Abstract
Children with sickle cell anemia have a higher-than-expected prevalence of poor educational attainment. We test two key hypotheses about educational attainment among students with sickle cell anemia, as measured by grade retention and use of special education services: (1) lower household per capita income is associated with lower educational attainment; (2) the presence of a silent cerebral infarct is associated with lower educational attainment. We conducted a multicenter, cross-sectional study of cases from 22 U.S. sites included in the Silent Infarct Transfusion Trial. During screening, parents completed a questionnaire that included sociodemographic information and details of their child’s academic status. Of 835 students, 670 were evaluable; 536 had data on all covariates and were used for analysis. The students’ mean age was 9.4 years (range: 5–15) with 52.2% male; 17.5% of students were retained one grade level and 18.3% received special education services. A multiple variable logistic regression model identified that lower household per capita income (odds ratio [OR] of quartile 1 = 6.36, OR of quartile 2 = 4.7, OR of quartile 3 = 3.87; P = 0.001 for linear trend), age (OR = 1.3; P < 0.001), and male gender (OR, 2.2; P = 0.001) were associated with grade retention; silent cerebral infarct (P = 0.31) and painful episodes (P = 0.60) were not. Among students with sickle cell anemia, household per capita income is associated with grade retention, whereas the presence of a silent cerebral infarct is not. Future educational interventions will need to address both the medical and socioeconomic issues that affect students with sickle cell anemia.
Introduction
Children with poor health are at risk for poor academic attainment [1]. Higher absenteeism is associated with grade retention and limited academic attainment among children with chronic diseases, including sickle cell anemia [2], cancer [3,4], renal disease [5], and asthma [6]. Poor academic attainment among children limits future earnings and financial independence [1,7]. Other risk factors for poor academic attainment include male gender, African–American race/ethnicity, low socioeconomic status [8–10], single-parent households, lower parental academic attainment, lack of parental encouragement, poor self-esteem [11,12], and lack of health insurance [13]. Children with lower socioeconomic status as measured by family income or maternal education are more likely to exhibit poorer performance on cognitive testing, to be retained a grade level, or to not complete high school [14,15]. The strong correlation of socioeconomic status with future earning potential and quality of life underscores the importance of educational attainment [16–19].
Children with sickle cell anemia have an especially high risk for poor academic attainment as compared with other children because of the high prevalence of cerebrovascular disease. Approximately 30% of untreated children with this condition will experience a cerebrovascular event [20,21]; 80% of these events will result in significant cognitive deficits [22]. Chronic anemia is associated with lower levels of cognition among children and adults [23–25]. Among children with sickle cell anemia both with and without silent cerebral infarcts, we previously found that lower family income per capita and absence of the head of household’s college education were associated with lower intelligence quotient scores [26]. We also found a grade-retention rate of 40% among 23 students with sickle cell anemia, cognitive deficits, and strokes [27]. Even students with sickle cell anemia and no stroke history had a 30% grade-retention rate [22]. Thus, grade retention among students with sickle cell anemia is common; causes are multifactorial and cannot be solely explained by the presence of strokes (Table I in Supporting Information).
Much literature regarding sickle cell anemia has focused on the prevention and treatment of cerebrovascular disease and anemia to address cognitive deficits and poor academic attainment. However, students with this chronic disease still face the same nonbiological issues as students without chronic disease: economic stress related to low income and reduced support from single-parent households or from parents with lower educational levels. On the basis of the observation that socioeconomic status is associated with poor academic performance, we explored the association between socioeconomic status as measured by household per capita income, silent cerebral infarcts, and educational attainment among students with sickle cell anemia. In light of our previous data [22], we conducted a cross-sectional analysis to test the hypothesis that among students with sickle cell anemia, those with a low household per capita income will have lower educational attainment as compared with students with a high household per capita income. Evidence to support that home environmental factors are associated with grade failure in children with sickle cell anemia will allow for resources to target the high-risk population to improve educational outcomes.
Methods
Study design
We performed a multicenter, cross-sectional study (N = 536) of 22 study sites included in the silent infarct transfusion (SIT) Trial. The SIT Trial (ClinicalTrials.gov: #NCT00072761) is a randomized, controlled, interventional trial for children with sickle cell anemia between 5- and 14-years-old. For study definition, sickle cell anemia included hemoglobin SS and sickle β0-thalassemia. A detailed description of the study design has been published [28]. Institutional review boards at each institution approved the study. All participants and their guardians provided assent, consent, or both. The current article describes screened participants; the randomized trial is ongoing.
For the SIT Trial and this cross-sectional study, children with the following conditions were ineligible: sickle cell anemia with history of a focal neurologic event lasting more than 24 h; history of overt stroke; transcranial Doppler study of more than 200 cm/s; comorbid neurologic disorders; human immunodeficiency virus infection; pregnancy; impaired renal function (i.e., creatinine >2_upper limit of normal); treatment with chronic blood transfusions; clinically significant alloimmunization; and metallic structures in the body that interfere with magnetic resonance imaging.
During screening, parents or guardians of children completed a comprehensive questionnaire with a section specifically created to complete this study. Sociodemographic information was obtained via questions from the National Health and Nutrition Examination Survey socioeconomic status measures [29]. Parents self-reported socioeconomic data as well as their children’s academic statuses (e.g., special education resources, grade retention). Children’s hematocrit levels were recorded from clinical records upon enrollment in the SIT Trial.
Initially, a total of 1,176 children met criteria for eligibility for the SIT Trial and completed screening. European children (N = 226) were excluded because those students are not retained in lower grades for poor performance. We excluded 135 American children because they were in kindergarten with no chance to repeat a grade level yet (or grade level was not recorded); 145 were then excluded because they did not have valid magnetic resonance imaging data; finally, 134 were missing data on covariates used in the model, chiefly household income (Fig. 1 in Supporting Information). An analysis of the differences between subjects with and without missing data is presented in Table II in the Supporting Information, showing no large or consistent differences.
Primary outcome
For the purpose of this report, academic attainment is measured as grade retention and receipt of special education services. Grade retention was defined as the student having repeated a grade level. Typically, children who have challenges in school that result in grade retention have an Individual Educational Program (IEP). An IEP provides special education services to meet a child’s unique needs, offering educational benefit. Children with sickle cell anemia and educational challenges would meet IEP criteria under the category titled “Other Health Impairment” [30].
Covariates of interest
Data about the following covariates were collected for their known association with our primary outcome. Parents or guardians reported their own highest completed level of education, which was classified as “less than high school graduate,” “GED/high school graduate,” or “any amount of college.” Marital statuses were collapsed into categories of “married” and “unmarried.” Total family income was reported in $5000 increments; the midpoint of each increment was divided by the total number of people in the household to create a per capita income variable. Type of health coverage was recorded as “Medicaid” (Medicaid or State Children’s Health Insurance Plan), “private insurance,” or “no coverage.”
Educational attainment
Grade retention was defined as the student having repeated a grade level. Receipt of an IEP constituted special education services. Receipt of a 504 Plan was recorded but not considered special education services. Section 504 is an antidiscrimination, civil rights statute (Rehabilitation Act of 1973) that requires the needs of students with disabilities be met as adequately as those of children without disabilities [31]. Children with sickle cell anemia may have a 504 Plan in place to provide more frequent restroom breaks, the chance to carry a water bottle, an extra set of books at home to reduce the physical burden of transporting books, or extra time to complete assignments as a result of fatigue.
Statistical analysis
Demographic features of the students with and without histories of grade retention were analyzed with the t-test for continuous variables and with the χ2 test or Fisher’s exact test for discrete variables, or the Mann–Whitney U for ordinal variables or those with skewed distributions. Multivariable logistic regression was used to model the risk of grade retention or receipt of an IEP. We used a combination of sociodemographic measures (i.e., household per capita income, level of education of head of household, age, gender, and type of health coverage) that was based on previously published studies [8–12] as well as biologically plausible measures, including silent cerebral infarcts, pain event rates, and hematocrit levels.
Results
Demographics
A total of 536 students, average age 9.4 years, were evaluated. Almost 48% were female, 98% were self-reported African Americans, 1.5% were Hispanic, 98.2% had hemoglobin SS, 17.5% had a history of grade retention, and 18.3% received an IEP. Students who were retained a grade level were more likely to be male (P = 0.002), to have slightly larger households (P = 0.05), to have lower per capita household income (P < 0.001), to be on Medicaid (P = 0.001), and to have a head of household with lower education (P = 0.01). There were no differences in the rate of grade failure by presence of silent cerebral infarct (P = 0.47) or pain event rate (P = 0.26), but hematocrit was lower in this group (P = 0.007) (Table I).
TABLE I.
Demographics of Children with Sickle Cell Anemia Screened in the Silent Infarct Transfusion Trial
| Variable | History of grade retention (N = 94) |
No history of grade retention (N = 442) |
P-value |
|---|---|---|---|
| Age of child at registration in years | 10.4 | 9.2 | <0.001 |
| Gender (male, %) | (67.0) | (49.1) | 0.002a |
| Silent infarct (%) | (28.7) | (32.6) | 0.466a |
| Hematocrit level | 22.2 | 23.2 | 0.007 |
| Pain event rate (episodes per 3 years) | 0.59 | 0.55 | 0.260b |
| Last grade that the child completed in school (median) | 3 | 2 | 0.095b |
| IEP in place (%) | (47.9) | (12.0) | <0.001a |
| 504 Plan in place (%) | (7.4) | (9.8) | 0.486a |
| Medicaid health care coverage for child (%) | (82.8) | (64.5) | 0.001a |
| Marital status of parents: unmarried (%) | (68.1) | (58.6) | 0.088a |
| Age of mother in years | 34.6 | 34.6 | 0.962 |
| Age of father in years | 38.4 | 37.2 | 0.225 |
| Number of people in household | 4.9 | 4.5 | 0.052 |
| Yearly household per capita income in U.S. dollars | 5668 | 9365 | <0.001 |
| Yearly household per capita income in U.S. dollars (median) | (IQR 5 5833) | (IQR 5 8929) | <0.001b |
| Head of household education (overall test) | 0.010a | ||
| Less than high school graduation (%) | 24.5 | 14.3 | |
| High school diploma or GED (%) | 36.2 | 31.0 | |
| Any amount of college (%) | 39.4 | 54.8 |
IEP, individualized education program; GED, general education development.
Chi-square test.
Mann–Whitney U test.
As household income was reported in ranges, for purposes of the logistic regressions, household income per capita was divided into quartiles (<$2999, $3000–$5834, $5835–$11,250, and >$11,251). There was a correlation between household per capita income and Medicaid (r = 0.49; P = 0.001).
Almost half of the students with a history of grade retention received educational support from school systems. A total of 47.9% (45 of 94) of students with grade retention had IEPs, and ~9.3% (50 of 536) of all students had 504 Plans. With regard to 504 Plans, there was no difference between those who had a history of grade retention as compared with those who did not.
Multivariable model of risks for grade retention
The multivariable logistic regression was conducted in two steps. First, a model was constructed with all characteristics and a significance of P < 0.20 was used to screen for inclusion in a reduced model. A second model was run with the screened set of characteristics.
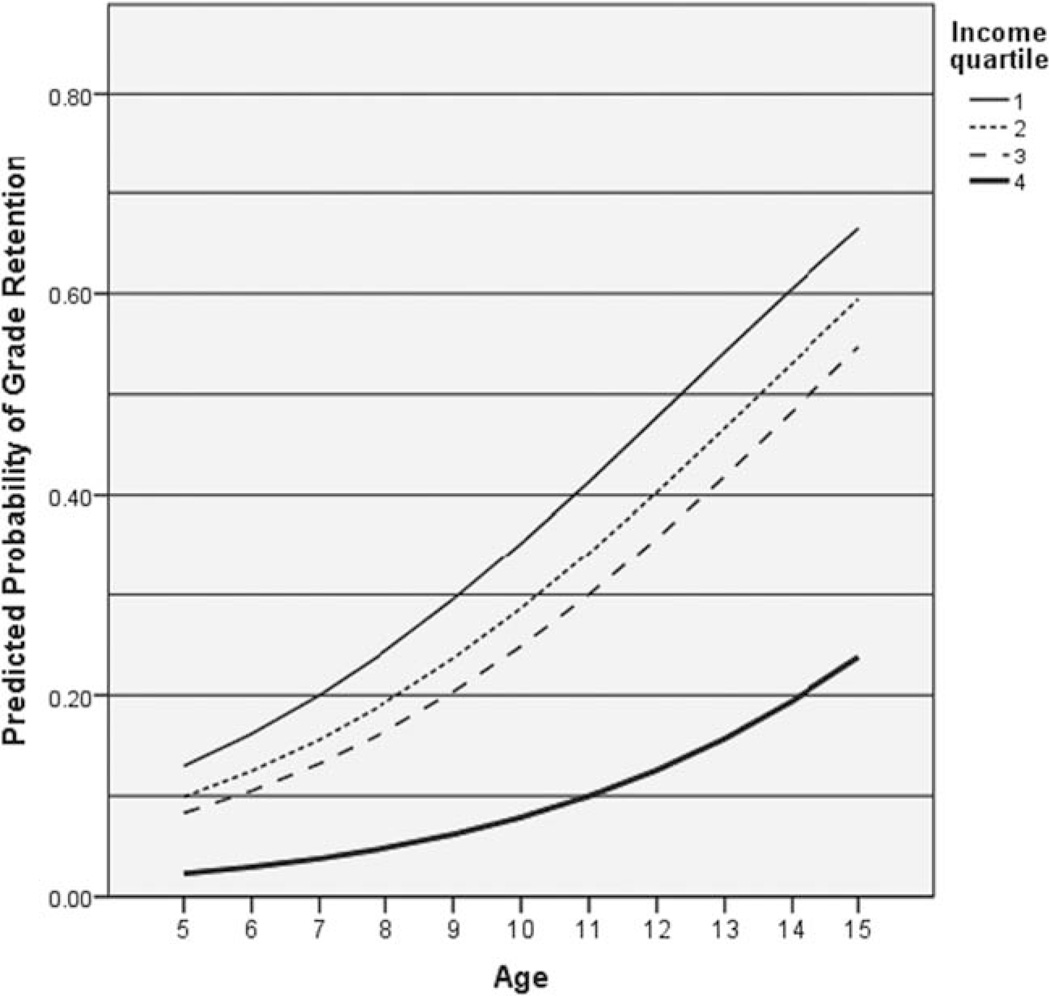
In the first stage, three characteristics met the screening criterion: age (P < 0.001), gender (P = 0.001), and household per capita income (P = 0.002). None of the biological factors, including silent cerebral infarct, met the screening criterion. In the reduced model with these three, all remained significantly associated with grade retention (model chi square = 56.0, 5 df, P < 0.001): male gender (OR 2.23, 95% CI 1.37–3.65, P = 0.006), age (OR 1.30, 95% CI 1.16– 1.44), and household per capita income (P < 0.001). Specifically, as compared with those in the highest quartile of household per capita income (>$11,251), those with household per capita incomes of $5835 to $11,250 (third quartile) had an OR of 3.87 for grade retention; those with household per capita incomes of $3000 to $5834 (quartile 2) had an OR of 4.70; and those in the lowest quartile (<$2999) had an OR of 6.36 (Table II). Figure 1 displays the predicted probability of grade retention for males based on the final model. By age 14 the model predicts an instance of grade retention is more likely than not for males in the lower three quartiles of income.
TABLE II.
Logistic Regression Model of Risk Factors for Grade Retention Among Children with Sickle Cell Anemia Screened in the Silent Infarct Transfusion Trial
| 95% CI for odds ratio |
||||
|---|---|---|---|---|
| Covariate | Odds ratio | Lower | Upper | P-value |
| Age at registration | 1.30 | 1.16 | 1.44 | <0.001 |
| Gender (male) | 2.23 | 1.37 | 3.65 | 0.001 |
| Household per capita income—Quartile 1a | 6.36 | 2.80 | 14.41 | <0.001 |
| Household per capita income—Quartile 3 | 4.70 | 2.08 | 10.62 | <0.001 |
| Household per capita income—Quartile 3 | 3.87 | 1.69 | 8.85 | 0.001 |
Household per capita income in US dollars; reference category is Quartile 4.
Figure 1.
Logistic regression predicted probabilities for grade retention for males, by age and household income.
Multivariable model of risks for individual education plan
In the first stage, three characteristics met the screening criterion: age (P = 0.001), grade retention (P < 0.001), and silent cerebral infarct (P = 0.12). In the reduced model, only two variables were significantly associated with IEP (model chi square = 69.6, 3 df, P < 0.001): age (OR 1.21, 95% CI 1.08–1.35, P = 0.001) and grade failure (OR 5.80, 95% CI 3.50–9.60, P < 0.001). Silent infarct was not (P = 0.120) (Table III).
TABLE III.
Logistic Regression Model of Risk Factors for Special Education Among Children with Sickle Cell Anemia Screened in the Silent Infarct Transfusion Trial
| 95% CI for odds ratio |
||||
|---|---|---|---|---|
| Covariate | Odds ratio | Lower | Upper | P-value |
| Age at registration | 1.21 | 1.08 | 1.35 | 0.001 |
| Grade failure | 5.80 | 3.50 | 9.60 | <0.001 |
Discussion
Sickle cell anemia is an inherited disease that most commonly occurs among people of African descent living in North America. Previous studies have documented lower levels of socioeconomic status among patients with this disease, but none have presented the overwhelming evidence of disparity as the current study [32–34]. A report from the Cooperative Study of Sickle Cell Disease showed that increasing age, lower reading achievement and lower family cohesion were associated with grade retention in 204 children with sickle cell disease [35]. By examining the largest sample of school-aged children with sickle cell anemia among a more recent cohort, our findings describe the striking educational challenges that these students face.
Household per capita income—not silent cerebral infarct or frequent painful episodes—was the dominant risk factor associated with grade retention among students with sickle cell anemia. Previous studies presented associations among silent cerebral infarcts, cognitive deficits, and poor academic achievement (i.e., grade retention and receipt of IEP or 504 plan); however, they failed to quantify the associations with household per capita income [22,35,36]. Recently, we published results from the SIT Trial that predicted lower intelligence scores with lower household income per capita, lower levels of head of household education, and increasing age of the child [26]. In fact, a lack of college education was associated with a decrease of 6.2 points while the presence of silent cerebral infarcts was associated with a 5.2 point decrease in intelligence quotient. These results may also be contributing to the increased risk of grade retention among the older children.
Multiple studies have documented lower intelligence quotient scores among children with silent cerebral infarcts as compared with sibling controls [22,36–38] and age-expected norms. Lower intelligence quotient scores have been documented among children with sickle cell anemia and overt strokes [22,36–38]. As a result of these sequelae, primary and secondary prevention of cerebral infarcts is a major component of care for children with sickle cell disease. Our data suggest that the focus on only preventing cerebral infarcts (primary or secondary) may not address the multifactorial issues that lead to poor educational attainment among these students.
An unexpected finding of the study was the degree of poverty of these children. According to the 1999 census, the average household per capita income in the United States was $21,587 [39]. Families that provided annual income information for our study had a median household per capita income of $6250. A total of 82% of families reported their annual incomes, and these reports skewed toward lower incomes. These data are correlated with the children’s health insurance coverage. Only 12 children (1.6%) had no health coverage at all, and the majority received health insurance from Medicaid (69%). Previous studies involving large national hospital databases estimated that 60–70% of children with sickle cell anemia have Medicaid coverage [40,41]. In 2009, the Department of Health and Human Services set the poverty guideline for the income of a family of four at $22,050 (i.e., $5512 per person) [39]. We believe that the household per capita income data obtained for this study are likely representative of the population of children with sickle cell anemia in the United States.
An inherent limitation of this study is that no temporal relationship can be established between household per capita income and academic attainment. Only one time-point is available, so we do not know when the silent cerebral infarcts occurred or the duration of the morbidity caused by cerebral ischemia. The average age of a child with a silent cerebral infarct in the Cooperative Study of Sickle Cell Disease cohort (n = 46) was 8 years (i.e., 6 years in girls, 10 years in boys). The mean age of children diagnosed with silent cerebral infarcts in the current study was 9.6 years. Given the similar age at diagnosis for silent cerebral infarcts in both cohorts, we believe the students in this study are representative of the population of children with sickle cell anemia in the United States with regard to this complication. The report from the Cooperative Study of Sickle Cell Disease found a similar rate of grade retention as our more modern cohort [35]. We were unable to obtain source documents for family income or school records. However, the data are consistent with previously published results among demographically similar families. For example, we confirmed previous findings that males are more likely to have lower levels of educational attainment than females. No clear etiology is identified in the literature, but self-esteem is less associated with academic performance among boys versus girls [42]. In addition, our previous work predicts a decrease in IQ for each year of age among students with sickle cell anemia [26]. The decreases in cognition may also contribute to the gradually increasing risk of grade retention and increasing age.
Another limitation of the study design included the inability to determine household income over time. Thus, we could not measure the length of exposure to silent cerebral infarct or to poverty. African Americans who are born into poverty tend to remain in or near poverty [43], so the exposure to poverty was likely longer than the exposure to the silent cerebral infarct. The U.S. Department of Education found that, among African-American students from kindergarten through eighth grade, 22.9% of those who were poor (<100% of poverty level), 10.9% of those who were near poor (100–199% of poverty level), and 5.1% of the nonpoor (≥200% of poverty level) had a history of grade retention [44]. When we categorized our family income data into these categories of poor, near poor, and nonpoor, 20%, 16.7%, and 5.9% of the groups, respectively, had failed a grade. These results are similar to those of Schatz’s study, which demonstrated a correlation of cognitive scores with lower socioeconomic status for both children with sickle cell anemia and controls; however, there was a “basement effect” of poverty for both groups [45].
Although silent cerebral infarcts were not a significant risk factor for grade retention in this model, their impact on cognition should not be discounted. Rather, our data suggest that silent cerebral infarcts alone may not be responsible for poor academic performance or even be the primary cause among this population. The impact of poverty on school systems is known. As minority enrollment and poverty increases, teacher quality decreases [46]. School districts with lower quality teachers are less likely to have students who are ready for college [47]. Perhaps the effect of poverty on educational attainment is so strong that the influence of other factors is difficult to detect.
In summary, the results of this study indicate that, among students with sickle cell anemia, a low household per capita income is associated with grade retention; whereas, the presence of a silent cerebral infarct is not. The majority of children with sickle cell anemia who receive care at 22 academic medical centers are living below or just above the poverty threshold. When designing studies to assess the medical determinants of cognition in children with sickle cell anemia, family household factors, and social determinants of health—including poverty—must be included. In all likelihood, only a multidisciplinary approach will allow us to make progress in our efforts to support these families. Our work and others suggest that interventions that target parenting skills and student specific health and educational issues are needed. The level of educational attainment is associated with the future earning potential and quality of life of these children [16–19]. Thus, the task of maximizing children’s educational attainment is an important public responsibility.
Supplementary Material
Acknowledgments
The contents of this paper are solely the responsibility of the authors and do not necessarily represent the official view of the NINDS, the NHLBI, or the NIH. Information about the NCATS is available at http://www.ncats.nih.gov/. Information about the Clinical and Translational Science Awards program can be found at http://www.ncats.nih.gov/research/cts/cts.html.
Contract grant sponsor: National Institute of Neurological Disorders and Stroke (NINDS); Contract grant numbers: U01NS042804, K23HL079073, 1 UL1 TR000448.
Contract grant sponsors: the National Heart, Lung and Blood Institute (NHLBI); and the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH).
Footnotes
Additional Supporting Information may be found in the online version of this article.
Conflict of interest: Nothing to report.
References
- 1.Haas SA. Health selection and the process of social stratification: the effect of childhood health on socioeconomic attainment. J Health Soc Behav. 2006;47:339–354. doi: 10.1177/002214650604700403. [DOI] [PubMed] [Google Scholar]
- 2.Fowler MG, Johnson MP, Atkinson SS. School achievement and absence in children with chronic health conditions. J Pediatr. 1985;106:683–687. doi: 10.1016/s0022-3476(85)80103-7. [DOI] [PubMed] [Google Scholar]
- 3.Gerhardt CA, Dixon M, Miller K, et al. Educational and occupational outcomes among survivors of childhood cancer during the transition to emerging adulthood. J Dev Behav Pediatr. 2007;28:448–455. doi: 10.1097/DBP.0b013e31811ff8e1. [DOI] [PubMed] [Google Scholar]
- 4.Mitby PA, Robison LL, Whitton JA, et al. Utilization of special education services and educational attainment among long-term survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. Cancer. 2003;97:1115–1126. doi: 10.1002/cncr.11117. [DOI] [PubMed] [Google Scholar]
- 5.Duquette PJ, Hooper SR, Wetherington CE, et al. Brief report: Intellectual and academic functioning in pediatric chronic kidney disease. J Pediatr Psychol. 2007;32:1011–1017. doi: 10.1093/jpepsy/jsm036. [DOI] [PubMed] [Google Scholar]
- 6.Blackman JA, Gurka MJ. Developmental and behavioral comorbidities of asthma in children. J Dev Behav Pediatr. 2007;28:92–99. doi: 10.1097/01.DBP.0000267557.80834.e5. [DOI] [PubMed] [Google Scholar]
- 7.Haas SA, Fosse NE. Health and the educational attainment of adolescents: evidence from the NLSY97. J Health Soc Behav. 2008;49:178–192. doi: 10.1177/002214650804900205. [DOI] [PubMed] [Google Scholar]
- 8.Risi S, Gerhardstein R, Kistner J. Children’s classroom peer relationships and subsequent educational outcomes. J Clin Child Adolesc Psychol. 2003;32:351–361. doi: 10.1207/S15374424JCCP3203_04. [DOI] [PubMed] [Google Scholar]
- 9.Smith-Maddox R. The social networks and resources of African American eighth graders: Evidence from the National Education Longitudinal Study of 1988. Adolescence. 1999;34:169–183. [PubMed] [Google Scholar]
- 10.Muijs RD. Predictors of academic achievement and academic self-concept; a longitudinal perspective. Br J Educ Psychol. 1997;67(Pt 3):263–277. doi: 10.1111/j.2044-8279.1997.tb01243.x. discussion 339–243. [DOI] [PubMed] [Google Scholar]
- 11.Lipman EL, Boyle MH, Dooley MD, et al. Child well-being in single-mother families. J Am Acad Child Adolesc Psychiatry. 2002;41:75–82. doi: 10.1097/00004583-200201000-00014. [DOI] [PubMed] [Google Scholar]
- 12.Garcia Bacete FJ, Rosel Remirez J. Family and personal correlates of academic achievement. Psychol Rep. 2001;88:533–547. doi: 10.2466/pr0.2001.88.2.533. [DOI] [PubMed] [Google Scholar]
- 13.Children’s Defense Fund. The State of America’s Children. Washington DC: Children’s Defense Fund; 2005. [Google Scholar]
- 14.National Institute of Child Health and Human Development Early Child Care Research Network. Duration and developmental timing of poverty and children’s cognitive and social development from birth through third grade. Child Dev. 2005;76:795–810. doi: 10.1111/j.1467-8624.2005.00878.x. [DOI] [PubMed] [Google Scholar]
- 15.Hernandez DJ. Double jeopardy: How third-grade reading skills and poverty influence high school graduation. Baltimore, MD: Annie E. Casey Foundation; 2011. Web site: http://www.aecf.org. [Google Scholar]
- 16.Duncan GJ, Magnuson KA. Can family socioeconomic resources account for racial and ethnic test dcore gaps? Future Child. 2005;15:35–54. doi: 10.1353/foc.2005.0004. [DOI] [PubMed] [Google Scholar]
- 17.Magnuson KA, Waldfogel J. Early childhood care and education: Effects on ethnic and racial gaps in school readiness. Future Child. 2005;15:169–196. doi: 10.1353/foc.2005.0005. [DOI] [PubMed] [Google Scholar]
- 18.Ou S-R. Do GED recipients differ from graduates and school dropouts?: Findings from an inner-city Cohort. Urban Edu. 2008;43:83–117. [Google Scholar]
- 19.Waanders C, Mendez JL, Downer JT. Parent characteristics, economic stress and neighborhood context as predictors of parent involvement in preschool children’s education. J School Psychol. 2007;45:619–636. [Google Scholar]
- 20.Pegelow CH, Macklin EA, Moser FG, et al. Longitudinal changes in brain magnetic resonance imaging findings in children with sickle cell disease. Blood. 2002;99:3014–3018. doi: 10.1182/blood.v99.8.3014. [DOI] [PubMed] [Google Scholar]
- 21.Ohene-Frempong K, Weiner SJ, Sleeper LA, et al. Cerebrovascular accidents in sickle cell disease rates and risk factors. Blood. 1998;91:288–294. [PubMed] [Google Scholar]
- 22.Schatz J, Brown RT, Pascual JM, et al. Poor school and cognitive functioning with silent cerebral infarcts and sickle cell disease. Neurology. 2001;56:1109–1111. doi: 10.1212/wnl.56.8.1109. [DOI] [PubMed] [Google Scholar]
- 23.Steen RG, Miles MA, Helton KJ, et al. Cognitive impairment in children with hemoglobin SS sickle cell disease: Relationship to MR imaging findings and hematocrit. AJNR Am J Neuroradiol. 2003;24:382–389. [PMC free article] [PubMed] [Google Scholar]
- 24.Hijmans CT, Grootenhuis MA, Oosterlaan J, et al. Neurocognitive deficits in children with sickle cell disease are associated with the severity of anemia. Pediatr Blood Cancer. 2011;57:297–302. doi: 10.1002/pbc.22892. [DOI] [PubMed] [Google Scholar]
- 25.Vichinsky EP, Neumayr LD, Gold JI, et al. Neuropsychological dysfunction and neuroimaging abnormalities in neurologically intact adults with sickle cell anemia. J Am Med Assoc. 2010;303:1823–1831. doi: 10.1001/jama.2010.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.King AA, Strouse JJ, Rodeghier MJ, et al. Parent education and biologic factors influence on cognition in sickle cell anemia. Am J Hematol. 2014;89:162–167. doi: 10.1002/ajh.23604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.King A, Herron S, McKinstry R, et al. A multi-disciplinary health care team’s efforts to improve educational attainment in children with sickle-cell anemia and cerebral infarcts. J Sch Health. 2006;76:33–37. doi: 10.1111/j.1746-1561.2006.00064.x. [DOI] [PubMed] [Google Scholar]
- 28.Casella JF, King AA, Barton B, et al. Design of the silent cerebral infarct transfusion (SIT) trial. Pediatr Hematol Oncol. 2010;27:69–89. doi: 10.3109/08880010903360367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rodriguez MA, Winkleby MA, Ahn D, et al. Identification of population subgroups of children and adolescents with high asthma prevalence: Findings from the Third National Health and Nutrition Examination Survey. Arch Pediatr Adolesc Med. 2002;156:269–275. doi: 10.1001/archpedi.156.3.269. [DOI] [PubMed] [Google Scholar]
- 30.Office of Special Education and Rehabilitation Services. A guide to the individualized education program. U.S. Department of Education; 2000. [Google Scholar]
- 31.United States Department of Education—Special Education and Rehabilitative Services. The Rehabilitation Act. 2004 [Google Scholar]
- 32.Panepinto JA, Pajewski NM, Foerster LM, et al. Impact of family income and sickle cell disease on the health-related quality of life of children. Qual Life Res. 2009;18:5–13. doi: 10.1007/s11136-008-9412-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boulet SL, Yanni EA, Creary MS, et al. Health status and healthcare use in a national sample of children with sickle cell disease. Am J Prevent Med. 2010;38:S528–S535. doi: 10.1016/j.amepre.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 34.Farber MD, Koshy M, Kinney TR. Cooperative study of sickle cell disease: Demographic and socioeconomic characteristics of patients and families with sickle cell disease. J Chronic Dis. 1985;38:495–505. doi: 10.1016/0021-9681(85)90033-5. [DOI] [PubMed] [Google Scholar]
- 35.Ladd RJ, Valrie CR, Walcott CM. Risk and resilience factors for grade retention in youth with sickle cell disease. Pediatr Blood Cancer. 2014;61:1252–1256. doi: 10.1002/pbc.24974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Armstrong FD, Thompson RJ, Jr, Wang W, et al. Cognitive functioning and brain magnetic resonance imaging in children with sickle Cell disease. Neuropsychology Committee of the Cooperative Study of sickle cell disease. Pediatrics. 1996;97:864–870. [PubMed] [Google Scholar]
- 37.Bernaudin F, Verlhac S, Freard F, et al. Multicenter prospective study of children with sickle cell disease: Radiographic and psychometric correlation. J Child Neurol. 2000;15:333–343. doi: 10.1177/088307380001500510. [DOI] [PubMed] [Google Scholar]
- 38.Brown RT, Buchanan I, Doepke K, et al. Cognitive and academic functioning in children with sickle-cell disease. J Clin Child Psychol. 1993;22:207–218. [Google Scholar]
- 39.United States Census Bureau. Washington, DC: 2000. Current Population Reports, P60-209, Money Income in the United States: 1999. [Google Scholar]
- 40.Brousseau DC, Owens PL, Mosso AL, et al. Acute care utilization and rehospitalizations for sickle cell disease. J Am Med Assoc. 2010;303:1288–1294. doi: 10.1001/jama.2010.378. [DOI] [PubMed] [Google Scholar]
- 41.McCavit TL, Xuan L, Zhang S, et al. National trends in incidence rates of hospitalization for stroke in children with sickle cell disease. Pediatr Blood Cancer. 2013;60:823–827. doi: 10.1002/pbc.24392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pegelow CH, Macklin EA, Moser FG, et al. Longitudinal changes in brain magnetic resonance imaging findings in children with sickle cell disease. Blood. 2002;99:3014–3018. doi: 10.1182/blood.v99.8.3014. [DOI] [PubMed] [Google Scholar]
- 43.Osborne JW. Race and academic disidentification. J Edu Psychol. 1997;89:728–735. [Google Scholar]
- 44.Ratcliffe C, McKernan S-M. Perspectives on Low-Income Working Families. Washington, DC: The Urban Institute; 2010. Childhood Poverty Persistence: Facts and Consequences; pp. 1–10. [Google Scholar]
- 45.Planty M, Hussar W, Snyder T, et al. National Center for Education Statistics. Jessup, MD: 2009. The Condition of Education 2009. NCES 2009-081. Available from: ED Pubs. P.O. Box 1398, Web site: http://nces.ed.gov/help/orderinfo.asp. [Google Scholar]
- 46.Schatz J, Finke R, Roberts CW. Interactions of biomedical and environmental risk factors for cognitive development: A preliminary study of sickle cell disease. J Dev Behav Pediatr. 2004;25:303–310. doi: 10.1097/00004703-200410000-00001. [DOI] [PubMed] [Google Scholar]
- 47.Presley JB, White BR, Gong Y. Policy Research Report: IERC 2005-2. Edwardsville, IL: Southern Illinois University, Edwardsville. Illinois Education Research Council; 2005. Examining the Distribution and Impact of Teacher Quality in Illinois. [Google Scholar]
- 48.Presley JB, Gong Y. Policy Research Report: IERC 2005-3. Edwardsville, IL: Southern Illinois University, Edwardsville. Illinois Education Research Council; 2005. The Demographics and Academics of College Readiness in Illinois. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.