Abstract
The main objective of this study was to characterize and find mechanisms to prevent acylation of therapeutic peptides encapsulated in glucose-star poly(D,L-lactic-co-glycolic acid) (PLGA) microspheres. The effect of addition of divalent cation salts CaCl2, MnCl2 as well as carboxymethyl chitosan (CMCS) on inhibition of acylation of octreotide (Oct), salmon calcitonin (sCT), and human parathyroid hormone (hPTH) was evaluated. Peptide content and integrity inside the degrading microspheres was monitored by reversed-phase high performance liquid chromatography (HPLC) and mass spectrometry during release incubation under physiological conditions. The extent of peptide acylation was strongly inhibited in the formulations containing divalent cations and/or CMCS as excipients, although specific effects were dependent on the specific peptide and excipient combinations. Both inorganic cations improved stability of Oct and hPTH but not sCT. Addition of CMCS alone was ineffective. Combining inorganic cations with CMCS improved stability of Oct and sCT but it had no effect on hPTH stability. The operative stabilization mechanisms are consistent with blocking peptide-PLGA interactions by a) directly competing for PLGA interactions with dications and/or b) increasing peptide affinity in the stabilizer phase within PLGA pores. Hence, inorganic multivalent cations are general stabilizers against peptide acylation, the effect of which may be augmented in certain instances with addition of CMCS.
Keywords: Acylation, Stability, Peptide, PLGA, Excipients, Sorption
1. Introduction
Biodegradable and biocompatible poly(D,L-lactic acid) (PLA) and poly(D,L-lactic-co-glycolic acid) (PLGA) microparticles and implants are among the most useful and promising platforms to provide long-acting systemic and/or local peptide and protein delivery [1–3]. However, among the most challenging tasks in successful product development is the stabilization of proteins and peptides during manufacture, storage and after drug administration [4–7]. In addition to potential deleterious effects resulting from accumulation of acidic degradation products [8–10], presence of moisture [11, 12] inside PLGA depots, and interaction between PLGA and peptide or protein [13, 14], chemical reactions between PLGA and peptides is also a significant obstacle. Nucleophilic primary amines, such as from the N-terminus and lysine side chain, can interact with the carboxylic acid end-groups of PLGA or PLGA degradation products to form acylated adducts, which could have potential detrimental effects, such as loss of activity, immunogenicity and toxicity. Peptide acylation has attracted significant attention recently [15–23]. It has been shown that the electrostatically driven sorption of the peptide to the PLGA is a common precursor to the acylation and followed by subsequent release of acylated peptide [18]. PEGylation of peptide to block labile primary amino groups of the peptide was also demonstrated to strongly inhibit acylation [18].
In previous studies from our group, we have demonstrated that soluble chloride salts of dications, such as Ca2+ and Mn2+, when placed in the presence of PLGA and octreotide (Oct), strongly inhibit octreotide adsorption to PLGA, the precursor to peptide acylation, and thus peptide acylation due to the competition with dicationic octreotide for ionic interaction with carboxylate end groups of PLGA [24]. Moreover, when these multivalent cationic salts were suitably co-encapsulated in injectable PLGA microspheres by an optimized double emulsion-solvent evaporation method, the salts were significantly retained and found to strongly inhibit acylation of the octreotide in degrading PLGA microspheres [25]. This finding suggested the use of soluble multivalent cationic salts as a viable strategy for minimizing the acylation of peptides.
The purpose of this study was to (a) test the generality of the water-soluble dicationic salts to stabilize two other peptides known to undergo acylation in PLGA, namely, salmon calcitonin (sCT) and human parathyroid hormone (hPTH), (b) to evaluate the feasibility to further inhibit acylation using polyelectrolyte as an additional excipient by inhibiting electrostatic interactions of the peptide with PLGA and (c) to explore the stabilizing mechanism of adding excipients.
2. Materials and Methods
2.1 Materials
Glucose star-type PLGA 50/50 is a star polymer with PLGA domains extending from hydroxyl groups of a D-glucose core [26]. The glucose star-type PLGA 50/50 with the acid number 4.2 mg KOH/g polymer was provided by Novartis Pharmaceutical Corp (Basel, Switzerland). Salmon calcitonin (sCT) and human parathyroid hormone, 1-34, (hPTH) were purchased from PolyPeptide Laboratories (Strasbourg, France). Octreotide acetate (Oct) was provided by Novartis Pharmaceutical Corp. Polyvinyl alcohol (PVA, 88 mol% hydrolyzed, MW 25,000) was obtained from Polysciences, Inc (Warrington, PA). Anhydrous manganese chloride (MnCl2) and calcium chloride (CaCl2) were obtained from Sigma (St. Louis, MO). O-Carboxymethyl chitosan (CMCS, MW 80–100 K, substitution degree: 60–80%) was supplied by Qingdao Heppe Biotechnology Co (Qingdao, China). Yttrium 1000 ppm, calcium 1000 ppm and manganese 1000 ppm ICP standard solutions were purchased from GFS Chemicals Company (Columbus, OH)). All other chemicals were of analytical grade or higher and were purchased from commercial suppliers.
2.2 Preparation of millicylinders encapsulating sCT and hPTH
PLGA was dissolved in methylene chlorine at 62.5% (wt/wt) concentration. sCT and hPTH were suspended in PLGA/methylene chloride solution at 5% theoretical loading (w/w). The suspension was then loaded into a 3 ml syringe and extruded into silicone rubber tubing (0.8 mm) by a syringe pump (Harvard Apparatus, Holliston, MA) at approximately 0.01 mL/min. The silicone tubing loaded with suspension was dried at room temperature for 24 h followed by further drying in a vacuum oven at 40 °C for another 48 h.
2.3 Preparation of microspheres
Microspheres were prepared using a modified double emulsion-solvent evaporation method [27]. Briefly 21–35 mg peptide was dissolved in 85 μl or 100 μl of methanol. PLGA was dissolved in 2 mL methylene chloride at 500 mg/ml concentration. Polymer and peptide solutions were mixed and homogenized at 10,000 rpm for 1 min in the ice bath using a homogenizer (VirTis Company, Gardiner, NY) to form W1/O emulsion. In some instances, 100 μl of aqueous solution of O-carboxymethyl chitosan (2 wt%) was added to the internal aqueous phase (W1) prior to homogenization. The emulsion stabilizer (2 ml of aqueous PVA solution at 1 or 3 wt%) was added and the mixture was vortexed with a Vortex Genie 2™ (Scientific Industries, Bohemia, NY) at maximum speed for 45 s. The 1 wt% PVA solution was used in preparation of Oct microspheres, while the 3 wt% PVA solution was used in order to obtain sCT and hPTH microspheres of a desired size distribution. The formed W1/O/W2 emulsion was immediately transferred to 100 ml 0.3% PVA aqueous solution under stirring at a constant rate. In the case of addition of divalent cationic salts, 25 mg CaCl2 or MnCl2 and 2.5 g corresponding salts were added to the organic phase and external aqueous phase (W2) simultaneously, as described in Table 1. After evaporation of methylene chloride (3 h), the microspheres were collected by sieve and washed repeatedly with deionized water. Finally, the microspheres were lyophilized for 24 h and stored in a desiccator at 4 °C before further investigation.
Table 1.
Formulation conditions of peptides loaded microspheres
| Formulation # | Peptide | CMCS added | Salt added | LTS w/w% | LAS w/w% | EES % | LTD w/w% | LAD w/w% | EED % |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Oct | − | − | − | − | − | 3.4 | 2.7±0.1 | 81±1 |
| 2 | Oct | + | − | − | − | − | 2.9 | 2.4±0.1 | 82±2 |
| 3 | Oct | − | CaCl2 | 2.4 | 2.2±0.2 | 94±6 | 3.4 | 3.3±0.2 | 98±3 |
| 4 | Oct | − | MnCl2 | 2.4 | 2.3±0.1 | 99±3 | 3.4 | 3.4±0.1 | 100±3 |
| 5 | Oct | + | CaCl2 | 2.4 | 2.0±0.2 | 84±7 | 3.4 | 3.8±0.2 | 111±4 |
| 6 | Oct | + | MnCl2 | 2.4 | 2.3±0.2 | 96±5 | 3.4 | 3.7±0.2 | 106±5 |
| 7 | sCT | − | − | − | − | − | 2.2 | 0.7±0.2 | 29±10 |
| 8 | sCT | + | − | − | − | − | 2.2 | 0.6±0.2 | 29±10 |
| 9 | sCT | − | CaCl2 | 2.4 | 1.5±0.1 | 62±2 | 2.1 | 0.7±0.1 | 33±2 |
| 10 | sCT | − | MnCl2 | 2.4 | 0.9±0.2 | 36±8 | 2.1 | 0.7±0.1 | 31±3 |
| 11 | sCT | + | CaCl2 | 2.4 | 1.8±0.1 | 73±1 | 2.1 | 1.5±0.1 | 73±2 |
| 12 | sCT | + | MnCl2 | 2.4 | 1.9±0.1 | 78±4 | 2.1 | 1.6±0.1 | 76±4 |
| 13 | hPTH | − | − | − | − | − | 2.1 | 0.8±0.1 | 37±3 |
| 14 | hPTH | + | − | − | − | − | 2.1 | 0.6±0.2 | 30±8 |
| 15 | hPTH | − | CaCl2 | 2.4 | 1.8±0.1 | 74±3 | 2.1 | 0.4±0.1 | 18±4 |
| 16 | hPTH | − | MnCl2 | 2.4 | 0.7±0.1 | 31±2 | 2.1 | 0.5±0.1 | 25±1 |
| 17 | hPTH | + | CaCl2 | 2.4 | 1.8±0.1 | 74.±1 | 2.1 | 0.5±0.1 | 22±1 |
| 18 | hPTH | + | MnCl2 | 2.4 | 1.9±0.1 | 79±2 | 2.1 | 0.4±0.1 | 21±1 |
Abbreviations: CMCS is carboxymethyl chitosan, Oct is octreotide, sCT is salmon calcitonin, hPTH is human parathyroid hormone 1-34, LTS is the theoretical loading of divalent cation salt, LAS is the actual loading of salt determined by ICP-OES, EES is the salt encapsulation efficiency, LTD is the theoretical loading of peptide, LAD is the actual loading of peptide, EED is the peptide encapsulation efficiency.
2.4 Microsphere characterization
2.4.1 Morphology of microspheres
The morphology of microspheres after freeze-drying was analyzed by scanning electron microscopy (SEM). Microspheres samples were sputter-coated with gold for analysis by Hitachi S3200 Variable Pressure SEM (Tokyo, Japan).
2.4.2 Drug loading and encapsulation efficiency
The microspheres were dissolved in methylene chloride and peptides were extracted with 0.1 M acetate buffer (pH 4.0). Peptide concentration and acylated peptide level were determined by high performance liquid chromatography (HPLC). Each formulation was analyzed in triplicate. HPLC conditions are described below.
2.4.3 Ca2+ and Mn2+ content in the microspheres
The content of divalent cations in the microspheres was analyzed by Inductively Coupled Plasma-Optical Emission Spectroscopy (ICP-OES, Perkin-Elmer Optima 2000 DV with Winlab software). Inorganic salts were extracted as previously described for peptides [25]. Solutions containing divalent cations were diluted with water prior to analysis and 1 ppm Yttrium was added as an internal standard. Ca2+ was detected at 396.85 nm in the radial plasma mode and Mn2+ was detected at 257.61 nm in the axial plasma mode. Each measurement was an average of three scans.
2.5 In vitro release and stability of peptide in PLGA microspheres
Release and acylation kinetics of peptides encapsulated in PLGA microspheres were examined during microsphere incubation in PBS (7.74 mM Na2HPO4, 2.26 mM NaH2PO4, 137 mM NaCl, and 3 mM KCl, pH 7.4) with 0.02 wt% Tween 80 (PBST) at 37 °C. Microspheres were placed in 1.0 ml of PBST under continuous agitation at a constant speed of 320 rpm. Release media was replaced at least weekly. At pre-selected time points, microspheres were lyophilized and their contents were extracted. The concentrations of peptide acylation products and divalent cations were assayed by HPLC and ICP-OES, respectively. Each formulation was examined in triplicate.
2.6 HPLC analysis of peptides
Oct, sCT and hPTH were analyzed by reversed phase HPLC (Waters Alliance system) using a Nova Pak 3.9×150 mm C-18 column (Waters, Milford, MA). A gradient elution method was utilized with mobile phases A: 0.1% (v/v) TFA in acetonitrile and B: 0.1% (v/v) TFA in water. The linear gradient of 25% to 35% A in 10 min were used for Oct, 30% to 35% A in 20 min for hPTH, and 32% to 37% A in 30 min for sCT. All gradients were delivered with 1.0 mL/min flow rate. UV absorbance was measured at 215 nm for Oct and 280 nm for sCT and hPTH.
2.7 Detection of peptide acylation products by RP-HPLC MS and MALDI-TOF MS
Oct and its degradation products were separated and identified previously by HPLC-MS [25]. hPTH and its degradation products were separated by HPLC and their corresponding fractions were collected and concentrated. Peptides in different fractions were identified by matrix-assisted laser desorption-ionization time-of-flight mass spectrometry (MALDI-TOF MS). sCT and its degradation were identified by MALDI-TOF MS without separation and fractionation. Samples were prepared by mixing 2 μl of peptide solution with 2μl of the matrix solution. Matrix solution contained 10 mg/ml solution of alpha-cyano-4-hydroxy-cinnamic acid (CHCA) in 50:50 of acetonitrile/ethanol mixture. One μl drops of peptide/matrix mixture were placed on the plate and dried prior to mass spectrometry analysis. The mass spectrometer, a Waters Tof Spec 2E™ (Waters, Milford, MA), was run in linear mode. The mass spectrometer was calibrated with a mixture of known peptides in the CHCA matrix (i.e., angiotensin I, substance P, renin substrate, ACTH Clip 18-39, insulin chain B oxidized, and insulin). The data was acquired and processed using MassLynx 3.5 software.
2.8 FTIR
Fourier transform infrared (FT-IR) spectra of KBr pellets were recorded in the 4000–400 cm−1 region using a Perkin-Elmer FT-IR spectrometer. Fifty scans were taken with a resolution of 4 cm−1.
2.9 EPR
Electron paramagnetic resonance (EPR) spectra of Mn2+ ion in aqueous solution were taken on a Bruker EMX Electron Spin Resonance Spectrometer at a frequency of 9.15 GHz and a power of 16.29 mW. All EPR measurements were done at lower temperature controlled by liquid nitrogen. The solution was placed in a 3 mm i.d. quartz tube. The 100 kHz field modulation width was 6 gauss. All the solutions contained 0.1 M NaCl.
2.10 Statistical Analysis
Analysis of covariance was conducted using Tukey pairwise comparisons (family error rate of α=0.05) to check for differences between formulations. If a p-value for a test or comparison is less than 0.05, then it can be concluded that a statistically significant difference exists. Minitab (v16) was used for the analysis.
3 Results and Discussion
3.1 Preparation and characterization of microspheres
Three therapeutic peptides were selected to examine the influence of divalent cations and carboxymethyl chitosan (CMCS) on inhibition of their acylation in PLGA implants: octreotide (Oct), salmon calcitonin (sCT) and human parathyroid hormone (hPTH). Octreotide is a 8-amino acid peptide with 2 labile primary amines (1 N-terminus and 1 lysine residue at position 5); salmon calcitonin (sCT) is a 32-amino acid peptide with 3 primary amines (1 N-terminus and 2 lysine residues at positions 11 and 18) and human parathyroid hormone (hPTH) is a 34-amino acid peptide with 4 primary amines (1 N-terminus and 3 lysine residues at positions 13, 26 and 27). All three peptides are basic. While the isoelectric points of Oct and hPTH are approximately 8.3 and 8.0, respectively, the isoelectric point of sCT is significantly higher at approximately 10.4. Peptides were encapsulated in microspheres using a modified double emulsion-solvent evaporation method. Peptides were encapsulated by with or without addition of CaCl2, MnCl2 and CMCS, and with combination of salt and CMCS. The resulting formulations are listed in Table 1. All microspheres were spherical with varying degree of surface porosity when analyzed by scanning electron microscopy (Fig. S1).
Whereas the encapsulation efficiency of Oct was high in all formulations, the encapsulation efficiencies of sCT and hPTH were typically low. This low encapsulation efficiency may have been caused by increased solubility of the peptides in the higher emulsifier concentration aqueous solution. Because sCT and hPHT strongly increased the organic phase viscosity, a 3% PVA emulsifier solution was used during microsphere preparation for these two peptides, instead of the 1% solution used for Oct encapsulation. This increase in emulsifier concentration allowed formation of sCT and hPTH microspheres with desirable size distributions. Control microsphere formulations prepared with lower PVA emulsifier concentrations for encapsulation of sCT and hPTH were much too large to be useful in this study. The low encapsulation efficiency of hPTH-loaded PLGA microparticles also has been reported elsewhere [28]. Interestingly, sCT-loaded formulations 11 and 12 containing both dication salt and CMCS displayed elevated encapsulation efficiency around 75%, in contrast with around 30% for other equivalent sCT-loaded formulations. The higher EE is consistent with the nonporous surface morphology of these microspheres relative to other sCT containing formulations (Fig S1). This improvement in EE and morphology was likely due to an interaction between the sCT with CMCS in presence of dication salts. This improvement was not observed when either dication (formulations 9 and 10) or CMCS (formulation 8) were added separately. Such an increase in loading efficiency was not observed for the hPTH formulation, strongly suggesting a lack of interaction between hPTH and CMCS-dicationic salt mixtures.
3.2 Identification of sCT and hPTH acylation products
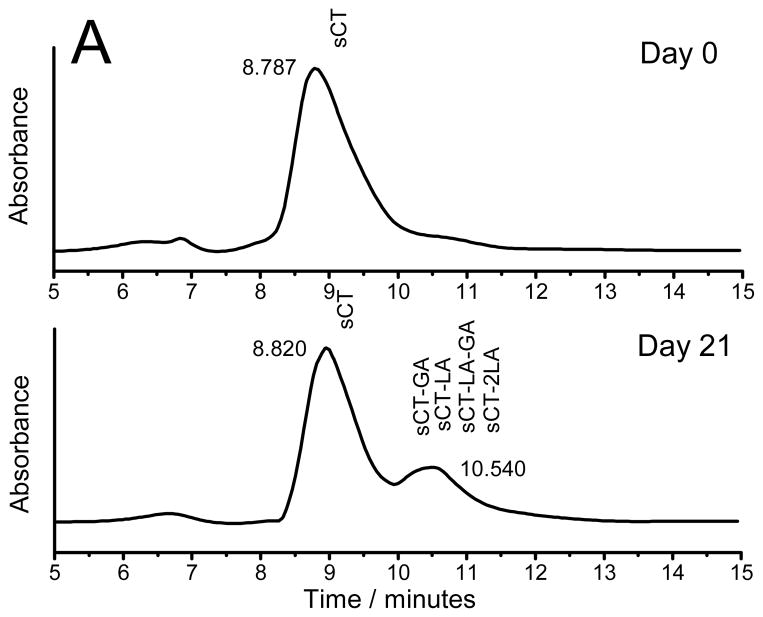
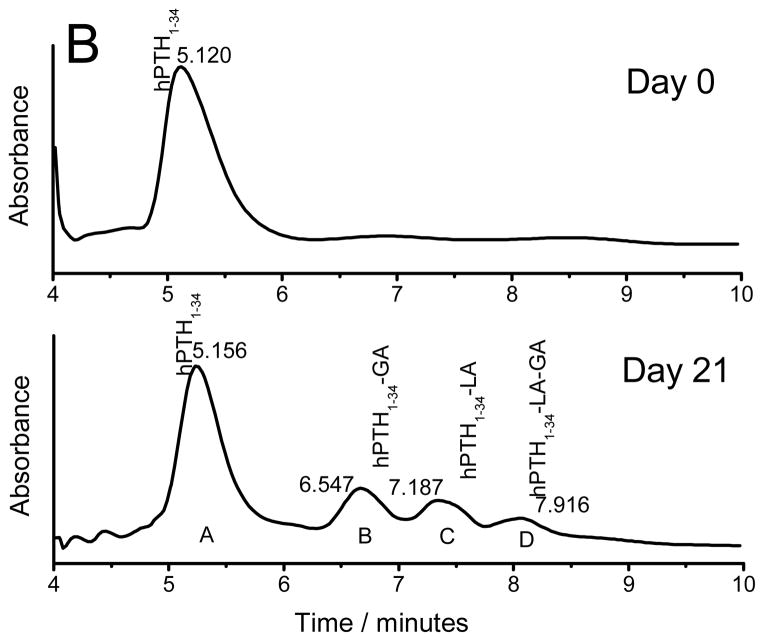
To examine the kinetics of chemical degradation of peptides inside PLGA implants, simple PLGA millicylinders were incubated in 97% RH at 37 °C, to rapidly cause instability of the peptide without drug release [4, 29]. Prior to incubation and at specified time intervals the peptides were extracted from millicylinders and analyzed by HPLC and MALDI-TOF MS. The HPLC chromatogram of extracted sCT and hPTH from millicylinders prior to incubation and after 21 days is displayed in Fig. 1. Only a main peptide peak was detected in the HPLC chromatograms before millicylinder incubation for both sCT and hPTH, indicating that both peptides were stable during encapsulation process. However, after millicylinders were incubated in 97% RH at 37 °C, peptide degradation products were detected in the HPLC chromatograms. MALDI-TOF MS analysis of sCT HPLC peak with 10.5 min retention time confirmed the presence of peptide acylation by single lactic (sCT-LA) and glycolic (sCT-GA) units and double acylation by either two lactic units (sCT-2LA) or one lactic and one glycolic unit (sCT-LA-GA). The HPLC profile of hPTH exhibited appearance of three peptide degradation peaks. The peaks were identified by MALDI-TOF MS to be different hPTH acylation products, i.e., hPTH1-34–GA, hPTH1-34–LA and hPTH1-34–LA–GA. The molecular masses and the expected modified peptides detected and MALDI-TOF MS spectra of extracted sCT and hPTH are provided in the supporting information (Table S1, Figures S2 and S3).
Figure 1.
HPLC chromatograms of sCT (A) and hPTH (B) extracted from the millicylinders incubated at 97% RH and 37 °C for 0 and 21 days.
3.3 Stabilization of octreotide by additives in PLCA microspheres
It has been reported that the ionic interaction between amino groups of Oct and the carboxylic acid end groups of degrading PLGA results in peptide adsorption to free-acid end group PLGA and subsequent acylation of peptide [30]. We demonstrated that co-encapsulation of water-soluble divalent cation salts efficiently inhibit the acylation of Oct in PLGA microspheres [24, 25]. However, the water-soluble cationic salts were not always retained in the polymer for an extended incubation period. Carboxylmethyl chitosan (CMCS), a known dication chelator, was co-encapsulated with Oct and water-soluble dicationic salts in PLGA microspheres in order to increase retention of the salts.
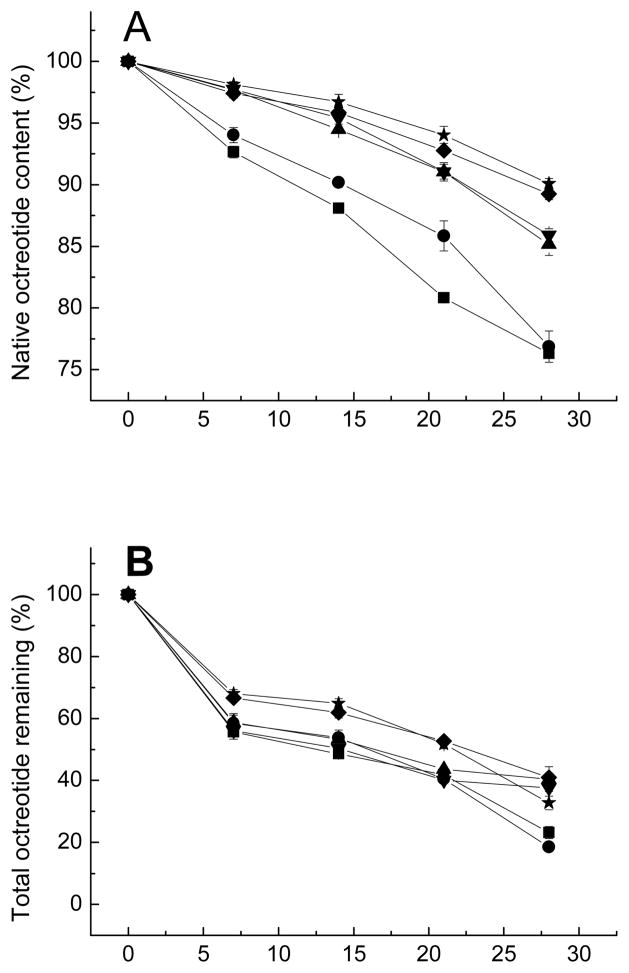
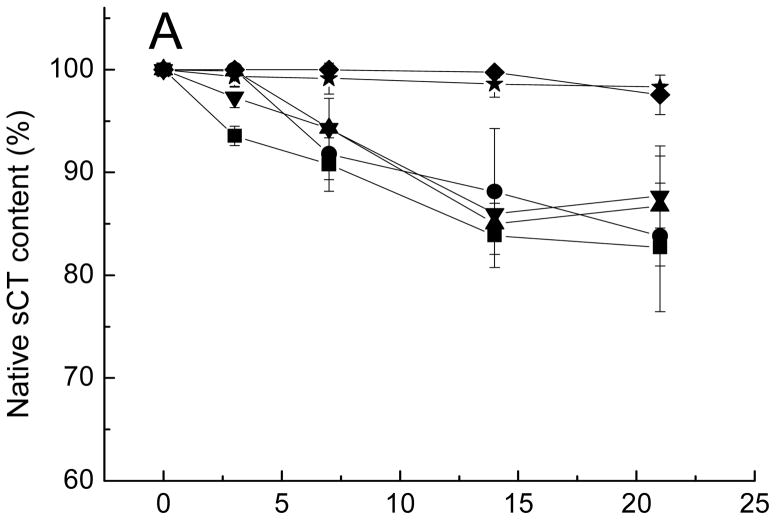
CMCS is the most frequently used derivative of chitosan. It has all the advantages of chitosan, including biocompatibility, biodegradability, antibacterial activity, nontoxicity, and excellent chelation capacity. In addition, CMCS is non-antigenic and has an excellent water-solubility [31–37]. CMCS contains carboxylic groups that could interact with dications providing chelator properties or could interact with amino groups of peptides, both could possibly be helpful to inhibit peptide acylation by minimizing dication loss from PLGA or increasing partitioning of peptide in the CMCS phase. CMCS was incorporated into microspheres by addition to the inner water phase during microspheres preparation. The effect of excipients on the stability of Oct in degrading microspheres are shown in Figure 2A. The differences in purity results from the other five formulations with either CMCS (formulation 2) or dications (formulation 3&4) or both (formulation 5&6) as excipients compared to control (formulation 1) were statistically significant. By simply adding a divalent cation (Ca2+ or Mn2+), the acylation of Oct was strongly inhibited, as was reported previously [25]. Adding both divalent cationic salts and CMCS further increased the extent of acylation inhibition. However, the purity increases caused by Ca2+ was not statistically significant from those caused by Mn2+, i.e. formulation 3 was not significant from formulation 4, and formulation 5 was not significant from formulation 6. Incorporation of CMCS without the divalent cationic salts also slightly improved the stability of Oct relative to the no additive formulation until 28 days.
Figure 2.
Kinetics of native octreotide content (A), total octreotide remaining (B), and divalent cation remaining (C) in PLGA microspheres after incubation in PBST at 37 °C.
■ Formulation 1, no additives; ● Formulation 2, CMCS; ▲ Formulation 3, CaCl2; ▼ Formulation 4, MnCl2; ◆ Formulation 5, CaCl2 and CMCS; ★ Formulation 6, MnCl2 and CMCS.
Divalent cations effectively inhibited acylation of octreotide likely due to competition with cationic Oct for ionic interactions with carboxyl groups of free-acid end-group PLGA [24, 25]. There are two potential mechanisms of how the addition carboxylmethyl chitosan can affect Oct acylation. CMCS is known to have good chelating affinity with metals [38]. Mn2+ mainly binds to the carboxymethyl groups of CMCS [39], whereas chelation of the biopolymer with Ca2+ is assisted by the acetamide and hydroxyl groups in addition to carboxyl groups [40]. This chelation ability might be helpful to prevent the peptide sorption competitor, divalent cations, from leaking from degrading PLGA microspheres. However, there is very limited effect of CMCS addition on retention of dications within PLGA microspheres as discussed later in Section 3.4 (Figure 2C). Another likely reason for decreasing the acylation is formation of polyanion/polycation complex between Oct and CMCS. The carboxyl residues of CMCS and the amino groups of Oct may interact to form the polyelectrolyte complex to reduce the availability of Oct for sorption to PLGA.
3.4 Chelation activity of CMCS
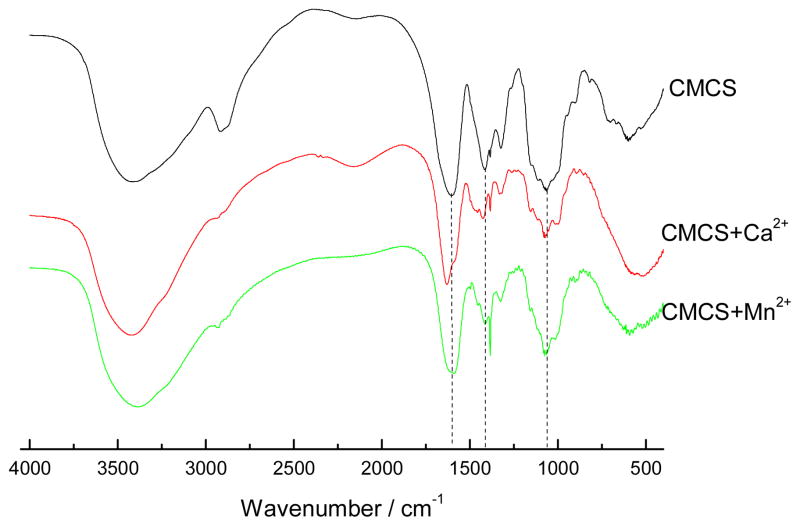
The chelation of affinity of CMCS to Ca2+ and Mn2+ ions was tested by FTIR and EPR. The FTIR spectra of CMCS in the presence and absence of Ca2+ or Mn2+ are shown in Figure 3. The spectrum of CMCS alone shows the characteristic absorption at 1602 and 1414 cm−1 due to asymmetric and symmetric stretching vibration of COO−, indicating a successful substitution of carboxymethyl groups in the biopolymer [41]. The strong, broad band at 3414 cm−1 is assigned to the stretching vibration of –OH. The C-O absorption peak of secondary hydroxyl group is at 1069 cm−1. The amino peak (N-H band) near 1589 cm−1 is almost not visible, which shows that the carboxymethyl group has substituted the amino group of chitosan. There was a change in the spectra upon addition of divalent cations. The absorption of asymmetric stretching vibration of COO− groups at 1602 cm−1 and 1414 cm−1, and hydroxyl groups at 1069 cm−1, both decreased and shifted to a higher wave number in the presence of Ca2+ or Mn2+. These changes are consistent with the chelation of CMCS with Ca2+ and Mn2+, which is assisted by hydroxyl and carboxyl groups.
Figure 3.
FTIR spectra of CMCS, CMCS+Ca2+ and CMCS+Mn2+.
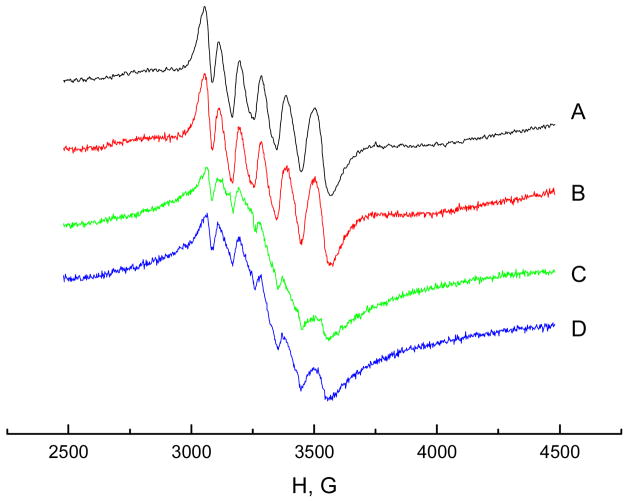
Electron paramagnetic resonance (EPR) is a powerful and sensitive technique often used for the identification and characterization of paramagnetic transition metals, such as Mn2+, which has unpaired d electrons. EPR was used in this study to identify the chelation affinity of CMCS to Mn2+. The EPR spectra of aqueous Mn2+ solution (A), Mn2+ solution containing Ca2+ (B), Mn2+ solution containing CMCS (C) and Mn2+ solution containing both Ca2+ and CMCS (D) are shown in Figure 4. The spectrum A for 0.25 mM MnCl2 aqueous solution (pH 6.2) exhibited a characteristic EPR signal of six lines, resulting from hyperfine interaction with the spin of the nucleus (I=5/2). In spectrum B, the same concentration of CaCl2 (0.25 mM) was added to the MnCl2 solution, which had no effect on Mn2+ EPR spectrum. In spectrum C, 0.015% CMCS was added to MnCl2 solution and the intensity of the EPR signal was reduced significantly, indicating bonding of Mn2+ ions to CMCS. It has been reported in the literature that the Mn2+ EPR signal disappears when the ion binds to macromolecules [42]. The intensity of the Mn2+ EPR signal was reduced less relative to spectrum C when CMCS was added to the solution containing both Ca2+ and Mn2+ ions (D) compared to 0.25 mM Mn2+ solution with CMCS only (C). These data strongly suggest that calcium ions are competitive for manganese binding to CMCS.
Figure 4.
EPR spectra for: 0.25 mM Mn2+ aqueous solution (A), 0.25 mM Mn2+ and 0.25mM Ca2+ solution (B), 0.25 mM Mn2+ with 0.015% CMCS (C) and 0.25 mM Mn2+ with 0.25 mM Ca2+ and 0.015% CMCS (D)
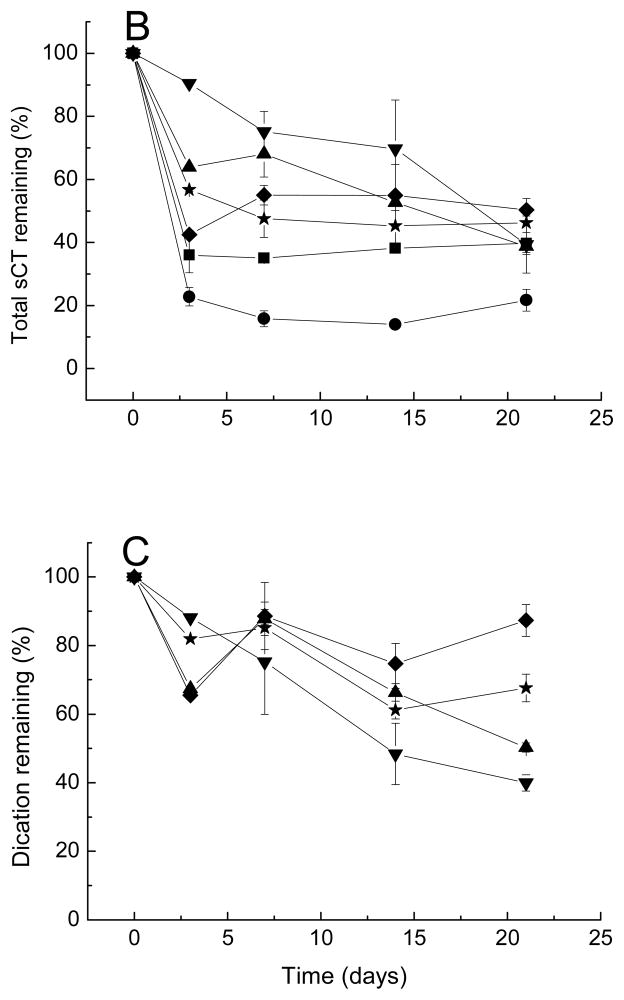
The amount of total peptide and divalent cation remaining in the microspheres during incubation in PBST 37°C is shown in Figures 2B and 2C. Since Oct is mildly unstable in phosphate buffer, the Oct remaining in the microspheres was used to evaluate the release kinetics [43]. The amount of Oct released from microspheres containing both CMCS and cationic salts was slightly reduced relative to microspheres prepared with either no additive, cationic salts of CMCS only, probably due to some interaction of peptide with CMCS in presence of dications. In contrast to our hypothesis, the presence of CMCS did not significantly affect cation retention in PLGA. Since the content of CMCS in each batch of microspheres was low (2 mg/1000 mg microspheres), there may have been insufficient CMCS to influence retention Ca2+ or Mn2+. In addition, development of acidic microclimate inside degrading PLGA microspheres may have interfered with the CMCS pH-dependent chelating ability [38]. The slight increase in Oct stability in presence of CMCS could be attributed to the charge interaction between the carboxylic groups of CMCS and the peptide amino groups, which would reduce Oct sorption to PLGA and its subsequent acylation. Further experiments would be required to test this hypothesis.
3.5 Acylation of sCT and hPTH in PLGA microspheres
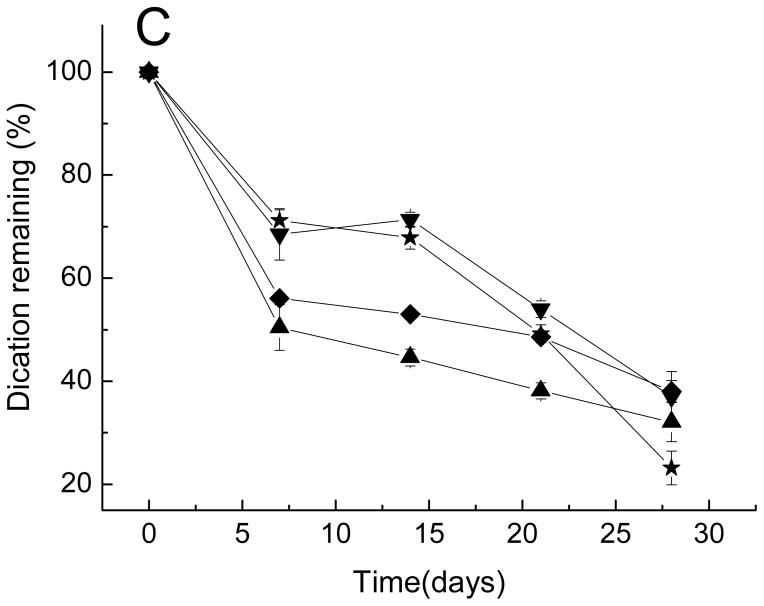
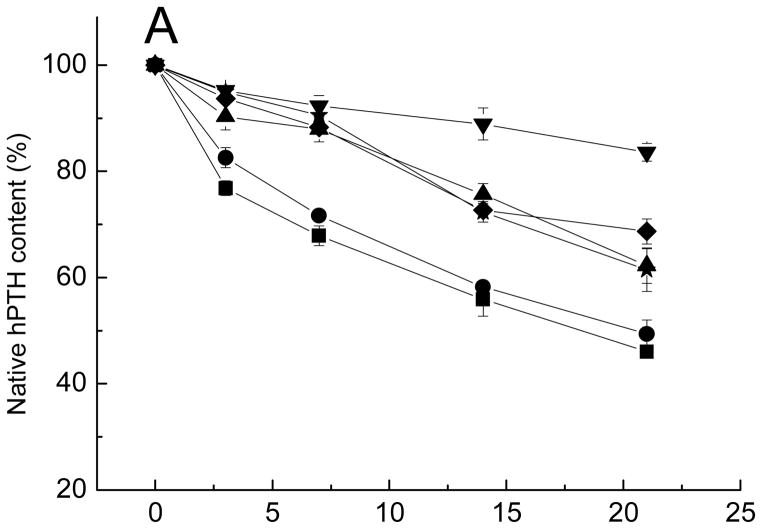
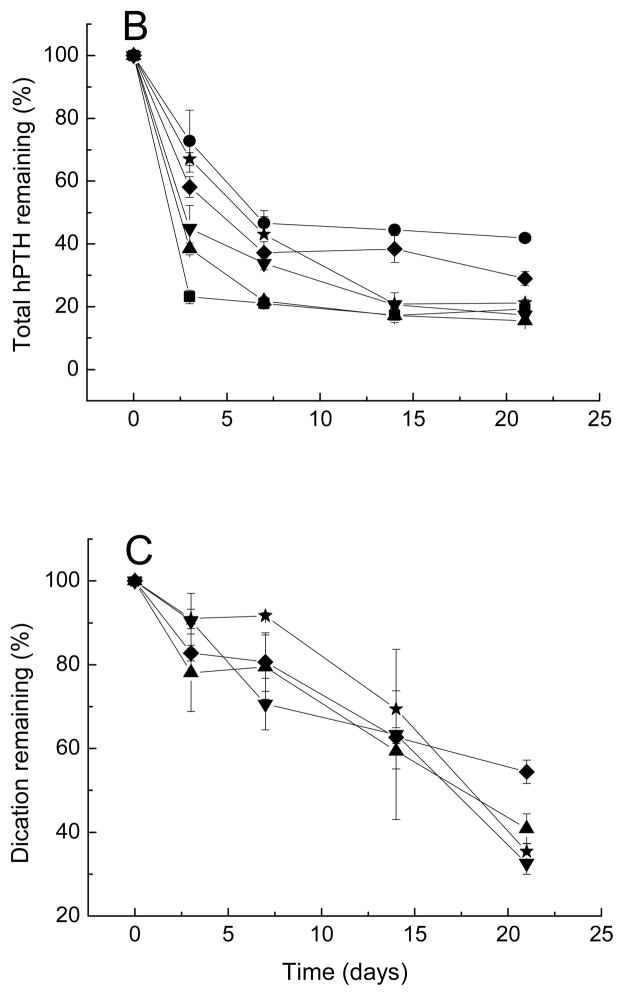
The effect of excipients on the stability of sCT and hPTH in degrading PLGA is displayed in Figs. 5A and 6A. The acylation was the most visible for the hPTH microspheres compared with Oct and sCT microspheres. Only 50% of native hPTH remain after 21-day incubation compared with 85% and 75% of intact sCT and Oct, respectively. This was probably due to the greater number of acylation target sites, i.e., primary amino groups of lysine residues and N-terminal amino acids. Similar to Oct, all five formulations with either CMCS or dications alone or both (formulations 14–18) improved the stability of PTH at different extents compared to control (formulation 13). Adding CMCS alone did not significantly inhibit PTH acylation. However, the addition of divalent cationic salts alone to hPTH PLGA microspheres decreased the acylation significantly. The dication stabilization effect is the most visible upon addition of MnCl2. Higher efficacy of Mn2+ ion relative to Ca2+ to inhibit sorption of amino groups of peptide to PLGA and their subsequent acylation has been reported previously [24]. Addition of CMCS in presence of dication salts did not further improve the stability of hPTH and had no effect on rate of release of dications for PLGA microspheres.
Figure 5.
Kinetics of native sCT content (A), total sCT remaining (B), and divalent cations (C) remaining in microspheres after incubation in PBST at 37 °C.
■ Formulation 7, no additives; ● Formulation 8, CMCS; ▲ Formulation 9, CaCl2; ▼ Formulation 10, MnCl2; ◆ Formulation 11, CaCl2 and CMCS; ★ Formulation 12, MnCl2 and CMCS.
Figure 6.
Kinetics of native hPTH content (A), total hPTH (B), and divalent cations (C) remaining in microspheres after incubation in PBST at 37 °C.
■ Formulation 13, no additives; ● Formulation 14, CMCS; ▲ Formulation 15, CaCl2; ▼ Formulation 16, MnCl2; ◆ Formulation 17, CaCl2 and CMCS; ★ Formulation 18, MnCl2 and CMCS.
Overall, the response of hPTH to co-encapsulation of different stabilizers in PLGA microspheres was similar to the response of Oct to the stabilizers. Addition of dication salts alone improved stability of these peptides, while addition of CMCS alone showed only very limited effect on stabilization of peptides or retention of dications in the polymer. By contrast, sCT exhibited very different behavior in presence of various stabilizers. Addition of dications alone or CMCS alone had no significant stabilization effect on sCT. By contrast, the co-encapsulation of both additives dication salt and CMCS fully stabilized sCT for the entire duration of incubation. This is consistent with increase in encapsulation efficiency of peptide upon addition of the divalent cation-CMCS mixture, strongly suggested peptide interaction with the divalent cation-CMCS combination, thereby inhibiting the precursory peptide-PLGA interaction to acylation. The potential reason for the different response of Oct and hPTH to addition of CMCS in comparison to sCT could be related to the difference in isoelectic points of these peptides. The sCT is the most basic of the three with isoelectic point of 10.4, while Oct and hPTH are less basic with isoelectic points of 8.3 and 8.0, respectively. Stronger basic properties of sCT might be favorable in its binding to a CMCS/dication phase and, hence, reduction of sorption to PLGA and prevention of acylation in presence of dication salts. The stabilizing effects of excipients on the three acylation-liable peptides are summarized in Table 2. In addition, a graphic describing the two proposed mechanisms of stabilization, namely, a) blocking peptide from combining with negatively charged sites on PLGA and b) increasing peptide partitioning into a CMCS/divalent cation phase is given in the Supporting Information (Fig. S4).
Table 2.
Summary of stabilizing effect of excipients on the three peptides
| Formulation# | Peptide | CMCS added | Salt added | % native peptide | ||||
|---|---|---|---|---|---|---|---|---|
| 3 day | 7 day | 14 day | 21 day | 28 day | ||||
| 1 | Oct | − | − | − | 92.7± 0.5 | 88.1 ± 0.3 | 80.8 ± 0.4 | 76.3 ± 0.4 |
| 2 | Oct | + | − | − | 94.0 ± 0.6 | 90.2 ± 0.4 | 86 ± 2 | 77 ± 2 |
| 3 | Oct | − | CaCl2 | − | 97.7 ± 0.2 | 94.5 ± 0.2 | 91.0 ± 0.7 | 85.2 ± 0.9 |
| 4 | Oct | − | MnCl2 | − | 97.7 ± 0.1 | 95.4 ± 0.5 | 91.1 ± 0.6 | 85.9 ± 0.5 |
| 5 | Oct | + | CaCl2 | − | 97.4 ± 0.4 | 95.9 ± 0.4 | 92.8 ± 0.1 | 89.2 ± 0.4 |
| 6 | Oct | + | MnCl2 | − | 98.1 ± 0.2 | 96.7 ± 0.6 | 94.0 ± 0.7 | 90.1 ± 0.4 |
| 7 | sCT | − | − | 93.5 ± 0.9 | 91 ± 3 | 84 ± 3 | 83 ± 7 | − |
| 8 | sCT | + | − | 100.0 ± 0.1 | 92 ± 3 | 88 ± 6 | 83.8 ± 0.8 | − |
| 9 | sCT | − | CaCl2 | 99.9 ± 0.2 | 94.3 ± 0.3 | 85 ± 2 | 87 ± 6 | − |
| 10 | sCT | − | MnCl2 | 97 ± 1 | 94 ± 3 | 86 ± 2 | 88 ± 4 | − |
| 11 | sCT | + | CaCl2 | 100.0 ± 0.1 | 100.0 ± 0.1 | 99.7 ± 0.5 | 98 ± 2 | − |
| 12 | sCT | + | MnCl2 | 99.3 ± 0.9 | 99 ± 2 | 99 ± 2 | 98.3 ± 0.3 | − |
| 13 | hPTH | − | − | 77 ± 2 | 68 ± 2 | 56 ± 3 | 46.1 ± 0.8 | − |
| 14 | hPTH | + | − | 83 ± 2 | 71.7 ± 0.5 | 58.2 ± 0.7 | 50 ± 3 | − |
| 15 | hPTH | − | CaCl2 | 90 ± 3 | 88 ± 3 | 76 ± 2 | 62 ± 4 | − |
| 16 | hPTH | − | MnCl2 | 95.2 ± 0.6 | 92 ± 2 | 89 ± 3 | 84 ± 2 | − |
| 17 | hPTH | + | CaCl2 | 93.7 ± 0.8 | 88.3 ± 0.8 | 73 ± 2 | 69 ± 3 | − |
| 18 | hPTH | + | MnCl2 | 94.9±0.7 | 90.5 ± 0.5 | 72 ± 2 | 61 ± 4 | − |
Addition of cation and/or CMCS affected the release of sCT and hPTH from microspheres due to a number of factors such as changes in porosity, water uptake, osmotic pressure and degradation of PLGA, as shown in Figs. 5B and 6B. Figs. 5C and 6C display the kinetics of divalent cation content remaining in sCT and hPTH loaded microspheres over incubation time, respectively. Addition of CMCS in the microspheres slightly retarded the leakage of cationic salts. This effect is more noticeable for sCT- than for hPTH-containing microspheres.
In addition to its advantages, further addition of CMCS with dications could also have potential negative effects on acylation, including: a) the formation of chemical complexes with dications may reduce cation availability for the electrostatic attractions with PLGA and the adsorption competition with peptide to PLGA; and b) the acceleration of water uptake upon incorporation of water-soluble CMCS and divalent cations could theoretically increase acylation. The large number of potential interactions with multiple excipients, peptide, and polymers and the resulting phase behavior that could exist, make difficult firm conclusions on the specific mechanism of acylation inhibition afforded by the unique combinations of excipients described here.
4 Conclusions
Although the mechanism of inhibition of peptide acylation is still uncertain, specific combinations of two types of excipients, divalent cationic salts and CMCS, were capable of strongly minimizing acylation of three separate peptides. The effects of excipients on inhibition of acylation varied depending on the properties of individual peptides. Selective addition these stabilizers to the PLGA microspheres for future peptide candidates will need to be considered on an individual peptide case-by-case basis.
Supplementary Material
Acknowledgments
This study was supported by Novartis Pharma AG and NIH HL 68345. We also gratefully acknowledge James Windak for assistance with MALDI-TOF MS analysis, and Anna Schwendeman for manuscript editing.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zhu GZ, Mallery SR, Schwendeman SP. Stabilization of proteins encapsulated in injectable poly (lactide-co-glycolide) Nat Biotechnol. 2000;18:52–57. doi: 10.1038/71916. [DOI] [PubMed] [Google Scholar]
- 2.Jiang WL, Gupta RK, Deshpande MC, Schwendeman SP. Biodegradable poly(lactic-co-glycolic acid) microparticles for injectable delivery of vaccine antigens. Adv Drug Delivery Rev. 2005;57:391–410. doi: 10.1016/j.addr.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 3.Sinha VR, Trehan A. Biodegradable microspheres for protein delivery. J Control Release. 2003;90:261–280. doi: 10.1016/s0168-3659(03)00194-9. [DOI] [PubMed] [Google Scholar]
- 4.Schwendeman SP. Recent advances in the stabilization of proteins encapsulated in injectable PLGA delivery systems. Crit Rev Ther Drug Carrier Syst. 2002;19:73–98. doi: 10.1615/critrevtherdrugcarriersyst.v19.i1.20. [DOI] [PubMed] [Google Scholar]
- 5.Fu K, Klibanov AM, Langer R. Protein stability in controlled-release systems. Nat Biotechnol. 2000;18:24–25. doi: 10.1038/71875. [DOI] [PubMed] [Google Scholar]
- 6.van de Weert M, Hennink WE, Jiskoot W. Protein instability in poly(lactic-co-glycolic acid) microparticles. Pharm Res. 2000;17:1159–1167. doi: 10.1023/a:1026498209874. [DOI] [PubMed] [Google Scholar]
- 7.Perez C, Castellanos IJ, Costantino HR, Al-Azzam W, Griebenow K. Recent trends in stabilizing protein structure upon encapsulation and release from bioerodible polymers. J Pharm Pharmacol. 2002;54:301–313. doi: 10.1211/0022357021778448. [DOI] [PubMed] [Google Scholar]
- 8.Zhu GZ, Schwendeman SP. Stabilization of proteins encapsulated in cylindrical poly(lactide-co-glycolide) implants: Mechanism of stabilization by basic additives. Pharm Res. 2000;17:351–357. doi: 10.1023/a:1007513425337. [DOI] [PubMed] [Google Scholar]
- 9.Estey T, Kang J, Schwendeman SP, Carpenter JF. BSA degradation under acidic conditions: A model for protein instability during release from PLGA delivery systems. J Pharm Sci. 2006;95:1626–1639. doi: 10.1002/jps.20625. [DOI] [PubMed] [Google Scholar]
- 10.Kang JC, Schwendeman SP. Comparison of the effects of Mg(OH)(2) and sucrose on the stability of bovine serum albumin encapsulated in injectable poly(D,L-lactide-co-glycolide) implants. Biomaterials. 2002;23:239–245. doi: 10.1016/s0142-9612(01)00101-6. [DOI] [PubMed] [Google Scholar]
- 11.Liu WR, Langer R, Klibanov AM. Moisture-Induced Aggregation of Lyophilized Proteins in the Solid-State. Biotechnol Bioeng. 1991;37:177–184. doi: 10.1002/bit.260370210. [DOI] [PubMed] [Google Scholar]
- 12.Schwendeman SP, Costantino HR, Gupta RK, Siber GR, Klibanov AM, Langer R. Stabilization of Tetanus and Diphtheria Toxoids against Moisture-Induced Aggregation. Proc Natl Acad Sci U S A. 1995;92:11234–11238. doi: 10.1073/pnas.92.24.11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oscarsson S. Factors affecting protein interaction at sorbent interfaces. J Chromatogr B. 1997;699:117–131. doi: 10.1016/s0378-4347(97)00224-7. [DOI] [PubMed] [Google Scholar]
- 14.Calis S, Jeyanthi R, Tsai T, Mehta RC, DeLuca PP. Adsorption of Salmon-Calcitonin to Plga Microspheres. Pharm Res. 1995;12:1072–1076. doi: 10.1023/a:1016278902839. [DOI] [PubMed] [Google Scholar]
- 15.Lucke A, Fustella E, Tessmar J, Gazzaniga A, Gopferich A. The effect of poly(ethylene glycol)-poly(D,L-lactic acid) diblock copolymers on peptide acylation. J Control Release. 2002;80:157–168. doi: 10.1016/s0168-3659(02)00020-2. [DOI] [PubMed] [Google Scholar]
- 16.Lucke A, Gopferich A. Acylation of peptides by lactic acid solutions. Eur J Pharm Sci. 2003;55:27–33. doi: 10.1016/s0939-6411(02)00138-8. [DOI] [PubMed] [Google Scholar]
- 17.Lucke A, Kiermaier J, Gopferich A. Peptide acylation by poly(alpha-hydroxy esters) Pharm Res. 2002;19:175–181. doi: 10.1023/a:1014272816454. [DOI] [PubMed] [Google Scholar]
- 18.Na DH, DeLuca PP. PEGylation of octreotide: I. Separation of positional isomers and stability against acylation by Poly(D,L-lactide-co-glycolide) Pharm Res. 2005;22:736–742. doi: 10.1007/s11095-005-2589-4. [DOI] [PubMed] [Google Scholar]
- 19.Na DH, Youn YS, Lee SD, Son MW, Kim WB, DeLuca PP, Lee KC. Monitoring of peptide acylation inside degrading PLGA microspheres by capillary electrophoresis and MALDI-TOF mass spectrometry. J Control Release. 2003;92:291–299. doi: 10.1016/s0168-3659(03)00366-3. [DOI] [PubMed] [Google Scholar]
- 20.Rothen-Weinhold A, Oudry N, Schwach-Abdellaoui K, Frutiger-Hughes S, Hughes GJ, Jeannerat D, Burger U, Besseghir K, Gurny R. Formation of peptide impurities in polyester matrices during implant manufacturing. Eur J Pharm Sci. 2000;49:253–257. doi: 10.1016/s0939-6411(00)00066-7. [DOI] [PubMed] [Google Scholar]
- 21.Houchin ML, Heppert K, Topp EM. Deamidation, acylation and proteolysis of a model peptide in PLGA films. J Control Release. 2006;112:111–119. doi: 10.1016/j.jconrel.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 22.Houchin ML, Neuenswander SA, Topp EM. Effect of excipients on PLGA film degradation and the stability of an incorporated peptide. J Control Release. 2007;117:413–420. doi: 10.1016/j.jconrel.2006.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Houchin ML, Topp EM. Chemical degradation of peptides and proteins in PLGA: A review of reactions and mechanisms. J Pharm Sci. 2008;97:2395–2404. doi: 10.1002/jps.21176. [DOI] [PubMed] [Google Scholar]
- 24.Sophocleous AM, Zhang Y, Schwendeman SP. A new class of inhibitors of peptide sorption and acylation in PLGA. J Control Release. 2009;137:179–184. doi: 10.1016/j.jconrel.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Y, Sophocleous AM, Schwendeman SP. Inhibition of Peptide Acylation in PLGA Microspheres with Water-soluble Divalent Cationic Salts. Pharm Res. 2009;26:1986–1994. doi: 10.1007/s11095-009-9914-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kang J, Lambert O, Ausborn M, Schwendeman SP. Stability of proteins encapsulated in injectable and biodegradable poly(lactide-co-glycolide)-glucose millicylinders. Int J Pharm. 2008;357:235–243. doi: 10.1016/j.ijpharm.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang J, Wang BM, Schwendeman SP. Mechanistic evaluation of the glucose-induced reduction in initial burst release of octreotide acetate from poly(D,L-lactide-co-glycolide) microspheres. Biomaterials. 2004;25:1919–1927. doi: 10.1016/j.biomaterials.2003.08.019. [DOI] [PubMed] [Google Scholar]
- 28.Wei GB, Pettway GJ, McCauley LK, Ma PX. The release profiles and bioactivity of parathyroid hormone from poly(lactic-co-glycolic acid) microspheres. Biomaterials. 2004;25:345–352. doi: 10.1016/s0142-9612(03)00528-3. [DOI] [PubMed] [Google Scholar]
- 29.Costantino HR, Langer R, Klibanov AM. Aggregation of a lyophilized pharmaceutical protein, recombinant human albumin: Effect of moisture and stabilization by excipients. Bio/Technology. 1995;13:493–496. doi: 10.1038/nbt0595-493. [DOI] [PubMed] [Google Scholar]
- 30.Murty SB, Goodman J, Thanoo BC, DeLuca PP. Identification of chemically modified peptide from poly(d,l-lactide-co-glycolide) microspheres under in vitro release conditions. AAPS Pharm Sci Tech. 2003;4:392–405. doi: 10.1208/pt040450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim TH, Jiang HL, Jere D, Park IK, Cho MH, Nah JW, Choi YJ, Akaike T, Cho CS. Chemical modification of chitosan as a gene carrier in vitro and in vivo. Prog Polym Sci. 2007;32:726–753. [Google Scholar]
- 32.Rinaudo M. Chitin and chitosan: Properties and applications. Prog Polym Sci. 2006;31:603–632. [Google Scholar]
- 33.Kumar MNVR. A review of chitin and chitosan applications. React Funct Polym. 2000;46:1–27. [Google Scholar]
- 34.Kumar MNVR, Muzzarelli RAA, Muzzarelli C, Sashiwa H, Domb AJ. Chitosan chemistry and pharmaceutical perspectives. Chem Rev. 2004;104:6017–6084. doi: 10.1021/cr030441b. [DOI] [PubMed] [Google Scholar]
- 35.Di Martino A, Sittinger M, Risbud MV. Chitosan: A versatile biopolymer for orthopaedic tissue-engineering. Biomaterials. 2005;26:5983–5990. doi: 10.1016/j.biomaterials.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 36.Crini G. Recent developments in polysaccharide-based materials used as adsorbents in wastewater treatment. Prog Polym Sci. 2005;30:38–70. [Google Scholar]
- 37.Zhao L, Xu L, Mitomo H, Yoshii F. Synthesis of pH-sensitive PVP/CM-chitosan hydrogels with improved surface property by irradiation. Carbohydr Polym. 2006;64:473–480. [Google Scholar]
- 38.Muzzarelli RAA. Carboxymethylated Chitins and Chitosans. Carbohydr Polym. 1988;8:1–21. [Google Scholar]
- 39.Tsutsumi A, Sasajima S, Hideshima T, Nishi N, Nishimura SI, Tokura S. Electron-Spin-Resonance Studies of Mn(Ii) Binding to Carboxymethyl and Phosphorylated Chitins in Aqueous-Solutions. Polym J. 1986;18:509–511. [Google Scholar]
- 40.Tokura S, Nishimura S, Nishi N. Studies on Chitin .9. Specific Binding of Calcium-Ions by Carboxymethyl-Chitin. Polym J. 1983;15:597–602. [Google Scholar]
- 41.Chen LY, Du YM. Aggregation behavior of 3,6-O-carboxymethylated chitin in aqueous solutions. J Appl Polym Sci. 2002;86:1838–1843. [Google Scholar]
- 42.Tsutsumi A, Ya D, Hiraoki T, Mochiku H, Yamaguchi R, Takahashi N. Esr Studies of Mn(Ii) Binding to Gellan and Carrageenan Gels. Food Hydrocolloids. 1993;7:427–434. [Google Scholar]
- 43.Wang J, Wang BA, Schwendeman SP. Characterization of the initial burst release of a model peptide from poly(D,L-lactide-co-glycolide) microspheres. J Control Release. 2002;82:289–307. doi: 10.1016/s0168-3659(02)00137-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.