regulate JMJD2B in hypoxia.
Epigenetic supression of gene transcription is potentially an important mechanism for cell survival under hypoxic conditions. Indeed, the number of genes suppressed by JMJD2B increases from 33 in normoxia to 137 in hypoxia although we do not understand how exactly JMJD2B initiates this program. Nevetheless, the activities of essential genes to ensure cell proliferation and survival should be maintained under either normal conditions or hypoxia, although extreme stress could significantly suppress cell proliferation or cause cell death. In addition to regulating important signaling pathways such as Wnt, Notch, TGF-beta and angiogensis, JMJD2B coordinately regulates cell cycle gene expression by upregulating CCNA1 expression and suppressing CCND1 and WEE1 expression in both normoxia and hypoxia. Therefore, JMJD2B seems to positively promote G2/M phase transition but negatively regulates the G1 phase. Since posttranslational modifcations such as proteasomal degradation play a critical role in regulating cell cycle, our findings that JMJD2B epigenetically regulates cyclin expression in breast cancer cells provides a new insight for understanding the functions of JMJD2 family members. However, this work does not provide direct evidence these genes are immeditely downstream of JMJD2B, which will need individual analysis of promoters.
Given that JMJD2B is highly expressed in ERα-positive breast cancers and is essential for breast cancer cell proliferation/survival under estrogen stimulation conditions, by directly or indirectly promoting cell cycle progression, targeting JMJD2B may be an alternative strategy to block the ERα signaling pathway. Importantly, tamoxifen-resistant ERα is still functional in driving JMJD2B expression in normoxia and hypoxia for breast cancer cell proliferation (data not shown). The availability of a crystal structure of the JmjC domain of JMJD2A (38), which exhibits high homology to that of JMJD2B, may accelerate the identification of small molecule inhibitors for this class of enzymes. Although JMJD2B is an ERα target gene, it is also highly expressed in other cancers such as prostate cancer and bladder cancer (data not shown)
By using JMJD2B and CA9 in combination, we have successfully stratified a subclass of breast cancers that show a worse outcome in overall survival. This suggests that the evaluation of JMJD2B and CA9 may be of value in patient selection if such therapies are developed.
Supplementary Material
Acknowledgements
We thank Professor Kristian Helin (Biotech Research & Innovation Centre, 2100 Copenhagen, Denmar) for providing HA-JMJD2B plasmid. Jun Yang was supported by a CRUK postdoctoral fellowship. Pathology analysis was supported by the Oxford NHS Biomedical Research Centre and the Experimental Cancer Medicine Centre.
Footnotes
Accession Numbers
The normalized data has been deposited in the GEO (GSE18384).
References
- 1.Enmark E, Gustafsson JA. Oestrogen receptors - an overview. J Intern Med. 1999;246:133–8. doi: 10.1046/j.1365-2796.1999.00545.x. [DOI] [PubMed] [Google Scholar]
- 2.McDonnell DP, Norris JD. Connections and regulation of the human estrogen receptor. Science. 2002;296:1642–4. doi: 10.1126/science.1071884. [DOI] [PubMed] [Google Scholar]
- 3.Carroll JS, Brown M. Estrogen receptor target gene: an evolving concept. Mol Endocrinol. 2006;20:1707–14. doi: 10.1210/me.2005-0334. [DOI] [PubMed] [Google Scholar]
- 4.Gordan JD, Simon MC. Hypoxia-inducible factors: central regulators of the tumor phenotype. Curr Opin Genet Dev. 2007;17:71–7. doi: 10.1016/j.gde.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Semenza GL. Regulation of mammalian O2 homeostasis by hypoxia-inducible factor 1. Annu Rev Cell Dev Biol. 1999;15:551–78. doi: 10.1146/annurev.cellbio.15.1.551. [DOI] [PubMed] [Google Scholar]
- 6.Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–32. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 7.Kaelin WG, Jr., Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell. 2008;30:393–402. doi: 10.1016/j.molcel.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 8.Kaelin WG., Jr. The von Hippel-Lindau protein, HIF hydroxylation, and oxygen sensing. Biochem Biophys Res Commun. 2005;338:627–38. doi: 10.1016/j.bbrc.2005.08.165. [DOI] [PubMed] [Google Scholar]
- 9.Hirota K, Semenza GL. Regulation of angiogenesis by hypoxia-inducible factor 1. Crit Rev Oncol Hematol. 2006;59:15–26. doi: 10.1016/j.critrevonc.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 10.Bos R, Zhong H, Hanrahan CF, et al. Levels of hypoxia-inducible factor-1 alpha during breast carcinogenesis. J Natl Cancer Inst. 2001;93:309–14. doi: 10.1093/jnci/93.4.309. [DOI] [PubMed] [Google Scholar]
- 11.Cho J, Bahn JJ, Park M, Ahn W, Lee YJ. Hypoxic activation of unoccupied estrogen-receptor-alpha is mediated by hypoxia-inducible factor-1 alpha. J Steroid Biochem Mol Biol. 2006;100:18–23. doi: 10.1016/j.jsbmb.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 12.Seifeddine R, Dreiem A, Tomkiewicz C, et al. Hypoxia and estrogen co-operate to regulate gene expression in T-47D human breast cancer cells. J Steroid Biochem Mol Biol. 2007;104:169–79. doi: 10.1016/j.jsbmb.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 13.Yi JM, Kwon HY, Cho JY, Lee YJ. Estrogen and hypoxia regulate estrogen receptor alpha in a synergistic manner. Biochem Biophys Res Commun. 2009;378:842–6. doi: 10.1016/j.bbrc.2008.11.142. [DOI] [PubMed] [Google Scholar]
- 14.Generali D, Buffa FM, Berruti A, et al. Phosphorylated ERalpha, HIF-1alpha, and MAPK signaling as predictors of primary endocrine treatment response and resistance in patients with breast cancer. J Clin Oncol. 2009;27:227–34. doi: 10.1200/JCO.2007.13.7083. [DOI] [PubMed] [Google Scholar]
- 15.Cloos PA, Christensen J, Agger K, et al. The putative oncogene GASC1 demethylates tri- and dimethylated lysine 9 on histone H3. Nature. 2006;442:307–11. doi: 10.1038/nature04837. [DOI] [PubMed] [Google Scholar]
- 16.Wigfield SM, Winter SC, Giatromanolaki A, Taylor J, Koukourakis ML, Harris AL. PDK-1 regulates lactate production in hypoxia and is associated with poor prognosis in head and neck squamous cancer. Br J Cancer. 2008;98:1975–84. doi: 10.1038/sj.bjc.6604356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Subramanian A, Tamayo P, Mootha VK, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102:15545–50. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van ’t Veer LJ, Dai H, van de Vijver MJ, et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002;415:530–6. doi: 10.1038/415530a. [DOI] [PubMed] [Google Scholar]
- 19.Elvidge GP, Glenny L, Appelhoff RJ, Ratcliffe PJ, Ragoussis J, Gleadle JM. Concordant regulation of gene expression by hypoxia and 2-oxoglutarate-dependent dioxygenase inhibition: the role of HIF-1alpha, HIF-2alpha, and other pathways. J Biol Chem. 2006;281:15215–26. doi: 10.1074/jbc.M511408200. [DOI] [PubMed] [Google Scholar]
- 20.Reich M, Liefeld T, Gould J, Lerner J, Tamayo P, Mesirov JP. GenePattern 2.0. Nat Genet. 2006;38:500–1. doi: 10.1038/ng0506-500. [DOI] [PubMed] [Google Scholar]
- 21.Subramanian A, Kuehn H, Gould J, Tamayo P, Mesirov JP. GSEA-P: a desktop application for Gene Set Enrichment Analysis. Bioinformatics. 2007;23:3251–3. doi: 10.1093/bioinformatics/btm369. [DOI] [PubMed] [Google Scholar]
- 22.Beyer S, Kristensen MM, Jensen KS, Johansen JV, Staller P. The histone demethylases JMJD1A and JMJD2B are transcriptional targets of hypoxia-inducible factor HIF. J Biol Chem. 2008;283:36542–52. doi: 10.1074/jbc.M804578200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pollard PJ, Loenarz C, Mole DR, et al. Regulation of Jumonji-domain-containing histone demethylases by hypoxia-inducible factor (HIF)-1alpha. Biochem J. 2008;416:387–94. doi: 10.1042/BJ20081238. [DOI] [PubMed] [Google Scholar]
- 24.Yang J, Ledaki I, Turley H, et al. Role of hypoxia-inducible factors in epigenetic regulation via histone demethylases. Ann N Y Acad Sci. 2009;1177:185–97. doi: 10.1111/j.1749-6632.2009.05027.x. [DOI] [PubMed] [Google Scholar]
- 25.Xia X, Lemieux ME, Li W, et al. Integrative analysis of HIF binding and transactivation reveals its role in maintaining histone methylation homeostasis. Proc Natl Acad Sci U S A. 2009;106:4260–5. doi: 10.1073/pnas.0810067106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cloos PA, Christensen J, Agger K, Helin K. Erasing the methyl mark: histone demethylases at the center of cellular differentiation and disease. Genes Dev. 2008;22:1115–40. doi: 10.1101/gad.1652908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carroll JS, Meyer CA, Song J, et al. Genome-wide analysis of estrogen receptor binding sites. Nat Genet. 2006;38:1289–97. doi: 10.1038/ng1901. [DOI] [PubMed] [Google Scholar]
- 28.Lin CY, Vega VB, Thomsen JS, et al. Whole-genome cartography of estrogen receptor alpha binding sites. PLoS Genet. 2007;3:e87. doi: 10.1371/journal.pgen.0030087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee DS, Mathur AK, Acker WB, 2nd, et al. Effects of smoking on survival for patients with end-stage liver disease. J Am Coll Surg. 2009;208:1077–84. doi: 10.1016/j.jamcollsurg.2009.01.050. [DOI] [PubMed] [Google Scholar]
- 30.Dressing G, Hagan C, Knutson T, Daniel A, Lange C. Progesterone receptors act as sensors for mitogenic protein kinases in breast cancer models. Endocr Relat Cancer. 2009 doi: 10.1677/ERC-08-0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sherr CJ. Cancer cell cycles. Science. 1996;274:1672–7. doi: 10.1126/science.274.5293.1672. [DOI] [PubMed] [Google Scholar]
- 32.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 33.Northcott PA, Nakahara Y, Wu X, et al. Multiple recurrent genetic events converge on control of histone lysine methylation in medulloblastoma. Nat Genet. 2009 doi: 10.1038/ng.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shi Y. Histone lysine demethylases: emerging roles in development, physiology and disease. Nat Rev Genet. 2007;8:829–33. doi: 10.1038/nrg2218. [DOI] [PubMed] [Google Scholar]
- 35.Wissmann M, Yin N, Muller JM, et al. Cooperative demethylation by JMJD2C and LSD1 promotes androgen receptor-dependent gene expression. Nat Cell Biol. 2007;9:347–53. doi: 10.1038/ncb1546. [DOI] [PubMed] [Google Scholar]
- 36.Northcott PA, Nakahara Y, Wu X, et al. Multiple recurrent genetic events converge on control of histone lysine methylation in medulloblastoma. Nat Genet. 2009;41:465–72. doi: 10.1038/ng.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Johnson AB, Denko N, Barton MC. Hypoxia induces a novel signature of chromatin modifications and global repression of transcription. Mutat Res. 2008;640:174–9. doi: 10.1016/j.mrfmmm.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ng SS, Kavanagh KL, McDonough MA, et al. Crystal structures of histone demethylase JMJD2A reveal basis for substrate specificity. Nature. 2007;448:87–91. doi: 10.1038/nature05971. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.